Abstract
Aim
Pediatric cancer has been associated with exposure to certain environmental carcinogens. The purpose of this work is to analyse the relationship between environmental pollution and pediatric cancer risk.
Method
We analysed all incidences of pediatric cancer (< 15) diagnosed in a Spanish region during the period 1998–2015. The place of residence of each patient and the exact geographical coordinates of main industrial facilities was codified in order to analyse the spatial distribution of cases of cancer in relation to industrial areas. Focal tests and focused Scan methodology were used for the identification of high-incidence-rate spatial clusters around the main industrial pollution foci.
Results
The crude rate for the period was 148.0 cases per 1,000,0000 children. The incidence of pediatric cancer increased significantly along the period of study. With respect to spatial distribution, results showed significant high incidence around some industrial pollution foci group and the Scan methodology identify spatial clustering. We observe a global major incidence of non Hodgkin lymphomas (NHL) considering all foci, and high incidence of Sympathetic Nervous System Tumour (SNST) around Energy and Electric and organic and inorganic chemical industries foci group. In the analysis foci to foci, the focused Scan test identifies several significant spatial clusters. Particularly, three significant clusters were identified: the first of SNST was around energy-generating chemical industries (2 cases versus the expected 0.26), another of NHL was around residue-valorisation plants (5 cases versus the expected 0.91) and finally one cluster of Hodgkin lymphoma around building materials (3 cases versus the expected 2.2)
Conclusion
Results suggest a possible association between proximity to certain industries and pediatric cancer risk. More evidences are necessary before establishing the relationship between industrial pollution and pediatric cancer incidence.
Keywords: Childhood cancer, Spatial analysis, Industrial pollution, Residential proximity, Urban pollution
1. Introduction
Pediatric Cancer (PC) was the leading cause of disease-related death in children under 15 in 2014 in Spain (INE, 2016). The most common tumours (age-adjusted rates per million for children aged 0–14) are leukaemia (Spain 47.0, Europe 44.0), central nervous system tumours (CNST) (Spain 33.2, Europe 29.9) and lymphoma (Spain 19.4, Europe 15.2) (Peris-Bonet et al., 2010; Stiller et al., 2006). The causes of PC are largely unknown, although in recent years an increase in incidence rates has been detected (Kaatsch, 2010; Ward et al., 2014). The reason for this increase is unknown, although it is believed that environmental changes are a contributing factor (Buka et al., 2007; Kaatsch, 2010).
Ambient air, especially in big cities and in the vicinity of industrial pollution foci, contains a wide variety of known human carcinogens, including polycyclic aromatic hydrocarbons, dioxins, arsenic, benzene, fine and ultrafine particles, asbestos and volatile organic compounds. In adults, it is estimated that 1–2% of cases of lung cancer can be associated with the presence of a high concentration of these compounds (Alberg and Samet, 2003). In Europe, national registers of polluting industries have increased awareness of the activities and emissions of the main foci of industrial pollution, which has facilitated the analysis of emissions and their effects. Several works have identified residential areas that are in the vicinity of industrial pollution foci as higher-risk cancer areas for adults (Bulka et al., 2013; Garcia-Perez et al., 2015a, 2015b; Morton-Jones et al., 1999; Ramis et al., 2009; Reynolds et al., 2003). Based on this empirical evidence, the World Health Organisation (WHO) has confirmed that air pollution is a human carcinogen, owing to the direct link between it and lung cancer (Loomis et al., 2013).
The presence of spatial clusters in pediatric cancer (PC) cases has been analysed in the search for etiological factors (Alexander et al., 1998; Demoury et al., 2012; McNally et al., 2009; Ramis et al., 2015). Observational studies that relate PC and air pollution are scarce (Garcia-Perez et al., 2016a; Reynolds et al., 2003), and in most cases focus on traffic density and the proximity of high-capacity roads; no consistent results on a global scale have been reached to date. Regarding industrial pollution, several works recently published in Spain argue for the relationship between exposure to industrial pollution and leukaemia, neuroblastoma, kidney and bone tumours in children aged 0–14 (Garcia-Perez et al., 2015b, 2016a, 2016b; García-Pérez et al., 2017). The low incidence of PC, the high degree of uncertainty associated with the variables under consideration, and the formation of micro-clusters emphasise the need to carry out spatial epidemiological studies, which can then be used to analyse the incidence of cancer in small urban units (Ortega-García et al., 2016).
The target of this work is to undertake a preliminary analysis, based on empirical evidence, of the spatial distribution of PC around industrial facilities in a European region (Region of Murcia, Spain).
2. Methods
2.1. Study area and population
Census track (CT) represents the smallest territorial unit for which population data are available in Spain. The region of Murcia (RM) is a European Region (NUTS II in Eurostat terminology) located in southeastern of Spain and is divided in 2011 into 1220 CT. We consider this spatial unit as reference to evaluate risk and spatial clusters. A latitude and longitude coordinates (centroid) was assigned to each CT and the distance between two CT was defined as the distance between centroids.
Reference population (risk population) came from Spanish Census 2001 and 2011. The total population (< 15 years) was 207,822 in 2001 and 259,083 in 2011. We performed linear interpolation to estimate the population between the censuses. For each CT we used the population at the census times immediately preceding and immediately following. For times before the first census time, the population size is set equal to the population size at that first census time, and for times after the last census time, the population is set equal to the population size at that last census time.
2.2. Cases
The subjects of analysis were PC (< 15) cases diagnosed in the RM between January 1998 and December 2015 by the MACAPEMUR (Environment and Pediatric Cancer in the RM) project. MACAPEMUR is a project for the compilation of Pediatric Environmental History in newly diagnosed cancer patients since 1998 in the RM (Cárceles-Álvarez et al., 2015; Ferris Tortajada et al., 2004; Ortega-García et al., 2011). The single-province character of the RM and the centralized care reference units of Pediatric Oncohematology and the Pediatric Environmental Health Speciality Unit site at the Clinical University Hospital Virgen of Arrixaca facilitated the access to medical records. The hospital registry from the Clinical University Hospital Virgen of Arrixaca register 100% of the children diagnosed with cancer in the RM. The classification of the cases is done by checking the clinical-pathological diagnosis with the international classification of diseases for oncology (ICD-O-3) (IARC, 2011) and the International Classification of Childhood Cancer (ICCC-3) (Steliarova-Foucher et al., 2005) within 0–2 months after of diagnosis. Over 99% of the cases are morphologically verified. Annually, a medical doctor performs an additional check of all cases to verify the correct classification and elimination of double registrations.
In all cases, the families were contacted by phone or in person. Once the diagnosis is made, a face-to-face interview is carried out by one doctor trained in pediatric cancer, environmental health and risk communication, which collect information on addresses at diagnosis; as well as another series of environmental data (Cárceles-Álvarez et al., 2015; Ferris Tortajada et al., 2004; Ortega-García et al., 2012). In this study, inclusion criteria comprised: children (< 15) diagnosed with cancer between 1998 and 2015 with an address in the RM corresponding to at-diagnosis residence. A total of 669 children were diagnosis with cancer during this period. Of these, we excluded 45 cases because they simply went to the RM in order to obtain a second opinion or complete the diagnostic and therapeutic process. Another 6 cases rejected to participate in the study. Finally, 624 cases were included in the study. Information on the residence at the time of diagnosis was then collected. These addresses were georeferenced and assigned a CT.
In order to reduce the border effects an exhaustive revision of hospital-based records of adjacent regions (Castilla-La Mancha and Comunidad Valenciana) was performed. This review did not provide new case studies.
The project was approved by the ethics research committee of Clinical University Hospital Virgen de la Arrixaca. Informed consent forms signed by all parents and children over 12 were collected.
2.3. Polluting industries
The identification of industrial pollution foci in the RM was carried out by studying the national emission and pollution register, which is maintained by the Spanish environment ministry (PRTR-Spain, http://www.prtr-es.es/). This register takes into consideration air pollution emitted by industrial facilities in the RM, and excludes the agricultural and animal-husbandry sectors. The geographical coordinates of industrial complexes were compiled using Google Maps and on-the-ground survey. Facilities located within 2 km of one another were grouped together in a single foci, which was, for analytical purposes, located in the geographic centroid. In total, the list includes 88 facilities, of which only 28 produce emissions; these were grouped in 12 foci. Fig. 1 illustrates the locations of these foci. Five types of industrial activity were defined (NACE Rev.2, http://ec.europa.eu/ eurostat/web/nace-rev2) and foci were assigned accordingly: energy industries (2 foci); organic and inorganic chemical industries (5 foci); pharmaceutical industries (3 foci); building material industries (2 foci); industries concerned with the incineration or valorisation of dangerous waste (4 foci). Some foci were assigned more than one category because of the presence of industrial facilities of different kinds. Table 1 provides further details about the facilities that make up each industrial foci and the exposure of the population under 15 in 2011.
Fig. 1.
