Abstract
Background
Hospital staff expressed health concerns after a surface disinfectant product containing hydrogen peroxide, peracetic acid, and acetic acid was introduced. We sought to determine if this product posed a health hazard.
Methods
An interviewer-administered questionnaire on work and health characteristics was completed by 163 current staff. Symptoms that improved away from work were considered work-related. Forty-nine air samples were taken for hydrogen peroxide, peracetic acid, and acetic acid. Prevalence ratios (PRs) were calculated using Poisson regression, and standardized morbidity ratios (SMRs) were calculated using nationally representative data.
Results
Product users reported higher prevalence of work-related wheeze and watery eyes than non-users (P < .05). Workers in the department with the highest air measurements had significantly higher prevalence of watery eyes (PR, 2.88; 95% confidence interval [CI], 1.18–7.05) than those in departments with lower air measurements, and they also had a >3-fold excess of current asthma (SMR, 3.47; 95% CI, 1.48–8.13) compared with the U.S. population.
Conclusions
This disinfectant product was associated with mucous membrane and respiratory health effects. Risks of mucous membrane irritation and asthma in health care workers should be considered in development of disinfection protocols to protect patients from hospital-acquired infections. Identification of optimal protocols that reduce worker exposures while maintaining patient safety is needed.
Keywords: Disinfectant, Cleaner, Occupational health, Asthma, Environmental services
Health care–associated infections (HAIs) remain a significant challenge to health care facilities in the United States. On any given day, approximately 1 in 25 hospital patients has at least 1 HAI.1 One of the most significant challenges to preventing HAIs is Clostridium difficile bacteria, which has replaced methicillin-resistant Staphylococcus aureus as the most common cause of HAI.2 Hospitalized patients acquire C difficile by ingesting spores transmitted from other patients through health care workers or from contact with contaminated surfaces in hospital rooms.3,4 Eliminating C difficile spores in the hospital environment requires the use of disinfectants that are sporicidal. Sporicides that are effective at reducing the environmental burden of C difficile may also contain chemicals that cause health effects for cleaning staff and other hospital workers.
Several studies have identified cleaning as an occupational risk factor for asthma among health care workers.5–8 There are a number of chemicals in cleaning and disinfecting products that can cause or exacerbate asthma because of their sensitizing or irritant properties, including quaternary ammonium compounds, ethanolamines, chlorhexidine, glutaraldehyde, ortho-phthalaldehyde, hexachlorophene, and chloramine-T.9–17 In addition, dermatitis and other adverse skin effects have been reported among hospital cleaning workers.18 However, some health care workers may underestimate their exposure or may lack knowledge of product components.19 There is no national surveillance of health effects related to cleaning and disinfection product use. In 4 states with occupational health surveillance, a total of 401 acute illnesses associated with work-related antimicrobial pesticide exposures in health care facilities were reported during 2002–2007. The most commonly reported health effects were eye irritation (55%), headaches or other neurologic symptoms (32%), respiratory symptoms (30%), and skin problems (24%). Among these reports, environmental service staff (EVS), who are largely responsible for cleaning and disinfection in health care facilities, was the most common occupation reporting health effects at 24%.20
In January 2015, the Centers for Disease Control and Prevention’s National Institute for Occupational Safety and Health (NIOSH) was notified through their Health Hazard Evaluation program of eye, respiratory, and skin problems among hospital EVS staff thought to be related to disinfectant use in a hospital. The hospital had introduced a new disinfectant product containing hydrogen peroxide, peracetic acid, and acetic acid in March 2014 to mitigate HAIs. We conducted a health hazard evaluation to assess if this disinfectant posed a health hazard to EVS and other hospital staff.
METHODS
An interviewer-administered questionnaire was offered to all EVS staff and an equal number of non-EVS staff on duty during the days of our visits. Non-EVS staff were recruited from the same departments of the hospital where EVS staff were located. Questions addressed self-reported respiratory and dermatologic symptoms, asthma and other diagnoses, smoking history, work history and practices, and demographic information. Participants ever having asthma were defined as those who were ever told by a physician or health care provider that they had asthma. Current asthma was defined as physician-diagnosed asthma that was still present. Some of these questions were taken from the Third National Health and Nutrition Examination Survey21 and the European Community Respiratory Health Survey.22 Asthma-like symptoms were defined as a response of yes to any of the following questions22:
Are you currently taking any medicine (including inhalers, aerosols, or tablets) for asthma?
Have you had wheezing or whistling in your chest at any time in the last 12 months?
Have you woken up with a feeling of tightness in your chest at any time in the last 12 months?
Have you been woken by an attack of asthma at any time in the last 12 months?
