Abstract
Background
The Khorana score is a predictive risk model for venous thromboembolism (VTE) in cancer patients planning to receive chemotherapy. Urothelial carcinoma and variant histologies (UC/VH) were underrepresented in the model. We sought to evaluate if the Khorana score predicts for VTE in a retrospective multinational dataset of metastatic UC/VH patients.
Methods
Patients diagnosed with metastatic UC/VH who received chemotherapy were eligible. Those with incomplete or miscoded data were excluded. Khorana scores were calculated based on pre-treatment data and categorized into high (≥3) or intermediate (1-2) VTE risk. Other patient, tumor, and therapy related factors were also analyzed. Chi-squared and logistic regression analyses were utilized to assess differences in VTE rates based on clinical characteristics. Subgroup analyses were performed to evaluate the Khorana score and associated variables for early (<3 months) and late (>3 months) VTE.
Results
943 patients were eligible for analysis. The cumulative VTE rate was 9.9%. There was no statistical difference in overall VTE rate between Khorana high and intermediate-risk groups (P=0.16). In the multivariate analysis, non-urothelial histology (OR=2.56,P=0.002) and the presence of cardiovascular (CVD) or CVD risk factors (OR=2.14,P=0.002) were associated with increased VTE risk. In the first 3 months from initiation of chemotherapy, Khorana high-risk (OR:2.08,P=0.04) was associated with higher VTE rates. White blood cell (WBC) count (OR:1.05,P=0.04) was the only significant Khorana variable for early VTE.
Conclusions
The Khorana score stratifies early, but not overall VTE risk in metastatic UC/VH patients. WBC count drives the increased early VTE risk seen with the Khorana score.
Introduction
Venous thromboembolism (VTE) is a common complication in the management of cancer patients, and is associated with increased morbidity and mortality.1-3 Compared to the general population, cancer patients are at a 4-7 fold increased risk of developing a VTE.3 However, the risk of developing a VTE is not uniform across all patients, and is dependent on various patient, tumor, and treatment-related factors. Nonetheless, individual risk factors alone are insufficient to identify a high-risk population who may benefit from primary thromboprophylaxis.
The Khorana score is a predictive risk assessment model for VTE in cancer patients.4 Khorana et al. identified 5 variables predictive for VTE: primary tumor site, body mass index (BMI), white blood cell (WBC) count, platelet count, and hemoglobin level or use of erythropoiesis-stimulating agents.4 The risk model then categorized patients into low (0), intermediate (1-2), or high-risk (≥3) based on these variables. Subsequently, the Khorana score was validated in the Vienna Cancer and Thrombosis Study (CATS), a prospective, observational cohort study of 819 patients representing 10 different malignancies.5
Although the Khorana score is now a validated tool to stratify VTE risk in cancer patients, urothelial carcinoma and variant histologies (UC/VH) were not well represented in these studies. In the original analysis, bladder cancer patients were grouped into the “other” category, which only represented 10% of the study population.4 Moreover, in the CATS study, UC/VH patients were not included in the analysis altogether.5 Yet, patients with genitourinary malignancies (excluding prostate cancer) are considered at “higher risk” and receive 1 point in the risk model. In this study, we sought to evaluate whether the Khorana score predicted for VTE in metastatic UC/VH patients treated with systemic chemotherapy.
Methods
Study Design and Patient Population
The Retrospective International Study of Cancers of the Urothelium (RISC) is a population-based, multi-center, international retrospective study of the management and outcomes of patients who develop at least muscle-invasive UC/VH. Clinical data was extracted from patient charts and compiled via a password-protected data capture platform from 29 international academic centers with previously described methods.6 All participating institutions were granted approval by their respective institutional review boards.
