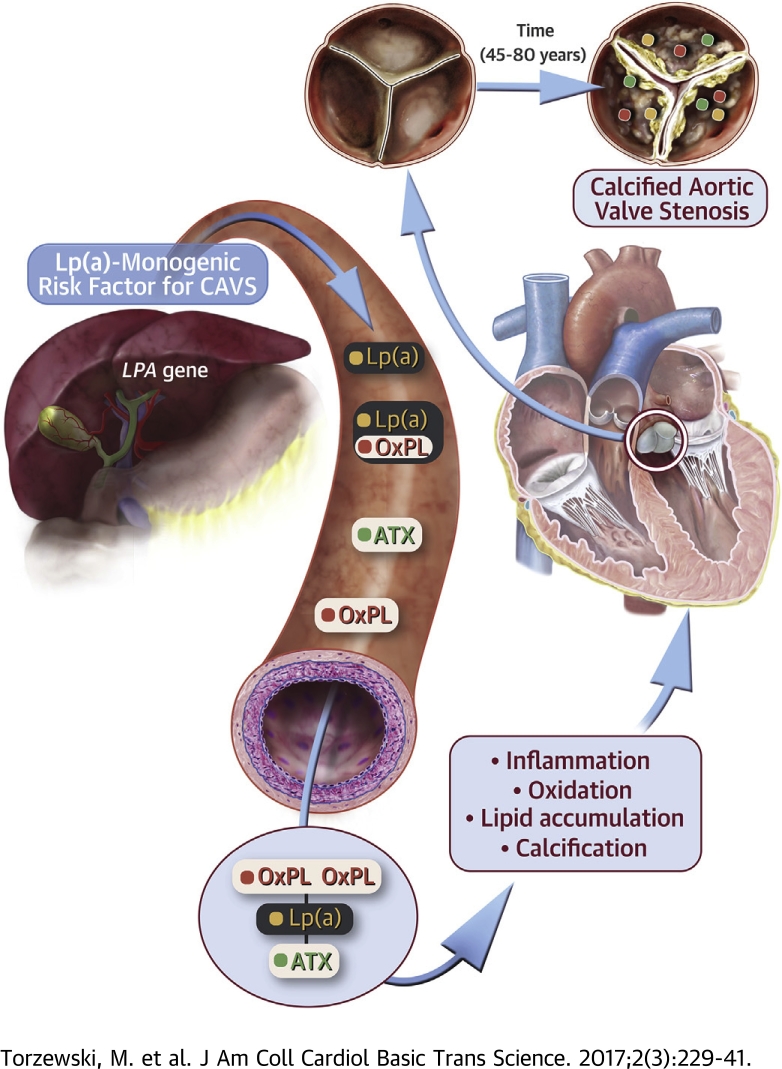
Visual Abstract
Key Words: aortic valve stenosis, autotaxin, inflammation, Lp(a), oxidation-specific epitopes
Abbreviations and Acronyms: apo(a), apolipoprotein(a); apoB, apolipoprotein B; ATX, autotaxin; AVR, aortic valve replacement; CAVS, calcific aortic valve stenosis; IgG, immunoglobulin G; Lp(a), lipoprotein(a); LysoPA, lysophosphatidic acid; LysoPC, lysophosphatidylcholine; MDA, malondialdehyde; OxPL, oxidized phospholipid; OxPL-apo(a), oxidized phospholipid on apolipoprotein(a); OxPL-apoB, oxidized phospholipid on apolipoprotein B-100; PC-OxPL, phosphocholine-containing oxidized phospholipids; RLU, relative light unit
Highlights
-
•
The LPA gene is the only monogenetic risk factor for CAVS, and OxPL and lysophosphatidic acid, generated by autotaxin from OxPL, are pro-inflammatory.
-
•
Both autotaxin–apolipoprotein B and autotaxin–apo(a) were measureable in plasma.
-
•
Immunohistochemistry revealed a strong presence of apo(a), OxPL, malondialdehyde-lysine, autotaxin, and macrophages, particularly in advanced lesions rich in cholesterol crystals and calcification.
-
•
Six species of OxPL and lysophosphatidic acid, with aldehyde-containing phosphocholine-based OxPL most abundant, were identified and quantified after extraction from valve leaflets.
-
•
We demonstrate the presence of a constellation of pathologically linked, Lp(a)-associated molecules in plasma and in aortic valve leaflets of patients with CAVS. These data are consistent with the hypothesis that Lp(a) is a key etiologic factor in patients with CAVS.
Summary
The LPA gene is the only monogenetic risk factor for calcific aortic valve stenosis (CAVS). Oxidized phospholipids (OxPL) and lysophosphatidic acid generated by autotaxin (ATX) from OxPL are pro-inflammatory. Aortic valve leaflets categorized pathologically from both ATX–apolipoprotein B and ATX–apolipoprotein(a) were measureable in plasma. Lipoprotein(a) (Lp[a]), ATX, OxPL, and malondialdehyde epitopes progressively increased in immunostaining (p < 0.001 for all). Six species of OxPL and lysophosphatidic acid were identified after extraction from valve leaflets. The presence of a constellation of pathologically linked, Lp(a)-associated molecules in plasma and in aortic valve leaflets of patients with CAVS suggest that Lp(a) is a key etiologic factor in CAVS.
With the extension of lifespan and aging of the population in the 21st century, calcific aortic valve disease (1), which encompasses early lesions as well as stenotic disease (clinically seen as calcific aortic valve stenosis [CAVS]), is becoming an increasingly prevalent disorder. It is estimated that hemodynamically significant CAVS is present in >1 million patients in the United States and that approximately 2.5 million cases will be present worldwide by 2020 and 4.5 million in 2030 (2). Aortic valve replacement (AVR) is the only option for palliation of symptoms and prevention of complications. Unfortunately, by the time AVR is needed, patients are often octogenarians and a large percentage are not eligible for AVR due to advanced frailty and other significant comorbidities. For example, a recent report comparing surgical AVR versus transcatheter AVR revealed 33% to 39% all-cause mortality, 12% to 19% rate of stroke, and 40% to 48% major adverse cardiac event rate at 3-year follow-up in both groups (3).
Lipoprotein(a) (Lp[a]) is a genetically determined, likely causal, and independent risk factor for the presence and progression of CAVS 4, 5, 6, 7, 8. Lp(a) is the major lipoprotein carrier of phosphocholine-containing oxidized phospholipids (PC-OxPLs) 9, 10 that may contribute to inflammation and induce calcification in valvular cells 11, 12. For example, in the ASTRONOMER (Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin) trial (4), patients with elevated Lp(a) and OxPL on apolipoprotein B-100 (OxPL-apoB) levels had the fastest progression rate and higher need for AVR. Autotaxin (ATX), which breaks down lysophosphatidylcholine (LysoPC) derived from OxPL to lysophosphatidic acid (LysoPA), was also recently shown to be strongly associated with CAVS (13). In turn, because OxPL and oxidized low-density lipoprotein have been shown to be present in CAVS 13, 14, 15, it is likely that a significant portion of the LysoPC is derived from hydrolysis of the truncated oxidized sn2 fatty acids found in OxPL present in oxidized low-density lipoprotein, which are generated by such enzymes as lipoprotein-associated phospholipase A2 and platelet-activating factor acetylhydrolase. In recent findings, ATX activity and either Lp(a) or OxPL-apoB strongly interacted to predict the presence of CAVS in patients undergoing AVR with concomitant coronary artery disease (8).
