Abstract
Purpose of review
Progress in stem cell research for blinding diseases over the past decade is now being applied to patients with retinal degenerative diseases and soon perhaps, glaucoma. However, the field still has much to learn about the conversion of stem cells into various retinal cell types, and the potential delivery methods that will be required to optimize the clinical efficacy of stem cells delivered into the eye.
Recent findings
Recent groundbreaking human clinical trials have demonstrated both the opportunities and current limitations of stem cell transplantation for retinal diseases. New progress in developing in vitro retinal organoids, coupled with the maturation of bio-printing technology, and non-invasive high-resolution imaging have created new possibilities for repairing and regenerating the diseased retina and rigorously validating its clinical impact in vivo.
Summary
While promising progress is being made, meticulous clinical trials with cells derived using good manufacturing practice, novel surgical methods, and improved methods to derive all of the neuronal cell types present in the retina will be indispensable for developing stem cell transplantation as a paradigm shift for the treatment of blinding diseases.
Keywords: Induced Pluripotent Stem Cells (iPSCs), Mesenchymal Stem Cells (MSCs), Retinal pigment epithelium (RPE), Retinal ganglion cells (RGCs), Photoreceptors, Age-related Macular Degeneration (AMD)
Introduction
After several decades of research, stem cell therapy is at a turning point with the current wave of clinical trials testing the efficacy of pluripotent stem cells for combating diseases of the nervous, cardiovascular, digestive, and endocrine systems. The eye and various ocular cell types have emerged as dominant targets for stem cell-based regenerative medicine mainly because the eye (a) the eye is easily accessible for stem cell delivery, (b) the eye is a small organ and the number of stem cells required for therapy would be lower compared to larger organs, (c) has an immuno-privileged environment that can tolerate foreign cells, (d) the eye can restrict and house the transplanted stem cells in a discrete environment, and (e) ocular cells can be non-invasively monitored for structural and functional changes during stem cell therapy.
Given that several ocular disorders ranging from age-related macular degeneration (AMD), Stargardt’s disease (macular dystrophy, STGD), and retinitis pigmentosa (RP) lead to the degeneration of the light-sensing photoreceptors and their underlying supportive retinal pigment epithelium (RPE) in the macula [1], much of the research in the past decade has extensively focused on replacing these ocular cell types with an autologous cell source and more recently on embryonic, fetal, and bone-marrow derived stem cells. Retinal diseases that result from progressive loss of neural cells (photoreceptors, ganglion cells) or microvascular cells (endothelial and pericytes) or supporting cells such as the RPE are potential targets of stem-cell therapy [2, 3]. Once photoreceptors are lost in cases of advanced retinal degeneration, the challenge of retinal stem cell therapy lies in the generation and re-introduction of photoreceptors at the ideal developmental stage to re-awaken the retinal circuits.
The recent success of generating a whole optic cup in vitro containing large number of photoreceptors from embryonic stem cells [4] provide compelling data supporting the need of exploring regenerative neural stem-cell therapies for the treatment of blinding retinal disorders. Many basic research programs and preclinical studies with animal models have used both embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) for differentiation into cells that display many features and morphological similarities with photoreceptors and RPE cells [5]. Since the source of ESCs is ethically contentious, other types of stem cells are being rigorously considered for reversing retinal pathology and accelerating retinal repair. Using pluripotential stem cells (PSC) and iPSC-derived RPEs, clinical trials are ongoing for tackling macular degeneration and other related retinopathies in humans [6]. In some trials, umbilical-tissue derived stem cells, fetal stem cells, and adult stem cells from adipose or bone marrow-derived stem cells are being directly transplanted into the eye with the goal of rescuing the retina. Although results of these trials are eagerly awaited, many of these clinical approaches were designed with the concept that transplanted stem cells would produce trophic factors and rescue or replace the dying neurons, photoreceptors and RPE in patients with AMD and other blinding disorders.
With the invention of 3D bioprinting technology, the stem cell field also has seen tremendous progress in tissue engineering and may prove to be a promising therapeutic approach to eye diseases [7]. In this review, we will discuss the various types of stem cells that are being used for RPE replacement to restore function and/or prevent loss of photoreceptors, summarize clinical advances in stem cell-based therapies for retinal diseases, and outline future directions and challenges for the field.
Intrinsic Retinal Regeneration in Vertebrates
The initial studies demonstrating isolation of neural stem/progenitor cells from adult ocular tissues and successful transplantation of these stem cells into the degenerating retina has sparked widespread interest amongst vision scientists [8–10]. However fundamental questions remained about the intrinsic and extrinsic properties of neural progenitor cells and the impact of environmental signals on migration and differentiation of these cell types. The retinas of amphibians and teleost fish have an unprecedented capacity to regenerate their retina after damage, demonstrating the existence of stem and progenitor cells in the eye [11–13]. Following damage, retinal cells express genes typical for embryonic retinal progenitors such as Pax6, Notch-3, and n-cadherin [14], and the relative spatiotemporal patterns of expression of these genes mimic the patterns observed during retinal neurogenesis.
The Müller glia responds to retinal injury by acquiring stem cell characteristics, allowing the generation of a proliferating population of multipotent Müller glia-derived progenitors, and promoting progenitor cell cycle exit and neuronal differentiation [15]. The initial injury response is reactive gliosis, which includes Müller glia proliferation. Reactive gliosis is initially beneficial to neurons because it protects the retina from glutamate neurotoxicity and releases growth factors necessary to promote neuronal survival [16, 17]. Recent studies also indicate that many of the epigenetic changes associated with stimulation of pluripotency in somatic cells are induced in Müller glia as they redirect into a stem cell phenotype [18]. Together, the robust regenerative capacity of fish and amphibians may help pave the way for a deeper understanding of potential retinal repair and regeneration approaches in mammals.
Stem Cell Programming in the Central Nervous System
In mammals, the radial glia function as stem cells in the developing central nervous system (CNS) and they produce neurons and glia [19, 20]. The Müller glia share a lineage with retinal neurons and possess a latent neurogenerative capacity, demonstrated in the post-natal chick retina. The appearance of markers specific for retinal neurons in Müller cells confirmed the source of retinal regeneration in this species. Likewise, Müller cells with stem/progenitor characteristics have been isolated from post-mortem adult human retina. These cells not only express Müller glial markers, but also neural progenitor markers such as Nestin, CHX10, SOX2, and others. Human Müller cells can grow indefinitely in culture and the presence of specific extracellular matrix and differentiation factors can trigger the expression of post-mitotic neuronal markers such as peripherin, recoverin, and S-opsin [21, 22]. Other putative progenitor cells in the adult human retina may be found in the ciliary body [23]. The ciliary marginal zone is a region of potential interest because expression of nestin, a stem cell marker [21]. However, other studies have shown that ciliary epithelial cells have limited expression of neuronal markers and do not appear to possess true retinal stem cell properties [24].
These endogenous human stem/progenitor cells are easy to differentiate because they have already undergone crucial stages of development within the eye and are committed towards a retinal fate. Transplantation of differentiated human Müller cells in the subretinal space of dystrophic Royal College of Surgeons (RCS) rats showed poor migration into the different retinal layers, whereas transplantation of neural stem cells from the brain have integrated with the host retina but failed to express retina-specific markers [25, 26]. This evidence suggests that while stem/progenitor cells can be coaxed to differentiate into retinal neurons and may be delivered to the eye to replace or repair damaged retinal cells, effective cell-based therapies for regenerating the adult human retina must still solve important unanswered problems such as successful targeting, synapse formation, and demonstration of meaningful visual function.