Patients diagnosed with metastatic disease of the bladder, urethra, or upper urinary tract (renal pelvis or ureter) who were subsequently treated with systemic chemotherapy were eligible for analysis. All patients were required to have complete information on all the Khorana score variables for inclusion in the analysis. Patients with miscoded data were excluded. Any patient without a date of diagnosis of metastatic disease or VTE data was also excluded. VTE incidence stratified by Khorana risk group was the primary endpoint of this analysis. A VTE was defined as a deep vein thrombosis, pulmonary embolism, catheter-associated thrombosis, or other venous thrombosis (eg splanchnic vein thrombosis); all diagnoses were assigned by local investigators without central radiographic adjudication. Khorana scores were calculated based on pre-treatment data and categorized into high (≥3) or intermediate (1-2) VTE risk. Low VTE risk score was not possible given the 1-point mandatory assignment for all patients in this analysis for their primary tumor site. Additionally, each individual Khorana score variable was independently assessed.
Other patient, tumor, and treatment-related factors were evaluated for their association with VTE risk. We have previously reported from the RISC database that non-urothelial histology, the presence of cardiovascular disease (CVD) (coronary artery disease, peripheral vascular disease, or a cerebrovascular accident) or CVD risk factors (hypertension, diabetes mellitus, or hyperlipidemia) and renal dysfunction are associated with development of a VTE in the metastatic setting.7 Given that the patient cohort for this analysis is a subset of the prior study, these clinical characteristics were chosen a priori to be included in the multivariate analysis. Comorbidities captured in the database were derived from the Charlson Comorbidity Index.8 Those with at least 2% prevalence were further assessed for their association with VTE. Age, gender, race, primary tumor location, and primary tumor treatment with either surgery or radiotherapy were also evaluated.
Lastly, since the median follow-up in the development and validation cohorts of the Khorana score was 73 days, we hypothesized that the Khorana score may be most effective in predicting early VTE risk.4 Therefore, we performed a subgroup analysis of Khorana high or intermediate-risk stratified by early (within the first three months) and late (after three months) VTE development following initiation of systemic chemotherapy.
Statistical Analysis
Cumulative VTE incidence was calculated from the time of initiation of first-line chemotherapy. For the univariate analysis, chi-square and fisher's exact test were utilized to evaluate differences in VTE rates when assessing categorical independent variables. When evaluating VTE rates based on continuous independent variables in the univariate analysis, simple logistic regression was applied. Khorana risk groups, a priori identified clinical characteristics, demographic variables (ie age, gender, and race), and any statistically significant independent covariates in the univariate analysis (P≤.05) were planned to be included in the multivariate logistic regression model. The subgroup analyses were carried out using the same multivariate logistic regression model.
Results
Study Population and Patient Characteristics
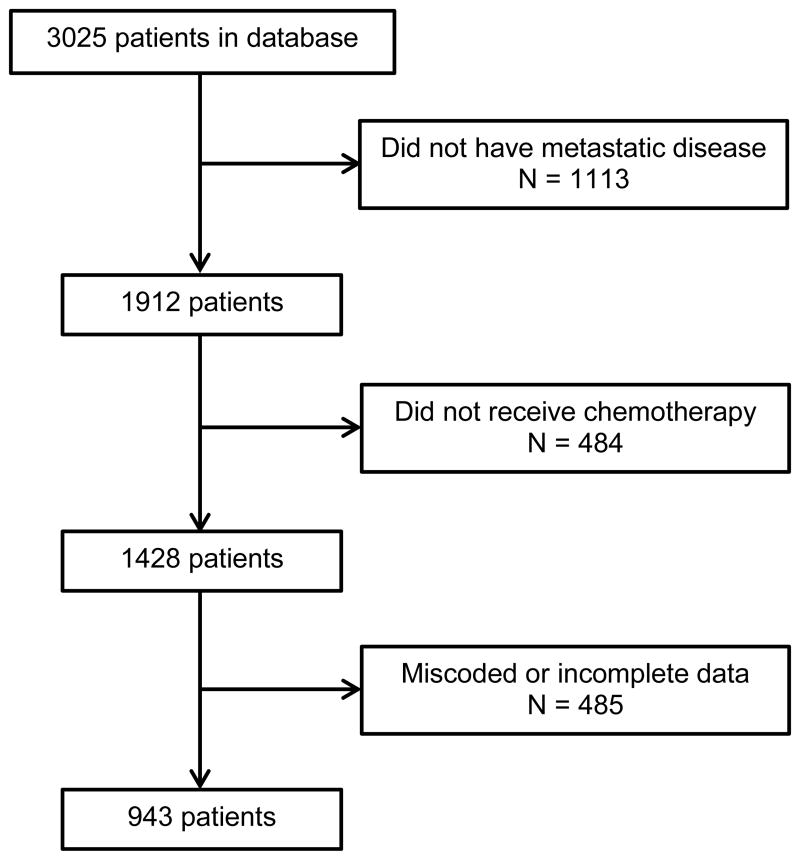
Of the 3025 patients that comprise the RISC database, 1912 developed metastatic disease (Figure 1). Of these, 484 did not receive chemotherapy in the metastatic setting and 485 had incomplete or miscoded data. Therefore, 943 patients were eligible for analysis. The baseline patient characteristics of the study cohort are shown in Table 1. The mean age was 66.0. Most patients' primary tumor was located in the bladder (82.4%), and the most common histologic subtype was urothelial (91.5%).
