Abstract
Objective
To develop an evidence-based guideline to help clinicians make decisions about when and how to safely taper, stop, or switch antihyperglycemic agents in older adults.
Methods
We focused on the highest level of evidence available and sought input from primary care professionals in guideline development, review, and endorsement processes. Seven clinicians (2 family physicians, 3 pharmacists, 1 nurse practitioner, and 1 endocrinologist) and a methodologist comprised the overall team; members disclosed conflicts of interest. We used a rigorous process, including the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach, for guideline development. We conducted a systematic review to assess evidence for the benefits and harms of deprescribing antihyperglycemic agents. We performed a review of reviews of the harms of continued antihyperglycemic medication use, and narrative syntheses of patient preferences and resource implications. We used these syntheses and GRADE quality-of-evidence ratings to generate recommendations. The team refined guideline content and recommendation wording through consensus and synthesized clinical considerations to address common front-line clinician questions. The draft guideline was distributed to clinicians and stakeholders for review and revisions were made at each stage. A decision-support algorithm was developed to accompany the guideline.
Recommendations
We recommend deprescribing antihyperglycemic medications known to contribute to hypoglycemia in older adults at risk or in situations where antihyperglycemic medications might be causing other adverse effects, and individualizing targets and deprescribing accordingly for those who are frail, have dementia, or have a limited life expectancy.
Conclusion
This guideline provides practical recommendations for making decisions about deprescribing antihyperglycemic agents. Recommendations are meant to assist with, not dictate, decision making in conjunction with patients.
Diabetes affects 20% of Canadians aged 65 to 69 years, increasing to 25% for those aged 75 to 79, and decreasing to 21% for those aged older than 85 years.1 It is helpful to consider age subgroups for context—young-old (> 65 to 74 years), middle-old (75 to 84 years), and old-old (≥ 85 years)2—and to remember that chronologic age is overshadowed by the importance of frailty. Appropriate glucose control reduces the risk of diabetes-related complications in adults and in the young-old.3,4 However, the intensity of glycemic control in older and frail adults has been controversial. Tight control increases the risk of hypoglycemia, with severe consequences such as impaired cognition, falls, fractures, motor vehicle accidents, and seizures.5 Persons with hypoglycemia have higher annual health care costs than those without do,6 placing a substantial economic burden on health care systems.7 As people move into the old-old age group or become frail, the risk-benefit balance context changes. Thus, individualized decisions regarding treatment goals and medications need to be made to minimize the risk of hypoglycemia. Accordingly, clinicians and patients would benefit from guidance in this area.
Deprescribing is the planned and supervised process of dose reduction or stopping of medication that might be causing harm or no longer providing benefit. The goal of deprescribing is to reduce medication burden and harm, while maintaining or improving quality of life. In an effort to provide guidelines and tools to aid clinicians in appropriately reducing or stopping medications, we initiated the Deprescribing Guidelines for the Elderly project. This project aims to rigorously develop, implement, and evaluate evidence-based recommendations and clinical considerations for deprescribing guidelines.
We selected antihyperglycemic agents as an important class for developing a deprescribing guideline to reduce the risks of hypoglycemia and related morbidity, and the burden of pill taking and complicated treatment regimens in older adults. As people age or become frail, their needs change, making safety an increasing priority over potential long-term treatment benefits. Prescribers and patients need to balance the potential for control of hyperglycemia symptoms and prevention of diabetes sequelae with the avoidance of hypoglycemia and its attendant risks of emergency department visits and hospitalization. Decisions about targets and treatment choices need to consider cognitive and physical function, treatment burden, frailty, quality of life, and life expectancy. In this guideline, we briefly outline treatment benefits; readers are encouraged to refer to diabetes treatment guidelines for a more fulsome overview of the benefits of individual medication classes. We provide a review of the harms of antihyperglycemic medications and synthesize literature regarding the effects of deprescribing antihyperglycemic medications. Deprescribing recommendations take into account what is known about patient values and preferences, as well as cost implications. Literature and guideline reviews provide the basis for clinical considerations, including individualized glycemic targets in various populations, deprescribing antihyperglycemic medications, and monitoring effects.
Scope
The target audience for this guideline includes physicians, pharmacists, nurse practitioners, registered nurses, and certified diabetes educators caring for older adults with type 2 diabetes who are receiving antihyperglycemic medications.
The target population includes adults older than 65 years of age who are receiving at least 1 antihyperglycemic medication to treat type 2 diabetes and who are at risk of hypoglycemia (eg, owing to advancing age, tight glycemic control, multiple comorbidities, drug interactions, hypoglycemia history or lack of awareness, impaired renal function, or receiving sulfonylureas or insulin); who are at risk of other antihyperglycemic adverse effects; or for whom benefit is uncertain owing to frailty, dementia, or limited life expectancy. The selection of an age threshold of 65 years is somewhat arbitrary given the lack of correlation between chronologic and biological age. We anticipate that clinicians will interpret the recommendations on an individualized basis for each of their patients.
METHODS
The methods used to develop this deprescribing guideline were designed using the checklist by Schünemann et al for a successful guideline enterprise8 and refined by the Deprescribing Guidelines for the Elderly project.9
The Guideline Development Team (GDT) comprised 7 clinicians (2 family physicians [R.U., S.S.], 3 pharmacists [B.F., C.R.F., L.M.], 1 nurse practitioner [M.B.], and 1 endocrinologist [H.L.]) and a clinical epidemiologist [V.W.]. A ninth GDT member resigned, as this person could not fully endorse the guideline. All clinician members had expertise in type 2 diabetes management in older people. The guideline chair (B.F.) was selected based on previous expertise developing deprescribing guidelines. A library scientist, a master’s student (W.T.), and a staff coordinator (C.B.) supported the GDT. Expertise, role descriptions, and conflicts of interest are available at CFPlus.*
We used the GRADE (Grading of Recommendations Assessment, Development and Evaluation) system for guideline development (Box 1).10,11 The GDT developed the following primary clinical question using the PICO (patient or problem, intervention, comparison, outcome) approach: In adults with type 2 diabetes, what are the effects (benefits and harms) of deprescribing (stopping, reducing dose, gradually tapering, and prescription substitution) antihyperglycemic agents compared with continued use of antihyperglycemic agents? Definitions specific to deprescribing antihyperglycemic medications were articulated by the GDT and are listed in Box 2. We conducted a preliminary literature search to identify studies examining deprescribing interventions that enrolled older adults without limitations on study designs or outcomes. Owing to the limited evidence identified for those older than 65 years of age in this literature review, the population for the systematic review included adults 18 years of age and older to ensure all deprescribing studies were identified. The systematic review search strategy was developed collaboratively with a librarian, and the protocol was registered with PROSPERO.11
Box 1. Notes on the GRADE framework for guideline development.