Types of Stem Cells
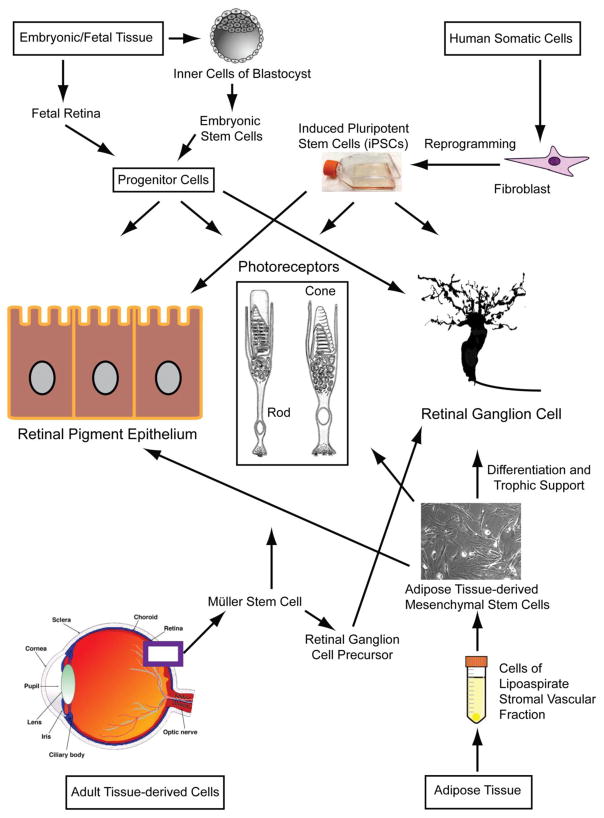
Stem cells have an intrinsic capacity to proliferate indefinitely, and by definition, they have the potential to differentiate into virtually any cell type. Based on their differentiation capabilities and origin, stem cells are broadly classified into (a) omnipotent stem cells that differentiate into embryonic and extra-embryonic tissues, (b) pluripotent stem cells that form embryonic tissue (ectoderm, endoderm, and mesoderm, iPSC), and (c) multipotent stem cells capable of differentiating into a limited number of cell types (e.g., mesenchymal stem cells) (Figure 1) [27]. During stem cell therapy, differentiated cells can physically replace the damaged cells in the tissue and, thereby, restore function. Alternatively, transplanted stem cells may secrete trophic, paracrine factors including cytokines, growth factors and extracellular matrix which can promote neuronal cell survival [28]. Although stem cells can be differentiated into photoreceptors, RPE, and retinal ganglion cells (RGCs), recent studies have also investigated the intrinsic regenerative potential of Müller glia and RPE, to differentiate into retinal cells [29]. The Müller stem cells isolated from the neural retina of human donor eyes can be differentiated into RGC precursors. Upon transplantation of these precursors, these cells expressed RGC markers in vivo demonstrating the possibility of deriving stem cells from adult tissues. On the other hand, transplantation of human Müller glia cells obtained from adult human retinas resulted in differentiation of these cells into rod photoreceptors. Several experimental approaches have shown that the embryonic or fetal retinal progenitors and iPSCs are capable of migrating into the degenerating retina and differentiating into mature retinal cell types including photoreceptors, RPE, and RGCs, supporting the concept that retinal progenitors and precursors are useful stem cell sources and can be used effectively for mechanistic studies of RGC and photoreceptor differentiation, as well as for RGC survival.
Fig. 1.
Schematic of the current sources of various types of stem cells under investigation and the retinal cells derived from them.
Embryonic stem cells (ESCs)
With their constitutive capacity to differentiate into all cell types and their migratory potential, pluripotent ESCs are ideal candidates for treating human retinal diseases [30–32]. Since studies suggest that ESCs can spontaneously differentiate into a neuronal fate in the absence of a specific intracellular signal [33], experimental manipulation of culture conditions by using a combination of growth factors including BMP antagonist, Wnt pathway inhibitor, and IGF-1 [34], or ectopic over-expression of retinal progenitor genes [35], or co-culturing with embryonic retinal explants [36] can lead to the induction of retinal fate in ESC cultures. These experimentally derived photoreceptors from ESCs have been shown to restore visual function in a mouse model of Leber’s Congenital Amaurosis [37]. Studies have shown that TrK receptor binding enhances ESC cell survival, suggesting the release of neurotrophins in an autocrine fashion [38], and transplantation of ESC-derived photoreceptors promotes the survival of nearby endogenous photoreceptors [39]. Emerging new data further demonstrate that ESCs can be directed towards a retinal ganglion cell fate by culturing cells on poly-L-lysine and laminin in the presence of serum followed by differentiation in the presence of FGF2 and sonic hedgehog (SHH). However, transplantation of these differentiated cells with RGC-like properties into rat eyes did not result in integration of these cells with the ganglion cell layer [40]. As an alternative, photoreceptors can be generated from hESCs-derived embryoid bodies when cultured in the presence of IGF-1, noggin, and DKK1 [34], or grown in the presence of LEFTY [41, 42]. To support transplantation of photoreceptors in AMD patients, it was conceptualized that the RPE should be concomitantly replaced, and a recent study confirmed that survival of photoreceptors significantly increased when co-cultured with hESC-derived RPE line [43], indicating that dual replacement is a promising therapeutic strategy. Additional new studies showed formation of entire optic cups containing a complex of neural retina and RPE from mouse ESCs [44] and hESCs [45].
Like ESC-derived photoreceptors, several experimental approaches have been proposed to direct the conversion of ESCs into RPE cells. These protocols include spontaneous differentiation of ESCs following removal of fibroblast growth factor from the culture medium [46], which results in overgrowth of stem cell colonies and concomitant appearance of pigmented areas, resembling RPE cells. Culturing of embryoid bodies in suspension followed by plating of adherent colonies in neural differentiation medium can also trigger spontaneous differentiation into RPE [47]. ESCs can be specifically directed towards RPE differentiation by inhibiting the Wnt and Nodal signaling pathways with dickkopf (DKK1) and left-right determination factor (LEFTY), respectively [48]. Also, sequential treatment with nicotinamide followed by activin A has been shown to induce ESC differentiation into RPE [49]. RPE cells derived from ESCs display many characteristics of the native RPE including a monolayer of hexagonal cells with apical microvilli, pigment-containing melanosome granules, apical orientation of Na+/K+ATPase confirming polarization [46] and associated tight junction proteins expressed on cell borders [43], apical secretion of PEDF, and capability of phagocytosis both in vitro and in vivo [49, 50]. Transplantation of ESC-derived terminally differentiated RPE cells into the subretinal space of RCS rats (slowed the degeneration of photoreceptors [51, 52]. Transplanted hESC-derived RPE cells (MA09-hRPE) into the submacular spaces of human eyes with dry AMD and Stargardt’s macular dystrophy (STGD) in Phase 1 clinical trials (Clinical Trials: NCT01469832, NCT01345006, others) found no evidence of teratoma formation or loss of vision in these individuals. New pigment was eventually detected in the patient with STGD demonstrating restoration of RPE function [53]. The London “Project to Cure Blindness” developed a hESC-derived RPE line that can be cultured as a monolayer on a sheet of polymer, mimicking Bruch’s membrane and designed to overcome the disorganized attachment of RPE cells to Bruch’s membrane when injected into the vitreous as a suspension [54]. The clinical trials are ongoing using hESC-derived RPE have demonstrated that transplanted RPE cells are well tolerated without evidence of tumorigenicity or adverse proliferation. Long-term data showed improved visual acuity in several patients with dry AMD and STGD in a Phase I trial [55]. These promising results represent the next therapeutic frontier for restoring sight through retinal tissue transplantation.
