Abstract
Telomeres are repetitive DNA sequences (TTAGGG) at the end of chromosomes. Cells with critically short telomeres enter replicative senescence and apoptosis. Several in vitro studies report that antimony causes cell apoptosis in human leukocyte cell lines. The goal of this analysis was to investigate whether there is an association between antimony exposure and leukocyte telomere length (LTL) among US adults aged 20 and older based on the National Health and Nutrition Examination Survey (NHANES) 1999–2002. We used multivariate linear regression to analyze the association of urinary antimony with LTL. LTL was log-natural transformed and the results were re-transformed and presented as percent differences. After adjustment for potential confounders, individuals in the 3rd and 4th quartiles of urinary antimony had statistically significantly shorter LTL (−4.78%, 95% CI: −8.42, −0.90; and −6.11%, 95% CI: −11.04, −1.00, respectively) compared to the lowest referent quartile, with evidence of a dose-response relationship (p-value for trend = 0.03). Shorter LTL with antimony was driven by middle aged (40–59 years) and older (60–85 years) adult groups. The association may be biologically plausible because of reported oxidative stress and apoptosis effects of antimony on blood cells, effects known to shorten telomere length.
Keywords: Antimony, Leukocyte Telomere Length, NHANES, heavy metals, aging
Introduction
The enzyme telomerase reverse transcriptase (TERT) is a cellular ribonucleoprotein with reverse transcriptase activity responsible for the maintenance of telomere length (Aubert and Lansdorp 2008). The main function of TERT is the addition of the hexamaric guanine-rich repetitive sequence (5′-TTAGGG-3′) to the chromosomal ends to compensate for the progressive loss of telomeric sequence, thereby maintaining chromosomal integrity and genetic stability (Aubert and Lansdorp 2008). Germ cells, certain white blood cells (e.g., lymphocyte), and cancer cells have active telomerase complexes, which make them relatively long-lived compared to other cell types (Finkel et al. 2007). In contrast, all other somatic cell types have a more finite life span that is regulated at the cell level; telomere erosion leads to loss of approximately 50–200 base pairs (bps) of telomeric DNA at each cell division until replicative senescence, a state of irreversible cell growth arrest and apoptosis, is reached (Chiu et al., 1997). The process of telomere-shortening can be accelerated by increased oxidative stress (von Zglinicki 2002). Independent of aging, telomere shortening is also associated with several health outcomes such as cardiovascular diseases (Haycock et al. 2014), atherosclerosis (Samani et al. 2001), hypertension (Demissie et al. 2006), diabetes (Zee et al. 2010), and mortality (Weischer et al. 2012).
Antimony is a silvery white metal that is commonly found within the Earth’s crust; it exists in either a trivalent or pentavalent state (ATSDR 1992). Antimony is a persistent, bio-accumulative, and toxic chemical. Exposure to and toxicity from antimony may arise due to occupational exposure, domestic use, or when it is used as a medical therapy (Sundar and Chakravarty 2010). Antimony is used to treat parasitic diseases such as leishmaniasis and schistosomiasis. Antimony is also used as a fire-retardant for plastics, and in textiles, rubber, adhesives, pigments in paints, lead storage batteries, pipe metals, semiconductors, and pewter (ATSDR 1992). Antimony is released into air and water from coal-burning power plants, incinerators, mines, and industrial facilities. In urban areas, the primary sources of exposure are likely to be vehicle exhaust and industrial emissions. The general population is constantly exposed to low levels of antimony in the environment through food, air, and drinking water (ATSDR 1992). People may also be exposed by skin contact with soil, water and other substances that contain antimony (ATSDR 1992).
The absorption, distribution, and excretion of antimony can vary depending on its oxidation state, with urinary excretion appearing to be greater for pentavalent antimony compounds than for trivalent compounds (Elinder and Friberg 1986). An elimination half-life of approximately 95 hours has been estimated after occupational exposures (Kentner et al. 1995). Human health effects from antimony at low environmental doses are not well characterized, although respiratory and cardiovascular effects have been reported (ATSDR 1992; Sundar and Chkravarty 2010). Studies have shown that most of the antimony that enters the body concentrates in the liver, lungs, intestines, and spleen (ATSDR 1992). It also rapidly accumulates in blood cells following in vivo administration (Winship 1987). Several in vitro studies report that antimony generates reactive oxygen species (ROS) and activates caspase-3 leading to cell apoptosis in human leukocyte cell lines (Lecureur et al. 2000a, b; Losler et al. 2009; Wyllie and Fairlamb 2006).
