Abstract
Background
Biomphalaria pfeifferi is highly compatible with the widespread human-infecting blood fluke Schistosoma mansoni and transmits more cases of this parasite to people than any other snail species. For these reasons, B. pfeifferi is the world’s most important vector snail for S. mansoni, yet we know relatively little at the molecular level regarding the interactions between B. pfeifferi and S. mansoni from early-stage sporocyst transformation to the development of cercariae.
Methodology/Principal findings
We sought to capture a portrait of the response of B. pfeifferi to S. mansoni as it occurs in nature by undertaking Illumina dual RNA-Seq on uninfected control B. pfeifferi and three intramolluscan developmental stages (1- and 3-days post infection and patent, cercariae-producing infections) using field-derived west Kenyan specimens. A high-quality, well-annotated de novo B. pfeifferi transcriptome was assembled from over a half billion non-S. mansoni paired-end reads. Reads associated with potential symbionts were noted. Some infected snails yielded fewer normalized S. mansoni reads and showed different patterns of transcriptional response than others, an indication that the ability of field-derived snails to support and respond to infection is variable. Alterations in transcripts associated with reproduction were noted, including for the oviposition-related hormone ovipostatin and enzymes involved in metabolism of bioactive amines like dopamine or serotonin. Shedding snails exhibited responses consistent with the need for tissue repair. Both generalized stress and immune factors immune factors (VIgLs, PGRPs, BGBPs, complement C1q-like, chitinases) exhibited complex transcriptional responses in this compatible host-parasite system.
Significance
This study provides for the first time a large sequence data set to help in interpreting the important vector role of the neglected snail B. pfeifferi in transmission of S. mansoni, including with an emphasis on more natural, field-derived specimens. We have identified B. pfeifferi targets particularly responsive during infection that enable further dissection of the functional role of these candidate molecules.
Author summary
Biomphalaria pfeifferi is the world’s most important snail vector for the widespread human-infecting blood fluke Schistosoma mansoni. Despite this, we know relatively little about the biology of this highly compatible African snail host of S. mansoni, especially for specimens from the field. Using an Illumina-based dual-seq approach, we captured a portrait of the transcriptional responses of Kenyan snails that were either uninfected with S. mansoni, or that harbored 1-day, 3-day, or cercariae-producing infections. Responses to infection were influenced both by the extent of schistosome gene expression and infection duration. We note and discuss several alterations in transcriptional activity in immune, stress and reproduction related genes in infected snails and the B. pfeifferi symbionts detected. Several host genes were highly up-regulated following infection and these might comprise excellent candidates for disruption to diminish compatibility. This study provides for the first time a large sequence dataset to help in interpreting the important vector role of B. pfeifferi in transmission of S. mansoni, including with an emphasis on more natural, field-derived specimens.
Introduction
Schistosomiasis is one of the world’s most prevalent neglected tropical diseases with over 218 million people worldwide requiring preventive chemotherapy in 2015, 92% of those occurring in 41 countries in Africa [1]. Human schistosomiasis has a greater public health impact than usually appreciated [2], often with a disproportionate impact on children, in whom it can cause both cognitive and physical impairments [3–6]. There is a growing consensus that we need to supplement chemotherapy with other control methods, including control of the obligatory molluscan intermediate host of schistosomes [7–10]. Snail control has been identified as an important component of the most successful control programs [11].
Among the most important schistosome species infecting humans and the one with the broadest geographical range is Schistosoma mansoni. Biomphalaria pfeifferi is one of 18 Biomphalaria species known to transmit S. mansoni. Biomphalaria pfeifferi has a broad geographic distribution in sub-Saharan Africa where the majority of cases of S. mansoni occur and exhibits a high degree of susceptibility to S. mansoni [12–16]. For instance, B. pfeifferi typically shows high infection rates (50%+) following exposure to S. mansoni from locations throughout Africa, but even to isolates originating from the Americas [12]. For these reasons, it can be argued that B. pfeifferi is the world’s most important intermediate host for S. mansoni. Understanding the role of B. pfeifferi in human schistosomiasis transmission becomes more critical because expanding agriculture and water development schemes [17] and climate change [18,19] threaten to alter the geographic range of both this snail species and of S. mansoni as well.
Given B. pfeifferi’s importance in transmission of S. mansoni, it is surprising we lack even the most basic information at the molecular level about its interactions with, and responses to, S. mansoni. Such responses could be particularly interesting in the case of B. pfeifferi because it differs from other major S. mansoni-transmitting snail species in that it is a strong preferential selfing species, a characteristic potentially resulting in low genetic diversity within populations [20–23]. Our relative ignorance regarding B. pfeifferi reflects the simple fact that it is often difficult to maintain this species in the laboratory, in contrast to the Neotropical snail B. glabrata which has been the standard model laboratory snail host for S. mansoni for decades [24]. Biomphalaria glabrata surely remains an important intermediate host of S. mansoni in the Neotropics, but given that the vast majority of S. mansoni cases occur in sub-Saharan Africa, it is critical that we extend more attention to the relevant African snail, B. pfeifferi.
The advent of genomics approaches including high throughput sequencing techniques have lead over the past decade to several studies of Biomphalaria snails and their interactions with S. mansoni and other trematodes including echinostomes. All of these studies have been undertaken with B. glabrata and have been amply reviewed and discussed [25–36]. In addition, the report of the international consortium on the Biomphalaria glabrata genome has now been published [37]. Ironically, the African Biomphalaria species that are responsible for transmitting the most S. mansoni infections by far have been largely ignored with respect to application of modern high-throughput sequence-based tools.
