Abstract
Biofilm formation is a key virulence factor for a wide range of microorganisms that cause chronic infections. The multifactorial nature of biofilm development and drug tolerance imposes great challenges for the use of conventional antimicrobials, and indicates the need for multi-targeted or combinatorial therapies. In this review, we focus on current therapeutic strategies and those that are under development that target vital structural and functional traits of microbial biofilms and drug tolerance mechanisms, including the extracellular matrix and dormant cells. We emphasize strategies that are supported by in vivo or ex vivo studies, highlight emerging biofilm-targeting technologies, and provide a rationale for multi-targeted therapies that are aimed at disrupting the complex biofilm microenvironment.
Initially reported as an arcane behaviour of bacterial populations, microbial biofilm formation is now recognized as a principle virulence factor in many localised chronic infections. Biofilm infections commonly recur after long periods of clinical quiescence. This is not primarily due to genetic resistance that arises by mutation, although the increased microbial cell density may favour transfer of resistance genes. Rather microorganisms that reside in biofilms may develop tolerance to traditional antibiotics or antimicrobial agents through metabolic dormancy or molecular persistence programmes. Moreover, the important role of the extracellular matrix in conferring antimicrobial tolerance to biofilms is being recognized1. Advances in multi-omic and imaging technologies have also revealed the remarkable complexity and spatial organization of polymicrobial biofilm infections2. Accordingly, our increased understanding of biofilms is rapidly changing the strategies used to treat these challenging infections (Fig. 1). Nonetheless, the control of biofilm formation and treating existing biofilms remains tenuous with few new therapeutic options currently available clinically.
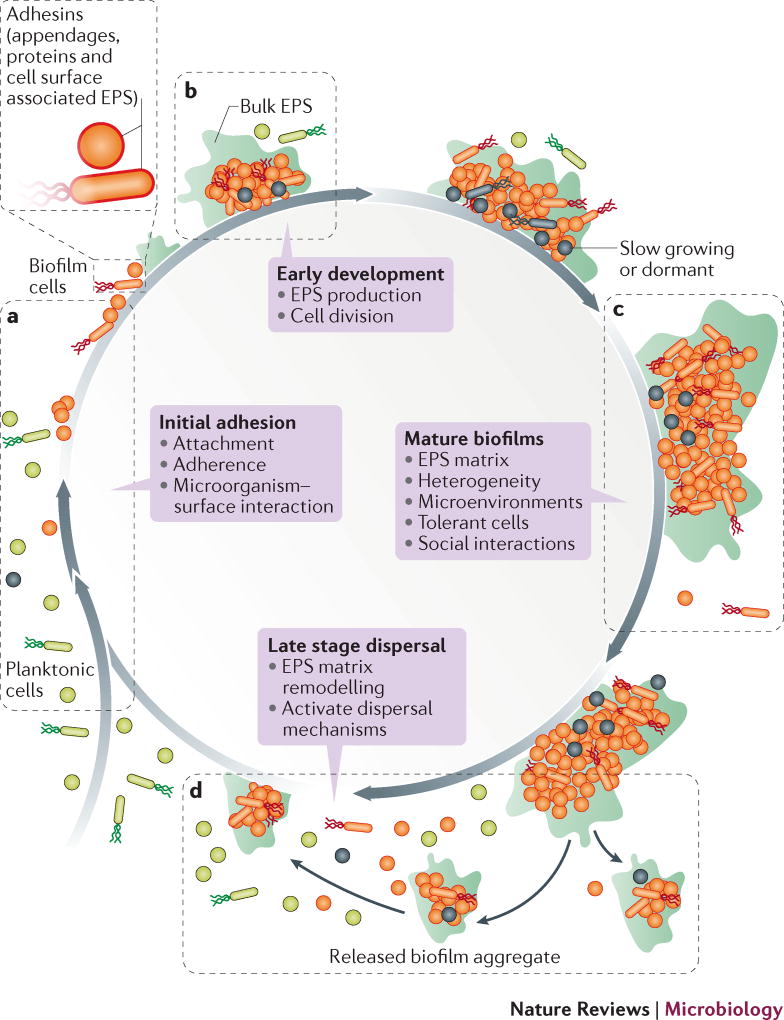
Figure 1. Opportunities for therapeutic intervention during various stages of the biofilm life-cycle.
Biofilm formation proceeds as a developmental process with distinct stages: “initial adhesion” where microorganisms bind to host or medical device surfaces through cell surface associated adhesins; “early biofilm formation” where they begin to divide and produce EPS which enhances adhesion, while forming the matrix that embeds the cells; “biofilm maturation” where 3D structures develop in which the EPS matrix provides a multi-functional and protective scaffold which allows heterogeneous chemical and physical microenvironments to form where microorganisms co-exist within polymicrobial and social interactions (competitive and synergistic); and finally “dispersal” where cells leave the biofilm to re-enter the planktonic phase. Biofilms can be targeted at these various stages. a) The initial phase of biofilm formation can be disrupted, for example, by preventing the attachment of microorganisms by interrupting the interactions between the microorganism and the surface, by targeting cell surface associated adhesins (appendages, proteins and EPS). b) The inhibition of early stages of biofilm development includes targeting the production of EPS and cellular division. c) Disruption of formed biofilms could be achieved by physical removal, the degradation of the EPS-matrix, targeting the establishment of pathogenic microenvironments (low pH or hypoxia) and social interactions (in polymicrobial biofilms) as well as elimination of dormant cells. d) Finally, biofilm dispersion can be induced by EPS matrix remodelling or activation to dispersal mechanisms.
Biofilm infections are not easily amenable to existing antimicrobial treatment or ‘single magic bullet’ approaches, because biofilm recalcitrance is a consequence of complex physical and biological properties with multiple microbial genetic and molecular factors, and also frequently involve multi-species interactions. A diverse range of microorganisms (Gram-positive and Gram-negative, motile and non-motile, aerobic, anaerobic and facultative bacteria, and fungi) form biofilms, which share many common features (Box 1). Although the ‘universal’ role of cell signalling in biofilm formation was revealed 20 years ago, signalling-based therapeutics have yet to be introduced for the clinical management of biofilm infections owing to the complexity in cell signalling networks. Similarly, the emergence of materials science, the development of surface modifications that incorporate technologies that target adhesion, as well as biomimicry or surface textures and chemistries from plants and animals3 were promising approaches to prevent microbial adherence and subsequent biofilm formation. Although many studies show statistical significant reductions in biofilm or alterations in biofilm structures in the laboratory, few were tested or validated using in vivo or human cell models to see if they translated to clinical significance. Many studies only report early time points, fail to use clinically relevant treatment regimens or do not consider the presence of molecularly complex host fluids or host cells at the site of biofilm infections. More recent approaches include targeting the extracellular polymeric substance (EPS) matrix. However, the variability in the composition of the EPS matrix and the interactions among the various components4 add new levels of complexity and provide challenges for the development of EPS-targeting therapeutics5.
Text Box 1. Common features of microbial biofilms.
| Chemical composition, physical properties |
| Adherence. Microorganisms adhere to virtually all man-made materials (i.e. plastics, metals, ceramics and hybrids) and biotic surfaces (i.e. tooth enamel, bone, skin, airway, intestinal, and vaginal mucosa, connective tissue, vascular endothelium), using both specific (bacterial adhesin-host receptor interactions) and non-specific adhesion (hydrophobic or electrostatic forces) mechanisms154. |
| Extracellular polymeric substance (EPS) matrix. Although the precise chemical and physical composition of the EPS (polysaccharides, proteins, nucleic acids) varies between species and growth conditions (i.e. nutrient type and abundance, hydrodynamics, temperature, oxygen concentration), the EPS provides a scaffold for mechanical stability, and creates compartmentalized chemical and physical microenvironments affording protection to the cells within a heterogeneous 3D structure. |
| Architecture. Although there is some variation in the structure of in vitro grown biofilms, there are a limited number of common forms (flat patches, mounds, mushrooms, towers, ripples, streamers) that are not generally species specific but largely dependent on biofilm maturity and the production of certain EPS components and growth conditions. Biofilms seen in many clinical specimens tend to consist of aggregates of cells of varying sizes and mixed-species in polymicrobial systems 155. |
| Viscoelasticity. A material property that allows biofilms to absorb and dissipate energy, rather than detach, when exposed to mechanical forces, such as hydrodynamic shear. The elastic component allows the biofilm to spring back into shape during intermittent perturbations, while the viscous component allows biofilms to flow like liquids when forces are sustained.5. |
| Heterogeneity. Biofilms are heterogeneous (non-uniform) in distribution, structure and physiology at various spatial scales. On the larger scale (mms to cms) they are generally not uniformly distributed on surfaces but occur in patches of cell-clusters (also called microcolonies or aggregates) in a range of sizes and shapes. Within the biofilm heterogeneous and compartmentalized microenvironments (10s to 100 µms) develop which modulate microbial activity, intercellular signalling and metabolic exchange locally, thus spatially organizing cellular and communal behaviour which is an important factor for enhanced tolerance and persistence. |
| Physiological and regulatory aspects |
| Developmental life cycle. Pseudomonas aeruginosa as a widely used model organism for studying microbial biofilms formation. Although many of the concepts identified in this organism, such as the mechanism of attachment, growth, maturation and dispersal, are widely conserved among other biofilm forming pathogens, there are fundamental differences. For example, many biofilm-forming species, such as staphylococci, unlike P. aeruginosa, are non-motile, many species have surface structures that are important for adhesion (capsule, pili or flagella) but many do not, and lastly not all species have known signalling systems. |
| Diffusible cell signals. These signals co-ordinate population behaviour156, metabolic activity, biofilm formation and dispersal. Families of homoserine lactones are produced by a number of Gram negative species while Gram positive organisms more commonly use autoinducing peptides (AIP). |
| Altered microenvironment formation. The development of gradients in nutrients, pH and oxygen as a consequence of metabolic activity of microorganisms that reside in a biofilm and diffusion limited mass transport of molecules into and out of the EPS matrix alter the microenvironment in the biofilm. |
| Dormant or slow growing sub-populations. Those include persisters and small colony variants (SCVs), which are tolerant to antibiotics. In addition, nutrient depletion in the interior of the biofilm can result in a stationary phase-like dormancy. |
Several excellent reviews discuss how microorganisms develop pathogenic biofilms and their protective mechanisms against antibiotics, antimicrobial agents and host innate immunity 1,6,7. This Review focusses on the challenges facing the development of biofilm-specific therapeutic strategies, how new insights into the chemical composition and structure of the EPS matrix, active biofilm dispersal pathways, and recognition of the role of dormant persister cells or slow-growing subpopulations in conferring antibiotic tolerance are being exploited to target biofilm infections. We also review developing technologies that promise to enhance the efficacy of current modalities or provide novel biofilm-targeting effects, including challenges to ensure biocompatibility and therapeutic efficacy, which are both critical for clinical translatability. Where possible we focus on technologies that have shown efficacy in preclinical trials, or robust animal or human cell infection models. However, as not all potential biofilm-targeting therapeutic strategies can be discussed in detail, we provide a comprehensive list of current and prospective biofilm-targeting strategies, their developmental stage and a brief list of advantages and disadvantages in Supplemental Table S1.
Finally, we contend that treating biofilm infections requires combination therapies or those that target more than one component of the complex biofilm microenvironment, similar to tumouregenesis8. Importantly, biofilm infections reflect an interplay between the host and opportunistic pathogens, often within a complex microbiota. Polymicrobial biofilms pose an additional challenge, requiring antimicrobials that are effective against all pathogenic microorganisms in the biofilm and limiting the efficacy of species-specific biofilm-targeting strategies. All of these challenges contribute to why so few therapies have yet to be translated clinically.
Current therapeutic approaches
Many biofilm management strategies being devised in the clinic and used by surgeons, are largely based on an approach from cancer treatment (Box 2): early and aggressive irrigation and debridement for physically removal and local delivery of high and sustained antimicrobial chemotherapy9. Given the devastating consequences if a biofilm infection persists, surgeons are undertaking earlier and more aggressive treatment, including revisiting ‘old’ last-resort antibiotics such as colistin10. Another established approach used for intravenous catheter-related infections is lock therapy11. Following a decision to treat rather than remove certain types of catheters, the potential to leave biofilms intact (but containing dead cells) includes the potential to promote colonization by other microorganisms. This illustrates a crucial point regarding biofilms: killing does not necessarily eradicate the biofilm. Therefore the challenge of using antimicrobial agents, which may kill microorganisms but leave behind other biofilm components, must be addressed.
Text Box 2. Lessons learned from cancer.
Over 100 years ago Paul Ehrlich, the German physician scientist used the term ‘magische kugel’ to describe an ideal hypothetical therapeutic agent that specifically targets and kills disease-causing cells157. In the context of cancer’ the target of such a magic bullet were the newly discovered receptors found on tumor cells. Another magic bullet was interferon, the cytokine that was discovered in 1957158. However, a magic bullet for cancer therapy remains elusive in part because over the last 40 years of intense molecular research, it has been shown that “cancer is not simply a single disease that affects many parts of the body”. Rather, it is many different diseases with common themes that can cause different kinds of disorders in many of our organs”159. In addition, individual tumors exhibit substantial chemical and clonal heterogeneity with distinct phenotypes which combined with the ability of cancer cells to rapidly adapt to chemotherapeutics and the microenvironment, challenge both broad spectrum and targeted therapies. In tumors, the structure and composition of the extracellular matrix is often altered, creating a favourable cellular niche for malignant transformation and cancer progression. Biofilms share similar common themes (Box 1), including the ability to create distinct microenvironments with unique chemical, physical, phylogenetic, genotypic and phenotypic heterogeneities. Early cancer therapy borrowed from approaches to treat acute bacterial and viral infections by targeting individual cells (with antibiotics and vaccination), with limited success. Our current understanding of biofilm biology is following a similar path to tumor biology. Rather than piled-up assemblages of clonal cells, microbial biofilms represents a dynamic self-constructed ecosystems within a matrix containing highly heterogeneous and compartmentalized milieu, and more effective biofilm therapies will likely need to target the complete microenvironment as well as the individual cells within144.
