Abstract
Objective
The onset of psychosis typically occurs during adolescence or early adulthood and can have a detrimental impact on social and cognitive development. Cognitive behavioural therapy (CBT) shows promise in reducing the risk of psychosis. Teaching families to apply CBT with their offspring may bolster therapeutic gains made in time-limited treatment. We developed a comprehensive group-and-family-based CBT (GFCBT) program that aims to facilitate psychosocial recovery, decrease symptoms and prevent transition to psychosis in youth at risk. GF-CBT is grounded in ecological systems and cognitive theories, resilience models and research on information processing in delusions. The theoretical rationale and description of GF-CBT are presented together with a pilot study that evaluated the program’s feasibility and explored participants’ outcomes.
Methods
Youth ages 16–21 at risk for psychosis and their families participated in an open trial with pre, post and 3-month follow-up assessments conducted by an independent evaluator. The Comprehensive Assessment of At-Risk Mental States was the primary clinical outcome measure.
Results
All enrolled participants (n = 6) completed GF-CBT and all remitted from at-risk mental state (ARMS). As a group participants showed statistically significant decreases in attenuated psychotic symptoms, negative symptoms, depression, cognitive biases and improvements in functioning. Family members showed significant improvements in use of CBT skills, enhanced communication with their offspring, and greater confidence in their ability to help. Gains were maintained at follow-up.
Conclusions
GF-CBT may delay or prevent transition to psychosis in youth at risk, and potentially facilitate recovery from ARMS. More rigorous, controlled research is needed to further evaluate this program.
Introduction
Early therapeutic interventions in most disorders, including schizophrenia, offer the greatest possibility for full recovery. Psychosis typically emerges in late adolescence or early adulthood, a vital stage in social and cognitive development, and can have a profoundly adverse impact on long-term functioning. Prior to the first full psychotic episode, 80–90% of patients experience attenuated psychotic symptoms of escalating severity over one to two years, referred to as a prodromal phase, ‘at risk mental state’ (ARMS), or ultra-high risk (UHR).1,2 About 30% of UHR individuals develop full-blown psychosis within 3 years,3 and about 40% of those who do not develop overt psychosis continue to experience ongoing attenuated psychotic symptoms and persistent functional disability.4,5 Recent meta-analyses indicate that individual cognitive behavior ral therapy (CBT) shows promise in reducing the risk of psychosis in the short-term (12 months). However, effects diminish over 2–4 years. More effective interventions are needed to prolong treatment gains and facilitate symptomatic and functional recovery in at-risk individuals.3,6
Targeted, symptom and stage of illness-specific CBT interventions have potential to produce strong therapeutic effects.7–9 Suspiciousness and social isolation are the most common symptoms,10 which tend to occur earlier in the prodromal period.11 Peer relationships are particularly important in adolescent development.12 Treatment modalities that target suspiciousness and facilitate peer support could therefore be especially beneficial.
A positive family environment predicts improvement in symptoms and social functioning among adolescents at-risk for psychosis.13 People experiencing paranoia may avoid others14 and become suspicious of their friends and family. Family members could benefit from learning CBT strategies for helping a relative who begins to isolate and exhibit suspiciousness. Family members could also play an important role in helping their relatives use CBT skills at home and other natural settings. Teaching family members CBT skills may serve as an additional protective factor for youth at risk for psychosis, as it can help create a more supportive family environment, and sustain therapeutic gains made in time-limited CBT.
In order to maximize potential benefits of different treatment modalities for delivering CBT to youth at risk for psychosis, we developed a program that combines group, family and individual formats: the group-and-family-based CBT program (GF-CBT). The primary aim of this study was to evaluate the feasibility of implementing GF-CBT program for youth at risk for psychosis. The secondary aim was to explore changes in participants’ symptoms, psychosocial functioning and cognitive biases, and in family members’ CBT skills and communication.
Methods
Study design and participants
The study used an open uncontrolled trial design with assessments conducted by an independent evaluator at baseline, post-treatment, and 3-month follow-up. Participants were recruited from New York City outpatient clinics and through advertisements. Inclusion criteria were 16–21 years old; English speaking; Comprehensive Assessment of At Risk Mental States (CAARMS)15 criteria met in one of three ways: (i) attenuated psychosis, (ii) brief limited intermittent psychotic symptoms (BLIPS), (iii) family history of psychosis and deterioration in functioning; Positive and Negative Syndrome Scale (PANSS) P6 (persecutory delusions) ≥3 (suspiciousness/paranoid ideation); one family member with at least 4 h/week contact with the participant (involvement of family member was not required); ability and willingness to give informed consent (if >18 years old) or assent with parent/legal guardian consent (if <18 years old). Exclusion criteria were moderate to severe learning disability; organic impairment known to affect brain; substance dependence disorder and use of street drugs within past 4 weeks. All participants continued to receive standard care, including the use of psychotropic medication (if prescribed). The trial was conducted between 2011 and 2013 and approved by the Weill Cornell Medical College Institutional Review Board.
Intervention
GF-CBT for Youth at Risk for Psychosis is a 15-week program that includes weekly CBT skills group and individual sessions for adolescents, and a weekly CBT skills group for family members.
The program aims to boost family and peer support, reduce isolation, normalize psychotic-like experiences, facilitate positive thinking, enhance reasoning and decision-making skills, and reduce cognitive biases.
GF-CBT is grounded in socio-cultural16 and ecological systems theories,17 psychosocial resilience models,18 a fuzzy-trace theory of emotion, memory and reasoning,19–21 and research on information processing in delusions.14,22,23 The guiding theory for the GF-CBT intervention is that gist memories (bottom-line meaning of past experiences), cognitive biases and other maladaptive cognitive processes may lead to the perception of neutral and anomalous experiences as threatening, which can increase stress and result in higher frequency of psychotic-like experiences. The perception of events as threatening may further lead to avoidance and reduced opportunities for reality testing, and therefore reinforce cognitive inaccuracies, promote the formation of delusions, and interfere with social functioning. The goal of GF-CBT is to reduce distress and therefore limit the impact of the interference by inculcating adaptive gist representations and enhancing adolescents’ ability to make reality based appraisals of their experiences (including anomalous experiences and intrusions). The Algoheuristic approach24,25 to teaching general methods of reasoning by facilitating the formation of specific metacognitive skills is utilized to help adolescents become aware of cognitive biases common in suspicious thoughts and delusions (e.g. jumping to conclusions, externalizing, personalizing) and to develop a less biased information-processing style. Central to the method is getting learners to realize the system of mental operations involved in the cognitive task (e.g. to collect an adequate amount of information in order to avoid jumping to a conclusion) through the following steps: (i) teaching an individual about a concept and/or procedure of thinking by explanation and demonstration (e.g. defining jumping to conclusions); (ii) formulating strategies to guide learners in selecting appropriate cognitive procedures (e.g. collecting sufficient information and generating hypotheses); (iii) introducing training exercises for acquisition of practical skills; (iv) assisting individuals in learning to use these strategies: being able to deliberately recall them, apply them, and eventually internalize them, so that reasoning tasks are carried out quickly and effortlessly.