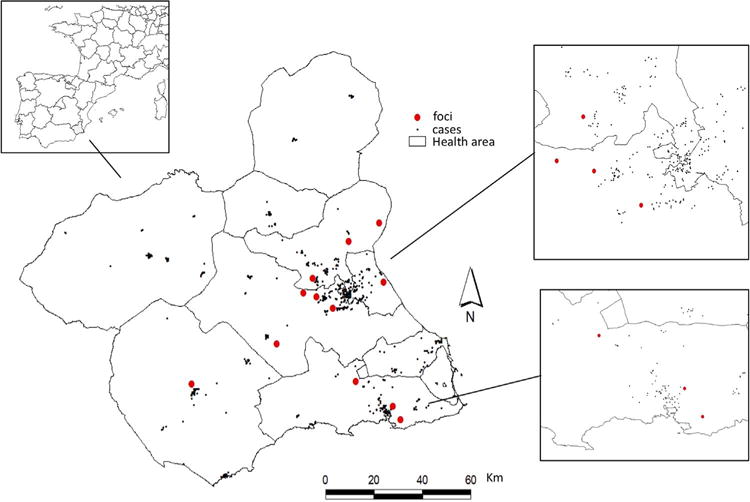
Cases and foci of air-polluting industries in RM (urban areas Murcia y Cartagena).
Table 1.
List of industrial associated with each focus, industrial activity and population (< 15 years) exposed.
| Industrial activitya
|
Population under 15 in 2011
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Foci | E | C | P | Ce | I | E-PRTRb (NACE Rev.2c) | 1 km | 3 km | 5 km | 10 km |
| Foci #1 | – | x | – | – | – | 6550 (20.53) | 0 | 6173 | 17732 | 38426 |
| Foci #2 | x | x | – | – | x | 722 (20.59); 1529 (19.20); 3442 (38.22); 3557 (20.59); 3589 (35.16); 3896 (20.14); 4791 (20.13); 5771 (52.10); 6515 (35.19); 6644 (35.16); 7733 (20.59); 7720 (20.14) | 0 | 1326 | 2461 | 17245 |
| Foci #3 | – | – | – | – | x | 3825 (38.22); 6251 (30.22); 7412 (81.22) | 0 | 6608 | 23118 | 28846 |
| Foci #4 | x | x | – | – | – | 1752 (20.16); 3965 (35.16) | 0 | 355 | 1927 | 2416 |
| Foci #5 | – | x | – | – | – | 6263 (20.41) | 0 | 4734 | 9847 | 20367 |
| Foci #6 | – | – | x | – | – | 4940 (21.10); 3490 (21.10) | 320 | 2365 | 4653 | 7930 |
| Foci #7 | – | – | – | – | x | 7750 (39.00) | 0 | 831 | 1629 | 2488 |
| Foci #8 | – | – | – | x | – | 9001 (23.51) | 0 | 110 | 624 | 729 |
| Foci #9 | – | – | – | x | – | 1574 (23.51) | 0 | 7750 | 11541 | 12192 |
| Foci #10 | – | x | x | – | – | 1702 (20.59); 1717 (21.10) | 426 | 6162 | 11406 | 17874 |
| Foci #11 | – | – | – | – | x | 3190 (38.21) | 0 | 414 | 682 | 5607 |
| Foci #12 | – | – | x | – | – | 6596 (21.10) | 0 | 0 | 3061 | 4021 |
E= energy industries; C= organic and inorganic chemical industries; P= pharmaceutical industries; Ce= building material industries; I= industries concerned with the incineration or valorisation of dangerous waste.
European Pollutant Release and Transfer Register. (http://prtr.ec.europa.eu/)
In brackets economic activity code.
2.4. Statistical analysis
In first place, a descriptive analysis of the main diagnostic groups according to age group and sub-period was carried out, which involved the calculation of standardised incidence ratio (SIR) and its confidence intervals (CI) by cancer type and sub-periods. In second place,1 we calculated several risk indicators (observed cases; expected cases and crude incidence rate; lowest number of observed cases which would be statistical significant at the one sided 5% type one level or less; exact value of the one sided type one error corresponding to this limit; power of the test based on this limit of statistical significance under the alternative hypothesis of a doubling of the incidence rate) for an area delimited by a buffer of 4 km (window) around each foci groups (E, C, P, Ce, I) and all foci of industry activity (Ramis et al., 2011). Moreover, under the hypothesis of Poisson distribution of cases, we computed a test of equal incidence rates inside and outside of each window. Additionally, similar results for each foci. Lastly, we used the focused-local spatial scan statistic in order to identify cluster of high incidence around each foci. The Scan methodology (Kulldorff et al., 1997) has been developed for different types of processes and in this paper we used the focused version under the hypothesis of Poisson distribution. The basic idea of this simple yet powerful methodology is using circular moving windows centred in any foci of different size to “scan” a study area. For each window the number of events within the windows and the risk population is counted. The likelihood of the observed spatial distribution of events in compared to the likelihood of distribution under the null hypothesis of no clustering (e.g. the relative risk inside an outside of window is the same). For more details about this methodology an annexed section is include.
The scan procedure identifies the window where the likelihood ratio is maximum (Most Likelihood Cluster. MLC). Furthermore, it is possible to attach a probability value to the MLC in order to support inferential statements using permutation bootstrapping. The model outputs were the location and radius of clusters, the number of CT in the cluster, the population in the clusters, the number of observed and expected cases. In our case, the local-focused scan statistic is used to determine high-risk areas around a pre-determined set of points (pollutant sources).
The analysis was carried out to examine overall cancer incidence and also, with the most common tumours: leukaemia, lymphoma (HL: Hodgkin lymphoma and NHL: non Hodgkin lymphoma), CNST and SNST. Information on other types was also shown by completeness.
3. Results
Table 2 illustrates the distribution of CR/ASRw and SIR, divided by type of tumour and period and compared the results with other Spanish and European studies (Peris Bonet et al., 2015; Steliarova-Foucher et al., 2002). A CR of 148.0 cases per million children under age of 15 years was obtained. Leukaemia and CNST were the most common types. The number of cases of NHL and SNST diagnosed in the six-year period 2010–2015 is twice that observed in the first six-year period (1998–2003). A significant increase in the incidence of overall cancer and the subtypes of SNST, NHL and leukaemia may also be observed in 2010–2015.
Table 2.
Distribution of diagnosed cases. CR and SIR by type and period.
| Cases by period
|
CRa/ASRw MACAPEMUR | Spain (00–13) | Europe (88–97) | SIR (CI 95%) by type and period in the RM
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 98–03 | 04–09 | 10–15 | All | % | 98–03 | 04–09 | 10–15 | |||||
| Leukaemia | 44 | 55 | 72 | 171 | 27.4 | 40.5/41.2 | 46.1/47.0 | 41.7/44.1 | 0.91 (0.66–1.22) | 1.14 (0.86–1.42) | 1.47 (1.15–1.85) | |
| ALL | 34 | 43 | 56 | 133 | 31.5/34.5 | 36.0/36.8 | 33.8/35.9 | 0.90 (0.62–1.26) | 1.14 (0.87–1.47) | 1.48 (1.17–1.85) | ||
| AML | 9 | 12 | 11 | 32 | 7.6/7.8 | 8.3/8.3 | 6.2/6.4 | 0.98 (0.45–1.86) | 1.31 (0.75–2.12) | 1.20 (0.67–1.98) | ||
| Others | 1 | – | 5 | 6 | 1.4/1.5 | 1.9/1.9 | 0.68 (0.01–3.79) | – | 2.72 (0.93–6.24) | |||
| Lymphomas | 19 | 11 | 35 | 65 | 10.4 | 15.4/16.1 | 19.6/19.2 | 15.9/15.2 | 1.01 (0.61–1.58) | 0.59 (0.29–1.05) | 1.76 (1.12–1.93) | |
| HL | 8 | 4 | 10 | 22 | 5.2/5.2 | 7.2/6.9 | 6.3/5.8 | 1.22 (0.52–2.40) | 0.61 (0.16–1.56) | 1.52 (0.73–2.80) | ||
| NHL | 11 | 7 | 25 | 43 | 10.2/11.0 | 12.4/12.4 | 9.6/9.3 | 0.90 (0.45–1.61) | 0.57 (0.23–1.18) | 1.88 (1.19–2.82) | ||
| CNST | 43 | 47 | 56 | 146 | 23.4 | 34.6/35.6 | 34.2/34.5 | 29.4/29.9 | 1.03 (0.74–1.38) | 1.12 (0.82–1.49) | 1.31 (0.99–1.71) | |
| SNST | 14 | 15 | 31 | 60 | 9.6 | 14.2/14.5 | 13.1/13.8 | 9.4/10.9 | 0.83 (0.46–1.40) | 0.89 (0.50–1.47) | 1.85 (1.25–2.62) | |
| Others | 52 | 62 | 68 | 182 | 29.2 | 43.2/43.8 | 40.8/41.3 | – | – | – | ||
| Total | 172 | 190 | 262 | 624 | 100 | 148.0/151.6 | 153.9/155.8 | 133.7/138.5 | 0.97 (0.83–1.12) | 1.06 (0.92–1.23) | 1.44 (1.27–1.63) | |
| CRa | 138.4 | 133.9 | 168.4 | 148.0 | ||||||||
CR = Crude rate 1000000 under 15; ASRw =age-world-standardized incidence rate; SIR = standardized incidence ratio; IC = confidence interval. ALL = acute lymphoblastic leukemias; AML = acute myeloblastic leukemias; HL = Hodking lymphomas; NHL = non Hodking lymphomas; CNST = central nervous system tumours; SNST = sympathetic nervous system tumours. Spanish data obtained from Peris Bonet el al. (2015). European data obtained from Steliarova-Foucher et al. (2002).