Symptoms that improved when the employees were away from work, either on their days off or when they were on vacation, were considered work-related.
We collected 49 full-shift air samples from the breathing zones of EVS staff while they performed their regular cleaning duties. Details of the air sampling and air sample results are reported in Hawley et al.23 All air samples were analyzed for the 3 chemicals found in the disinfectant: hydrogen peroxide, peracetic acid, and acetic acid.
Statistical analyses were conducted using SAS software version 9.3 (SAS Institute, Cary, NC). Statistically significant differences between demographic characteristics, symptoms, and diagnoses were assessed using Student t test for continuous variables and χ2 test for categorical variables. We used Fisher exact test when cell sizes were <5. We considered results to be statistically significant when P = ≤.05 using a 2-sided test, and 95% confidence intervals were calculated.
Incidence densities of self-reported adult-onset asthma diagnosed by a physician before and after hire at the hospital were estimated using birth date, hire date, and diagnosis date. Asthma incidence density before hire was calculated by adding the number of adult-onset asthma diagnoses that occurred before hire and dividing by the sum of participants’ years at risk before hire. Asthma incidence density after hire was calculated by adding the number of adult-onset asthma diagnoses that occurred after hire and dividing by the sum of participants’ time at risk after hire. An incidence density ratio was calculated using Poisson regression.
We compared the observed prevalence of shortness of breath, cough, wheeze, watery eyes, and doctor-diagnosed asthma among participants to expected values for the general U.S. adult population obtained from the Third National Health and Nutrition Examination Survey. For these comparisons, we calculated standardized morbidity ratios (SMRs) using indirect standardization for race (white, black, or Mexican-American), sex, age (17–39 years or ≥40 years), and cigarette smoking status (ever vs never smoker).21
Among the participants who worked in a department where air sampling was performed, we evaluated associations between symptoms and department-level air measurements by calculating prevalence ratios using Poisson regression. Concentrations of hydrogen peroxide, peracetic acid, and acetic acid from personal air samples were used in the American Conference of Governmental Industrial Hygienists’ (ACGIH) additive mixture formula. We used this formula to categorize departments by their total mixture of hydrogen peroxide, peracetic acid, and acetic acid.24 This formula takes the measured parts per million (ppm) concentrations of hydrogen peroxide and acetic acid and divides them by their established Occupational Safety and Health Administration (OSHA) permissible exposure limits (PELs) and NIOSH recommended exposure limits (RELs) of 1 ppm for hydrogen peroxide and 10 ppm for acetic acid. Measured ppm concentrations of peracetic acid were divided by the occupational exposure limits proposed by multiple researchers, of 0.2 ppm.25–27 Hydrogen peroxide [HP], peracetic acid [PAA], and acetic acid [AA] represent the measured full-shift time weighted average. ACGIH mixture formula results <1 are considered to be below the threshold limit value where adverse effects may occur.
The mixture values derived from the formula were averaged using arithmetic means calculated by the hospital department where the survey participant was assigned. Based on this department-level value of the ACGIH mixture formula, 9 departments were categorized into tertiles of low (≤0.075), medium (>0.075 to ≤0.190), and high (>0.190) exposure categories. Prevalence ratios were calculated by comparing the single department with the highest ACGIH value, departments in the high category, and departments in the medium category to departments in the low category.
RESULTS
A total of 163 current employees, including 78% (n = 79/101) of EVS staff who were working on the days of the survey, completed the questionnaire. Five EVS staff refused to participate. Hospital supervisors and charge nurses assisted in pulling non-EVS staff from their duties to participate in the survey. As a result, there were no non-EVS staff who refused to participate. Non-EVS staff included nursing staff (n = 27); other patient care staff (n = 25), such as patient care technicians, respiratory therapists, and nursing assistants; administrative staff (n = 13), such as business managers and unit clerks; and other hospital staff (n = 19), such as cooks, dietitians, and laboratory staff. Table 1 describes the demographic and work characteristics of questionnaire participants.
Table 1.