There are no effective medical therapies to prevent the development or progression of CAVS, and statins have failed to reduce progression of AVR in 4 randomized trials 16, 17, 18, 19, 20. Statins additionally may significantly raise plasma Lp(a) and OxPL-apoB (21), which may be counterproductive in preventing CAVS. For example, rosuvastatin raised Lp(a) levels 20% in the ASTRONOMER trial (4). However, novel Lp(a)-lowering agents may be used in the near future to test the hypothesis that lowering Lp(a) reduces progression of CAVS 22, 23, 24, 25.
The objective of this study was 3-fold: 1) to develop plasma measures of autotaxin carried by apoB and Lp(a); 2) to define lysophophatidic and phosphocholine-containing oxidized phospholipids within aortic valve leaflets; and 3) to document the presence of Lp(a) and oxidation-specific epitopes in aortic valve leaflets obtained following aortic valve replacement.
Methods
A variety of techniques were used to study patients with mild to moderate CAVS and severe CAVS undergoing AVR. These techniques included novel enzyme-linked chemiluminescent assay of plasma components, as well as immunohistochemistry and liquid chromatography-tandem mass spectroscopy of extracts of aortic valve leaflets. Full details are presented in the Supplemental Appendix.
Antibodies to Lp(a), ATX, and oxidation-specific epitopes
LPA4 is a murine monoclonal immunoglobulin G (IgG) antibody to apo(a) that was generated by immunizing mice with the apo(a) sequence TRNYCRNPDAEIRP. E06 is a natural immunoglobulin M murine monoclonal antibody that binds to the phosphocholine head group of oxidized but not native phospholipids. MDA2 is a murine IgG monoclonal antibody that recognizes malondialdehyde-modified proteins and lipid adducts. An alkaline phosphatase–labeled goat anti-human ATX polyclonal antibody was purchased from Life Technologies (Carlsbad, California).
Novel chemiluminescent enzyme-linked immunoadsorbent assay to detect lipoprotein-associated ATX
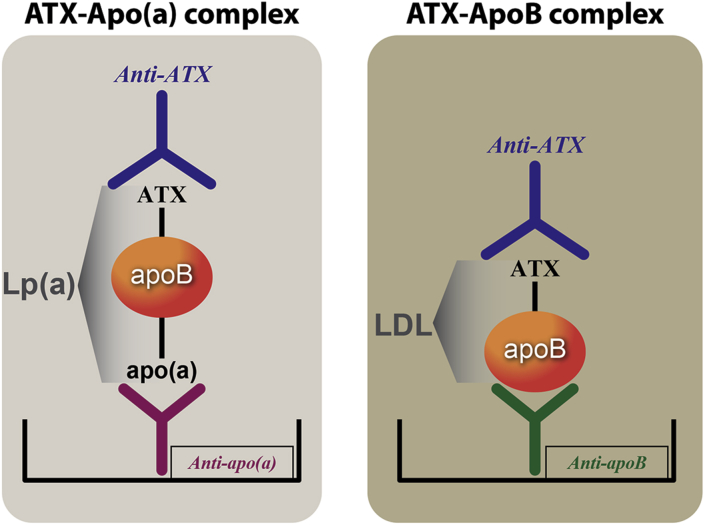
A sensitive and quantitative sandwich-based chemiluminescent enzyme-linked immunoadsorbent assay was used to measure ATX associated with plasma lipoproteins containing apolipoprotein B (apoB)-100, which includes apoB on very low density lipoprotein, intermediate-density lipoprotein, low-density lipoprotein, and Lp(a) (ATX-apoB) and also specifically only on Lp(a) (ATX-apo[a]) (Figure 1). Microtiter 96-well plates were coated overnight at 4°C with antibodies MB47 to bind apoB-100 and LPA4 to bind Lp(a) (all at 5 μg/ml antigen of 40 μl/well). Conditions were established to ensure that the amount of plasma added was sufficient to provide a saturating and equal amount of each lipoprotein captured in each well. Excess material was washed off and the plates blocked with 1% tris buffered saline/bovine serum albumin for 45 min. After the plates were washed, ethylenediaminetetraacetic acid plasma was added at 1:50 dilution (40 μl/well) for 75 min to bind apoB-100, Lp(a), and high-density lipoprotein, respectively. After the plates were again washed, goat anti-human ATX antibody at 1 μg/ml (40 μl/well) was incubated with the plates for 60 min. After washing excess material off the plates, alkaline phosphatase–labeled goat anti-rabbit IgG (Sigma, St. Louis, Missouri) (40 μl/well) was added for 60 min. After a final washing, Lumi-phos 530 (Lumigen, Inc., Southfield, Michigan) (25 μl/well) was added for 75 min and luminescence read on a Dynex luminometer (Chantilly, Virginia). The results are reported as relative light units (RLUs) in 100 ms after the background (tris buffered saline/bovine serum albumin) RLUs are subtracted. High and low values were added to each 96-well plate as internal controls.
Figure 1.
ELISAs to Measure ATX-Lp(a) and ATX-apoB
Methodology of novel chemiluminescent enzyme-linked immunoadsorbent assay (ELISA) to measure autotaxin (ATX)–apolipoprotein(a) (apo[a]) and ATX–apolipoprotein B (apoB) complexes. ApoB-100 and lipoprotein(a) (Lp[a]) lipoproteins were captured from plasma with specific antibodies bound to microtiter well plates. Conditions were established so that the added plasma contains saturating amounts of lipoproteins to be captured. The content of ATX mass on each lipoprotein was then detected with a goat anti-human ATX antibody.
Patients with CAVS
The first group of patients comprised 14 patients with mild to moderate CAVS who had available ethylenediaminetetraacetic acid plasma samples stored frozen at –70°C. The second group comprised 68 patients undergoing AVR for symptomatic CAVS with prospective collection of aortic valve leaflets and were recently described (26). Formalin-fixed nonrheumatic aortic valves with varying degrees of macroscopic disease were analyzed for the presence of apolipoprotein(a) (apo[a]), ATX, oxidation-specific epitopes, and macrophages. The third group comprised 4 patients who underwent AVR and liquid chromatography-tandem mass spectroscopy analysis of valve leaflets for the presence of specific species of OxPL, and LysoPA was performed.
Histological and immunohistochemical analysis of valve leaflets
Although all patients had a clinical diagnosis of CAVS, there was pathological variability in the extent of involvement of the aortic valve leaflets. For this reason, valve leaflets were classified pathologically as grade 1 to 4, with a pathological classification as previously described 26, 27. In addition, a semi-quantitative method was used to determine the proportion of area stained by each antibody. The proportion of the areas stained for each epitope relative to the area occupied by the various pathological grades of CAVS was estimated and assigned to 1 of 5 scores: 0, <5%; 1, 6% to 25%; 2, 26% to 50%; 3, 51% to 75%; or 4, 76% to 100%. A single aortic valve specimen usually comprises >1 pathological grade.