In the population exposed less than 4 kms, the most relevant results (Table 3) shows an increase in the incidence of NHL around all foci group (p=0.07). A significant increase in the incidence of SNST related to energy industries (0.02) and the group other tumours with the building material industries. By foci to foci analysis (Table 4), we observe a significant increase of PC (all types) around Foci #4, due to the significant increase of SNST and others tumours. Additionally, we found around the foci #3 and #9 a significant increase and NHL and HL, respectively.
Table 3.
List of industrial activity and population (< 15 years) exposed less than 4 km.
| Foci Group | Population (< 15) in 2011 around focus | L | HL | NHL | CNST | SNST | Other | All | |
|---|---|---|---|---|---|---|---|---|---|
| All | 62473 | Observed (Expected) | 29 (35.87) | 5 (4.61) | 13 (8.58) | 30 (30.53) | 13 (12.71) | 36 (37.94) | 126 (130.24) |
| SIR | 0.81 | 1.08 | 1.52 | 0.98 | 1.02 | 0.95 | 0.97 | ||
| CI 95% (SIR) | (0.54,1.16) | (0.35,2.53) | (0.81,2.59) | (0.66,1.40) | (0.54,1.75) | (0.66,1.31) | (0.81,1.15) | ||
| p-valuea | 0.91 | 0.50 | 0.07 | 0.57 | 0.50 | 0.64 | 0.65 | ||
| CI 95% ratio λy/λx | (0.536,+∞) | (0.39,+∞) | (0.933,+∞) | (0.679,+∞) | (0.578,+∞) | (0.679,+∞) | (0.813,+∞) | ||
| Lowest (p-value) | 46 (0.03) | 9 (0.02) | 14 (0.03) | 40 (0.03) | 19 (0.03) | 48 (0.03) | 147 (0.05) | ||
| Power | 0.999 | 0.442 | 0.732 | 0.997 | 0.883 | 1.000 | 1.000 | ||
| E | 4388 | Observed (Expected) |
1 (1.68) | 1 (0.21) | 0 (0.38) | 1 (1.41) | 3 (0.61) | 3 (1.75) | 9 (6.04) |
| SIRc | 0.6 | 4.82 | – | 0.71 | 4.95 | 1.71 | 1.49 | ||
| CId 95% (SIR) | (0.02,3.33) | (0.12,26.84) | – | (0.02,3.95) | (1.02,14.48) | (0.35,5.00) | (0.68,2.83) | ||
| p-valuea | 0.80 | 0.19 | – | 0.75 | 0.02 | 0.24 | 0.14 | ||
| CIb 95% ratio λy/λx | (0.031,+∞) | (0.242,+∞) | – | (0.037,+∞) | (1.445,+∞) | (0.477,+∞) | (0.796,+∞) | ||
| Lowest (p-value)e | 5 (0.02) | 2 (0.01) | 3 (0.00) | 5 (0.01) | 3 (0.02) | 5 (0.03) | 11 (0.03) | ||
| Power | 0.124 | 0.009 | 0.008 | 0.067 | 0.036 | 0.142 | 0.548 | ||
| C | 31967 | Observed (Expected) |
9 (12.77) | 2 (1.62) | 5 (3.02) | 10 (10.83) | 8 (4.55) | 18 (13.47) | 52 (46.26) |
| SIR | 0.70 | 1.23 | 1.66 | 0.92 | 1.76 | 1.34 | 1.12 | ||
| CI 95% (SIR) | (0.32,1.34) | (0.15,4.45) | (0.54,3.87) | (0.44,1.70) | (0.76,3.46) | (0.79,2.11) | (0.84,1.47) | ||
| p-valuea | 0.89 | 0.49 | 0.18 | 0.64 | 0.07 | 0.11 | 0.19 | ||
| CI 95% ratio λy/λx | (0.358,+∞) | (0.208,+∞) | (0.647,+∞) | (0.491,+∞) | (0.911,+∞) | (0.881,+∞) | (0.889,+∞) | ||
| Lowest (p-value) | 19 (0.04) | 5 (0.02) | 7 (0.03) | 17 (0.04) | 9 (0.03) | 20 (0.04) | 58 (0.04) | ||
| Power | 0.887 | 0.110 | 0.262 | 0.813 | 0.426 | 0.897 | 1.000 | ||
| P | 14467 | Observed (Expected) |
12 (9.69) | 2 (1.24) | 3 (2.32) | 9 (8.24) | 2 (3.43) | 8 (10.24) | 36 (35.16) |
| SIR | 1.24 | 1.61 | 1.30 | 1.09 | 0.58 | 0.78 | 1.02 | ||
| CI 95% (SIR) | (0.64,2.16) | (0.19,5.80) | (0.27,3.78) | (0.50,2.07) | (0.07,2.11) | (0.34,1.54) | (0.72,1.42) | ||
| p-valuea | 0.25 | 0.35 | 0.41 | 0.43 | 0.86 | 0.80 | 0.45 | ||
| CI 95% ratio λy/λx | (0.715,+∞) | (0.277,+∞) | (0.344,+∞) | (0.563,+∞) | (0.1,+∞) | (0.382,+∞) | (0.759,+∞) | ||
| Lowest (p-value) | 16 (0.03) | 4 (0.03) | 6 (0.02) | 14 (0.03) | 8 (0.02) | 16 (0.04) | 46 (0.03) | ||
| 0.736 | 0.106 | 0.187 | 0.676 | 0.253 | 0.808 | 0.999 | |||
| Ce | 12165 | Observed (Expected) |
3 (7.24) | 3 (0.92) | 0 (1.72) | 8 (6.13) | 3 (2.57) | 13 (7.59) | 30 (26.17) |
| SIR | 0.41 | 3.27 | – | 1.3 | 1.17 | 1.71 | 1.15 | ||
| CI 95% (SIR) | (0.09,1.21) | (0.67,9.56) | – | (0.56,2.57) | (0.24,3.41) | (0.91,2.93) | (0.77,1.64) | ||
| p-valuea | 0.97 | 0.06 | – | 0.26 | 0.46 | 0.03 | 0.22 | ||
| CI 95% ratio λy/λx | (0.111,+∞) | (0.908,+∞) | – | (0.652,+∞) | (0.319,+∞) | (1.039,+∞) | (0.834,+∞) | ||
| Lowest (p-value) | 13 (0.02) | 4 (0.01) | 5 (0.02) | 11 (0.04) | 6 (0.04) | 13 (0.03) | 35 (0.04) | ||
| Power | 0.585 | 0.039 | 0.135 | 0.568 | 0.259 | 0.654 | 0.993 | ||
| I | 9847 | Observed (Expected) |
8 (15.05) | 1 (1.95) | 6 (3.63) | 11 (12.85) | 4 (5.31) | 11 (15.97) | 41 (54.76) |
| SIR | 0.53 | 0.51 | 1.65 | 0.86 | 0.75 | 0.69 | 0.75 | ||
| CI 95% (SIR) | (0.23,1.05) | (0.01,2.85) | (0.61,3.60) | (0.43,1.53) | (0.21,1.93) | (0.34,1.23) | (0.54,1.02) | ||
| p-valuea | 0.98 | 0.87 | 0.15 | 0.74 | 0.79 | 0.92 | 0.98 | ||
| CI 95% ratio λy/λx | (0.249,+∞) | (0.024,+∞) | (0.722,+∞) | (0.463,+∞) | (0.243,+∞) | (0.371,+∞) | (0.549,+∞) | ||
| Lowest (p-value) | 22 (0.04) | 5 (0.04) | 8 (0.02) | 20 (0.03) | 10 (0.03) | 23 (0.04) | 68 (0.03) | ||
| Power | 0.922 | 0.199 | 0.305 | 0.848 | 0.494 | 0.938 | 1.000 |
Abbreviations: L = leukaemia; HL = Hodking lymphomas; NHL = non Hodking lymphomas; CNST = central nervous system tumours. SNST = sympathetic nervous system tumours.
E= energy industries; C= organic and inorganic chemical industries; P= pharmaceutical industries; Ce= building material industries; I= industries concerned with the incineration or valorisation of dangerous waste.
p-value (H0: λy/λx≤1; HA: λy/λx > 1). Assume that Y ∼ Poisson(nλy) and X ∼ Poisson(mλx). n=total population around (< 4 km) Foci; m=total population outside (≥4 km) Foci. Y = Cases around (< 4 km) Foci; X = Cases outside (≥4 km) Foci influence.
Exact confidence interval α=0.05 to the ratio λy/λx.
SIR=Standardize Incidence Ratio by age group with respect to Murcia Region.
Exact Confidence Interval α=0.05.
Lowest number of observed cases which would be statistical significant at the one sided 5% type one level or less test using this test. In brackets: Exact value of the one sided type one error corresponding to this limit.
Table 4.
