Abstract
Purpose
Mantle cell lymphoma (MCL) is a mature B-cell lymphoma considered to be incurable with current treatments, including first-line rituximab in combination with multiagent chemotherapy and for those eligible, high-dose chemotherapy and stem cell support or rituximab maintenance. On the other hand, achieving a complete remission by high-sensitive flow cytometry is associated with prolonged duration of remission, stressing the need to develop and/or incorporate novel agents into the management of MCL. To this end, we examined the activity of ofatumumab, an anti-CD20 monoclonal antibody with distinct binding and immunologic properties compared to rituximab, in MCL preclinical models.
Experimental Design
MCL cells were labeled with 51Cr before incubation with rituximab or ofatumumab (10 μg/mL) plus human serum or effector cells. 51Cr-release was measured and the percentage of lysis was calculated. Surface CD20, CD55, and CD59 were measured by Imagestream analysis. SCID mice inoculated subcutaneously with Z138 cells were assigned to control versus four doses of ofatumumab or rituximab (10 mg/kg/dose).
Results
Ofatumumab exhibited enhanced in vitro complement-dependent cytotoxicity activity compared with rituximab in MCL cell lines, despite a high degree of in vitro resistance to rituximab associated with low CD20 levels and/or high expression of complement inhibitory proteins. Ofatumumab also delayed tumor progression and prolonged survival in a murine model of MCL.
Conclusions
Our results demonstrate that ofatumumab is more effective than rituximab in MCL preclinical models, including in the presence of rituximab resistance, and support the clinical investigation of ofatumumab in combination with standard systemic chemotherapy in MCL (NCT01527149
Introduction
Mantle cell lymphoma (MCL) is a mature B-cell non-Hodgkin lymphoma (B-NHL) characterized by overexpression of cyclin D1 resulting from the translocation t(11;14; ref. 1). MCL represents about 2% to 10% of B-NHL. Frequently presenting with advanced stage disease, MCL is generally considered to be incurable with current treatment regimens that incorporate high-dose cytarabine, rituximab, anthracycline-based multiagent chemotherapy regimens (i.e., CHOP) and, for those eligible patients, high-dose chemotherapy with autologous stem cell support (HDC-ASCS). Despite a high response rate observed to upfront therapy and significant prolongation in survival with modern treatment regimens, the disease is still characterized by continuous relapses and acquirement of resistance to subsequent treatments resulting in a median survival of only 3 to 5 years postdiagnosis (2).
The addition of the anti-CD20 mAb rituximab to chemotherapy regimens has improved overall survival (OS) in indolent and aggressive B-NHL, though patients frequently relapse with disease that demonstrates variable degrees of sensitivity to retreatment with rituximab containing regimens (3–6). The addition of rituximab to chemotherapy in treating MCL has also been shown to improve clinical outcomes, including OS (7). Despite the survival benefit with the addition of rituximab, nearly all MCL patients eventually relapse and novel treatment approaches are needed to further improve outcomes.
In order to improve on the advances achieved in the era of rituximab in treating B-NHL, it is important to gain an understanding of factors that contribute to the antitumor activity of or to the development of resistance to rituximab. Potential factors that affect rituximab activity include genetic polymorphisms in the Fc receptor, decreased CD20 expression, and increased complement inhibitory protein (CIP) expression (8–11). Multiple next-generation anti-CD20 mAbs with variable mechanisms of activity have been developed and are currently under evaluation in the treatment of B-NHL.
Ofatumumab is a humanized anti-CD20 mAb that is currently Federal Drug Administration (FDA) approved for the treatment of refractory chronic lymphocytic leukemia (CLL; ref. 12). Ofatumumab binds to a unique, more membrane-proximal epitope on the CD20 antigen (13). In preclinical models, ofatumumab has demonstrated similar antibody-dependent cellular cytotoxicity (ADCC) activity with enhanced complement-dependent cytotoxicity (CDC) activity compared with rituximab and more efficiently induces B-cell depletion in vivo (14–16). Ofatumumab is currently under investigation in clinical trials in both treatment naïve and relapsed/refractory indolent and aggressive B-NHL, including MCL.
We have previously reported on the enhanced in vitro and in vivo efficacy of ofatumumab against Burkitt’s lymphoma (BL) and diffuse large B-cell lymphoma (DLBCL) preclinical models, including in the setting of acquired rituximab resistance (17). To further characterize the activity of ofatumumab against MCL cells, we performed preclinical testing in cytarabine-sensitive and -resistant MCL cell lines, primary patient-derived MCL cells and in in vivo SCID mouse xenograft models of human MCL.