This guideline was informed by a systematic review and developed in accordance with methods from the GRADE Working Group10:
We focused our review and recommendations on outcomes important to patients, such as harms or benefits resulting from deprescribing an antihyperglycemic agent, quality of life, and pill burden. Outcomes were proposed and agreed upon at the first Guideline Development Team face-to-face meeting
Ratings of the quality in evidence profile tables included high, moderate, low, or very low based on the GRADE certainty criteria and indicate our certainty in estimates of effect. As only controlled before-and-after studies were identified, they received a low-quality rating, but could be rated up or down by limitations or strengths in any of 4 domains: risk of bias, inconsistency, indirectness, and imprecision. Publication bias could not be rated owing to the paucity of studies11
The GRADE Working Group outlines appropriate wording for recommendations depending on the rating of strength and confidence in the evidence, as well as inclusion of other areas to consider when formulating a final rating, including harms, patient values and preferences, and resource use. A strong recommendation with implications for patients (phrased as “we recommend ...”) implies that all patients in the given situation would want the recommended course of action, and only a slight proportion would not. A weak recommendation (phrased as “we suggest ...”) implies that most patients would wish to follow the recommendation, but some patients would not. Clinicians must help patients make management decisions consistent with values and preferences. Implications for clinicians are similar such that a strong recommendation implies all or most patients should receive the intervention. A weak recommendation should prompt a clinician to recognize that different choices will be appropriate for individual patients
GRADE—Grading of Recommendations Assessment, Development and Evaluation.
Box 2. Definitions of antihyperglycemic medication deprescribing.
Deprescribing can include stopping the medication, dose reduction, or prescription substitution:
Stopping can be done either via abrupt discontinuation or a tapering regimen
Dose reduction involves using a lower dose of antihyperglycemic medication compared with baseline, including gradual tapering regimens that require multiple dose reduction steps
Prescription substitution involves discontinuing the antihyperglycemic medication causing harm or placing the patient at higher risk, and replacing the medication with an alternative antihyperglycemic agent with lower associated risk
The systematic review focused on outcomes relevant to patients, caregivers, and health care providers.12 Primary outcomes included rates of hypoglycemic and hyperglycemic events, changes in hemoglobin A1c (HbA1c) level, and the proportion of patients experiencing cardiovascular complications. Secondary outcomes included outcomes associated with hypoglycemia or hyperglycemia (eg, falls, emergency department visits, hospitalizations, seizures), quality of life, patient satisfaction measures, pill burden, and death.
Draft recommendations were formulated by the guideline lead (B.F.) with assistance from the coordinator (C.B.) and master’s student (W.T.). These recommendations were synthesized from systematic review data (effect estimates and certainty of evidence), literature on patient values and preferences, resource implications (costs of continued use of antihyperglycemic agents in older persons and costs of deprescribing), and clinical experience. Recommendations were refined after discussion with the GDT via teleconference and e-mail, and voting on the final wording was conducted. The cut-off for consensus on the final recommendation wording was set at 80% agreement. Eight members of the GDT agreed with the final recommendations.
RECOMMENDATIONS
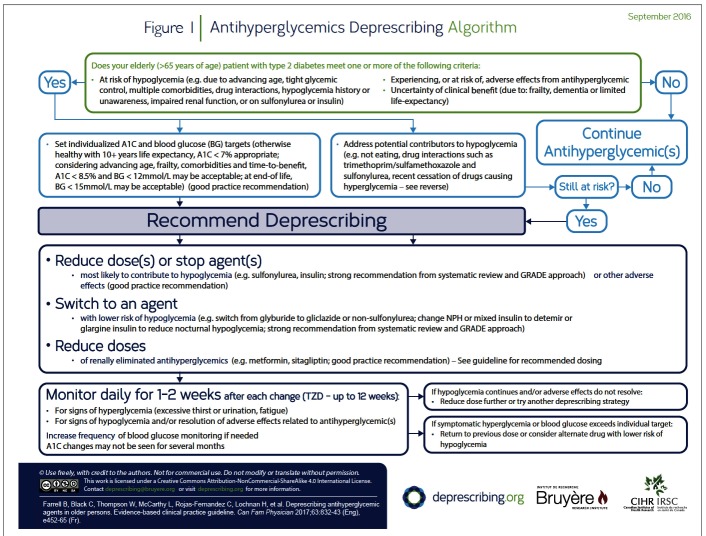
In this section of the guideline, we summarize the evidence reviews (systematic review of deprescribing studies, review of reviews of the harms of antihyperglycemic agents, patient values and preferences, cost and resource implications literature) that support the GRADE-based recommendations. (Additional details of the evidence review and references are available at CFPlus.*) The decision-support algorithm developed for this guideline is provided in Figure 1. The rationale for the recommendations is summarized below and outlined briefly in Table 1.
Figure 1.
Antihyperglycemics Deprescribing Algorithm
Table 1.
Evidence to recommendations table: Does deprescribing (complete cessation of all antihyperglycemic agents, reduction of 50% of insulin dose [in patients with a total insulin dose > 20 units/d], cessation of glyburide, switching from glyburide to another SU) antihyperglycemic medications compared with continuous antihyperglycemic medication use result in benefit or harms in adults > 65 y (community dwelling and in long-term care)?