Since understanding the mechanisms of biofilm formation derives primarily from how they form on solid surfaces, most clinical trials testing biofilm-targeting approaches or FDA-approved therapeutics have focussed on indwelling medical devices. Current biofilm-targeting technologies can be divided into two groups: physical-mechanical approaches (for example, high velocity spray and jet irrigators) that are aimed at biofilm disruption and removal; and surface-coating or eluting substrates, which can be impregnated with antibiotics and/or antimicrobials (for example, acrylic beads with absorbable antibiotic-loaded bone cement to prevent orthopaedic infection12) for biofilm prevention; where higher localised antibiotic concentrations can be achieved for longer periods by in situ antibiotic delivery compared to systemic administration. Several antimicrobial metal or inorganic coatings have also reached clinical application to prevent biofilm formation13. These include silver coating in endotracheal tubes, catheters, megaprostheses, wound dressings and copper alloys in hospital surfaces (supplemental Table S1). With respect to treating prexisiting biofilms, laboratory studies show that statistically significant reductions in biofilm viability can require extended incubation periods with high antibiotic concentrations. In situ release offers an important approach to achieve such high concentrations over long treatment periods 14,15. Although antibiotic-impregnated beads in bone cements or dental restorative materials were used before biofilm formation was recognized as a distinct etiological factor, they represent a class of technologies that are now being re-examined to gain better understanding of their effect in controlling biofilm infections.16
Mechanical disruption using water sprays and jets have been developed and used for pathogenic biofilm removal and for irrigation, including debridement of surgical site infections to remove necrotic tissue, exudates or dental biofilms. High-speed imaging has provided important information on fluid-biofilm-surface interactions and show that although a statistically significant amount of biofilm is removed from the area, the biofilm becomes fluidized and spreads across the surface17. The ability of biofilms to become fluidized probably explains the tenacity of bacteria on surfaces after pulsed lavage18 and may contribute to the low success rate of irrigation and debridement alone in treating periprosthetic infections. An advantage of water-based jets is that antimicrobial agents can be readily added so that the fluid doubles as a delivery device as well as creating mechanical forces acting on the biofilm. However, despite advances in biofilm specific-clinical therapies, particularly for indwelling devices, most approaches still entail conventional antibiotic-based therapy or topical broad-spectrum antimicrobials.
EPS-targeting strategies
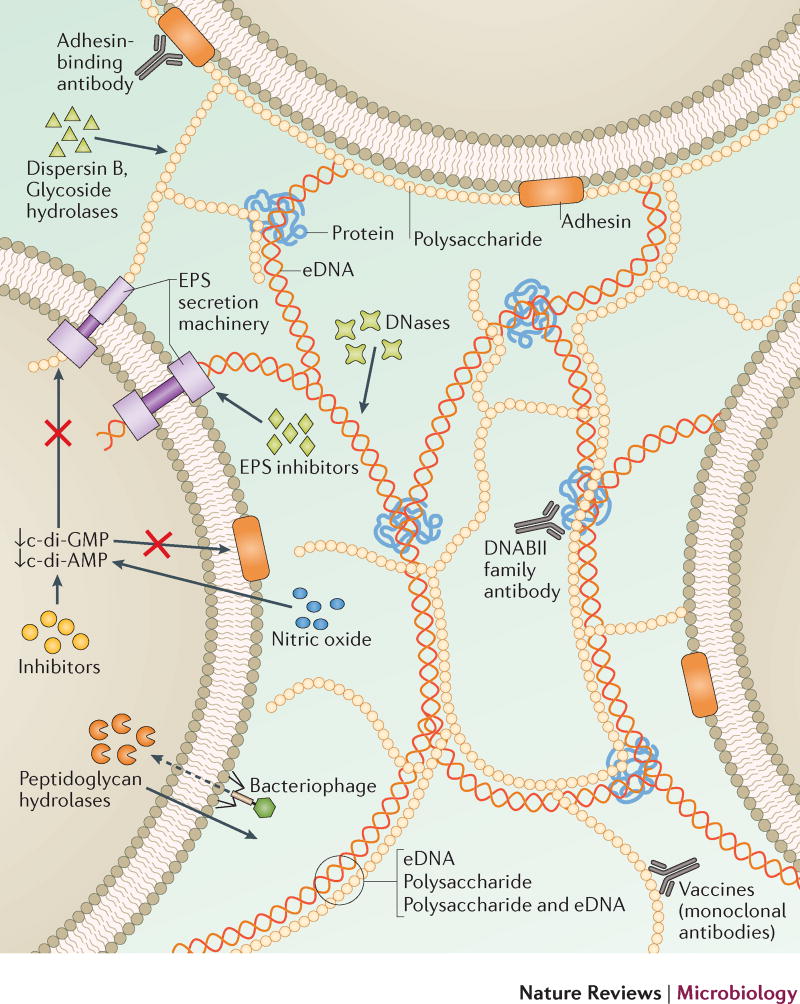
The composition of the EPS matrix is temporally and structurally variable depending on the type of microorganism, local mechanical shear forces, substrate availability and the host environment. The EPS matrix promotes microbial adherence to a surface, cell-cell adhesion and aggregation19, and functions as a 3D scaffold that provides cohesiveness, mechanical stability and protection against host effectors and antimicrobial therapies. In addition, the EPS matrix can dynamically modulate chemical and nutrient gradients, and delineate pathogenic environments (such as acidic pH and hypoxia), which contribute to key virulence attributes, including recalcitrance1,4. Thus, targeting the EPS may be an effective strategy to remove biofilm, disaggregate bacteria and disrupt the pathogenic environment20. Targeting can be achieved by: inhibiting EPS production, binding EPS adhesins on the microbial surfaces to block adhesion, or by degrading EPS in established biofilms (Fig 2).
Figure 2. Targeting the EPS.
Disruption of EPS components, and the underlying mechanisms that are responsible for the production and secretion of EPS components, represent attractive targets for the development of biofilm-targeting strategies, some of which have potential efficacy across microbial species. One approach includes the degradation of the EPS. Treatments have been developed that directly target the eDNA (DNases), exopolysaccharides (dispersin B, glycoside hydrolases, monoclonal antibody vaccines), and protein (DNABII family antibodies) components of the matrix. EPS adhesin-binding antibodies or inhibitors and phage-encoded peptidoglycan hydrolases have been developed to target bacterial adhesion and biofilm initiation. Inhibitors of EPS synthesis and the secretion systems have also shown promise to disrupt biofilm accumulation. Endogenous pathways that induce biofilm dispersal can also be targeted, including the regulation of c-di-GMP and c-di-AMP levels using exogenous NO and inhibitors, or targeting quorum sensing using various inducing peptides and messenger molecules. Importantly, all of these treatment strategies, alone or in combination, can lead to inhibition of biofilm formation, disrupt biofilm integrity and/or promote the release of individual bacterial cells that are more susceptible to conventional antibiotic treatment enhancing clinical efficacy.
Disrupting EPS synthesis and secretion, and binding of EPS adhesins
Several extracellular and intracellular signalling networks as well as non-signalling mechanisms that promote the production of EPS have been identified. In general, cyclic-di-GMP and cyclic-di-AMP21 control various EPS-producing exoenzymes, polysaccharides and adhesins that are potential candidates that can be targeted to inhibit or disrupt EPS22,23. These nucleotide-signalling molecules regulate glucan-producing exoenzymes (for example, glucosyltransferase) in Gram-positive Streptococcus mutans as well as the aggregative exopolysaccharides Psl and Pel in Gram-negative P. aeruginosa. Several potential small inhibitors of di-guanylate or di-adenylyl cyclase have been identified through library screening or in silico drug discovery combined with bioactivity assessment using in vitro biofilm models,24,25 although their efficacy against biofilms awaits further in vivo validation.
The inhibition of EPS glucan synthesis by glucosyltransferase using small-molecule inhibitors reduced the accumulation of pathogenic biofilms on teeth, and supressed the onset of oral diseases in vivo without disturbing the resident microbiota.26,27 These small-molecule inhibitors alone are not superior than current chemical modalities for oral biofilm control (chlorhexidine) or tooth decay prevention (fluoride), however, when used in combination, EPS inhibitors can greatly enhance their therapeutic effects26. Inhibitors of adhesin production and adhesin-binding antibodies or peptides have also been developed to disrupt bacterial binding to host surfaces. Small molecules (for example, peptides or mannosides) that target host- EPS matrix interactions have shown efficacy in prevention and treatment of both bacterial and fungal biofilm infections in vivo.28,29 Mannosides that target the bacterial adhesin FimH (alone or combined with trimethoprim-sulfamethoxazole) prevented catheter-associated urinary tract infection (UTI) in mice by reducing Escherichia coli colonization 2-log, and treated chronic cystitis by reducing the E. coli population 3-log28,30,31. A recent study has also attempted to address the low half-life and bioavailability of O-mannosides by generating C-mannosides, which have increased metabolic stability and in vivo efficacy. Prophylactic treatment with this compound reduced the E. coli burden 2-log and treatment of chronic infection resulted in a 4-log reduction in a UTI mouse model32. Similarly, ring-fused 2-pyridones, which inhibit the biogenesis of curli and type-I pili, reduced uropathogenic E. coli bladder colonisation more than 10-fold and the establishment of intracellular bacterial communities in an in vivo mouse UTI model33. Several other biomolecules that bind to EPS adhesins are discussed in detail elsewhere34.
Targeting EPS chemical composition and structure
Exopolysaccharide-degrading enzymes such as glucanohydrolases (dextranase and mutanase) and dispersin B can disrupt the matrix of pathogenic oral biofilms, and glycoside hydrolases have been used to degrade a mixed-species S. aureus and P. aeruginosa biofilm grown in a mouse model of chronic wounds 35–37, although poor retention and enzymatic stability (for example, susceptibility to proteolysis) may compromise efficacy in vivo36. Nevertheless, a purified serine protease, Esp, from S. epidermidis inhibited S. aureus biofilm formation and eradicated pre-existing biofilms in vitro, while the susceptibility to the antimicrobial β-defensin 2 was enhanced and S. aureus nasal colonization in humans was reduced38. Another approach used endolysins (bacteriophage-encoded peptidoglycan hydrolases), which enzymatically degraded the bacterial cell wall peptidoglycan 39. Engineered peptidoglycan hydrolase constructs with distinct antimicrobial activities degraded multiple unique bonds in the peptidoglycan structure specific to S. aureus40, and increased killing and biofilm removal in animal models. Fusion proteins derived from multiple bacteriophage-encoded endolysins, each with unique actions, all added simultaneously may also reduce the risk of resistance, yet show sufficient specificity to avoid targeting commensal strains. Glycoside hydrolases were recently shown to both disrupt pre-existing P. aeruginosa biofilms and potentiate neutrophil mediated killing41.
DNases have also shown efficacy in disrupting biofilms42. Consistent with the role of eDNA in the EPS matrix and in early biofilm development, DNase I is effective in disrupting early biofilms in vitro and in vivo42,43. Notably, other biomolecules, including polysaccharides and various proteins that are associated with eDNA contribute to biofilm structural integrity, which may explain the efficacy of DNase I in treating nascent biofilms. Few studies have used DNases to specifically target biofilms in vivo, however, it was shown to statistically significantly decrease Gardnerella vaginalis colonization on vaginal mucosal epithelial cells in a mouse model44. Therapeutic use of recombinant human DNase I (dornase alfa) degrades neutrophil and microorganism-derived DNA in the sputum of patients with cystic fibrosis, thus reducing sputum viscosity45. An intervention study with dornase alfa in patients with cystic fibrosis and early lung disease showed significantly improved lung function and lower risk of exacerbation compared to placebo groups, with a potential decrease in the rate of lung function decline in children46. A clinical trial investigating the efficacy of dornase alfa for the treatment of chronic otitis media, at the time of tympanostomy tube insertion to promote bacterial clearance from the middle ear combined with antibiotic drops, is under evaluation47,48.
Matrix-degrading enzymes can help disperse bacteria in biofilms for more effective killing when combined with antimicrobial agents. Targeting EPS can also disrupt the viscoelastic properties to further weaken biofilm cohesiveness and enhance antimicrobial efficacy, including host mediated antimicrobial responses. Recent studies showed that glucano-hydrolases, glycoside-hydrolases and DNases enhanced antimicrobial delivery and potentiated killing by antibiotics or antimicrobial peptides when used in combination against pre-formed biofilms in vitro49,50. Overall, EPS synthesis inhibitors or EPS-degrading enzymes, which lack intrinsic antibacterial activity, seem to be a promising adjunctive approach for biofilm control that could potentially enhance the killing efficacy of antimicrobial agents and promote biofilm removal when co-administered.