List of industrial associated with each focus, industrial activity and population (< 15 years) exposed less than 4 km.
| Foci | Number Facilities | Types of Emission§ |
Population (< 15) around focus 2011 |
L | HL | NHL | CNST | SNST | Others | All | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Foci #1 | 1 | C | 17732 | Observed (Expected) | 5 (7.27) | 1 (0.93) | 2 (1.74) | 7 (6.19) | 5 (2.58) | 10 (7.68) | 30 (26.39) |
| SIRc(CId 95%) | 0.69 (0.22,1.60) | 1.07(0.03,5.97) | 1.15 (0.14,4.16) | 1.13(0.45,2.33) | 1.94 (0.63,4.52) | 1.30 (0.62,2.39) | 1.14 (0.77,1.62) | ||||
| p-valuea | 0.85 | 0.61 | 0.52 | 0.42 | 0.11 | 0.23 | 0.25 | ||||
| CIb 95% ratio λy/λx | (0.26,+∞) | (0.05,+∞) | (0.19,+∞) | (0.52,+∞) | (0.77,+∞) | (0.71,+∞) | (0.82,+∞) | ||||
| Lowest (p-value)e | 13 (0.03) | 4 (0.01) | 5 (0.02) | 11 (0.04) | 6 (0.04) | 13 (0.04) | 36 (0.03) | ||||
| Power | 0.592 | 0.041 | 0.140 | 0.581 | 0.262 | 0.670 | 0.992 | ||||
| Foci #2 | 12 | E; C; I | 2461 | Observed (Expected) |
0 (0.90) | 0 (0.11) | 0 (0.21) | 0 (0.76) | 1 (0.32) | 0 (0.95) | 1 (3.25) |
| SIR (CId 95%) | – | – | – | – | 3.12 (0.08,17.40) | – | 0.31 (0.01,1.71) | ||||
| p-valuea | – | – | – | – | 0.26 | – | 0.96 | ||||
| CI 95% ratio λy/λx | – | – | – | – | (0.16,+∞) | – | (0.01,+∞) | ||||
| Lowest (p-value) | 4 (0.01) | 2 (0.00) | 2 (0.01) | 3 (0.04) | 2 (0.03) | 4 (0.01) | 7 (0.04) | ||||
| Power | 0.036 | 0.002 | 0.009 | 0.068 | 0.027 | 0.044 | 0.327 | ||||
| Foci #3 | 3 | I | 23118 | Observed (Expected) |
7 (13.07) | 1 (1.70) | 5 (2.06) | 10 (11.16) | 3 (4.61) | 9 (13.87) | 35 (47.57) |
| SIR (CId 95%) | 0.54 (0.22,1.10) | 0.59 (0.01,3.28) | 2.43 (0.79,5.68) | 0.90 (0.43,1.65) | 0.65 (0.13,1.90) | 0.65 (0.30,1.23) | 0.74 (0.51,1.02) | ||||
| p-valuea | 0.98 | 0.82 | 0.05 | 0.68 | 0.85 | 0.93 | 0.98 | ||||
| CI 95% ratio λy/λx | (0.23,+∞) | (0.02,+∞) | (0.98,+∞) | (0.47,+∞) | (0.16,+∞) | (0.32,+∞) | (0.52,+∞) | ||||
| Lowest (p-value) | 20 (0.03) | 5 (0.02) | 6 (0.01) | 18 (0.03) | 9 (0.04) | 21 (0.03) | 60 (0.03) | ||||
| Power | 0.867 | 0.129 | 0.124 | 0.787 | 0.442 | 0.885 | 1.000 | ||||
| Foci #4 | 2 | E; C | 1927 | Observed (Expected) |
1 (0.78) | 1 (0.09) | 0 (0.17) | 1 (0.65) | 2 (0.29) | 3 (0.81) | 8 (2.79) |
| SIR (CId 95%) | 1.28 (0.03,7.15) | 10.7 (0.27,59.60) | – | 1.54 (0.04,8.59) | 7.01 (0.85,25.33) | 3.72 (0.77,10.86) | 2.87(1.24,5.66) | ||||
| p-valuea | 0.52 | 0.09 | – | 0.46 | 0.02 | 0.04 | 0.00 | ||||
| CI 95% ratio λy/λx | (0.06,+∞) | (0.53,+∞) | – | (0.08,+∞) | (1.3,+∞) | (1.05,+∞) | (1.48,+∞) | ||||
| Lowest (p-value) | 3 (0.03) | 2 (0.00) | 2 (0.01) | 3 (0.02) | 2 (0.02) | 3 (0.04) | 7 (0.02) | ||||
| Power | 0.073 | 0.001 | 0.005 | 0.043 | 0.021 | 0.082 | 0.198 | ||||
| Foci #5 | 1 | C | 9847 | Observed (Expected) |
3 (3.82) | 0 (0.48) | 3 (0.89) | 2 (3.24) | 0 (1.37) | 5 (4.03) | 13 (13.84) |
| SIR (CId 95%) | 0.78 (0.16,2.29) | – | 3.35 (0.69,9.80) | 0.62 (0.07,2.23) | – | 1.24 (0.40,2.89) | 0.94 (0.50,1.61) | ||||
| p-valuea | 0.72 | – | 0.06 | 0.83 | – | 0.36 | 0.61 | ||||
| CI 95% ratio λy/λx | (0.21,+∞) | – | (0.91,+∞) | (0.10,+∞) | – | (0.49,+∞) | (0.55,+∞) | ||||
| Lowest (p-value) | 8 (0.03) | 3 (0.01) | 4 (0.01) | 7 (0.04) | 4 (0.04) | 8 (0.04) | 21 (0.03) | ||||
| Power | 0.357 | 0.017 | 0.035 | 0.324 | 0.143 | 0.416 | 0.880 | ||||
| Foci #6 | 2 | P | 4653 | Observed (Expected) |
3 (2.56) | 0 (0.33) | 0 (0.61) | 3 (2.18) | 0 (0.91) | 1 (2.71) | 7 (9.30) |
| SIR (CId 95%) | 1.17 (0.24,3.43) | – | – | 1.38 (0.28,4.02) | – | 0.37 (0.01,2.06) | 0.75 (0.30,1.55) | ||||
| p-valuea | 0.47 | – | – | 0.37 | – | 0.93 | 0.81 | ||||
| CI 95% ratio λy/λx | (0.31,+∞) | – | – | (0.37,+∞) | – | (0.01,+∞) | (0.35,+∞) | ||||
| Lowest (p-value) | 6 (0.04) | 2 (0.04) | 3 (0.02) | 6 (0.02) | 4 (0.01) | 7 (0.01) | 15 (0.04) | ||||
| 0.256 | 0.029 | 0.036 | 0.151 | 0.038 | 0.181 | 0.758 | |||||
| Foci #7 | 1 | I | 1629 | Observed (Expected) |
1 (1.06) | 0 (0.14) | 0 (0.26) | 1 (0.90) | 0 (0.37) | 2 (1.12) | 4 (3.85) |
| SIR (CId 95%) | 0.95 (0.02,5.27) | – | – | 1.11 (0.03,6.16) | – | 1.79 (0.22,6.46) | 1.04 (0.28,2.66) | ||||
| p-valuea | 0.65 | – | – | 0.59 | – | 0.30 | 0.53 | ||||
| CI 95% ratio λy/λx | (0.04,+∞) | – | – | (0.05,+∞) | – | (0.31,+∞) | (0.35,+∞) | ||||
| Lowest (p-value) | 4 (0.02) | 2 (0.00) | 2 (0.02) | 4 (0.01) | 3 (0.00) | 4 (0.02) | 8 (0.04) | ||||
| 0.064 | 0.003 | 0.016 | 0.036 | 0.007 | 0.077 | 0.366 | |||||
| Foci#8 | 1 | Ce | 624 | Observed (Expected) |
0 (0.38) | 0 (0.05) | 0 (0.09) | 0 (0.32) | 0 (0.13) | 0 (0.40) | 0 (1.38) |
| SIR (CId 95%) | – | – | – | – | – | – | – | ||||
| CI 95% (SIR) | – | – | – | – | – | – | – | ||||
| p-valuea | – | – | – | – | – | – | – | ||||
| CI 95% ratio λy/λx | – | – | – | – | – | – | – | ||||
| Lowest (p-value) | 3 (0.00) | 1 (0.04) | 2 (0.00) | 2 (0.04) | 2 (0.00) | 3 (0.00) | 5 (0.01) | ||||
| Power | 0.008 | 0.005 | 0.001 | 0.027 | 0.002 | 0.009 | 0.062 | ||||
| Foci#9 | 1 | Ce | 11541 | Observed (Expected) |
3 (6.86) | 3 (0.87) | 0 (1.62) | 8 (5.81) | 3 (2.44) | 13 (7.20) | 30 (24.79) |
| SIR (CId 95%) | 0.44 (0.09,1.28) | 3.46 (0.71,10.10) | – | 1.38 (0.59,2.71) | 1.23 (0.25,3.59) | 1.81 (0.96,3.09) | 1.21 (0.82,1.73) | ||||
| p-valuea | 0.96 | 0.05 | – | 0.22 | 0.42 | 0.02 | 0.15 | ||||
| CI 95% ratio λy/λx | (0.11,+∞) | (0.96,+∞) | – | (0.69,+∞) | (0.33,+∞) | (1.09,+∞) | (0.88,+∞) | ||||
| Lowest (p-value) | 12 (0.04) | 4 (0.01) | 5 (0.02) | 11 (0.03) | 6 (0.03) | 13 (0.02) | 34 (0.03) | ||||
| Power | 0.613 | 0.032 | 0.110 | 0.494 | 0.220 | 0.577 | 0.987 | ||||
| Foci#10 | 2 | C; P | 11406 | Observed (Expected) |
8 (6.16) | 2 (0.80) | 3 (1.49) | 5 (5.26) | 2 (2.17) | 7 (6.52) | 27 (22.41) |
| SIR (CId 95%) | 1.3 (0.56,2.56) | 2.5 (0.30,9.04) | 2.01 (0.41,5.87) | 0.95 (0.31,2.22) | 0.92 (0.11,3.33) | 1.07 (0.43,2.21) | 1.2 (0.79,1.75) | ||||
| p-valuea | 0.27 | 0.18 | 0.18 | 0.60 | 0.64 | 0.47 | 0.18 | ||||
| CI 95% ratio λy/λx | (0.64,+∞) | (0.44,+∞) | (0.54,+∞) | (0.36,+∞) | (0.15,+∞) | (0.50,+∞) | (0.85,+∞) | ||||
| Lowest (p-value) | 11 (0.04) | 3 (0.04) | 5 (0.01) | 10 (0.03) | 6 (0.02) | 12 (0.03) | 31 (0.04) | ||||