Figure 1.
Flow chart of patient inclusion and exclusion for analysis.
Table 1. Baseline characteristics of study cohort.
| Characteristic | N (%) |
|---|---|
|
| |
| Age: Mean +/- SD | 66.0 +/- 10.0 |
|
| |
| Gender | |
| Male | 731 (77.5) |
| Female | 207 (22.0) |
| Unknown | 5 (0.5) |
|
| |
| Race | |
| Caucasian | 863 (91.5) |
| Black | 36 (3.8) |
| Asian | 15 (1.6) |
| Hispanic | 19 (2.0) |
| Unknown | 10 (1.1) |
|
| |
| Tumor location | |
| Bladder | 777 (82.4) |
| Other | 149 (15.8) |
| Unknown | 17 (1.8) |
|
| |
| Histology | |
| Urothelial | 863 (91.5) |
| Non-urothelial | 80 (8.5) |
|
| |
| Primary treatment* | |
| Surgery | 539 (57.2) |
| Radiotherapy | 48 (5.1) |
| None/Unknown | 375 (39.8) |
19 patients treated with both surgery and radiotherapy
Cumulative Incidence of VTE and Association with Clinical Characteristics
Unadjusted VTE incidence rates based on patient demographics and clinical characteristics are shown in Table 2. In all, there were 93 VTEs for a cumulative incidence of 9.9%. The VTE incidence rates for the high-risk and intermediate-risk groups were 13.0% (95%CI:8.2%-19.1%) and 9.2% (95%CI:7.3%-11.5%), respectively. However, there was not a statistically significant difference in cumulative VTE incidence between the two groups (P=0.15). Additionally, none of the independent Khorana score variables were associated with increased VTE risk. Non-urothelial histology (P<0.001), the presence of CVD or CVD risk factors (P=0.001) and renal dysfunction (P=0.03) were associated with development of a VTE, as previously reported.7 No other clinical characteristics assessed achieved statistical significance in the univariate analysis.
Table 2. Unadjusted venous thromboembolism incidence rates by clinical characteristics.