EPS-targeted antibodies and nucleic acid-binding proteins
Vaccine approaches pose several challenges as a strategy to target biofilms , as vaccines are specific to a microorganism and clinical isolates from biofilm infections show considerable variability in genotype and/or the phenotypic expression of vaccine-targeted epitopes51. A more effective approach may be to use antibodies targeted specific EPS components. Monoclonal antibodies against P. aeruginosa-derived EPS identified epitopes that bound to the polysaccharide Psl, which is widely present in P. aeruginosa clinical isolates52. Psl was shown to be a serotype-independent, antibody-accessible antigen, and anti-Psl antibodies increased opsonophagocytic killing of P. aeruginosa, inhibited attachment to lung epithelial cells in vitro, and showed prophylactic protection in multiple animal models of P. aeruginosa infection. Additionally, vaccine-elicited antibodies to Enterococcus faecalis pilus tip (EbpA) abrogated bacterial binding to fibrinogen and biofilm formation in a mouse model of catheter-associated urinary tract infections (CAUTI)53. Notably, EbpA did not mediate E. faecalis adhesion directly to the catheter material, but rather inhibited binding to fibrinogen deposited on the catheter surface. The EbpA antibody response prevented EbpA-mediated fibrinogen-dependent bacterial aggregation and biofilm formation on catheters. This approach highlights why using a complex host-microorganism model can reveal additional targets. In another approach, a multivalent vaccine exploiting both planktonic and biofilm-expressed polypeptides from S. aureus showed increased efficacy in combination with antibiotics compared to antibiotic treatment alone in a rabbit model of osteomyelitis54.
Nonetheless, targeting broadly conserved components in EPS is desirable. The DNABII family of DNA-binding proteins have a key role in providing structural integrity to eDNA55. The high binding affinity of integration host factor (IHF) has specifically been exploited to target nucleoproteins in biofilms and been widely tested in animal models. Antibodies against E. coli IHF are cross-reactive and bind to DNABII in multiple bacterial species, resulting in biofilm destabilisation and the release of individual bacteria. When combined with antibiotic therapy, immunotherapy targeting DNABII has shown efficacy in vivo against biofilms in numerous types of bacteria, including oral bacteria56, uropathogenic E. coli57 and P. aeruginosa in a mouse lung infection model58. This approach has also shown efficacy against MRSA biofilms compared with antibiotic treatment alone in mouse models59,60. In a combinatorial approach without using antibiotics, DNABII antibodies were combined with a vaccine strategy. A study with nontypeable H. influenzae (NTHi) in an animal model of otitis media used IHF and recombinant soluble type IV pili (rsPilA) co-administered with an adjuvant and delivered by transcutaneous immunization to achieve early NTHi eradication and prevention of disease 61. This approach also resulted in the disassembly of NTHi biofilms that were established prior to immunization, thus leading to resolution of existing disease.
Inducing biofilm dispersal
Biofilm dispersal has been shown to be a regulated process that involves the degradation of the EPS matrix, and the triggering of this response has provided research strategies designed to promote biofilm self-disassembly. These approaches, for the most part, assume that dispersed bacteria have returned to an active state akin to their planktonic phenotype, rendering them more susceptible to conventional antibiotics. Furthermore, liberated inactive cells will also have lost a degree of protection conferred by their association with the biofilm community and structural organization. Regardless of their dispersed state, it remains vitally important in the clinical setting that dispersive or exogenous EPS-degrading agents are administered alongside systemic antibiotics to avoid recolonization or bacteremia, and potentially septicaemia.
Targeting cyclic-di-GMP pathway
The intracellular secondary messenger nucleotide c-di-GMP has a key role in the biofilm lifecycle of both Gram-positive and Gram-negative bacteria, whereby increased levels promote biofilm formation and reduced levels disassembly62. The enzymes governing c-di-GMP levels, diguanylate cyclases (synthesis) and phosphodiesterases (breakdown), possess GGDEF, EAL and HD-GYP domains that are found in numerous bacterial phyla. This signalling pathway therefore offers an attractive strategy to target multiple species, although the complexity of c-di-GMP regulation makes it challenging to control63. However, few studies that show biofilm dispersal have used relevant cell models in vitro or in vivo animal models. One study used a P. aeruginosa construct containing an exogenous E. coli phosphodiesterase. When expression was induced in vivo it resulted in reduced c-di-GMP and dispersal of biofilms on silicone implants in a mouse foreign body infection model64. Although, in principle, this study supports the potential use of such phosphodiesterases as a strategy to modulate c-di-GMP and target biofilms, the authors noted limitations of their findings, including an increased bacterial burden in the spleen. C-di-GMP is also a potent stimulator of host immunity via interferon responses, and therefore it may be difficult to attribute effects on biofilms specifically in vivo65.
A well-characterized approach to modulate c-di-GMP levels is though nitric oxide (NO). NO was first shown to regulate c-di-GMP levels and mediate biofilm dispersal in Pseudomonas aeruginosa66 at low concentrations, and these results have since been reproduced in several other bacterial species67. However, the use of gaseous NO or spontaneous NO-donors presents clinical challenges owing to potential cytotoxicity from systemic exposure, lack of specificity in targeting biofilm infections and cost. In addition, as NO is labile, the optimal concentration to disperse biofilms is difficult to measure;. however NO microelectrodes are highly sensitive and offer excellent spatial and temporal resolution in tissues or body fluids. A proof-of-concept preclinical study using low-dose gaseous NO in the pM to nM range, has recently shown to reduce the size of the P. aeruginosa biofilm aggregate in sputum as a primary clinical outcome in a small number of patients with cystic fibrosis68. Patients did not exhibit adverse effects to NO therapy. Although the biofilm aggregate size was significantly decreased, NO did not reduce CFU as seen in another study69, perhaps because patients continued to receive antibiotic therapy throughout the study period. However a Phase I clinical trial is ongoing to study the efficacy and safety of NO in patients with cystic fibrosis70.
To address the cost of administering gaseous NO and potential systemic cytotoxicity issues, cephalosporin-3´-diazeniumdiolates (C3Ds), composed of a stabilized diazeniumdiolate NO-donor attached to the 3’-position of cephalosporin, have recently been developed to selectively deliver NO to bacterial biofilms71. These pro-drug candidates are designed to specifically release NO upon cleavage of the cephalosporin β-lactam ring via bacterial β-lactamases and have been shown to be effective in dispersing in vitro P. aeruginosa biofilms71. NTHi biofilms grown on primary ciliated epithelia also showed enhanced sensitivity to the antibiotic azithromycin, reducing viability 2-log when a specific C3D, PYRRO-C3D, was used as an adjuvant; this response is possibly attributable to dispersal and modulation of metabolic activity72. This effect was also demonstrated in a study using primary epithelial cells from patients with primary ciliary dyskinesia (PCD), a disease that compromises mucociliary clearance. Airway cells from patients with PCD showed increased susceptibility to NTHi biofilm formation compared to epithelial cells from healthy individuals, and PYRRO-C3D in combination with antibiotic significantly decreased NTHi viability 2-log compared to antibiotic treatment alone73. Treatment of infected healthy airway cells and infected airway cells from unhealthy individuals had no effect on transepithelial electrical resistance, which suggests that epithelial barrier function was unaffected. Although this alone is not a sufficient assessment of toxicity, the targeted release of low NO concentrations (48 – 90 nM) should improve the safety of patients.
NO-donor instability is also an issue that is being addressed by developing nitroxides (sterically hindered NO analogues) that exert biological responses via NO-mimetic properties74. These molecules (carboxy-TEMPO, CTMIO, DCTEIO) elicited biofilm dispersal in P. aeruginosa and E. coli similar to NO, with carboxy-TEMPO also reducing tolerance to ciprofloxacin74,75. However, treatment with carboxy-TEMPO failed to disperse MRSA biofilms, which indicates that this approach may be restricted to biofilms formed by certain species. This highlights an ongoing concern for polymicrobial biofilms infections. Other drugs that are currently under development include ciprofloxacin-nitroxide conjugates, which similar to C3Ds, combine antibiotic activity with a donor compound76, and fimbrolide-NO donor hybrids, which simultaneously target quorum sensing (QS) and NO pathways77.
Targeting quorum sensing
The role of QS systems in biofilm development and dispersal offers another intensely studied strategy for the development of novel therapeutics. QS requires the binding of a signalling molecule to a corresponding transcriptional regulator, which activates the downstream transcription of select targets. As the production of many virulence determinants in pathogenic bacteria requires cell-cell communication, QS inhibitors (QSI) that target the AHL-QS system in Gram-negative bacteria or the QS systems in Gram-positive bacteria have been extensively evaluated for efficacy on clinically relevant bacterial biofilms using in vitro and in vivo models. For example, the QS autoinducer, AI-2, functions as a chemorepellent in Helicobacter pylori by regulating the proportion and spatial organisation of biofilm cells78. Treatment of in vitro biofilms with exogenous AI-2 resulted in both a reduction in the proportion of adherent cells and dispersal78. The autoinducing peptide type I (AIP-I) also triggered dispersal in MRSA biofilms on titanium disks rendering detached MRSA more susceptible to treatment with rifampicin and levofloxacin79. Additionally, the use of a RNAIII-inhibiting peptide (RIP) resulted in a 7-log reduction in MRSA compared to 5-log reductions observed with RIP-soaked Allevyn or teicoplanin treatments alone in a mouse wound model80. A recent study used a high-throughput screen to identify a benzamide-benzimidazole derivative, termed M64, that interferes with the Pseudomonas quinolone signal (PQS) quorum sensing system, which regulates biofilm formation and the production of virulence factors in P. aeruginosa81. Interestingly, M64 reduced both the virulence and persistence of the P. aeruginosa strain PA14 in a mouse model of burn and lung infections when used alone, and it reduced the bacterial load further when used in combination with ciprofloxacin. M64 also did not exhibit cytotoxicity in mouse macrophages and was shown to reduce the number of persister cells in the population.
Although the increased efficacy of antibiotic treatment with QSI in vivo is promising, reduced bacterial loads often depend on the strain and biofilm model82. Furthermore, QS molecules can be washed away during biofilm initiation, whereas the EPS matrix can bind and sequester QS molecules and the effects may be limited to highly localized areas within the biofilm structure1. Therefore, inhibitors need access and specific targeting to site of active QS-signalling. These factors in addition to the complexity in cell signalling networks, make it a challenging therapeutic approach albeit such inhibitors can be used in combination with other strategies.
Metabolic interference
The potential of exogenous amino acids in the treatment of biofilms has garnered considerable interest, with specific amino acids having been shown to affect both biofilm metabolism and development. L-arginine (L-Arg) functions as a substrate for alkali production by arginolytic bacteria (for example, Streptococcus gordonii), which can neutralize acids and modulate pH homeostasis within oral biofilms clinically83. Treatment of polymicrobial biofilms comprising Streptococcus mutans, S. gordonii and Actinomyces naeslundii with L-Arg suppressed S. mutans growth and resulted in substantial reduction in insoluble EPS and altered biofilm architecture84. In addition to pH modulatory effects83, L-Arg also repressed genes involved in the production of insoluble EPS and bacteriocin in S. mutans, while increasing hydrogen peroxide (used against S. mutans) production by S. gordonii84 L-Arg reduced biomass and altered EPS architecture in S. gordonii biofilms85, and also destabilized multispecies oral biofilms, thus reducing viability and increasing susceptibility to cetylpyridinium chloride86. An alternative amino acid, L-methionine, was also identified as a promising adjuvant for treating P. aeruginosa biofilms, triggering disassembly and increasing sensitivity towards ciprofloxacin in a mouse model of chronic pneumonia, and enhancing survival of infected mice87. This activity was attributed to up-regulation of four different DNase genes and the subsequent degradation of eDNA in the EPS matrix, although the exact pathways that regulate this response were not determined. Interestingly, L-Met seems to have been chosen for this study following screening of a selection of D- amino acids and L- amino acids for their activity against P. aeruginosa biofilms. Given the diversity in amino acid utilization between bacterial species it is unlikely that a single amino acid would have a universal function, however, their importance, and that of bacterial metabolism in general, should not be underestimated in the development of future treatment strategies.
Another approach is based on evidence that iron metabolism is important in biofilm formation in several pathogens88–91. Iron acquisition is crucial for pathogens to establish infection, and epithelial cells containing the ΔF508 cystic fibrosis transmembrane conductance regulator (CFTR) mutation showed that increased biofilm formation by P.aeruginosa was linked to increased availability of iron92. Host defences normally actively sequester iron to limit the growth of infecting bacteria since iron is an essential nutrient. However, Pseudomonas aeruginosa possesses multiple redundant iron receptor and uptake systems such as production of the siderophore pyoverdin (an iron-chelating molecule).However gallium, which is chemically similarto iron , is be taken up by bacteria but not replace its functionality, thus inhibiting the iron-dependent pathways required for cell growth and biofilm formation. This “Trojan horse” strategy interfered with P. aeruginosa growth and iron metabolism, killed planktonic bacteria in an acute mouse pneumonia model and reduced bacterial counts in established biofilms by 3 logs in a chronic biofilm lung infection model93. Gallium was administered via inhalation and importantly uptake was independent of the P. aeruginosa siderophore pyoverdin in vitro. However in vivo it was not clear if gallium may have anti-inflammatory effects other than directly inhibiting biofilm formation. Nonetheless, using iron chelators adjunctively with tobramycin reduced P. aeruginosa in a co-culture model of human bronchial epithelial cells from a patient with cystic fibrosis that carried the CFTR ΔF508 mutation, resulted in a 7-log reduction in viable bacteria and also prevented biofilm formation on these cells94. More recently, the oxidation state of iron was shown to be important95. This study examined mucus from the airways of patients with cystic fibrosis and found that ferrous iron was the primary form of bioavailable iron, which also correlated with the severity of cystic fibrosis lung disease, whereas ferric iron did not. This study highlights the importance of directly investigating the phenotypic state of bacteria in situ in human infections and its potential translational relevance in informing new therapeutic approaches.
