Abstract
Mucosal barrier injury laboratory-confirmed bloodstream infections (MBI-LCBIs) lead to significant morbidity, mortality, and healthcare resource utilization in hematopoietic stem cell transplant (HSCT) patients. Determination of the healthcare burden of MBI-LCBIs and identification of patients at risk of MBI-LCBIs will allow researchers to identify strategies to reduce MBI-LCBI rates. The objective of our study was to describe the incidence, risk factors, timing, and outcomes of MBI-LCBIs in hematopoietic stem cell transplant patients. We performed a retrospective analysis of 374 patients who underwent HSCT at a large free-standing academic children’s hospital to determine the incidence, risk factors, and outcomes of patients that developed a bloodstream infection (BSI) including MBI-LCBI, central line–associated BSI (CLABSI), or secondary BSI in the first year after HSCT. Outcome measures included nonrelapse mortality (NRM), central venous catheter removal within 7 days of positive culture, shock, admission to the pediatric intensive care unit (PICU) within 48 hours of positive culture, and death within 10 days of positive culture. One hundred seventy BSIs were diagnosed in 100 patients (27%): 80 (47%) MBI-LCBIs, 68 (40%) CLABSIs, and 22 (13%) secondary infections. MBI-LCBIs were diagnosed at a significantly higher rate in allogeneic HSCT patients (18% versus 7%, P = .007). Reduced-intensity conditioning (OR, 1.96; P = .015) and transplant-associated thrombotic microangiopathy (OR, 2.94; P = .0004) were associated with MBI-LCBI. Nearly 50% of all patients with a BSI developed septic shock, 10% died within 10 days of positive culture, and nearly 25% were transferred to the PICU. One-year NRM was significantly increased in patients with 1 (34%) and more than 1 (56%) BSIs in the first year post-HSCT compared with those who did not develop BSIs (14%) (P ≤ .0001). There was increased 1-year NRM in patients with at least 1 MBI-LCBI (OR, 1.94; P = .018) and at least 1 secondary BSI (OR, 2.87; P = .0023) but not CLABSIs (OR, 1.17; P = .68). Our data demonstrate that MBI-LCBIs lead to substantial use of healthcare resources and are associated with significant morbidity and mortality. Reduction in frequency of MBI-LCBI should be a major public health and scientific priority.
Keywords: Bloodstream infection, Mucosal barrier injury, laboratory-confirmed, bloodstream infection, Central line-associated, bloodstream infection, CLABSI, MBI-LCBI, Transplant-associated, thrombotic microangiopathy, Hematopoietic stem cell, transplantation
INTRODUCTION
Patients undergoing hematopoietic stem cell transplantation (HSCT) are at high risk for bloodstream infections (BSIs) and associated morbidity and mortality [1]. BSIs in the healthcare setting are classified as a primary BSI, related to a central venous catheter (CVC) or other hospital-acquired source, or secondary BSI, such as those associated with abscess or pneumonia [2]. Central line–associated BSIs (CLABSIs) are among the most serious complications in HSCT recipients and lead to prolonged hospitalization, intensive care admissions, prolonged antibiotic treatment, and increased mortality [1,3,4]. CVC maintenance care has been shown to be effective in reducing CLABSIs in a broad range of healthcare delivery settings [5–7].
It has become evident that some BSIs that occur in patients with CVCs do not arise from the catheter but instead are derived from other sources, such as translocation of bacteria through nonintact mucosa [2,8]. The Centers for Disease Control and Prevention developed a modification of the CLABSI definition, termed “mucosal barrier injury laboratory-confirmed BSI” (MBI-LCBI), on the basis of literature review and expert opinion. In 2013 this definition was integrated into National Healthcare Safety Network (NHSN) methods for primary BSI surveillance to aid in identifying a subset of BSIs reported as CLABSIs that are likely related to mucosal barrier injury and not the presence of a central line [2]. Currently, primary BSIs in patients with CVCs are now defined as either LCBI (hereafter referred to as CLABSI) or MBI-LCBI [9]. Additionally, the 2016 NHSN guidelines require additional validation steps to categorize a BSI as a secondary infection, thus decreasing the amount of subjectivity in primary and secondary BSI determination [9].
Unlike CLABSIs, MBI-LCBIs are not expected to be prevented by improved CVC maintenance care. Because this is a recent classification of BSI, few data describe the incidence, timing, and consequences of MBI-LCBIs after HSCT, and no data are found regarding healthcare resource utilization due to MBI-LCIs after HSCT. Moreover, there are currently no potential strategies to prevent bacteremia secondary to MBI after HSCT.