| VTE/N | VTE rate (%) | 95% CI | p-value | |
|---|---|---|---|---|
|
| ||||
| Total | 93/943 | 9.9 | 8.0,12.0 | -- |
|
| ||||
| Age§ | 0.99* | 0.97,1.01 | 0.41 | |
|
| ||||
| Gender | ||||
| Male | 66/731 | 9.0 | 7.0,11.3 | 0.09 |
| Female | 27/207 | 13.0 | 8.8,18.4 | |
|
| ||||
| Race | ||||
| Caucasian | 83/863 | 9.6 | 7.7,11.8 | 0.38 |
| Other | 9/70 | 12.9 | 6.1,23.0 | |
|
| ||||
| Primary tumor location | ||||
| Bladder | 81/777 | 10.4 | 8.4,12.8 | 0.26 |
| Other | 11/149 | 7.4 | 3.7,12.8 | |
|
| ||||
| Histology | ||||
| Urothelial | 76/863 | 8.8 | 7.0,10.9 | <0.001 |
| Non-urothelial | 17/80 | 21.3 | 12.9,31.8 | |
|
| ||||
| Primary treated with surgery | ||||
| Surgery | 48/539 | 8.9 | 6.6,11.6 | 0.26 |
| No surgery | 45/404 | 11.1 | 8.2,14.6 | |
|
| ||||
| Primary treated with radiation | ||||
| Radiation | 5/48 | 10.4 | 3.5,22.7 | 0.81 |
| No radiation | 88/895 | 9.8 | 8.0,12.0 | |
|
| ||||
| CVD or CVD risk factors | ||||
| Yes | 64/496 | 12.9 | 10.1,16.2 | 0.001 |
| No | 29/447 | 6.5 | 4.4,9.2 | |
|
| ||||
| Renal dysfunction | ||||
| Yes | 13/77 | 16.9 | 9.3,27.1 | 0.03 |
| No | 80/866 | 9.2 | 7.4,11.4 | |
|
| ||||
| COPD | ||||
| Yes | 6/82 | 7.3 | 2.7,15.3 | 0.42 |
| No | 87/861 | 10.1 | 8.2,12.3 | |
|
| ||||
| Congestive heart failure | ||||
| Yes | 2/26 | 7.7 | 1.0, 25.1 | 1.00 |
| No | 91/917 | 9.9 | 8.1,12.0 | |
|
| ||||
| Khorana risk group | ||||
| High-risk (≥3) | 21/162 | 13.0 | 8.2,19.1 | 0.15 |
| Intermediate-risk (1-2) | 72/781 | 9.2 | 7.3,11.5 | |
|
| ||||
| WBC count (WBC/ul)§ | 1.00* | 0.96,1.05 | 0.92 | |
|
| ||||
| Hemoglobin (g/dl)§ | 1.01* | 0.91,1.13 | 0.82 | |
|
| ||||
| Platelet count (plt/ul)§ | 1.00* | 0.998, 1.001 | 0.67 | |
|
| ||||
| Body mass index (kg/m2)§ | 1.04* | 1.00, 1.08 | 0.08 | |
Calculated as continuous variables
Results indicate odds ratios for the given variables
Multivariate Analysis of Clinical Characteristics and VTE
As previously reported7, on multivariate analysis, non-urothelial histology (OR=2.56, 95%CI:1.40,4.70; P=0.002) and CVD or CVD risk factors (OR=2.14, 95%CI:1.31,3.48, P=0.002) were associated with an increased incidence of VTE (Table 3). Patients stratified into the Khorana high-risk group did not have a statistically significant increase in VTE compared to patients with an intermediate-risk Khorana score (OR=1.48, 95%CI:0.86,2.53; P=0.16). Renal dysfunction (OR=1.69, 95%CI:0.86,3.29: P=0.13) also did not reach statistical significance. This may have been a result of a low absolute event rate and low prevalence of renal dysfunction in this study cohort, resulting in insufficient power to demonstrate a difference.
Table 3. Multivariate logistic regression of venous thromboembolic risk based on clinical characteristics.
| Clinical characteristic | OR | 95% CI | p-value |
|---|---|---|---|
| Khorana high-risk | 1.48 | 0.86, 2.53 | 0.16 |
| Non-urothelial histology | 2.56 | 1.40, 4.70 | 0.002 |
| CVD or CVD risk factors | 2.14 | 1.31, 3.48 | 0.002 |
| Renal dysfunction | 1.69 | 0.86, 3.29 | 0.13 |
Multivariate Analysis of Association of Khorana score with Early (<3 months) and Late (>3 months) VTE
On subgroup analysis, a high-risk Khorana score was associated with a statistically significant increase in early VTE (OR=2.08, 95%CI:1.04,4.15; P=0.04) but not late VTE (OR=0.94, 95%CI:0.42,2.10; P=0.89) (Table 4). When assessing each variable comprising the Khorana score independently, only WBC count (OR=1.05, 95%CI:1.00, 1.10; P=0.04) was associated with a higher frequency of early VTE.