Fetal stem cells
Fetal retina is a potential source of isolating retinal precursors that can be used for restoring vision in individuals with AMD and the many forms of retinitis pigmentosa (RP) because these precursors have the potential to reintegrate into the mature retina and differentiate into rod photoreceptors. Transplantation of these precursors from fetal retina improved visual function in mice and humans with photoreceptor loss [56–58]. The use of fetal progenitor/stem cells remains controversial due to ethical concerns and is currently limited to existing cell lines in the United States. These advanced strategies can be pursued for developing a dual graft of RPE/photoreceptors, which can be transplanted for repairing and regenerating laminated human retinal tissue. Towards this goal, the National Eye Institute recently announced the “3-D Retina Organoid Challenge”, a prize competition designed to generate lab-grown, reproducible 3-D retina organoids that can be used to speed the discovery of treatments for blinding diseases.
Mesenchymal stem cells (MSCs)
Adult stem cells such as MSCs are one of the promising cell types with high potential for regenerative properties in various diseases [59–61]. Of the many sources of MSCs, bone marrow, adipose tissue, dental pulp, peripheral blood, cord blood and fetal liver and lung are well known. Because MSCs comprise only a minor fraction of bone marrow (BM) tissues (0.0001%–0.01%) [62] adipose tissue derived stem cells (ASC) have gained more interest recently [61]. ASC are easy to isolate (mechanical and collagenase digestion) and in abundant supply since 500,000-1 million cells can be isolated from 1 gram of isolated adipose tissue [63]. Furthermore, fat is a medical “waste” tissue, minimizing ethical limitations for its use. Purity, characterization, and scale up manufacturing of cell lines for clinical use are well documented [64], giving ASCs an advantage over BM-MSCs and other less well-defined stem cell populations. MSCs are shown to express RPE markers upon induction with RPE-conditioned medium [65], and have been shown to differentiate into neurons, rods, Müller cells, and retinal ganglion cells [66–68], however, their use in vivo is less established at this time. In some elegant studies, neuroretinal organotypic cultures and co-cultures have been developed to understand the pathophysiology of retinal degenerative diseases. These included MSCs, RPE, and neuroretinal explants, but demonstrated limited or no neuroprotective effects on the retina [69, 70]. Animal studies conducted in RCS rats or p23H rats have shown promising results with MSCs [71–75]. Multimodal mechanisms for MSC-mediated retinal protection that differ by administration route and may synergize in combination [76]. These data come from the RCS rat model, additional studies using other retinal dystrophy models are needed for further translational evaluation of MSCs.
The challenges of using live stem cells for regenerative therapies include issues of cell viability in vivo, differentiation and function in hostile potentially pro-inflammatory tissue environment following transplantation, and poor cell retention and integration into the target retinal tissue [77]. An alternative approach available with MSCs is to use MSC-conditioned media as a neurotrophic, paracrine product to protect the retina. To this end, several studies have been conducted that support the trophic effects of paracrine factors released by MSCs in the regeneration of retina [78–81], similar to the implant (NT-501 ECT, Neurotech) which elutes ciliary neurotrophic factor from encapsulated RPE cells as a neuroprotective agent. Recent advances in 3-dimensional (3D) bioprinting technology, where using a printer device one can deposit and layer building blocks of bioink (a composition of cells and structural scaffolds). Mesenchymal stem cells were used in this 3D bioprinting technology for cardiovascular diseases [7] and has been proposed for retinal use [82, 83].
Induced pluripotent stem cells (iPSCs)
Programming of differentiated somatic cells (including fibroblasts) by forced expression of specific transcription factors including Oct4, Sox2, plus (Myc, Klf4 or Nanog, Lin28) can induce the conversion of somatic cells to ESC-like cells with pluripotent qualities, termed iPSCs [84–88]. Several protocols have been developed demonstrating conversion of iPSCs to RPE-like cells with cobblestone morphology, pigmentation, formation of tight junctions, expression of RPE-specific protein RPE65, and intrinsic capacity to phagocytose shed photoreceptor outer segment tips [89]. iPSCs were used to derive RPE [89–91], and transplantation of iPSC-derived RPE improved visual function in RCS rats [91–93]. iPSCs have been differentiated into photoreceptors [41, 89, 94] by culturing ES cell clumps on matrigel in the presence of Noggin, Dickkopf-1, IGF-1, SHH, followed by supplementation of culture medium with human Activin-A to encourage the exit of photoreceptor progenitor cells from the cell cycle and induce further maturation and differentiation. In recent studies, both hESCs and iPSCs have been differentiated into mature retinal ganglion cells (RGCs) capable of transmitting action potentials [95, 96].
Human iPSCs have been used as models for the study of many different diseases such as monogenic disorders, complex disorders, and early-onset and late-onset diseases. They are an indispensable tool in translational research because reprogramming of any patient-derived somatic cell ranging from fibroblasts to blood cells with wide range of donor ages enables the generation of new iPSC lines modeling human genetic disorders [97–99]. Of note, residual somatic epigenetic memory may persist in iPSC after differentiation, thereby affecting utility in disease models [100, 101]. Reprogramming somatic cells to pluripotent cells has been driven by concerns related to immunological incompatibility, which has been resolved by developing banks of undifferentiated cells that can be differentiated and transplanted to recipients based on matching of human leukocyte-antigens [102]. Nonetheless, iPSC-based therapies are rapidly emerging as exciting avenues of personalized medicine and ongoing studies are using iPSC cells to model retinal diseases ranging from Stargardt disease to AMD. The use of an iPSC line will be an asset for organ module integration into a human-on-a-chip [103]. Human-on-a-chip technology will allow a robust model for personalized drug responses, provide valuable insight into an individual’s reaction to specific treatment regimens and compound tolerability. More in-depth knowledge should be gained with regard to various population differences, including genetics, gender, and demographics. Even in special cases, such as patients with rare diseases, tissue samples can be obtained and examined to learn about mechanisms and potential therapeutics. iPSCs are being investigated for developing patient-specific therapies and providing cell-based models for neurodegenerative disorders as well. For instance, iPSCs derived from a patient with glaucoma were used to determine the contribution of TBK1 gene in ganglion cell death through autophagy [104], and iPSCs from patients with retinitis pigmentosa were used as a cell-based platform to screen for drugs that could reduce the harmful consequences of rhodopsin point mutations [105].
Although iPSCs represent exciting opportunities in disease modeling and drug screening, caution must be taken in extending iPSC-based therapies to the clinic [5] because (a) iPSCs harbor subtle differences in gene expression and DNA methylation and the epigenetic differences can potentially impact differentiation capacity and their utility as disease models [106], (b) somatic point mutations and copy number variations have been reported in iPSCs [107], and (c) chromosomal telomeres may be significantly shortened in iPSC lines compared with native RPE [108].