Materials and Methods
MCL cell lines and cytarabine-resistant MCL cell lines
Experiments were conducted in several cytarabine-sensitive and cytarabine-resistant MCL cell lines. The sensitive cell lines Granta, HBL-2, Jeko-1, Mino, and Rec-1 were purchased from DSMZ and the cytarabine-resistant MCL cell lines Granta-AraCR, HBL-2-AraCR, Jeko-1-AraCR, Mino-AraCR, and Rec-1-AraCR generated by Dr. Pavel Klener (Charles University, Prague, Czech Republic) by culturing each respective parental cell line in escalating doses of cytarabine (18). The Z-138 and Raji cell lines were obtained from the ATCC. Cell lines were authenticated by short tandem repeat analysis. All cell lines were routinely tested for mycoplasma contamination by PCR, microbial presence by microscopic observation, and antibiotic deprivation and morphology by microscopic observation. Cytarabine-sensitive cells were maintained in RPMI-1640 supplemented with Hepes 5 mmol/L, sodium pyruvate 1 mmol/L, penicillin and streptomycin (100 IU/mL), and 10% heat-inactivated FBS (HI-FBS; RPMI-1640). Cytarabine-resistant cell lines were initially defrosted and cultured in RPMI-1640 with cytarabine 50 μmol/L for 2 months then maintained in RPMI-1640. The rituximab-resistant Raji 4RH cell line was developed within our laboratory as previously described (8, 19).
Primary tumor cells derived from patients with mantle cell lymphoma
Neoplastic B cells were isolated by MACS sorting (negative selection) from pretreatment biopsy tissue obtained from patients with B-cell NHL treated at Roswell Park Cancer Institute (RPCI, Buffalo, NY) procured under Institutional Review Board (IRB) RPCI protocols I42804 and I42904. Tissue specimens were placed in PBS-containing collagenase type IV (1 mg/mL; Sigma-Aldrich) and incubated for 15 minutes at 37°C, including manual agitation for five minutes. Next, samples were diluted with RPMI-1640–containing 10% FBS and the cell suspension filtered through a 100-μm cell strainer to remove large clumps. Subsequently, lymphocytes were enriched by histopaque density centrifugation. B cells were then isolated from enriched lymphocytes by MACS separation using a human B-cell Isolation Kit II (Miltenyi Biotec). Cells were incubated with ofatumumab, rituximab, isotype, or media with 25% human serum. After 48 hours, cell viability was determined by Cell-Titer Glo assay (Promega).
Functional cytotoxicity assays of ofatumumab and rituximab-induced ADCC and CDC
Standard 51Cr release assays were performed to assess anti-CD20 mAb-mediated CDC and ADCC. For CDC and ADCC assays, 5 × 106 MCL cells were labeled for 2 hours at 37°C with 3.7MBq of 51Cr (100 μCi). Subsequently, 1 × 105 cells/well or 1 × 104 cells/well were placed in 96-well plates for CDC or ADCC assays, respectively. MCL cells were then exposed to RPMI-1640, ofatumumab, rituximab, or isotype control at a final concentration of 10 μg/mL in combination with human serum (dilution 1:4, CDC assays) or peripheral blood mononuclear cells (PBMC) at an effector:target ration of 40:1 (ADCC assays). Pooled human serum and PBMCs were collected from healthy donors under RPCI IRB-approved protocol CIC 01–16. PBMCs were obtained by Ficoll-Histopaque centrifugation of whole blood as previously described (20). Cells were incubated at 37°C, 5% CO2 for 6 hours. A set of 51Cr-labeled NHL cells was incubated in RPMI-1640 and then treated with 50 μL of a 5% Triton solution to determine maximum cell lysis. Finally, the 96-well plates were centrifuged at 2,000 rpm for 5 minutes and the supernatant of each well was collected; gamma emission was measured by the Packard Auto-Gamma Cobra II series counting system (IBM Inc.). Percentage of 51Cr release (lysis) was calculated using the standard formula: %lysis = [(test sample release-background release)/(maximum release − background release)] × 100. All samples were run in triplicate in three different sets of experiments. Results are reported as mean values with standard error bars.
Direct killing assays
The direct antitumor effect was assayed using an alamarBlue (Invitrogen) reduction assay. MCL cell lines (5 × 10ˆ4/well) were incubated with rituximab, ofatumumab, or isotype control at 10 μg/mL in the presence of a cross-linking IgG antibody (1 μg/mL). Cell viability and the induction of apoptosis were measured after 24, 48, and 72 hours of incubation.
Surface expression of CD20 and complement inhibitory proteins (CD55 and CD59)
To correlate anti-CD20 antibody activity in MCL cell lines to expression of CD20, CD55, and CD59, surface expression of each protein was measured by quantitative flow cytometry and surface density was determined by flow cytometry using Image-stream (Amnis). Flow-cytometric analysis was performed using the FCS express software version 3 for windows (De Novo Software).
Antibodies
Rituximab (Biogen Idec and Genentech) was obtained from the RPCI Pharmacy Department at a stock concentration of 10 mg/mL. Chimeric anti-human Her-2 neu (trastuzumab) provided by Genentech was used as an isotype control. Ofatumumab was provided by Genmab or obtained from the RPCI Pharmacy Department. Unless otherwise specified, antibodies were used at a final concentration of 10 μg/mL.
For phenotypic analysis of lymphoma cells, FITC-conjugated mouse anti-human CD20, phycoerythin (PE)-conjugated mouse anti-human CD59, and Cy-Chrome-conjugated mouse anti-human CD55 mAbs were purchased from BD Pharmingen Inc. FITC-goat anti-mouse and PE-goat anti-human and APC-goat anti-human monoclonal antibodies were used as isotype controls (BD Pharmingen Inc.
