Abstract
Polyamidoamine (PAMAM) dendrimers have been investigated as a potential platform for a number of ocular drugs, but only in aqueous solution. In this work we have developed fast dissolving dendrimer-based nanofibers (DNF) as a topical delivery vehicle for the glaucoma drug brimonidine tartrate (BT). The safety and drug release kinetics of these nanofiber mats were evaluated in vitro and in vivo. DNF caused no toxicity at therapeutic levels in cultured cells or ocular irritation in animal tests using a normotensive rat model. Intra-ocular pressure response was equivalent between DNF and BT solution in a single dose test, but DNF showed improved efficacy with daily dosing over a 3-week test period. This study indicates electrospun dendrimer nanofibers are a viable alternative to aqueous solutions as a more efficient method of administering antiglaucoma drug topically.
Keywords: Electrospinning, glaucoma, dendrimer, drug delivery
Graphical Abstract
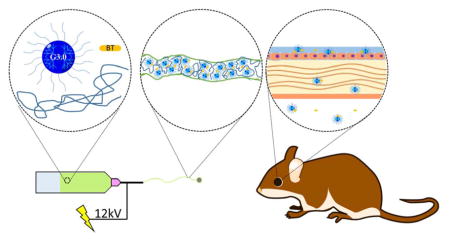
INTRODUCTION
Among the available routes of administration for ocular drugs, topical application is considered the most desirable due to its combination of good tissue specificity and minimal invasiveness.1 However, delivery of drugs using conventional saline eye drops is severely limited by anatomical and physiologic factors unique to the eye. Following administration, the entire drop is drained into the systemic circulation in a matter of minutes, typically resulting in less than 5% of the drug reaching the desired tissue.2 This increases costs and creates the risk of off-target effects. Patient non-compliance is a major issue for glaucoma medications because most eye drops must be applied at least two or three times per day, and they are difficult to administer. Combined, these drawbacks lead many glaucoma patients to skip doses or even stop medication treatment.3–4 Estimates of patient non-compliance vary, but electronic monitoring studies have consistently shown that a major portion of glaucoma patients struggle to adhere to this dosing regimen for more than a few weeks.5 For these patients, long-term treatment of high intraocular pressure (IOP) then relies on more invasive therapies such as surgical intervention. Despite the clear benefits of a more efficient topically applied ocular drug delivery vehicle, significant research investment has so far borne no optimal solution.6–9
A true replacement for saline eye drops would increase the bioavailability of therapeutic compounds and reduce the dosing frequency while still having a simple application procedure and minimal invasiveness. While conventional materials have failed to deliver these features, novel nanocarriers such as dendrimers may offer new opportunities.10–14 Dendrimers are a class of polymers with a well-defined branching structure. Each branch has a terminal end group that can be functionalized with solubilizing polymers, targeting domains, imaging molecules, or therapeutic compounds.13 The ability to incorporate these agents into a single nanoparticle makes dendrimers extremely flexible drug delivery platforms.15
Polyamidoamine (PAMAM) dendrimers have already demonstrated promise as vehicles for a number of ocular drugs.16–20 These previous studies have delivered drug-loaded dendrimers as nanoparticles in solution, where they rely solely on the mucoadhesive properties of the polymer to extend residence time on the corneal surface, but solid dosage forms such as erodible inserts extend corneal residence time using mechanisms independent of particle charge.7 We also believe solid dendrimer-based materials will have other advantages over dendrimers in solution including superior storage ability, ease of administration and a more reliable application procedure.21
Previously, we developed dendrimer based nanofibers.17 In this work, we sought to further develop this material platform as well as investigate it as an ocular drug delivery vehicle. To this end, modified PAMAM dendrimers were co-spun with polyethylene oxide and brimonidine tartrate into nanofiber mats. The drug release kinetics of these fiber mats and the permeability of the drug across live corneas was quantified. The in vivo efficacy and biocompatibility of these nanofibers was evaluated in a normotensive rat model by intra-ocular pressure measurement and examination over a three-week trial. Disposition of dissolved dendrimers in various ocular organs was determined by using fluorescently labeled nanofibers. Success of using these fiber mats for glaucoma medications has been demonstrated by their ability to match or exceed the performance of conventional saline eye drops in each of these tests.
MATERIALS AND METHODS
Materials
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. PAMAM dendrimers (G3.0) were purchased from Dendritech (Midland, MI). Hexafluoroisopropanol (HFIP) was purchased from Oakwood Chemicals (Estill, South Carolina). Cellulose dialysis membranes were purchased from Spectrum Labs (Rancho Dominguez, California). Fresh rabbit eyes were purchased from Pel-Freeze Biologicials (Rogers, Arkansas).
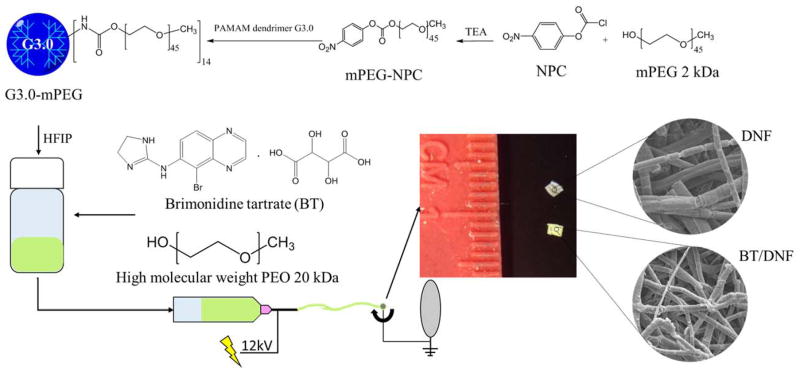
G3.0-mPEG synthesis
PAMAM dendrimers generation 3.0 were conjugated with methoxy polyethylene glycol (mPEG) (Mn=2 kDa) as previously described (Scheme 1).22 Briefly, mPEG (1 equiv) was dissolved in tetrahydrofuran, followed by addition of 4-nitrophenol chloroformate (NPC) (1.5 equiv) and triethylamine (TEA) (20 equiv). The reaction was run for 24 h at room temperature and the salt was filtered off. The resulting mPEG-NPC was collected by precipitation in diethyl ether and vacuum dried. PAMAM dendrimer G3.0 and mPEG-NPC were dissolved in DMSO separately. The mPEG-NPC solution was added dropwise to the dendrimer solution at a feed molar ratio of 16:1 for mPEG-NPC:G3.0. After 72 h, the solvent was removed under vacuum. The resulting product G3.0-mPEG was purified via dialysis in deionized water using a 7000 MWCO dialysis membrane.