Conclusions
Although stem cell based therapies for retinal regeneration continues to advance, there are many hurdles to overcome. The first challenge for ongoing clinical trials is to determine whether the transplanted cells successfully integrate into the degenerating retina. This is important because the degenerating retina continues to remodel leading to the formation of unconventional synapses [109]. While neural stem cells transplanted into the adult retina have shown evidence of being capable to integrate into the host retina, they have failed to differentiate into retinal phenotypes [26]. On the other hand, stem cells easily differentiate into retinal phenotypes, but have difficulty migrating and integrating with the host adult retina [110]. Furthermore, the survival of transplanted cells depends on environmental cues in the eye, which may be altered by disease progression [111]. Second, it is unclear whether delivery of cells in suspension, autologous RPE-choroid grafts, sheets of fetal RPE, or as a retrievable permeable capsule loaded with stem cells [112], will be appropriate for rescuing retinal function in patients with retinal degenerative disease [113]. The disadvantage of using RPE cell suspensions is that the RPE cells are distributed with an uncertain fate [114], as they need to attach to the (diseased) Bruch’s membrane [115] often forming clumped rosettes [46] or undergoing anoikis [116]. Patients transplanted with autologous RPE-choroid sheets also did not exhibit better outcomes in part because optical coherence tomography imaging showed irregularities in the cell layers within the graft [112]. Third, with regard to neuronal transplantation including photoreceptors, it is still unclear how to generate appropriate GMP manufactured donor cells for transplantation so that they are genetically similar enough to the recipient to avoid rejection. Culturing and differentiation of patient-derived stem cells/somatic cells or genetically modifying a laboratory-derived stem cell to neurons will require a deeper understanding of developmental biology and the identification of key regulators in the neuronal differentiation pathway. Fourth, we need irrefutable donor cell labeling since the interpretation of cell transplantation experiments is dependent on the presence of an exogenous label for the identification of implanted cells.
To accurately assess cell survival after injection to the living subject, we will need a high resolution, non-invasive imaging and monitoring strategy. Several studies have successfully used quantum dot bioconjugates to label stem cells with high efficiency and to track stem cells longitudinally in live rats [117–119]. Though, the transplanted cells may demonstrate integration and evidence of function, more validation studies are required [120–122]. Finally, one of the most difficult challenges will be the precise product characterization of a stem cell, iPSC, or its derived product that meets the standards of regulatory authorities, methods of manufacturing and scale up potential that will meet FDA requirements for human use.
In conclusion, many challenges remain for the implementation of stem cell therapy for degenerative retinal diseases. These include the delivery and integration of functional retinal neurons and RPE, reconnection of the transplanted cells to circuits within with the degenerating retina, potentially reviving endogenous stem cells in the retina, and restoring visual function. While the therapeutic use of stem cells for retinal diseases is still in its infancy, based upon the progress seen over the past decade, one can reasonably expect that this approach will ultimately yield an effective treatment strategy for restoring visional function in the near future.
Acknowledgments
This study was supported in part by grants from the National Eye Institute (EY023427), Department of Defense (W81XWH-16-1-0778), the Shulsky Foundation (New York, NY), the Plough Foundation (Memphis, TN), the Lions of Arkansas Foundation, and an unrestricted departmental grant from Research to Prevent Blindness (New York, NY).
Footnotes
Conflict of interests: SB: None; EC: None; RG is a scientific consultant and has financial interest in Cell Care Therapeutics, Inc.
Conflict of Interest
Sujoy Bhattacharya and Edward Chaum each declare no potential conflicts of interest.
Rajashekhar Gangaraju reports grants from National Eye Institute, grants from Department of Defense, during the conduct of the study; other from Cell Care Therapeutics, Inc, outside the submitted work; In addition, Dr. Gangaraju has a patent U.S. Provisional Patent Application No. US20150377908A1. pending, and a patent U.S. Provisional Patent Application No. 62/294,489 pending.
Human and Animal Rights and Informed Consent
This article contains no studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
* Of importance
** Of major importance
- 1.Moshiri A, Close J, Reh TA. Retinal stem cells and regeneration. The International journal of developmental biology. 2004;48(8–9):1003–14. doi: 10.1387/ijdb.041870am. [DOI] [PubMed] [Google Scholar]
- 2.MacLaren RE, Pearson RA. Stem cell therapy and the retina. Eye. 2007;21(10):1352–9. doi: 10.1038/sj.eye.6702842. [DOI] [PubMed] [Google Scholar]
- 3.Mellough CB, Steel DH, Lako M. Genetic basis of inherited macular dystrophies and implications for stem cell therapy. Stem cells. 2009;27(11):2833–45. doi: 10.1002/stem.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh MS, MacLaren RE. Stem cells as a therapeutic tool for the blind: biology and future prospects. Proceedings Biological sciences. 2011;278(1721):3009–16. doi: 10.1098/rspb.2011.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *5.Ramsden CM, Powner MB, Carr AJ, Smart MJ, da Cruz L, Coffey PJ. Stem cells in retinal regeneration: past, present and future. Development. 2013;140(12):2576–85. doi: 10.1242/dev.092270. This comprehensive review is focused on the various stem-cell based approaches for treating retinal diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **6.Reardon S, Cyranoski D. Japan stem-cell trial stirs envy. Nature. 2014;513(7518):287–8. doi: 10.1038/513287a. This review article highlighted Japan’s regulatory authorities allowing a clinical study of iPS cells for the treatment of age-related macular degeneration at the RIKEN Center for Developmental Biology. [DOI] [PubMed] [Google Scholar]
- **7.Ameri K, Samurkashian R, Yeghiazarians Y. Three-Dimensional Bioprinting: Emerging Technology in Cardiovascular Medicine. Circulation. 2017;135(14):1281–3. doi: 10.1161/CIRCULATIONAHA.116.024945. This review article discussed the revolutionary use of 3-dimensional bioprinting (using bioink composed of cells and structural scaffolds) for stem cell therapy in cardiovascular medicine. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad I, Tang L, Pham H. Identification of neural progenitors in the adult mammalian eye. Biochemical and biophysical research communications. 2000;270(2):517–21. doi: 10.1006/bbrc.2000.2473. [DOI] [PubMed] [Google Scholar]
- 9.Haruta M, Kosaka M, Kanegae Y, Saito I, Inoue T, Kageyama R, et al. Induction of photoreceptor-specific phenotypes in adult mammalian iris tissue. Nature neuroscience. 2001;4(12):1163–4. doi: 10.1038/nn762. [DOI] [PubMed] [Google Scholar]
- 10.Chacko DM, Das AV, Zhao X, James J, Bhattacharya S, Ahmad I. Transplantation of ocular stem cells: the role of injury in incorporation and differentiation of grafted cells in the retina. Vision research. 2003;43(8):937–46. doi: 10.1016/s0042-6989(02)00688-0. [DOI] [PubMed] [Google Scholar]