In vivo effects of ofatumumab and rituximab in mantle cell lymphoma
For in vivo experiments, 6- to 8-week-old SCID mice were bred and maintained at the Department of Laboratory Animal Resource (DLAR) facility at RPCI. The Institutional Animal Care and Use Committee (IACUC) at RPCI approved the experimental design under protocol P966M. All animals were housed and maintained in laminar flow cabinets or micro isolator units and provided with sterilized food and water. Six- to 8-week-old SCID mice were inoculated subcutaneously on the posterior flank with 10 × 106 Z-138 cells suspended in Matrigel. Mice were monitored for establishment of a subcutaneous tumor nodule. Tumor volume was determined by caliper measurement as previously described (21). Once the tumor volume reached approximately 250 mm3, mice were assigned to either a control group, ofatumumab (10 mg/kg), or rituximab (10 mg/kg). Monoclonal antibodies were delivered IV via tail vein injection on days +0, +3, +7, and +10 following tumor engraftment. Tumor volume was monitored by serial caliper measurement. Mice with a tumor exceeding 2 cm in largest diameter were sacrificed as per RPCI IACUC requirements. Tumor size >2 cm in largest diameter was used as the survival endpoint in a Kaplan–Meier analysis. Differences in outcomes between treatment groups were compared by log-rank analysis.
Statistical analysis
In vitro experiments were performed in triplicates on three separate occasions. Data were plotted and analyzed using SPSS 16.0 for windows 2003 software (IBM). For in vitro studies (ADCC and CDC assays), statistical differences between treatment groups and controls were determined by unpaired Students t test. In addition, in vivo survival curves were generated using the Kaplan–Meier method using SPPS software. Differences in survival were compared by log-rank testing. A P value of less than 0.05 was defined as having statistical significance.
Results
Ofatumumab activates complement and results in more potent mab-CDC than rituximab in MCL cells
Assays of CDC and ADCC activity were performed in a panel of MCL cell lines. Similar to previously reported results, ofatumumab exhibited greater induction of CDC-induced cell lysis when compared with rituximab (17). Ofatumumab (10 μg/mL) induced a higher percentage of cell lysis compared with rituximab (10 μg/mL) in all but one of the cell lines tested (Granta cells), including in multiple cell lines (Mino, Rec-1, and Jeko-1) in which rituximab elicited minimal activity (Fig. 1). Both rituximab and ofatumumab (10 μg/mL) demonstrated lower levels of in vitro ADCC (effector:target 40:1) with little difference noted between the two antibodies (Fig. 2). Cytarabine resistance was not associated with any degree of resistance to either antibody.
Figure 1.
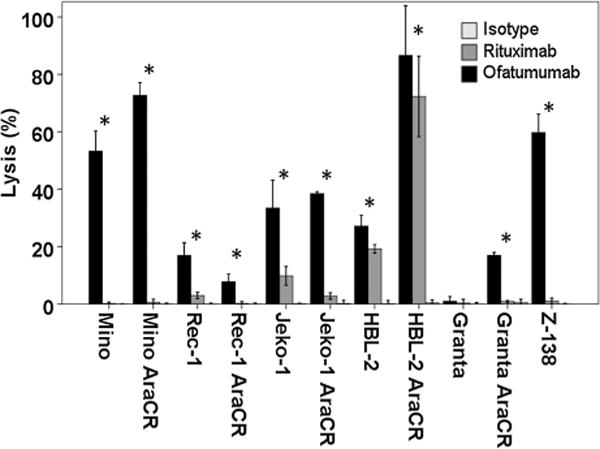
Ofatumumab induces significantly higher levels of CDC-associated cell lysis than rituximab. MCL tumor cell lines were tested for CDC cell lysis using a 51Cr release assay with human serum from normal healthy donors as a source of complement. In all MCL cell lines tested, with the exception of Granta, ofatumumab was more effective than rituximab at inducing CDC lysis at a dose of 10 μg/mL. (*, P < 0.05).
Figure 2.
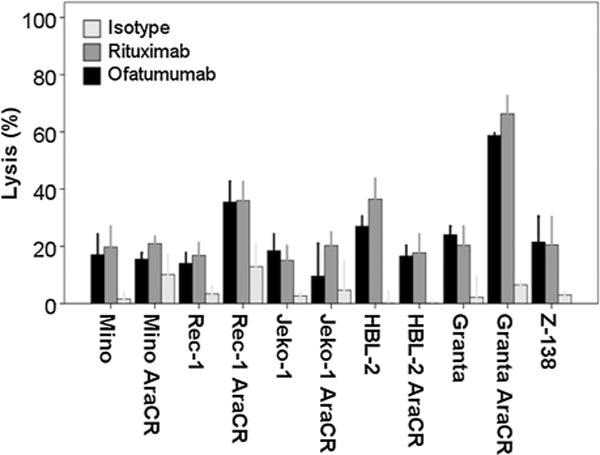
Ofatumumab and rituximab induce a similar degree of ADCC-associated cell lyis. MCL tumor cell lines were tested for ADCC cell lysis using a 51Cr release assay using effector cells from normal healthy donors at a ratio of 40:1. In all MCL cell lines tested, ofatumumab and rituximab induced similar levels of ADCC lysis at a dose of 10 μg/mL.