Scheme 1.
Synthesis and fabrication of DNF and BT/DNF mats. Following the synthesis of PEGylated PAMAM dendrimer G3.0 (i.e., G3.0-mPEG), G3.0-mPEG is dissolved in HFIP with high molecular weight PEO in the absence or presence of the antiglaucoma drug BT and electrospun into DNF or BT/DNF mats.
Electrospinning
HFIP electrospinning solution containing G3.0-mPEG (120 mg/mL) and polyethylene oxide (PEO) (Mn= 90 kDa, 20 mg/mL) was prepared. Brimonidine tartrate (BT) was added to the electrospinning solution at the final concentration of 20 mg/mL prior to electrospinning. One mL of electrospinning solution (with or without BT) was loaded into a custom electrospinning machine and spun onto a rectangular aluminum mandrel using the following parameters: 12 kV DC offset, 20 cm airgap distance, and a 1.5 mL/h solvent flow rate. The obtained dendrimer nanofibers (DNF) and BT-containing DNF (i.e., BT/DNF) were degassed overnight at atmospheric pressure (i.e., ATM degassing) and removed from the mandrel. Any residual solvent was further removed under vacuum for 1 h (i.e.., vacuum degassing).
Fiber characterization
To assess fiber morphology, the samples of electrospun fiber mats were mounted and platinum sputter coated for imaging using a Hiatachi SU-70 scanning electron microscope. Fiber mechanical properties were measured by using uniaxial tensile testing. “Dogbone” punches (19.0×3.2 mm) were taken and deformed at a 10 %/min stain rate until failure using an MTS Bionix 200 Mechanical Testing System.
In vitro drug release
Cellulose dialysis membranes (MWCO 3.5 kDa) were sealed on one end and filled with 10 mg BT/DNF dissolved in 100 μL simulated tear fluid (STF).23 The tube was placed in 2 mL of STF in a quartz cuvette. The concentration of the drug in the bulk solution was quantified using absorbance of 320 nm light at 5 min intervals for 90 min. This procedure was repeated for neat brimonidine solution (40 μg) and unmodified dendrimers mixed with an equivalent drug amount.
Cornea permeability
Brimonidine transport across live corneas was measured using Franz diffusion cells (PermeGear, Hellertown, PA). Corneas from fresh rabbit eyes were excised and placed immediately into diffusion cells with the endothelial surface facing the acceptor chamber and the epithelial surface facing the donor chamber. The acceptor chamber was filled with 5 mL of glutathione buffered Ringer’s solution and the donor chamber with 100 μL of solution containing 40 μg of the drug. The entire cell was placed in a water bath at 37 ºC. At various time points 250 μL samples were taken from the acceptor chamber solution and drug concentration was measured using a reverse phase HPLC equipped with a UV detector (96v:4v water: acetonitrile mobile phase, 50×150 mm C18 column with 5 μm pore size).
In vitro cytocompatibility
NIH 3T3 fibroblasts were seeded into 96 well plates at a density of 5000 cells/well and allowed to attach overnight. The cells were incubated with DNF at various concentrations (on the basis of dendrimer) for an additional 24 h and assessed for viability using WST-1 viability assay.
IOP measurement
Normotensive adult brown Norway rats (Charles River Labs, Wilmington MA) were used for all animal experiments in this study. They were housed under proper conditions at Virginia Commonwealth University (VCU) and Medical University of South Carolina (MUSC). The rats were kept under a cycle of 12-h light and 12-h dark for all the studies. All animal procedures were approved by the VCU and MUSC IACUC. Rat IOP measurements were taken with a TonoLab rebound tonometer (ICare, Finland) as described earlier.24 No anesthetics or artificial restraints were employed during measurement. All measurements were taken by the same operator at the same location using the hand corresponding to that eye. Time 0 for all experiments (and baseline IOP readings) occurred at approximately 10 a.m. All IOP values reported represent the average of minimum 18 and maximum 30 individual instrument readings of each eye.
In vivo single dose response
Rats (n=4) were conditioned for at least 1 week to establish baseline IOP. On the day of the experiment the animals had either 5 μL BT solution (40 μg), or an equivalent dose of BT/DNF placed in the right eye (experimental eye). At time points 2, 4, 6, and 24 h post-dosing the IOP in both the experimental and contralateral eye was measured. Animals were closely monitored for irritation and inflammation for the duration of the test.
Chronic use safety and efficacy
Normotensive rats (n=3) received 40 μg BT delivered via saline solution or BT/DNF mat in the right eye (experimental eye) daily. IOP was measured daily in both eyes immediately prior to drug administration. After 21 d, the animals were euthanized and the eyes enucleated. Eyes were immediately fixed with Davidson’s solution and 5 μm sections prepared. Sections were stained with hematoxylin and eosin and imaged.
Ocular disposition
Fluorescently tagged nanofiber mats were synthesized by covalently linking fluorescein isothiocyanate (FITC) to G3.0-mPEG prior to electrospinning. Normotensive rats (n=3) had fluorescently tagged nanofiber mats (without drug) applied to the right eye. At various time points, the animals were euthanized by carbon dioxide asphyxiation and the ocular tissues harvested. Ocular globes were fixed in 4% paraformaldehyde, perfused with glucose, and frozen for cryosectioning. Nanoparticle contents in the cornea, ciliary body, and retina were visualized using fluorescence imaging.
Statistical analysis
The data are expressed as mean ±standard deviation. The single dose response data were analyzed using one-way analysis of variance (ANOVA) for multiple comparisons versus control group (time point zero) (Holm-Sidak method) in each treatment. T-test was used for comparison of 3-week IOP reduction effect between BT saline solution and BT/DNF. A p value <0.05 is considered statistically significant.