Table 4. Multivariate logistic regression of early (< 3 months) and late (> 3 months) venous thromboembolism from initiation of systemic therapy based on clinical characteristics.
| Clinical Characteristic | VTE/N (%) | OR | 95% CI | p-value |
|---|---|---|---|---|
|
| ||||
| Early VTE (< 3 months) | ||||
|
| ||||
| Khorana risk group | ||||
| High-risk | 13/162 (8.0) | 2.08 | 1.04, 4.15 | 0.04 |
| Intermediate-risk | 31/781 (4.0) | 1.0 | ref | ref |
|
| ||||
| WBC count (WBC/ul)§ | N/A | 1.05 | 1.00, 1.10 | 0.04 |
|
| ||||
| Hemoglobin (g/dl)§ | N/A | 0.97 | 0.82, 1.15 | 0.73 |
|
| ||||
| Platelet count (plt/ul)§ | N/A | 1.00 | 0.999, 1.003 | 0.38 |
|
| ||||
| Body mass index (kg/m2)§ | N/A | 1.04 | 0.99, 1.09 | 0.17 |
|
| ||||
| Histology | ||||
| Non-urothelial | 6/80 (7.5) | 1.60 | 0.64, 4.01 | 0.32 |
| Urothelial | 38/863 (4.4) | 1.0 | ref | ref |
|
| ||||
| CVD or CVD risk factors | ||||
| Yes | 30/496 (6.1) | 2.03 | 1.03, 4.03 | 0.04 |
| No | 14/447 (3.1) | 1.0 | ref | ref |
|
| ||||
| Renal dysfunction | ||||
| Yes | 5/77 (6.5) | 1.26 | 0.46, 3.41 | 0.65 |
| No | 39/866 (4.5) | 1.0 | ref | ref |
|
| ||||
| Late VTE (> 3 months) | ||||
|
| ||||
| Khorana risk group | ||||
| High-risk | 8/162 (4.9) | 0.94 | 0.42, 2.10 | 0.89 |
| Intermediate-risk | 41/781 (5.3) | 1.0 | ref | ref |
|
| ||||
| WBC count (WBC/ul)§ | N/A | 0.93 | 0.84, 1.02 | 0.12 |
|
| ||||
| Hemoglobin (g/dl)§ | N/A | 1.12 | 0.97, 1.31 | 0.13 |
|
| ||||
| Platelet count (plt/ul)§ | N/A | 1.00 | 0.996, 1.001 | 0.15 |
|
| ||||
| Body mass index (kg/m2)§ | N/A | 1.01 | 0.95, 1.06 | 0.85 |
|
| ||||
| Histology | ||||
| Non-urothelial | 11/80 (13.8) | 3.17 | 1.52, 6.60 | 0.002 |
| Urothelial | 38/863 (4.4) | 1.0 | ref | ref |
|
| ||||
| CVD or CVD risk factors | ||||
| Yes | 34/496 (6.9) | 2.06 | 1.07, 3.97 | 0.03 |
| No | 15/447 (3.4) | 1.0 | ref | ref |
|
| ||||
| Renal dysfunction | ||||
| Yes | 8/77 (10.4) | 1.98 | 0.86, 4.56 | 0.11 |
| No | 41/866 (4.7) | 1.0 | ref | ref |
Calculated as continuous variables
Discussion
The Khorana score was developed to improve stratification of VTE risk in ambulatory cancer patients planning on receiving chemotherapy.4 One potential application of the Khorana score is to identify a subset of cancer patients at particularly high risk of VTE for enrollment in prospective primary thromboprophylaxis studies. Two randomized clinical trials of primary thromboprophylaxis in multiple solid tumors have demonstrated a statistically significant decrease in VTE rates.9, 10 However, the absolute benefit from thromboprophylaxis was only approximately 2% in both studies, with the placebo group in both studies having a VTE rate of less than 4% at a median follow-up of 3.5 months. Consequently, primary thromboprophylaxis has not been adopted into routine clinical practice nor recommended by consensus guidelines for VTE prevention.11, 12 In their original analysis, Khorana et al. demonstrated that patients with a high-risk score had a VTE rate of 7.1% and 6.7% whereas the intermediate-risk score patients had a VTE rate of 1.8% and 2.0% in the development and validation cohorts, respectively.4 Subsequently, the CATS study showed a VTE incidence rate of 17.7% at 6 months in patients with a high-risk Khorana score.5 These studies suggest that patients with high-risk Khorana scores may be ideal for clinical trial enrollment in primary thromboprophylaxis studies.