GF-CBT follows principles of the individual CBT for UHR approach,26–28 and group CBT for delusions and paranoia,29,30 but combines individual and group with family CBT modalities. The involvement of family members is designed to support, encourage and maintain use of CBT skills at home, and to help family members cope with their own distress. We adapted CBT for psychosis clinician training program to teach family members CBT skills that they can continue implementing beyond the 15-week program to bolster gains made during the time-limited GF-CBT. To teach family members how to apply CBT skills with their offspring, we use a combination of didactic learning (skills are described and demonstrated via video examples) and practice (skills are role-played in group sessions with an actor trained to play an adolescent prone to paranoia). CBT lessons combine a PowerPoint presentation with participant workbooks that include didactic materials, exercises and homework.31,32 (Detailed description of GF-CBT in Supporting Information Table S1).
Measures
The feasibility was evaluated by the rate of consent (percentage of potentially eligible individuals who signed consent form and completed screening), session attendance, dropout rate, and participants’ satisfaction with group, individual and family CBT modalities.
Primary clinical outcome measure was the CAARMS, used to determine if an individual meets criteria for an ARMS for the onset of a first-episode psychotic disorder and to assess related psychopathology. 15 The CAARMS consists of seven subscales, each with global and frequency ratings: positive symptoms, cognitive change-attention/concentration, emotional disturbance, negative symptoms, behavioural change, motor/physical change and general psychopathology. The CAARMS also includes the Social and Occupational Functioning Scale (SOFAS),33 which measures overall functioning in a single score (0–100).
Secondary measures covered four domains: (i) symptoms: PANSS;34 Peter’s Delusion Inventory (PDI);35 Beck Depression Inventory, 2nd Edition (BDI-II);36 The State Trait Anxiety Inventory (STAI);37 (ii) cognitive processes and biases known to play a role in the formation of delusions: The Beads Task (an experimental task measuring data gathering reasoning bias);38–40 The Davos Assessment of Cognitive Biases (DACOBS; a self-report measure of cognitive biases);41 (iii) psychosocial functioning and communication: The Social Functioning Scale (SFS);42 Global Functioning Role (GFR) and Global Functioning Social (GFS) Scales;43 The Empathy Scale-F (ES-F; adapted from a scale measuring clinician empathy44 for adolescents to rate perceived empathy from participating family members); The Cognitive Behavioral Therapy Skills for Families Scale (CBTSF-S; developed to measure family members’ proficiency in using CBT skills with their at-risk relatives, Supporting Information Table S3); (iv) therapeutic alliance and group therapeutic factors: The Working Alliance Inventory (WAI);45 The Empathy Scale (ES);44 Group Cohesiveness Scale (CS);46 and Therapeutic Factors Scale.47 Additionally, qualitative interviews were conducted with adolescents and family members to evaluate their experience with the program.
Descriptive characteristics of the sample were obtained using a demographic questionnaire, the Wechsler Test of Adult Reading (WTAR)48 and the Prodromal Questionnaire (PQ).49
Data Analysis
Feasibility outcomes were reported using means and standard deviations for continuous variables and percentages for categorical variables. Mixed models methodology for repeated measures was used to explore treatment effects on symptom severity, functioning, cognitive processes and biases at post-treatment and separately at 3-month follow up. Effect sizes (Cohen’s d) were computed to assess the magnitude of effects at both time points relative to baseline. The analyses were exploratory and were not adjusted for inflated Type I error due to testing multiple hypotheses.
Interrater Reliability
All assessments were videotaped. A blind, independent second rater assessed 50% of all interviews. There was 100% agreement for CAARMS diagnosis at all time points. Intraclass correlation coefficients (ICC) for CAARMS total and subscales were generally high, ranging from 0.80 to 0.98. Similar results were observed for PANSS and CBTSF-S (Supporting Information Table S2).
Results
Participant flow, recruitment, retention and session attendance
Twenty-one individuals and their family members were referred to the study. After telephone prescreening, 11 potential participants were invited to attend an informed consent session, nine consented and completed screening (82% consent rate), six met eligibility criteria. Six participants and five family members were allocated to GF-CBT and completed it. The mean number of individual adolescent sessions attended was 10 (SD = 2.6), group adolescent sessions 8.6 (SD = 3.9), and group family sessions 12 (SD = 2.9). Five participants and four family members completed the 3-month follow-up assessments (Fig. 1).
Figure 1.
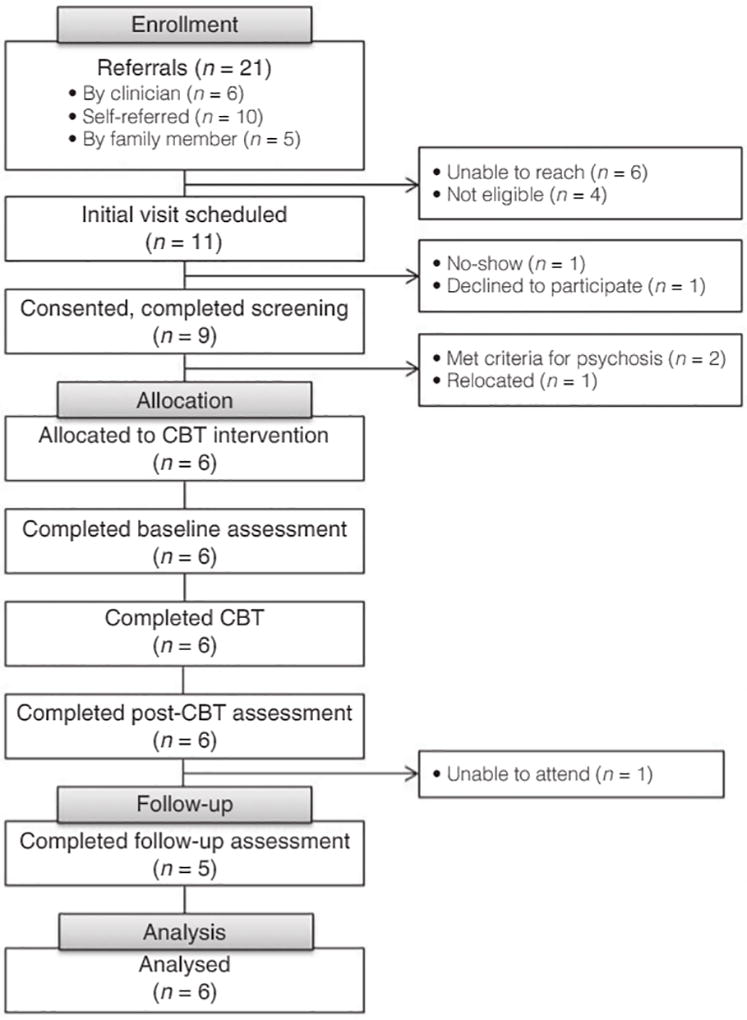
Consort diagram.