| Power | 0.574 | 0.079 | 0.082 | 0.482 | 0.149 | 0.541 | 0.981 | ||||
| Foci#11 | 1 | I | 682 | Observed (Expected) |
0 (0.27) | 0 (0.04) | 1 (0.07) | 0 (0.23) | 0 (0.09) | 0 (0.29) | 1 (0.97) |
| SIR(CId 95%) | – | – | 15.33 (0.39,85.43) | – | – | – | 1.03 (0.03,5.72) | ||||
| p-valuea | – | – | 0.06 | – | – | – | 0.62 | ||||
| CI 95% ratio λy/λx | – | – | (0.783,+∞) | – | – | – | (0.05,+∞) | ||||
| Lowest (p-value) | 2 (0.031) | 1 (0.03) | 2 (0.00) | 2 (0.02) | 2 (0.00) | 2 (0.03) | 4 (0.01) | ||||
| Power | 0.018 | 0.003 | 0.000 | 0.012 | 0.001 | 0.021 | 0.047 | ||||
| Foci#12 | 1 | P | 3061 | Observed (Expected) |
1 (0.96) | 0 (0.12) | 0 (0.21) | 1 (0.80) | 0 (0.35) | 0 (1.00) | 2 (3.45) |
| SIR(CId 95%) | 1.04 (0.03,5.78) | – | – | – | – | – | 0.58 (0.07,2.09) | ||||
| p-valuea | 0.60 | – | – | – | – | – | 0.84 | ||||
| CI 95% ratio λy/λx | (0.05,+∞) | – | – | – | – | – | (0.10,+∞) | ||||
| Lowest (p-value) | 4 (0.01) | 2 (0.00) | 2 (0.02) | 3 (0.04) | 2 (0.04) | 4 (0.01) | 8 (0.02) | ||||
| Power | 0.046 | 0.002 | 0.009 | 0.079 | 0.034 | 0.053 | 0.258 |
Abbreviations: L = leukaemia; HL = Hodking lymphomas; NHL = non Hodking lymphomas; CNST = central nervous system tumours. SNST = sympathetic nervous system tumours.
p-valuea (H0: λy/λx≤1; HA: λy/λx > 1). Assume that Y ∼ Poisson(nλy) and X ∼ Poisson(mλx). n=total population around (< 4 km) Foci; m=total population outside (≥4 km) Foci. Y = Cases around (< 4 km) Foci; X = Cases outside (≥4 km) Foci influence.
Exact confidence interval α=0.05 to the ratio λy/λx.
SIR=Standardize Incidence Ratio by age group with respect to Murcia Region.
Exact Confidence Interval α=0.05.
Lowest number of observed cases which would be statistical significant at the one sided 5% type one level or less test using this test. In brackets: Exact value of the one sided type one error corresponding to this limit.
E= energy industries; C= organic and inorganic chemical industries; P= pharmaceutical industries; Ce= building material industries; I= industries concerned with the incineration or valorisation of dangerous waste.
Table 5 shows the result of using the Scan test to look for high-incidence clusters around the 12 foci identified. Overall results for all types of tumours indicate significant values in only one cluster, near energy industries in Foci #4, where 8 cases within a radius of 3.39 km were noted; these results should be compared to the expected incidence, based on population, of 2.99 cases. Results per type of tumour for leukaemia, CNST and SNST did not result in the formation of significant clusters, in contrast with lymphomas. Five cases of NHL have been observed around Foci #3 (influence radius of 2.69 km). The population is 5218 and the number of expected cases was 0.91. Foci #3 is at the centre of a cluster of waste-valorisation industries. A cluster with three cases of Hodgkin lymphoma has been observed around foci #9. Results in others foci yielded p-values < 0.1: foci #4 in SNST, foci #2 and foci #10 in NHL.
Table 5.
Focused space Scan test results by tumour type and type of industry.
| Energy | Chemical | Pharmaceutical | Building material | Dangerous waste | All foci | ||
|---|---|---|---|---|---|---|---|
| All | #CT en MLC | 4 | 4 | 5 | 25 | 6 | 4 |
| Foci | Foci #4 | Foci #4 | Foci #10 | Foci #9 | Foci #3 | Foci #4 | |
| Population | 1017 | 1017 | 5993 | 6093 | 1786 | 1017 | |
| Km | 3.39 | 3.62 | 2.75 | 2.23 | 2.00 | 3.39 | |
| Number of cases | 8 | 8 | 25 | 22 | 5 | 8 | |
| Expected cases | 2.99 | 2.68 | 15.77 | 16.03 | 4.7 | 2.68 | |
| Annual cases/106 | 43.7 | 43.7 | 23.2 | 20.1 | 15.6 | 43.7 | |
| p-value | 0.02 ** | 0.13 | 0.21 | 0.26 | 0.99 | 0.27 | |
| Leukaemia | # CT en MLC | 7 | 25 | 15 | – | – | 15 |
| Foci | Foci #4 | Foci #10 | Foci #12 | – | – | Foci #12 | |
| Population | 1750 | 5993 | 2807 | – | – | 2807 | |
| Km | 4.80 | 2.75 | 4.74 | – | – | 4.74 | |
| Number of cases | 2 | 7 | 6 | – | – | 6 | |
| Expected cases | 1.27 | 4.34 | 2.03 | – | – | 2.03 | |
| Annual cases/106 | 6.3 | 6.5 | 11.9 | – | – | 11.9 | |
| p-value | 0.58 | 0.88 | 0.15 | – | – | 0.45 | |
| CNST | #CT en MLC | – | 17 | 22 | 8 | 62 | 17 |
| Foci | – | Foci #1 | Foci #10 | Foci #9 | Foci #3 | Foci#1 | |
| Population | – | 3418 | 5487 | 1725 | 10458 | 3418 | |
| Km | – | 2.04 | 2.65 | 2.30 | 3.20 | 2.04 | |
| Number of cases | – | 5 | 5 | 3 | 8 | 5 | |
| Expected cases | – | 2.11 | 3.39 | 1.07 | 6.46 | 2.11 | |
| Annual cases/106 | – | 8.1 | 5.1 | 2.86 | 4.3 | 8.1 | |
| p-value | – | 0.62 | 0.79 | 0.25 | 0.82 | 0.84 | |
| SNST | #CT en MLC | 4 | 58 | 37 | 24 | 9 | 4 |
| Foci | Foci #4 | Foci #1 | Foci #10 | Foci #9 | Foci #2 | Foci #4 | |
| Population | 1017 | 12453 | 8991 | 6093 | 2019 | 1017 | |
| Km | 3.39 | 4.44 | 4.27 | 2.99 | 4.73 | 3.39 | |
| Number of cases | 2 | 7 | 3 | 3 | 2 | 2 | |
| Expected cases | 0.26 | 3.18 | 2.20 | 1.56 | 0.52 | 0.26 | |
| Annual cases/106 | 10.9 | 3.1 | 1.9 | 2.7 | 5.5 | 10.9 | |
| p-value | 0.06 * | 0.41 | 0.74 | 0.30 | 0.43 | 0.46 | |
| NHL | #CT en MLC | 12 | 4 | 15 | – | 24 | 26 |
| Foci | Foci #2 | Foci #10 | Foci #10 | – | Foci #3 | Foci #3 | |
| Population | 2447 | 775 | 3838 | – | 5218 | 5218 | |
| Km | 5.00 | 1.16 | 2.36 | – | 2.69 | 2.69 | |
| Number of cases | 2 | 2 | 3 | – | 5 | 5 | |
| Expected cases | 0.43 | 0.14 | 0.67 | – | 0.91 | 0.91 | |
| Annual cases/106 | 4.5 | 14.3 | 4.3 | – | 5.3 | 5.3 | |
| p-value | 0.09 * | 0.08 * | 0.151 | – | 0.01 ** | 0.06 * | |
| HL | #CT en MLC | – | 13 | 13 | 18 | 80 | 32 |
| Foci | – | Foci #10 | Foci #10 | Foci #9 | Foci #3 | Foci #9 | |
| Population | – | 3462 | 3462 | 7493 | 20640 | 7493 | |
| Km | – | 2.26 | 2.26 | 2.67 | 4.35 | 3.37 | |
| Number of cases | – | 2 | 2 | 3 | 2 | 3 | |
| Expected cases | – | 0.32 | 0.32 | 0.70 | 1.93 | 0.7 | |
| Annual cases/106 | – | 3.2 | 3.2 | 2.2 | 0.5 | 2.2 | |
| p-value | – | 0.26 | 0.127 | 0.04 ** | 0.612 | 0.360 |
CT = Census track; MLC =Most Likelihood Cluster; Population = Population (< 15) inside of MLC; Km = radius of MLC around foci; Number of cases= Number of cases inside of cluster; Expected cases= Expected cases inside of cluster.