RESULTS AND DISCUSSION
Synthesis and fabrication
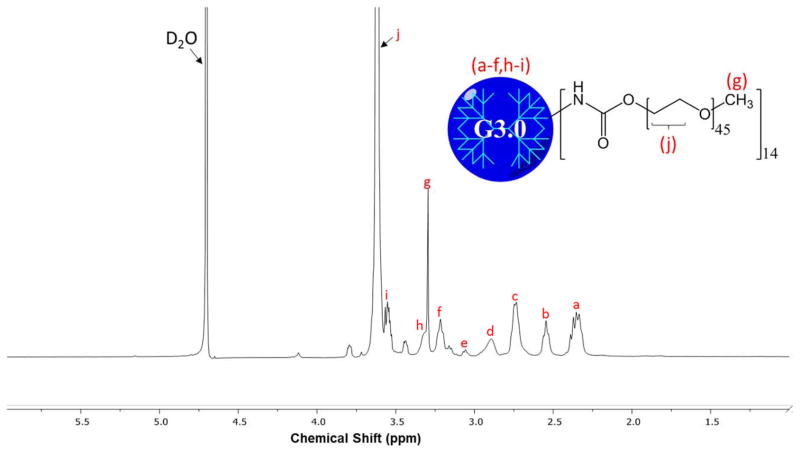
The 1H NMR spectrum confirms that the partial modification of G3.0 PAMAM dendrimers with mPEG was successful (Fig. 1). Integration of the peak at 3.72 ppm, the methylene protons on PEG, and 2.4–3.5 ppm, assorted dendrimer proton peaks, showed the 16:1 molar feed ratio mPEG:G3.0 resulted in a final dendrimer surface coverage of approximately 40%.
Figure 1. 1H NMR spectrum of G3.0-mPEG.
Degree of PEGylation was determined by integrating proton peak j (mPEG) and comparing with integration of proton peaks a–f, h, and i (G3.0). The analysis determined around 40% of G3.0 surface groups were PEGylated.
PEG modification of PAMAM dendrimers has been widely studied by our lab and others, and has a major impact on the physical and chemical properties of the nanoparticles. 25–26 A higher degree of PEGylation (the percentage of surface groups modified) results in easier electrospinning by lowering the charge density and breaking up the highly compact structure of dendrimers. Both of these changes help encourage the polymer chain entanglements that differentiate electrospinning nanofibers from electrospraying nanoparticles.27 These property changes also greatly increase the biocompatibility of PAMAM dendrimers, which are cytotoxic in their unmodified form. However, for drug delivery applications full PEGylation is not desirable as it neutralizes the dendrimers’ charge to a degree where cell permeation is inhibited, and it leaves no remaining surface groups for later modification.28 Approximately half PEGylation was selected for this project to balance efficient nanofiber production and leave sufficient available groups to later adapt the material to delivery alternate therapeutic compounds, targeting moieties, or imaging tags.
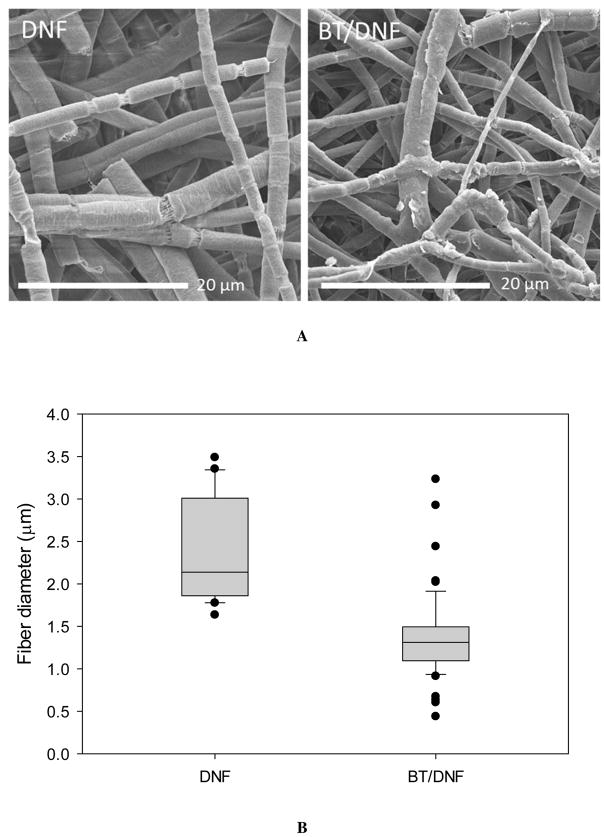
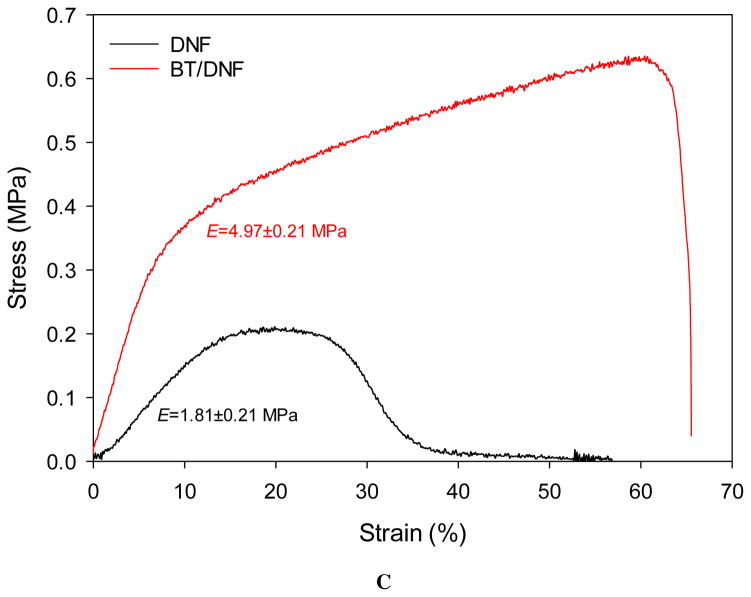
Electrospinning parameters were designed to optimize efficiency while still producing dry nanofibers. The most significant determining factor of fiber yield and morphology was expected to be the quantity of high molecular weight PEO added to the electrospinning solution.29 While 1% wt/vol was sufficient to produce fibers a higher quantity of 2% was selected to ensure the final material would dissolve rapidly when placed onto the ocular surface. Parameters such as flow rate, mandrel speed, and DC offset were minimized at the given air gap distance to maintain a stable pull of fibers. No preferred fiber morphology was directed with this approach. The SEM images show DNF mats had anisotropic fiber alignment (Fig. 2A). The median fiber diameter was around 2 μm for plain DNF with the distribution skewed toward smaller fibers (Fig. 2B). The addition of BT decreased median fiber diameter to less than 1.5 μm and balanced the distribution. Mechanical properties were not robust, with a modulus of just 1.81 and 4.97 MPa for DNF and BT/DNF respectively, but these values were sufficient to cut and manipulate the mats without breakage (Fig 2C).