Sample demographic and clinical characteristics
The majority of participants were female (n = 4, 66.7%) and single (n = 5, 83.3%). The participants were ethnically diverse, with ages ranging from 17 to 21 years (M = 19.50, SD = 1.52). Four individuals (67%) met CAARMS criteria for attenuated psychosis, one for BLIPS, and one met both attenuated psychosis criteria and was in CAARMS – vulnerability group. Participants had varying levels of educational attainment, and all experienced disruptions in education and work due to symptoms. Demographic and clinical characteristics of the sample are presented in Table 1.
Table 1.
Baseline Clinical and Demographic Characteristics
| Characteristic | n=6 |
|---|---|
| Age, mean (SD) | 19.50 (1.52) |
| Gender, Male, № (%) | 2 (33.3%) |
| Education | |
| Finished high school | 5 (83.3%) |
| Homeschooled (high school) | 1 (16.7%) |
| Started college | 3 (50%) |
| Dropped classes | 2 (33.3%) |
| Leave of absence | 1 (16.7%) |
| Currently not in school or working | 3 (50%) |
| Relationship status: Single № (%) | 5 (83.3%) |
| Ethnic group | |
| African American | 3 (50%) |
| Hispanic | 1 (16.7%) |
| Caucasian | 1 (16.7%) |
| Mixed | 1 (16.7%) |
| Disadvantaged background (recent immigrants, adopted) | 6 (100%) |
| Parents’ marital status, single | 6 (100%) |
| Living situation, Lives with mother | 6 (100%) |
| Mother’s employment status, employed | 6 (100%) |
| IQ, WTAR, Mean (SD) | 98.67 (14.17) |
| Prodromal Questionnaire, Positive Symptoms, Mean (SD) (0, 45) | 15.33 (3.67) |
| CAARMS Criteria | |
| CAARMS, At-Risk Mental State № (%) | 6 (100%) |
| CAARMS Attenuated Psychosis | 4 (66.7%) |
| CAARMS Vulnerability & Attenuated Psychosis | 1 (16.7%) |
| CAARMS BLIPS | 1 (16.7%) |
| SOFAS, Mean (SD) (1, 100) | 48.50 (3.67) |
| BDI-II, Mean (SD) (0, 63) | 19.5 (15.35) |
| STAI, Mean (SD) (20, 80) | 50.17 (15.43) |
| Previously hospitalized | 1 (16.7%) |
| Using psychotropic medications* | 4 (66.7%) |
| Antipsychotics | 1 (16.7%) |
| Antipsychotics and mood stabilizer | 1 (16.7%) |
| Antipsychotics and anti-anxiety and anti-depressants | 1 (16.7%) |
At post-CBT and 3-months follow up, only three participants continued to be prescribed antipsychotics.
Abbreviations: WTAR = Wechsler Test of Adult Reading (Wechsler, 2001), Prodromal Questionnaire (PQ; Loewey et al., 2005); CAARMS = Comprehensive Assessment of At-Risk Mental States, BLIPS = Brief limited intermittent psychotic symptoms, SOFAS = Social and Occupational Functioning Assessment Scale, BDI-II = Beck Depression Inventory-II; STAI, State-Trait Anxiety Inventory; SD= Standard Deviation.
Effect of treatment intervention on ARMS and severity of symptoms
At post-treatment, attenuated psychotic symptoms in all participants were reduced to a level of remission from ARMS as defined by the CAARMS. Participants demonstrated significant decreases in CAARMS Positive Symptoms (Global P < 0.01, d = 1.64 and frequency P < 0.01, d = 1.58), and improvements in functioning (SOFAS, P < 0.001, d = 2.30), as well as significant decreases in CAARMS Total Global and Frequency scales (P < 0.01, d = 1.34 and P < 0.005, d = 1.25, respectively). Improvements were also observed in negative symptoms, general psychopathology, behavioral changes and emotional disturbance (Table 2). A similar pattern of results was observed for changes in psychopathology as measured by PANSS total score (P < 0.05, d = 1.64), with significant decreases in delusions (P1), suspiciousness/persecution (P6), disorientation (G10) and active social avoidance (G16). Likewise, participants self-reported significant decreases in delusional distress, conviction and preoccupation (PDI), as well as in anxiety (STAI) and depression (BDI-II; Table 3). The vast majority of gains were maintained at 3-month follow-up (Tables 2,3).
Table 2.