Results
Presence of ATX in circulating plasma in patients with mild to moderate CAVS
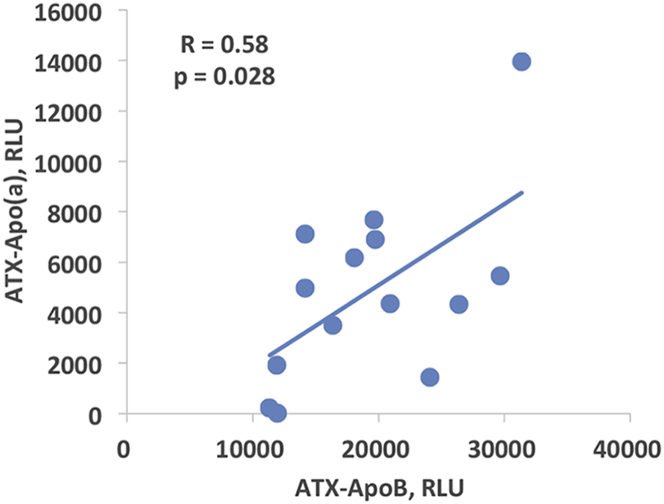
Lipoprotein-associated ATX, measured as ATX-apoB and ATX-apo(a), were quantitated in 14 patients with mild to moderate CAVS. The Vpeak was 3.30 ± 0.48 m/s, mean and peak gradients were 24.4 ± 8.0 mm Hg and 44.9 ± 12.7 mm Hg, respectively, and aortic valve area was 1.32 ± 0.34 cm2. ATX-apoB and ATX-apo(a) could be directly measured on apoB and Lp(a), respectively, and they exhibited a modest correlation (r = 0.58; p = 0.028) (Figure 2). There were no significant correlations of ATX-apoB or ATX-apo(a) with Lp(a) and OxPL-apoB or oxidized phospholipid on apolipoprotein(a) (OxPL-apo[a]) (not shown).
Figure 2.
Correlation Between ATX-Apo(a) and ATX-apoB
Spearman correlation between plasma levels of ATX-apoB and ATX-apo(a) complexes in patients with mild to moderate calcific aortic valve stenosis. RLU = relative light units; other abbreviations as in Figure 1.
Clinical characteristics of patients with CAVS
Table 1 presents the clinical characteristics of the 68 patients with CAVS undergoing AVR. Baseline characteristics included 26.8% former or current smokers, 92.5% with hypertension, and 27.9% with diabetes. Levels of low-density lipoprotein cholesterol tended to be in the normal range; Lp(a) levels were not available because they are not generally measured in patients with CAVS. CAVS was severe, with a mean gradient of 51.0 ± 14.87 mm Hg and an aortic valve area of 0.71 ± 0.19 cm2.
Table 1.
Baseline Clinical, Laboratory and Echocardiography Variables of the CAVS Study Group
| Age, yrs | 76 (54–86) |
| BMI, kg/m2 | 27.3 (20.2–40.5) |
| Smoking | |
| Never | 41 (73.2) |
| Past | 10 (17.9) |
| Present | 5 (8.9) |
| Hypertension | |
| No | 5 (7.5) |
| Yes | 62 (92.5) |
| Diabetes | |
| No | 49 (72.1) |
| Yes | 19 (27.9) |
| Total cholesterol, mg/dl | 183 (114–297) |
| LDL-C, mg/dl | 103 (56–220) |
| Creatinine, μmol/l | 79.6 (53.0–176.8) |
| hsCRP, mg/l | 2.0 (0.0–29.0) |
| HbA1c, %Hb | 5.8 (4.6–9.7) |
| NT-proBNP, pg/ml | 547 (21–19,799) |
| Mean aortic valve gradient, mm Hg∗ | 51.0 (14.9) |
| Mean aortic valve area, cm2† | 0.71 (0.19) |
Values are median (min–max) or n (%).
CAVS = calcific aortic valve stenosis; HbA1c = glycosylated hemoglobin; hsCRP = high-sensitivity C-reactive protein; LDL-C = low-density lipoprotein cholesterol; NT-proBNP = N-terminal pro–B-type natriuretic peptide.
Only 26 patients had available echocardiography data.
Only 36 patients had available echocardiography data.
Presence of apo(a), oxidation-specific epitopes, macrophages, and calcification in aortic valve leaflets in severe CAVS
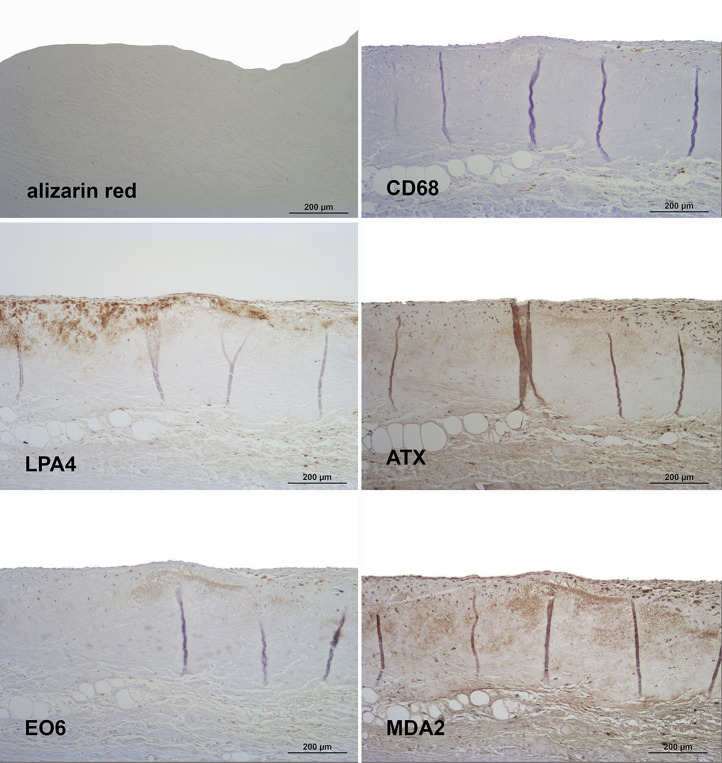
Aortic valve leaflet lesions from 68 patients fulfilling the pathological criteria of grades 1 to 4 were examined. Figures 3, 4, 5, and 6 display representative examples of grades 1 to 4 of aortic valve calcification, as detected by alizarin red staining and structural damage 26, 27. With the use of the specific monoclonal antibody LPA4, apo(a) was detectable in every lesion examined.
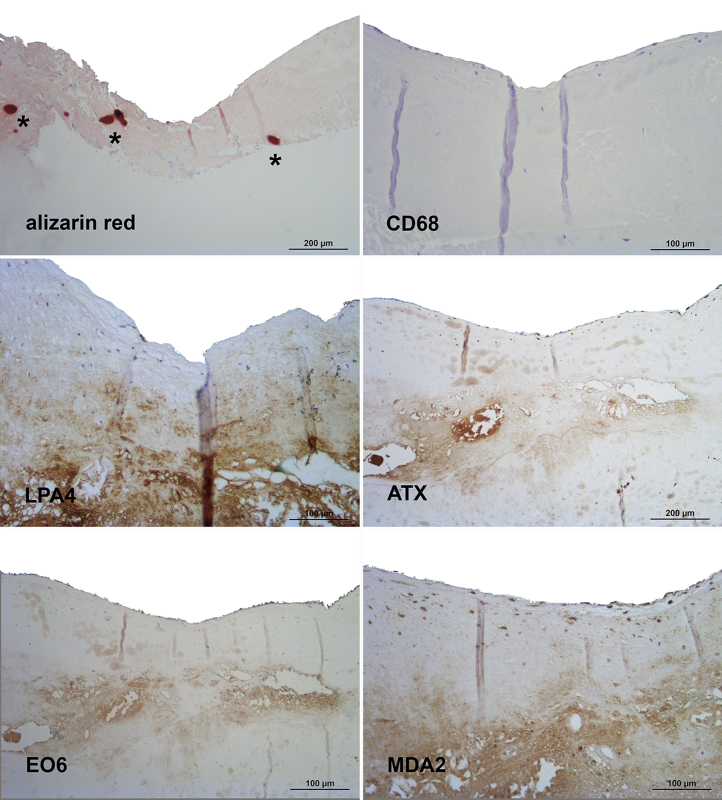
Figure 3.