Figure 2.
DNF characterization. (A) High magnification electron micrographs of DNF and BT/DNF. (B) Histograms of fiber measurements show BT caused an overall decrease in fiber diameter. (C) Uniaxial tensile testing shows BT/DNF has higher elastic modulus, peak stress, and strain at break than DNF.
In vitro assessment
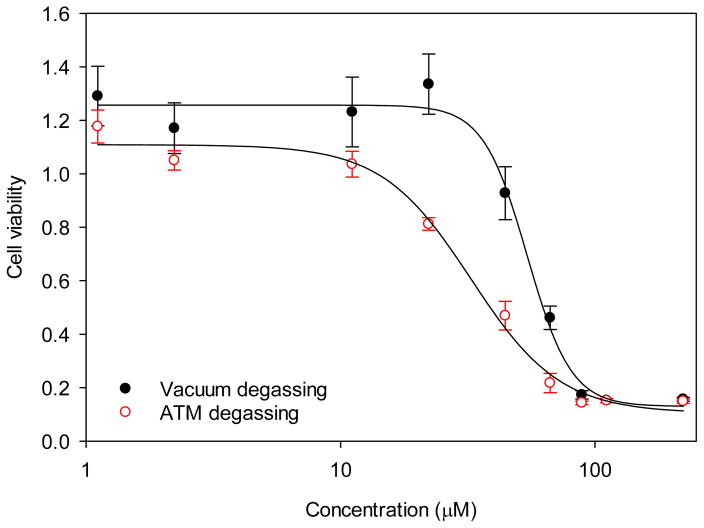
Conversion of the tetrazolium salt WST-1 to formazan was used as an indirect measure of cytotoxicity by quantifying the metabolic activity of viable cells. As the fibers are not expected to have an impact on the mitochondrial activity of cells, this can be used to approximate the number of living cells quickly. Preliminary experiments on fibroblasts showed a 50% reduction in cell viability at a concentration of 32.5 μM (IC50). To determine if this was caused by residual electrospinning solvent another batch of fibers were dried by vacuum oven prior to testing. Its IC50 increased by 67% to 54.2 μM (Fig. 3). This is well above the expected maximum concentration in vivo, roughly 30 μM (the molar concentration of dissolving one DNF mat in 10 μL of solution). Because of this finding, all samples later used in animal experiments were first vacuum dried.
Figure 3. DNF cytotoxicity.
NIH 3T3 fibroblasts were incubated with varying concentrations of DNF for 24 h and cell viability measured via WST-1 assay. Degassing fibers under vacuum (vacuum degassing) was shown to be more efficient in removing residual electrospinning solvent and increasing DNF cytocompatibility than degassing fibers at atmospheric pressure (ATM degassing).
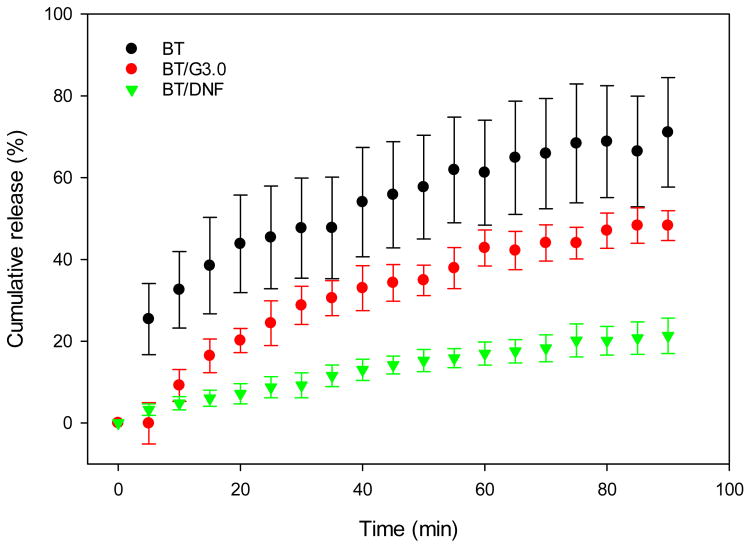
As proof of concept before animal testing, the release kinetics of BT from DNF mats was evaluated under static in vitro conditions, as well as permeation using ex vivo rabbit corneas. Static BT release due to diffusion was determined by the interaction of the drug with G3.0-mPEG in solution. Release was significantly slower from G3.0-mPEG fibers compared to both neat BT solution and unmodified G3.0 dendrimers (Fig. 4). Due to the number of constituents present, there are several mechanisms which may be responsible for this result. Slowed drug release in the G3.0 group indicates there is some interaction between PAMAM dendrimers and BT in solution. This may be with the terminal or core groups on the polymer. More importantly, the PEGylation of the dendrimers and the addition of PEO to the fibers appear to form a loose network in solution.
Figure 4. In vitro brimonidine release.
BT delivery vehicles were dissolved and drug diffusion across a 3.5 kDa dialysis membrane was measured for 90 min. Unmodified dendrimers and DNF slowed initial drug diffusion in STF. DNF further slowed the rate of drug release for the entire duration of the test.
Corneal permeability was investigated in an ex vivo rabbit model. Dendrimers have previously been shown to increase permeability of epithelial cell layers, but BT eye drops have a relatively high corneal permeability compared to most ocular drugs.30 Typical permeability coefficients for brimonidine range between 2.1×10−7 and 3.6×10−7 cm/s.31 At the doses used in our tests G3.0-mPEG fibers had no difference in permeability compared to brimonidine solution and all groups agreed with the literature values for live rabbit corneas (Table 1). While increased permeability would be beneficial to drug delivery efficiency, it is also known to cause numerous potential problems, most commonly irritation.32 The fact that DNF did not affect corneal permeability indicates that they are not disrupting the structure of the native epithelium, an important consideration for the long term biocompatibility of the material.33–34
Table 1.