CAARMS as Primary Outcome Measure
| Variable (min, max possible score) |
Baseline | Post-CBT | 3 Month follow-up |
Analysis (pre to post) |
Effect size§ |
Analysis (pre to follow-up) |
Effect size§ |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| Mean | SD | Mean | SD | Mean | SD | F | df | p | F | df | p | |||
| CAARMS Glob. Tot. (0, 168) ‡ | 37.67 | 20.91 | 13.33 | 14.98 | 7.00 | 4.06 | 31.47 | 6 | 0.001 | 1.34 | 20.97 | 5.16 | 0.006 | 2.04 |
| CAARMS Freq. Tot.(0, 168) ‡ | 43.50 | 26.89 | 15.33 | 16.92 | 10.40 | 5.94 | 20.71 | 6 | 0.004 | 1.25 | 16.44 | 5.25 | 0.009 | 1.70 |
| SOFAS (1, 100) † | 48.50 | 3.67 | 60.83 | 6.64 | 74.00 | 5.48 | 58.67 | 6 | <0.001 | 2.30 | 116.26 | 1.78 | 0.013 | 5.49 |
| CAARMS Pos. Glob. (0, 24)‡ | 7.67 | 5.09 | 1.50 | 1.52 | 1.20 | 1.30 | 12.58 | 6 | 0.012 | 1.64 | 10.88 | 6.13 | 0.016 | 1.74 |
| CAARMS Pos. Freq. (0, 24) $ | 10.33 | 7.34 | 1.83 | 1.94 | 1.00 | 1.23 | 12.07 | 6 | 0.013 | 1.58 | 10.62 | 6.05 | 0.017 | 1.77 |
| CAARMS Neg. Glob. (0, 18) $ | 5.83 | 1.94 | 2.00 | 3.10 | 0.40 | 0.55 | 10.41 | 6 | 0.018 | 1.48 | 67.94 | 6.15 | <0.001 | 3.81 |
| CAARMS Neg. Freq. (0, 18) $ | 7.50 | 3.56 | 2.67 | 4.18 | 1.40 | 2.19 | 12.58 | 6 | 0.012 | 1.24 | 31.78 | 6.20 | 0.001 | 2.06 |
| CAARMS Gen. Glob. (0, 48) $ | 9.83 | 8.01 | 3.17 | 3.49 | 2.40 | 1.67 | 10.30 | 6 | 0.018 | 1.08 | 8.60 | 5.72 | 0.028 | 1.28 |
| CAARMS Gen. Freq. (0, 48)‡ | 12.17 | 11.34 | 3.33 | 2.58 | 4.40 | 3.65 | 6.52 | 6 | 0.043 | 1.07 | 6.34 | 5.55 | 0.049 | 0.92 |
| CAARMS Emot. Glob. (0, 18) ‡ | 3.67 | 2.73 | 1.33 | 1.21 | 0.60 | 0.89 | 3.81 | 6 | 0.099 | 1.11 | 9.85 | 6.13 | 0.020 | 1.51 |
| CAARMS Emot. Freq. (0, 18) ‡ | 1.67 | 1.86 | 1.17 | 2.04 | 0.40 | 0.89 | 0.20 | 6 | 0.672 | 0.26 | 8.50 | 5.66 | 0.029 | 0.87 |
| CAARMS Behav. Glob. (0, 24) ‡ | 6.17 | 6.18 | 3.00 | 5.90 | 1.20 | 1.30 | 6.83 | 6 | 0.040 | 0.52 | 6.64 | 5.13 | 0.048 | 1.11 |
| CAARMS Behav. Freq. (0, 24)‡ | 7.17 | 7.03 | 3.83 | 7.47 | 1.20 | 1.30 | 4.67 | 6 | 0.074 | 0.46 | 6.03 | 5.34 | 0.054 | 1.18 |
| CAARMS Cognitive Glob. (0, 24) ‡ | 2.17 | 1.84 | 1.83 | 1.33 | 1.40 | 0.55 | 1.20 | 6 | 0.315 | 0.21 | 1.20 | 5.07 | 0.323 | 0.57 |
| CAARMS Cognitive Freq. (0, 24) ‡ | 2.67 | 2.16 | 1.67 | 1.86 | 2.00 | 1.41 | 1.29 | 6 | 0.300 | 0.50 | 0.46 | 5.50 | 0.526 | 0.37 |
CAARMS motor/physical changes subscale was not included in the analysis due to lack of variation in scores as the majority of study participants showed no symptoms.
Increase in scores is improvement.
Decrease in scores is improvement.
Cohen’s d = (Mpre-Mpost)/s.
Beh., behavioural changes; CAARMS, Comprehensive Assessment of At Risk Mental States; CBT, cognitive behavioural therapy; df, degrees of freedom; Emot., emotional disturbance; F, F-test; Freq, frequency;
Gen., general psychopathology; Glob., global; Neg., negative; P, P-value; Pos., positive; SD, standard deviation; SOFAS, Social and Occupational Functioning Assessment Scale; Tot., total.
Table 3.
Changes in Severity of Symptoms
| Variable (min, max possible score) |
Baseline | Post-CBT | 3 Month follow-up |
Analysis (pre to post) |
Effect size ‡ | Analysis (pre to follow-up) |
Effect size ‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| Mean | SD | Mean | SD | Mean | SD | F | df | p | F | df | p | |||
| PANSS Total (30, 210) † | 57.67 | 11.91 | 41.50 | 7.26 | 38.40 | 4.62 | 11.69 | 6 | 0.014 | 1.64 | 26.15 | 6.23 | 0.002 | 2.13 |
| PANSS Positive (7, 49) † | 14.33 | 2.25 | 8.33 | 1.51 | 8.00 | 1.00 | 40.50 | 6 | <0.001 | 3.13 | 127.16 | 6.77 | <0.001 | 3.64 |
| PANSS Negative (7, 49) † | 14.83 | 5.04 | 12.67 | 2.58 | 9.60 | 2.61 | 0.92 | 6 | 0.373 | 0.54 | 25.32 | 6.09 | 0.002 | 1.30 |
| PANSS General (7, 112) † | 30.00 | 7.46 | 20.17 | 4.71 | 20.40 | 3.78 | 29.29 | 6 | 0.002 | 1.58 | 10.79 | 5.34 | 0.020 | 1.62 |
| PANSS Persecution (1, 7) † | 3.50 | 1.76 | 2.00 | 1.10 | 1.80 | 0.84 | 23.14 | 6 | 0.003 | 1.02 | 13.89 | 5.96 | 0.010 | 1.23 |
| PDI Total (0, 336) † | 45.00 | 36.44 | 10.17 | 12.40 | 9.00 | 12.06 | 8.95 | 6 | 0.024 | 1.28 | 12.52 | 5.94 | 0.012 | 1.33 |
| PDI Distress (0, 105) † | 13.00 | 10.83 | 2.17 | 3.92 | 1.40 | 1.67 | 10.04 | 6 | 0.019 | 1.33 | 10.97 | 6 | 0.016 | 1.50 |
| PDI Preoccupation (0, 105) † | 13.33 | 9.61 | 3.00 | 3.80 | 2.60 | 3.44 | 12.59 | 6 | 0.012 | 1.41 | 15.24 | 5.69 | 0.009 | 1.49 |
| PDI Conviction (0, 105) † | 16.00 | 12.74 | 3.83 | 3.71 | 11.40 | 5.96 | 7.83 | 6 | 0.031 | 1.30 | 14.21 | 6.05 | 0.009 | 0.46 |
| BDI (0, 63) † | 19.5 | 15.35 | 8.83 | 9.52 | 6.80 | 10.13 | 13.16 | 6 | 0.011 | 0.84 | 5.86 | 4.23 | 0.069 | 0.98 |
| STAI (20, 80) † | 50.17 | 15.43 | 36.83 | 9.41 | 41.60 | 12.44 | 5.23 | 6 | 0.062 | 1.04 | 1.23 | 3.94 | 0.315 | 0.56 |
Decrease in scores is improvement.
Cohen’s d = (Mpre-Mpost)/s.
BDI-II, Beck Depression Inventory-II; CBT, cognitive behavioural therapy; df, degrees of freedom; F, F-test; P, P-value; PANSS, Positive and Negative Syndrome Scale; PDI, Peters’ Delusions Inventory; SD, standard deviation; STAI, State-Trait Anxiety Inventory.