Demographic and work characteristics of the survey participants at a hospital, August 2015
| Characteristic | All participants (N = 163) | EVS (n = 79) | Non-EVS (n = 84) | P value* | Disinfectant product use (n = 78) | No disinfectant product use (n = 85) | P value* |
|---|---|---|---|---|---|---|---|
| Age, y | 43 (19–67) | 45 (20–67) | 40 (19–67) | .40 | 43 (20–67) | 43 (19–67) | .43 |
| Tenure, y | 5 (0.13–43) | 5 (0.21–36) | 5 (0.13–43) | .83 | 5 (0.21–36) | 5 (0.13–43) | .57 |
| Male | 50 (31) | 39 (49) | 11 (13) | <.05 | 33 (42) | 17 (20) | <.05 |
| Race | |||||||
| White | 92 (56) | 21 (27) | 71 (85) | <.05 | 26 (33) | 66 (78) | <.05 |
| Black | 57 (35) | 47 (59) | 10 (12) | 41 (53) | 16 (19) | ||
| Other or unknown† | 14 (9) | 11 (14) | 3 (4) | 11 (14) | 3 (4) | ||
| Smoking status | |||||||
| Current | 27 (17) | 16 (20) | 11 (13) | .36 | 16 (21) | 11 (13) | .42 |
| Former | 32 (20) | 13 (16) | 19 (23) | 14 (18) | 18 (21) | ||
| Never | 104 (64) | 50 (63) | 54 (64) | 48 (62) | 56 (66) | ||
NOTE. Values are median (range), n (%), or as otherwise indicated. EVS, Environmental services staff.
P values calculated using χ 2 test or Fisher exact test for categorical variables or Student t test for continuous variables.
Includes participants who indicated >1 race or did not indicate a race.
EVS staff represented 48% (n = 79/163) of questionnaire participants. EVS and Non-EVS staff were similar regarding age, tenure, and smoking status. Men were significantly (P < .05) more represented among EVS staff than non-EVS staff, representing roughly half (49%, n = 39/79) of EVS participants. Most non-EVS staff were white (85%, n = 71/84), whereas most EVS staff were black (59%, n = 47/ 79). Differences in race were statistically significant (P < .05) (Table 1).
Although most EVS staff were users of the disinfectant product containing hydrogen peroxide, acetic acid, and peracetic acid, 11 (14%, n = 11/79) indicated that they did not use the disinfectant. Ten (12%, n = 10/84) non-EVS staff members indicated that they used the disinfectant. Disinfectant users and nonusers were similar regarding age, tenure, and smoking status. Disinfectant users were significantly (P < .05) more likely to be men than disinfectant nonusers, with 42% of disinfectant users being men. Black participants were more represented among disinfectant users, representing 53% (n = 41/ 78) of disinfectant users and 19% (n = 16/85) of disinfectant nonusers. Seventy-eight percent (n = 66/85) of disinfectant nonusers were white. This difference in race was statistically significant (P < .05) (Table 1).
The most commonly reported symptoms among all participants were nasal problems, such as rhinitis, (42%, n = 68/163) and watery eyes (40%, n = 65/163) (Table 2). Other commonly reported symptoms included asthma-like symptoms (28%, n = 46/ 163), skin problems (19%, 31/163), and wheeze (16%, n = 26/163). The prevalence of shortness of breath, cough, wheeze, and eye symptoms were not significantly different than the expected prevalence for the U.S. adult population (data not shown). A number of reported symptoms were described to be work-related, including 61% (n = 19/ 31) of skin problems, 50% (n = 4/8) of asthma attacks, 48% (n = 31/ 65) of watery eyes, 44% (n = 4/9) of cough, and 43% (n = 29/68) of nasal problems. Use of allergy medication was reported by 29% (n = 48/163) of participants.
Table 2.
Symptoms and self-reported diagnoses of the survey participants, by disinfectant product use at a hospital, August 2015
| Health effect | All participants (N = 163) | Work-related symptoms* | Disinfectant product use (n = 78) | No disinfectant product use (n = 85) | P value† |
|---|---|---|---|---|---|
| Symptom | |||||
| Nasal problems‡ | 68 (42) | 29 (18) | 31 (40) | 37 (44) | .64 |
| Watery eyes‡ | 65 (40) | 31 (18) | 35 (45) | 30 (35) | .26 |
| Asthma-like symptoms§ | 46 (28) | 16 (10) | 24 (31) | 22 (26) | .60 |
| Skin problems‡ | 31 (19) | 19 (11) | 12 (15) | 19 (22) | .32 |
| Wheeze‡ | 26 (16) | 6 (4) | 12 (15) | 14 (16) | >.99 |
| Shortness of breath | 21 (13) | 7 (4) | 11 (14) | 10 (12) | .82 |
| Chest tightness‡ | 18 (11) | 4 (2) | 10 (13) | 8 (9) | .62 |
| Cough | 9 (6) | 4 (2) | 5 (6) | 4 (5) | .74 |
| Asthma attack‡ | 8 (5) | 4 (2) | 3 (4) | 5 (6) | .72 |
| Medication use | |||||
| Allergy medicine | 48 (29) | 9 (6) | 16 (21) | 32 (38) | <.05 |
| Asthma medicine | 18 (11) | 6 (4) | 10 (13) | 8 (9) | .62 |
| Diagnosis | |||||
| Asthma | |||||
| Ever | 32 (20) | 14 (18) | 18 (21) | .69 | |
| Current | 23 (14) | 12 (15) | 11 (13) | .66 | |
| Nasal allergies | 37 (23) | 11 (14) | 26 (31) | <.05 | |
NOTE. Values are n (%) or as otherwise indicated.