Permeability coefficient of rabbit corneas to brimonidine.
| Formulation | P (10−6 cm/s) | Q-R2 |
|---|---|---|
| BT solution | 6.90 | 0.93 |
| BT/G3.0 | 7.77 | 0.97 |
| BT/DNF | 6.43 | 0.91 |
Permeability coefficient (P) was calculated for formulation. There was no significant difference in P between groups and results were consistent with literature values.31
In vivo efficacy and safety
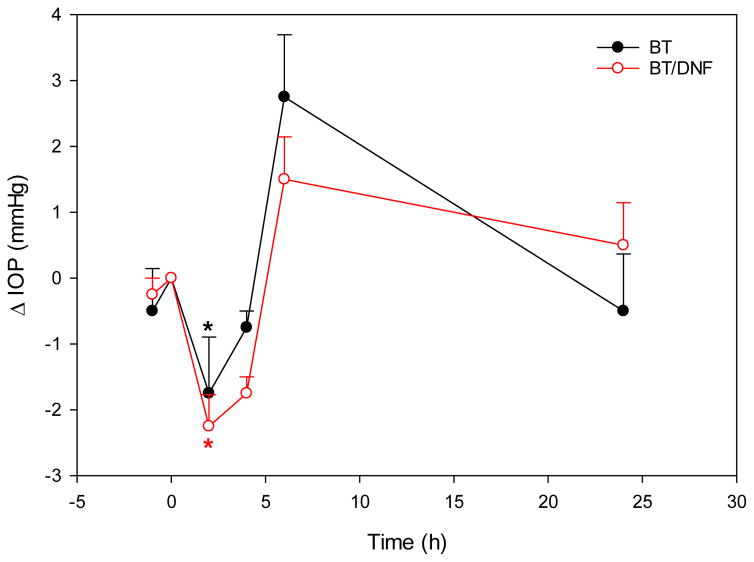
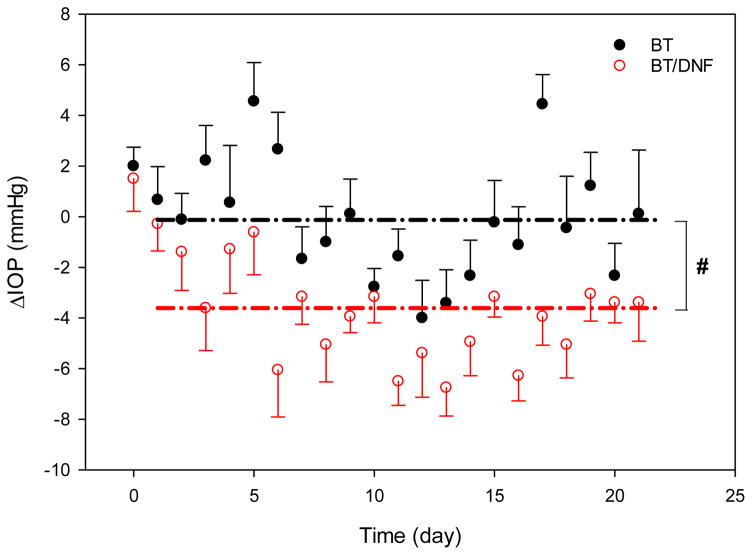
In vivo experiments indicate BT drug delivery efficiency may be improved by DNF, but the effect is difficult to capture in normotensive animals. Drug efficacy was equivalent between DNF and saline eye drops in terms of single dose IOP response (Fig. 5). In those tests, both formulations induced an approximately 2 mmHg (~15%) drop in IOP at 2 h, followed by a rebound above baseline at 6 h, and finally a return to baseline values by 24 h. While DNF values were slightly lower than eye drops at all these time points, the difference was not significant. It took several days of repeated application to achieve a differential effect with DNF. The nature of IOP measurement on fully conscious animals produces considerable noise, but over the three-week test period DNF experimental eyes recorded significantly lower average pressure values than conventional eye drops (Fig. 6). Taken together, these results suggest DNF mats deliver BT with a similar efficiency to conventional eye drops with each individual dose, but over repeated applications they build an additive effect, and are able to achieve a significant drop in IOP using a sub-therapeutic drug dosage.
Figure 5. In vivo single dose response.
Brown Norway rats (n=4) received a single dose of BT via saline eye drops or DNF topically. One dose of BT/DNF and one dose BT saline eye drops are equivalently effective in reducing IOP response (* indicates significantly different, P<0.01).
Figure 6. In vivo 3-week daily dose response.
Brown Norway rats (n=4) received a daily dose of brimonidine via saline eye drops or DNF for three weeks. IOP was recorded immediately prior to drug application. Values expressed are the difference between the experimental and contralateral eyes after normalizing individual eyes to baseline levels. The dash lines represent the mean IOP reduction values. DNF was able to sustain reduced IOP over the test period compared to saline eye drops (# indicates significantly different, P<0.001).