Presence of Apolipoprotein(a), Oxidation-Specific Epitopes, and Autotaxin in Grade 1 of Aortic Valve Disease
Sequential sections stained for calcification with alizarin red S and macrophages (CD68) (upper panel), apolipoprotein(a) (LPA4) and autotaxin (ATX) (middle panel) as well as oxidized phospholipids (EO6) and malondialdehyde epitopes (MDA2) (lower panel). Note the lack of calcified areas as well as the predominantly extracellular co-localization of the different antigens. In all panels, the aortic side of the valve is at the top.
Figure 4.
Presence of Apolipoprotein(a), Oxidation-Specific Epitopes, and Autotaxin in Grade 2 of Aortic Valve Disease
Sequential sections stained for alizarin red S and macrophages (CD68) (upper panel), apolipoprotein (a) (LPA4), and autotaxin (ATX) (middle panel), as well as oxidized phospholipids (EO6) and malondialdehyde epitopes (MDA2) (lower panel). Note the small calcified areas (asterisks), the lack of CD68 staining in this individual lesion (high variation of macrophages staining in aortic valve sclerosis), as well as the predominantly extracellular co-localization of the different antigens. In all panels, the aortic side of the valve is at the top.
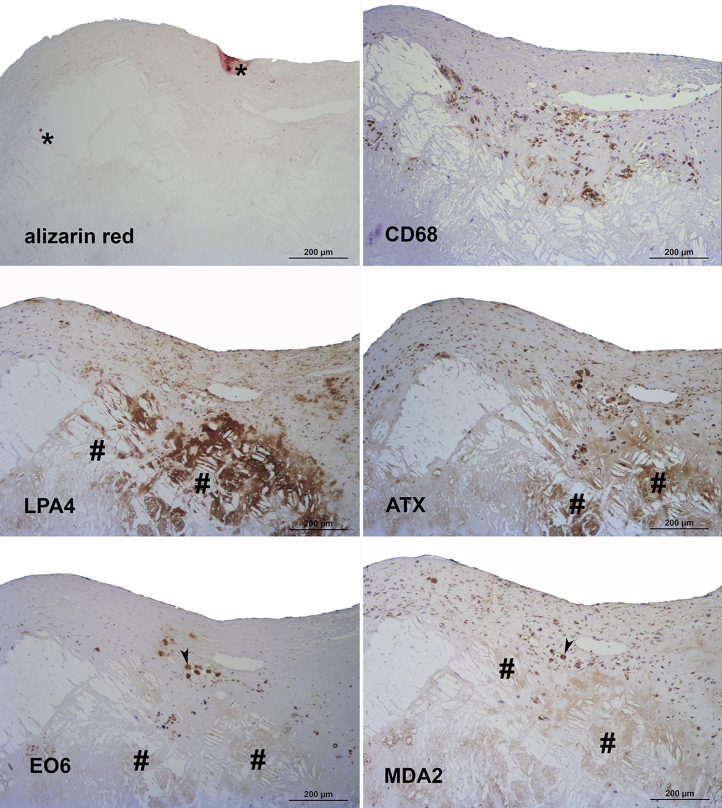
Figure 5.
Presence of Apolipoprotein(a), Oxidation-Specific Epitopes, and Autotaxin in Grade 3 of Aortic Valve Disease
Sequential sections stained for alizarin red S and macrophages (CD68) (upper panel), apolipoprotein (a) (LPA4) and autotaxin (ATX) (middle panel), as well as oxidized phospholipids (EO6) and malondialdehyde epitopes (MDA2) (lower panel). Note the predominance of cholesterol crystals compared to calcified areas (∗) in this individual lesion and co-localization of the different antigens around cholesterol crystal deposits (#). Sometimes additional intracellular oxidized phospholipid staining is also evident (arrowheads). In all panels, the aortic side of the valve is at the top.
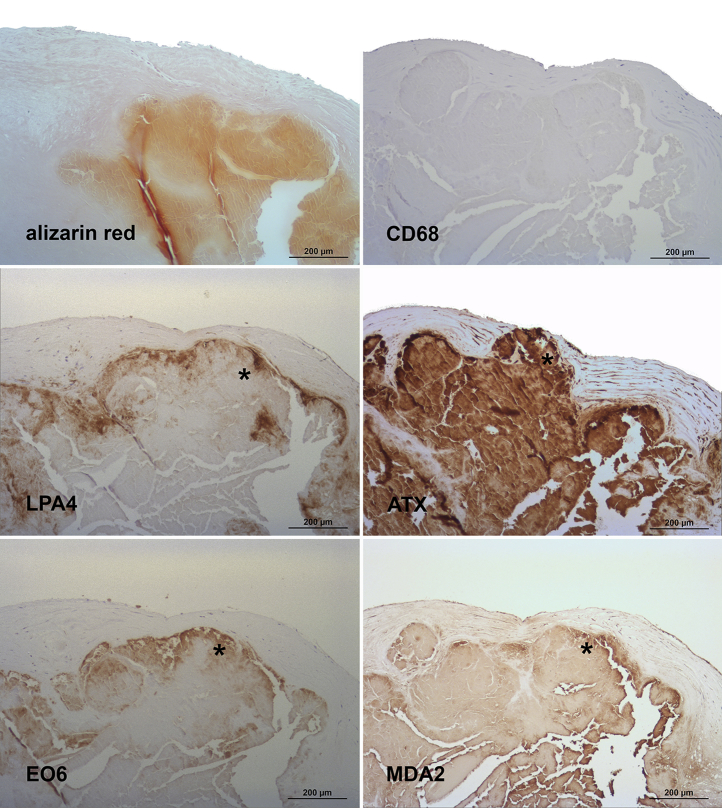
Figure 6.
Presence of Apolipoprotein(a), Oxidation-Specific Epitopes, and Autotaxin in Grade 4 of Aortic Valve Disease
Sequential sections stained for alizarin red S and macrophages (CD68) (upper panel), apolipoprotein (a) (LPA4), and autotaxin (ATX) (middle panel), as well as oxidized phospholipids (EO6) and malondialdehyde epitopes (MDA2) (lower panel). Note the co-localization of the different antigens around and within heavily calcified areas (∗) as well as the lack of CD68 staining in this individual lesion (high variation of macrophages staining in aortic valve sclerosis). In all panels, the aortic side of the valve is at the top.