The aim of this study was to describe the incidence, risk factors, timing, and outcomes of MBI-LCBIs, CLABSI, and secondary BSIs in HSCT recipients. Determination of the healthcare burden of MBI-LCBI and identification of patients at risk for MBI-LCBIs are essential to allow researchers the ability to identify strategies to reduce MBI-LCBI rates in HSCT patients.
METHODS
We retrospectively reviewed the records of 374 consecutive patients who underwent HSCT at Cincinnati Children’s Hospital Medical Center from May 2011 to January 2015. Patient data were collected through the first year post-HSCT. The study was approved by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center.
Patient Demographics
Seventy-five percent of patients (282/374) were allogeneic HSCT recipients and 25% (92/374) were autologous. The median patient age was 5.9 years (interquartile range, IQR 2.9 to 13.7), and 206 (55%) were boys. Most patients underwent transplant for an underlying malignancy (n = 170; 45%) or immune deficiency (n = 113; 30%). A myeloablative conditioning regimen was used in 256 patients (68%) and a reduced-intensity regimen in 118 (32%). All autologous HSCT recipients underwent transplant for a solid tumor malignancy and received a myeloablative preparative regimen. The median time to neutrophil engraftment was 12 days (IQR, 10 to 15). We retrospectively applied the most recent (2016) NHSN criteria in the classification CLABSIs, MBI-LCBIs, and secondary BSIs to all infections occurring in HSCT recipients through the first year post-transplant (both inpatient and outpatient) [9].
BSI Definitions
BSIs were classified as a CLABSI if they were a common commensal organism, isolated from a blood culture on 2 occasions, or a recognized pathogen isolated from 1 blood culture, AND did not meet criteria for an MBI-LCBI or secondary BSI. BSIs were classified as an MBI-LCBI if both the organism and patient criteria described below were met. Eligible organisms include those commonly found in the gastrointestinal tract, such as Enterococcus species and Enterobacteriaceae species [9]. In addition, the BSI had to occur in a patient with 1 of the following:
-
○
An allogeneic HSCT recipient with grades III to IV graft-versus-host disease (GVHD) diagnosed within the same hospitalization
-
○
An allogeneic HSCT recipient with ≥1 L of diarrhea (20 mL/kg for those < 18 years of age) in a 24-hour period within 7 days of the positive blood culture
-
○
Neutropenia, defined as an absolute neutrophil count or total WBC count < 500 cells/mm3 within 3 days before or after the positive blood culture
Secondary BSIs (ie, related to an infection at another site, such that the primary site of infection may have seeded the bloodstream secondarily) were determined according to NHSN criteria [9].
Patients did not receive routine prophylactic antibacterial agents. Empiric antibiotics, including piperacillin/tazobactam, meropenem, or cefepime, were used empirically during febrile episodes or when shock was present. All patients received prophylactic antifungal therapy. Patients with positive blood cultures were treated for a minimum of 10 to 14 days.
Covariates
Patient clinical data were collected from the electronic medical record, including demographics, disease and therapy characteristics, transplant complications, and outcomes. The following variables were evaluated: gender, age at transplant, diagnosis, donor relationship, HLA match, stem cell graft source, conditioning regimen, and neutrophil engraftment defined as an absolute neutrophil count > 500/mm3 daily for 3 days. Currently accepted clinical criteria were used for diagnosis of acute GVHD [10], chronic GVHD, transplant-associated thrombotic microangiopathy (TA-TMA) [11,12], and engraftment syndrome in allogeneic stem cell recipients [13]. One-year nonrelapse mortality (NRM) was defined as death without progression or relapse of disease during the first year post-HSCT.
Infection-Specific Outcomes
Outcome variables were obtained related to each infection including CVC removal within 7 days of the positive culture; shock, defined as requirement of at least 40 mL/kg of fluid resuscitation and/or inotropic medication within 24 hours of the positive culture; admission to the pediatric intensive care unit (PICU) within 48 hours of the positive culture; length of stay in the PICU after admission; and death within 10 days of positive culture.