Cytotoxicity assays were also performed in tumor cells isolated from MCL patient lymph node biopsy samples. A total of 4 MCL patients with lymph node tissue were available. In two patient samples, ofatumumab induced a slightly higher though overall similar degree of decrease in cell viability compared with rituximab when primary MCL cells were exposed to each antibody (10 mg/mL) in the presence of human serum (Fig. 3). In the other two samples, ofatumumab exposure resulted in a statistically significant decrease in viability when compared with rituximab, though one of the two had a dramatic response to both antibodies and thus, though statistically significant, the difference would be unlikely to be clinically relevant.
Figure 3.
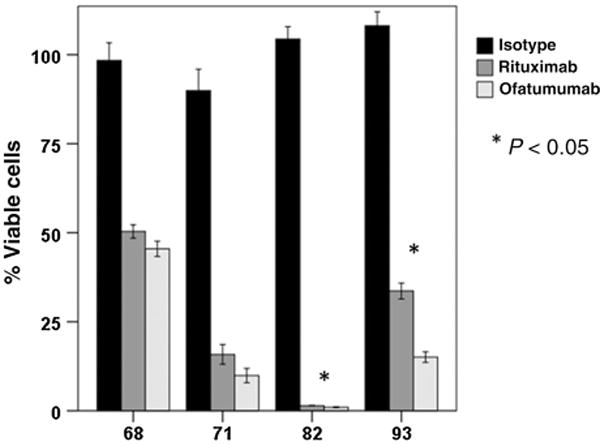
In MCL cells derived from patient samples, ofatumumab induced similar or more cell lysis by CDC compared with rituximab. B cells were isolated from pretreatment tumor samples (n = 4) obtained from MCL patients and tested for monoclonal antibody response by CellTiter Glo assay using rituximab or ofatumumab at a concentration of 10 μg/mL in the presence of human serum as a source of complement. Two of the four samples exhibit a statistically significant decrease in viability following ofatumumab exposure as compared to rituximab. The remaining samples show a similar response to each antibody.
Ofatumumab and rituximab exposure led to a similar degree of direct antilymphoma effect
In MCL cell lines exposed to ofatumumab or rituximab, there was a time-dependent decrease in cell proliferation when compared with an isotype control. Overall, the direct effects of antibody exposure were modest and similar between ofatumumab and rituximab-treated cells with the only statistically significant difference being noted in one of the cell lines tested (Mino)at the 72-hour time point (Fig. 4).
Figure 4.
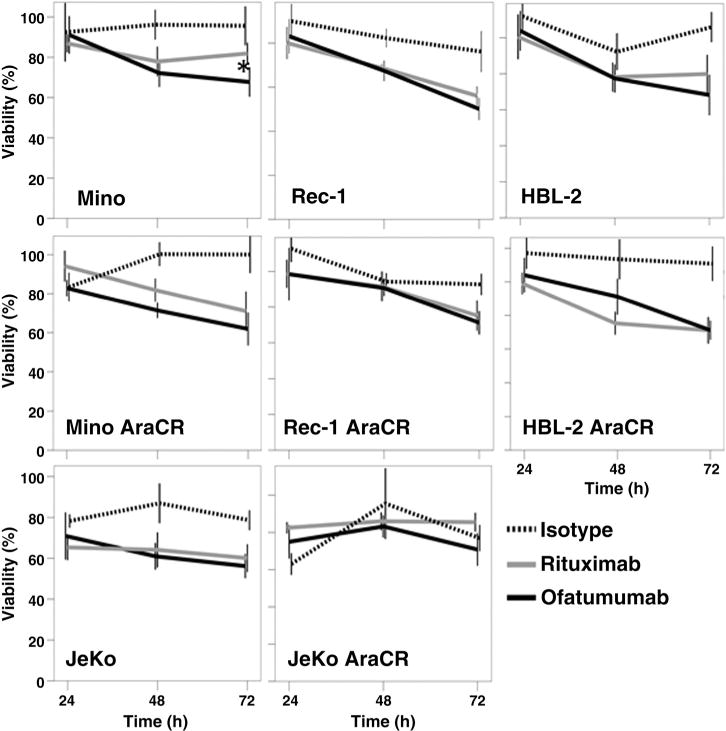
Ofatumumab and rituximab exhibit modest direct antitumor activity against MCL cell lines. MCL cell lines were utilized in alamarBlue reduction assays to investigate the direct antilymphoma activity of each antibody. Each antibody exhibited a time-dependent decrease in MCL cell viability following exposure to 10 μg/mL of each respective antibody in the presence of a cross-linking IgG antibody. With the exception of the Mino cell line at the 72-hour time point, all cell lines demonstrated similar degrees of direct antitumor activity with ofatumumab and rituximab at the dose tested (*, P < 0.05).