Targeting dormant cells in biofilms
Targeting pathways to induce processes such as dispersal requires that cells are metabolically active. However, available evidence also shows that dormant cells or persisters residing within biofilms have a key role for drug tolerance (Box 3). It is therefore attractive to consider antimicrobial approaches that physically or chemically disrupt cells rather than interfering with cellular processes. Non-discriminating oxidizing agents such as hypochlorite and hydrogen peroxide have been used as irrigants in wound96 and endodontic debridement97. However studies reveal that even strong oxidizers such as sodium hypochlorite fail to eradicate biofilms98 probably because long-term exposure is not possible due to cytotoxicity concerns. Broad-spectrum cationic biguanides such as chlorhexidine or quaternary ammonium adhere to cell walls and disrupts cell membranes. However, penetration was limited over the expected timescales used in ex-vivo dental biofilms99 with longer term exposure increasing cytotoxicity, thus making this approach clinically impractical.
Text Box 3. Persistence, resistance and tolerance.
The terms persistence, resistance and tolerance, are often used interchangeably when used to describe the inability of antibiotics (and antimicrobial agents) to inhibit or kill bacteria within a biofilm to the same extent as planktonic cultures 160. Resistance usually has an underlying heritable genetic basis that might be acquired through point mutation or horizontal gene transfer and is defined through standardized MIC and MBC assays. Tolerance is less-well defined and is arguably more appropriately used when antibiotic-susceptible strains (by MIC and MBC) require much higher concentrations to obtain similar log-reductions when growing in the biofilm phenotype. Importantly, tolerance can be lost when biofilms are dispersed into single cells, thus dispersal strategies are normally considered as adjuvants for antimicrobial therapy. However, dispersed planktonic aggregates of cells may still retain tolerance. Persistence (and persistent biofilm) is a term that is loosely used to describe a clinically protracted unabated biofilm infection despite treatment. However, persistence in this sense should not be confused with ‘persisters’ 161 or sub-populations of cells with a distinct dormant phenotype affording them protection against antibiotics, which kill the metabolically active population. Persister cells can occur in both planktonic and biofilm cultures, but the stressful conditions, physical stability and protection from host phagocytes afforded by the biofilm microenvironment appear to contribute to harbouring microbial populations, which grow and repopulate once the antibiotic stress is removed. It is thought that these populations are tolerant of conventional antibiotics because there are no active cellular processes to interrupt. These subpopulations can form spontaneously or be induced from environmental stresses in the biofilm microenvironment162 (see Ref.161 for a Review).
Other exploratory avenues include antibiotics that are used for the treatment of infections caused by slow-growing bacteria. Rifampin, used to treat staphylococcal orthopaedic-implant infections, raises concern about the development of rifampin resistance. However, used in combination with other antibiotics, rifampin and fosfomycin enhanced efficacy in treating foreign body MRSA biofilm infections in vivo100. Likewise, disrupting a cellular target in dormant cells can kill persisters. The acyldepsipeptide antibiotic (ADEP4) can activate the ClpP protease in dormant persister cells in Gram-positive bacteria so the cells effectively ‘digest’ themselves. Although it is an elegant concept to endogenously activating cytoplasmic enzymes for proteolytic degradation in biofilms, it should be noted that ClpP is not an essential enzyme and ClpP-null mutants are not affected by ADEP4. To address this, treatment with both ADEP4 and rifampin showed good efficacy in a chronic biofilm mouse deep abscess-like infection model101 using various S. aureus species. However, this study illustrates that careful consideration needs to be given to antibiotic pairings. Particularly in this case since rifampin resistance occurs at high frequency and so it is usually combined with other active antimicrobials. In the case of ClpP-null mutants ADEP4 would be ineffective and rifampin would in effect be acting as a monotherapy
Antimicrobial peptides (AMP) represent another approach in treating biofilms independent of the presence of microbial activity. An important advantage of AMPs is that they are widely conserved and therefore attractive as broad-acting antimicrobial agents that may be useful against both bacterial and fungal biofilms102,103. Conversely, species-specific targeting is also possible with synthetic AMPs that consist of dual functionally independent moieties (a broad-spectrum AMP with a killing moiety, and a species-specific binding peptide with target specificity). This approach may remove specific pathogens such as S. mutans from oral multispecies biofilm communities to promote a ‘healthy-like microbiome’ as was shown in vitro104. Another advantage is that the pore-forming activity of an AMP targets respiring cells as well as persister and dormant populations, which might reduce the potential for bacteria to develop AMP resistance. Therefore, AMPs have potential as therapeutics to target biofilms. Synthetic peptides that modify specific AMP sequences were designed that showed both inhibitory activity and, when used together with antibiotics, enhanced killing of P. aeruginosa biofilms in invertebrate infection models105. Specific peptides also triggered the degradation of ppGpp, preventing the accumulation of this secondary messenger and abrogating biofilm formation of several G-positive and G-negative pathogens103. However, more pre-clinical efficacy studies are required as AMPs can bind to EPS matrix components and to other host molecules, which reduces their effectiveness, and microbial proteases may further diminish AMP potency106. Additionally, the high cost of AMPs synthesis is a barrier for clinical development and commercialization, although using chloroplast-based technology for large-scale production in automated greenhouses may mitigate costs50. Nevertheless, AMPs can be immobilized onto solid surfaces to enhance efficacy or specificity. This was particularly effective as a polymer-based approach on catheters, as AMP coatings greatly reduced P. aeruginosa adhesion and infection over 7 days in a mouse UTI model107. Furthermore, structurally nanoengineered AMP polymers exhibited potent killing activity against several Gram-negative, colistin-resistant and MDR pathogens, and they exhibited low toxicity and efficacy in an animal model of Acinetobacter baumannii infection108. The recent completion of two Phase II clinical studies of brilacidin (a membrane-acting AMP mimetic) as an intravenous agent for skin infections demonstrate the feasibility of AMPs for systemic therapeutics109.
AMPs can also enhance conventional antimicrobial activity, and the combination with strategies that target the EPS matrix may further increase both the access and permeabilizing properties of AMPs once in the biofilm50,103. Although targeting tolerant cells using AMPs is a promising approach, reaching the target cells that are embedded within a biofilm either topically or systemically and for the compound to be active across a spatially and chemically heterogeneous microenvironment remain important challenges in vivo. The stability and durability of AMP coatings within the body is also an issue that needs to be further addressed, particularly where wear might be expected due to shear caused by moving tissues and fluids as well as proteolytic degradation.
The promise of new technologies
While our understanding of biofilm microenvironments is evolving, technological advances have provided unprecedented avenues to develop multi-targeted therapeutic approaches that prevent and disrupt biofilms or enhance drug efficacy (Fig. 3). Nano- and chemical engineering approaches provide unparalleled flexibility to control the composition, size, shape, surface area and surface chemistry, and functionality of nanostructures that can be used to develop a new generation of modified materials or to coat existing solid surfaces for biofilm prevention. Functionalized nanoparticles, including stimuli-triggered activation, can be designed to enhance penetration and selectively target or release drugs locally after bacterial attachment or within biofilms. In this Review we focus on overall concepts and provide insights into their clinical potential based on recent studies using in vivo models. We have provided a full list of current and prospective technologies and additional references in Supplemental Table S1.
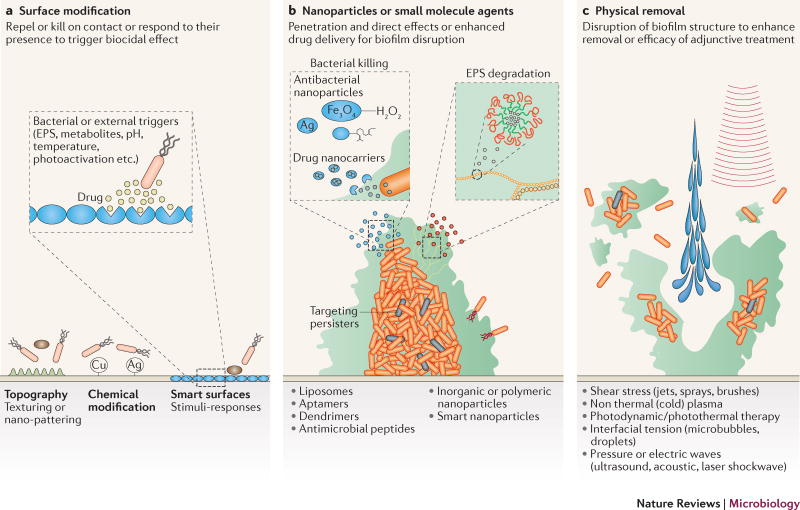
Figure 3. Technological approaches to combat biofilms.
Recent advances in material science and nanotechnology enabled the engineering of a wide array of biofilm-targeting strategies. a) The material and surface properties of medical devices, such as surface charge, hydrophobicity, roughness, topography and chemistry among others, can be modified to prevent bacterial attachment and therefore attenuate or block biofilm formation. Additionally, ‘smart’ or stimuli-triggered responsive surfaces can be constructed that elicit their effect only in response to physical contact with cell-wall or membrane associated adhesins or chemical cues (i.e. secreted EPS, metabolites) of the bacteria. b) Advancement in nanoparticle synthesis has led to the development of diverse approaches to combat biofilms. Inorganic metallic (silver, copper etc.) and organic nanoparticles (liposomes, aptamers etc.), have been increasingly evaluated to improve their anti-biofilm efficacy, as well as their biocompatibility to reduce toxic effects on the host. Nanoparticles can be used to form nanocoatings, be incorporated into materials as composites or fillings or combined together with conventional antimicrobials and other approaches designed to physically disrupt or remove the biofilm. Furthermore, antimicrobial peptides (AMPs) and aptamers also display specific biofilm-targeting properties that can be also used to enhance specificity and efficacy of nanoparticles (hybrid nanoparticles). c) New technologies for physical biofilm removal, including mechanical, energy- and light-based disruption, may further improve biofilm intervention strategies. Given the multifaceted nature of biofilm formation and the complex microbial interactions with the surrounding physical and chemical environment, a combination of these approaches may be required to successfully combat biofilm-mediated disease.
Surface modifications
Surface-tethering or the incorporation of an antibiotic or biocide within a “reservoir” coating has long been studied as a possible approach to inhibit bacterial adhesion and biofilm formation110. However, sustaining efficacy and therefore justifying their progression to wide scale use into the clinic has been challenging. The amount of biocide required to achieve efficacy as well as its chemical composition are often limited by potential deleterious effects, exemplified by silver nanoparticles which were shown to be toxic to rat hosts111. Additionally, antimicrobial reservoir coatings are subject to progressive decreases in efficacy as the active agent is depleted and thus have a limited lifetime of activity. Further, nonspecific absorption of exogenous surfactants and proteins may mask the engineered surface or hinder release.
Advances in material and surface engineering have led to the development of well-defined topographic surface patterns that can control biofilm formation without including antimicrobial agents112. The most well-established ordered topography is the Sharklet™ surface. Inspired by shark skin and its inherent anti-biofouling properties, microscale ribs of various lengths are combined into a repeating diamond micropattern, creating a textured surface that prevents macro and micro biofouling113 as well as bacterial colonisation and biofilm formation when incorporated into the surfaces of medical devices114. Surface modifications are mostly focused on nonspecific protein repulsion and the inhibition of bacterial colonization. This can be challenging due to the structural and physio-chemical diversity of the numerous proteins in biological fluids surrounding a surface in a biomedical setting 115. Hydrophilic polymer brushes or tethered polymers such as poly(ethylene glycol) (PEG) are widely used in the prevention of medical device fouling116. While early bacterial adhesion is attenuated, probably due to the inhibition of an initial protein priming layer, the multifaceted nature of bacterial colonization (often involving non-proteinaceous adhesins) can lead to eventual biofilm formation117. Further studies with ‘super-hydrophilic’ (super-wet) or ‘super-hydrophobic’ surfaces have decreased protein deposition and bacterial attachment of clinically relevant surfaces115,118.
The incorporation of these materials into medical devices shows promising, but variable results. While recently greater sustainability of super-hydrophobic surfaces upon mechanical abrasion has been demonstrated119, the antibiofilm effects of these surfaces are often transient or subject to species bias120. Short to medium-term biofilm suppression may be sufficient however to permit effective immune and prophylactic responses and tissue integration of a foreign body.
The development of functionalized medical implant surfaces with a vast array of antimicrobial and antibiofilm properties has been intensely studied, particularly with respect to titanium implants. To use these surfaces in biomedical applications, modern surface design has been largely driven by top-down methods such as lithography, imprinting and others121 to produce a vast array of antibacterial coatings, including but not limited to, silver, copper, titanium dioxide and chitosan. Furthermore, emerging bottom-up approaches using nanomaterials as ‘building blocks’122 and surface attachment and immobilization of biomolecules, including antimicrobial peptides or proteins and polysaccharides123,124, have also resulted in the development of antibacterial surface coatings. Moreover, the unexpected discovery that certain bacterial polysaccharides can inhibit biofilm formation124, has led to the development of strategies to counter biofilm formation‥ Hyaluronic acid (which is one of the most studied polysaccharides) reduced the adhesion of S. aureus to hyaluronic acid-coated titanium surfaces125 and poly(methyl methacrylate) intraocular lenses126.
Bottom-up surface-assemblies can also be combined with top-down surface processing127 to generate nanocoatings with biofilm-targeting properties and biocompatibility128. Recent in vivo studies demonstrated the feasibility and efficacy of tunable multi-layer nanocoatings that released different combinations of antibiotics129 or sequential delivery of gentamicin and an osteoinductive growth factor130 in a time-staggered manner for prevention of biofilm-associated infection and bone tissue repair around implants. Both studies demonstrated the ability to prevent biofilm formation on the device surface, relative to uncoated controls, with the nanocoatings able to clear infiltrating bacteria and prevented colonization of the implant while promoting bone formation and osseointegration. Importantly, biocompatibility, as well as long-term host retention and release kinetics were also demonstrated, thus providing promise for their more wide-spread application in orthopaedics.