Statistical Analysis
Descriptive statistics were presorted as medians, IQRs, and frequencies. Univariate patient demographic and transplant-related factors were compared between groups using the Fisher exact test for categorical variables and the Wilcoxon rank sum test for continuous variables. The incidences of BSI (any type) and MBI-LCBI for the first infection event were determined using the cumulative incidence function with death as a competing risk and were censored at the earlier of 1-year or second transplant and compared using Gray’s test. A multivariate competing risk regression using backward selection was performed to assess risk factors for MBI-LCBI and CLABSI infection separately. Gray’s competing risk method was used to compute the 1-year NRM cumulative incidence curve in patients who developed at least 2 BSIs and 1 BSI versus those who did not develop a BSI in the first year post-transplant. NRM was calculated with relapse as competing risk and was censored at 1 year or the time of second transplant. A multivariate competing risk regression model was used to compare overall NRM by type of infection, with acute GVHD and TA-TMA included in the model. All statistical tests conducted were 2-sided, and P < .05 were considered significant. Cumulative incidence was calculated in R [14,15].
RESULTS
Bloodstream Infections
One hundred seventy BSIs were diagnosed in 100 patients (27%). The median time to first BSI was 49 days post-transplant (IQR, 7 to 103 days), and 46% of 100 patients had at least 1 BSI before neutrophil engraftment. Eighty infections (47%) were classified as MBI-LCBIs, 68 (40%) as CLABSIs, and 22 (13%) as secondary infections.
Allogeneic stem cell recipients developed BSIs at a significantly higher rate than autologous recipients. Thirty-one percent (88/282) of allogeneic and 13% (12/92) of autologous HSCT recipients developed at least 1 BSI after transplant (P = .0007) (Figure 1A). In addition, MBI-LCBIs were diagnosed at a significantly higher rate in allogeneic stem cell recipients (18% versus 7%, P = .007) (Figure 1B). The median time to first MBI-LCBI was 61 days post-HSCT (IQR, 6 to 102 days), and 38% of MBI-LCBIs occurred before engraftment. In light of the lower number of infections in the autologous HSCT population, we focused our analysis of risk factors on the allogeneic HSCT recipients, and autologous recipients are not considered further in this article.
Figure 1.
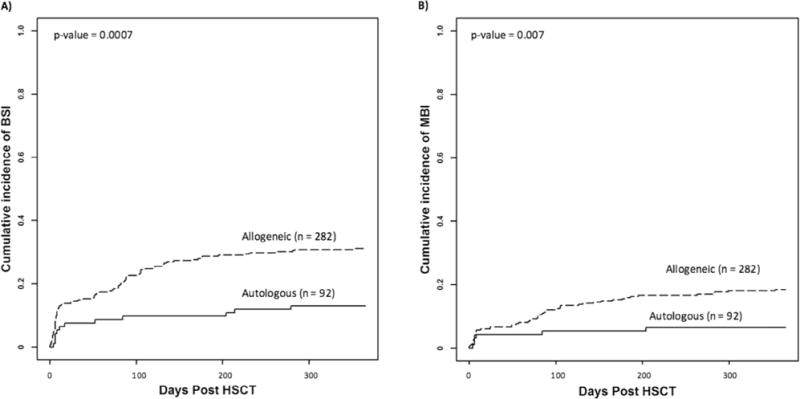
Cumulative incidence of BSIs in autologous and allogeneic stem cell recipients (n = 374). Cumulative incidence of the first (A) BSI of any type and (B) MBI-LCBI in allogeneic and autologous stem cell transplant recipients. MBI indicates mucosal barrier injury laboratory-confirmed BSI.
Risk Factors for MBI-LCBIs in Allogeneic HSCT Recipients
Eighteen percent (52/282) of allogeneic stem cell recipients developed at least 1 MBI-LCBI, 15% (43/282) developed at least 1 CLABSI, and 6% (16/282) developed at least 1 secondary infection. Table 1 details the pretransplant and post-transplant variables associated with each type of infection. In univariate analysis, reduced-intensity conditioning was associated with development of an MBI-LCBI, and patients transplanted for an immune deficiency were more likely to develop CLABSIs, whereas CLABSIs were less frequent in those with marrow failure syndromes. Graft source, age, and gender did not alter risk of any category of BSI.
Table 1.