Work-related symptoms were defined as symptoms that improved away from the facility, either on days off or on vacation.
P values calculated usingχ 2 or Fisher exact test.
In the last 12 months.
Asthma-like symptoms were defined as current use of asthma medicine or ≥ 1 of the following symptoms in the last 12 months: wheezing or whistling in the chest, awakening with a feeling of chest tightness, or attack of asthma.
Among the 68 employees who said they had nasal problems, 50% (n = 34/68) said that something at work brought on or made their nasal problems worse. Of those, 50% (n = 17/34) specified that the disinfectant product brought on or made their nasal problems worse. Fifty-two percent (n = 34/65) reported that something at work brought on or made their watery eye symptoms worse, and among those, 68% (n = 23/34) specified the disinfection product. Fifty-three percent (n = 9/17) of staff who reported that something at work brought on or made their skin problems worse specified the disinfection product.
Twenty percent (n = 32/163) of participants reported ever being diagnosed with asthma (Table 2), which was 2.4 times higher (SMR, 2.4; 95% CI, 1.6–3.4) than expected when compared with the U.S. adult population. Current asthma was reported by 14% (n = 23/ 163) of participants and was 2.3 times higher (SMR, 2.3; 95% CI, 1.5–3.6) than expected for the U.S. adult population. A total of 15 (9%, n = 15/163) participants described asthma that met the definition for adult-onset asthma. The prehire adult-onset asthma incidence density was 4.8 cases per 1,000 person years. The posthire adult-onset asthma incidence density was 5.5 cases per 1,000 person years; these incidence densities were not significantly different. Similarly, we evaluated the asthma incidence densities before and after the introduction of the disinfection product; these incidence densities were also not significantly different.
A comparison of health symptoms between disinfectant product users and nonusers can be seen in Table 2. Use of allergy medication and self-reported doctor diagnosis of nasal allergies were significantly less common among disinfectant product users than nonusers (P < .05). There were no other significant differences between disinfectant product users and nonusers. No significant associations were found between the prevalence of health effects and other hospital cleaning products (results not shown).
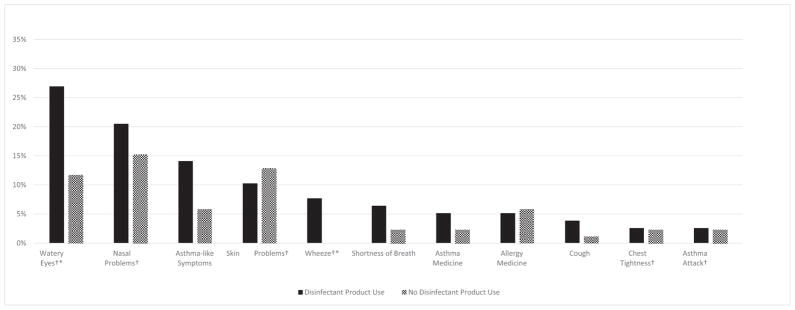
Comparing disinfectant users and nonusers who reported symptoms, some symptoms were described to be work-related. Figure 1 shows that disinfectant product users reported higher prevalence of work-related watery eyes, nasal problems, asthma-like symptoms, wheeze, shortness of breath, use of asthma medicine, cough, chest tightness, and asthma attack, with statistically significant differences for wheeze and watery eyes (P < .05).
Fig 1.
Prevalence of work-related symptoms by disinfectant product use at a hospital, August 2015. Work-related symptoms were defined as symptoms that improved away from the facility, either on days off or on vacation. *Statistically significant differences using χ2 test (P < .05). †All symptoms specific to the last 12 months.