Effect of treatment intervention on cognitive processes and biases
Targeted cognitive biases also decreased at post-treatment (DACOBS, P < 0.001, d = 1.19), specifically in the domains of cognitive inflexibility, external attribution bias, social cognition, and subjective cognitive problems (domains that were elevated above norms for healthy controls at baseline). Reductions, though not statistically significant, were also observed for jumping to conclusions bias (The Beads Task; Table 4).
Table 4.
Changes in Information Processing Style
| Variable (min, max possible score) |
Baseline | Post-CBT | 3 Month Follow-up |
Analysis (pre to post) |
Effect size§ | Analysis (pre to follow-up) |
Effect size§ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| Mean | SD | Mean | SD | Mean | SD | F | df | p | F | df | p | |||
| DACOBS Total (42, 294) ‡ e | 155.67 | 33.61 | 111.00 | 41.25 | 104.60 | 47.19 | 44.54 | 6 | <0.001 | 1.19 | 4.76 | 3.74 | 0.099 | 1.25 |
| DACOBS Jump (6, 42) ‡ | 22.83 | 4.17 | 20.67 | 6.13 | 17.60 | 9.40 | 0.84 | 6 | 0.394 | 0.41 | 1.64 | 4.91 | 0.258 | 0.72 |
| DACOBS CogInfx(6, 42) ‡ e | 18.67 | 5.09 | 14.17 | 6.76 | 12.20 | 4.09 | 16.02 | 6 | 0.007 | 0.75 | 10.75 | 3.69 | 0.034 | 1.40 |
| DACOBS Attn (6, 42) ‡ | 23.00 | 10.52 | 18.00 | 6.68 | 17.60 | 9.40 | 2.20 | 6 | 0.189 | 0.57 | 0.16 | 4.99 | 0.709 | 0.54 |
| DACOBS ExtAtt (6, 42) ‡ e | 20.67 | 6.77 | 16.17 | 6.91 | 17.60 | 9.13 | 132.55 | 6 | <0.001 | 0.66 | 1.23 | 5.41 | 0.314 | 0.38 |
| DACOBS SocCog (6, 42) ‡ e | 26.00 | 4.73 | 14.67 | 6.25 | 16.60 | 9.66 | 34.17 | 6 | 0.001 | 2.04 | 1.28 | 5.00 | 0.309 | 1.24 |
| DACOBS Safe (6, 42) ‡ e | 19.17 | 9.68 | 14.67 | 8.38 | 14.40 | 11.17 | 1.22 | 6 | 0.312 | 0.50 | 0.97 | 4.24 | 0.377 | 0.56 |
| DACOBS SubCog (6, 42) ‡ e | 23.50 | 8.22 | 12.67 | 7.34 | 8.60 | 2.97 | 58.91 | 6 | <0.001 | 1.39 | 35.32 | 5.93 | 0.001 | 2.41 |
| Beads Task 85/15 † | 7.00 | 4.52 | 8.67 | 5.54 | 0.66 | 6 | 0.447 | 0.33 | ||||||
| Beads Task 60/40† e | 8.83 | 3.97 | 13.00 | 5.14 | 5.01 | 6 | 0.067 | 0.91 | ||||||
| Beads Task Salient † | 9.17 | 6.21 | 9.50 | 6.06 | 0.03 | 6 | 0.865 | 0.05 | ||||||
Increase in scores is improvement.
Decrease in scores is improvement.
Cohen’s d = (Mpre-Mpost)/s.
Attn, attention to threat bias; BEADS, a task used to measure a ‘jump-to-conclusions’ reasoning bias or making decisions on the basis of limited evidence; CBT, cognitive behavioural therapy; CogInfx, cognitive inflexibility bias; DACOBS, Davos Assessment of Cognitive Biases; df, degrees of freedom; e, high or very high levels at baseline, compared with normal population; ExtAtt, external attribution bias; F, F-test; Jump, jumping to conclusions bias; P, P-value; Safe, safety behaviours; SD, standard deviation; SocCog, social cognition problems; SubCog, subjective cognitive problems.
Effect of treatment intervention on adolescents’ psychosocial functioning and family communication
Improvements in adolescents’ social functioning were evident at post-treatment, (GFS P < 0.05, d = 0.85). Family members’ reports of adolescents’ social functioning concurred with these results (SFS P = 0.061, d = 1.02), particularly regarding social engagement and independence performance at 3-month follow-up (P < 0.05, d = 0.96 and P < 0.05, d = 1.51, respectively). Family members’ empathy, as perceived by adolescents, also increased but did not reach statistical significance (ES-F, P = > 0.05, d = 0.96). Gains were largely maintained at the 3-month follow-up. Likewise, there were significant improvements in family members’ communication and use of CBT skills at post-treatment (CBTSF-S, P < 0.05, d = 0.56; Table 5).
Table 5.