Univariate Analysis of Allogeneic HSCT Recipients Who Did or Did Not Develop ≥ 1 MBI-LCBI, CLABSI, or Secondary Infection in the First Year Post-Transplant (n = 282)
| Pretransplant Variables | MBI-LCBIs
|
CLABSIs
|
Secondary Infections
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| ≥1 MBI-LCBI (n = 52) |
No MBI-LCBI (n=230) |
P | ≥1 CLABSI (n = 43) |
No CLABSI (n=239) |
P | ≥1 Secondary Infection (n = 16) |
No Secondary Infections (n=266) |
P | |
| Median age, yr (IQR) | 9.6 (2.9–16.3) | 6.1 (2.2–12.4) | .132 | 5.3 (2.4–13.2) | 6.4 (2.3–12.9) | .795 | 7.0 (2.7–18.7) | 6.2 (2.2–12.9) | .517 |
| Male (n = 179) | 36 (69%) | 143 (62%) | .560 | 29 (67%) | 150 (63%) | .609 | 11 (69%) | 168 (63%) | .792 |
| Diagnosis | .118 | .001 | 1.000 | ||||||
| Immunodeficiency (n = 113) | 26 (50%) | 87 (38%) | 27 (63%) | 86 (36%) | 6 (38%) | 107 (40%) | |||
| Malignancy (n = 78) | 11 (21%) | 67 (29%) | 9 (21%) | 69 (29%) | 4 (25%) | 74 (28%) | |||
| Marrow failure (n = 59) | 7 (13%) | 52 (23%) | 4 (9%) | 55 (23%) | 4 (25%) | 55 (21%) | |||
| Benign hematology (n = 17) | 5 (10%) | 12 (5%) | 2 (5%) | 15 (6%) | 1 (6%) | 16 (6%) | |||
| Genetic (n = 15) | 3 (6%) | 12 (5%) | 1 (2%) | 14 (6%) | 1 (6%) | 14 (5%) | |||
| Preparative regimen | .029 | .064 | 1.000 | ||||||
| Reduced Intensity (n = 118) | 29 (56%) | 89 (39%) | 24 (56%) | 94 (39%) | 7 (44%) | 111 (42%) | |||
| Myeloablative (n = 164) | 23 (44%) | 141 (61%) | 19 (44%) | 145 (61%) | 9 (56%) | 155 (58%) | |||
| Donor | .612 | .716 | .570 | ||||||
| Unrelated donor (n = 201) | 39 (75%) | 162 (70%) | 32 (74%) | 169 (71%) | 13 (81%) | 188 (71%) | |||
| Related donor (n = 81) | 13 (25%) | 68 (30%) | 11 (26%) | 70 (29%) | 3 (19%) | 78 (29%) | |||
| Full match (n = 200) | 34 (65%) | 166 (72%) | .398 | 31 (72%) | 169 (71%) | 1.000 | 11 (69%) | 189 (71%) | .784 |
| Graft | .693 | 1.000 | .756 | ||||||
| Bone marrow (n = 221) | 41 (79%) | 180 (78%) | 34 (79%) | 187 (78%) | 12 (75%) | 209 (79%) | |||
| PBSCs (n = 38) | 6 (12%) | 32 (14%) | 6 (14%) | 32 (13%) | 2 (3%) | 36 (14%) | |||
| Cord (n = 23) | 5 (9%) | 18 (8%) | 3 (7%) | 20 (9%) | 2 (3%) | 21 (7%) | |||
| Post-Transplant Variables | |||||||||
| Grades II–IV acute GVHD (n = 83) | 23 (44%) | 60 (26%) | .012 | 18 (42%) | 65 (27%) | .068 | 9 (56%) | 74 (28%) | .023 |
| TA-TMA (n = 125) | 35 (67%) | 90 (39%) | .0003 | 30 (70%) | 95 (40%) | .0004 | 12 (75%) | 113 (42%) | .017 |
| Chronic GVHD (n = 18) | 4 (8%) | 14 (6%) | .759 | 5 (12%) | 13 (5%) | .166 | 3 (19%) | 15 (6%) | .072 |
| Engraftment syndrome (n = 35) | 7 (13%) | 28 (12%) | .817 | 5 (12%) | 30 (13%) | 1.000 | 4 (25%) | 31 (12%) | .122 |
Infection classification is not mutually exclusive. PBSCs indicates peripheral blood stem cells. Values in bold indicate P < .05
In univariate analysis, MBI-LCBIs and secondary infections were significantly associated with grades II to IV acute GVHD in allogeneic recipients (P = .012 and .023, respectively), whereas CLABSIs were not (P = .07). In contrast, MBI-LCBIs (P = .0003), CLABSIs (P = .0004), and secondary BSIs (P = .017) were all associated with TA-TMA. Chronic GVHD and engraftment syndrome were not associated with increased risk of a BSI.