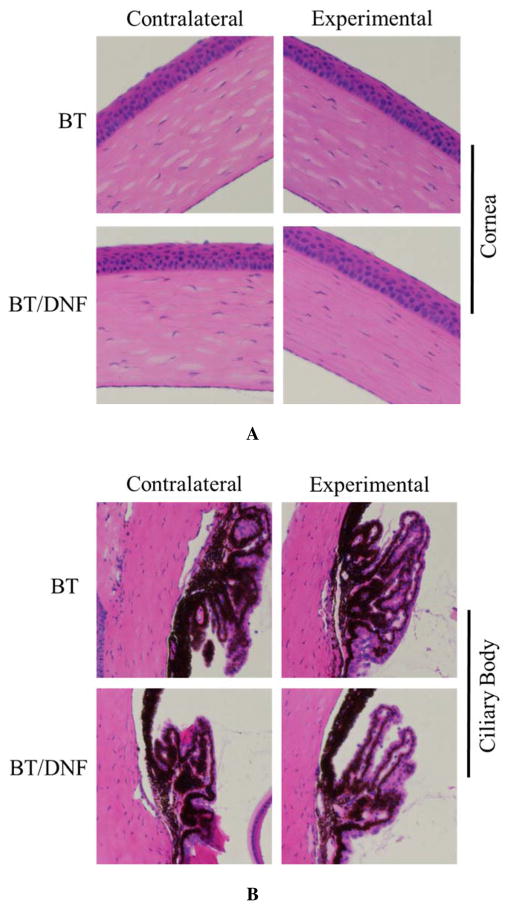
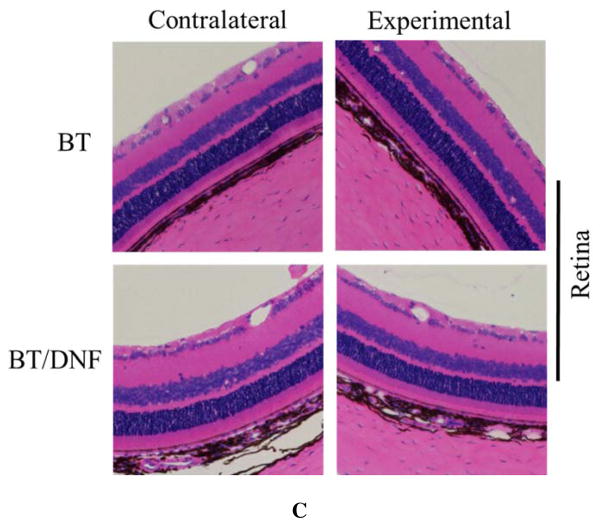
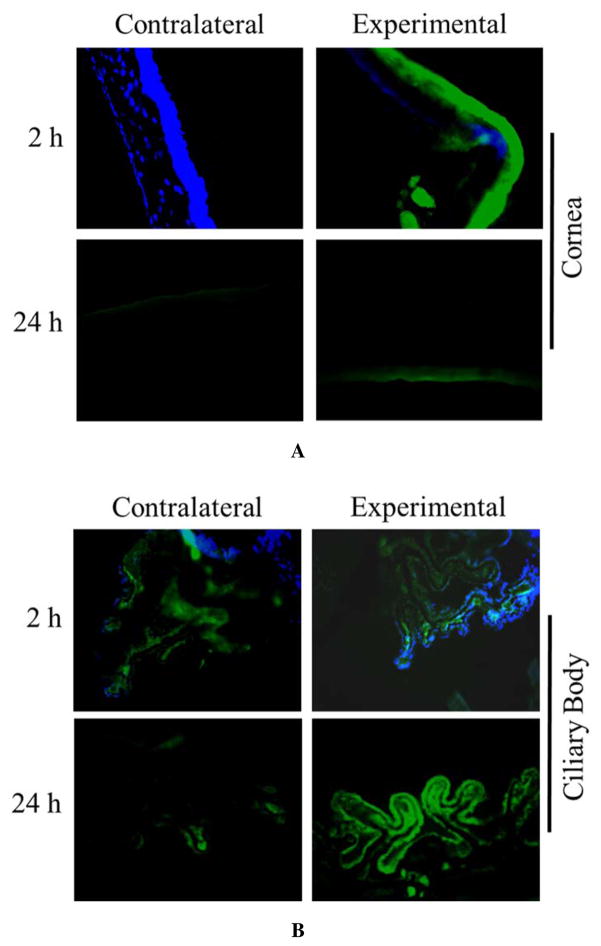
In vivo ocular tolerance of DNF was excellent. DNF dissolved virtually instantly (less than 1 s by video analysis (Movie S1 in Supporting Information). Animals reacted noticeably less to direct DNF application than BT solution. No outwardly visible signs of irritation or inflammation, such as redness, excessive blinking, or ocular discharge were observed in any animals in the single dose response experiments. During the chronic use trial several of the animals exhibited known side effects of BT in the experimental eye by the second week, namely redness of the surrounding skin. Histopathology of these eyes showed no gross morphological changes in the eyes exposed to either BT solution or DNF (Fig. 8). Taken together, DNF appears to be a safe material for ocular applications.
Figure 8. Ocular histopathology of chronic DNF application.
Brown Norway rats (n=3) received a single dose of BT via saline eye drops or DNF every 24 h for 21 days. No changes in cornea morphology could be observed between the experimental groups. Other ocular structures such as the ciliary body and retina showed the same result.
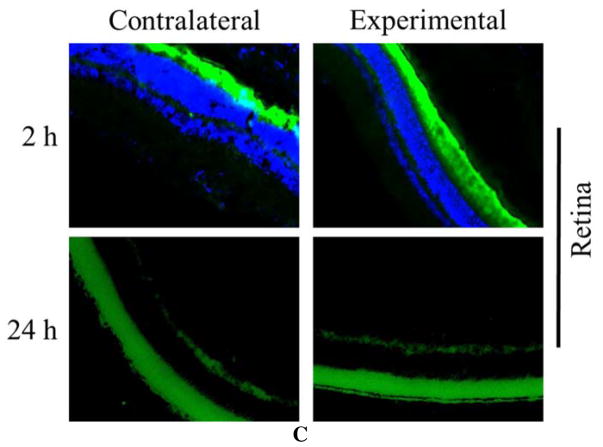
A potential mechanism for this additive effect can be found in the results of the disposition experiments. Fluorescence images indicated that while few dendrimers could still be observed in the cornea 24 h after fiber application, quantities in the ciliary body had noticeably increased compared to eyes harvested just 2 h after fiber application (Fig. 7). This means in the chronic use trial, some of the previous day’s dendrimers were still present in the target organ when that day’s BT was at its peak concentration. If these latent dendrimers were able to increase the residence time of that next dose of BT, it could account for the gradual increase and plateau in potency that we observed. It also agrees with the response of the contralateral eye, which saw a slight IOP drop from baseline in the eye drop group, but no change with DNF. If the dendrimers are holding the drug closer to the site of application, then they may be able to reduce the bleed-over effects common with BT.
Figure 7. Ocular disposition of DNF.
Brown Norway rats (n=3) received a DNF-FITC mat topically in the right eye (experimental eye) while the left eye received no treatment (contralateral eye). Animals were euthanized at 2 or 24 h and the ocular tissues harvested and immediately processed for cryosectioning. Fluorescent imaging of sections from various ocular organs showed most dendrimers were flushed from the cornea in 24 h. Over the same time period, FITC-G3.0-mPEG accumulated in the ciliary body of the experimental eye.