| DECISION DOMAIN | SUMMARY OF REASON FOR DECISION | SUBDOMAINS INFLUENCING DECISION |
|---|---|---|
| QoE: Is there high-or moderate-quality evidence? Yes ☑ No ☑ |
QoE: very low
|
NA |
| Balance of benefits and harms: Is there certainty that the benefits of deprescribing outweigh the harms? Yes ☑ No □ Is there certainty that the benefits of continued use outweigh the harms? Yes ☑ No ☑ |
Our target population is individuals > 65 y, specifically those at high risk of hypoglycemia (eg, owing to overly intense glycemic control, multiple comorbidities, drug interactions, hypoglycemia history or lack of awareness), those at high risk of other adverse effects (eg, owing to reduced renal function), and those in whom benefit is uncertain owing to frailty, dementia, or limited life expectancy. Our systematic review demonstrated that deprescribing antihyperglycemic agents does not result in clinically concerning increases in blood glucose levels in nursing home patients and community-dwelling elderly patients, although there is no evidence that deprescribing reduces incidence of hypoglycemia
|
Is the baseline risk for benefit of deprescribing similar across subgroups? Yes ☑ No □
Is the baseline risk for harm from deprescribing similar across subgroups? Yes ☑ No □ Should there be separate recommendations for subgroups based on risk levels for harm from deprescribing? Yes ☑ No ☑
Is the baseline risk for benefit of continued use similar across subgroups? Yes ☑ No □ Is the baseline risk for harm from continued use similar across subgroups? Yes ☑ No □
|
| Values and preferences: Is there confidence in the estimate of relative importance of outcomes and patient preferences? Yes ☑ No ☑ (See references 119–122, 124, 127, 129, and 132 in the evidence reviews at CFPlus*) |
Patients view insulin therapy and frequent self-monitoring of blood glucose as burdensome. Older patients with diabetes tend to have goals focused on maintaining independence and social function vs risk control or prevention of complications; therefore, burden of care is more important than strict adherence and intensive therapy. Hypoglycemia can negatively affect QoL and function. Goals and preferences surrounding intensity of treatment appear to be variable, and patient goals, time to benefit, and complexity should be discussed when developing treatment plans. Many patients will prefer less intensive treatment, although some might prefer more aggressive treatment |
Perspective taken: patient perspective—we have taken the view that many older patients see intensive treatment as burdensome and the consequences of intensive treatment such as hypoglycemia negatively affect QoL. Older persons tend to value independence, function, and QoL over risk reduction and reduced mortality Source of values and preferences: nonsystematic literature review Source of variability, if any: cannot estimate Method for determining values satisfactory for this recommendation? Yes ☑ No □ All critical outcomes measured? Yes ☑ No □
|
| Resource implications: Are the resources worth the expected net benefit? Yes ☑ No □ (See references 136 and 137 in the evidence reviews at CFPlus*) |
Patients experiencing hypoglycemia show increased annual diabetes-related (and overall) health costs vs those without hypoglycemia (MD = $5024, P < .0001). Decision tree analysis suggests insulin and SUs might not be cost effective in older persons owing to the risk of hypoglycemia and associated events |
Feasibility: is this intervention generally available? Yes ☑ No □
Opportunity cost: is this intervention and its effects worth withdrawing or not allocating resources from other interventions? Yes ☑ No □
|
| Strength of main recommendation: strong | Our systematic review showed that deprescribing did not produce a clinically concerning increase in blood glucose levels (as shown by HbA1c outcomes), although the QoE was very low. Evidence suggests no benefit associated with intensive blood glucose control in the elderly and potential for harm (increased risk of hypoglycemia). Older persons (specifically, frail elderly) are at higher risk of hypoglycemia. Survey data and interviews suggest hypoglycemia is associated with lower QoL in older persons. The elderly tend to value lower treatment burden, independence, and function above risk reduction from intensive therapy. Patients with hypoglycemia produce increased diabetes-related health care costs vs those without hypoglycemia, and insulin or SUs might not be cost effective in older persons owing to hypoglycemia | |
HbA1c—hemoglobin A1c, MD—mean difference, MI—myocardial infarction, NA—not applicable, QoE—quality of evidence, QoL—quality of life, RR—relative risk, SU—sulfonylurea.
Recommendations (Box 3) apply to people older than 65 years of age receiving at least 1 antihyperglycemic medication to treat type 2 diabetes and who are at risk of hypoglycemia (eg, owing to advancing age, overly intense glycemic control, multiple comorbidities, drug interactions, hypoglycemia history or lack of awareness, impaired renal function, or taking a sulfonylurea or insulin); who are at risk of other antihyperglycemic adverse effects; or in whom benefit is uncertain owing to frailty, dementia, or limited life expectancy. These recommendations do not apply to people who do not have type 2 diabetes.
Box 3. Recommendations.
These recommendations apply to all elderly (> 65 y) adults taking ≥ 1 antihyperglycemic medications to treat type 2 diabetes and meeting ≥ 1 of the following criteria:
at risk of hypoglycemia (eg, owing to advancing age, overly intense glycemic control, multiple comorbidities, drug interactions, hypoglycemia history or lack of awareness, impaired renal function, or taking a sulfonylurea or insulin);
at risk of other antihyperglycemic adverse effects; or
in whom benefit is uncertain owing to frailty, dementia, or limited life expectancy
We recommend the following:
Deprescribing antihyperglycemic agents that are known to contribute to hypoglycemia (strong recommendation, very low-quality evidence)
Deprescribing antihyperglycemic agents in patients experiencing or at risk of adverse effects (good practice recommendation)
Individualizing glycemic targets to goals of care and time to benefit according to the Canadian Diabetes Association guidelines and other guidelines that specifically address frailty, dementia, and the end of life (good practice recommendation), and deprescribing accordingly (strong recommendation, very low-quality evidence)
Our systematic review identified 2 controlled before-and-after deprescribing studies.12 One study found that an educational intervention delivered to pharmacists decreased glyburide use (by stopping it or switching it to a safer agent) without compromising glycemic control. The other study demonstrated that cessation of antihyperglycemic agents in older nursing home residents did not result in clinically meaningful HbA1c level increases and that monitoring was beneficial to identify hyperglycemia in a subset of residents. Hypoglycemia risk was not reduced in either study. Both studies suggest deprescribing antihyperglycemic agents is feasible and likely safe, but the quality of evidence was very low and the studies had limitations. (A summary of systematic review findings is available at CFPlus.*) Box 1 contains definitions of the strength and quality of deprescribing trial evidence and Table 1 contains evidence regarding recommendation considerations across all decision domains (quality of evidence, balance of benefits and harms, patient values and preferences, and resource implications).
Given the recent relaxation of targets for those with dementia, frailty, or limited life expectancy in most clinical guidelines, the risks and costs of hypoglycemia and other adverse effects, treatment burden, and lack of evidence of harm from deprescribing, we rated the recommendation to deprescribe antihyperglycemic medications in individualized situations for patients meeting these criteria as strong.