In our patient cohort, there was no statistically significant difference in overall VTE rates between Khorana high-risk and intermediate-risk patients. However, when we evaluated the Khorana score's ability to predict early VTE risk, the Khorana score was able to stratify VTE risk in metastatic UC/VH patients. In the first 3 months, metastatic UC/VH patients with a high-risk Khorana score had a VTE rate of 8.0%, comparable to the VTE rate in the original Khorana analysis and higher than the rates seen in unselected randomized clinical trials. Therefore, the Khorana score could be utilized to identify metastatic UC/VH patients who could be candidates for primary thromboprophylaxis clinical trials.
The Khorana score's ability to predict for early VTE may come as a result of the variables in the model also being prognostic for worse outcomes. For example, a hemoglobin level <10 g/dl has been validated as a poor prognostic factor in patients with metastatic urothelial carcinoma who have progressed on platinum-based chemotherapy.13 Of all the Khorana variables, leukocytosis has the strongest evidence for association with early mortality. Connolly et al. reported that patients with leukocytosis initiating chemotherapy had a 2.2-fold increased risk of early mortality, with a median time to death of 38 days.14 Furthermore, a case series of 9 urothelial carcinoma patients with unexplained leukocytosis and neutrophilia demonstrated a median overall survival of 71 days from development of leukocytosis to death.15 Thus, early VTE may be associated with aggressive disease and patients with late VTE may have a better prognosis malignancy, with VTE likely driven by other factors.
In our multivariate analysis, only WBC count was found to be significantly associated with increased risk for early VTE. Leukocytosis, particularly neutrophilia, has been previously associated with an increased risk of VTE.14 The mechanism underlying the association of leukocytosis, neutrophilia and VTE is unclear. One possible explanation is through tumor-derived granulocyte colony-stimulating factor (G-CSF) leading to production of neutrophilic extracellular traps (NETs).16 NETs are released from highly activated neutrophils and shown to be harmful to the vasculature through promotion of thrombus formation by providing the nidus for platelet adhesion, activation, and aggregation.17, 18 Furthermore, in a G-CSF producing mouse model of mammary carcinoma, administration of G-CSF neutralizing antibody reduced the peripheral blood neutrophil count and the ability of neutrophils to undergo NET generation, suggesting that activation of neutrophils with G-CSF is important for NET formation.19 Lastly, NET formation coincided with development of venous thrombi in the lung. Taken together, these studies suggest that NETs may play a role in VTE development in cancer patients with leukocytosis and neutrophilia.
There were several limitations to our study. First, the study is limited by its retrospective nature and the inherent confounders associated with such analyses. In addition, clinical characteristics that may predispose patients to VTE, such as poor ECOG performance status or inherited thrombophilia, had significant missing data and were not included in the analysis. Although VTE for this analysis utilized a uniform definition, each event was ascribed by an independent investigator, and central radiographic adjudication was not performed. Lastly, the ability to find statistical significance in some of our variables in the early and late subgroup analyses may have been limited by absolute incidence rates for patients with specific variables, in particular renal dysfunction.
In summary, the Khorana score does not adequately stratify cumulative VTE risk in metastatic UC/VH patients overall. However, it appears to be able to predict early VTE, thus, it is reasonable to use the Khorana score to identify a high risk population for a primary thromboprophylaxis clinical trial of patients initiating first-line chemotherapy. It is possible, however, that the reason the Khorana score is able to predict for early VTE is because the variables within the model also likely predict for early mortality, and VTE may be associated with poor prognosis disease. Finally, in metastatic UC/VH patients, further investigation into the association between leukocytosis and VTE should be undertaken to better understand and treat the underlying biologic mechanism.