Other phenomenon could also be responsible for the result. The ocular bioactivity of topically applied PAMAM dendrimers themselves is somewhat unknown, but no strong effect has been detected in the limited studies conducted to this point.35–38 It could also be that fiber application is simply more reliable. Great care was taken to ensure that drug dosage was consistent between the groups (as confirmed by HPLC), but eye drop instillation is prone to inaccuracy in both animals and humans. Since the fibers are applied dry it is easy to ensure that all of the material successfully makes it into the eye, where it is not uncommon for some amount of a drop to spill onto the face of the animal. This could point to a possible pediatric or veterinarian use for the material, where topical drops problematic not just for their low efficiency, but also their awkward application procedure.
CONCLUSIONS
In this work, electrospun dendrimer based fibers have been developed as a novel vehicle for topical administration of therapeutic compounds to the eye. The drug delivery efficacy and ocular tolerance of the material has been evaluated in vitro, ex vivo, and in vivo using a model ocular drug, brimonidine tartrate. Results were equivalent between DNF and saline eye drops in terms of safety and single dose drug delivery efficacy, but DNF outperformed the control when applied daily. Disposition experiments showed dendrimer accumulation in the anterior chamber, suggesting the platform can function as a powerful ocular drug delivery vehicle with further study and development. The studies suggest that drug loaded dendrimer nanofiber mats can be a promising alternative to drug saline eye drops.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (R01EY024072). Tissue sectioning and H& E staining services in support of the research project were generated by the VCU Massey Cancer Center Cancer Mouse Model Shared Resource, supported, in part, with funding from NIH-NCI Cancer Center Support Grant P30CA016059.
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Demonstration of topical administration of fast dissolving BT/DNF on a rat’s eye (avi).
References
- 1.Ibrahim MM, Abd-Elgawad A-EH, Soliman OA, Jablonski MM. Novel topical ophthalmic formulations for management of glaucoma. Pharm Res. 2013;30(11):2818–2831. doi: 10.1007/s11095-013-1109-1. [DOI] [PubMed] [Google Scholar]
- 2.Liu S, Jones L, Gu FX. Nanomaterials for ocular drug delivery. Macromol Biosci. 2012;12(5):608–620. doi: 10.1002/mabi.201100419. [DOI] [PubMed] [Google Scholar]
- 3.Sleath B, Blalock S, Covert D, Stone JL, Skinner AC, Muir K, Robin AL. The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmol. 2011;118(12):2398–2402. doi: 10.1016/j.ophtha.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel SC, Spaeth GL. Compliance in patients prescribed eyedrops for glaucoma. Ophthalmic Surg Lasers Imaging Retina. 1995;26(3):233–236. [PubMed] [Google Scholar]
- 5.Sayner R, Carpenter DM, Blalock SJ, Robin AL, Muir KW, Hartnett ME, Giangiacomo AL, Tudor G, Sleath B. Accuracy of patient-reported adherence to glaucoma medications on a visual analog scale compared with electronic monitors. Clin Ther. 2015;37(9):1975–1985. doi: 10.1016/j.clinthera.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coursey TG, Henriksson JT, Marcano DC, Shin CS, Isenhart LC, Ahmed F, De Paiva CS, Pflugfelder SC, Acharya G. Dexamethasone nanowafer as an effective therapy for dry eye disease. J Controlled Release. 2015;213:168–174. doi: 10.1016/j.jconrel.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Di Colo G, Zambito Y. A study of release mechanisms of different ophthalmic drugs from erodible ocular inserts based on poly (ethylene oxide) Eur J Pharm Biopharm. 2002;54(2):193–199. doi: 10.1016/s0939-6411(02)00086-3. [DOI] [PubMed] [Google Scholar]
- 8.Fedorchak MV, Conner IP, Medina CA, Wingard JB, Schuman JS, Little SR. 28-day intraocular pressure reduction with a single dose of brimonidine tartrate-loaded microspheres. Exp Eye Res. 2014;125:210–216. doi: 10.1016/j.exer.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng C-C, Burke MT, Carbia BE, Plummer C, Chauhan A. Extended drug delivery by contact lenses for glaucoma therapy. J Controlled Release. 2012;162(1):152–158. doi: 10.1016/j.jconrel.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Bravo-Osuna I, Woodward A, Argueso P, Martínez ITM, Gómez R, de la Mata FJ, Navarro MM, Noiray M, Ponchel G, Herrero-Vanrell R. Linear polymers versus PAMAM dendrimers in the interaction with transmembrane ocular mucins: Analysis by biosensor technology. Invest Ophthalmol Visual Sci. 2012;53(14):1845. [Google Scholar]
- 11.Holden CA, Tyagi P, Thakur A, Kadam R, Jadhav G, Kompella UB, Yang H. Polyamidoamine dendrimer hydrogel for enhanced delivery of antiglaucoma drugs. Nanomedicine (NY, NY, US) 2012;8(5):776–783. doi: 10.1016/j.nano.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Imayasu M, Ito I, Fukuchi H, Cavanagh HD. Effects of multipurpose care solutions for RGP contact lenses on corneal epithelial tight junctions. Contact Lens Anterior Eye. 2015;(38):e15. [Google Scholar]
- 13.Yang H, Kao WJ. Dendrimers for pharmaceutical and biomedical applications. J Biomater Sci, Polym Ed. 2006;17(1–2):3–19. doi: 10.1163/156856206774879171. [DOI] [PubMed] [Google Scholar]
- 14.Yang H, Tyagi P, Kadam RS, Holden CA, Kompella UB. Hybrid dendrimer hydrogel/PLGA nanoparticle platform sustains drug delivery for one week and antiglaucoma effects for four days following one-time topical administration. ACS Nano. 2012;6(9):7595. doi: 10.1021/nn301873v. [DOI] [PubMed] [Google Scholar]
- 15.Yang H, Leffler CT. Hybrid dendrimer hydrogel/poly(lactic-co-glycolic acid) nanoparticle platform: an advanced vehicle for topical delivery of antiglaucoma drugs and a likely solution to improving compliance and adherence in glaucoma management. J Ocul Pharmacol Ther. 2013;29(2):166–172. doi: 10.1089/jop.2012.0197. [DOI] [PubMed] [Google Scholar]