In this study, we conducted a cross-sectional analysis to examine the association of urinary antimony concentrations with leukocyte telomere length (LTL) in a representative sample of US adults (20 years of age and older) who participated in the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2002.
Methods
Study population
NHANES is a cross-sectional, nationally representative survey of the non-institutionalized civilian population of the United States conducted by the National Center for Health Statistics, Centers for Disease Control and Prevention (NCHS, CDC). Beginning in 1999, the survey has been conducted continuously and released in 2-year cycles. For our study we merged the publicly available files for NHANES cycles 1999–2000 and 2001–2002 using the NCHS recommendations (Johnson et al. 2013). The survey employs a multistage stratified probability sample based on selected counties, blocks, households, and persons within households.
NCHS-trained professionals conducted interviews in participants’ homes, and extensive physical examinations, including blood and urine collection, were conducted at mobile exam centers (MECs). All procedures were approved by the NCHS Research Ethics Review Board (Protocol #98–12 http://www.cdc.gov/nchs/nhanes/irba98.htm), and all participants provided written informed consent. The response rates for all of the examined person were 76.3% for NHANES 1999–2000 and 79.6% for NHANES 2001–2002 (http://www.cdc.gov/nchs/data/nhanes/analytic_guidelines_11_12.pdf).
Leukocyte telomere length (LTL) measurements
Aliquots of purified DNA, isolated from whole blood, were provided by NCHS. The LTL assay was performed in the laboratory of Dr. Elizabeth Blackburn at the University of California, San Francisco, using the quantitative polymerase chain reaction. Briefly, the method measures the ratio of telomere length (T) relative to standard (S) single-copy gene reference DNA, known as the T/S ratio (Cawthon 2002; Lin et al. 2010). Each sample was assayed three times on three different days. The samples were assayed in duplicate wells, resulting in six data points. Control DNA values were used to normalize between-run variability. Runs with more than four control DNA values falling outside 2.5 standard deviations from the mean for all assay runs were excluded from further analysis (< 6% of runs). For each sample, potential outliers were identified and excluded from the calculations (< 2% of samples). The CDC conducted a quality control review before linking the LTL data to the NHANES public-use data files. The formula 3,274 + 2,413 * (T/S) was used to convert T/S ratio to base pairs (bps) (http://wwwn.cdc.gov/Nchs/Nhanes/2001-2002/TELO_B.htm).
Urinary Biomarkers
Urinary antimony and urinary lead levels were measured by inductively coupled plasma-mass spectrometry using a multi-element analytical technique at the CDC, National Center for Environmental Health (NCEH), Division of Laboratory Sciences (DLS). The level of detection (LOD) for urinary antimony and urinary lead were 0.04 and 0.10 ng/mL, respectively. Urinary concentrations of antimony and lead below the LOD were assigned the LOD divided by the square root of 2, as recommended by NCHS (Johnson et al. 2013). Only 5% of individuals in the population had non-detectable levels of urinary antimony. Urinary antimony was categorized as weighted quartiles based on the distribution of urinary levels among the study population, resulting in approximately the same number of participants within each quartile.
Urinary lead (as natural log-transformed variable) was entered into the models because it has been associated with antimony exposures (ATSDR 1992; Sundar and Chkravarty 2010). To account for variation in dilution in spot urinary samples, urinary creatinine was entered into the analyses as an independent variable in all the models as suggested (Barr et al. 2005).Urinary creatinine was determined using a Jaffe rate reaction measured with a CX3 analyzer and was entered into the model as a natural log-transformed variable.
Statistical analysis
LTL was not normally distributed, thus it was natural log-transformed. Analyses were performed using the weights from the urinary heavy metals subsample as recommended by NCHS and were calculated according to NHANES guidelines (Johnson et al. 2013). SAS-Callable SUDAAN 10 (Research Triangle Institute, Research Triangle Park, NC) was used to account for the NHANES complex sampling design. All tests were two sided, and p ≤0.05 was the level of significance. We ran three models: Model 1 was adjusted for urinary creatinine, age (years) and age squared; Model 2 was further adjusted for sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, or Others), education (less than high-school, high school graduate, or some college and above), alcohol consumption, self-reported smoking status (current, former, or never smoker), serum cotinine (natural log-transformed), and body weight status (underweight/normal, overweight, or obese); and, Model 3 was further adjusted for urinary lead (natural log-transformed).