Abbreviations: L = leukaemia; HL = Hodking lymphomas; NHL = non Hodking lymphomas; CNST = central nervous system tumours. SNST = sympathetic nervous system tumours
p-value < 0.05.
p-value < 0.1.
4. Discussion
This study described the incidence of PC according to period and age in the RM and analysed the geographical pattern of the cases examining the relationship between these patterns and industrial pollution foci.
The results from the descriptive analysis show that the incidence of PC in the RM was similar to that in other European regions (Gatta et al., 2014; Peris-Bonet et al., 2015). The significant increase in PC, and specifically in leukaemia, lymphoma and neuroblastoma is a common trend that has been observed over the last decade (Kaatsch, 2010; Ward et al., 2014). Although a major effort is being made in recent years to reduce industrial emissions in Europe, some hazardous pollutants such as methane or benzopyrene continue to increase (European Environment Agency, 2016).
The results from the geographical analysis suggest some spatial clustering around certain industrial pollution foci. Globally, the results show a high incidence of NHL around the industrial facilities with some spatial clustering around certain industrial pollution foci. Also, the overall results (for all types of tumour) indicated a cluster around energy industries. Results for NHL revealed one cluster near waste-valorisation industries and HL close to building material industries.
Most publications on the relationship between PC and environmental pollution focus on leukaemia, CNST and all cancer types combined. In recent years, spatial epidemiological studies have linked PC with several environmental risk factors, including pesticides (McNally et al., 2014; Wheeler et al., 2011), or industrial pollutants (García Pérez et al., 2015b; García-Pérez et al., 2016a). However, we should not rule out the hypothesis infectious aetiology as more plausible (Kreis et al., 2016; McNally and Eden, 2004; Ortega-García et al., 2016). The evaluation of exposure is generally limited, and the majority of studies focus on the proximity of highways or on traffic density, and results are disparate. A European study observed an increase in the number of cases of leukaemia (Harrison et al., 1999), but numerous studies carried out in the USA (Alexander et al., 1998; Harrison et al., 1999; Puett et al., 2010; Reynolds et al., 2002; Selvin et al., 2004; Von Behren et al., 2008) and Denmark (Raaschou-Nielsen et al., 2001) led to different conclusions. Crosignani et al. (2004) estimated the relationship between traffic-related benzene emissions and the incidence of pediatric leukaemia and observed that concentrations above 10 micrograms per cubic metre increased the risk by 3.91 (95% 1.36, 11.7). Concerning CNST, several previous studies have found spatial patterns. A British study showed evidence of overall spatio-temporal clustering among cases of primitive neuroectodermal tumours (McNally et al., 2012). Another British study found evidence of space-time clustering in cases of astrocytoma and ependymoma (McNally et al., 2002). In the literature, there are very few studies of cluster of cases of lymphomas in children. A British space-time clustering study found space-time clustering for HL and NHL (Goodman et al., 2014). Proximity to high-capacity thoroughfares has also been associated with the incidence of NHL among Danish children (Raaschou-Nielsen et al., 2001).
Concerning industrial foci, a higher incidence of leukaemia has been observed in the vicinity of petrol stations (Harrison et al., 1999; Steffen et al., 2004; Weng et al., 2009), repair workshops (Steffen et al., 2004), nuclear plants (Kaatsch et al., 2008; Spix et al., 2008), mineral-treatment plants, and galvanisation and metallurgical industries (Garcia-Perez et al., 2015b). A higher incidence of neuroblastoma has also been reported in areas in proximity to metal plants and mines (Garcia-Perez et al., 2016b). Moreover, it has been reported that proximity to industrial facilities, especially those dealing with metals and organic chemistry, increases the incidence of kidney tumours (Garcia-Perez et al., 2016a). A higher risk of CNST has been associated with intrauterine exposure to carcinogenic industrial substances, although no direct association between this and specific industrial foci has yet been established (McKean-Cowdin et al., 1998). A recent Spanish study detected a cluster of four cases of NHL within a radius of 643 m (Ramis et al., 2015).
The findings in our study showed a global higher incidence of NHL around foci of industrial pollution. The aetiology of most NHLs is unknown. Some analyses link the higher risk of NHL to exposure to chemicals, pesticides, organochlorines, alkylphenols and organic solvents (De Roos et al., 2010). A higher risk of NHL has also been related to proximity to paper industries (Ramis et al., 2009), rubber/plastic refineries, metallic-derivate industries (De Roos et al., 2010), benzene-emitting foci (Bulka et al., 2013) and cement kilns (Pronk et al., 2013). In certain professions, an increased risk of NHL has been noted: rubber-industry employees, vets, uranium miners, workers exposed to asbestos, timber-, metal- and textile-industry workers, farmers and cleaners (Baris and Zahm, 2000; t Mannetje et al., 2008).
Concerning our research, a higher overall risk of cancer is observed around Foci #4. The chemical/energy complex located on Foci #4 is related to the manufacture of bisphenol-A derived polycarbonate plastics and resins. This statistically significant result concerns all types of tumour combined, which limits the value of these results. However, it is important to highlight that reviewing MACAPEMUR database we found 4 out of the 8 cases observed are neuroectodermal or derived from the neural crest, and there are previous studies that have found associations between SNST and exposures to environmental toxicants such as bisphenol A, polycyclic aromatic hydrocarbons or carbon tetrachloride (Heck et al., 2013; Zheng et al., 2015). Muirhead et al. (2015) describe in their study that neuroblastic tumours occur in mini-epidemics where transient environmental exposures, such as infections or air pollution, are involved. These chemical products have motivated studies regarding child health effects, mainly birth outcomes and neurodevelopment but not cancer (Rochester, 2013). More research about exposure to bisphenol A and childhood health is needed to fill the gap in cancer aetiology.
In the proximity of Foci #3 (industries concerned incineration and valorisation of dangerous waste), there are more environmental hazards that could be associated with the high incidence of NHL, such as an urban area and the Foci #2. Also, it is very close an area of over 20 ha, in which thousands of tons of soil rich in heavy metals (lead, cadmium, arsenic) and phosphogypsum-derived radioactive elements (uranium, thorium, and polonium), sit in the open air.
In the case of Foci #9 (building material industries), 3 cases of HL have been observed. The presence of aggregated or clustered cases of LH is a known risk factor for this type of tumour. Some authors have proposed infectious aetiologies, such as EBV and herpesvirus (Cader et al., 2010; Linabery et al., 2014).
Finally, we must discuss the main limitations of this study. The approach used in the paper may have at least three important short-comings. Firstly, the low incidence of PC and the small size of the population of children studied hamper attempts to obtain consistent evidence, although the time-span of the project is relatively long (18 years), which makes the analysis more consistent. Additionally, two complementary methods have been used in the analysis, obtaining the same results. Secondly, the use of Focused Scan methodology, based on an isotropic model with circular windows, may lead to a false classification, as exposure depends on prevailing winds, topography and water pollution. This limits the possibility of attaining positive results, but does not invalidate the associations found. Thirdly, the non-inclusion of possible confounding factors, both indoor (professional-related exposure, lifestyle of the parents) and outdoor (non-atmospheric and non-identified pollution foci), can also lead to spurious results. Finally, another limitation is that the results of this study are based on the address at the time of diagnosis and do not allow us to explore the effects of the latency period of PC and the changes of residence between the pregnancy and the time of diagnosis, among other aspects.
All cases are being carefully monitored by project MACAPEMUR (Ortega-García et al., 2011) in the hope that the etiopathogenesis of these clusters can be clarified further (Ortega-Garcia et al., 2016; Ortega-García et al., 2012). This study is part of an ongoing research project that aims at improving the environmental health and quality of life of PC survivors in the RM. We expect to be able to offer more evidence in future works.
5. Conclusion
The results of this study suggest a possible association between proximity to certain industries and increased risk of PC. Globally, living in the vicinity of any industrial activity seem to increase the incidence of NHL. Furthermore, the energy/chemical industries seems to increase the overall risk of PC (taking all types of tumour into consideration), specifically neuroectodermics tumours. While residing near waste-valorisation industries and building material industries appear to increase the risk of NHL and HL, respectively. These results stress the need to carry out detailed assessments on health hazards to children who have been exposed to industrial toxic emissions.
In the clusters detected, near foci #3, #4 and #9, a detailed integrative cancer risk-assessment, including an environmental clinical history of each case and the potentially relevant community-related data, may contribute to achieving two targets: improving our understanding of the etiopathogenesis behind these cases and to give the opportunities to improve public health decision making under complex problems.
Supplementary Material
Acknowledgments
The authors wish to express their gratitude to all the participants of the study. This work was supported by Mount Sinai International Exchange Program for Minority Students (grant MD001452) from the National Center on Minority Health and Health Disparities of the U.S. National Institutes of Health; and Prof. Fernando A. López, grateful for the financial support offered by the projects from Programa de Ayudas a Grupos de Excelencia de la Región de Murcia, Fundación Seneca (#19884-GERM-15) and Ministry of Economy and Competiveness (ECO2015-651758-P).