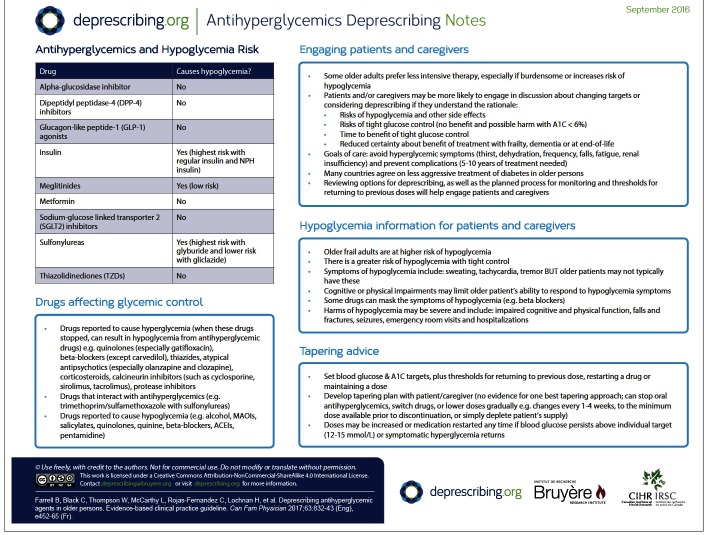
Consideration of harm includes hypoglycemia and other side effects experienced with each class of antihyperglycemic medications. In older adults, hypoglycemia might manifest as dizziness, weakness, delirium, or confusion rather than diaphoresis, tremor, and palpitations, as typically seen in younger individuals. Older adults, particularly frail adults, are at higher risk of hypoglycemia and its consequences (eg, impaired cognitive and physical function, falls, fractures, motor vehicle accidents, seizures, emergency department visits, hospitalization, mortality). Insulin and long-acting sulfonylureas are most commonly associated with hypoglycemia. (Ranges of frequency ratios of harms are available at CFPlus.*)
Considering patient values and preferences, older people tend to value lower treatment burden, independence, and function over the potential risk reduction gained from intensive therapy. Survey and interview data suggest hypoglycemia is associated with a lower quality of life in older persons. Heterogeneity in patient preferences suggests treatment decisions must be individualized. (The evidence reviews and related references are available at CFPlus.*)
Diabetes medication expenditure for older people in Canada was approximately $670 million in 2008. The economic burden of hypoglycemia, particularly with sulfonylureas and insulin, is high, although difficult to quantify; those with hypoglycemia have been shown to have substantially higher health care costs. (The evidence reviews and related references are available at CFPlus.*)
Clinical considerations
This guideline is a tool to be used together with consideration of a patient’s personal and medical context and care goals. As patients might be less accustomed to dialogue about changing diabetes targets or reducing or stopping their diabetes medications, heightened health care provider awareness of potential concerns might help foster improved patient uptake and outcomes. The following questions were articulated by the GDT as important to consider when making decisions about the steps for deprescribing antihyperglycemic agents.
How should benefits and harms be weighed in determining need for continued use of antihyperglycemic medications?
Antihyperglycemic medications are prescribed with an assumption of potential benefit, and patients might have been taking them for many years or have had them recently added when the question of deprescribing arises.
Deprescribing decisions are made as a result of a shift toward 1 or more medications potentially causing more harm than benefit, or providing no benefit. Such a shift in balance might occur in any older patient; those at risk of falls, hypoglycemia, or other adverse effects; those with cognitive impairment or dementia; the vulnerable (ie, pre-frail) or frail elderly; and those with a limited lifespan (eg, < 5 years) or who are terminally ill.
To assess benefit, we suggest that clinicians consider the time frame for avoidance of complications (eg, retinopathy, nephropathy, cardiovascular morbidity and mortality) and symptomatic hyperglycemia control (eg, avoidance of polydipsia, polyuria, dehydration, fatigue, falls, renal insufficiency). The immediate concern is to balance medication safety with avoidance of osmotic diuresis. With regard to time to benefit for avoiding diabetic complications, 5 to 10 years of treatment has been shown to reduce risk or progression of microvascular disease and nonfatal myocardial infarction.3 A reduction in the composite end point of cardiovascular mortality, nonfatal myocardial infarction and stroke, and heart failure hospitalization was shown after 3 years in patients with established cardiovascular disease.13 Clinicians could take into account the benefit the patient is likely to continue to accrue depending on the number of years invested in treatment. Avoiding symptomatic hyperglycemia is an important benefit for all older patients who are otherwise healthy, frail, or have a limited lifespan. Hyperglycemic symptoms can generally be avoided if fasting or preprandial blood glucose levels are maintained below 12 mmol/L. Further, there is no evidence to suggest that asymptomatic hyperglycemia is harmful in frail patients or in those with a limited lifespan.14–18
To assess harm, we suggest that clinicians carefully consider potential adverse effects and the risks associated with hypoglycemia, which are compounded by frailty and cognitive impairment or dementia. Additionally, the burden of treatment and its effect on quality of life and adherence must also be assessed and considered.
Considering factors affecting benefit and harm and aligning the patient’s goals with treatment intensity is essential and will guide potential deprescribing. Once it has been determined that the potential for harm from antihyperglycemic agents outweighs the potential for benefit, consideration might be given to deprescribing in a manner that is consistent with the patient’s values and care goals. While this can be difficult in a highly morbid population, frameworks such as the Minimally Disruptive Medicine care model have been proposed to address complex patients’ overall health goals. In these models, the focus is placed on achieving the patient’s goals for life and health, with the smallest treatment burden possible.19
How should the benefits and harms be weighed in the frail elderly?
Frail older adults, those with dementia, and those at the end of life deserve special mention given their precarious benefit-risk balance. Specifically, Mallery et al developed guidelines for severely frail individuals (ie, those with Clinical Frailty Scale [CFS] scores > 7 and who require assistance performing basic activities of daily living, such as bathing or dressing).18 The guidelines highlight 4 important considerations: current diabetes studies have not included frail individuals; the time to benefit is not relevant for those who are frail; microvascular outcomes are inconsequential with frailty; and achieving tight glycemic control is of uncertain benefit. To illustrate, consider that the 4-year survival probability is approximately 45% for individuals with CFS scores of 6 to 7 (ie, moderately to severely frail).20 Further, a median survival time of 23 months was noted in frail older patients with diabetes, while a mean life expectancy of 3.4 years was noted in non-frail people.21,22 In the context of frailty or limited lifespan, diabetes is well suited for deprescribing.
What are appropriate HbA1c and blood glucose targets for those who are frail, have cognitive impairment or dementia, or have a limited life expectancy?
The Canadian Diabetes Association guidelines adopted an individualized approach to targeting HbA1c level in older people.23,24 (Targets from various diabetes guidelines are available at CFPlus.*) Opinions differ among national and international guidelines with regard to appropriate glycemic targets, even in frail individuals, those with dementia, or those at the end of life. (Glycemic targets by patient frailty status are available at CFPlus.*) To avoid symptoms, blood glucose targets of 5 to 12 mmol/L (fasting and preprandial) are considered acceptable, corresponding to an HbA1c level below 8.5%.24–30 Some guidelines recommend lower limits for HbA1c levels. In end-of-life care, blood glucose levels between 9 and 15 mmol/L are considered appropriate.25 Guideline development team members agreed that for some patients, targeting blood glucose control primarily to minimize risk of dehydration and impaired wound healing is important but that such decisions need to be made on an individual basis, taking the patient’s full medical context into account. Additional information about HbA1c and blood glucose targets is described in the section summarizing other guidelines. Tools that help estimate life expectancy are available, but do not provide exact time-to-death values, and to our knowledge have not been used to guide diabetes treatment.