Grade 1 lesions were characterized by a lack of calcified areas, and a predominant focal deposition of apo(a) was present in the fibrosa (Figure 3). ATX, as well as OxPL epitopes, stained by antibody E06, were less evident but where present, they did co-localize with apo(a). MDA-lysine epitopes, stained by antibody MDA2, were diffusely present in the leaflet, but macrophages stained by antibody to CD68 were sparse.
Grade 2 lesions were characterized by early development of calcified nodules and a more prominent deposition of apo(a) in the fibrosa as well as in the spongiosa (Figure 4). ATX was present in areas of apo(a) staining. OxPL epitopes as well as MDA-lysine epitopes were also more prominent than in grade 1 but less evident than apo(a).
Grade 3 lesions were characterized by large calcified nodules and/or cholesterol crystal deposits, and a more abundant deposition of apo(a) as well as ATX compared with grade 1 and 2 lesions, mainly around cholesterol crystal deposits (Figure 5) and/or calcified areas (not shown). OxPL and MDA-lysine epitopes were much less prominent than apo(a).
Grade 4 lesions were characterized by significant fibrosis and calcification grossly destroying the structural integrity of the valvular cusp, as well as more abundant localization of apo(a) as well as ATX, throughout the lesions, including around calcified areas (Figure 6). OxPL epitopes in grade 4 lesions were highly co-localized with apo(a) epitopes. MDA-lysine epitopes also co-localized with apo(a) and MDA-lysine but were much more prominent.
Of note, macrophage staining exhibited a high interindividual variation ranging from none (Figures 4 and 6) or only sparse (Figure 3) to abundant (Figure 5) staining. Nondiseased regions did not show any macrophage staining (not shown).
As evidenced from Figures 3, 4, 5, and 6, Lp(a) staining largely overlapped with both OxPL and MDA-lysine staining. Furthermore, additional intracellular OxPL staining was evident (Figure 5).
Table 2 displays the mean area of immunostaining represented by Lp(a), ATX, OxPL, and MDA epitopes relative to the entire area occupied for each of the pathologically defined grades of CAVS. There is a positive correlation between the epitope positive area and the pathological grade of the valves analyzed. Each of the aforementioned epitopes progressively increased in immunostaining, ranging from <25% of the area occupied for each epitope in grade 1 to 50% to 100% occupied in grade 4 (p < 0.01 for all).
Table 2.
Area of Involvement (Mean Positive Area) of Each Epitope According to Pathological Grades of CAVS
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | p Value for Trend | |
|---|---|---|---|---|---|
| Apo(a) | 1.3 (n = 32) | 1.9 (n = 9) | 1.6 (n = 11) | 2.3 (n = 11) | <0.001 |
| Autotaxin | 1.0 (n = 2) | 1.0 (n = 3) | 3.0 (n = 4) | 3.6 (n = 5) | <0.0001 |
| OxPL | 0.7 (n = 32) | 2.0 (n = 8) | 2.7 (n = 11) | 2.8 (n = 11) | <0.0001 |
| MDA | 1.6 (n = 25) | 2.4 (n = 7) | 3.1 (n = 9) | 4.0 (n = 7) | <0.0001 |
n represents the number of pathological grades analyzed within each category. Some of the leaflets harbored >1 different pathological grade, which is why the numbers analyzed are different from those of leaflets/patients.
Apo(a) = apolipoprotein(a); CAVS = calcific aortic valve stenosis; MDA = malondialdehyde; OxPL = oxidized phospholipid.
Presence of LysoPA and PC-containing OxPL in aortic valve leaflets by liquid chromatography-tandem mass spectrometry in severe CAVS
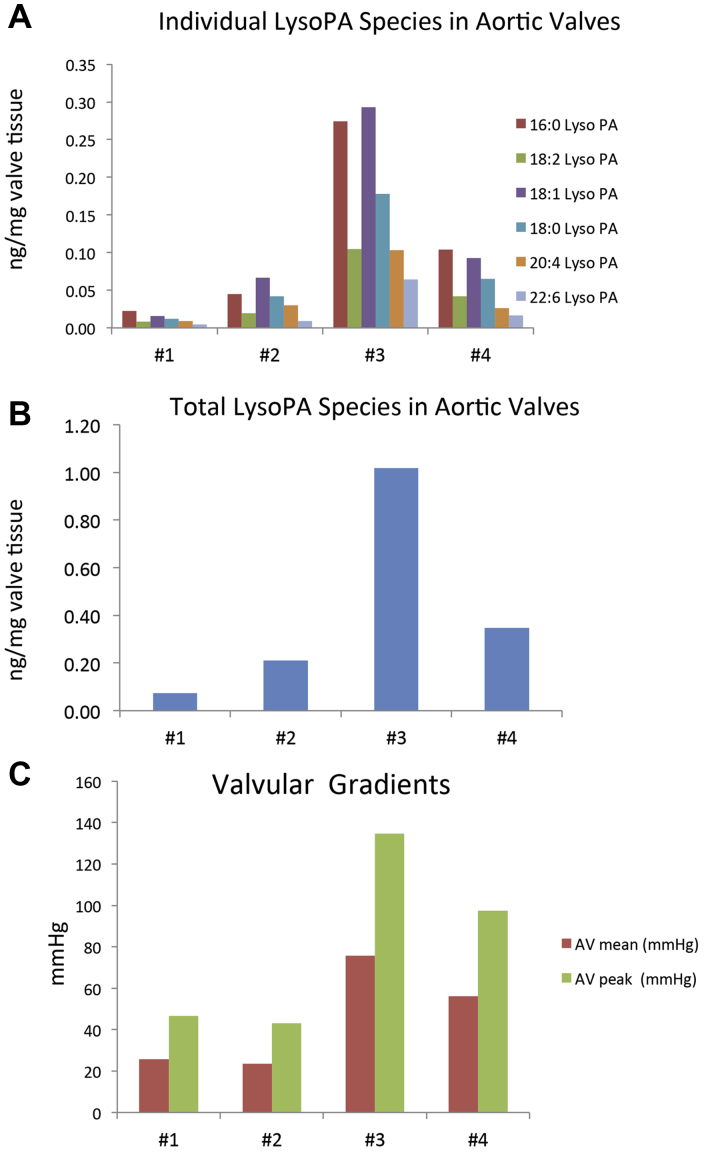
Supplemental Figure 1 shows the single ion multiple reaction monitoring chromatogram of LysoPA extracted from human stenotic aortic valves, representing the most common LysoPA species seen in human plasma. In the 4 different stenotic aortic valves, 16:0 and 18:1 LysoPA species were shown to be the most abundant species (Figure 7A). Variation in total amounts of LysoPA based on amount of valve tissue extracted was observed despite using similar tissue wet weights (Figure 7B). Comparing LysoPA levels in each stenotic valve versus valvular gradients, there was a general trend of increased LysoPA levels and both mean and peak valvular gradients (Figure 7C).
Figure 7.
Presence of LysoPA Species in Valve Leaflets and Relationship to Valve Gradients
Prevalence of (A) individual lysophosphatidic acid (LysoPA) species and (B) total LysoPA mass and (C) mean and peak valvular gradients in 4 different stenotic aortic valves. Reverse-phase separation followed by tandem mass spectrometry detection of (A) LysoPA species 16:0, 18:2, 18:1, 18:0, 20:4 and 22:6 in 4 aortic valves and (B) the total LysoPA amounts per milligram of valve tissue extracted. (C) Represents the mean and peak valvular gradients for each of the study valves.