Preliminary analyses found a significant interaction term between antimony and age-groups (p=0.03); therefore, we performed analyses by age-group stratification. The following groups were used: young adults (ages 20–39 years), middle aged adults (age 40–59 years), and older adults (age 60 and older). A sensitivity analysis was conducted using adjustment for lymphocyte and granulocyte proportions in whole blood because telomere length in the leukocyte population differs by cell subset (Weng et al. 2001). Since our dependent variable LTL was log-transformed, the results were re-transformed by exponentiation of the β coefficients and presented as percent differences estimated by comparing each of the upper three quartiles to the lowest quartile using the formula 100*(eβ −1); statistical tests for linear trends were conducted by modeling quartiles as an ordinal variable using integer values. Moreover, to further characterize the shape of the relationship between antimony and LTL we used urinary antimony as restricted cubic spline. We used a modified SAS macro written by Desquilbet and Mariotti (2010) to account for NHANES weight and sample design and the knots used for restricted cubic spline were placed at the 5th, 35th, 65th and 95th percentile as recommended by Harrell (2010). As sensitivity analyses, we also characterized the shape of the relationship between antimony and LTL using urinary antimony normalized by urinary creatinine (with antimony expressed as μg/g of creatinine) in restricted cubic spline analyses.
Results
The geometric mean (SE) LTL of the study population (n=2307) was 1.03 (0.01). The weighted distributions of study population characteristics are shown in Table 1. Women represented slightly over 51% of the sample; the geometric mean age of participants was approximately 43 years old. The geometric mean (SE) of urinary antimony was 0.12 ng/mL (0.0003 ng/mL).
Table 1.
Sample size and weighted characteristics of the NHANES 1999–2002 participants 20 years of age and older
| ALL | ||
|---|---|---|
| n. | Weighted | |
| Antimony, Urine (ng/mL), GM (SE) | 2307 | 0.12 (0.003) |
| Lead, Urine (ng/mL), GM (SE) | 2307 | 0.71 (0.02) |
| Age (Years), GM (SE) | 2307 | 42.82 (0.57) |
| Urinary Creatinine (mg/dL), GM (SE) | 2036 | 102.73 (2.22) |
| BMI (kg/m2), GM (SE) | 2307 | 27.38 (0.22) |
| Serum Cotinine (ng/mL), GM (SE) | 2307 | 0.59 (0.09) |
| c-reactive protein (mg/dL), GM (SE) | 2307 | 0.19 (0.01) |
| Leukocyte Telomere length, GM (SE) | 2307 | 1.03 (0.01) |
| Sex | ||
| Men, % (SE) | 1122 | 48.72 (1.36) |
| Women, % (SE) | 1185 | 51.28 (1.36) |
| Body Weight | ||
| Underweight/Normal weight,% (SE) | 727 | 34.52 (1.46) |
| Overweight, % (SE) | 857 | 36.36 (1.47) |
| Obese, % (SE) | 723 | 29.12 (1.67) |
| Smoking Status | ||
| Current Smoker, % (SE) | 530 | 25.45 (1.70) |
| Former Smoker, % (SE) | 611 | 25.04 (1.44) |
| Never Smoked, % (SE) | 1166 | 49.51 (1.90) |
| Alcohol Consumption | ||
| No Alcohol, % (SE) | 806 | 29.84 (2.01) |
| 1–4 drinks per week, % (SE) | 1326 | 62.27 (2.09) |
| >4 drinks per week, % (SE) | 175 | 7.88 (0.94) |
| Education Level | ||
| Less than High School % (SE) | 775 | 21.66 (1.08) |
| Completed High School % (SE) | 578 | 27.98 (1.29) |
| More than High School % (SE) | 954 | 50.36 (1.84) |
| Race/Ethnicity | ||
| White (Non-Hispanic) % (SE) | 1159 | 73.62 (1.84) |
| Non-Hispanic Black % (SE) | 394 | 9.51 (1.20) |
| Mexican-American % (SE) | 583 | 6.88 (0.876)) |
| Other % (SE) | 171 | 9.99 (1.652) |
Table 2 shows the results of the multivariable linear regression. Briefly, in all three models, urinary antimony was statistically significantly associated with shorter LTL. Individuals in the 3rd and 4th quartiles of urinary antimony had statistically significantly shorter LTL (−4.78%, 95% CI: −8.42, −0.90; and −6.11, 95% CI: −11.04, −1.00, respectively) compared to the lowest referent quartile with evidence of a dose-response (p trend = 0.03) (Table 2 model 3). Furthermore, analyses using restricted cubic spline confirmed the dose-relationship between urinary antimony and LTL (Supplemental Figure 1a). Sensitivity analyses using antimony standardized by creatinine (expressed as μg/g of creatinine) showed similar dose-response relationships (Supplemental Figure 2a).