MCL cell lines express high levels of surface CD20 and complement inhibitory proteins (CIP) CD55 and CD59
In order to investigate the possible mechanism(s) that could explain the differences in the antitumor activity between rituximab and ofatumumab in MCL cell lines, we tested the density of surface CD20 expression using Imagestream technology. Burkitt lymphoma cell lines known to be sensitive (Raji) or resistant (Raji-4RH) to rituximab were included for comparison. The Raji-4RH cell line was generated in our laboratory by the serial exposure of Raji cells to escalating doses of rituximab and human serum as previously described (8). The MCL cell lines Mino, Jeko-1, Rec-1, and Z-138 all had surface CD20 density similar to the rituximab-sensitive Raji cell line as opposed to Raji-4RH cells known to have a low surface CD20 density, suggesting CD20 expression levels did not play a significant role in their intrinsic resistance to rituximab when compared with ofatumumab (Fig. 5A). Meanwhile, each of the MCL cell lines demonstrated an increased level of surface CD55 and CD59 density compared with Raji cells. The degree of CD55/CD59 surface expression in MCL was comparable with that observed in Raji-4RH cells, suggesting that higher levels of CIPs may contribute to the rituximab-resistant phenotype in the MCL cell lines on in vitro testing (Fig. 5B and C). Of note, Granta, the one cell line that was resistant to both rituximab and ofatumumab induced CDC, exhibited very high levels of CD55 and the highest levels of CD59, even exceeding those demonstrated in the rituximab-resistant Raji-4RH cells, potentially explaining its high level of resistance to either of these antibodies.
Figure 5.
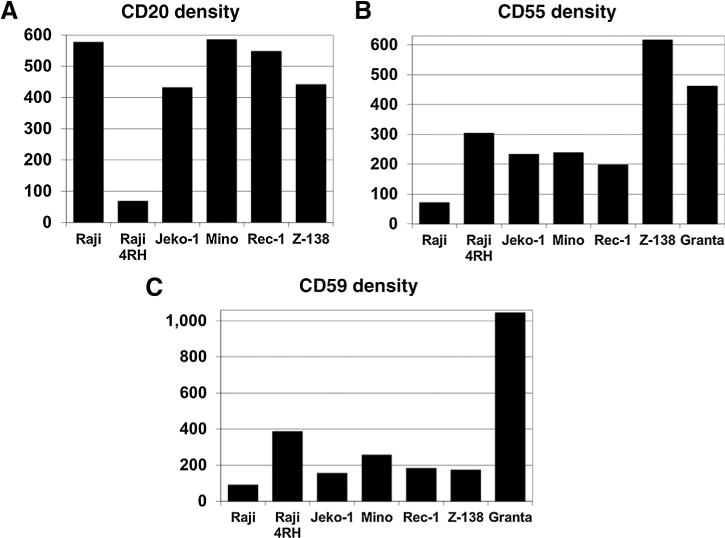
Expression of CD20, CD55, and CD59 in MCL cell lines compared with rituximab-sensitive Raji and rituximab-resistant Raji 4RH cell lines. The surface density of CD20, CD55, and CD59 was determined using Imagestream. MCL cell lines exhibit similar density of CD20 expression on their cell membrane when compared with the rituximab-sensitive Raji cell line. However, the complement inhibitory proteins CD55 and CD59 are present at increased surface density compared with Raji cells, similar to the rituximab-resistant Raji 4RH cell line.
Ofatumumab more effectively controlled tumor growth and prolonged survival in an in vivo SCID mouse model of MCL
Using a subcutaneous tumor model of MCL, the activity of ofatumumab and rituximab was investigated in Z-138 cell bearing SCID mice. Mice with established subcutaneous tumors treated with four doses of ofatumumab (10 mg/kg) exhibited delayed early tumor growth compared with mice treated with rituximab at the same dose and schedule (Fig. 6A). All mice in both the ofatumumab and rituximab groups had tumors that responded to their respective anti-CD20 therapy with a decrease in tumor volume. However, ofatumumab-treated mice demonstrated a sustained response, whereas rituximab-treated mice eventually developed progressive disease as evidenced by the development of progressively larger tumors. Using a subcutaneous tumor size of 2 cm in any dimension as the endpoint for survival, ofatumumab significantly prolonged survival as compared with rituximab in this subcutaneous SCID mouse model at the dosing regimen tested (Fig. 6B). Median survival in the rituximab-treated mice was 127 days compared with the ofatumumab mice where the median survival was not reached (P = 0.044).
Figure 6.
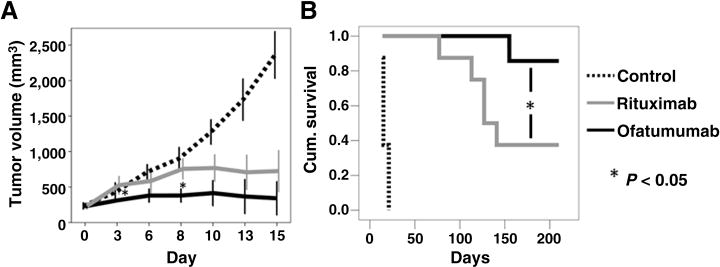
Ofatumumab delayed tumor growth and prolonged survival in an in vivo SCID mouse model of MCL. SCID mice were inoculated subcutaneously on the posterior flank with 10 × 106 Z-138 cells. Mice with established tumors were assigned to control, ofatumumab 10 mg/kg or rituximab 10 mg/kg for 4 doses on days 0, 3, 7, and 10 following tumor establishment. Tumor volume was determined by caliper measurement. Ofatumumab slowed early tumor growth when compared with rituximab with a significant difference in tumor volume noted days 3 and 8 following the start of treatment. Mice were sacrificed when the subcutaneous tumor grew to 2 cm in its largest diameter. Using this time-point as the survival endpoint in a Kaplan–Meier analysis, ofatumumab (median survival not reached) significantly prolonged survival compared with rituximab (median = 127 days; *, P < 0.05).