In multivariate analysis (Table 2), use of reduced-intensity conditioning (odds ratio [OR], 1.96; P = .015) was associated with increased risk of at least 1 MBI-LCBI. The only pre-HSCT variable associated with increased risk of CLABSI in multivariate analysis was transplant for immune deficiency (OR, 4.42; P = .0061). TA-TMA was associated with both MBI-LCBI and CLABSI (OR, 2.94; P = .0004 and OR, 2.97; P = .0019, respectively). There were too few secondary infections to support multivariate analysis of that endpoint.
Table 2.
Multivariate Analysis Comparing Allogeneic HSCT Recipients (n = 282) Who Developed >1 MBI-LCBI and CLABSI in the First Year Post-Transplant versus Those Who Did Not
| Variable | Allogeneic Recipients Who Developed ≥1 MBI-LCBI
|
Allogeneic Recipients Who Developed ≥1 CLABSI
|
||
|---|---|---|---|---|
| Multivariate
|
Multivariate
|
|||
| OR | P | OR | P | |
| Diagnosi | ||||
| Marrow failure | 1 | – | 1 | – |
| Immunodeficiency | 1.93 | .15 | 4.42 | .0061 |
| Malignancy | 1.24 | .68 | 2.14 | .21 |
| Benign hematology | 2.59 | .14 | 1.74 | .54 |
| Genetic | 2.62 | .19 | 1.61 | .67 |
| RIC | 1.96 | .015 | 1.27 | .51 |
| Unrelated donor | 1.07 | .86 | .88 | .73 |
| Full match | .68 | .23 | .98 | .96 |
| Grades II–IV acute GVHD | 1.67 | .07 | 1.20 | .57 |
| TA-TMA | 2.94 | .0004 | 2.97 | .0019 |
Values in bold type indicate variables included in final model. RIC indicates reduced-intensity conditioning.
Diagnosis variables were considered for exclusion or inclusion together.
Healthcare Resource Utilization
Table 3 describes outcomes of patients who developed a BSI after HSCT. Forty-six percent of patients with an MBI-LCBI, 50% of those with a CLABSI, and 45% of those with a secondary infection developed septic shock at the time of the infection (P = .88), and almost one-fourth of children with MBI-LCBIs or CLABSIs were transferred to the PICU in association with their infection. Similar numbers of patients with each category of infections underwent line removal as a consequence of their infection (P = .75). The median number of PICU days was similar between patients developing an MBI-LCBI (6 days) and CLABSI (5 days); however, patients developing a secondary infection spent a median of 32 days in the intensive care unit.
Table 3.
Outcomes and Resource Utilization of Patients Who Developed a BSI after HSCT (n = 170)
| MBI-LCBI (n = 80) |
CLABSI (n = 68) |
Secondary Infections (n = 22) |
P | |
|---|---|---|---|---|
| Septic shock within 24 hours of infection | 37 (46%) | 34 (50%) | 10 (45%) | .88 |
| Central line removed within 7 days | 31 (39%) | 30 (44%) | 10 (45%) | .75 |
| Death within 10 days | 7 (9%) | 7 (10%) | 3 (14%) | .79 |
| Transfer to PICU within 48 hours | 17 of 73 (23%) | 14 of 59 (24%) | 2 of 13 (15%) | .80 |
| Patients in PICU at time of infectio | 7 | 9 | 9 | – |
| Median days in the PICU in patients transferred from floor (IQR) | 6 (3–10) | 5 (3–15) | 32 (18–46) | – |
Patients already in PICU not included in days calculation.
Nonrelapse Mortality
One-year NRM was significantly increased in patients with 1 (34%) and more than 1 (56%) BSI in the first year post-HSCT compared with those who did not develop an infection (14%) (P ≤ .0001) (Figure 2). Multivariate analysis revealed increased risk of 1-year NRM in patients with at least 1 MBI-LCBI (OR, 1.94; P = .018) and at least 1 secondary BSI (OR, 2.87; P = .0023) but not CLABSIs (OR, 1.17; P = .68) (Table 4). When acute GVHD is included in the multivariate analysis, risk of NRM remained significantly increased in those with secondary BSIs (OR, 2.35; P = .02) and increased in those with MBI-LCBIs (OR, 1.73; P = .055) (Table 4). Finally, NRM was similar in autologous stem cell recipients who did and did not develop at least 1 BSI and/or MBI-LCBI.
Figure 2.

NRM in allogeneic stem cell transplant recipients (n = 282) who did not develop a BSI, who developed 1 BSI, or who developed 2 or more BSIs in the first year after stem cell transplant.