Appendix A. Supporting information
Supporting information associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.envres.2017.03.009.
Footnotes
Thanks to anonymous referee for this suggestion.
References
- Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest. 2003;123:21s–49s. doi: 10.1378/chest.123.1_suppl.21s. [DOI] [PubMed] [Google Scholar]
- Alexander FE, Boyle P, Carli PM, Coebergh JW, Draper GJ, Ekbom A, Levi F, McKinney PA, McWhirter W, Michaelis J, Peris-Bonet R, Petridou E, Pompe-Kirn V, Plisko I, Pukkala E, Rahu M, Storm H, Terracini B, Vatten L, Wray N. Spatial clustering of childhood leukaemia: summary results from the EUROCLUS project. Br J Cancer. 1998;77:818–824. doi: 10.1038/bjc.1998.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baris D, Zahm SH. Epidemiology of lymphomas. Curr Opin Oncol. 2000;12:383–394. doi: 10.1097/00001622-200009000-00002. [DOI] [PubMed] [Google Scholar]
- Buka I, Koranteng S, Osornio Vargas AR. Trends in childhood cancer incidence: review of environmental linkages. Pediatr Clin N Am. 2007;54:177–203. doi: 10.1016/j.pcl.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Bulka C, Nastoupil LJ, McClellan W, Ambinder A, Phillips A, Ward K, Bayakly AR, Switchenko JM, Waller L, Flowers CR. Residence proximity to benzene release sites is associated with increased incidence of non-Hodgkin lymphoma. Cancer. 2013;119:3309–3317. doi: 10.1002/cncr.28083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cader FZ, Kearns P, Young L, Murray P, Vockerodt M. The contribution of the Epstein-Barr virus to the pathogenesis of childhood lymphomas. Cancer Treat Rev. 2010;36:348–353. doi: 10.1016/j.ctrv.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Cárceles-Álvarez A, Ortega-García JA, Fuster-Soler JL, Rivera-Pagán GA, Bermúdez-Cortés M, Gomariz-Peñalver V, Monzó-Nuñez E, López-Hernández FA. Long-term follow up of childhood cancer survivors in the Murcia Region: preferences and attitudes of Primary Care professionals. An Pediatr. 2015;83:264–271. doi: 10.1016/j.anpedi.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Crosignani P, Tittarelli A, Borgini A, Codazzi T, Rovelli A, Porro E, Contiero P, Bianchi N, Tagliabue G, Fissi R, Rossitto F, Berrino F. Childhood leukemia and road traffic: a population-based case-control study. Int J Cancer. 2004;108:596–599. doi: 10.1002/ijc.11597. [DOI] [PubMed] [Google Scholar]
- De Roos AJ, Davis S, Colt JS, Blair A, Airola M, Severson RK, Cozen W, Cerhan JR, Hartge P, Nuckols JR, Ward MH. Residential proximity to industrial facilities and risk of non-Hodgkin lymphoma. Environ Res. 2010;110:70–78. doi: 10.1016/j.envres.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demoury C, Goujon-Bellec S, Guyot-Goubin A, Hemon D, Clavel J. Spatial variations of childhood acute leukaemia in France, 1990–2006: global spatial heterogeneity and cluster detection at ‘living-zone’ level. Eur J Cancer Prev. 2012;21:367–374. doi: 10.1097/CEJ.0b013e32834e31d8. [DOI] [PubMed] [Google Scholar]
- European Environment Agency. EEA Report No 28/2016. Luxembourg: 2016. Air Quality in Europe –2016 report. [Google Scholar]
- Ferris Tortajada J, Ortega-García JA, Marco Macián A, García Castell J. Environment and pediatric cáncer. An Pediatr. 2004;61:42–50. doi: 10.1016/s1695-4033(04)78352-6. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez J, Lopez-Abente G, Castello A, Gonzalez-Sanchez M, Fernandez-Navarro P. Cancer mortality in towns in the vicinity of installations for the production of cement, lime, plaster, and magnesium oxide. Chemosphere. 2015a;128:103–110. doi: 10.1016/j.chemosphere.2015.01.020. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez J, Lopez-Abente G, Gomez-Barroso D, Morales-Piga A, Romaguera EP, Tamayo I, Fernandez-Navarro P, Ramis R. Childhood leukemia and residential proximity to industrial and urban sites. Environ Res. 2015b;140:542–553. doi: 10.1016/j.envres.2015.05.014. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez J, Morales-Piga A, Gomez J, Gomez-Barroso D, Tamayo-Uria I, Pardo Romaguera E, Fernandez-Navarro P, Lopez-Abente G, Ramis R. Association between residential proximity to environmental pollution sources and childhood renal tumors. Environ Res. 2016a;147:405–414. doi: 10.1016/j.envres.2016.02.036. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez J, Morales-Piga A, Gomez-Barroso D, Tamayo-Uria I, Pardo Romaguera E, Fernandez-Navarro P, Lopez-Abente G, Ramis R. Risk of neuroblastoma and residential proximity to industrial and urban sites: a case-control study. Environ Int. 2016b:92–93. 269–275. doi: 10.1016/j.envint.2016.04.023. [DOI] [PubMed]
- García-Pérez J, Morales-Piga A, Gómez-Barroso D, Tamayo-Uria I, Pardo Romaguera E, López-Abente G, Ramis R. Risk of bone tumors in children and residential proximity to industrial and urban areas: new findings from a case-control study. Sci Total Environ. 2017;579:1333–1342. doi: 10.1016/j.scitotenv.2016.11.131. [DOI] [PubMed] [Google Scholar]
- Gatta G, Botta L, Rossi S, Aareleid T, Bielska-Lasota M, Clavel J, Dimitrova N, Jakab Z, Kaatsch P, Lacour B, Mallone S, Marcos-Gragera R, Minicozzi P, Sánchez-Pérez MJ, Sant M, Santaquilani M, Stiller C, Tavilla A, Trama A, Visser O, Peris-Bonet R, EUROCARE Working Group Childhood cancer survival in Europe 1999–2007: results of EUROCARE-5-a population-based study. Lancet Oncol. 2014;15:35–47. doi: 10.1016/S1470-2045(13)70548-5. [DOI] [PubMed] [Google Scholar]
- Goodman M, LaKind JS, Fagliano JA, Lash TL, Wiemels JL, Winn DM, Patel C, Van Eenwyk J, Kohler BA, Schisterman EF, Albert P, Mattison DR. Cancer cluster investigations: review of the past and proposals for the future. Int J Environ Res Public Health. 2014;11:1479–1499. doi: 10.3390/ijerph110201479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RM, Leung PL, Somervaille L, Smith R, Gilman E. Analysis of incidence of childhood cancer in the West Midlands of the United Kingdom in relation to proximity to main roads and petrol stations. Occup Environ Med. 1999;56:774–780. doi: 10.1136/oem.56.11.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck JE, Park AS, Qiu J, Cockburn M, Ritz B. An exploratory study of ambient air toxics exposure in pregnancy and the risk of neuroblastoma in offspring. Environ Res. 2013;127:1–6. doi: 10.1016/j.envres.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC. International Classification of Diseases for Oncology ICD-O-3. Lyon, France: 2011. [Google Scholar]
- INE. Defunciones por causa de muerte. Madrid, Spain: 2016. Available at: 〈 http://www.ine.es/jaxiT3/Tabla.htm?T=7947〉 (accessed on 12 December 2016) [Google Scholar]
- Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev. 2010;36:277–285. doi: 10.1016/j.ctrv.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Kaatsch P, Spix C, Schulze-Rath R, Schmiedel S, Blettner M. Leukaemia in young children living in the vicinity of German nuclear power plants. Int J Cancer. 2008;122:721–726. doi: 10.1002/ijc.23330. [DOI] [PubMed] [Google Scholar]
- Kreis C, Grotzer M, Hengartner H, Spycher BD. Space-time clustering of childhood cancers in Switzerland: a nationwide study. Int J Cancer. 2016;138:2127–2135. doi: 10.1002/ijc.29955. [DOI] [PubMed] [Google Scholar]
- Kulldorff M, Feuer EJ, Miller BA, Freedman LS. Breast cancer clusters in the northeast United States: a geographic analysis. Am J Epidemiol. 1997;146:161–170. doi: 10.1093/oxfordjournals.aje.a009247. [DOI] [PubMed] [Google Scholar]
- Linabery AM, Erhardt EB, Fonstad RK, Ambinder RF, Bunin GR, Ross JA, Spector LG, Grufferman S. Infectious, autoimmune and allergic diseases and risk of Hodgkin lymphoma in children and adolescents: a Children’s Oncology Group study. Int J Cancer. 2014;135:1454–1469. doi: 10.1002/ijc.28785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis D, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Baan R, Mattock H, Straif K. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013;14:1262–1263. doi: 10.1016/s1470-2045(13)70487-x. [DOI] [PubMed] [Google Scholar]
- McKean-Cowdin R, Preston-Martin S, Pogoda JM, Holly EA, Mueller BA, Davis RL. Parental occupation and childhood brain tumors: astroglial and primitive neuroectodermal tumors. J Occup Environ Med. 1998;40:332–340. doi: 10.1097/00043764-199804000-00007. [DOI] [PubMed] [Google Scholar]
- McNally RJ, Eden TO. An infectious aetiology for childhood acute leukaemia: a review of the evidence. Br J Haematol. 2004;127:243–263. doi: 10.1111/j.1365-2141.2004.05166.x. [DOI] [PubMed] [Google Scholar]