Discussion
Our results indicate that ofatumumab exhibits more potent antilymphoma activity against human MCL cells and MCL mouse models when compared with rituximab. As previously reported, ofatumumab demonstrated a significantly higher degree of CDC-associated cytotoxicity. It has been proposed that ofatumumab’s enhanced CDC activity may be related to its unique epitope binding, positioning the antibody more proximally to the cell membrane allowing it to more efficiently activate complement (15). Our findings also indicate that MCL cell lines may exhibit resistance to rituximab related to higher levels of CD55 and CD59 on the lymphoma cell surface. Differences between mAbs-targeting CD20 in their capacity to activate the complement cascade, could explain why MCL with higher levels of CIP are sensitive to ofatumumab but not rituximab. On the other hand, ADCC activity in vitro was noted to be comparable between the two antibodies. Using in vivo models of MCL, we demonstrated that ofatumumab was more effective than rituximab in controlling lymphoma growth. While, this could be explained by differences in complement activation in vivo, we previously demonstrated that the predominant mechanism of rituximab activity in our SCID mouse model is likely ADCC. Thus, although no significant differences in terms of ADCC was observed in vitro between rituximab and ofatumumab, it is possible that in vivo induction of ADCC is higher among ofatumumab-treated MCL bearing SCID mice when compared with those animals treated with rituximab.
Although ofatumumab has increased potency in preclinical testing when compared with rituximab, it is currently unclear whether these results will translate into improved clinical responses or outcomes in lymphoma patients. In the initial phase I/II trial of ofatumumab as a single agent in relapsed/refractory follicular lymphoma (FL), Hagenbeek and colleagues reported that ofatumumab was well tolerated and adverse events were primarily infusion-related toxicities following the first antibody infusion (22). The best response rate was 43%, with an overall response rate (ORR) of 64% observed in patients previously treated with rituximab, including 3 out of 4 patients previously deemed to be rituximab refractory (22). In recently reported results from Czuczman and colleagues, ofatumumab monotherapy resulted in an ORR of only 11%, though these patients were very heavily pretreated and there was an ORR of 22% in patients refractory to rituximab monotherapy (23). There is limited data currently available on the clinical use of ofatumumab in MCL. Furtado and colleagues recently reported on a phase II trial of single-agent ofatumumab in relapsed/refractory MCL (24). In 12 patients, the overall response rate was only 8.3% with the best response being a PR in 1 patient, whereas 6 patients (50%) had stable disease. Hunstig and colleagues also recently published a single case report of a patient with refractory MCL who achieved a complete response following single-agent ofatumumab despite failing five prior treatment regimens (25).
When combined with chemotherapy, ofatumumab has shown more promising response rates though with mixed results and no evidence of superiority over rituximab in the relapsed setting, albeit with limited data available. In a study of ofatumumab in combination with CHOP chemotherapy in treatment naïve follicular lymphoma patients, the O-CHOP combination exhibited a 90% ORR in the lower-dose 500 mg group with 100% ORR in the 1,000 mg group, including a 62% complete remission (CR) rate (26). In the setting of aggressive B-NHL, ofatumumab in combination with dexamethasone, cytarabine, and cisplatin or ifosfamide, carboplatin and etoposide (DHAP or ICE) resulted in an ORR of 61% in patients with relapsed/primary refractory disease following at least one prior rituximab containing regimen (27). However, in a recently reported large international trial investigating ofatumumab head to head with rituximab in combination with DHAP in relapsed/refractory DLBCL, the ORR to O-DHAP was significantly lower at 38% and was similar to that of R-DHAP (42%) with no difference in progression free or overall survival between groups (28). Of note, in 19 previously untreated older (>65 years) patients with MCL, the combination of ofatumumab, bendamustine, and dexamethasone resulted in an ORR of 94% with 89% of those achieving a CR (29).
On the basis of our results, ofatumumab represents a promising novel agent for the treatment of patients with MCL either upfront or in the setting of rituximab-resistant disease; however, the role of ofatumumab in treating MCL is unclear when considering other novel agents with potential promise in treating MCL such as other anti-CD20 mAb (e.g., obinutuzumab), proteasome inhibitors (e.g., bortezomib or carfilzomib), mTOR inhibitors (e.g., temsirolimus), or inhibitors of the B-cell receptor pathway (e.g., ibrutinib). Although results from early-phase trials have been somewhat mixed, ofatumumab continues to be investigated in trials incorporating de novo and relapsed/refractory B-NHL patients, including an ongoing clinical trial sponsored by our institution investigating ofatumumab in combination with hyperfractionated cyclophosphamide, doxorubicin, vincristine, and dexamethasone (O-HyperCVAD) alternating with ofatumumab high-dose cytarabine and methotrexate (O-MA) for patients with newly diagnosed MCL (NCT01527149).
Translational Relevance.
Mantle cell lymphoma (MCL) is generally considered incurable with modern treatment regimens that incorporate rituximab with combination chemotherapy regimens. In preclinical testing, novel anti-CD20 monoclonal antibodies have demonstrated variable degrees of increased activity, when compared with rituximab, against a variety of CD20-expressing malignancies. In this study, we found that the anti-CD20 monoclonal antibody ofatumumab exhibits more potent activity compared with rituximab against MCL cells in vitro and in vivo, including in cell lines with significant in vitro resistance to rituximab associated with high levels of expression of the complement inhibitory proteins CD55 and CD59, which have previously been correlated with decreased rituximab-associated cytotoxicity. Our work supports the further development of ofatumumab in the treatment of MCL.