Health effects and air measurements
There were 85 survey participants who worked in 9 departments where air sampling was performed. These departments included labor and delivery (n = 28), a neonatal intensive care unit (n = 18), an outpatient clinic (n = 4), 2 antepartum-postpartum units (n = 14), 2 medical-surgical units (n = 11), an oncology unit (n = 5), and an intensive care unit (n = 5). All air measurements for hydrogen peroxide and acetic acid were below their respective OSHA PELs.27 There is currently no established PEL or REL for peracetic acid. The labor and delivery department had the highest average measurements of all 3 chemicals: 168.7 ppb for hydrogen peroxide, 25.0 ppb for peracetic acid, and 249.8 ppb for acetic acid. This department also had the highest average ACGIH mixture result. ACGIH mixture formula results for all departments were well below the threshold value level of 1. ACGIH mixture categories were comprised of the following departments: low (outpatient clinic at 0.055, medical-surgical unit at 0.064, and postpartum-antepartum unit at 0.075), medium (intensive care unit at 0.151, oncology unit at 0.151, and postpartum unit at 0.188), and high (neonatal intensive care unit at 0.192, a medical-surgical unit at 0.246, and labor and delivery unit at 0.315).
Prevalence ratios comparing the labor and delivery department, departments with high ACGIH mixture formula results, and departments with medium ACGIH mixture formula results with the departments with low formula results are in Figure 2. Statistically significant results were found for watery eyes in the labor and delivery department (prevalence ratio, 2.88; 95% CI, 1.18–7.05) and departments with high ACGIH mixture formula results (prevalence ratio, 2.58; 95% CI, 1.07–6.26) when compared with departments with low ACGIH mixture formula results.
Fig 2.
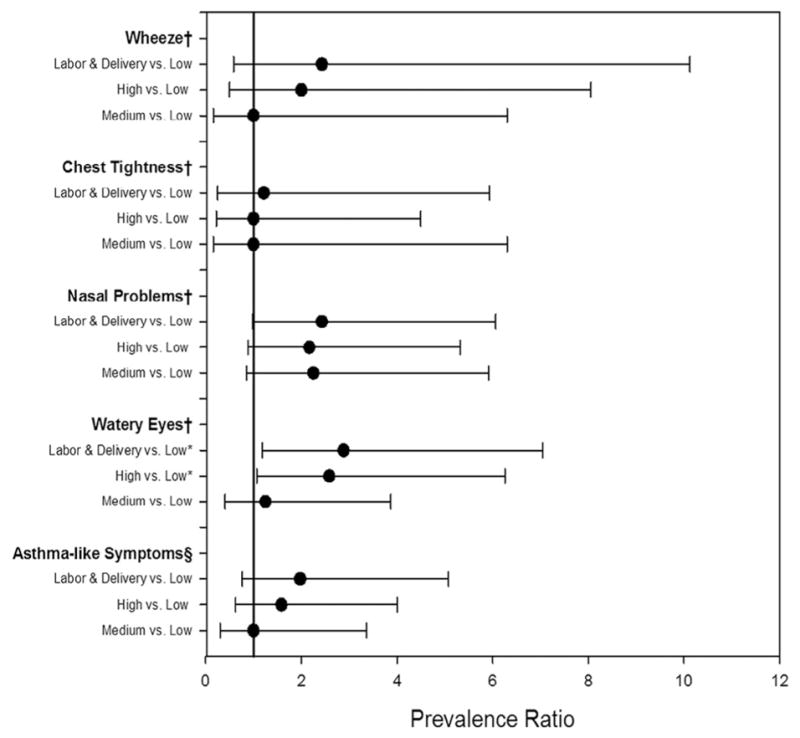
Prevalence ratios and 95% confidence intervals comparing the labor and delivery department, departments with high and medium ACGIH mixture formula results, and departments with low ACGIH mixture formula results. *Statistically sig-nificant results. †All symptoms specific to the last 12 months. §Asthma-like symptoms, defined as current use of asthma medicine or ≥ 1 of the following symptoms in the last 12 months: wheezing or whistling in the chest, awakening with a feeling of chest tightness, or attack of asthma. ACGIH, American Conference of Governmental Industrial Hygienists
We compared the prevalence of shortness of breath, cough, wheeze, watery eyes, and asthma diagnosis among workers in the labor and delivery department with the U.S. adult population. The prevalence of watery eyes (SMR, 1.70; 95% CI, 1.06–2.72), lifetime asthma diagnosis (SMR, 2.50; 95% CI, 1.07–5.85), and current asthma (SMR, 3.47; 95% CI, 1.48–8.13) was significantly higher than expected when compared with the U.S. adult population. Prevalence of watery eyes and current asthma among workers in all other departments where air sampling was performed was not significantly higher than expected. However, lifetime asthma diagnosis was significantly higher than expected (SMR, 2.52; 95% CI, 1.41–4.51) in these other departments.