TABLE 2.
Percent Difference (95% CI) in Leukocyte Telomere Length by Urinary Antimony Level in NHANES 1999–2002 in all participants and stratified by age group.
| Model 1* | Model 2** | Model 3*** | |
|---|---|---|---|
| All Participants (20–85 | |||
| n. | 2421 | 2306 | 2306 |
| Urinary Antimony Q1 | Referent | Referent | Referent |
| Urinary Antimony Q2 | −2.18 (−5.82, 1.71) | −1.78 (−5.07, 1.51) | −1.98 (−5.16, 1.31) |
| Urinary Antimony Q3 | −4.59 (−8.79, −0.30) | −4.59 (−8.24, −0.70) | −4.78 (−8.42, −0.90) |
| Urinary Antimony Q4 | −6.93 (−11.84, −0.80) | −5.92 (−10.95, −0.50) | −6.11 (−11.04, −1.00) |
| p trend | 0.023 | 0.031 | 0.027 |
| 20 – 39 years old | |||
| n | 840 | 807 | 807 |
| Urinary Antimony Q1 | Referent | Referent | Referent |
| Urinary Antimony Q2 | 5.13 (0.60, 9.86) | 5.87 (0.90, 11.07) | 5.65 (0.70, 10.74) |
| Urinary Antimony Q3 | 1.11 (−5.16, 7.68) | 1.71 (−4.02, 7.79) | 1.41 (−4.40, 7.57) |
| Urinary Antimony Q4 | −0.30 (−7.60, 7.57) | 1.31 (−6.39, 9.53) | 0.90 (−6.76, 9.09) |
| p trend | 0.050 | 0.071 | 0.076 |
| Model R-Square | 0.033812 | 0.084509 | 0.085112 |
| 40 – 59 years old | |||
| n | 746 | 720 | 720 |
| Urinary Antimony Q1 | Referent | Referent | Referent |
| Urinary Antimony Q2 | −4.69 (−13.41, 1.82) | −4.50 (−9.79, 1.11) | −4.40 (−9.70, 1.21) |
| Urinary Antimony Q3 | −7.13 (−12.54, −1.39) | −6.85 (−12.37, −1.09) | −6.76 (−12.37, −0.80) |
| Urinary Antimony Q4 | −10.33 (−15.89, −4.50) | −10.06 (−16.05, −3.63) | −9.97 (−15.89, −3.63) |
| p trend | 0.002 | 0.021 | 0.024 |
| Model R-Square | 0.057553 | 0.097438 | 0.097629 |
| 60 – 85 years old | |||
| n | 835 | 779 | 779 |
| Urinary Antimony Q1 | Referent | Referent | Referent |
| Urinary Antimony Q2 | −7.04 (−11.40, −2.47) | −6.95 (−10.95, −2.86) | −7.04 (−11.04, −2.96) |
| Urinary Antimony Q3 | −7.50 (−14.62, −0.20) | −8.88 (−15.97, −1.19) | −8.97 (−15.97, −1.39) |
| Urinary Antimony Q4 | −8.52 (−15.97, −0.50) | −9.24 (−16.47, −1.39) | −9.43 (−16.64, −1.69) |
| p trend | 0.033 | 0.019 | 0.015 |
| Model R-Square | 0.076975 | 0.140211 | 0.141873 |
Model 1: adjusted for urinary creatinine (natural log-transformed), age (years, continuous), age squared.
Model 2: adjusted for the variables in model 1 plus education (less than high-school, high school graduate, some college and above), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, and Other), alcohol consumption, self-reported smoking status (current, former, or never smoker), serum cotinine (natural log-transformed), body weight status, and sex.
Model 3: adjusted for the variables in model 2 plus urinary lead (natural log-transformed).