Table 4.
Univariate and Multivariate NRM in Allogeneic HSCT Recipients by Infection Category, TA-TMA, and Acute GVHD (n = 282)
| Univariate
|
Multivariate without Acute GVHD
|
Multivariate with Acute GVHD
|
||||
|---|---|---|---|---|---|---|
| OR | P | OR | P | OR | P | |
| MBI-LCBI (n = 52) | 3.04 | .0005 | 1.94 | .018 | 1.73 | .055 |
| CLABSI (n = 43) | 2.57 | .0014 | 1.17 | .68 | 1.08 | .83 |
| Secondary (n = 16) | 5.06 | <.0001 | 2.87 | .0023 | 2.35 | .02 |
| Grades II–IV acute GVHD (n = 83) | 3.43 | <.0001 | – | – | 2.25 | .0036 |
| TA-TMA (n = 130) | 6.07 | <.0001 | 4.72 | .0003 | 4.23 | .0009 |
Values in bold type indicate those included in the final model.
DISCUSSION
In this study we evaluated all BSI events in 374 consecutive children and young adults undergoing HSCT at a single center and retrospectively applied the 2016 NHSN criteria. This is the first study to describe the incidence, risk factors, and outcomes of patients who develop MBI-LCBIs after HSCT. The MBI-LCBI definition was first developed in 2013 to define BSIs among immune-compromised patients that may not be related to central lines [2]. Our data show that nearly 50% of BSIs in our HSCT population met the MBI-LCBI definition and constitute a significant burden to this high-risk patient population. We were particularly interested to determine whether the definition was effective in identifying groups of transplant recipients with different outcomes and in need of different strategies to reduce infections and healthcare resource utilization. Our data show clear differences in risk factors, morbidity, and mortality in transplant recipients with CLABSIs, MBI-LCIs, and secondary infections, suggesting that the definitions are clinically meaningful and identify categories of patients in whom different strategies are needed to prevent infection.
An important goal of our study was to examine risk factors for MBI-LCBI and identify high-risk patients for future intervention studies. Surprisingly, univariate and multivariate analyses show that risk of MBI-LCBI is almost doubled in allogeneic recipients treated with a reduced-intensity preparative regimen. In contrast, the intensity of the preparative regimen did not modify risk of CLABSIs or secondary infections. Reduced-intensity preparative regimes are generally believed to reduce tissue damage and mucositis [16,17] and might be expected to reduce risk of MBI-LCBIs. In our clinical practice, reduced-intensity preparative regimens are typically used for immune deficiency and bone marrow failure patients and myeloablative regimens are used for children with malignancy. It is possible that differences in risk by diagnosis are confounding the association with reduced-intensity conditioning. In support of this, univariate analysis in children with immune deficiency, who often have severe enteropathy before transplant, were at increased risk of CLABSIs, and there was a nonsignificant increase in the number of MBI-LCBIs. However, inclusion of diagnosis in the multivariate model did not favor this hypothesis. The reduced-intensity preparative regimen most frequently used at our center includes the alkylating agent melphalan, whereas the myeloablative regimens generally include busulfan, cyclophosphamide, and radiation. It is possible that differential effect of melphalan on bowel permeability facilitates MBI-LCBI. Alternatively, the gut microbiome might differ in the 2 populations, modifying risk of translocation with over-representation of pathogenic bacteria, and studies are underway to address this question.
Univariate and multivariate analyses also showed a strong association between GVHD and MBI-LCBI. This finding is not surprising, because occurrence of GVHD is 1 criterion for definition of MBI-LCBI [9]. Perhaps less intuitively, the occurrence of TA-TMA was also strongly associated with MBI-LCBI. Recent work from our own center has shown that a degree of TA-TMA is frequent after HSCT, occurring in about one-third of patients if monitored carefully [12,18]. Moreover, it is evident that TA-TMA leads to systemic vascular injury and widespread tissue injury, including the intestine [19,20]. It is therefore not surprising that translocation of bacteria occurs in these patients, and transplant recipients with symptoms or signs of TA-TMA are a high-risk group who would benefit from strategies to reduce the frequency of MBI-LCBI.