- 11.Braisted JE, Raymond PA. Regeneration of dopaminergic neurons in goldfish retina. Development. 1992;114(4):913–9. doi: 10.1242/dev.114.4.913. [DOI] [PubMed] [Google Scholar]
- 12.Wu DM, Schneiderman T, Burgett J, Gokhale P, Barthel L, Raymond PA. Cones regenerate from retinal stem cells sequestered in the inner nuclear layer of adult goldfish retina. Investigative ophthalmology & visual science. 2001;42(9):2115–24. [PubMed] [Google Scholar]
- 13.Cameron DA, Gentile KL, Middleton FA, Yurco P. Gene expression profiles of intact and regenerating zebrafish retina. Molecular vision. 2005;11:775–91. [PubMed] [Google Scholar]
- 14.Ghosh S, Hui SP. Regeneration of Zebrafish CNS: Adult Neurogenesis. Neural plasticity. 2016;2016:5815439. doi: 10.1155/2016/5815439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powell C, Grant AR, Cornblath E, Goldman D. Analysis of DNA methylation reveals a partial reprogramming of the Muller glia genome during retina regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(49):19814–9. doi: 10.1073/pnas.1312009110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bringmann A, Iandiev I, Pannicke T, Wurm A, Hollborn M, Wiedemann P, et al. Cellular signaling and factors involved in Muller cell gliosis: neuroprotective and detrimental effects. Progress in retinal and eye research. 2009;28(6):423–51. doi: 10.1016/j.preteyeres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Goldman D. Muller glial cell reprogramming and retina regeneration. Nature reviews Neuroscience. 2014;15(7):431–42. doi: 10.1038/nrn3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramachandran R, Fausett BV, Goldman D. Ascl1a regulates Muller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nature cell biology. 2010;12(11):1101–7. doi: 10.1038/ncb2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(50):17528–32. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sottile V, Li M, Scotting PJ. Stem cell marker expression in the Bergmann glia population of the adult mouse brain. Brain research. 2006;1099(1):8–17. doi: 10.1016/j.brainres.2006.04.127. [DOI] [PubMed] [Google Scholar]
- 21.Bhatia B, Singhal S, Jayaram H, Khaw PT, Limb GA. Adult retinal stem cells revisited. The open ophthalmology journal. 2010;4:30–8. doi: 10.2174/1874364101004010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrence JM, Singhal S, Bhatia B, Keegan DJ, Reh TA, Luthert PJ, et al. MIO-M1 cells and similar muller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem cells. 2007;25(8):2033–43. doi: 10.1634/stemcells.2006-0724. [DOI] [PubMed] [Google Scholar]
- 23.Tropepe V, Coles BL, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, et al. Retinal stem cells in the adult mammalian eye. Science. 2000;287(5460):2032–6. doi: 10.1126/science.287.5460.2032. [DOI] [PubMed] [Google Scholar]
- 24.Cicero SA, Johnson D, Reyntjens S, Frase S, Connell S, Chow LM, et al. Cells previously identified as retinal stem cells are pigmented ciliary epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(16):6685–90. doi: 10.1073/pnas.0901596106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi M, Palmer TD, Takahashi J, Gage FH. Widespread integration and survival of adult-derived neural progenitor cells in the developing optic retina. Molecular and cellular neurosciences. 1998;12(6):340–8. doi: 10.1006/mcne.1998.0721. [DOI] [PubMed] [Google Scholar]
- 26.Young MJ, Ray J, Whiteley SJ, Klassen H, Gage FH. Neuronal differentiation and morphological integration of hippocampal progenitor cells transplanted to the retina of immature and mature dystrophic rats. Molecular and cellular neurosciences. 2000;16(3):197–205. doi: 10.1006/mcne.2000.0869. [DOI] [PubMed] [Google Scholar]
- *27.Trounson A, DeWitt ND. Pluripotent stem cells progressing to the clinic. Nature reviews Molecular cell biology. 2016;17(3):194–200. doi: 10.1038/nrm.2016.10. This review article emphasized the need for gathering additonal data demonstrating the function and mechanisms of action of pluripotent stem cells in treating a range of eye diseases including age-related macular degeneration, Stargardt disease, and myopic macular degeneration. [DOI] [PubMed] [Google Scholar]
- 28.Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regenerative medicine. 2010;5(1):121–43. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeon S, Oh IH. Regeneration of the retina: toward stem cell therapy for degenerative retinal diseases. BMB reports. 2015;48(4):193–9. doi: 10.5483/BMBRep.2015.48.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 31.Bibel M, Richter J, Schrenk K, Tucker KL, Staiger V, Korte M, et al. Differentiation of mouse embryonic stem cells into a defined neuronal lineage. Nature neuroscience. 2004;7(9):1003–9. doi: 10.1038/nn1301. [DOI] [PubMed] [Google Scholar]
- 32.Hirano M, Yamamoto A, Yoshimura N, Tokunaga T, Motohashi T, Ishizaki K, et al. Generation of structures formed by lens and retinal cells differentiating from embryonic stem cells. Developmental dynamics: an official publication of the American Association of Anatomists. 2003;228(4):664–71. doi: 10.1002/dvdy.10425. [DOI] [PubMed] [Google Scholar]
- 33.Hemmati-Brivanlou A, Melton D. Vertebrate embryonic cells will become nerve cells unless told otherwise. Cell. 1997;88(1):13–7. doi: 10.1016/s0092-8674(00)81853-x. [DOI] [PubMed] [Google Scholar]
- 34.Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(34):12769–74. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabata Y, Ouchi Y, Kamiya H, Manabe T, Arai K, Watanabe S. Specification of the retinal fate of mouse embryonic stem cells by ectopic expression of Rx/rax, a homeobox gene. Molecular and cellular biology. 2004;24(10):4513–21. doi: 10.1128/MCB.24.10.4513-4521.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugie Y, Yoshikawa M, Ouji Y, Saito K, Moriya K, Ishizaka S, et al. Photoreceptor cells from mouse ES cells by co-culture with chick embryonic retina. Biochemical and biophysical research communications. 2005;332(1):241–7. doi: 10.1016/j.bbrc.2005.04.125. [DOI] [PubMed] [Google Scholar]
- 37.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell stem cell. 2009;4(1):73–9. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pyle AD, Lock LF, Donovan PJ. Neurotrophins mediate human embryonic stem cell survival. Nature biotechnology. 2006;24(3):344–50. doi: 10.1038/nbt1189. [DOI] [PubMed] [Google Scholar]
- 39.Meyer JS, Katz ML, Maruniak JA, Kirk MD. Embryonic stem cell-derived neural progenitors incorporate into degenerating retina and enhance survival of host photoreceptors. Stem cells. 2006;24(2):274–83. doi: 10.1634/stemcells.2005-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jagatha B, Divya MS, Sanalkumar R, Indulekha CL, Vidyanand S, Divya TS, et al. In vitro differentiation of retinal ganglion-like cells from embryonic stem cell derived neural progenitors. Biochemical and biophysical research communications. 2009;380(2):230–5. doi: 10.1016/j.bbrc.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 41.Mellough CB, Sernagor E, Moreno-Gimeno I, Steel DH, Lako M. Efficient stage-specific differentiation of human pluripotent stem cells toward retinal photoreceptor cells. Stem cells. 2012;30(4):673–86. doi: 10.1002/stem.1037. [DOI] [PubMed] [Google Scholar]