Efforts to engineer surfaces with even more control over the specificity and sensitivity of their antibacterial or antibiofilm capabilities have led to so-called smart surfaces, which are also known as stimuli-responsive or triggered biofilm-targeting surfaces. Triggers, including pH, temperature, salt concentration, metabolites, electrical currents and photoactivation, induce topographical and chemical changes in the surface area as well as generating heat or induce drug release to kill or repel bacterial attachment (Supplemental Table S1). The design principles for controlling bacterial adhesion or biofilm removal mechanisms that may be triggered on demand are intriguing. However, the effectiveness of these approaches has been evaluated largely in vitro. Consequently, as with all surface modifications, whether functionality will remain in vivo upon binding of endogenous host proteins in saliva, blood, synovial fluid and urine is unclear. Another consideration is that bacteria that associate with the surface may be killed and remain attached, thus masking the underlying technology and even provide a nutrient source for other bacteria. Therefore, it is important to not only have a killing effect but also a ‘self-cleaning’ mechanism, possibly facilitated by mechanical shear of surrounding body fluids or tissues. Furthermore, challenges to enhance mechanochemical stability and overcome coating deterioration and dissolution, and non-adverse host reactions to the coating itself will need to be addressed in future studies to facilitate clinical application131–133.
Nanoparticles
Nanoparticles are versatile and bioactive and they are becoming increasingly popular as an biofilm-targeting approach. Nanoparticles with intrinsic antimicrobial activity, primarily inorganic materials such as silver, can act as biofilm-targeting agents or as nanocoatings (as described above). Due to their flexible chemical structures, they can also function as drug delivery vehicles (nanocarriers) with organic nanoparticles accounting for over two-thirds of the systems approved for use in humans134. Furthermore, both inorganic and organic nanoparticles can be combined or modified by adding molecules (hybrid nanoparticles) to enhance their biological properties or provide multifunctionality. As excellent in-depth reviews on the principles and current applications of nanoparticles, particularly silver, are available13,135, we focus on clinically used liposomal nanoparticles for drug delivery and emerging technologies, including stimuli-triggered activation, that have shown efficacy in vivo.
Liposomes are physiologically compatible vesicles that are composed of one or more phospholipid bilayers, and they represent one of the most widely developed organic nanoparticles for drug delivery . They are able to penetrate the biofilm well, are biocompatible and show efficacy against biofilms of a wide variety of bacterial species for a diverse number of antibiotics136,137. These nanocarriers can protect the antimicrobial agent from deleterious interactions with the matrix, or enzymatic inactivation and degradation at the infection site by other bacterial and host components. The lipid structure can also fuse with the bacterial outer membrane releasing the drug directly into the cell, thereby potentially maximizing therapeutic effects while reducing host cytotoxicity137. Furthermore, liposomes can carry more than one drug by co-encapsulation and can be also functionalized by linking biomolecules (for example, peptides, pH-responsive polymers) on the nanoparticle surface to increase targeting specificity and triggered release. Importantly, however, some studies have reported a reduced efficacy of liposomal-encapsulated antimicrobials dependent on the environment in which the biofilm resides; for example: host- and microorganism-derived substances such as mucus and alginate could inhibit bacteria-liposome interactions138. Nevertheless, several formulations are currently in preclinical studies and clinical trials, and some are commercially available 139. For example, liposomal ciprofloxacin and amikacin have shown promise in the management of chronic lung infection in cystic fibrosis140,141. The potential of liposomes to function as delivery agents for other antimicrobials, such as NO, has demonstrated significantly reduced S. aureus biofilm mass compared to controls in a sheep model of chronic rhinosinusitis142. Whilst this study did not note any negative clinical symptoms, a transient increase in heart rate and decrease in mean arterial pressure was observed in the animals which require further investigation before this strategy can advance into human trials.
Nanoparticles with multi-functionality or on-demand activation upon specific stimuli similar to smart surfaces represent the most widely developed class of nanoparticles currently under development (Supplemental Table S1). Recent studies with inorganic nanoparticles such as iron oxide (Fe3O4) with a peroxidase-like function catalyzed hydrogen peroxide (H2O2) at concentrations ranging from 0.1–1% H2O2 in a dose- and pH-dependent manner, and showed potent effects against virulent oral biofilms in vivo143. Under acidic (pathological) conditions, nanoparticles activated the generation of free radicals from H2O2 in situ, which induced the degradation of the biofilm matrix and rapid killing of the embedded bacteria (>5-log reduction of viable cells compared to control cells within 5 min, and 5000-fold more effective than 1% H2O2 alone)144. Daily topical treatments effectively reduced the onset and severity of dental caries (tooth decay), preventing cavitation altogether in a rodent model of the disease. The pH-dependent functionality prevents catalytic reaction at physiological pH and unmitigated free-radical production, thus improving biocompatibility.
Stimuli-triggered mechanisms by nanoparticles can also enhance the selectivity of drug activation or delivery to cells within a biofilm, protecting host tissues and the commensal microbiota while targeting infective agents within pathological microniches143,145,146. Delivery of the antibacterial agent farnesol via acidic pH-triggered polymeric nanoparticles enhanced its biofilm-targeting activity 4-fold (compared to farnesol alone); thus, the delivery system greatly improved the drug efficacy against an oral biofilm infection in vivo following topical treatment146. These water-soluble polymeric nanocarriers can encapsulate hydrophobic and apolar drugs into aqueous solution, which is a crucial issue in product development. Similarly, nanoparticles that are conjugated with a pH-responsive element145 or pH-sensitive surface charge switching147 were developed to increase biofilm penetration and selective bacterial binding for targeted delivery and antibacterial activity in acidic conditions.
Another exciting area of development is in increasing the specificity of the nanoparticles by selectively targeting biofilm matrix constituents or through the introduction of bacteria-specific ligands, to improve both efficacy and biocompatibility. Nanoparticles functionalized with biofilm EPS matrix-digesting enzymes (DNase) and loaded with ciprofloxacin eradicated established P. aeruginosa biofilms without cytotoxicity against macrophages49. Likewise, tobramycin alginate-chitosan nanoparticles functionalised by conjugation to dornase alfa (DNase) demonstrated better biofilm penetration and DNA degradation in sputum from patient with cystic fibrosis and increased protection against bacteria in an invertebrate infection model148. Furthermore, nanoparticles that were designed to release and activate NO in situ had antifungal and antibacterial effects, inhibited biofilm formation and promoted the degradation of the EPS matrix in vitro and in vivo149,150. Recently, reports showed that linking antimicrobial peptides102 or aptamers151 to nanoparticle surfaces enhanced their killing efficacy, specificity or functionality.
Overall, nanoparticles offer a promising therapeutic platform for the development of new effective biofilm-targeting approaches. However, whilst the development of novel nanoparticles has continued apace, there is a continually widening gap between the number of new formulations under laboratorial investigation and those in clinical use. Further advances in this field should focus on enhancing in vivo efficacy (compared to current treatment modalities) and biocompatibility, and on understanding the potential toxicity and the metabolism of nanoparticles in the body. Affordable large scale manufacturing would be also required for product development to the healthcare market. Nevertheless, the availability of previously FDA-approved nanoparticles demonstrates their potential for more wide-spread future clinical use.
Future directions
The initiation of a biofilm involves complex and dynamic interactions among the surface, the microorganism and the EPS. Upon biofilm establishment, the adhesive strength and viscoelastic properties make the removal of a biofilm from surfaces difficult, and resident microorganisms become tolerant to antimicrobials. Although tolerance is a common feature of biofilms, the mechanisms underlying tolerance as a microbial survival strategy are multifaceted. Likewise a reciprocal multifaceted approach to control biofilms is far more likely to achieve clinical success than a futile search for a magic bullet (Box 2). Understanding the complexity of biofilm biology highlights the role of complementary strategies that target both the microorganisms and the surrounding EPS matrix to either prevent the initiation of a biofilm or to disrupt existing biofilms. The challenge of using antimicrobials alone, which may kill microorganisms, but leave behind biodegradable substrates for microbial reutilization, must be addressed. Thus, eliminating existing biofilms may require simultaneous degradation of the protective EPS matrix, and targeting and killing both resident microorganisms and dispersed cells. The complexity of polymicrobial interactions (synergistic, cooperative or antagonistic), spatial organization and community behaviour with host immunity factors further reinforces the need of a combinatorial therapy. Rapid advances in drug discovery methods should accelerate the identification of EPS-inhibitors, inducers of biofilm dispersal and agents that target dormant cells, as well as combinations thereof with host immunomodulation therapies152,153. However, further validation of proof-of-concept studies using clinically relevant animal models as well as clinical trials are needed for rigorous evaluation. Bacterial co-cultures with primary human cells to evaluate host-microbial response can be valuable to investigate, for example, the role of genetic mutations such as found in patients with cystic fibrosis or PCD on the establishment and treatment of biofilms in patients suffering from these diseases. Opportunities to create physico-chemical and biological structures, including organ-on-chip, within microfluidic devices or using 3D printed tissues may also help assess treatment efficacy by mimicking in vivo-like environments.
Furthermore, new technologies , including ‘smart-release’ or ‘on-demand activation’ of bioactive agents when triggered by pathogenic microenvironments (e.g. acidic pH or hypoxia), have been developed for enhanced selectivity and controlled in situ drug delivery. However, the vast majority of the studies were conducted in vitro using non-clinically relevant models or treatment regimens, with many failing to progress to in vivo studies and even fewer to clinical application. The complexity of the host microbiota, where commensals co-exist with potential pathogens, provide a great challenge in developing antimicrobial agents against a particular microbial species. The presence of biological fluids that change surface chemistries poses yet another challenge. The ability of a drug to penetrate existing biofilms should be also considered, as this feature affects both potential cytotoxicity and antibacterial efficacy, and the potential for de novo emergence of antimicrobial resistance (owing to bacteria being subjected to sub-lethal antibiotic concentrations). Antibodies, aptamers or peptides that are linked to nanoparticles greatly enhance specificity, although higher costs and additional chemistry to produce multi-component structures may be limiting factors. A key approach may be to trigger antimicrobial activity in response to pathogenic microenvironments (for example, acidic pH, hypoxia or pathogen-derived metabolites). Thus, the biological effects can be tuned to specifically target the biofilm microenvironment, degrade the matrix and kill resident bacteria, thereby eradicating the pathogenic niche with precision and minimal cytotoxicity to surrounding tissues. Nevertheless, we noted a discrepancy between the research efforts on new technologies and commercialization. A concerted effort of chemists, engineers and biomedical researchers combined with toxicology and safety studies will help clinicians to assess the efficacy of these new technologies in clinical trials. However, the successful translation into the clinic is not just dependent on efficacy of the technology, but also on regulatory agencies and industry efforts to bring it to the market. Future directions should focus on achieving maximal efficacy and specificity with minimal toxicity and long-term therapeutic effects along with industry partnerships to develop low-cost and practical formulations for clinical use.
Supplementary Material
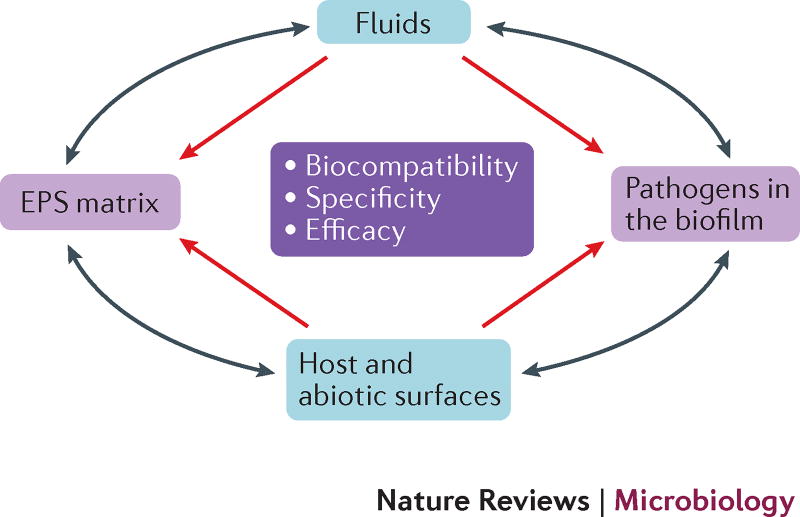
Figure 4. Multi-targeting approach to combat biofilms.
The physical and biological complexity of biofilms and tolerance to antimicrobials render them less susceptible to conventional therapeutic approaches. Biofilm targets include microbial cells (often polymicrobial communities) and the EPS matrix, and therapeutics can be delivered from the overlying surrounding biological fluid as well as the surfaces of the medical devices themselves. We envision exogenous approaches (such as adhesion-targeting materials and coatings, and adhesin-blocking agents) to complement or synergize with endogenous activation (such as immunity modulation) to prevent microbial attachment to host or abiotic surfaces in patients. Likewise, a combination of approaches that degrade the protective matrix, activate dispersal, and target the resident pathogens, persisters and dispersed cells without affecting commensals may be required to eliminate existing biofilms. Long-term effects of modified surfaces in the presence of biological fluids as well as enhanced drug penetration properties and a decrease in toxicity or allergic reactions are required for in vivo efficacy. These combined with clinically relevant treatment regimen (either topical or systemic) and long-term effect assessment should help successfully translate the hypothetical concepts into the clinic. The grey arrows indicate that biofilm bacteria and EPS can move or interact between the surface and fluid phases.
Acknowledgments
Work in the authors laboratory is supported in part by the National Institute for Dental and Craniofacial Research grants DE018023, DE025220 and DE025848 (HK); The Ohio State University Infectious Disease Discovery Theme- Public Health Preparedness for Infectious Disease Transdisciplinary Team Grant (PS).
P.S. has received research funding from and/or has consulted from Philips Oral Healthcare, Smith & Nephew, Biocomposites Ltd., Zimmer-Biomet, Colgate-Palmolive. H.K. has received funding from Johnson&Johnson, Colgate-Palmolive and DENTSPLY. RPH has consulted for Biocomposites Ltd.