Our data demonstrate that BSIs in HSCT recipients lead to increased use of healthcare resources, including treatment for septic shock, transfer to the PICU and need for central line removal and replacement. Interestingly, we saw a similar frequency of septic shock in children with MBI-LCBIs, CLABSIs, and secondary infections, although PICU utilization was markedly higher in those with secondary infections. In multivariate analysis, both MBI-LCBIs and secondary infections were associated with a significant increase in risk of NRM, whereas CLABSIs were not, increasing the imperative to reduce the frequency of MBI-LCBIs and secondary infections. It might be argued that MBI-LCBIs are simply a “marker” for sicker patients and do not directly cause mortality, but clinical experience and our data showing frequent transfer to the PICU support a more direct role in mortality.
One of the original incentives for defining MBI-LCBI was to separate infections that could be reduced by attention to line care from those that could not. A recent study demonstrated no change in MBI-LCBI rates with CLABSI prevention standard compliance, whereas the interventions did impact CLABSI rates, further supporting the value of the definition [21]. Unfortunately, this has led to the belief in some quarters that MBI-LCBI cannot be prevented. We believe it will be possible to reduce the frequency of MBI-LCBIs but that novel and innovative strategies are needed to achieve this goal. Our data show for the first time an association of BSIs with TA-TMA, and our group has recently described genetic markers that allow prediction of patients at high risk of TA-TMA before transplantation [22]. The frequency of TA-TMA (and perhaps MBI-LCBI) in high-risk patients could be reduced by use of alternative transplant regimes (eg, reduced use of sirolimus and calcineurin inhibitors) or careful screening for TA-TMA and early institution of complement blockade [23]. In alternative strategies, we are currently studying dietary approaches to improve gut permeability and reduce MBI-LCBIs, including vitamins A and D, use of nondigestible oligosaccharides to maintain bowel microbiome diversity, and human behavior strategies to improve compliance with mouth care, another potential site of translocation [24,25].
Our current study has some limitations. Our data are a retrospective review of BSIs in a single pediatric institution. Our practice has a particular focus on care of immune deficiencies and bone marrow failure syndromes, and these data may not be applicable to all HSCT populations. In particular, our data may underestimate the morbidity and mortality seen in adult transplant programs because older patients may tolerate chemotherapy and subsequent sepsis more poorly. Despite these limitations, our data clearly show a heavy burden on healthcare utilization from BSIs, and in particular MBI-LCBIs, that merits further attention to potential strategies to reduce these infections.
In summary, our data show that about half of the BSIs at our pediatric HSCT center meet the definition of MBI-LCBIs and that this definition is successful in identifying a different category of patient from those with CLABSIs or secondary infections. Today, more than 50,000 HSCTs are carried out annually worldwide, and these numbers increase each year. MBI-LCBIs lead to significant morbidity and mortality and healthcare resource utilization. Reduction in frequency of MBI-LCBIs should be a major public health and scientific priority.
Acknowledgments
The authors thank Joshua Schaffzin, MD, for manuscript edits and Carol Frese, RN, and Deborah Hacker, RN, for assistance in data acquisition.
Footnotes
Financial disclosure: The authors have nothing to disclose.
Conflict of interest statement: There are no conflicts of interest to report.
References
- 1.Poutsiaka DD, Price LL, Ucuzian A, Chan GW, Miller KB, Snydman DR. Blood stream infection after hematopoietic stem cell transplantation is associated with increased mortality. Bone Marrow Transplant. 2007;40:63–70. doi: 10.1038/sj.bmt.1705690. [DOI] [PubMed] [Google Scholar]
- 2.See I, Iwamoto M, Allen-Bridson K, Horan T, Magill SS, Thompson ND. Mucosal barrier injury laboratory-confirmed bloodstream infection: results from a field test of a new National Healthcare Safety Network definition. Infect Control Hosp Epidemiol. 2013;34:769–776. doi: 10.1086/671281. [DOI] [PubMed] [Google Scholar]
- 3.Wilson MZ, Rafferty C, Deeter D, Comito MA, Hollenbeak CS. Attributable costs of central line-associated bloodstream infections in a pediatric hematology/oncology population. Am J Infect Control. 2014;42:1157–1160. doi: 10.1016/j.ajic.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 4.Cecinati V, Brescia L, Tagliaferri L, Giordano P, Esposito S. Catheter-related infections in pediatric patients with cancer. Eur J Clin Microbiol Infect Dis. 2012;31:2869–2877. doi: 10.1007/s10096-012-1652-4. [DOI] [PubMed] [Google Scholar]
- 5.Bundy DG, Gaur AH, Billett AL, et al. Preventing CLABSIs among pediatric hematology/oncology inpatients: national collaborative results. Pediatrics. 2014;134:e1678–e1685. doi: 10.1542/peds.2014-0582. [DOI] [PubMed] [Google Scholar]
- 6.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 7.Miller MR, Griswold M, Harris JM, et al. Decreasing PICU catheter-associated bloodstream infections: NACHRI’s quality transformation efforts. Pediatrics. 2010;125:206–213. doi: 10.1542/peds.2009-1382. [DOI] [PubMed] [Google Scholar]
- 8.Freeman JT, Elinder-Camburn A, McClymont C, et al. Central line-associated bloodstream infections in adult hematology patients with febrile neutropenia: an evaluation of surveillance definitions using differential time to blood culture positivity. Infect Control Hosp Epidemiol. 2013;34:89–92. doi: 10.1086/668431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Center for Disease Control and Prevention. Bloodstream infection event (central line-associated bloodstream infection and non-central line-associated bloodstream infection) 2016 Available at: http://www.cdc.gov/nhsn/PDFs/pscManual/4PSC_CLABScurrent.pdf. Accessed Match 26, 2016.