- 42.Meyer JS, Howden SE, Wallace KA, Verhoeven AD, Wright LS, Capowski EE, et al. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem cells. 2011;29(8):1206–18. doi: 10.1002/stem.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu D, Deng X, Spee C, Sonoda S, Hsieh CL, Barron E, et al. Polarized secretion of PEDF from human embryonic stem cell-derived RPE promotes retinal progenitor cell survival. Investigative ophthalmology & visual science. 2011;52(3):1573–85. doi: 10.1167/iovs.10-6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eiraku M, Sasai Y. Mouse embryonic stem cell culture for generation of three-dimensional retinal and cortical tissues. Nature protocols. 2011;7(1):69–79. doi: 10.1038/nprot.2011.429. [DOI] [PubMed] [Google Scholar]
- 45.Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell stem cell. 2012;10(6):771–85. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Vugler A, Carr AJ, Lawrence J, Chen LL, Burrell K, Wright A, et al. Elucidating the phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantation. Experimental neurology. 2008;214(2):347–61. doi: 10.1016/j.expneurol.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Meyer JS, Shearer RL, Capowski EE, Wright LS, Wallace KA, McMillan EL, et al. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(39):16698–703. doi: 10.1073/pnas.0905245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ikeda H, Osakada F, Watanabe K, Mizuseki K, Haraguchi T, Miyoshi H, et al. Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(32):11331–6. doi: 10.1073/pnas.0500010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Idelson M, Alper R, Obolensky A, Ben-Shushan E, Hemo I, Yachimovich-Cohen N, et al. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell stem cell. 2009;5(4):396–408. doi: 10.1016/j.stem.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 50.Carr AJ, Vugler A, Lawrence J, Chen LL, Ahmado A, Chen FK, et al. Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell-derived RPE cells using a novel human retinal assay. Molecular vision. 2009;15:283–95. [PMC free article] [PubMed] [Google Scholar]
- 51.Lu B, Malcuit C, Wang S, Girman S, Francis P, Lemieux L, et al. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem cells. 2009;27(9):2126–35. doi: 10.1002/stem.149. [DOI] [PubMed] [Google Scholar]
- 52.Lund RD, Wang S, Klimanskaya I, Holmes T, Ramos-Kelsey R, Lu B, et al. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning and stem cells. 2006;8(3):189–99. doi: 10.1089/clo.2006.8.189. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379(9817):713–20. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 54.Carr AJ, Smart MJ, Ramsden CM, Powner MB, da Cruz L, Coffey PJ. Development of human embryonic stem cell therapies for age-related macular degeneration. Trends in neurosciences. 2013;36(7):385–95. doi: 10.1016/j.tins.2013.03.006. [DOI] [PubMed] [Google Scholar]
- **55.Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, et al. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. The New England journal of medicine. 2017;376(11):1038–46. doi: 10.1056/NEJMoa1608368. This paper assessed the feasibility of transplanting RPE cells differentiated from induced pluripotent cells in a patient with neovascular AMD, and showed that after 1 year of surgery, the transplanted RPE sheet remained intact and visual acutiy had not improved or worsened. [DOI] [PubMed] [Google Scholar]
- 56.Radtke ND, Aramant RB, Seiler MJ, Petry HM, Pidwell D. Vision change after sheet transplant of fetal retina with retinal pigment epithelium to a patient with retinitis pigmentosa. Archives of ophthalmology. 2004;122(8):1159–65. doi: 10.1001/archopht.122.8.1159. [DOI] [PubMed] [Google Scholar]
- 57.Pearson RA, Barber AC, Rizzi M, Hippert C, Xue T, West EL, et al. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485(7396):99–103. doi: 10.1038/nature10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGill TJ, Cottam B, Lu B, Wang S, Girman S, Tian C, et al. Transplantation of human central nervous system stem cells - neuroprotection in retinal degeneration. The European journal of neuroscience. 2012;35(3):468–77. doi: 10.1111/j.1460-9568.2011.07970.x. [DOI] [PubMed] [Google Scholar]
- 59.De Miguel MP, Fuentes-Julian S, Blazquez-Martinez A, Pascual CY, Aller MA, Arias J, et al. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Current molecular medicine. 2012;12(5):574–91. doi: 10.2174/156652412800619950. [DOI] [PubMed] [Google Scholar]
- 60.Laroni A, Novi G, Kerlero de Rosbo N, Uccelli A. Towards clinical application of mesenchymal stem cells for treatment of neurological diseases of the central nervous system. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2013;8(5):1062–76. doi: 10.1007/s11481-013-9456-6. [DOI] [PubMed] [Google Scholar]
- *61.Rajashekhar G. Mesenchymal stem cells: new players in retinopathy therapy. Frontiers in endocrinology. 2014;5:59. doi: 10.3389/fendo.2014.00059. This review paper discussed the feasibility of using readily available adipose stromal cells (ASC) from liposuction for treating patients with retinal diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 63.Hong SJ, Traktuev DO, March KL. Therapeutic potential of adipose-derived stem cells in vascular growth and tissue repair. Current opinion in organ transplantation. 2010;15(1):86–91. doi: 10.1097/MOT.0b013e328334f074. [DOI] [PubMed] [Google Scholar]
- 64.Casteilla L, Planat-Benard V, Laharrague P, Cousin B. Adipose-derived stromal cells: Their identity and uses in clinical trials, an update. World journal of stem cells. 2011;3(4):25–33. doi: 10.4252/wjsc.v3.i4.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vossmerbaeumer U, Ohnesorge S, Kuehl S, Haapalahti M, Kluter H, Jonas JB, et al. Retinal pigment epithelial phenotype induced in human adipose tissue-derived mesenchymal stromal cells. Cytotherapy. 2009;11(2):177–88. doi: 10.1080/14653240802714819. [DOI] [PubMed] [Google Scholar]
- 66.Jin W, Xing YQ, Yang AH. Epidermal growth factor promotes the differentiation of stem cells derived from human umbilical cord blood into neuron-like cells via taurine induction in vitro. In vitro cellular & developmental biology Animal. 2009;45(7):321–7. doi: 10.1007/s11626-009-9184-7. [DOI] [PubMed] [Google Scholar]
- 67.Moviglia GA, Blasetti N, Zarate JO, Pelayes DE. In vitro differentiation of adult adipose mesenchymal stem cells into retinal progenitor cells. Ophthalmic research. 2012;48(Suppl 1):1–5. doi: 10.1159/000339839. [DOI] [PubMed] [Google Scholar]
- *68.Bray AF, Cevallos RR, Gazarian K, Lamas M. Human dental pulp stem cells respond to cues from the rat retina and differentiate to express the retinal neuronal marker rhodopsin. Neuroscience. 2014;280:142–55. doi: 10.1016/j.neuroscience.2014.09.023. This study demonstrated that human adult dental pulp stem cells respond to conditional media from rat retinal organotypic explants and differentiate to express a mature photoreceptor marker, rhodopsin. [DOI] [PubMed] [Google Scholar]
- 69.Singh AK, Srivastava GK, Garcia-Gutierrez MT, Pastor JC. Adipose derived mesenchymal stem cells partially rescue mitomycin C treated ARPE19 cells from death in co-culture condition. Histology and histopathology. 2013;28(12):1577–83. doi: 10.14670/HH-28.1577. [DOI] [PubMed] [Google Scholar]
- 70.Rodriguez-Crespo D, Di Lauro S, Singh AK, Garcia-Gutierrez MT, Garrosa M, Pastor JC, et al. Triple-layered mixed co-culture model of RPE cells with neuroretina for evaluating the neuroprotective effects of adipose-MSCs. Cell and tissue research. 2014;358(3):705–16. doi: 10.1007/s00441-014-1987-5. [DOI] [PubMed] [Google Scholar]