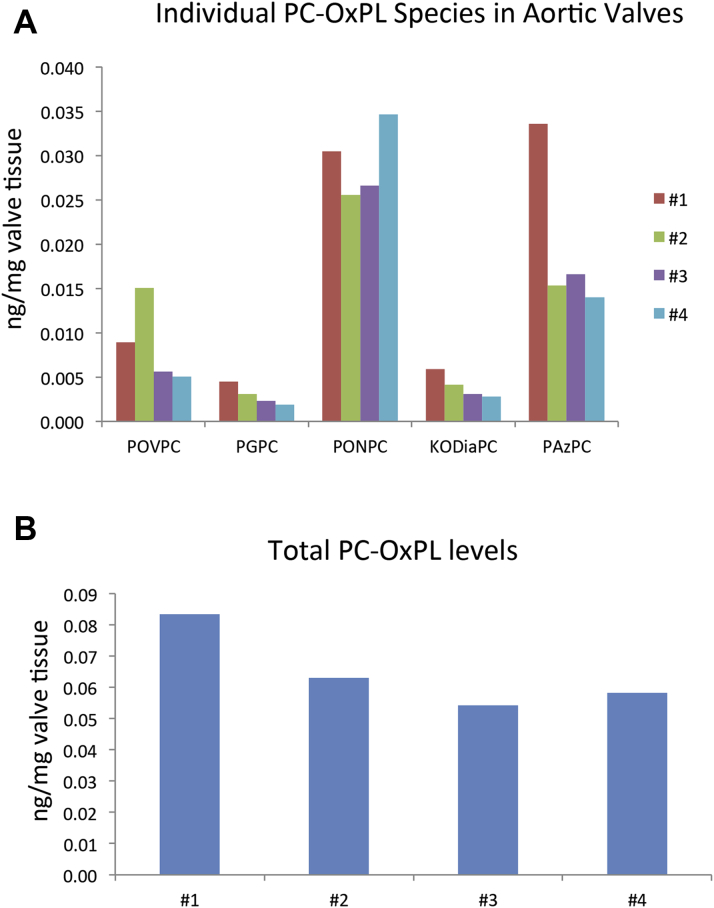
We also identified and quantitated the most abundant fragmented PC-containing OxPL based on known OxPL standards as described in the Methods section. Supplemental Figure 2 shows the multiple reaction monitoring single ion plots for fragmented PC-OxPL extracted from human stenotic aortic valve leaflets. The most abundant OxPL were PONPC and PAzPC, which were identified in all stenotic valves studied (Figure 8A). The aldehyde-containing PC-OxPL, which include POVPC and PONPC, were the most abundant. The acid containing PC-OxPL consisting of PAzPC, PGPC, and KODiaPC were the next most prevalent fragmented species. Total PC-OxPL levels varied among the 4 valves examined (Figure 8B).
Figure 8.
Presence of Individual PC-OxPL Species in Valve Leaflets
Liquid chromatography-tandem mass spectrometry analysis of the most abundant fragmented phosphocholine-containing oxidized phospholipid (PC-OxPLs) compounds extracted from human stenotic aortic valves and represented as (A) individual PC-OxPL compounds or (B) as total PC-OxPL levels within each of the valves. Reverse phase separation coupled with tandem mass spectrometry detection was used to detect POVPC (1-palmitoyl-2-[5'-oxo-valeroyl]-sn-glycero-3-phosphocholine), PGPC (1-palmitoyl-2-glutaryl-sn-glycero-3-phosphocholine), PONPC (1-palmitoyl-2-[9'-oxononanoyl]-sn-glycero-3-phosphocholine), KODiA-PC (1-[palmitoyl]-2-[5-keto-6-octene-dioyl]-sn-glycero-3-phosphocholine), and PAzPC (1-palmitoyl-2-azelaoyl-sn-glycero-3-phosphocholine).
Discussion
The present study showed that Lp(a)-associated molecules are present in plasma and aortic valve leaflets in patients with CAVS. Specifically, ATX could be detected on apo(a) and apoB circulating in plasma, suggesting that it can be transported by Lp(a) and delivered to aortic valve leaflets. Apo(a), the defining component of Lp(a), was also present in aortic valve leaflets from subjects with symptomatic CAVS who underwent AVR. In particular, apo(a), OxPL, MDA-lysine epitopes, and ATX that generates LysoPA were particularly present in the most advanced, pathologically defined grade 4 lesions. Importantly, apo(a), OxPL, MDA-lysine, and ATX were located adjacent to prominent areas of extracellular aortic valve calcification. Finally, targeted analysis of specific lipid species in stenotic valves by using liquid chromatography-tandem mass spectrometry directly confirmed the presence of the major LysoPA species and a variety of PC-containing OxPL. Overall, these data suggest that after a latent period of prolonged plasma and leaflet exposure, these complexes may induce inflammation, calcification, and fibrosis and lend strong support to the hypothesis that the Lp(a)–ATX–OxPL axis is a key determinant of CAVS.
Lp(a) levels >30 to 50 mg/dl have been associated with CAVS in >10 epidemiological studies from North American, European, and Asian populations (reviewed by Yeang et al. [11]). Moreover, the single nucleotide polymorphism rs10455872 in the LPA gene studied in Mendelian randomization studies 5, 6, 7, and which is associated with elevated Lp(a) levels, is the only monogenetic risk factor for CAVS (7), suggesting a causal role for Lp(a) in this disease. Aortic valve calcification predicts progression of disease in humans, and pathways driving aortic valve calcification are key risk factors for CAVS 28, 29. The apo(a) moiety of Lp(a), via its lysine-binding domains, can bind to fibrin on denuded or injured endothelium 30, 31, such as that on aortic valves subjected to mechanical stress in vivo, and accumulate in valve leaflets. Thereafter, the pro-inflammatory and pro-calcific cargo on Lp(a) such as OxPL, LysoPC, and LysoPA (implicated in ectopic calcification) may promote CAVS.
Another important Lp(a)-associated molecule is ATX, as shown in this study according to enzyme-linked immunoadsorbent assay and also by Bouchareb et al. (13) in ultracentrifugally purified Lp(a)-containing fractions. ATX is a secreted enzyme that is a member of the ecto-nucleotide pyrophosphatase/phosphodiesterase family of ectoenzymes (ENPP) that hydrolyzes phosphodiester bonds of various nucleotides. Unlike other ENPPs, ATX also possesses phospholipase D activity and catalyzes the hydrolysis of LysoPC into LysoPA. ATX seems to be the predominant phospholipase D activity responsible for the generation of LysoPA levels in vivo, as heterozygous ATX knockout mice had one-half the LysoPA levels compared with their wild-type counterparts. Therefore, ATX is likely an important contributor of LysoPA found in aortic valve leaflets either via hydrolysis of LysoPC on Lp(a) ultimately retained in aortic valve leaflets or LysoPC from other sources.