Acknowledgments
Grant Support
The Flow and Image Cytometry Facility is funded by the NCI Cancer Center Support Grant P30CA16056. The ImageStream Cytometer was acquired with support from the NIH 1S10ODOD018048-01.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors’ Contributions
Conception and design: M.J. Barth, M.S. Czuczman, F.J. Hernandez-Ilizaliturri
Development of methodology: M.J. Barth, F.J. Hernandez-Ilizaliturri
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): M.J. Barth, C. Mavis, M.S. Czuczman, F.J. Hernandez-Ilizaliturri
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): M.J. Barth, C. Mavis, M.S. Czuczman, F.J. Hernandez-Ilizaliturri
Writing, review, and/or revision of the manuscript: M.J. Barth, C. Mavis, M.S. Czuczman, F.J. Hernandez-Ilizaliturri
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): M.J. Barth, C. Mavis, F.J. Hernandez-Ilizaliturri
Study supervision: F.J. Hernandez-Ilizaliturri
References
- 1.Bertoni F, Rinaldi A, Zucca E, Cavalli F. Update on the molecular biology of mantle cell lymphoma. Hematol Oncol. 2006;24:22–7. doi: 10.1002/hon.767. [DOI] [PubMed] [Google Scholar]
- 2.Herrmann A, Hoster E, Zwingers T, Brittinger G, Engelhard M, Meusers P, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009;27:511–8. doi: 10.1200/JCO.2008.16.8435. [DOI] [PubMed] [Google Scholar]
- 3.Czuczman MS, Weaver R, Alkuzweny B, Berlfein J, Grillo-Lopez AJ. Prolonged clinical and molecular remission in patients with low-grade or follicular non-Hodgkin’s lymphoma treated with rituximab plus CHOP chemotherapy: 9-year follow-up. J Clin Oncol. 2004;22:4711–6. doi: 10.1200/JCO.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 4.Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–32. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–33. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 6.Davis TA, Grillo-Lopez AJ, White CA, McLaughlin P, Czuczman MS, Link BK, et al. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin’s lymphoma: safety and efficacy of re-treatment. J Clin Oncol. 2000;18:3135–43. doi: 10.1200/JCO.2000.18.17.3135. [DOI] [PubMed] [Google Scholar]
- 7.Schulz H, Bohlius JF, Trelle S, Skoetz N, Reiser M, Kober T, et al. Immunochemotherapy with rituximab and overall survival in patients with indolent or mantle cell lymphoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2007;99:706–14. doi: 10.1093/jnci/djk152. [DOI] [PubMed] [Google Scholar]
- 8.Czuczman MS, Olejniczak S, Gowda A, Kotowski A, Binder A, Kaur H, et al. Acquirement of rituximab resistance in lymphoma cell lines is associated with both global CD20 gene and protein down-regulation regulated at the pretranscriptional and posttranscriptional levels. Clin Cancer Res. 2008;14:1561–70. doi: 10.1158/1078-0432.CCR-07-1254. [DOI] [PubMed] [Google Scholar]
- 9.Jazirehi AR, Vega MI, Bonavida B. Development of rituximab-resistant lymphoma clones with altered cell signaling and cross-resistance to chemotherapy. Cancer Res. 2007;67:1270–81. doi: 10.1158/0008-5472.CAN-06-2184. [DOI] [PubMed] [Google Scholar]
- 10.Macor P, Tripodo C, Zorzet S, Piovan E, Bossi F, Marzari R, et al. In vivo targeting of human neutralizing antibodies against CD55 and CD59 to lymphoma cells increases the antitumor activity of rituximab. Cancer Res. 2007;67:10556–63. doi: 10.1158/0008-5472.CAN-07-1811. [DOI] [PubMed] [Google Scholar]
- 11.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–8. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 12.Cheson BD. Ofatumumab, a novel anti-CD20 monoclonal antibody for the treatment of B-cell malignancies. J Clin Oncol. 2010;28:3525–30. doi: 10.1200/JCO.2010.27.9836. [DOI] [PubMed] [Google Scholar]
- 13.Teeling JL, Mackus WJ, Wiegman LJ, van den Brakel JH, Beers SA, French RR, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177:362–71. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]
- 14.Teeling JL, French RR, Cragg MS, van den Brakel J, Pluyter M, Huang H, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004;104:1793–800. doi: 10.1182/blood-2004-01-0039. [DOI] [PubMed] [Google Scholar]
- 15.Pawluczkowycz A, Beurskens F, Beum P, Lindorfer M, van de Winkel J, Parren P, et al. Binding of submaximal C1q promotes complement-dependent cytotoxicity (CDC) of B cells opsonized with anti-CD20 mAbs ofatumumab (OFA) or rituximab (RTX): considerably higher levels of CDC are induced by OFA than by RTX. J Immunol. 2009;183:749–58. doi: 10.4049/jimmunol.0900632. [DOI] [PubMed] [Google Scholar]