- 71.Inoue Y, Iriyama A, Ueno S, Takahashi H, Kondo M, Tamaki Y, et al. Subretinal transplantation of bone marrow mesenchymal stem cells delays retinal degeneration in the RCS rat model of retinal degeneration. Experimental eye research. 2007;85(2):234–41. doi: 10.1016/j.exer.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 72.Lund RD, Wang S, Lu B, Girman S, Holmes T, Sauve Y, et al. Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem cells. 2007;25(3):602–11. doi: 10.1634/stemcells.2006-0308. [DOI] [PubMed] [Google Scholar]
- *73.Jayaram H, Jones MF, Eastlake K, Cottrill PB, Becker S, Wiseman J, et al. Transplantation of photoreceptors derived from human Muller glia restore rod function in the P23H rat. Stem cells translational medicine. 2014;3(3):323–33. doi: 10.5966/sctm.2013-0112. This paper showed that human mesenchymal stem cells can be regarded as a potential cell source for treating human photoreceptor degenerations and offer the possibility for the development of autologous transplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tzameret A, Sher I, Belkin M, Treves AJ, Meir A, Nagler A, et al. Epiretinal transplantation of human bone marrow mesenchymal stem cells rescues retinal and vision function in a rat model of retinal degeneration. Stem cell research. 2015;15(2):387–94. doi: 10.1016/j.scr.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 75.Wang S, Lu B, Girman S, Duan J, McFarland T, Zhang QS, et al. Non-invasive stem cell therapy in a rat model for retinal degeneration and vascular pathology. PloS one. 2010;5(2):e9200. doi: 10.1371/journal.pone.0009200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **76.Bakondi B, Girman S, Lu B, Wang S. Multimodal Delivery of Isogenic Mesenchymal Stem Cells Yields Synergistic Protection from Retinal Degeneration and Vision Loss. Stem cells translational medicine. 2017;6(2):444–57. doi: 10.5966/sctm.2016-0181. This paper reported that MSC-mediated retinal protection differ by adminitration route (intravenous versus intravitreal) and synergize when combined, in the RCS rat model. Data from this study showed that MSCs provide trophic support of visual function in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Newell KA. Clinical transplantation tolerance. Seminars in immunopathology. 2011;33(2):91–104. doi: 10.1007/s00281-011-0255-y. [DOI] [PubMed] [Google Scholar]
- 78.Johnson TV, DeKorver NW, Levasseur VA, Osborne A, Tassoni A, Lorber B, et al. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain: a journal of neurology. 2014;137(Pt 2):503–19. doi: 10.1093/brain/awt292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PloS one. 2014;9(10):e109305. doi: 10.1371/journal.pone.0109305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsuruma K, Yamauchi M, Sugitani S, Otsuka T, Ohno Y, Nagahara Y, et al. Progranulin, a major secreted protein of mouse adipose-derived stem cells, inhibits light-induced retinal degeneration. Stem cells translational medicine. 2014;3(1):42–53. doi: 10.5966/sctm.2013-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *81.Yu B, Shao H, Su C, Jiang Y, Chen X, Bai L, et al. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Scientific reports. 2016;6:34562. doi: 10.1038/srep34562. This study showed that intravitreally injected MSCs from either mouse adipose tissue or human umbilical cord, and their exosomes, reduced laser-induced retinal damage through inhibition of apoptosis and suppression of inflammatory responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jha BS, Bharti K. Regenerating Retinal Pigment Epithelial Cells to Cure Blindness: A Road Towards Personalized Artificial Tissue. Current stem cell reports. 2015;1(2):79–91. doi: 10.1007/s40778-015-0014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *83.Soleimannejad M, Ebrahimi-Barough S, Nadri S, Riazi-Esfahani M, Soleimani M, Tavangar SM, et al. Retina tissue engineering by conjunctiva mesenchymal stem cells encapsulated in fibrin gel: Hypotheses on novel approach to retinal diseases treatment. Medical hypotheses. 2017;101:75–7. doi: 10.1016/j.mehy.2017.02.019. This paper provided a novel 3D-scaffold approach for differentiation of conjunctiva mesesenchymal stem cells into photoreceptors in fibrin gel with induction medium containing taurine. [DOI] [PubMed] [Google Scholar]
- 84.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 85.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 86.Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proceedings of the Japan Academy Series B, Physical and biological sciences. 2009;85(8):348–62. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell stem cell. 2009;4(6):472–6. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell stem cell. 2010;7(5):618–30. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hirami Y, Osakada F, Takahashi K, Okita K, Yamanaka S, Ikeda H, et al. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neuroscience letters. 2009;458(3):126–31. doi: 10.1016/j.neulet.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 90.Buchholz DE, Hikita ST, Rowland TJ, Friedrich AM, Hinman CR, Johnson LV, et al. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem cells. 2009;27(10):2427–34. doi: 10.1002/stem.189. [DOI] [PubMed] [Google Scholar]
- 91.Carr AJ, Vugler AA, Hikita ST, Lawrence JM, Gias C, Chen LL, et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PloS one. 2009;4(12):e8152. doi: 10.1371/journal.pone.0008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liao JL, Yu J, Huang K, Hu J, Diemer T, Ma Z, et al. Molecular signature of primary retinal pigment epithelium and stem-cell-derived RPE cells. Human molecular genetics. 2010;19(21):4229–38. doi: 10.1093/hmg/ddq341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Osakada F, Jin ZB, Hirami Y, Ikeda H, Danjyo T, Watanabe K, et al. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. Journal of cell science. 2009;122(Pt 17):3169–79. doi: 10.1242/jcs.050393. [DOI] [PubMed] [Google Scholar]
- 94.Lamba DA, McUsic A, Hirata RK, Wang PR, Russell D, Reh TA. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PloS one. 2010;5(1):e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *95.Tanaka T, Yokoi T, Tamalu F, Watanabe S, Nishina S, Azuma N. Generation of retinal ganglion cells with functional axons from human induced pluripotent stem cells. Scientific reports. 2015;5:8344. doi: 10.1038/srep08344. This study showed that combining 3D suspension culture of iPSCs followed by a period of 2D adhesive culture in the presence of retinal differentiation and maturation culture medium resulted in axonal elongation of RGCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Riazifar H, Jia Y, Chen J, Lynch G, Huang T. Chemically induced specification of retinal ganglion cells from human embryonic and induced pluripotent stem cells. Stem cells translational medicine. 2014;3(4):424–32. doi: 10.5966/sctm.2013-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamada Y, Haga H, Yamada Y. Concise review: dedifferentiation meets cancer development: proof of concept for epigenetic cancer. Stem cells translational medicine. 2014;3(10):1182–7. doi: 10.5966/sctm.2014-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Avior Y, Sagi I, Benvenisty N. Pluripotent stem cells in disease modelling and drug discovery. Nature reviews Molecular cell biology. 2016;17(3):170–82. doi: 10.1038/nrm.2015.27. [DOI] [PubMed] [Google Scholar]