Changes in Adolescent’s Functioning and Family Communication
| Variable (min, max possible score) |
Baseline | Post-CBT | 3 Month follow-up |
Analysis (pre to post) |
Effect size‡ | Analysis (pre to follow-up) |
Effect size‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| Mean | SD | Mean | SD | Mean | SD | F | df | p | F | df | p | |||
| Adolescents | ||||||||||||||
| GFR (1, 10) † | 6.33 | 2.25 | 7.17 | 1.60 | 8.00 | 0.71 | 5.17 | 6 | 0.063 | 0.43 | 5.17 | 2.02 | 0.150 | 1.00 |
| GFS (1, 10) † | 5.50 | 2.35 | 7.17 | 1.47 | 7.60 | 1.34 | 7.50 | 6 | 0.034 | 0.85 | 7.20 | 4.21 | 0.052 | 1.10 |
| SFS Family Total (0, 223) † | 106.00 | 21.06 | 125.00 | 15.62 | 133.00 | 9.83 | 5.81 | 5 | 0.061 | 1.02 | 6.29 | 2.50 | 0.104 | 1.65 |
| SFS Social (0, 15) † | 9.80 | 2.39 | 10.80 | 1.30 | 12.00 | 2.16 | 0.96 | 5 | 0.372 | 0.52 | 12.40 | 4.36 | 0.021 | 0.96 |
| SFS Independence (0, 39) † | 22.60 | 7.16 | 26.40 | 3.78 | 30.25 | 0.50 | 1.61 | 5 | 0.261 | 0.66 | 7.57 | 4.99 | 0.040 | 1.51 |
| Family Members | ||||||||||||||
| ES-F ( −15,15 ) † | 9.60 | 5.03 | 13.50 | 2.81 | 12.40 | 2.88 | 4.21 | 5 | 0.090 | 0.96 | 7.03 | 4.55 | 0.050 | 0.68 |
| CBTFS-S Emot. Expression | 2.00 | 1.58 | 3.40 | 1.14 | 2.75 | 1.71 | 9.42 | 5 | 0.028 | 1.02 | 2.34 | 4 | 0.197 | 0.46 |
| CBTFS-S Collect. Evidence | 1.20 | 0.84 | 2.40 | 1.34 | 1.50 | 1.29 | 12.86 | 5 | 0.016 | 1.07 | 1.33 | 4 | 0.312 | 0.28 |
| CBTFS-S Elicit Alternative | 2.00 | 1.00 | 3.60 | 1.14 | 3.50 | 1.29 | 53.33 | 5 | 0.001 | 1.49 | 71.88 | 4 | 0.001 | 1.30 |
| CBTFS-S Offer Alternative | 2.20 | 1.10 | 3.60 | 1.14 | 3.25 | 1.50 | 40.83 | 5 | 0.001 | 1.25 | 39.08 | 4 | 0.002 | 0.80 |
| CBTFS-S Planning Future | 1.20 | 1.10 | 2.60 | 1.14 | 2.00 | 1.83 | 54.47 | 5 | 0.001 | 1.25 | 1.26 | 4 | 0.323 | 0.53 |
| CBTFS-S Empathic Respond | 2.40 | 0.89 | 3.40 | 0.55 | 2.75 | 0.96 | 12.50 | 5 | 0.017 | 1.35 | 1.00 | 4 | 0.338 | 0.38 |
| CBTFS-S Interpersonal | 2.60 | 1.14 | 3.80 | 1.10 | 3.00 | 1.41 | 45.00 | 5 | 0.001 | 1.07 | 1.26 | 4 | 0.323 | 0.31 |
Increase in scores is improvement.
Cohen’s d = (Mpre-Mpost)/s.
CBT, cognitive behavioural therapy; CBTSF-S, CBT Skills for Families Scale; Collect. Evidence, collecting evidence for beliefs; df, degrees of freedom; Emot. Expression, encouraging emotional expression; Empathic Respond, empathically respond; Encourage Alternative, encourage alternative explanation; ES-F, Empathy Scale Family; GFR, Global Functioning Role; GFS, Global Functioning Social; Interpersonal, Interpersonal Effectiveness; Offer Alternative, offer alternative explanation; P, P-value; Planning Future, planning to prevent future reoccurrence; SD, standard deviation F, F-test; SFS, Social Functioning Scale.
Therapeutic alliance and group therapeutic factors
Participants reported high levels of therapeutic alliance with the therapist (WAI adolescents, M = 6.33, SD = 0.91; WAI family members, M = 6.63, SD = 0.50; Supporting Information Table S4). Perceived therapist’s empathy and group cohesiveness were in high ranges for both adolescents (M = 14.17, SD = 1.17 and M = 59.50, SD = 2.43, respectively) and family members (M = 13.50, SD = 2.81 and M = 55.20, SD = 3.56, respectively). Adolescents reported ‘altruism’ and ‘installation of hope’ as the most important therapeutic factors, whereas family members emphasized ‘universality’ and group ‘cohesion’ (‘I’m not the only one,’ ‘others had solved problems similar to mine’; Supporting Information Table S5).
Discussion
The uniqueness of the GF-CBT program is in harnessing group, family and individual CBT modalities, the theoretical basis of the intervention, and innovative nature of teaching CBT skills to families. The present study supports the feasibility of the GF-CBT program, as evidenced by the lack of dropout high levels of therapeutic alliance and satisfaction with the program. All study participants had largely positive comments about the set-up of the program and recommended maintaining all three modalities. Both adolescents and family members found the group format beneficial for learning, feedback and social support. They enjoyed the parallel learning that took place in adolescent and family groups. The group format appears to be particularly beneficial for adolescents prone to psychosis as it provides an opportunity for corrective interpersonal experiences and enhances psychosocial functioning. The majority of adolescents felt that 15 weeks was the correct length for the program and reported that classroom format, where didactic material is alternated with written responses to questions followed by discussion, was particularly appealing.
As expected, none of the participants developed a psychotic episode and all remitted from at-risk mental state as defined by the CAARMS by the end of the intervention. As a group, participants showed statistically significant decreases in symptoms and improvements in functioning, suggesting clinical benefits of the program. All program participants reported increases in positive thoughts and enhanced ability to cope with paranoid or stressful thoughts, problem-solve and modulate emotional experiences. Similar to other studies on group CBT for psychosis,50,51 the majority of adolescents reported improved self-esteem, decreased social isolation and reduced feelings of loneliness. These improvements were consistent with reported feelings of increased connection with and support from other group members.
This study also demonstrated the feasibility of teaching family members CBT skills. All participants reported improved family communication. Family members reported increased empathy and understanding of their offspring’s experiences, and greater confidence in their ability to help. Adolescents reported improvements in family members’ empathy and an increased willingness to share distressing emotional experiences with family members.
Consistent with other studies,27,52,53 these results suggest that learning about the cognitive model and psychosis-specific cognitive biases is beneficial for preventing onset and escalation of symptomatology among at-risk individuals. At baseline, participants self-reported elevated belief inflexibility and external attribution biases, as measured by DACOBS, and, consistent with Broome et al.,54 demonstrated JTC bias on the harder version of the Beads Task (60/40), but not on the easy version (80/20). At post-treatment, these biases normalized. There was a discrepancy between self-reported JTC, measured by DACOBS, which was below average41 at all time points and experimental JTC, measures by the harder version of the Beads Task, suggesting participants’ lack of awareness about the JCT bias. Freeman et al.55 recommend that intervention for JCT should address working memory deficits. Our preliminary findings support the notion that increasing awareness of JCT bias through explicit instruction may also be beneficial.
The GF-CBT pilot study was a small open trial, with significant limitations, including lack of control group, unblinded assessments and potential confounds, such as the use of psychotropic medications. Another limitation is the short follow-up of 3 months; however, a 2-year follow-up of the study cohort is taking place to further assess symptom status and social function. Although there was no dropout during the course of the study and the intervention was well received, the intervention is intensive and requires a considerable time commitment on the part of families. The number of sessions in this protocol might also pose a financial challenge, especially for those who receive socialized or insurance-managed healthcare. Recruitment for the study was challenging. We solicited referrals from all major New York City hospitals and clinics, but received a relatively small number of inquires, and about one quarter were on behalf of individuals who had already experienced their first psychotic episode. Administering a prodromal screening questionnaire at intake in mental health clinics, modelled after recruitment practices in the Netherlands, 27 may be beneficial in future studies.