Urinary antimony quartiles: Q1 ≤0.08 ng/mL; Q2 0.09 – 0.12 ng/mL; Q3 0.13 – 0.18 ng/mL; Q4 >0.18 ng/mL
Analyses stratified by age group indicated that the statistically significantly association of shorter LTL with antimony were driven by middle aged (40–59 years) and older (60–85 years) adult groups. Conversely, in young adults (20–39 years) there was a statistically significantly higher LTL in individuals in the 2nd antimony quartile compared to the referent quartile (Table 2). Figure 1 summarize the LTL percent difference by antimony quartile for all participants and by age group. Sensitivity analyses including lymphocytes and granulocytes proportion in whole blood yielded results similar to those from the primary analyses (data not shown). Further analyses using restricted cubic spline confirmed the dose-response relationship between urinary antimony and LTL in the different age groups (Supplemental Figures 1b–d). Sensitivity analyses using antimony standardize by creatinine (expressed as μg/g of creatinine) showed similar dose-relationship in the different age groups (Supplemental Figures 2b–d).
Figure 1.
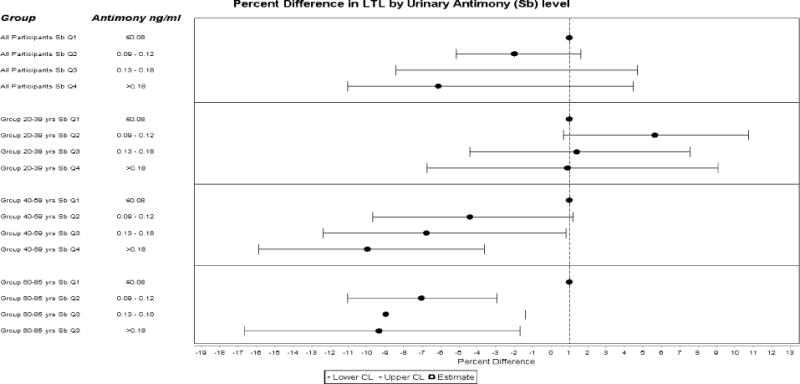
Adjusted (percent difference and 95% CIs for Leukocyte Telomere Length (T/S ratio) in association with quartile of urinary antimony among all adults participants and by age group (20 – 39, 40 – 59, and 60 – 85 years) Data are based on the beta estimated adjusted for urinary creatinine (natural log-transformed), age (years, continuous), age squared, sex, education (less than high-school, high school graduate, some college and above), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, and Other), alcohol consumption, self-reported smoking status (current, former, or never smoker), serum cotinine (natural log-transformed), body weight status and urinary lead.
Discussion
To our knowledge, this is the first study reporting on urinary antimony and leukocyte telomere length. In this general population study, we found that environmental exposure to urinary antimony was associated with shorter LTL. Specifically, participants in the 3rd and 4th quartiles of urinary antimony had a decreased leukocyte telomere length of 4.78% and 6.11%, respectively, compared to those in the referent lowest quartile. Telomere erosion by age (as calculated by the multivariate analyses in this cohort) leads on average to a loss of 12 bps per year. Participants in the third and fourth quartile of urinary antimony concentration had, on average, a loss of LTL of approximately 115 and 147 bps, respectively. If these findings reflect a relationship measured without bias, then the difference between individuals of the same chronological age in the highest quartile versus the lowest quartile would be equivalent to 12.25 calendar years.
The underlying biological mechanism of how antimony may play a role in telomere shortening could potentially involve the promotion of oxidative stress and generation of reactive oxygen species (ROS). Telomeres are particularly sensitive to ROS because they are rich in guanine residues, and guanine can be oxidized to 8 hydroxyguanine, which is unstable (Mason and Perdigones 2013). Both pentavalent and trivalent antimonial compounds induce the generation of ROS in human and murine cell line (Mann et al. 2006; Mookerjee Basu et al. 2006; Rais et al. 2000; Wyllie and Fairlamb 2006).
The mechanisms of apoptosis are highly complex and sophisticated, involving an energy-dependent cascade of molecular events. There are two main apoptotic pathways: the extrinsic or death receptor pathway and the intrinsic or mitochondrial pathway. Both of these pathways converge on the same terminal pathway involving cleavage of caspase-3. This results in DNA fragmentation, leading to formation of apoptotic bodies, expression of ligands for phagocytic cell receptors, and terminal uptake by phagocytic cells (Elmore 2007). Therefore, once caspase-3 is activated, there seems to be an irreversible commitment towards cell death.