DISCUSSION
We found a higher prevalence of work-related wheeze and watery eyes among users of the disinfectant product. Among workers who reported that something at work brought on or made their nasal symptoms worse, half stated that the disinfectant product brought on or made their symptoms worse. The types of symptoms reported by staff in our survey were similar to the symptoms reported in a 2007 case study that described cough, wheezing, and shortness of breath among 2 endoscopy nurses exposed to a mixture of peracetic acid and hydrogen peroxide.28
We also found a higher prevalence of several health effects among product users and nonusers working in the labor and delivery department, which had the highest mixture of chemicals found in the disinfectant product. The prevalence of watery eyes and current asthma in this department, but not other departments, was significantly higher than what would be expected for the U.S. adult population. Higher air measurements in labor and delivery were consistent with our observation of increased frequency and duration of cleaning tasks, described in the NIOSH report23 The labor and delivery department had short patient stays, requiring consecutive terminal cleaning of rooms to accept new admissions.
The prevalence of asthma in this hospital was significantly higher than what would be expected when compared with the U.S. adult population. We did not find a significant difference in the prevalence of asthma among disinfectant users when compared with nonusers. Several case studies have described new onset asthma or asthma exacerbations among individuals who encounter cleaning and disinfecting chemicals through the air in their work environment, but do not use these products.9,29 The bystander effect, whereby nonproduct users are exposed to vapors from cleaning products, may explain why we saw no significant differences in asthma prevalence between product users and nonusers, a large asthma burden overall in this facility, and increases in health effects in departments with higher measures of cleaning chemicals in the air.
There are a number of limitations to our study. Survey participants were asked which cleaning and disinfection products they used using an inventory of all known cleaning products in the hospital. Although we did not find significant results between other products and health effects, air samples for other cleaning chemicals such as quaternary ammonium compounds were not taken. At the time of our survey, no effective air sampling method for quaternary ammonium compounds had been developed, and our observations during air sampling showed that the disinfectant product was being used as the primary disinfectant for most surfaces throughout the hospital, and use of other products was less frequent.23 We also relied on self-reports of health concerns and whether symptoms were work-related, which may be subject to recall or reporting bias. In addition, the cross-sectional nature of our evaluation makes it difficult to determine temporal relationships between health effects and use of the disinfectant; however, anecdotally, reports of symptoms increased after the product was introduced at the hospital. Finally, the questionnaire was administered to current workers only. These workers might have been healthier than all workers who had ever been employed at the facility because workers who were too ill to work might have resigned, possibly resulting in an underestimation of symptoms and diagnoses.
In 2015, this disinfectant product was listed as an asthmagen, or a substance that causes asthma, by the Association of Occupational and Environmental Clinics.30 Although the air measurements were well below the OSHA PEL and ACGIH mixture threshold, we did observe increased health effects in the departments with higher measures of chemicals in the air. For this reason, exposure reduction should be considered even when chemicals are measured at low levels. In 2008, the Centers for Disease Control and Prevention’s Healthcare Infection Control Practices Advisory Committee (HICPAC) developed “Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008.”31 This guideline acknowledges that occupational diseases among cleaning personnel have been associated with disinfectant use at levels below OSHA or NIOSH exposure limits. HICPAC recommends that controls be used to minimize exposure to disinfectants, including elimination or substitution of the chemical, engineering or administrative controls, or use of personal protective equipment.
Although cleaning and disinfecting agents are an important part of reducing HAIs, these products must be used judiciously and used in a way that reflects the level of risk in acquiring an HAI. HICPAC states that detergent and water are adequate for cleaning surfaces in nonpatient care areas. We recommend that the disinfectant product not be used in nonpatient care areas, which may include public bathrooms or administrative o3ces. It is also important for employees to report any symptoms they feel are related to their work so controls may be put into place to reduce worker exposure. Implementing a reporting system for employees to report work-related symptoms directly to occupational health staff, with the option to remain anonymous, may help facilitate this communication. HICPAC recommends this measure as a performance indicator for disinfection and sterilization.31 For those workers experiencing work-related health effects associated with cleaning or disinfection products, occupational health staff should develop policies and practices that would allow these workers to be reassigned to an area with less disinfectant exposure. Finally, it is important that employees understand the potential hazards from exposure to cleaning products and how to protect themselves. HICPAC recommends that each worker be informed of the possible health effect(s) of his or her exposure to chemicals.31 These recommendations may help to address worker concerns regarding cleaning and disinfection products and encourage communication regarding occupational illness.
CONCLUSIONS
Some infection control practices that require the use of products containing hydrogen peroxide, peracetic acid, and acetic acid may result in adverse health effects in exposed workers. HAIs remain a significant threat to patient safety, and environmental cleaning and disinfection is a key aspect in a comprehensive HAI prevention plan. However, by taking worker concerns about occupational illness seriously and implementing controls to reduce exposure to cleaning and disinfection products, health care facilities may provide a safer environment for workers while protecting patients from HAIs. This study highlights the importance of considering disinfectant exposure when evaluating health care workers for occupational illness. More research is needed to characterize product exposure, associated health effects, and control methods.