This trial established the feasibility and acceptability of the intervention and provided preliminary exploratory information about treatment gains. Currently, GF-CBT is tested in a pilot randomized controlled trial (GF-CBT vs. monitoring), with a 2-year follow-up. Upon completion of this trial, we will examine the size of treatment gains to inform future comparative studies. Although results of this study must be interpreted with caution due to study limitations, our findings support the hypothesis that group and family-based preventative CBT, which targets attenuated psychotic symptoms and aims to preserve psychosocial development, may be beneficial for younger individuals prone to psychosis.
Supplementary Material
Acknowledgments
The authors thank all study participants and the research team members for their hard work, invaluable insight and dedication to this project.
Funding
This work was supported by grant KL2-RR0249997 of the Clinical and Translational Science Center at Weill Cornell Medical College and Sidney R. Baer, Jr. Foundation.
References
- 1.Yung AR, Phillips LJ, Yuen HP, et al. Psychosis prediction: 12-month follow up of a high-risk (‘prodromal’) group. Schizophr Res. 2003;60:21–32. doi: 10.1016/s0920-9964(02)00167-6. [DOI] [PubMed] [Google Scholar]
- 2.Olsen KA, Rosenbaum B. Prospective investigations of the prodromal state of schizophrenia: review of studies. Acta Psychiatr Scand. 2006;113:247–72. doi: 10.1111/j.1600-0447.2005.00697.x. [DOI] [PubMed] [Google Scholar]
- 3.Fusar-Poli P, Borgwardt S, Bechdolf A, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70:107–20. doi: 10.1001/jamapsychiatry.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Addington J, Cornblatt BA, Cadenhead K, et al. Conversion in Napls: those who do not convert to psychosis. Schizophr Bull. 2011;37:1. [Google Scholar]
- 5.Cornblatt BA, Carrion RE, Addington J, et al. Risk factors for psychosis: impaired social and role functioning. Schizophr Bull. 2012;38:1247–57. doi: 10.1093/schbul/sbr136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Gaag M, Smit F, Bechdolf A, et al. Preventing a first episode of psychosis: meta-analysis of randomized controlled prevention trials of 12month and longer-term follow-ups. Schizophr Res. 2013;149:56–62. doi: 10.1016/j.schres.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Birchwood M, Todd P, Jackson C. Early intervention in psychosis. The critical period hypothesis. Br J Psychiatry Suppl. 1998;172:53–9. [PubMed] [Google Scholar]
- 8.Garety PA, Fowler DG, Freeman D, Bebbington P, Dunn G, Kuipers E. Cognitive-behavioural therapy and family intervention for relapse prevention and symptom reduction in psychosis: randomised controlled trial. Br J Psychiatry. 2008;192:412–23. doi: 10.1192/bjp.bp.107.043570. [DOI] [PubMed] [Google Scholar]
- 9.Fusar-Poli P, Yung AR, McGorry P, van Os J. Lessons learned from the psychosis high-risk state: towards a general staging model of prodromal intervention. Psychol Med. 2013;44(1):1–8. doi: 10.1017/S0033291713000184. [DOI] [PubMed] [Google Scholar]
- 10.McGlashan TH, Zipursky RB, Perkins D, et al. The PRIME North America randomized double-blind clinical trial of olanzapine versus placebo in patients at risk of being prodromally symptomatic for psychosis I. Study rationale and design. Schizophr Res. 2003;61:7–18. doi: 10.1016/s0920-9964(02)00439-5. [DOI] [PubMed] [Google Scholar]
- 11.Gourzis P, Katrivanou A, Beratis S. Symptomatology of the initial prodromal phase in schizophrenia. Schizophr Bull. 2002;28(3):415–29. doi: 10.1093/oxfordjournals.schbul.a006950. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan S, Lewis G, Wiles N, Thompson A, Evans J. Psychotic experiences and social functioning: a longitudinal study. Soc Psychiatry Psychiatr Epidemiol. 2013;48:1053–65. doi: 10.1007/s00127-013-0715-x. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien MP, Gordon JL, Bearden CE, Lopez SR, Kopelowicz A, Cannon TD. Positive family environment predicts improvement in symptoms and social functioning among adolescents at imminent risk for onset of psychosis. Schizophr Res. 2006;81:269–75. doi: 10.1016/j.schres.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Freeman D, Garety PA, Kuipers E. Persecutory delusions: developing the understanding of belief maintenance and emotional distress. Psychol Med. 2001;31:1293–306. doi: 10.1017/s003329170100455x. [DOI] [PubMed] [Google Scholar]
- 15.Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust N Z J Psychiatry. 2005;39:964–71. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- 16.Vygotsky LS. Development of higher psychological functions. Sov Psychol. 1977;15:60–73. [Google Scholar]
- 17.Bronfenbrenner U. Young-children in context – impact of self, family and society on development – Mcloughlin,Cs, Gullo,Df. Contemp Psychol. 1986;31:527–8. [Google Scholar]
- 18.Luthar SS, Cicchetti D, Becker B. The construct of resilience: a critical evaluation and guidelines for future work. Child Dev. 2000;71:543–62. doi: 10.1111/1467-8624.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reyna VF, Brainerd CJ. Dual processes in decision making and developmental neuroscience: a fuzzy-trace model. Dev Rev. 2011;31:180–206. doi: 10.1016/j.dr.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reyna VF, Farley F. Risk and rationality in adolescent decision making – implications for theory, practice, and public policy. Psychol Sci. 2006;7:1–44. doi: 10.1111/j.1529-1006.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- 21.Reyna VF. A theory of medical decision making and health: fuzzy trace theory. Med Decis Making. 2008;28:850–65. doi: 10.1177/0272989X08327066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garety PA, Kuipers E, Fowler D, Freeman D, Bebbington PE. A cognitive model of the positive symptoms of psychosis. Psychol Med. 2001;31:189–95. doi: 10.1017/s0033291701003312. [DOI] [PubMed] [Google Scholar]
- 23.Garety PA, Freeman D, Jolley S, et al. Reasoning, emotions, and delusional conviction in psychosis. J Abnorm Psychol. 2005;114:373–84. doi: 10.1037/0021-843X.114.3.373. [DOI] [PubMed] [Google Scholar]
- 24.Landa LN. Algorithmization in Learning and Instruction. Englewood Cliffs, NJ: Educational Technology Publications; 1974. [Google Scholar]