Several in vitro studies have described the induction of apoptosis by trivalent antimony through activation of caspase-3 (Lecureur et al. 2002a, b; Losler et al. 2009; Mann et al. 2006; Muller et al. 1998; Wyllie and Fairlamb 2006). Lecureur et al. (2002b) pointed out that the cytotoxic potassium antimonyl tartrate concentrations action on human lymphoma Daudi cells and on B lymphocytes from leukemia patients were in micromolar concentrations; concentrations that are likely to be achievable in vivo in humans (Schulert et al. 1966).
Loss of telomere length induces the same Sb2O3-induced cellular apoptotic pathways. In an elegant experimental design, Bermudez et al.(2006) using normal ovarian epithelial cell lines (that are telomerase-negative) transfected with a plasmid construct containing human telomerase reverse transcriptase (hTERT) cDNA, found that expression of hTERT was sufficient and specific to reduce caspase-mediated cellular apoptosis. Cells transfected with hTERT had increased mean telomere length, increased growth capacity, and increased cell survival. Furthermore, exposure of hTERT-transfected cells to staurosporine, an inducer of apoptosis, resulted in suppressed caspase-3 activation. Conversely, cells transfected with dominant-negative (DN) hTERT had restored caspase-3 activation after staurosporine treatment, at a similar level to that found in control cells. Because antimony exposure and telomere loss induce the same pathways, and telomere loss throughout life may also be due to cumulative oxidative damage (d’Adda di Fagagna et al. 2003; von Zglinicki et al. 2005), it may be biologically plausible that antimony causes shortened LTL through the induction of cellular oxidative stress. Alternatively, antimony toxicity may accelerate apoptosis of cells already undergoing telomere loss and senescence.
After stratification by age groups, we found that there was a statistically significant association of antimony with shorter LTL in middle age and older adults groups but not in young adults. These differences may be due to the fact that older adults may undergo telomere loss due to repeated activation of immune cells – particularly CD8 T lymphocytes – from persistent viral infection, lifelong exposure to various pathogens, as well as oxidative stress (Effros 2011). Reactivation of latent viruses, such as several herpes viruses, frequently occurs in older people that are more likely to be under conditions of immunosuppression; whereas herpes viruses are infrequently re-activated in younger adults, particularly in those with normally functioning immune systems (Effros 2011). There are several reports of antimony activating latent varicella zoster virus among patients treated for leishmaniasis or schistosomiasis with antimonial compounds (Barros et al. 2014; Hartzell et al. 2006; Ritchken and Kantor 1947). Since antimony accumulates highly and rapidly in blood cells after in vivo administration (Winship 1987) and also induces oxidative damage in leukocyte cells (Muller et al. 1998; Wyllie and Fairlamb 2006), antimony exposure may contribute to cumulative oxidative damage. Therefore, it might increase cell senescence that will be manifested to a greater extent in older adults compared to younger adults.
Although the strength of our study is that it is based on a nationally representative survey, the main study limitation is the cross-sectional design of NHANES. Thus, we cannot infer a causal association. Although we entered the percentage of lymphocytes and granulocytes as separate variables in our analyses, we could not exclude the confounding effect of the proportion of different leukocyte subtypes. In younger individuals, telomeres are generally shorter in granulocytes compared to T lymphocytes; whereas, in older individuals there is a reverse, with granulocytes having longer telomeres than T lymphocytes, possibly reflecting an increase of memory T cells, which have shorter telomeres compared to naïve T lymphocytes (Weng 2001). An additional limitation is that we could not exclude the effect of co-exposure to arsenic. Antimony, which belongs to the same group (Group V) of the periodic table as arsenic, shares numerous biological features with this metalloid (Gebel 1997). Arsenic trioxide induces oxidative damage, activates caspase-3, and induces apoptosis in human leucocyte cells, and the toxic cellular effects are greater than that induced by a corresponding dose of antimonial compounds (Losler et al. 2009). In the NHANES 1999–2000 and 2001–2002 cycles, arsenic was not measured, thus we may not know whether the observed associations might be due to arsenic co-exposure.
In conclusion, in the largest study to date of the association between antimony exposure and telomere length, we found an independent association between environmental exposures to antimony and shorter LTL after adjustment for potential confounders. Further studies are needed to confirm our findings of antimony on telomere shortening and the potential role in chronic diseases involved in cellular senescence and aging.
Supplementary Material
Acknowledgments
DISCLAIMER: The findings and conclusion in this report are those of the authors and do not necessarily represent the views of CDC/ATSDR.