Glossary
- Lock therapy
An approach whereby a high concentration of antibiotics are injected into the catheter lumen for an extended period to eradicate bacteria. Catheter locks have been used to treat sepsis since the 1980s; however with the understanding that infecting microorganisms are present as biofilms on medical device materials, this approach is now specifically tailored to improve efficacy
- EPS
The EPS can contain exopolysaccharides, fibrous and globular proteins (including extracellular enzymes), lipids and nucleic acids (eDNA). Those components form a matrix that can be surface-associated or secreted locally or deposited on abiotic and biotic surfaces. The EPS-matrix acts as a ‘multifunctional scaffold’ that supports and protects embedded bacteria
- Nitric oxide
Nitric oxide (NO) is a ubiquitous signalling molecule found in both prokaryotic and eukaryotic systems. NO is toxic in the mM range, but in the pM and nM range it can be used to form oxidative and nitrosative reactive species that interact with proteins, DNA and metabolic enzymes. As NO is labile, the optimal concentration to disperse biofilms is difficult to measure; however NO microelectrodes are highly sensitive and offer excellent spatial and temporal resolution in tissues or body fluids
- Antimicrobial peptides
A subset of host defence peptides with antibiotic activity. Peptides such as LL-37 (cathelicidin) and human β-defensins are rapidly-acting, small-molecule effectors as part of the innate immune response of the host
- Topographic surface patterns
Patterns include protruding squares, cone-shapes, wrinkle and ridge-like patterning or nanopores that disrupt bacterial adhesion
- Super-hydrophobic surfaces
Surfaces that maintain air at the solid-liquid interface when hydrated. This leads to improved functionality via water repellency or reduced drag
- Smart surfaces
Smart surfaces elicit their effect only upon contact with certain physiological or physiochemical cues to provide targeted application, thus increasing therapeutic precision and reducing the risk of cytotoxicity
- Nanoparticles
Structures with a size range between 1–1000 nm. They can be classified as organic or inorganic and can exhibit antibacterial properties or can be used as drug delivery systems
- Adhesins
Bacterial or fungal surface-associated determinants that mediate adherence to living cells or attachment to abiotic surfaces and can promote virulence
- Antimicrobial chemotherapy
The clinical treatment of microbial infections with antimicrobial agents
- Mannosides
A mannose glycoside consisting of a carbohydrate bound to the hydroxyl group of another compound by O-, N-, S- or C-glycosidic bonds, each with different susceptibilities to hydrolysis
- Curli
A class of bacterial amyloid (aggregates of proteins that form insoluble fibres) produced by many Enterobacteriaceae and a major component of the extracellular matrix, promoting surface adhesion, cell aggregation, and biofilm formation
- Type-I pili
Filamentous surface structures possessing a FimH adhesin at the pilus tip, mediating adherence to host cells and uropathogenic E. coli invasion of bladder epithelial cells
- Cystic fibrosis transmembrane conductance regulator
(CFTR). A transmembrane protein and ion transport channel that regulates epithelial fluid homeostasis central to airway mucociliary clearance and defence against inhaled pathogens
- Biguanides
Class of organic compounds (C2H7N5) used as oral antihyperglycemic drugs. Derivatives of this compound with bactericidal activity are commonly used as antiseptic and disinfecting agents such as chlorhexidine
- Surfactants
Compounds that lower the surface tension between liquids and solids. Surfactants are used as cleaning detergents and some biofilm bacteria produce their own surfactants in order to disperse from a surface
- Biofouling
The unwanted accumulation of micro and macro-organisms on surfaces. Microbial biofilms are often considered ‘biofouling’, particularly in the context of industrial surfaces;
Footnotes
Author contributions
Hyun Koo. Contribution of writing and editing to all sections and figures. Preparation of Fig. 4.
Raymond N Allan. Contribution of writing and editing to all sections and figures. Preparation of first draft of Fig. 2.
Robert P Howlin. Contribution of writing and editing to all sections and figures. Preparation of first draft of Fig. 3.
Luanne Hall-Stoodley. Conceptualized the original outline of the article. Contribution of writing and editing to all sections and figures.
Paul Stoodley. Conceptualized the original outline of the article. Preparation of first draft of Fig. 1. Contribution of writing and editing to all sections and figures.
Competing Interests Statement
The authors declare competing interests.
References
- 1.Flemming HC, et al. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 2016;14:563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 2.Stacy A, McNally L, Darch SE, Brown SP, Whiteley M. The biogeography of polymicrobial infection. Nat Rev Microbiol. 2016;14:93–105. doi: 10.1038/nrmicro.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magin CM, Cooper SP, Brennan AB. Non-toxic antifouling strategies. Materials Today. 2010;13:36–44. [Google Scholar]
- 4.Hobley L, Harkins C, MacPhee CE, Stanley-Wall NR. Giving structure to the biofilm matrix: an overview of individual strategies and emerging common themes. FEMS Microbiol Rev. 2015;39:649–669. doi: 10.1093/femsre/fuv015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson BW, et al. Viscoelasticity of biofilms and their recalcitrance to mechanical and chemical challenges. FEMS Microbiol Rev. 2015;39:234–245. doi: 10.1093/femsre/fuu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Acker H, Van Dijck P, Coenye T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 2014;22:326–333. doi: 10.1016/j.tim.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Lebeaux D, Ghigo J-M, Beloin C. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiology and Molecular Biology Reviews. 2014;78:510–543. doi: 10.1128/MMBR.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koo H, Yamada KM. Dynamic cell-matrix interactions modulate microbial biofilm and tissue 3D microenvironments. Curr Opin Cell Biol. 2016;42:102–112. doi: 10.1016/j.ceb.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoiby N, et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect. 2015;21(Suppl 1):S1–25. doi: 10.1016/j.cmi.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 10.Velkov T, Roberts KD, Li J. Rediscovering the octapeptins. Nat Prod Rep. 2017;34:295–309. doi: 10.1039/c6np00113k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raad I, et al. Successful Salvage of Central Venous Catheters in Patients with Catheter-Related or Central Line-Associated Bloodstream Infections by Using a Catheter Lock Solution Consisting of Minocycline, EDTA, and 25% Ethanol. Antimicrob Agents Chemother. 2016;60:3426–3432. doi: 10.1128/AAC.02565-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mistry S, et al. A novel, multi-barrier, drug eluting calcium sulfate/biphasic calcium phosphate biodegradable composite bone cement for treatment of experimental MRSA osteomyelitis in rabbit model. Journal of Controlled Release. 2016;239:169–181. doi: 10.1016/j.jconrel.2016.08.014. doi: http://dx.doi.org/10.1016/j.jconrel.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Micro. 2013;11:371–384. doi: 10.1038/nrmicro3028. http://www.nature.com/nrmicro/journal/v11/n6/abs/nrmicro3028.html-supplementary-information. [DOI] [PubMed] [Google Scholar]
- 14.Castaneda P, McLaren A, Tavaziva G, Overstreet D. Biofilm Antimicrobial Susceptibility Increases With Antimicrobial Exposure Time. Clin Orthop Relat Res. 2016;474:1659–1664. doi: 10.1007/s11999-016-4700-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howlin RP, et al. Antibiotic-loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections. Antimicrob Agents Chemother. 2015;59:111–120. doi: 10.1128/AAC.03676-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Besinis A, De Peralta T, Tredwin CJ, Handy RD. Review of nanomaterials in dentistry: interactions with the oral microenvironment, clinical applications, hazards, and benefits. ACS nano. 2015;9:2255–2289. doi: 10.1021/nn505015e. [DOI] [PubMed] [Google Scholar]
- 17.Fabbri S, et al. Streptococcus mutans biofilm transient viscoelastic fluid behaviour during high-velocity microsprays. J Mech Behav Biomed Mater. 2016;59:197–206. doi: 10.1016/j.jmbbm.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Urish KL, DeMuth PW, Craft DW, Haider H, Davis CM., 3rd Pulse lavage is inadequate at removal of biofilm from the surface of total knee arthroplasty materials. J Arthroplasty. 2014;29:1128–1132. doi: 10.1016/j.arth.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Flemming H-C, Wingender J. The biofilm matrix. Nature Reviews Microbiology. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 20.Gunn JS, Bakaletz LO, Wozniak DJ. What’s on the Outside Matters: The Role of the Extracellular Polymeric Substance of Gram-negative Biofilms in Evading Host Immunity and as a Target for Therapeutic Intervention. J Biol Chem. 2016;291:12538–12546. doi: 10.1074/jbc.R115.707547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng X, Zhang Y, Bai G, Zhou X, Wu H. Cyclic di-AMP mediates biofilm formation. Molecular microbiology. 2016 doi: 10.1111/mmi.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mann EE, Wozniak DJ. Pseudomonas biofilm matrix composition and niche biology. FEMS Microbiol Rev. 2012;36:893–916. doi: 10.1111/j.1574-6976.2011.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teschler JK, et al. Living in the matrix: assembly and control of Vibrio cholerae biofilms. Nat Rev Microbiol. 2015;13:255–268. doi: 10.1038/nrmicro3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernicola S, et al. In Silico Discovery and In Vitro Validation of Catechol-Containing Sulfonohydrazide Compounds as Potent Inhibitors of the Diguanylate Cyclase PleD. J Bacteriol. 2015;198:147–156. doi: 10.1128/jb.00742-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambanthamoorthy K, et al. Identification of small molecules that antagonize diguanylate cyclase enzymes to inhibit biofilm formation. Antimicrob Agents Chemother. 2012;56:5202–5211. doi: 10.1128/aac.01396-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falsetta ML, et al. Novel antibiofilm chemotherapy targets exopolysaccharide synthesis and stress tolerance in Streptococcus mutans to modulate virulence expression in vivo. Antimicrob Agents Chemother. 2012;56:6201–6211. doi: 10.1128/aac.01381-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren Z, et al. Molecule Targeting Glucosyltransferase Inhibits Streptococcus mutans Biofilm Formation and Virulence. Antimicrob Agents Chemother. 2015;60:126–135. doi: 10.1128/aac.00919-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Totsika M, et al. A FimH inhibitor prevents acute bladder infection and treats chronic cystitis caused by multidrug-resistant uropathogenic Escherichia coli ST131. J Infect Dis. 2013;208:921–928. doi: 10.1093/infdis/jit245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nett JE, Cabezas-Olcoz J, Marchillo K, Mosher DF, Andes DR. Targeting Fibronectin To Disrupt In Vivo Candida albicans Biofilms. Antimicrob Agents Chemother. 2016;60:3152–3155. doi: 10.1128/aac.03094-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guiton PS, et al. Combinatorial small-molecule therapy prevents uropathogenic Escherichia coli catheter-associated urinary tract infections in mice. Antimicrob Agents Chemother. 2012;56:4738–4745. doi: 10.1128/aac.00447-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spaulding CN, et al. Selective depletion of uropathogenic E. coli from the gut by a FimH antagonist. Nature. 2017;546:528–532. doi: 10.1038/nature22972. http://www.nature.com/nature/journal/v546/n7659/abs/nature22972.html-supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mydock-McGrane L, et al. Antivirulence C-Mannosides as Antibiotic-Sparing, Oral Therapeutics for Urinary Tract Infections. J Med Chem. 2016;59:9390–9408. doi: 10.1021/acs.jmedchem.6b00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cegelski L, et al. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nat Chem Biol. 2009;5:913–919. doi: 10.1038/nchembio.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cozens D, Read RC. Anti-adhesion methods as novel therapeutics for bacterial infections. Expert Rev Anti Infect Ther. 2012;10:1457–1468. doi: 10.1586/eri.12.145. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan JB. Biofilm matrix-degrading enzymes. Methods Mol Biol. 2014;1147:203–213. doi: 10.1007/978-1-4939-0467-9_14. [DOI] [PubMed] [Google Scholar]
- 36.Pleszczynska M, Wiater A, Janczarek M, Szczodrak J. (1-->3)-alpha-D-Glucan hydrolases in dental biofilm prevention and control: A review. International journal of biological macromolecules. 2015;79:761–778. doi: 10.1016/j.ijbiomac.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 37.Fleming D, Chahin L, Rumbaugh K. Glycoside Hydrolases Degrade Polymicrobial Bacterial Biofilms in Wounds. Antimicrob Agents Chemother. 2016 doi: 10.1128/AAC.01998-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwase T, et al. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature. 2010;465:346–349. doi: 10.1038/nature09074. [DOI] [PubMed] [Google Scholar]
- 39.Schmelcher M, et al. Evolutionarily distinct bacteriophage endolysins featuring conserved peptidoglycan cleavage sites protect mice from MRSA infection. J Antimicrob Chemother. 2015;70:1453–1465. doi: 10.1093/jac/dku552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Becker SC, et al. Triple-acting Lytic Enzyme Treatment of Drug-Resistant and Intracellular Staphylococcus aureus. Sci Rep. 2016;6:25063. doi: 10.1038/srep25063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baker P, et al. Exopolysaccharide biosynthetic glycoside hydrolases can be utilized to disrupt and prevent Pseudomonas aeruginosa biofilms. Science advances. 2016;2:e1501632. doi: 10.1126/sciadv.1501632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okshevsky M, Regina VR, Meyer RL. Extracellular DNA as a target for biofilm control. Curr Opin Biotechnol. 2015;33:73–80. doi: 10.1016/j.copbio.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Kaplan JB, et al. Low levels of beta-lactam antibiotics induce extracellular DNA release and biofilm formation in Staphylococcus aureus. MBio. 2012;3:e00198–00112. doi: 10.1128/mBio.00198-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hymes SR, Randis TM, Sun TY, Ratner AJ. DNase inhibits Gardnerella vaginalis biofilms in vitro and in vivo. J Infect Dis. 2013;207:1491–1497. doi: 10.1093/infdis/jit047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manzenreiter R, et al. Ultrastructural characterization of cystic fibrosis sputum using atomic force and scanning electron microscopy. J Cyst Fibros. 2012;11:84–92. doi: 10.1016/j.jcf.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Konstan MW, Ratjen F. Effect of dornase alfa on inflammation and lung function: potential role in the early treatment of cystic fibrosis. J Cyst Fibros. 2012;11:78–83. doi: 10.1016/j.jcf.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thornton RB, et al. Neutrophil extracellular traps and bacterial biofilms in middle ear effusion of children with recurrent acute otitis media--a potential treatment target. PLoS One. 2013;8:e53837. doi: 10.1371/journal.pone.0053837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Institute WATK. Dissolving the glue in glue ear. 2017 < https://www.telethonkids.org.au/our-research/early-environment/infection-and-vaccines/vaccine-trials-group/dissolving-the-glue-in-glue-ear/>.