Acknowledgments
We thank the participating hospital staff members, the National Institute for Occupational Safety and Health field team members (Michael Beaty, Randy Boylstein, Matt Duling, Ethan Fechter-Leggett, Reid Harvey, Alyson Johnson, Robert B. Lawrence, Tia McClelland, Christopher Mugford, Randall Nett, Anand Ranpara, Marcia Stanton, M. Abbas Virji, and Sandy White), and technical reviewer Laura Kurth.
Footnotes
Conflicts of interest: None to report.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health. Mention of product names does not imply endorsement by the National Institute for Occupational Safety and Health/Centers for Disease Control and Prevention.
References
- 1.Centers for Disease Control and Prevention (CDC) [Accessed August 3, 2016];HAI data and statistics. 2016 Available from: http://www.cdc.gov/hai/surveillance/
- 2.Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32:387–90. doi: 10.1086/659156. [DOI] [PubMed] [Google Scholar]
- 3.Shaughnessy MK, Micielli RL, DePestel DD, Arndt J, Strachan CL, Welch KB, et al. Evaluation of hospital room assignment and acquisition of Clostridium difficile infection. Infect Control Hosp Epidemiol. 2011;32:201–6. doi: 10.1086/658669. [DOI] [PubMed] [Google Scholar]
- 4.McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320:204–10. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 5.Arif AA, Delclos GL. Association between cleaning-related chemicals and work-related asthma and asthma symptoms among healthcare professionals. Occup Environ Med. 2012;69:35–40. doi: 10.1136/oem.2011.064865. [DOI] [PubMed] [Google Scholar]
- 6.Arif AA, Delclos GL, Serra C. Occupational exposures and asthma among nursing professionals. Occup Environ Med. 2009;66:274–8. doi: 10.1136/oem.2008.042382. [DOI] [PubMed] [Google Scholar]
- 7.Delclos GL, Gimeno D, Arif AA, Burau KD, Carson A, Lusk C, et al. Occupational risk factors and asthma among health care professionals. Am J Respir Crit Care Med. 2007;175:667–75. doi: 10.1164/rccm.200609-1331OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinn MM, Henneberger PK, Braun B, Delclos GL, Fagan K, et al. National Institute for Occupational Safety and Health (NIOSH), National Occupational Research Agenda (NORA) Cleaning and Disinfecting in Healthcare Working Group. Cleaning and disinfecting environmental surfaces in health care: toward an integrated framework for infection and occupational illness prevention. Am J Infect Control. 2015;43:424–34. doi: 10.1016/j.ajic.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 9.Burge PS, Richardson MN. Occupational asthma due to indirect exposure to lauryl dimethyl benzyl ammonium chloride used in a floor cleaner. Thorax. 1994;49:842–3. doi: 10.1136/thx.49.8.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Purohit A, Kopferschmitt-Kubler MC, Moreau C, Popin E, Blaumeiser M, Pauli G. Quaternary ammonium compounds and occupational asthma. Int Arch Occup Environ Health. 2000;73:423–7. doi: 10.1007/s004200000162. [DOI] [PubMed] [Google Scholar]
- 11.Savonius B, Keskinen H, Tuppurainen M, Kanerva L. Occupational asthma caused by ethanolamines. Allergy. 1994;49:877–81. doi: 10.1111/j.1398-9995.1994.tb00791.x. [DOI] [PubMed] [Google Scholar]
- 12.Beaudouin E, Kanny G, Morisset M, Renaudin JM, Mertes M, Laxenaire MC, et al. Immediate hypersensitivity to chlorhexidine: literature review. Eur Ann Allergy Clin Immunol. 2004;36:123–6. [PubMed] [Google Scholar]
- 13.Gannon PF, Bright P, Campbell M, O’Hickey SP, Burge PS. Occupational asthma due to glutaraldehyde and formaldehyde in endoscopy and x ray departments. Thorax. 1995;50:156–9. doi: 10.1136/thx.50.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujita H, Ogawa M, Endo Y. A case of occupational bronchial asthma and contact dermatitis caused by ortho-phthalaldehyde exposure in a medical worker. J Occup Health. 2006;48:413–6. doi: 10.1539/joh.48.413. [DOI] [PubMed] [Google Scholar]