- **99.Kimbrel EA, Lanza R. Current status of pluripotent stem cells: moving the first therapies to the clinic. Nature reviews Drug discovery. 2015;14(10):681–92. doi: 10.1038/nrd4738. This review article summarized the timeline of the key dates in the development of pluripotent stem cell-based therapies for AMD, and outlines the ongoing clinical trials for treating AMD using hESC and iPSC-derived RPE. [DOI] [PubMed] [Google Scholar]
- 100.Kim K, Zhao R, Doi A, Ng K, Unternaehrer J, Cahan P, et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nature biotechnology. 2011;29(12):1117–9. doi: 10.1038/nbt.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ohi Y, Qin H, Hong C, Blouin L, Polo JM, Guo T, et al. Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nature cell biology. 2011;13(5):541–9. doi: 10.1038/ncb2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zimmermann A, Preynat-Seauve O, Tiercy JM, Krause KH, Villard J. Haplotype-based banking of human pluripotent stem cells for transplantation: potential and limitations. Stem cells and development. 2012;21(13):2364–73. doi: 10.1089/scd.2012.0088. [DOI] [PubMed] [Google Scholar]
- **103.Fabre KM, Livingston C, Tagle DA. Organs-on-chips (microphysiological systems): tools to expedite efficacy and toxicity testing in human tissue. Experimental biology and medicine. 2014;239(9):1073–7. doi: 10.1177/1535370214538916. This important review highlighted the development of 3-dimensional organ systems from human cells on bioengineered platforms that mimic in vivo tissue architecture and physiological conditions. This approach can monitor key organ-level functions in response to stem cell transplantation and is an innovative tool in translational science. [DOI] [PubMed] [Google Scholar]
- *104.Tucker BA, Solivan-Timpe F, Roos BR, Anfinson KR, Robin AL, Wiley LA, et al. Duplication of TBK1 Stimulates Autophagy in iPSC-derived Retinal Cells from a Patient with Normal Tension Glaucoma. Journal of stem cell research & therapy. 2014;3(5):161. doi: 10.4172/2157-7633.1000161. The authors reported the development and characterization of iPSC and retinal ganglion cell-like neurons from unaffected controls and normotensive glaucoma (NTG) patients with TBK1 gene duplications to investigate the role of autophagy in the pathogenesis of NTG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yoshida T, Ozawa Y, Suzuki K, Yuki K, Ohyama M, Akamatsu W, et al. The use of induced pluripotent stem cells to reveal pathogenic gene mutations and explore treatments for retinitis pigmentosa. Molecular brain. 2014;7:45. doi: 10.1186/1756-6606-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Doi A, Park IH, Wen B, Murakami P, Aryee MJ, Irizarry R, et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nature genetics. 2009;41(12):1350–3. doi: 10.1038/ng.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471(7336):63–7. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kokkinaki M, Sahibzada N, Golestaneh N. Human induced pluripotent stem-derived retinal pigment epithelium (RPE) cells exhibit ion transport, membrane potential, polarized vascular endothelial growth factor secretion, and gene expression pattern similar to native RPE. Stem cells. 2011;29(5):825–35. doi: 10.1002/stem.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Haq W, Arango-Gonzalez B, Zrenner E, Euler T, Schubert T. Synaptic remodeling generates synchronous oscillations in the degenerated outer mouse retina. Frontiers in neural circuits. 2014;8:108. doi: 10.3389/fncir.2014.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Van Hoffelen SJ, Young MJ, Shatos MA, Sakaguchi DS. Incorporation of murine brain progenitor cells into the developing mammalian retina. Investigative ophthalmology & visual science. 2003;44(1):426–34. doi: 10.1167/iovs.02-0269. [DOI] [PubMed] [Google Scholar]
- *111.Casaroli-Marano RP, Nieto-Nicolau N, Martinez-Conesa EM, Edel M, ABA-P Potential Role of Induced Pluripotent Stem Cells (IPSCs) for Cell-Based Therapy of the Ocular Surface. Journal of clinical medicine. 2015;4(2):318–42. doi: 10.3390/jcm4020318. The review article highlighted the emerging roles of autologous mesenchymal and iPSCs for ocular surface reconstruction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Falkner-Radler CI, Krebs I, Glittenberg C, Povazay B, Drexler W, Graf A, et al. Human retinal pigment epithelium (RPE) transplantation: outcome after autologous RPE-choroid sheet and RPE cell-suspension in a randomised clinical study. The British journal of ophthalmology. 2011;95(3):370–5. doi: 10.1136/bjo.2009.176305. [DOI] [PubMed] [Google Scholar]
- 113.Sieving PA, Caruso RC, Tao W, Coleman HR, Thompson DJ, Fullmer KR, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(10):3896–901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zarbin MA. Analysis of retinal pigment epithelium integrin expression and adhesion to aged submacular human Bruch’s membrane. Transactions of the American Ophthalmological Society. 2003;101:499–520. [PMC free article] [PubMed] [Google Scholar]
- 115.Tsukahara I, Ninomiya S, Castellarin A, Yagi F, Sugino IK, Zarbin MA. Early attachment of uncultured retinal pigment epithelium from aged donors onto Bruch’s membrane explants. Experimental eye research. 2002;74(2):255–66. doi: 10.1006/exer.2001.1123. [DOI] [PubMed] [Google Scholar]
- 116.Tezel TH, Del Priore LV, Kaplan HJ. Reengineering of aged Bruch’s membrane to enhance retinal pigment epithelium repopulation. Investigative ophthalmology & visual science. 2004;45(9):3337–48. doi: 10.1167/iovs.04-0193. [DOI] [PubMed] [Google Scholar]
- 117.Jayagopal A, Russ PK, Haselton FR. Surface engineering of quantum dots for in vivo vascular imaging. Bioconjugate chemistry. 2007;18(5):1424–33. doi: 10.1021/bc070020r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jayagopal A, Su YR, Blakemore JL, Linton MF, Fazio S, Haselton FR. Quantum dot mediated imaging of atherosclerosis. Nanotechnology. 2009;20(16):165102. doi: 10.1088/0957-4484/20/16/165102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barnett JM, Penn JS, Jayagopal A. Imaging of endothelial progenitor cell subpopulations in angiogenesis using quantum dot nanocrystals. Methods in molecular biology. 2013;1026:45–56. doi: 10.1007/978-1-62703-468-5_4. [DOI] [PubMed] [Google Scholar]
- **120.Kilian T, Fidler F, Kasten A, Nietzer S, Landgraf V, Weiss K, et al. Stem cell labeling with iron oxide nanoparticles: impact of 3D culture on cell labeling maintenance. Nanomedicine. 2016;11(15):1957–70. doi: 10.2217/nnm-2016-0042. This paper demonstrated that M4E nanoparticle labeling of human MSCs could serve as a graft for regenerative therapies. [DOI] [PubMed] [Google Scholar]
- *121.Nicholls FJ, Liu JR, Modo M. A Comparison of Exogenous Labels for the Histological Identification of Transplanted Neural Stem Cells. Cell transplantation. 2017;26(4):625–45. doi: 10.3727/096368916X693680. This paper showed that Hoechst 3342 is unreliable, whereas PKH26 and Q tracker are reliable labels for the identification of transplanted cells during cell therapy, without exerting major cellular effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **122.Santos-Ferreira TF, Borsch O, Ader M. Rebuilding the Missing Part-A Review on Photoreceptor Transplantation. Frontiers in systems neuroscience. 2016;10:105. doi: 10.3389/fnsys.2016.00105. This review discussed that transplantation of iPSC/ESC and retinal organoid-derived photoreceptors as evolving replacement approaches for the treatment of late-stage AMD. [DOI] [PMC free article] [PubMed] [Google Scholar]