IRB approval: CDC/ATSDR has determined that our research did not meet the criteria for human research as per federal regulation and therefore did not require review.
Footnotes
Competing Financial Interests: The authors declare they have no actual or potential competing financial interests.
References
- ATSDR. Agency for Toxic Substances and Disease Registry. Toxicological Profile for Antimony. Atlanta, GA: US Department of Health and Human Services, Public Health Service; 1992. [Google Scholar]
- Aubert G, Lansdorp PM. Telomeres and aging. Physiological reviews. 2008;88:557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the u.S. Population: Implications for urinary biologic monitoring measurements. Environmental health perspectives. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros AB, Rodrigues AM, Batista MP, Munhoz Junior S, Hueb M, Fontes CJ. Varicella zoster virus reactivation during or immediately following treatment of tegumentary leishmaniasis with antimony compounds. Mem Inst Oswaldo Cruz. 2014;109:499–501. doi: 10.1590/0074-0276130563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez Y, Erasso D, Johnson NC, Alfonso MY, Lowell NE, Kruk PA. Telomerase confers resistance to caspase-mediated apoptosis. Clinical interventions in aging. 2006;1:155–167. doi: 10.2147/ciia.2006.1.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CP, Harley CB. Replicative senescence and cell immortality: the role of telomeres and telomerase. Proc Soc Exp Biol Med. 1997;214:99–106. doi: 10.3181/00379727-214-44075. [DOI] [PubMed] [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative pcr. Nucleic acids research. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the framingham heart study. Aging cell. 2006;5:325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–1057. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- Effros RB. Telomere/telomerase dynamics within the human immune system: Effect of chronic infection and stress. Experimental gerontology. 2011;46:135–140. doi: 10.1016/j.exger.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinder CG, Friberg L. Antimony. In: Friberg L, Nordberg GF, Vouk VB, editors. Handbook on the toxicology of metals. 2nd. New York: Elsevier; 1986. pp. 26–42. [Google Scholar]
- Elmore S. Apoptosis: A review of programmed cell death. Toxicologic pathology. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448:767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- Gebel T. Arsenic and antimony: Comparative approach on mechanistic toxicology. Chemico-biological interactions. 1997;107:131–144. doi: 10.1016/s0009-2797(97)00087-2. [DOI] [PubMed] [Google Scholar]
- Harrell F. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis (Springer Series in Statistics) Springer; 2010. [Google Scholar]
- Hartzell JD, Aronson NE, Nagaraja S, Whitman T, Hawkes CA, Wortmann G. Varicella zoster virus meningitis complicating sodium stibogluconate treatment for cutaneous leishmaniasis. Am J Trop Med Hyg. 2006;74:591–2. [PubMed] [Google Scholar]
- Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta-analysis. Bmj. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, et al. National health and nutrition examination survey: Analytic guidelines, 1999–2010. Vital and health statistics Series 2, Data evaluation and methods research. 2013:1–24. [PubMed] [Google Scholar]
- Kentner M, Leinemann M, Schaller KH, Weltle D, Lehnert G. External and internal antimony exposure in starter battery production. International archives of occupational and environmental health. 1995;67:119–123. doi: 10.1007/BF00572235. [DOI] [PubMed] [Google Scholar]
- Lecureur V, Lagadic-Gossmann D, Fardel O. Potassium antimonyl tartrate induces reactive oxygen species-related apoptosis in human myeloid leukemic hl60 cells. International journal of oncology. 2002a;20:1071–1076. [PubMed] [Google Scholar]
- Lecureur V, Le Thiec A, Le Meur A, Amiot L, Drenou B, Bernard M, et al. Potassium antimonyl tartrate induces caspase- and reactive oxygen species-dependent apoptosis in lymphoid tumoral cells. British journal of haematology. 2002b;119:608–615. doi: 10.1046/j.1365-2141.2002.03863.x. [DOI] [PubMed] [Google Scholar]