How should patients and families be engaged?
When patients and families understand the rationale for deprescribing, expected benefits, and the planned deprescribing process, they are more likely to feel confident about deprescribing.31 Clinicians should therefore consider discussing the following with patients and families: the benefit of tight blood glucose control might take several years to manifest; the risk of severe hypoglycemia increases with age and frailty; hypoglycemia might lead to adverse effects such as falls and fractures; many countries around the world agree on less aggressive treatment of diabetes in some older persons (eg, frail persons); deprescribing involves gradually reducing doses, discontinuing medications, or switching to safer alternatives; and patients and families are involved in choosing goals and how to taper and monitor the effects of changes (such as symptoms to watch for and follow-up frequency).
What deprescribing should happen?
Deprescribing can involve dose reduction, switching to a safer medication, or stopping a medication altogether.
If a patient is experiencing or is at high risk of hypoglycemia, 4 approaches must be considered:
Is the patient taking medications known to cause hypoglycemia? Reduce or stop medications that are known to cause hypoglycemia (eg, insulin, sulfonylureas, and less commonly, meglitinides). (A list of antihyperglycemic agents and their effects is available at CFPlus.*) With regard to insulin, reducing the dose (particularly of prandial insulin) might minimize hypoglycemia risk, or using insulin detemir or glargine instead of isophane or mixed insulin might reduce risk of nocturnal hypoglycemia.32–35 Within the sulfonylurea class, switching glyburide to short-or long-acting gliclazide might reduce, but not eliminate, the risk of hypoglycemia36–38; options other than a sulfonylurea should be considered.
Could a drug interaction be increasing the hypoglycemic effect of antihyperglycemic medications? If so, consider reducing the dose or stopping the offending interacting drug, or reducing the dose of antihyperglycemic medications. (A list of drug interactions and a list of drugs that might be associated with hypoglycemia are available at CFPlus.*)
Has a medication that causes hyperglycemia recently been stopped? If so, doses of antihyperglycemic agents might need to be reduced. (A list of drugs that might be associated with hyperglycemia is available at CFPlus.*)
Is renal impairment a consideration? As the clearance of certain medications and metabolism of insulin is impaired with renal impairment, doses of such medications should be reduced as appropriate.39
If a patient has or develops signs or symptoms related to antihyperglycemic medications (eg, diarrhea with metformin, heart failure worsening with thiazolidinediones), the dose could be lowered or stopped, and if needed, the medication could be replaced.
How should tapering be approached?
A deprescribing plan should be developed with the patient and family. The plan might include goal blood glucose and HbA1c levels, and thresholds for returning to a previous dose or restarting a drug, or maintaining a dose or drug. Our systematic review did not identify trials that provided optimal tapering approaches. Antihyperglycemic medications might be discontinued without dose reduction40 if the HbA1c level is below target or the risk of hypoglycemia is high. If clinicians or patients feel more comfortable reducing doses gradually, then a tapering approach might be used, with changes every 1 to 4 weeks to the minimum dose available before final discontinuation. A plan that is often convenient is for the patient to gradually deplete the current medication supply. Medication doses might be increased or medication restarted at any time if blood glucose levels persist above 12 to 15 mmol/L or if hyperglycemia is symptomatic.
What monitoring is necessary and how frequently should it be done?
Effects of medication changes on blood glucose levels are typically seen within a few days of dose changes of insulin and most antihyperglycemic agents (although effects of changes in thiazolidinediones might take up to 12 weeks). Blood glucose might be monitored more frequently for the first 1 to 2 weeks following dose reduction or stopping. The frequency of monitoring will depend on patient-specific factors such as concomitant medications and hypoglycemia or hyperglycemia risk (eg, patients using insulin might require more frequent testing than those not using insulin).
Patients and families should be educated regarding signs and symptoms of hyperglycemia and asked to report them if they occur.
Changes in HbA1c level might not be seen for several months. Hemoglobin A1c measurements might be misleading in conditions such as iron, vitamin B12, and folate deficiency anemias,41 and might be falsely low in patients with renal failure.
Once a patient’s blood glucose level is stable and hypoglycemia is no longer a risk or is substantially diminished, the frequency of blood glucose testing can be reduced or stopped in accordance with the Canadian Agency for Drugs and Technology in Health recommendations, which recommend that regular blood glucose testing is not routinely required with oral antihyperglycemic agents except in circumstances of dose changes or concurrent illness.42
What should be done if hyperglycemia occurs?
If hyperglycemic symptoms develop after medications are tapered or discontinued, a medication with the lowest risk of hypoglycemia (eg, metformin) might be restarted at the lowest possible dose. If glucose levels above the individualized target persist, but do not result in symptoms, consideration should be given to re-evaluating goals and targets with the patient and family to guide further dosage decisions. For example, if the patient is frail and asymptomatic, it has been suggested that plasma glucose levels between 9 and 15 mmol/L are acceptable.25
When should an endocrinologist be consulted?
Consider consulting an endocrinologist when targets cannot be achieved (the patient continues to have hypoglycemia or hyperglycemia despite medication or dose changes), when patients are receiving dialysis, or when changing doses of glucocorticoids.
When should a diabetes educator be engaged?
Consider referral to a diabetes educator for patients taking insulin who require instruction about avoiding, recognizing, and treating hypoglycemia, or for patients who might require closer supervision during deprescribing than can be carried out by the prescriber.
Clinical and stakeholder review
External clinical review of the guideline was conducted by 3 pharmacists, a geriatrician, a family physician, and an endocrinologist using the AGREE II (Appraisal of Guidelines for Research and Evaluation II) Global Rating Scale tool.43 Relevant stakeholder organizations (ie, for diabetes care providers, family physicians, pharmacists, and nurse practitioners) were invited to review and endorse the guidelines. Modifications were made to the original guideline draft to address reviewer comments. This evidence-based clinical practice guideline for deprescribing antihyperglycemic agents has been endorsed by the Canadian Nurses Association and the College of Family Physicians of Canada.
How this deprescribing guideline relates to other clinical practice guidelines for antihyperglycemic agents
Various international diabetes management guidelines advocate individualized determination of glycemic targets and provide recommendations for the general geriatric population and those with dementia, limited life expectancy, or various degrees of frailty.18,23–25,29,44,45 Guidelines generally advise avoiding hypoglycemia altogether and avoiding an HbA1c level below 6.5%,18,45 and stress the importance of assessing functional status and frailty to determine individualized targets. Other than considering ages older than 60 years or 65 years, treatment targets are not aligned with chronologic age.