Acknowledgments
J.D.R is funded by T32 CA009515 NIH Training Grant.
Footnotes
Disclosures: The authors have no relevant disclosures.
The authors listed below have made substantial contributions to the intellectual content of the paper in the various sections described below:
Conception and design: J.D. Ramos, M.D. Galsky, E.Y. Yu
Analysis or interpretation of data: J.D. Ramos, M.F. Casey, M.D. Galsky, E.Y. Yu
Critical writing or revising the intellectual content: J.D. Ramos, M.F. Casey, A. Bamias, U. De Giorgi, J. Bellmunt, L.C. Harshman, S. Ladoire, Y. Wong, A.S. Alva, J.E. Rosenberg, M.D. Galsky, E.Y. Yu
Final approval of the version to be published: J.D. Ramos, M.F. Casey, A. Bamias, U. De Giorgi, J. Bellmunt, L.C. Harshman, S. Ladoire, Y. Wong, A.S. Alva, J.E. Rosenberg, M.D. Galsky, E.Y. Yu
References
- 1.Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 2.Sorensen HT, Mellemkjaer L, Olsen JH, et al. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 3.Timp JF, Braekkan SK, Versteeg HH, et al. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122:1712–1723. doi: 10.1182/blood-2013-04-460121. [DOI] [PubMed] [Google Scholar]
- 4.Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377–5382. doi: 10.1182/blood-2010-02-270116. [DOI] [PubMed] [Google Scholar]
- 6.Galsky MD, Pal SK, Chowdhury S, et al. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer. 2015;121:2586–2593. doi: 10.1002/cncr.29387. [DOI] [PubMed] [Google Scholar]
- 7.Ramos JD, Casey MF, Crabb SJ, et al. Impact of chemotherapy on venous thromboembolism and prognostic implications in patients with metastatic urinary tract tumors. Cancer Med in press. [Google Scholar]
- 8.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 9.Agnelli G, George DJ, Kakkar AK, et al. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med. 2012;366:601–609. doi: 10.1056/NEJMoa1108898. [DOI] [PubMed] [Google Scholar]
- 10.Agnelli G, Gussoni G, Bianchini C, et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009;10:943–949. doi: 10.1016/S1470-2045(09)70232-3. [DOI] [PubMed] [Google Scholar]
- 11.Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2189–2204. doi: 10.1200/JCO.2013.49.1118. [DOI] [PubMed] [Google Scholar]
- 12.Streiff MB, Holmstrom B, Ashrani A, et al. Cancer-Associated Venous Thromboembolic Disease, Version 1.2015. J Natl Compr Canc Netw. 2015;13:1079–1095. doi: 10.6004/jnccn.2015.0133. [DOI] [PubMed] [Google Scholar]
- 13.Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28:1850–1855. doi: 10.1200/JCO.2009.25.4599. [DOI] [PubMed] [Google Scholar]
- 14.Connolly GC, Khorana AA, Kuderer NM, et al. Leukocytosis, thrombosis and early mortality in cancer patients initiating chemotherapy. Thromb Res. 2010;126:113–118. doi: 10.1016/j.thromres.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izard JP, Gore JL, Mostaghel EA, et al. Persistent, Unexplained Leukocytosis Is a Paraneoplastic Syndrome Associated With a Poor Prognosis in Patients With Urothelial Carcinoma. Clin Genitourin Cancer. 2015;13:e253–258. doi: 10.1016/j.clgc.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Hisada Y, Geddings JE, Ay C, et al. Venous thrombosis and cancer: from mouse models to clinical trials. J Thromb Haemost. 2015;13:1372–1382. doi: 10.1111/jth.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinod K, Wagner DD. Thrombosis: tangled up in NETs. Blood. 2014;123:2768–2776. doi: 10.1182/blood-2013-10-463646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demers M, Krause DS, Schatzberg D, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci U S A. 2012;109:13076–13081. doi: 10.1073/pnas.1200419109. [DOI] [PMC free article] [PubMed] [Google Scholar]