- 49.Baelo A, et al. Disassembling bacterial extracellular matrix with DNase-coated nanoparticles to enhance antibiotic delivery in biofilm infections. J Control Release. 2015;209:150–158. doi: 10.1016/j.jconrel.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 50.Liu Y, et al. Topical delivery of low-cost protein drug candidates made in chloroplasts for biofilm disruption and uptake by oral epithelial cells. Biomaterials. 2016;105:156–166. doi: 10.1016/j.biomaterials.2016.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhattacharya M, Wozniak DJ, Stoodley P, Hall-Stoodley L. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev Anti Infect Ther. 2015;13:1499–1516. doi: 10.1586/14787210.2015.1100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DiGiandomenico A, et al. Identification of broadly protective human antibodies to Pseudomonas aeruginosa exopolysaccharide Psl by phenotypic screening. J Exp Med. 2012;209:1273–1287. doi: 10.1084/jem.20120033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flores-Mireles AL, Pinkner JS, Caparon MG, Hultgren SJ. EbpA vaccine antibodies block binding of Enterococcus faecalis to fibrinogen to prevent catheter-associated bladder infection in mice. Sci Transl Med. 2014;6:254ra127. doi: 10.1126/scitranslmed.3009384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brady RA, et al. Resolution of Staphylococcus aureus biofilm infection using vaccination and antibiotic treatment. Infect Immun. 2011;79:1797–1803. doi: 10.1128/iai.00451-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goodman SD, et al. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoid-associated proteins. Mucosal immunology. 2011;4:625–637. doi: 10.1038/mi.2011.27. [DOI] [PubMed] [Google Scholar]
- 56.Rocco CJ, Davey ME, Bakaletz LO, Goodman SD. Natural antigenic differences in the functionally equivalent extracellular DNABII proteins of bacterial biofilms provide a means for targeted biofilm therapeutics. Mol Oral Microbiol. 2016 doi: 10.1111/omi.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Devaraj A, Justice SS, Bakaletz LO, Goodman SD. DNABII proteins play a central role in UPEC biofilm structure. Mol Microbiol. 2015;96:1119–1135. doi: 10.1111/mmi.12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Novotny LA, Jurcisek JA, Goodman SD, Bakaletz LO. Monoclonal antibodies against DNA-binding tips of DNABII proteins disrupt biofilms in vitro and induce bacterial clearance in vivo. EBioMedicine. 2016;10:33–44. doi: 10.1016/j.ebiom.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Estelles A, et al. A High-Affinity Native Human Antibody Disrupts Biofilm from Staphylococcus aureus Bacteria and Potentiates Antibiotic Efficacy in a Mouse Implant Infection Model. Antimicrob Agents Chemother. 2016;60:2292–2301. doi: 10.1128/aac.02588-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Freire MO, et al. A bacterial-biofilm-induced oral osteolytic infection can be successfully treated by immuno-targeting an extracellular nucleoid-associated protein. Mol Oral Microbiol. 2017;32:74–88. doi: 10.1111/omi.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Novotny LA, et al. Antibodies against the majority subunit of type IV Pili disperse nontypeable Haemophilus influenzae biofilms in a LuxS-dependent manner and confer therapeutic resolution of experimental otitis media. Mol Microbiol. 2015;96:276–292. doi: 10.1111/mmi.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McDougald D, Rice SA, Barraud N, Steinberg PD, Kjelleberg S. Should we stay or should we go: mechanisms and ecological consequences for biofilm dispersal. Nat Rev Microbiol. 2011;10:39–50. doi: 10.1038/nrmicro2695. [DOI] [PubMed] [Google Scholar]
- 63.Romling U, Balsalobre C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J Intern Med. 2012;272:541–561. doi: 10.1111/joim.12004. [DOI] [PubMed] [Google Scholar]
- 64.Christensen LD, et al. Clearance of Pseudomonas aeruginosa foreign-body biofilm infections through reduction of the cyclic Di-GMP level in the bacteria. Infect Immun. 2013;81:2705–2713. doi: 10.1128/iai.00332-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burdette DL, et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barraud N, et al. Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-GMP levels, and enhanced dispersal. J Bacteriol. 2009;191:7333–7342. doi: 10.1128/JB.00975-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barraud N, Kelso MJ, Rice SA, Kjelleberg S. Nitric oxide: a key mediator of biofilm dispersal with applications in infectious diseases. Curr Pharm Des. 2015;21:31–42. doi: 10.2174/1381612820666140905112822. [DOI] [PubMed] [Google Scholar]
- 68.Howlin RP, et al. Low dose nitric oxide as targeted anti-biofilm adjunctive therapy to treat chronic Pseudomonas aeruginosa infection in cystic fibrosis. Molecular Therapy. 2017 doi: 10.1016/j.ymthe.2017.06.021. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deppisch C, et al. Gaseous nitric oxide to treat antibiotic resistant bacterial and fungal lung infections in patients with cystic fibrosis: a phase I clinical study. Infection. 2016;44:513–520. doi: 10.1007/s15010-016-0879-x. [DOI] [PubMed] [Google Scholar]
- 70.Novoteris L. Efficacy and Safety of Inhaled Nitric Oxide (NO) in Cystic Fibrosis (CF) Patients. < https://clinicaltrials.gov/ct2/show/NCT02498535> (
- 71.Barraud N, et al. Cephalosporin-3’-diazeniumdiolates: targeted NO-donor prodrugs for dispersing bacterial biofilms. Angew Chem Int Ed Engl. 2012;51:9057–9060. doi: 10.1002/anie.201202414. [DOI] [PubMed] [Google Scholar]
- 72.Collins SA, et al. Cephalosporin-3’ -diazeniumdiolate NO-donor prodrug PYRRO-C3D enhances azithromycin susceptibility of Non-typeable Haemophilus influenzae biofilms. Antimicrob Agents Chemother. 2016 doi: 10.1128/AAC.02086-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walker WT, et al. Primary Ciliary Dyskinesia Ciliated Airway Cells Show Increased Susceptibility to Haemophilus influenzae Biofilm Formation. Eur Respir J. 2017 doi: 10.1183/13993003.00612-2017. In Press. [DOI] [PubMed] [Google Scholar]
- 74.de la Fuente-Nunez C, Reffuveille F, Fairfull-Smith KE, Hancock RE. Effect of nitroxides on swarming motility and biofilm formation, multicellular behaviors in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2013;57:4877–4881. doi: 10.1128/AAC.01381-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reffuveille F, Fuente-Nunez Cde L, Fairfull-Smith KE, Hancock RE. Potentiation of ciprofloxacin action against Gram-negative bacterial biofilms by a nitroxide. Pathog Dis. 2015;73 doi: 10.1093/femspd/ftv016. [DOI] [PubMed] [Google Scholar]
- 76.Verderosa AD, Mansour SC, de la Fuente-Nunez C, Hancock RE, Fairfull-Smith KE. Synthesis and Evaluation of Ciprofloxacin-Nitroxide Conjugates as Anti-Biofilm Agents. Molecules. 2016;21 doi: 10.3390/molecules21070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kutty SK, et al. Design, synthesis, and evaluation of fimbrolide-nitric oxide donor hybrids as antimicrobial agents. J Med Chem. 2013;56:9517–9529. doi: 10.1021/jm400951f. [DOI] [PubMed] [Google Scholar]
- 78.Anderson JK, et al. Chemorepulsion from the Quorum Signal Autoinducer-2 Promotes Helicobacter pylori Biofilm Dispersal. MBio. 2015;6:e00379. doi: 10.1128/mBio.00379-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lauderdale KJ, Malone CL, Boles BR, Morcuende J, Horswill AR. Biofilm dispersal of community-associated methicillin-resistant Staphylococcus aureus on orthopedic implant material. J Orthop Res. 2010;28:55–61. doi: 10.1002/jor.20943. [DOI] [PubMed] [Google Scholar]
- 80.Simonetti O, et al. RNAIII-inhibiting peptide enhances healing of wounds infected with methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2008;52:2205–2211. doi: 10.1128/AAC.01340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Starkey M, et al. Identification of Anti-virulence Compounds That Disrupt Quorum-Sensing Regulated Acute and Persistent Pathogenicity. PLoS Pathogens. 2014;10:e1004321. doi: 10.1371/journal.ppat.1004321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brackman G, Cos P, Maes L, Nelis HJ, Coenye T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob Agents Chemother. 2011;55:2655–2661. doi: 10.1128/aac.00045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nascimento MM, et al. The effect of arginine on oral biofilm communities. Mol Oral Microbiol. 2014;29:45–54. doi: 10.1111/omi.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.He J, et al. l-Arginine Modifies the Exopolysaccharide Matrix and Thwarts Streptococcus mutans Outgrowth within Mixed-Species Oral Biofilms. J Bacteriol. 2016;198:2651–2661. doi: 10.1128/JB.00021-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jakubovics NS, et al. Critical roles of arginine in growth and biofilm development by Streptococcus gordonii. Mol Microbiol. 2015;97:281–300. doi: 10.1111/mmi.13023. [DOI] [PubMed] [Google Scholar]
- 86.Kolderman E, et al. L-arginine destabilizes oral multi-species biofilm communities developed in human saliva. PLoS One. 2015;10:e0121835. doi: 10.1371/journal.pone.0121835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gnanadhas DP, Elango M, Datey A, Chakravortty D. Chronic lung infection by Pseudomonas aeruginosa biofilm is cured by L-Methionine in combination with antibiotic therapy. Sci Rep. 2015;5:16043. doi: 10.1038/srep16043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Banin E, Vasil ML, Greenberg EP. Iron and Pseudomonas aeruginosa biofilm formation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11076–11081. doi: 10.1073/pnas.0504266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oglesby-Sherrouse AG, Djapgne L, Nguyen AT, Vasil AI, Vasil ML. The complex interplay of iron, biofilm formation, and mucoidy affecting antimicrobial resistance of Pseudomonas aeruginosa. Pathog Dis. 2014;70:307–320. doi: 10.1111/2049-632X.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin MH, Shu JC, Huang HY, Cheng YC. Involvement of iron in biofilm formation by Staphylococcus aureus. PLoS One. 2012;7:e34388. doi: 10.1371/journal.pone.0034388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garcia CA, Alcaraz ES, Franco MA, Passerini de Rossi BN. Iron is a signal for Stenotrophomonas maltophilia biofilm formation, oxidative stress response, OMPs expression, and virulence. Front Microbiol. 2015;6:926. doi: 10.3389/fmicb.2015.00926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moreau-Marquis S, et al. The DeltaF508-CFTR mutation results in increased biofilm formation by Pseudomonas aeruginosa by increasing iron availability. Am J Physiol Lung Cell Mol Physiol. 2008;295:L25–37. doi: 10.1152/ajplung.00391.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaneko Y, Thoendel M, Olakanmi O, Britigan BE, Singh PK. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. The Journal of clinical investigation. 2007;117:877–888. doi: 10.1172/JCI30783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moreau-Marquis S, O’Toole GA, Stanton BA. Tobramycin and FDA-approved iron chelators eliminate Pseudomonas aeruginosa biofilms on cystic fibrosis cells. Am J Respir Cell Mol Biol. 2009;41:305–313. doi: 10.1165/rcmb.2008-0299OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hunter RC, et al. Ferrous iron is a significant component of bioavailable iron in cystic fibrosis airways. MBio. 2013;4 doi: 10.1128/mBio.00557-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lu M, Hansen EN. Hydrogen Peroxide Wound Irrigation in Orthopaedic Surgery. J Bone Joint Infect. 2017;2:3–9. doi: 10.7150/jbji.16690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ordinola-Zapata R, Bramante C, Aprecio R, Handysides R, Jaramillo D. Biofilm removal by 6% sodium hypochlorite activated by different irrigation techniques. International endodontic journal. 2014;47:659–666. doi: 10.1111/iej.12202. [DOI] [PubMed] [Google Scholar]
- 98.Liu H, Wei X, Ling J, Wang W, Huang X. Biofilm formation capability of Enterococcus faecalis cells in starvation phase and its susceptibility to sodium hypochlorite. Journal of endodontics. 2010;36:630–635. doi: 10.1016/j.joen.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 99.von Ohle C, et al. Real-time microsensor measurement of local metabolic activities in ex vivo dental biofilms exposed to sucrose and treated with chlorhexidine. Appl Environ Microbiol. 2010;76:2326–2334. doi: 10.1128/AEM.02090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mihailescu R, et al. High activity of Fosfomycin and Rifampin against methicillin-resistant staphylococcus aureus biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother. 2014;58:2547–2553. doi: 10.1128/AAC.02420-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Conlon BP, et al. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature. 2013;503:365–370. doi: 10.1038/nature12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pletzer D, Coleman SR, Hancock RE. Anti-biofilm peptides as a new weapon in antimicrobial warfare. Curr Opin Microbiol. 2016;33:35–40. doi: 10.1016/j.mib.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Batoni G, Maisetta G, Esin S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim Biophys Acta. 2016;1858:1044–1060. doi: 10.1016/j.bbamem.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 104.Guo L, et al. Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc Natl Acad Sci U S A. 2015;112:7569–7574. doi: 10.1073/pnas.1506207112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de la Fuente-Nunez C, et al. D-enantiomeric peptides that eradicate wild-type and multidrug-resistant biofilms and protect against lethal Pseudomonas aeruginosa infections. Chem Biol. 2015;22:196–205. doi: 10.1016/j.chembiol.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jones EA, McGillivary G, Bakaletz LO. Extracellular DNA within a nontypeable Haemophilus influenzae-induced biofilm binds human beta defensin-3 and reduces its antimicrobial activity. J Innate Immun. 2013;5:24–38. doi: 10.1159/000339961000339961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu K, et al. Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials. 2017;116:69–81. doi: 10.1016/j.biomaterials.2016.11.047. [DOI] [PubMed] [Google Scholar]