Diabetes management guidelines generally consider blood glucose targets of 5 to 12 mmol/L (fasting and preprandial) as acceptable in moderately to severely frail people.24–30 However, the guidelines differ in how frailty is defined, and on the range of glucose and HbA1c targets. Such variation makes it challenging to clearly and consistently identify appropriate glucose and HbA1c targets in these populations. In contrast to most other guidelines that recommend less stringent targets for the frail in the range of HbA1c levels below 8% or below 8.5%, the Diabetes Care Program of Nova Scotia recommends avoiding stringent targets altogether and discontinuing drugs that result in blood glucose levels less than 7.0 mmol/L or HbA1c levels below 8% in the severely frail.18 Blood glucose targets below 20 mmol/L (as long as the patient is asymptomatic) and HbA1c levels below 12% are advocated in severely frail individuals.
A position statement from the American Diabetes Association comprehensively outlines approaches to managing diabetes in long-term care, including the importance of avoiding hypoglycemia; it reiterates an HbA1c target below 8.5% as appropriate for frail individuals and suggests that there is no role for HbA1c measurement in patients at the end of life.46
Collectively, these management guidelines provide guidance for less stringent, individualized targets and appropriate medication prescribing for older people. However, none addresses evidence for the central issue of deprescribing antihyperglycemic agents as a method of lowering the risk of hypoglycemia in older people, and no evidence-based guidelines for antihyperglycemic deprescribing were identified in our review of the literature.
An antihyperglycemic deprescribing guideline works in conjunction with current management guidelines because it offers clinicians recommendations and clinical considerations to help them deprescribe antihyperglycemic medications when potential harm might outweigh continued benefit in older people.
Gaps in knowledge
Further research is necessary to assess the potential benefit of antihyperglycemic treatment in older people, those with dementia, those who are frail, and those at the end of life. In particular, our knowledge of acutely or chronically high glycemic levels that could cause harm, and over what period of time, in these populations is limited. The short-and medium-term consequences of high blood glucose levels on outcomes important to patients (eg, cognition, infections) are largely unknown and warrant further investigation to help identify evidence-informed treatment targets.47 Investigations of treatment burden and quality of life for patients and caregivers are important, as are studies in different settings such as assisted-living and long-term care facilities. Studies with clear and appropriate frailty assessments would be helpful, particularly as there is no guidance for those with CFS scores between 4 and 5.
Little deprescribing research for antihyperglycemic agents has been completed. Only 2 controlled before-and-after studies were eligible for our systematic review and neither study addressed outcomes important to patients, such as falls, emergency department visits, hospitalizations, pill burden, quality of life, and patient satisfaction. Appropriately designed studies are needed that evaluate deprescribing in relevant populations (eg, those with different degrees of frailty, those in long-term care), measure outcomes important to patients, and evaluate patient preferences and values with regard to diabetes treatment and deprescribing. The optimal approach to deprescribing antihyperglycemic agents has not been evaluated, and details regarding monitoring and follow-up have not been hitherto well described. Deprescribing trials must be powered to detect clinically important differences on reducing hypoglycemia events and effects.
Pharmacoeconomic studies to illustrate the effect of various approaches to diabetes treatment and for management of adverse effects in these populations are needed to further guide recommendations.
Finally, research is necessary to investigate optimal methods of delivering this proposed intervention, and a dialogue needs to be opened between clinicians and policy makers to educate them on how and why treatment paradigms are changing in this aging population, especially in the regulated environment of long-term care settings.
Next steps
The GDT will provide routine guideline updates as new evidence emerges that might change recommendations. Prospective evaluation of the effect of adoption of this and other deprescribing guidelines will be part of our research strategy.
Conclusion
While treatment of diabetes has long-term benefit for younger adults and the young-old, older people at risk of hypoglycemia or falls, or those with frailty, dementia, or limited life expectancy might experience more harm than benefit. A systematic review identified 2 studies that demonstrated antihyperglycemic agents (ie, glyburide and insulin) might be safely deprescribed (eg, stopped or substituted with a different sulfonylurea) in these populations. This guideline is aimed at helping clinicians make decisions with older patients and their caregivers about individualizing glycemic targets and determining when and how to deprescribe antihyperglycemic medications to minimize the potential for harm.
Acknowledgments
Funding was provided by a Canadian Institutes of Health Research Partnerships for Health System Improvement grant and the Chair of the Michel Saucier Endowed Fund in Geriatric Pharmacology, Health and Aging of the Faculty of Pharmacy at the University of Montreal in Quebec.
EDITOR’S KEY POINTS
No evidence demonstrates benefits of tight glycemic control for older adults who are frail, have dementia, or have a limited life expectancy. Individualizing therapy, including glycemic targets, to goals of care and time to benefit is good practice.
A systematic review suggests that deprescribing antihyperglycemic agents is feasible and safe in those with a low hemoglobin A1c level or those taking glyburide, with monitoring and response to blood glucose levels that risk rising above target, although the quality of evidence was very low.
Deprescribing antihyperglycemic agents might involve lowering doses, switching to a safer medication, or stopping medications. Antihyperglycemic medications known to contribute to hypoglycemia should be deprescribed in older adults at risk, or in situations where antihyperglycemic medications might be causing other adverse effects.
Future research should address the level of chronic hyperglycemia that causes harm in this population; treatment effectiveness; optimal glycemic targets; patient preferences regarding glycemic control and treatment burden; consistent approaches to measuring patient-relevant outcomes; optimal deprescribing regimens; short-and long-term benefits and harms of continuing versus deprescribing antihyperglycemic medications; and cost effects.
Footnotes
This article is eligible for Mainpro+ certified Self-Learning credits. To earn credits, go to www.cfp.ca and click on the Mainpro+ link.
This article has been peer reviewed.
La traduction en français de cet article se trouve à www.cfp.ca dans la table des matières du numéro de novembre 2017 à la page e452.
Descriptions of contributors’ expertise, roles, and conflicts of interest; evidence reviews and related references; summary of findings tables; ranges of frequency ratios of harms; targets from various diabetes guidelines; glycemic targets for different patient frailty statuses; antihyperglycemic agents and their effects; drug interactions that might lead to hypoglycemia; drugs that might be associated with hypoglycemia or hyperglycemia; and an easy-to-print version of the algorithm are available at www.cfp.ca. Go to the full text of the article online and click on the CFPlus tab.