- McNally RJ, Cairns DP, Eden OB, Alexander FE, Taylor GM, Kelsey AM, Birch JM. An infectious aetiology for childhood brain tumours? Evidence from space-time clustering and seasonality analyses. Br J Cancer. 2002;86:1070–1077. doi: 10.1038/sj.bjc.6600228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally RJ, Alexander FE, Vincent TJ, Murphy MF. Spatial clustering of childhood cancer in Great Britain during the period 1969–1993. Int J Cancer. 2009;124:932–936. doi: 10.1002/ijc.23965. [DOI] [PubMed] [Google Scholar]
- McNally RJ, James PW, Picton SV, McKinney PA, Laar M, van Feltbower RG. Space-time clustering of childhood central nervous system tumours in Yorkshire, UK. BMC Cancer. 2012;12:13. doi: 10.1186/1471-2407-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally RJ, Stiller C, Vincent TJ, Murphy MF. Cross-space-time clustering of childhood cancer in Great Britain: evidence for a common aetiology. Int J Cancer. 2014;134:136–143. doi: 10.1002/ijc.28332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton-Jones T, Diggle P, Elliott P. Investigation of excess environmental risk around putative sources: stone’s test with covariate adjustment. Stat Med. 1999;18:189–197. doi: 10.1002/(sici)1097-0258(19990130)18:2<189::aid-sim7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Muirhead CR, Tweddle DA, Basta NO, McNally RJ. Temporal clustering of neuroblastic tumours in children and young adults from Northern England. Environ Health. 2015;4 doi: 10.1186/s12940-015-0058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Garcia JA, López-Hernández FA, Carceles-Alvarez A, Santiago-Rodriguez EJ, Sanchez AC, Bermudez-Cortes M, Fuster-Soler JL. Analysis of small areas of pediatric cancer in the municipality of Murcia (Spain) An Pediatr. 2016;84:154–162. doi: 10.1016/j.anpedi.2015.04.021. [DOI] [PubMed] [Google Scholar]
- Ortega-García JA, López-Hernández FA, Sobrino-Najul E, Febo I, Fuster-Soler JL. Environment and paediatric cancer in the Region of Murcia (Spain): integrating clinical and environmental history in a geographic information system. An Pediatr. 2011;74:255–260. doi: 10.1016/j.anpedi.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-García JA, Soldin OP, López-Hernández FA, Trasande L, Ferris-Tortajada J. Congenital fibrosarcoma and history of prenatal exposure to petroleum derivatives. Pediatrics. 2012;130:e1019–e1025. doi: 10.1542/peds.2011-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris Bonet R, Felipe García S, Valero Poveda S, Pardo Romaguera E. Registro Español de Tumores Infantiles (RETI-SEHOP) Valencia, Spain: 2015. Cáncer infantil en España. Estadísticas 1980–2014. [Google Scholar]
- Peris-Bonet R, Felipe García S, Martínez Ruiz N, Pardo Romaguera E, Valero Poveda S. Childhood cancer incidence and survival in Spain. Ann Oncol. 2010;21(Suppl 3) doi: 10.1093/annonc/mdq092. [DOI] [PubMed] [Google Scholar]
- Pronk A, Nuckols JR, De Roos AJ, Airola M, Colt JS, Cerhan JR, Morton L, Cozen W, Severson R, Blair A, Cleverly D, Ward MH. Residential proximity to industrial combustion facilities and risk of non-Hodgkin lymphoma: a case-control study. Environ Health. 2013;12:20. doi: 10.1186/1476-069X-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puett R, Lawson A, Clark A, Hebert J, Kulldorff M. Power evaluation of focused cluster tests. Environ Ecol Stat. 2010;17:303–316. doi: 10.1007/s10651-009-0108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaschou-Nielsen O, Hertel O, Thomsen BL, Olsen JH. Air pollution from traffic at the residence of children with cancer. Am J Epidemiol. 2001;153:433–443. doi: 10.1093/aje/153.5.433. [DOI] [PubMed] [Google Scholar]
- Ramis R, Vidal E, Garcia-Perez J, Lope V, Aragones N, Perez-Gomez B, Pollan M, Lopez-Abente G. Study of non-Hodgkin’s lymphoma mortality associated with industrial pollution in Spain, using Poisson models. BMC Public Health. 2009;9:26. doi: 10.1186/1471-2458-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramis R, Diggle P, Cambra K, López-Abente G. Prostate cancer and industrial pollution risk around putative focus in a multi-source scenario. Environ Int. 2011;37:577–585. doi: 10.1016/j.envint.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Ramis R, Gomez-Barroso D, Tamayo I, Garcia-Perez J, Morales A, Pardo Romaguera E, Lopez-Abente G. Spatial analysis of childhood cancer: a case/control study. PLoS One. 2015;10:e0127273. doi: 10.1371/journal.pone.0127273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P, Von Behren J, Gunier RB, Goldberg DE, Hertz A, Smith D. Traffic patterns and childhood cancer incidence rates in California, United States. Cancer Causes Control. 2002;13:665–673. doi: 10.1023/a:1019579430978. [DOI] [PubMed] [Google Scholar]
- Reynolds P, Von Behren J, Gunier RB, Goldberg DE, Hertz A, Smith DF. Childhood cancer incidence rates and hazardous air pollutants in California: an exploratory analysis. Environ Health Perspect. 2003;111:663–668. doi: 10.1289/ehp.5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester JR. Bisphenol A and human health: a review of the literature. Reprod Toxicol. 2013;42:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Selvin S, Ragland KE, Chien EYL, Buffler PA. Spatial analysis of childhood leukemia in a case/control study. Int J Hyg Environ Health. 2004;207:555–562. doi: 10.1078/1438-4639-00327. [DOI] [PubMed] [Google Scholar]
- Spix C, Schmiedel S, Kaatsch P, Schulze-Rath R, Blettner M. Case-control study on childhood cancer in the vicinity of nuclear power plants in Germany 1980– 2003. Eur J Cancer. 2008;44:275–284. doi: 10.1016/j.ejca.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Steffen C, Auclerc MF, Auvrignon A, Baruchel A, Kebaili K, Lambilliotte A, Leverger G, Sommelet D, Vilmer E, Hemon D, Clavel J. Acute childhood leukaemia and environmental exposure to potential sources of benzene and other hydrocarbons; a case-control study. Occup Environ Med. 2004;61:773–778. doi: 10.1136/oem.2003.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steliarova-Foucher E, Berrino F, Coebergh JW, Kaatsch P, Lacour B, Michaelis J, Mitton N, Stiller CA, Parkin DM. European Network of Cancer Registries. International Agency for Research on Cancer; Lyon, France: 2002. ACCISpass1.01, Software for Analysis and Presentation of Data on Incidence and Survival of Children and Adolescents in Europe. [Google Scholar]
- Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International classification of childhood cancer. (Third Edition)Cancer. 2005;103:1457–1467. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- Stiller CA, Marcos-Gragera R, Ardanaz E, Pannelli F, Almar Marques E, Canada Martinez A, Steliarova-Foucher E. Geographical patterns of childhood cancer incidence in Europe, 1988–1997. Report from the automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:1952–1960. doi: 10.1016/j.ejca.2006.05.017. [DOI] [PubMed] [Google Scholar]
- ’t Mannetje A, Dryson E, Walls C, McLean D, McKenzie F, Maule M, Cheng S, Cunningham C, Kromhout H, Boffetta P, Blair A, Pearce N. High risk occupations for non-Hodgkin’s lymphoma in New Zealand: case-control study. Occup Environ Med. 2008;65:354–363. doi: 10.1136/oem.2007.035014. [DOI] [PubMed] [Google Scholar]
- Von Behren J, Reynolds P, Gunier RB, Rull RP, Hertz A, Urayama KY, Kronish D, Buffler PA. Residential traffic density and childhood leukemia risk. Cancer Epidemiol Biomark Prev. 2008;17:2298–2301. doi: 10.1158/1055-9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- Weng HH, Tsai SS, Chiu HF, Wu TN, Yang CY. Childhood leukemia and traffic air pollution in Taiwan: petrol station density as an indicator. J Toxicol Environ Health A. 2009;72:83–87. doi: 10.1080/15287390802477338. [DOI] [PubMed] [Google Scholar]
- Wheeler DC, De Roos AJ, Cerhan JR, Morton LM, Severson R, Cozen W, Ward MH. Spatial-temporal analysis of non-Hodgkin lymphoma in the NCI-SEER NHL case-control study. Environ Health. 2011;10:63. doi: 10.1186/1476-069X-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JC, Qi SQ, Yang L, Ma YY, Dong KR, Zhu HT, Yang SB, Xu T, Zheng S, Xiao XM. Effects of bisphenol A on decreasing the percentage and promoting the growth of stem cell-like cells from SK-N-SH human neuroblastoma cells. Genet Mol Res. 2015;14:2986–2993. doi: 10.4238/2015.April.10.8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