- 16.Carta F, Osman SM, Vullo D, Gullotto A, Winum JY, AlOthman Z, Masini E, Supuran CT. Poly(amidoamine) dendrimers with carbonic anhydrase inhibitory activity and antiglaucoma action. J Med Chem. 2015;58(9):4039–4045. doi: 10.1021/acs.jmedchem.5b00383. [DOI] [PubMed] [Google Scholar]
- 17.Aduba DC, Overlin JW, Frierson CD, Bowlin GL, Yang H. Electrospinning of PEGylated polyamidoamine dendrimer fibers. Mater Sci Eng C. 2015;56:189–194. doi: 10.1016/j.msec.2015.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yavuz B, Bozdag Pehlivan S, Sumer Bolu B, Nomak Sanyal R, Vural I, Unlu N. Dexamethasone - PAMAM dendrimer conjugates for retinal delivery: preparation, characterization and in vivo evaluation. J Pharm Pharmacol. 2016;68(8):1010–1020. doi: 10.1111/jphp.12587. [DOI] [PubMed] [Google Scholar]
- 19.Yao W, Sun K, Mu H, Liang N, Liu Y, Yao C, Liang R, Wang A. Preparation and characterization of puerarin–dendrimer complexes as an ocular drug delivery system. Drug Dev Ind Pharm. 2010;36(9):1027–1035. doi: 10.3109/03639041003610799. [DOI] [PubMed] [Google Scholar]
- 20.Mishra V, Jain NK. Acetazolamide encapsulated dendritic nano-architectures for effective glaucoma management in rabbits. Int J Pharm. 2014;461(1–2):380–390. doi: 10.1016/j.ijpharm.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 21.Boateng JS, Popescu AM. Composite bi-layered erodible films for potential ocular drug delivery. Colloids Surf B. 2016;145:353–361. doi: 10.1016/j.colsurfb.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Yang H, Morris JJ, Lopina ST. Polyethylene glycol-polyamidoamine dendritic micelle as solubility enhancer and the effect of the length of polyethylene glycol arms on the solubility of pyrene in water. J Colloid Interface Sci. 2004;273(1):148–154. doi: 10.1016/j.jcis.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 23.Marques MRC, Loebenberg R, Almukainzi M. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011;18(3):15–28. [Google Scholar]
- 24.Husain S, Abdul Y, Singh S, Ahmad A, Husain M. Regulation of nitric oxide production by delta-opioid receptors during glaucomatous injury. PLoS One. 2014;9(10):e110397. doi: 10.1371/journal.pone.0110397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang W, Xiong W, Wan J, Sun X, Xu H, Yang X. The decrease of PAMAM dendrimer-induced cytotoxicity by PEGylation via attenuation of oxidative stress. Nanotechnology. 2009;20(10):e105103. doi: 10.1088/0957-4484/20/10/105103. [DOI] [PubMed] [Google Scholar]
- 26.Madaan K, Kumar S, Poonia N, Lather V, Pandita D. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. J Pharm Bio Allied Sci. 2014;6(3):139–150. doi: 10.4103/0975-7406.130965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shenoy SL, Bates WD, Frisch HL, Wnek GE. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: good solvent, non-specific polymer–polymer interaction limit. Polymer. 2005;46(10):3372–3384. [Google Scholar]
- 28.Sweet DM, Kolhatkar RB, Ray A, Swaan P, Ghandehari H. Transepithelial transport of PEGylated anionic poly (amidoamine) dendrimers: implications for oral drug delivery. J Controlled Release. 2009;138(1):78–85. doi: 10.1016/j.jconrel.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu JH, Fridrikh SV, Rutledge GC. The role of elasticity in the formation of electrospun fibers. Polymer. 2006;47(13):4789–4797. [Google Scholar]
- 30.Kitchens KM, Kolhatkar RB, Swaan PW, Eddington ND, Ghandehari H. Transport of poly (amidoamine) dendrimers across Caco-2 cell monolayers: influence of size, charge and fluorescent labeling. Pharm Res. 2006;23(12):2818–2826. doi: 10.1007/s11095-006-9122-2. [DOI] [PubMed] [Google Scholar]
- 31.Prausnitz MR, Noonan JS. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J Pharm Sci. 1998;87(12):1479–1488. doi: 10.1021/js9802594. [DOI] [PubMed] [Google Scholar]
- 32.Kaur IP, Smitha R. Penetration enhancers and ocular bioadhesives: two new avenues for ophthalmic drug delivery. Drug Dev Ind Pharm. 2002;28(4):353–370. doi: 10.1081/ddc-120002997. [DOI] [PubMed] [Google Scholar]
- 33.Souza JG, Dias K, Silva SAM, de Rezende LCD, Rocha EM, Emery FS, Lopez RFV. Transcorneal iontophoresis of dendrimers: PAMAM corneal penetration and dexamethasone delivery. J Controlled Release. 2015;200:115–124. doi: 10.1016/j.jconrel.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 34.Xiang CD, Batugo M, Gale DC, Zhang T, Ye J, Li C, Zhou S, Wu EY, Zhang EY. Characterization of human corneal epithelial cell model as a surrogate for corneal permeability assessment: metabolism and transport. Drug Metab Dispos. 2009;37(5):992–998. doi: 10.1124/dmd.108.026286. [DOI] [PubMed] [Google Scholar]
- 35.Wang W-Y, Yao C, Shao Y-F, Mu H-J, Sun K-X. Determination of puerarin in rabbit aqueous humor by liquid chromatography tandem mass spectrometry using microdialysis sampling after topical administration of puerarin PAMAM dendrimer complex. J Pharm Biomed Anal. 2011;56(4):825–829. doi: 10.1016/j.jpba.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 36.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discovery. 2009;8(2):129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richichi B, Baldoneschi V, Burgalassi S, Fragai M, Vullo D, Akdemir A, Dragoni E, Louka A, Mamusa M, Monti D. A divalent PAMAM-based matrix metalloproteinase/carbonic anhydrase inhibitor for the treatment of dry eye syndrome. Chem– Eur J. 2016;22(5):1714–1721. doi: 10.1002/chem.201504355. [DOI] [PubMed] [Google Scholar]
- 38.Vandamme TF, Brobeck L. Poly (amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. J Controlled Release. 2005;102(1):23–38. doi: 10.1016/j.jconrel.2004.09.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.