- Lin J, Epel E, Cheon J, et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. Journal of immunological methods. 2010;352:71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losler S, Schlief S, Kneifel C, Thiel E, Schrezenmeier H, Rojewski MT. Antimony-trioxide- and arsenic-trioxide-induced apoptosis in myelogenic and lymphatic cell lines, recruitment of caspases, and loss of mitochondrial membrane potential are enhanced by modulators of the cellular glutathione redox system. Annals of hematology. 2009;88:1047–1058. doi: 10.1007/s00277-009-0736-4. [DOI] [PubMed] [Google Scholar]
- Mann KK, Davison K, Colombo M, Colosimo AL, Diaz Z, Padovani AM, et al. Antimony trioxide-induced apoptosis is dependent on sek1/jnk signaling. Toxicology letters. 2006;160:158–170. doi: 10.1016/j.toxlet.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Mason PJ, Perdigones N. Telomere biology and translational research. Transl Res. 2013;162:333–42. doi: 10.1016/j.trsl.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookerjee Basu J, Mookerjee A, Sen P, Bhaumik S, Sen P, Banerjee S, et al. Sodium antimony gluconate induces generation of reactive oxygen species and nitric oxide via phosphoinositide 3-kinase and mitogen-activated protein kinase activation in leishmania donovani-infected macrophages. Antimicrobial agents and chemotherapy. 2006;50:1788–1797. doi: 10.1128/AAC.50.5.1788-1797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S, Miller WH, Jr, Dejean A. Trivalent antimonials induce degradation of the pml-rar oncoprotein and reorganization of the promyelocytic leukemia nuclear bodies in acute promyelocytic leukemia nb4 cells. Blood. 1998;92:4308–4316. [PubMed] [Google Scholar]
- Rais S, Perianin A, Lenoir M, Sadak A, Rivollet D, Paul M, et al. Sodium stibogluconate (pentostam) potentiates oxidant production in murine visceral leishmaniasis and in human blood. Antimicrobial agents and chemotherapy. 2000;44:2406–2410. doi: 10.1128/aac.44.9.2406-2410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchken J, Kantor F. Herpes zoster, a toxic manifestation resulting from the therapeutic administration of antimony and potassium tartrate in the treatment of schistosomiasis. Clin Proc. 1947;6:125–9. [PubMed] [Google Scholar]
- Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001;358:472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- Schulert AR, Rassoul AA, Mansour M, Girgis N, McConnell E, Farid Z. Biological disposition of antibilharzial antimony drugs. Ii. Antimony fate and uptake by schistosoma haematobium eggs in man. Experimental parasitology. 1966;18:397–402. doi: 10.1016/0014-4894(66)90040-3. [DOI] [PubMed] [Google Scholar]
- Shen HM, Liu ZG. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free radical biology & medicine. 2006;40:928–939. doi: 10.1016/j.freeradbiomed.2005.10.056. [DOI] [PubMed] [Google Scholar]
- Sundar S, Chakravarty J. Antimony toxicity. Int J Environ Res Public Health. 2010;7:4267–77. doi: 10.3390/ijerph7124267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends in biochemical sciences. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, Martin-Ruiz CM. Telomeres as biomarkers for ageing and age-related diseases. Current molecular medicine. 2005;5:197–203. doi: 10.2174/1566524053586545. [DOI] [PubMed] [Google Scholar]
- Wang ZG, Rivi R, Delva L, et al. Arsenic trioxide and melarsoprol induce programmed cell death in myeloid leukemia cell lines and function in a PML and PML-RARalpha independent manner. Blood. 1998;92:1497–1504. [PubMed] [Google Scholar]
- Weischer M, Bojesen SE, Cawthon RM, Freiberg JJ, Tybjaerg-Hansen A, Nordestgaard BG. Short telomere length, myocardial infarction, ischemic heart disease, and early death. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:822–829. doi: 10.1161/ATVBAHA.111.237271. [DOI] [PubMed] [Google Scholar]
- Weng N. Interplay between telomere length and telomerase in human leukocyte differentiation and aging. Journal of leukocyte biology. 2001;70:861–867. [PubMed] [Google Scholar]
- Winship KA. Toxicity of antimony and its compounds. Adverse drug reactions and acute poisoning reviews. 1987;6:67–90. [PubMed] [Google Scholar]
- Wyllie S, Fairlamb AH. Differential toxicity of antimonial compounds and their effects on glutathione homeostasis in a human leukaemia monocyte cell line. Biochemical pharmacology. 2006;71:257–267. doi: 10.1016/j.bcp.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Zee RY, Castonguay AJ, Barton NS, Germer S, Martin M. Mean leukocyte telomere length shortening and type 2 diabetes mellitus: A case-control study. Translational research: the journal of laboratory and clinical medicine. 2010;155:166–169. doi: 10.1016/j.trsl.2009.09.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