Contributors
All authors made substantial contributions to the conception and design of the guideline; the acquisition, analysis, and interpretation of data; and drafting the article, revising it critically for important intellectual content, and approving the final version.
Competing interests
Dr Farrell received research funding for the purposes of developing this guideline; received financial payments from the Institute for Healthcare Improvement and the Commonwealth Fund for the deprescribing guidelines summary; and received payment from the Ontario Pharmacists Association for speaking engagements. Dr McCarthy is a former member of the Diabetes Educator Section of Diabetes Canada. Dr Lochnan is a member of Diabetes Canada and has received funding and participated in multicentre diabetes clinical trials with sponsorship from pharmaceutical companies that produce agents for management of diabetes. None of the other authors has any competing interests to declare.
References
- 1.Public Health Agency of Canada . Diabetes in Canada. Facts and figures from a public health perspective. Ottawa, ON: Public Health Agency of Canada; 2011. [Google Scholar]
- 2.Pkrl JJ. The demographics of aging.... Transgenerational Design Matters. 2011. Available from: http://transgenerational.org/aging/demographics.htm. Accessed 2017 Jul 5.
- 3.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–53. Erratum in: Lancet 1999;354(9178):602. [PubMed] [Google Scholar]
- 4.ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72. doi: 10.1056/NEJMoa0802987. Epub 2008 Jun 6. [DOI] [PubMed] [Google Scholar]
- 5.Abdelhafiz AH, Rodríguez-Mañas L, Morley JE, Sinclair AJ. Hypoglycemia in older people—a less well recognized risk factor for frailty. Aging Dis. 2015;6(2):156–67. doi: 10.14336/AD.2014.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bron M, Marynchenko M, Yang H, Yu AP, Wu EQ. Hypoglycemia, treatment discontinuation, and costs in patients with type 2 diabetes mellitus on oral antidiabetic drugs. Postgrad Med. 2012;124(1):124–32. doi: 10.3810/pgm.2012.01.2525. [DOI] [PubMed] [Google Scholar]
- 7.Veronese G, Marchesini G, Forlani G, Saragoni S, Degli Esposti L, Centis E, et al. Costs associated with emergency care and hospitalization for severe hypoglycemia. Nutr Metab Cardiovasc Dis. 2016;26(4):345–51. doi: 10.1016/j.numecd.2016.01.007. Epub 2016 Jan 18. [DOI] [PubMed] [Google Scholar]
- 8.Schünemann HJ, Wiercioch W, Etxeandia I, Falavigna M, Santesso N, Mustafa R, et al. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. CMAJ. 2014;186(3):E123–42. doi: 10.1503/cmaj.131237. Epub 2013 Dec 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrell B, Pottie K, Rojas-Fernandez CH, Bjerre LM, Thompson W, Welch V. Methodology for developing deprescribing guidelines: using evidence and GRADE to guide recommendations for deprescribing. PLoS One. 2016;11(8):e0161248. doi: 10.1371/journal.pone.0161248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. doi: 10.1016/j.jclinepi.2010.04.026. Epub 2010 Dec 31. [DOI] [PubMed] [Google Scholar]
- 11.Black C, Thompson W, Lochnan H, McCarthy L, Rojas-Fernandez C, Shamji S, et al. Benefits and harms of deprescribing versus continuing antihyperglycemics for treatment of type 2 diabetes mellitus: a systematic review protocol. PROSPERO. 2015. CRD4201502. Available from: www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015025727. Accessed 2017 Sep 13.
- 12.Black CD, Thompson W, Welch V, McCarthy L, Rojas-Fernandez C, Lochnan H, et al. Lack of evidence to guide deprescribing of antihyperglycemics: a systematic review. Diabetes Ther. 2017;8(1):23–31. doi: 10.1007/s13300-016-0220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. doi: 10.1056/NEJMoa1504720. Epub 2015 Sep 17. [DOI] [PubMed] [Google Scholar]
- 14.Brown AF, Mangione CM, Saliba D, Sarkisian CA, California Healthcare Foundation/ American Geriatrics Society Panel on Improving Care for Elders with Diabetes Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003;51(5 Suppl Guidelines):S256–80. doi: 10.1046/j.1532-5415.51.5s.1.x. [DOI] [PubMed] [Google Scholar]
- 15.Araki A, Ito H. Diabetes mellitus and geriatric syndromes. Geriatr Gerontol Int. 2009;9(2):105–14. doi: 10.1111/j.1447-0594.2008.00495.x. [DOI] [PubMed] [Google Scholar]
- 16.Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care. 2011;34(6):1329–36. doi: 10.2337/dc10-2377. Epub 2011 Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox DJ, Kovatchev BP, Gonder-Frederick LA, Summers KH, McCall A, Grimm KJ, et al. Relationships between hyperglycemia and cognitive performance among adults with type 1 and type 2 diabetes. Diabetes Care. 2005;28(1):71–7. doi: 10.2337/diacare.28.1.71. [DOI] [PubMed] [Google Scholar]
- 18.Mallery LH, Ransom T, Steeves B, Cook B, Dunbar P, Moorhouse P. Evidence-informed guidelines for treating frail older adults with type 2 diabetes: from the Diabetes Care Program of Nova Scotia (DCPNS) and the Palliative and Therapeutic Harmonization (PATH) Program. J Am Med Dir Assoc. 2013;14(11):801–8. doi: 10.1016/j.jamda.2013.08.002. Epub 2013 Sep 24. [DOI] [PubMed] [Google Scholar]
- 19.Leppin AL, Montori VM, Gionfriddo MR. Minimally disruptive medicine: a pragmatically comprehensive model for delivering care to patients with multiple chronic conditions. Health Care (Basel) 2015;3(1):50–63. doi: 10.3390/healthcare3010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubbard RE, Andrew MK, Fallah N, Rockwood K. Comparison of the prognostic importance of diagnosed diabetes, co-morbidity and frailty in older people. Diabet Med. 2010;27(5):603–6. doi: 10.1111/j.1464-5491.2010.02977.x. [DOI] [PubMed] [Google Scholar]
- 22.Hamada S, Gulliford MC. Antidiabetic and cardiovascular drug utilisation in patients diagnosed with type 2 diabetes mellitus over the age of 80 years: a population-based cohort study. Age Ageing. 2015;44(4):566–73. doi: 10.1093/ageing/afv065. Epub 2015 May 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Imran SA, Rabasa-Lhoret R, Ross S. Targets for glycemic control. Can J Diabetes. 2013;37(Suppl 1):S31–4. doi: 10.1016/j.jcjd.2013.01.016. Epub 2013 Mar 26. [DOI] [PubMed] [Google Scholar]