Bone formation within the diseased aortic valve leaflets is driven by the differentiation of vascular cells into osteoblasts (32), via bone morphogenic protein signaling and up-regulation of osteoblastic transcription factors, including RUNX2 and MSX2, which are up-regulated after exposure to oxidized low-density lipoprotein (reviewed by Yeang et al. [11]). Both exogenously added OxPL 33, 34 and LysoPA (35) were sufficient in differentiating vascular cells or mesenchymal stem cells, respectively, into osteoblasts in culture. Interestingly, LysoPC also promoted osteoblast differentiation in vitro, but this outcome was completely dependent on ATX activity (13), implying that LysoPC is only an intermediary to LysoPA with respect to development of CAVS. Moreover, intraperitoneal LysoPA administration potentiated aortic valve calcification in a mouse model of aortic stenosis (13), whereas the role of OxPL in CAVS in animal models remains to be studied (36). Our findings that OxPL was detected immunologically and by using mass spectrophotometry in calcified aortic valve leaflets, in conjunction with the observation by Bouchareb et al. (13) that LysoPA was highly enriched in human aortic valve leaflets from subjects with CAVS, further supports the importance of these 2 Lp(a)-associated lipids as biologically active mediators in the pathogenesis of CAVS.
Clinical data further exemplify the relevance of Lp(a), OxPL, and ATX toward the development and progression of CAVS. A secondary analysis of the ASTRONOMER trial showed that elevated Lp(a) and OxPL were predictive of a worse outcome in 220 subjects with mild to moderate CAVS followed up for 3.5 years. Those with the highest tertile of baseline Lp(a) (>58.5 mg/dl) had faster progression rates (average peak velocity, 0.26 ± 0.03 m/s/year vs. 0.17 ± 0.02 m/s/year) and had an approximately 2-fold increased risk of a composite outcome of AVR and cardiac death, which increased to 5.5-fold if they were younger than the median age of 57 years (36). In concert with the Lp(a) findings, those with the highest tertile of OxPL-apoB (>5.50 nM) as well as OxPL-apo(a) (>33.5 nM) had an increased rate of progression and need for AVR consistent with the thesis that OxPL carried by Lp(a) participates in the pathogenesis of CAVS. In addition, in a case-control analysis of 150 subjects with CAD and CAVS compared with 150 individuals with CAD alone, an increased risk of CAVS was associated with elevated ATX mass and ATX activity (37). Subjects with elevated ATX activity (≥84 RFU/min) and either higher Lp(a) (≥50 mg/dl) or OxPL-apoB (≥2.02 nM) had a dramatically higher risk of CAVS (3.46 [interquartile range: 1.40 to 8.58] nM; p = 0.007) and (5.48 [interquartile range: 2.45 to 12.27] nM; p < 0.0001), respectively (8). Overall, these findings suggest that Lp(a), OxPL, and ATX interact in mediating progression of CAVS.
Clinical implications
This study reinforces the roles of Lp(a), OxPL, and ATX as pathogenic risk factors for CAVS. The hypothesis that Lp(a) lowering may slow the progression of CAVS and need for AVR may now be tested with the development of antisense oligonucleotides that potently lower Lp(a) levels (23). It also implies that targeting OxPL or ATX may also be viable therapeutic approaches to inhibiting the development or progression of CAVS. The data from these 3 distinct group of subjects complement each other, showing that: ATX can be transported by Lp(a) in plasma; is present in similar locations to apo(a), OxPL, and areas of calcification in diseased aortic valve leaflets; and that its product, LysoPA, can be directly detected in valve leaflets from subjects with CAVS.
Study limitations
This study included tissue and/or blood samples from 3 distinct populations, and therefore direct comparison of plasma levels of ATX-apoB and ATX-apo(a) with histological assessment of aortic valve apo(a), ATX content, and spectrophotometric quantitation of OxPL and LysoPA content was not feasible. Also, plasma levels of Lp(a) were not available in patients undergoing AVR due to lack of prior evidence of its etiologic importance to correlate the plasma levels with histological findings. Finally, the number of samples available for ATX assays and for aortic valve leaflets available for liquid chromatography-tandem mass spectrometry analysis of OxPL and LysoPC was small, and these results will require validation in larger studies. Future studies should address whether elevated plasma levels of ATX-apo(a) and ATX-apo(a) correlate with clinical severity of CAVS and the presence of ATX, OxPL, and LysoPA valve content.
Conclusions
The observations in this study provide further evidence that an Lp(a)–OxPL-ATX–LysoPA axis may be important for the pathogenesis of CAVS. These observations provide a rationale for therapeutic attempts to reduce entry of Lp(a) into valve leaflets, or to inactivate its attendant OxPL (37), to reduce the risk of developing CAVS or to reduce its rate of progression and attendant complications and need for AVR.
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: Elevated Lp(a), OxPL-apoB, and ATX are abundant in aortic valve leaflets obtained during AVR and may contribute to the development and progression of CAVS.
TRANSLATIONAL OUTLOOK: Targeting elevated Lp(a), OxPL-apoB, and ATX with therapeutic agents may be a viable approach to prevent or reduce the rate of progression of CAVS.
Acknowledgment
The authors thank Kerstin Winter for expert technical assistance.
Footnotes
This work was supported by the innovation fund of the Robert-Bosch-Hospital and the Robert Bosch Foundation. Drs. Tsimikas and Witztum are supported by grants from the National Institutes of Health (R01 grants HL119828, P01-HL088093, P01 HL055798, R01-HL106579, R01-HL078610, and R01-HL124174). Drs. Tsimikas and Witztum are co-inventors of and receive royalties from patents or patent applications owned by the University of California San Diego on antibodies used in biotheranostic applications. Dr. Tsimikas has a dual appointment at the University of California San Diego and Ionis Pharmaceuticals, Inc. Dr. Witztum has received honoraria for consulting for Ionis, CymaBay, and Prometheus Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Rajamannan N.M., Evans F.J., Aikawa E. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: calcific aortic valve disease-2011 update. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yutzey K.E., Demer L.L., Body S.C. Calcific aortic valve disease: a consensus summary from the Alliance of Investigators on Calcific Aortic Valve Disease. Arterioscler Thromb Vasc Biol. 2014;34:2387–2393. doi: 10.1161/ATVBAHA.114.302523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deeb G.M., Reardon M.J., Chetcuti S. 3-Year outcomes in high-risk patients who underwent surgical or transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;67:2565–2574. doi: 10.1016/j.jacc.2016.03.506. [DOI] [PubMed] [Google Scholar]
- 4.Capoulade R., Chan K.L., Yeang C. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J Am Coll Cardiol. 2015;66:1236–1246. doi: 10.1016/j.jacc.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Kamstrup P.R., Tybjaerg-Hansen A., Nordestgaard B.G. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63:470–477. doi: 10.1016/j.jacc.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 6.Arsenault B.J., Boekholdt S.M., Mora S. Impact of high-dose atorvastatin therapy and clinical risk factors on incident aortic valve stenosis in patients with cardiovascular disease (from TNT, IDEAL, and SPARCL) Am J Cardiol. 2014;113:1378–1382. doi: 10.1016/j.amjcard.2014.01.414. [DOI] [PubMed] [Google Scholar]