- 108.Lam SJ, et al. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. 2016;1:16162. doi: 10.1038/nmicrobiol.2016.162. http://dharmasastra.live.cf.private.springer.com/articles/nmicrobiol2016162-supplementary-information. [DOI] [PubMed] [Google Scholar]
- 109.Scott RW, Tew GN. Mimics of Host Defense Proteins; Strategies for Translation to Therapeutic Applications. Curr Top Med Chem. 2017;17:576–589. doi: 10.2174/1568026616666160713130452. [DOI] [PubMed] [Google Scholar]
- 110.Busscher HJ, et al. Biomaterial-associated infection: locating the finish line in the race for the surface. Sci Transl Med. 2012;4:153rv110. doi: 10.1126/scitranslmed.3004528. [DOI] [PubMed] [Google Scholar]
- 111.De Jong WH, et al. Systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials. 2013;34:8333–8343. doi: 10.1016/j.biomaterials.2013.06.048. [DOI] [PubMed] [Google Scholar]
- 112.Swartjes JJ, et al. Current Developments in Antimicrobial Surface Coatings for Biomedical Applications. Curr Med Chem. 2015;22:2116–2129. doi: 10.2174/0929867321666140916121355. [DOI] [PubMed] [Google Scholar]
- 113.Schumacher JF, et al. Species-specific engineered antifouling topographies: correlations between the settlement of algal zoospores and barnacle cyprids. Biofouling. 2007;23:307–317. doi: 10.1080/08927010701393276. [DOI] [PubMed] [Google Scholar]
- 114.May RM, et al. An engineered micropattern to reduce bacterial colonization, platelet adhesion and fibrin sheath formation for improved biocompatibility of central venous catheters. Clinical and Translational Medicine. 2015;4:9. doi: 10.1186/s40169-015-0050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Falde EJ, Yohe ST, Colson YL, Grinstaff MW. Superhydrophobic materials for biomedical applications. Biomaterials. 2016;104:87–103. doi: 10.1016/j.biomaterials.2016.06.050. doi: http://dx.doi.org/10.1016/j.biomaterials.2016.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Damodaran VB, Murthy NS. Bio-inspired strategies for designing antifouling biomaterials. Biomaterials Research. 2016;20:18. doi: 10.1186/s40824-016-0064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zeng G, Ogaki R, Meyer RL. Non-proteinaceous bacterial adhesins challenge the antifouling properties of polymer brush coatings. Acta biomaterialia. 2015;24:64–73. doi: 10.1016/j.actbio.2015.05.037. doi: http://dx.doi.org/10.1016/j.actbio.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 118.Wen L, Tian Y, Jiang L. Bioinspired super-wettability from fundamental research to practical applications. Angew Chem Int Ed Engl. 2015;54:3387–3399. doi: 10.1002/anie.201409911. [DOI] [PubMed] [Google Scholar]
- 119.Bai X, Xue CH, Jia ST. Surfaces with Sustainable Superhydrophobicity upon Mechanical Abrasion. ACS Appl Mater Interfaces. 2016 doi: 10.1021/acsami.6b08672. [DOI] [PubMed] [Google Scholar]
- 120.Fadeeva E, et al. Bacterial Retention on Superhydrophobic Titanium Surfaces Fabricated by Femtosecond Laser Ablation. Langmuir. 2011;27:3012–3019. doi: 10.1021/la104607g. [DOI] [PubMed] [Google Scholar]
- 121.Gilabert-Porres J, et al. Design of a Nanostructured Active Surface against Gram-Positive and Gram-Negative Bacteria through Plasma Activation and in Situ Silver Reduction. ACS Applied Materials & Interfaces. 2016;8:64–73. doi: 10.1021/acsami.5b07115. [DOI] [PubMed] [Google Scholar]
- 122.Paula AJ, Koo H. Nanosized Building Blocks for Customizing Novel Antibiofilm Approaches. J Dent Res. 2017;96:128–136. doi: 10.1177/0022034516679397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bayramov DF, Neff JA. Beyond conventional antibiotics - New directions for combination products to combat biofilm. Adv Drug Deliv Rev. 2016 doi: 10.1016/j.addr.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Junter G-A, Thébault P, Lebrun L. Polysaccharide-based antibiofilm surfaces. Acta biomaterialia. 2016;30:13–25. doi: 10.1016/j.actbio.2015.11.010. doi: http://dx.doi.org/10.1016/j.actbio.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 125.Palumbo FS, et al. A polycarboxylic/amino functionalized hyaluronic acid derivative for the production of pH sensible hydrogels in the prevention of bacterial adhesion on biomedical surfaces. Int J Pharm. 2015;478:70–77. doi: 10.1016/j.ijpharm.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 126.Ciofu O, Tolker-Nielsen T, Jensen PO, Wang H, Hoiby N. Antimicrobial resistance, respiratory tract infections and role of biofilms in lung infections in cystic fibrosis patients. Adv Drug Deliv Rev. 2015;85:7–23. doi: 10.1016/j.addr.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 127.Liu W, et al. Synthesis of TiO2 nanotubes with ZnO nanoparticles to achieve antibacterial properties and stem cell compatibility. Nanoscale. 2014;6:9050–9062. doi: 10.1039/c4nr01531b. [DOI] [PubMed] [Google Scholar]
- 128.Jia Z, et al. Bioinspired anchoring AgNPs onto micro-nanoporous TiO2 orthopedic coatings: Trap-killing of bacteria, surface-regulated osteoblast functions and host responses. Biomaterials. 2016;75:203–222. doi: 10.1016/j.biomaterials.2015.10.035. doi: http://dx.doi.org/10.1016/j.biomaterials.2015.10.035. [DOI] [PubMed] [Google Scholar]
- 129.Ashbaugh AG, et al. Polymeric nanofiber coating with tunable combinatorial antibiotic delivery prevents biofilm-associated infection in vivo. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1613722113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Min J, et al. Designer Dual Therapy Nanolayered Implant Coatings Eradicate Biofilms and Accelerate Bone Tissue Repair. ACS Nano. 2016;10:4441–4450. doi: 10.1021/acsnano.6b00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Holzapfel BM, et al. How smart do biomaterials need to be? A translational science and clinical point of view. Adv Drug Deliv Rev. 2013;65:581–603. doi: 10.1016/j.addr.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 132.Zhuang J, Gordon MR, Ventura J, Li L, Thayumanavan S. Multi-stimuli responsive macromolecules and their assemblies. Chemical Society reviews. 2013;42:7421–7435. doi: 10.1039/c3cs60094g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shchukin D, Mohwald H. Materials science. A coat of many functions. Science. 2013;341:1458–1459. doi: 10.1126/science.1242895. [DOI] [PubMed] [Google Scholar]
- 134.Schütz CA, Juillerat-Jeanneret L, Mueller H, Lynch I, Riediker M. Therapeutic nanoparticles in clinics and under clinical evaluation. Nanomedicine. 2013;8:449–467. doi: 10.2217/nnm.13.8. [DOI] [PubMed] [Google Scholar]
- 135.Natan M, Banin E. From Nano to Micro: using nanotechnology to combat microorganisms and their multidrug resistance. FEMS Microbiol Rev. 2017;41:302–322. doi: 10.1093/femsre/fux003. [DOI] [PubMed] [Google Scholar]
- 136.Rukavina Z, Vanić Ž. Current Trends in Development of Liposomes for Targeting Bacterial Biofilms. Pharmaceutics. 2016;8:18. doi: 10.3390/pharmaceutics8020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Forier K, et al. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J Control Release. 2014;190:607–623. doi: 10.1016/j.jconrel.2014.03.055. [DOI] [PubMed] [Google Scholar]
- 138.Alipour M, Suntres ZE, Halwani M, Azghani AO, Omri A. Activity and interactions of liposomal antibiotics in presence of polyanions and sputum of patients with cystic fibrosis. PLoS One. 2009;4:e5724. doi: 10.1371/journal.pone.0005724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zazo H, Colino CI, Lanao JM. Current applications of nanoparticles in infectious diseases. J Control Release. 2016;224:86–102. doi: 10.1016/j.jconrel.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 140.Cipolla D, Blanchard J, Gonda I. Development of Liposomal Ciprofloxacin to Treat Lung Infections. Pharmaceutics. 2016;8 doi: 10.3390/pharmaceutics8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Clancy JP, et al. Phase II studies of nebulised Arikace in CF patients with Pseudomonas aeruginosa infection. Thorax. 2013;68:818–825. doi: 10.1136/thoraxjnl-2012-202230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jardeleza C, et al. An in vivo safety and efficacy demonstration of a topical liposomal nitric oxide donor treatment for Staphylococcus aureus biofilm-associated rhinosinusitis. Transl Res. 2015;166:683–692. doi: 10.1016/j.trsl.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 143.Gao L, et al. Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials. 2016;101:272–284. doi: 10.1016/j.biomaterials.2016.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Benoit DS, Koo H. Targeted, triggered drug delivery to tumor and biofilm microenvironments. Nanomedicine. 2016;11:873–879. doi: 10.2217/nnm-2016-0014. [DOI] [PubMed] [Google Scholar]
- 145.Liu Y, et al. Surface-Adaptive, Antimicrobially Loaded, Micellar Nanocarriers with Enhanced Penetration and Killing Efficiency in Staphylococcal Biofilms. ACS Nano. 2016;10:4779–4789. doi: 10.1021/acsnano.6b01370. [DOI] [PubMed] [Google Scholar]
- 146.Horev B, et al. pH-activated nanoparticles for controlled topical delivery of farnesol to disrupt oral biofilm virulence. ACS Nano. 2015;9:2390–2404. doi: 10.1021/nn507170s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Radovic-Moreno AF, et al. Surface charge-switching polymeric nanoparticles for bacterial cell wall-targeted delivery of antibiotics. ACS Nano. 2012;6:4279–4287. doi: 10.1021/nn3008383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Deacon J, et al. Antimicrobial efficacy of tobramycin polymeric nanoparticles for Pseudomonas aeruginosa infections in cystic fibrosis: formulation, characterisation and functionalisation with dornase alfa (DNase) J Control Release. 2015;198:55–61. doi: 10.1016/j.jconrel.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 149.Ahmadi MS, et al. Sustained Nitric Oxide-Releasing Nanoparticles Induce Cell Death in Candida albicans Yeast and Hyphal Cells, Preventing Biofilm Formation In Vitro and in a Rodent Central Venous Catheter Model. Antimicrob Agents Chemother. 2016;60:2185–2194. doi: 10.1128/aac.02659-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mihu MR, et al. Sustained Nitric Oxide-Releasing Nanoparticles Interfere with Methicillin-Resistant Staphylococcus aureus Adhesion and Biofilm Formation in a Rat Central Venous Catheter Model. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/aac.02020-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jo H, Ban C. Aptamer-nanoparticle complexes as powerful diagnostic and therapeutic tools. Experimental & molecular medicine. 2016;48:e230. doi: 10.1038/emm.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Watters C, Everett JA, Haley C, Clinton A, Rumbaugh KP. Insulin Treatment Modulates the Host Immune System To Enhance Pseudomonas aeruginosa Wound Biofilms. Infection and immunity. 2014;82:92–100. doi: 10.1128/iai.00651-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hajishengallis G, et al. Complement inhibition in pre-clinical models of periodontitis and prospects for clinical application. Seminars in immunology. 2016;28:285–291. doi: 10.1016/j.smim.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 155.Bjarnsholt T, et al. The in vivo biofilm. Trends in microbiology. 2013;21:466–474. doi: 10.1016/j.tim.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 156.Papenfort K, Bassler BL. Quorum sensing signal-response systems in Gram-negative bacteria. Nature Reviews Microbiology. 2016;14:576–588. doi: 10.1038/nrmicro.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Strebhardt K, Ullrich A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8:473–480. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 158.Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147:258–267. [PubMed] [Google Scholar]
- 159.NIH MedlinePlus: the magazine [Internet] 2013;7:2–3. [Google Scholar]
- 160.Brauner A, Fridman O, Gefen O, Balaban NQ. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol. 2016;14:320–330. doi: 10.1038/nrmicro.2016.34. [DOI] [PubMed] [Google Scholar]
- 161.Conlon BP, Rowe SE, Lewis K. Persister cells in biofilm associated infections. Adv Exp Med Biol. 2015;831:1–9. doi: 10.1007/978-3-319-09782-4_1. [DOI] [PubMed] [Google Scholar]
- 162.Stewart PS, et al. Contribution of stress responses to antibiotic tolerance in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2015;59:3838–3847. doi: 10.1128/AAC.00433-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.