- 24.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Meneilly GS, Knip A, Tessier D. Diabetes in the elderly. Can J Diabetes. 2013;37(Suppl 1):S184–90. doi: 10.1016/j.jcjd.2013.01.045. Epub 2013 Mar 26. [DOI] [PubMed] [Google Scholar]
- 25.Sinclair A, Dunning T, Colagiuri S. Managing older people with type 2 diabetes. Global guideline. Brussels, Belgium: International Diabetes Federation; 2013. [Google Scholar]
- 26.Canadian Geriatrics Society . Geriatrics. Five things physicians and patients should question. Toronto, ON: Choosing Wisely Canada; 2014. Available from: https://choosingwiselycanada.org/geriatrics. Accessed 2017 Sep 13. [Google Scholar]
- 27.McMillan J, Holroyd-Leduc JM. Management of diabetes among frail older adults. Can Geriatr Soc J CME. 2014;4(2):8–13. [Google Scholar]
- 28.Turner D. HgA1c targets and use of agents for diabetes in older adults. Edmonton, AB: College of Physicians and Surgeons of Alberta; 2015. [Google Scholar]
- 29.American Diabetes Association Older adults. Diabetes Care. 2015;38(Suppl):S67–9. doi: 10.2337/dc15-S013. [DOI] [PubMed] [Google Scholar]
- 30.Lee SJ, Boscardin WJ, Stijacic Cenzer I, Huang ES, Rice-Trumble K, Eng C. The risks and benefits of implementing glycemic control guidelines in frail older adults with diabetes mellitus. J Am Geriatr Soc. 2011;59(4):666–72. doi: 10.1111/j.1532-5415.2011.03362.x. Epub 2011 Apr 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reeve E, To J, Hendrix I, Shakib S, Roberts MS, Wiese MD. Patient barriers to and enablers of deprescribing: a systematic review. Drugs Aging. 2013;30(10):793–807. doi: 10.1007/s40266-013-0106-8. [DOI] [PubMed] [Google Scholar]
- 32.Coscelli C, Calabrese G, Fedele D, Pisu E, Calderini C, Bistoni S, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care. 1992;15(11):1628–30. doi: 10.2337/diacare.15.11.1628. [DOI] [PubMed] [Google Scholar]
- 33.Janka HU, Plewe G, Busch K. Combination of oral antidiabetic agents with basal insulin versus premixed insulin alone in randomized elderly patients with type 2 diabetes mellitus. J Am Geriatr Soc. 2007;55(2):182–8. doi: 10.1111/j.1532-5415.2007.01043.x. [DOI] [PubMed] [Google Scholar]
- 34.Garber AJ, Clauson P, Pedersen CB, Kølendorf K. Lower risk of hypoglycemia with insulin detemir than with neutral protamine Hagedorn insulin in older persons with type 2 diabetes: a pooled analysis of phase III trials. J Am Geriatr Soc. 2007;55(11):1735–40. doi: 10.1111/j.1532-5415.2007.01414.x. [DOI] [PubMed] [Google Scholar]
- 35.Rys P, Wojciechowski P, Rogoz-Sitek A, Niesyczyński G, Lis J, Syta A, et al. Systematic review and meta-analysis of randomized clinical trials comparing efficacy and safety outcomes of insulin glargine with NPH insulin, premixed insulin preparations or with insulin detemir in type 2 diabetes mellitus. Acta Diabetol. 2015;52(4):649–62. doi: 10.1007/s00592-014-0698-4. Epub 2015 Jan 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30(2):389–94. doi: 10.2337/dc06-1789. [DOI] [PubMed] [Google Scholar]
- 37.Skoff RA, Waterbury NV, Shaw RF, Egge JA, Cantrell M. Glycemic control and hypoglycemia in Veterans Health Administration patients converted from glyburide to glipizide. J Manag Care Pharm. 2011;17(9):664–71. doi: 10.18553/jmcp.2011.17.9.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clemens KK, McArthur E, Dixon SN, Fleet JL, Hramiak I, Garg AX. The hypoglycemic risk of glyburide (glibenclamide) compared with modified-release gliclazide. Can J Diabetes. 2015;39(Suppl 4):32–40. doi: 10.1016/j.jcjd.2015.09.087. [DOI] [PubMed] [Google Scholar]
- 39.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. McFarlane P, Gilbert RE, MacCallum L, Senior P. Chronic kidney disease in diabetes. Can J Diabetes. 2013;37(Suppl 1):S129–36. doi: 10.1016/j.jcjd.2013.01.037. Epub 2013 Mar 26. [DOI] [PubMed] [Google Scholar]
- 40.Sjöblom P, Tengblad A, Löfgren UB, Lannering C, Anderberg N, Rosenqvist U, et al. Can diabetes medication be reduced in elderly patients? An observational study of diabetes drug withdrawal in nursing home patients with tight glycaemic control. Diabetes Res Clin Pract. 2008;82(2):197–202. doi: 10.1016/j.diabres.2008.08.014. Epub 2008 Sep 26. [DOI] [PubMed] [Google Scholar]
- 41.Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388–94. doi: 10.1007/s11606-013-2595-x. Epub 2013 Sep 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canadian Optimal Medication Prescribing and Utilization Service . Optimal therapy recommendations for the prescribing and use of blood glucose test strips. Ottawa, ON: Canadian Agency for Drugs and Technology in Health; 2009. [Google Scholar]
- 43.Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839–42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sinclair A, Morley JE, Rodríguez-Mañas L, Paolisso G, Bayer T, Zeyfang A, et al. Diabetes mellitus in older people: position statement on behalf of the International Association of Gerontology and Geriatrics (IAGG), the European Diabetes Working Party for Older People (EDWPOP), and the International Task Force of Experts in Diabetes. J Am Med Dir Assoc. 2012;13(6):497–502. doi: 10.1016/j.jamda.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 45.American Geriatrics Society Expert Panel on the Care of Older Adults with Diabetes Mellitus. Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society guidelines for improving the care of older adults with diabetes mellitus: 2013 update. J Am Geriatr Soc. 2013;61(11):2020–6. doi: 10.1111/jgs.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munshi MN, Florez H, Huang ES, Kalyani RR, Mupanomunda M, Pandya N, et al. Management of diabetes in long-term care and skilled nursing facilities: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(2):308–18. doi: 10.2337/dc15-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pearson-Stuttard J, Blundell S, Harris T, Cook DG, Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 2016;4(2):148–58. doi: 10.1016/S2213-8587(15)00379-4. Epub 2015 Dec 3. [DOI] [PubMed] [Google Scholar]