- 16.Beum PV, Lindorfer MA, Beurskens F, Stukenberg PT, Lokhorst HM, Pawluczkowycz AW, et al. Complement activation on B lymphocytes opsonized with rituximab or ofatumumab produces substantial changes in membrane structure preceding cell lysis. J Immunol. 2008;181:822–32. doi: 10.4049/jimmunol.181.1.822. [DOI] [PubMed] [Google Scholar]
- 17.Barth MJ, Hernandez-Ilizaliturri FJ, Mavis C, Tsai PC, Gibbs JF, Deeb G, et al. Ofatumumab demonstrates activity against rituximab-sensitive and -resistant cell lines, lymphoma xenografts and primary tumour cells from patients with B-cell lymphoma. Br J Haematol. 2012;156:490–8. doi: 10.1111/j.1365-2141.2011.08966.x. [DOI] [PubMed] [Google Scholar]
- 18.Klanova M, Lorkova L, Vit O, Maswabi B, Molinsky J, Pospisilova J, et al. Downregulation of deoxycytidine kinase in cytarabine-resistant mantle cell lymphoma cells confers cross-resistance to nucleoside analogs gemcitabine, fludarabine and cladribine, but not to other classes of anti-lymphoma agents. Mol Cancer. 2014;13:159. doi: 10.1186/1476-4598-13-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olejniczak SH, Hernandez-Ilizaliturri FJ, Clements JL, Czuczman MS. Acquired resistance to rituximab is associated with chemotherapy resistance resulting from decreased Bax and Bak expression. Clin Cancer Res. 2008;14:1550–60. doi: 10.1158/1078-0432.CCR-07-1255. [DOI] [PubMed] [Google Scholar]
- 20.Ottonello L, Morone P, Dapino P, Dallegri F. Monoclonal Lym-1 antibody-dependent lysis of B-lymphoblastoid tumor targets by human complement and cytokinine-exposed mononuclear and neutrophilic polymorphonuclear leukocytes. Blood. 1996;87:5171–8. [PubMed] [Google Scholar]
- 21.Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24:148–54. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- 22.Hagenbeek A, Gadeberg O, Johnson P, Pedersen LM, Walewski J, Hellmann A, et al. First clinical use of ofatumumab, a novel fully human anti-CD20 monoclonal antibody in relapsed or refractory follicular lymphoma: results of a phase 1/2 trial. Blood. 2008;111:5486–95. doi: 10.1182/blood-2007-10-117671. [DOI] [PubMed] [Google Scholar]
- 23.Czuczman MS, Fayad L, Delwail V, Cartron G, Jacobsen E, Kuliczkowski K, et al. Ofatumumab monotherapy in rituximab-refractory follicular lymphoma: results from a multicenter study. Blood. 2012;119:3698–704. doi: 10.1182/blood-2011-09-378323. [DOI] [PubMed] [Google Scholar]
- 24.Furtado M, Dyer MJS, Johnson R, Berrow M, Rule S. Ofatumumab monotherapy in relapsed/refractory mantle cell lymphoma—a phase II trial. Br J Haematol. 2014;165:575–8. doi: 10.1111/bjh.12769. [DOI] [PubMed] [Google Scholar]
- 25.Hunstig F, Hammersen J, Kunert C, Petersen I, Merz H, Glaser A, et al. Complete remission after treatment with single-agent ofatumumab in a patient with high-risk leukemic mantle-cell lymphoma. J Clin Oncol. 2013;31:e312–e5. doi: 10.1200/JCO.2012.45.9438. [DOI] [PubMed] [Google Scholar]
- 26.Czuczman MS, Hess G, Gadeberg OV, Pedersen LM, Goldstein N, Gupta I, et al. Chemoimmunotherapy with ofatumumab in combination with CHOP in previously untreated follicular lymphoma. Br J Haematol. 2012;157:438–45. doi: 10.1111/j.1365-2141.2012.09086.x. [DOI] [PubMed] [Google Scholar]
- 27.Matasar MJ, Czuczman MS, Rodriguez MA, Fennessy M, Shea TC, Spitzer G, et al. A Phase II Study of Ofatumumab in Combination with ICE or DHAP Chemotherapy in Relapsed or Refractory Aggressive B-Cell Lymphoma Prior to Autologous Stem Cell Transplantation (ASCT) ASH Annual Meeting Abstracts. 2011;118:957. [Google Scholar]
- 28.van Imhoff GW, McMillan A, Matasar MJ, Radford J, Ardeshna KM, Kuliczkowski K, et al. Ofatumumab Versus Rituximab Salvage Chemoim-munotherapy in Relapsed or Refractory Diffuse Large B-Cell Lymphoma: The Orcharrd Study (OMB110928) ASH Annual Meeting Abstracts. 2014;124:630. doi: 10.1200/JCO.2016.69.0198. [DOI] [PubMed] [Google Scholar]
- 29.Magni M, Di Nicola M, Carlo-Stella C, Devizzi L, Guidetti A, Matteucci P, et al. Safety, Tolerability and Activity of Ofatumumab, Bendamustine and Dexamethasone Combination As First-Line Treatment of Mantle-Cell Lymphoma in the Elderly: A Multicenter Study. ASH Annual Meeting Abstracts. 2011;118:1647. [Google Scholar]