- 15.Nagy L, Orosz M. Occupational asthma due to hexachlorophene. Thorax. 1984;39:630–1. doi: 10.1136/thx.39.8.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reilly MJ, Davis LK, Tumpowsky C, Flattery J, Harrison R, Reinisch F, et al. Work-related asthma among health care workers: surveillance data from California, Massachusetts, Michigan and New Jersey, 1993–1997. Am J Ind Med. 2005;47:265–75. doi: 10.1002/ajim.20138. [DOI] [PubMed] [Google Scholar]
- 17.Kujala VM, Reijula KE, Ruotsalainen EM, Heikkinen K. Occupational asthma due to chloramine-T solution. Respir Med. 1995;89:693–5. doi: 10.1016/0954-6111(95)90137-x. [DOI] [PubMed] [Google Scholar]
- 18.Stingeni L, Lapomarda V, Lisi P. Occupational hand dermatitis in hospital environments. Contact Dermatitis. 1995;33:172–6. doi: 10.1111/j.1600-0536.1995.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 19.Donnay C, Denis MA, Magis R, Fevotte J, Massin N, Dumas O, et al. Underestimation of self-reported occupational exposure by questionnaire in hospital workers. Occup Environ Med. 2011;68:611–7. doi: 10.1136/oem.2010.061671. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) Acute antimicrobial pesticide-related illnesses among workers in health-care facilities—California, Louisiana, Michigan, and Texas, 2002–2007. MMWR Morb Mortal Wkly Rep. 2010;59:551–6. [PubMed] [Google Scholar]
- 21.Department of Health and Human Services (DHHS), National Center for Health Statistics. Third national health and nutrition examination survey, 1988–1994, NHANES III adult and examination data files (CD-ROM) Hyattsville (MD): Centers for Disease Control and Prevention; 1996. Public use data file documentation number 76200. [Google Scholar]
- 22.Grassi M, Rezzani C, Biino G, Marinoni A. Asthma-like symptoms assessment through ECRHS screening questionnaire scoring. J Clin Epidemiol. 2003;56:238–47. doi: 10.1016/s0895-4356(02)00613-3. [DOI] [PubMed] [Google Scholar]
- 23.Hawley B, Casey M, Cummings K, Edwards N, Johnson A, Cox-Ganser J, editors. National Institute for Occupational Safety and Health (NIOSH) Evaluation of exposure to a new cleaning and disinfection product and symptoms in hospital employees. Morgantown, WV: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; 2017. NIOSH HHE Report No. 2015–0053-3269. [Google Scholar]
- 24.American Conference of Governmental Industrial Hygienists (ACGIH) 2016 TLVs® and BEIs®: threshold limit values for chemical substances and physical agents and biological exposure indices. Cincinnati (OH): American Conference of Governmental Industrial Hygienists (ACGIH); 2016. [Google Scholar]
- 25.Gagnaire F, Marignac B, Hecht G, Héry M. Sensory irritation of acetic acid, hydrogen peroxide, peroxyacetic acid and their mixture in mice. Ann Occup Hyg. 2002;46:97–102. doi: 10.1093/annhyg/mef005. [DOI] [PubMed] [Google Scholar]
- 26.Pechacek N, Osorio M, Caudill J, Peterson B. Evaluation of the toxicity data for peracetic acid in deriving occupational exposure limits: a minireview. Toxicol Lett. 2015;233:45–57. doi: 10.1016/j.toxlet.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 27.National Institute for Occupational Safety and Health (NIOSH) NIOSH pocket guide to chemical hazards. Cincinnati (OH): Centers for Disease Control and Prevention, US Department of Health and Human Services; 2010. [Google Scholar]
- 28.Cristofari-Marquand E, Kacel M, Milhe F, Magnan A, Lehucher-Michel MP. Asthma caused by peracetic acid-hydrogen peroxide mixture. J Occup Health. 2007;49:155–8. doi: 10.1539/joh.49.155. [DOI] [PubMed] [Google Scholar]
- 29.Shelton D, Urch B, Tarlo SM. Occupational asthma induced by a carpet fungicide–tributyl tin oxide. J Allergy Clin Immunol. 1992;90:274–5. doi: 10.1016/0091-6749(92)90085-g. [DOI] [PubMed] [Google Scholar]
- 30.Association of Occupational and Environmental Clinics (AOEC) [Accessed August 17, 2016];Comprehensive occupational & environmental exposure database. 2015 Available from: http://www.aoecdata.org/ExpCodeLookup.aspx.
- 31.Centers for Disease Control and Prevention (CDC) Guideline for disinfection and sterilization in healthcare facilities, 2008. Atlanta (GA): Infection Control Practices Advisory Committee (HICPAC); 2008. [Google Scholar]