- 25.Landa LN. Landamatics instructional design theory and methodology for teaching general methods of thinking. In: Reigeluth CME, editor. Instructional Design Theories and Models: A New Paradigm of Instructional Theory Mahwah. NJ: Lawrence Erlbaum; 1999. pp. 341–70. [Google Scholar]
- 26.Morrison AP, French P, Walford L, et al. Cognitive therapy for the prevention of psychosis in people at ultra-high risk: randomised controlled trial. Br J Psychiatry. 2004;185:291–7. doi: 10.1192/bjp.185.4.291. [DOI] [PubMed] [Google Scholar]
- 27.van der Gaag M, Nieman DH, Rietdijk J, et al. Cognitive behavioral therapy for subjects at ultrahigh risk for developing psychosis: a randomized controlled clinical trial. Schizophr Bull. 2012;38:1180–8. doi: 10.1093/schbul/sbs105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Addington J, Epstein I, Liu L, French P, Boydell KM, Zipursky RB. A randomized controlled trial of cognitive behavioral therapy for individuals at clinical high risk of psychosis. Schizophr Res. 2011;125:54–61. doi: 10.1016/j.schres.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Landa Y, Chadwick P, Beck AT, et al. Targeting Information processing biases and social avoidance in group cognitive behavioral therapy for paranoia: a pilot randomized controlled clinical trial. Schizophr Bull. 2011;37:271. [Google Scholar]
- 30.Landa Y, Silverstein S, Schwartz F, Savitz A. Group cognitive behavioral therapy for delusions: helping patients improve reality testing. J Contemp Psychother. 2006;36(1):9–17. [Google Scholar]
- 31.Landa Y. Cognitive behavioral therapy for the prevention of paranoia. Workbook. 2010 [Google Scholar]
- 32.Landa Y. Cognitive behavioral skills for families. 2011 [Google Scholar]
- 33.Goldman HH, Skodol AE, Lave TR. Revising axis V for DSM-IV: a review of measures of social functioning. Am J Psychiatry. 1992;149:1148–56. doi: 10.1176/ajp.149.9.1148. [DOI] [PubMed] [Google Scholar]
- 34.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 35.Peters ER, Joseph SA, Garety PA. Measurement of delusional ideation in the normal population: introducing the PDI (Peters et al. Delusions Inventory) Schizophr Bull. 1999;25:553–76. doi: 10.1093/oxfordjournals.schbul.a033401. [DOI] [PubMed] [Google Scholar]
- 36.Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 37.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 38.Dudley REJ, John CH, Young AW, Over DE. Normal and abnormal reasoning in people with delusions. Br J Clin Psychol. 1997;36:243–58. doi: 10.1111/j.2044-8260.1997.tb01410.x. [DOI] [PubMed] [Google Scholar]
- 39.Garety PA, Hemsley DR, Wessely S. Reasoning in deluded schizophrenic and paranoid patients – biases in performance on a probabilistic inference task. J Nerv Ment Dis. 1991;179:194–201. doi: 10.1097/00005053-199104000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Peters E, Garety P. Cognitive functioning in delusions: a longitudinal analysis. Behav Res Ther. 2006;44:481–514. doi: 10.1016/j.brat.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 41.van der Gaag M, Schutz C, ten Napel A, et al. Development of the Davos Assessment of Cognitive Biases Scale (DACOBS) Schizophr Res. 2013;144:63–71. doi: 10.1016/j.schres.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The social functioning scale – the development and validation of a new scale of social-adjustment for use in family intervention programs with schizophrenic-patients. Br J Psychiatry. 1990;157:853–9. doi: 10.1192/bjp.157.6.853. [DOI] [PubMed] [Google Scholar]
- 43.Cornblatt BA, Auther AM, Niendam T, et al. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophr Bull. 2007;33:688–702. doi: 10.1093/schbul/sbm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burns DD, Auerbach A. Therapeutic Empathy in Cognitive–Behavioral Therapy: Does it Really Make a Difference? New York: Guilford Press; 1996. [Google Scholar]
- 45.Horvath AO, Greenberg LS. Development and validation of the working alliance inventory. J Couns Psychol. 1989;36:223–33. [Google Scholar]
- 46.Stokes JP. Toward an understanding of cohesion in personal change groups. Int J Group Psychother. 1983;33:449–67. doi: 10.1080/00207284.1983.11491345. [DOI] [PubMed] [Google Scholar]
- 47.Bloch S, Reibstein J, Crouch E, Holroyd P, Themen J. A method for the study of therapeutic factors in group psychotherapy. Br J Psychiatry. 1979;134:257–63. doi: 10.1192/bjp.134.3.257. [DOI] [PubMed] [Google Scholar]
- 48.Wechsler D. Wechsler Test of Adult Reading. San Antonio, TX: Harcourt Assessment; 2001. [Google Scholar]
- 49.Loewy RL, Bearden CE, Johnson JK, Raine A, Cannon TD. The prodromal questionnaire (PQ): preliminary validation of a self-report screening and psychotic measure for prodromal syndromes. Schizophr Res. 2005;79:117–25. [PubMed] [Google Scholar]
- 50.Granholm E, Ben-Zeev D, Link PC. Social disinterest attitudes and group cognitive-behavioral social skills training for functional disability in schizophrenia. Schizophr Bull. 2009;35:874–83. doi: 10.1093/schbul/sbp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lecomte T, Leclerc C, Corbiere M, Wykes T, Wallace CJ, Spidel A. Group cognitive behavior therapy or social skills training for individuals with a recent onset of psychosis? Results of a randomized controlled trial. J Nerv Ment Dis. 2008;196:866–75. doi: 10.1097/NMD.0b013e31818ee231. [DOI] [PubMed] [Google Scholar]
- 52.Moritz S, Kerstan A, Veckenstedt R, et al. Further evidence for the efficacy of a metacognitive group training in schizophrenia. Behav Res Ther. 2011;49:151–7. doi: 10.1016/j.brat.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 53.Gaweda L, Moritz S, Kokoszka A. The metacognitive training for schizophrenia patients: description of method and experiences from clinical practice. Psychiatr Pol. 2009;43:683–92. [Epub 2010/03/10]; Trening metapoznawczy dlachorych na schizofrenie. opis metody I doswiadczen klinicznych. [PubMed] [Google Scholar]
- 54.Broome MR, Johns LC, Valli I, et al. Delusion formation and reasoning biases in those at clinical high risk for psychosis. Br J Psychiatry. 2007;191:S38–42. doi: 10.1192/bjp.191.51.s38. [DOI] [PubMed] [Google Scholar]
- 55.Freeman D, Startup H, Dunn G, et al. Understanding jumping to conclusions in patients with persecutory delusions: working memory and intolerance of uncertainty. Psychol Med. 2014;44(14):1–8. doi: 10.1017/S0033291714000592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