- 10.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Laskin BL, Goebel J, Davies SM, Jodele S. Small vessels, big trouble in the kidneys and beyond: hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Blood. 2011;118:1452–1462. doi: 10.1182/blood-2011-02-321315. [DOI] [PubMed] [Google Scholar]
- 12.Jodele S, Davies SM, Lane A, et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood. 2014;124:645–653. doi: 10.1182/blood-2014-03-564997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith AR, Majhail NS, MacMillan ML, et al. Hematopoietic cell transplantation comorbidity index predicts transplantation outcomes in pediatric patients. Blood. 2011;117:2728–2734. doi: 10.1182/blood-2010-08-303263. [DOI] [PubMed] [Google Scholar]
- 14.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2006. [Google Scholar]
- 15.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40:381–387. doi: 10.1038/sj.bmt.1705727. [DOI] [PubMed] [Google Scholar]
- 16.Ringdén O, Erkers T, Aschan J, et al. A prospective randomized toxicity study to compare reduced-intensity and myeloablative conditioning in patients with myeloid leukaemia undergoing allogeneic haematopoietic stem cell transplantation. J Intern Med. 2013;274:153–162. doi: 10.1111/joim.12056. [DOI] [PubMed] [Google Scholar]
- 17.Bornhäuser M, Kienast J, Trenschel R, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13:1035–1044. doi: 10.1016/S1470-2045(12)70349-2. [DOI] [PubMed] [Google Scholar]
- 18.Dandoy CE, Davies SM, Hirsch R, et al. Abnormal echocardiography 7 days after stem cell transplantation may be an early indicator of thrombotic microangiopathy. Biol Blood Marrow Transplant. 2015;21:113–118. doi: 10.1016/j.bbmt.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Bietar J, Warren M, Dandoy C, et al. Histologic features of intestinal thrombotic microangiopathy in pediatric and young adult patients after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21:1994–2001. doi: 10.1016/j.bbmt.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jodele S, Laskin BL, Dandoy CE, et al. A new paradigm: diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev. 2015;29:191–204. doi: 10.1016/j.blre.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metzger KE, Rucker Y, Callaghan M, et al. The burden of mucosal barrier injury laboratory-confirmed bloodstream infection among hematology, oncology, and stem cell transplant patients. Infect Control Hosp Epidemiol. 2015;36:119–124. doi: 10.1017/ice.2014.38. [DOI] [PubMed] [Google Scholar]
- 22.Jodele S, Zhang K, Zou F, et al. The genetic fingerprint of susceptibility for transplant-associated thrombotic microangiopathy. Blood. 2016;127:989–996. doi: 10.1182/blood-2015-08-663435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jodele S, Fukuda T, Mizuno K, et al. Variable eculizumab clearance requires pharmacodynamic monitoring to optimize therapy for thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2016;22:307–315. doi: 10.1016/j.bbmt.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Best D, Osterkamp E, Demmel K, et al. Increasing activities of daily living is as easy as 1-2-3. J Pediatr Oncol Nurs. 2015 doi: 10.1177/1043454215616607. pii:1043454215616607. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Wallace G, Jodele S, Myers KC, et al. Vitamin D deficiency in pediatric hematopoietic stem cell transplantation patients despite both standard and aggressive supplementation. Biol Blood Marrow Transplant. 2016 doi: 10.1016/j.bbmt.2016.03.026. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