- 7.Thanassoulis G., Campbell C.Y., Owens D.S. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–512. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nsaibia M.J., Mahmut A., Boulanger M.C. Autotaxin interacts with lipoprotein(a) and oxidized phospholipids in predicting the risk of calcific aortic valve stenosis in patients with coronary artery disease. J Intern Med. 2016;280:509–517. doi: 10.1111/joim.12519. [DOI] [PubMed] [Google Scholar]
- 9.Bergmark C., Dewan A., Orsoni A. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res. 2008;49:2230–2239. doi: 10.1194/jlr.M800174-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Leibundgut G., Scipione C., Yin H. Determinants of binding of oxidized phospholipids on apolipoprotein(a) and lipoprotein(a) J Lipid Res. 2013;54:2815–2830. doi: 10.1194/jlr.M040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeang C., Wilkinson M.J., Tsimikas S. Lipoprotein(a) and oxidized phospholipids in calcific aortic valve stenosis. Curr Opin Cardiol. 2016;31:440–450. doi: 10.1097/HCO.0000000000000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Brien K.D., Reichenbach D.D., Marcovina S.M., Kuusisto J., Alpers C.E., Otto C.M. Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of 'degenerative' valvular aortic stenosis. Arterioscler Thromb Vasc Biol. 1996;16:523–532. doi: 10.1161/01.atv.16.4.523. [DOI] [PubMed] [Google Scholar]
- 13.Bouchareb R., Mahmut A., Nsaibia M.J. Autotaxin derived from lipoprotein(a) and valve interstitial cells promotes inflammation and mineralization of the aortic valve. Circulation. 2015;132:677–690. doi: 10.1161/CIRCULATIONAHA.115.016757. [DOI] [PubMed] [Google Scholar]
- 14.Cote N., Pibarot P., Pepin A. Oxidized low-density lipoprotein, angiotensin II and increased waist circumference are associated with valve inflammation in prehypertensive patients with aortic stenosis. Int J Cardiol. 2010;145:444–449. doi: 10.1016/j.ijcard.2009.05.054. [DOI] [PubMed] [Google Scholar]
- 15.Mahmut A., Boulanger M.C., El Husseini D. Elevated expression of lipoprotein-associated phospholipase A2 in calcific aortic valve disease: implications for valve mineralization. J Am Coll Cardiol. 2014;63:460–469. doi: 10.1016/j.jacc.2013.05.105. [DOI] [PubMed] [Google Scholar]
- 16.Cowell S.J., Newby D.E., Prescott R.J. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 17.Rossebo A.B., Pedersen T.R., Boman K. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 18.Chan K.L., Teo K., Dumesnil J.G., Ni A., Tam J., ASTRONOMER Investigators Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121:306–314. doi: 10.1161/CIRCULATIONAHA.109.900027. [DOI] [PubMed] [Google Scholar]
- 19.Dichtl W., Alber H.F., Feuchtner G.M. Prognosis and risk factors in patients with asymptomatic aortic stenosis and their modulation by atorvastatin (20 mg) Am J Cardiol. 2008;102:743–748. doi: 10.1016/j.amjcard.2008.04.060. [DOI] [PubMed] [Google Scholar]
- 20.Teo K.K., Corsi D.J., Tam J.W., Dumesnil J.G., Chan K.L. Lipid lowering on progression of mild to moderate aortic stenosis: meta-analysis of the randomized placebo-controlled clinical trials on 2344 patients. Can J Cardiol. 2011;27:800–808. doi: 10.1016/j.cjca.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Yeang C., Hung M.Y., Byun Y.S. Effect of therapeutic interventions on oxidized phospholipids on apolipoprotein B100 and lipoprotein(a) J Clin Lipidol. 2016;10:594–603. doi: 10.1016/j.jacl.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Graham M.J., Viney N., Crooke R.M., Tsimikas S. Antisense inhibition of apolipoprotein (a) to lower plasma lipoprotein (a) levels in humans. J Lipid Res. 2016;57:340–351. doi: 10.1194/jlr.R052258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsimikas S., Viney N.J., Hughes S.G. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet. 2015;386:1472–1483. doi: 10.1016/S0140-6736(15)61252-1. [DOI] [PubMed] [Google Scholar]
- 24.Raal F.J., Giugliano R.P., Sabatine M.S. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol. 2014;63:1278–1288. doi: 10.1016/j.jacc.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Gaudet D., Kereiakes D.J., McKenney J.M. Effect of alirocumab, a monoclonal proprotein convertase subtilisin/kexin 9 antibody, on lipoprotein(a) concentrations (a pooled analysis of 150 mg every two weeks dosing from phase 2 trials) Am J Cardiol. 2014;114:711–715. doi: 10.1016/j.amjcard.2014.05.060. [DOI] [PubMed] [Google Scholar]
- 26.Twardowski L., Cheng F., Michaelsen J. Enzymatically modified low-density lipoprotein is present in all stages of aortic valve sclerosis: implications for pathogenesis of the disease. J Am Heart Assoc. 2015;4:e002156. doi: 10.1161/JAHA.115.002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warren B.A., Yong J.L. Calcification of the aortic valve: its progression and grading. Pathology. 1997;29:360–368. doi: 10.1080/00313029700169315. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins W.S., Vesey A.T., Shah A.S. Valvular (18)F-Fluoride and (18)F-fluorodeoxyglucose uptake predict disease progression and clinical outcome in patients with aortic stenosis. J Am Coll Cardiol. 2015;66:1200–1201. doi: 10.1016/j.jacc.2015.06.1325. [DOI] [PubMed] [Google Scholar]
- 29.Dweck M.R., Pawade T.A., Newby D.E. Aortic stenosis begets aortic stenosis: between a rock and a hard place? Heart. 2015;101:919–920. doi: 10.1136/heartjnl-2015-307519. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen L.B., Stender S., Kjeldsen K., Nordestgaard B.G. Specific accumulation of lipoprotein(a) in balloon-injured rabbit aorta in vivo. Circulation Res. 1996;78:615–626. doi: 10.1161/01.res.78.4.615. [DOI] [PubMed] [Google Scholar]
- 31.Hughes S.D., Lou X.J., Ighani S. Lipoprotein(a) vascular accumulation in mice. In vivo analysis of the role of lysine binding sites using recombinant adenovirus. J Clin Invest. 1997;100:1493–1500. doi: 10.1172/JCI119671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohler E.R., 3rd, Gannon F., Reynolds C., Zimmerman R., Keane M.G., Kaplan F.S. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 33.Parhami F., Morrow A.D., Balucan J. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–687. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 34.Mody N., Parhami F., Sarafian T.A., Demer L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Rad Biol Med. 2001;31:509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y.B., Kharode Y., Bodine P.V., Yaworsky P.J., Robinson J.A., Billiard J. LPA induces osteoblast differentiation through interplay of two receptors: LPA1 and LPA4. J Cell Biochem. 2010;109:794–800. doi: 10.1002/jcb.22471. [DOI] [PubMed] [Google Scholar]
- 36.Yeang C., Cotter B., Tsimikas S. Experimental animal models evaluating the causal role of lipoprotein(a) in atherosclerosis and aortic stenosis. Cardiovasc Drugs Ther. 2016;30:75–85. doi: 10.1007/s10557-015-6634-1. [DOI] [PubMed] [Google Scholar]
- 37.Leibundgut G., Witztum J.L., Tsimikas S. Oxidation-specific epitopes and immunological responses: translational biotheranostic implications for atherosclerosis. Curr Opin Pharmacol. 2013;13:168–179. doi: 10.1016/j.coph.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.