Projects going beyond the study of individual genes or gene families of B. glabrata began with studies of expressed sequence tags [38–40], ORESTES studies [41,42], and then microarrays [43,44]. These studies showed B. glabrata has the capacity for more diverse immune responsiveness than previously known, including production of diversified molecules like FREPs (fibrinogen-related proteins) [28,45,46]. Hanington et al. [47] examined the transcriptional responses of B. glabrata during the intramolluscan development of both S. mansoni and Echinostoma paraensei, and showed snail defense-related transcripts were generally down-regulated starting shortly after infection. A later generation array including ~31,000 ESTs from B. glabrata provided new insights into how the APO or amebocyte-producing organ of B. glabrata responds to immune challenge [48], and to the effects on B. glabrata transcriptional responses of the molluscicide niclosamide that is commonly used for snail control operations [49].
Additional recent studies of the interactions between B. glabrata and S. mansoni have focused on genetic linkage studies to identify chromosome regions of interest that contain genes influencing resistance to infection [32,50,51]. Functional studies have also used RNAi to knock-down particular B. glabrata gene products shown to influence susceptibility to S. mansoni [30–32,52].
Relevant to the present study, Deleury et al. [53] published the first Illumina sequencing study with B. glabrata, and identified 1,685 genes that exhibited differential expression after immune challenge. More recent studies employing RNA-Seq have identified B. glabrata genes associated with a state of heightened innate immunity [54] or with differential response of FREPs in B. glabrata strains differing in their susceptibility to S. mansoni [34]. Despite the fairly extensive efforts with respect to gene and genomic sequencing, gene profiling, or transcriptomics for B. glabrata and to a lesser extent for Oncomelania hupensis [55,56], the snail host of Schistosoma japonicum, to date there have been no equivalent studies published for B. pfeifferi, or for other schistosome-transmitting planorbid snails, including species of Bulinus, several of which transmit members of the Schistosoma haematobium species group in Africa, southern Europe and southwest Asia.
With this in mind, we have undertaken an Illumina RNA-Seq study of B. pfeifferi, and of B. pfeifferi infected with S. mansoni for 1 or 3 days, or with naturally acquired cercariae-shedding or “patent” infections. The intramolluscan transcriptional responses of S. mansoni will be the subject of a separate paper. The challenge of parsing S. mansoni sequences from the aggregate of reads obtained from infected B. pfeifferi has been aided by availability of the S. mansoni genome [57] and stage-specific transcriptional studies for S. mansoni [58–60].
Our view of schistosome-snail encounters has also been largely formed by studies of lab-reared snails and schistosomes. RNA-Seq offers a way to bridge and expand upon these traditional views by revealing the detailed molecular and cellular mechanisms taking place in genetically diverse hosts and parasites. This is the first Illumina study performed on samples of both field-derived vector snails and their corresponding schistosome parasites, adding a unique perspective to our understanding of schistosome transmission “in the wild” in endemic regions. This approach also serves to remind us that the snails targeted for infection by schistosome miracidia in the field are best considered as holobionts with potentially complex sets of symbiotic associates [61,62]. Finally, we note that this study will add to the literature a considerable amount of new data for B. pfeifferi, an important neglected vector species that has hitherto been understudied. Included among the snail genes highlighted are several that relate to stress, immune or reproductive functions, or that may be key players in influencing the noteworthy widespread ability of this snail to support schistosomiasis transmission.
Methods
Ethics and permissions statements
We enrolled human subjects who provided fecal samples containing Schistosoma mansoni eggs that were hatched to obtain miracidia used to infect some of the Biomphalaria pfeifferi snails used in this study. Fecal samples were obtained and pooled from five S. mansoni-positive primary school children aged 6–12 years from Obuon primary school in Asao, Nyakach area, Nyanza Province, western Kenya (00°19’01”S, 035°00’22”E). Written and signed consent was given by parents/guardians for all children. The KEMRI Ethics Review Committee (SSC No. 2373) and the UNM Institution Review Board (IRB 821021–1) approved all aspects of this project involving human subjects. All children found positive for S. mansoni were treated with praziquantel following standard protocols. Details of recruitment and participation of human subjects for fecal collection are described in Mutuku et al. [15]. This project was undertaken with approval of Kenya’s National Commission for Science, Technology, and Innovation (permit number NACOSTI/P/15/9609/4270), National Environment Management Authority (NEMA/AGR/46/2014) and an export permit has been granted by the Kenya Wildlife Service (0004754).
Sample collection and experimental exposures
Biomphalaria pfeifferi used in Illumina sequencing were collected from Kasabong stream in Asembo Village, Nyanza Province, western Kenya (34.42037°E, 0.15869°S) in November 2013. Snails were transferred to our field lab at The Centre for Global Health Research (CGHR) at Kisian, western Kenya. Snails sized 6-9mm in shell diameter were placed into 24-well culture plates and exposed to natural light to check for the shedding of digenetic trematode cercariae, including cercariae of S. mansoni [15]. Snails found to be shedding cercariae of other digenetic trematode species were excluded from this study.
Snails shedding S. mansoni cercariae and non-shedding snails (controls) were separated and held for one day in aerated aquaria containing dechlorinated tap water and boiled leaf lettuce. After cleaning shells with 70% EtOH, whole shedding and control snails were placed individually into 1.5ml tubes with 1ml of TRIzol (Invitrogen, Carlsbad CA) and stored at -80°C until extraction.
Biomphalaria pfeifferi confirmed to be uninfected were exposed to S. mansoni using standard methods to hatch the parasite eggs [15]. Snails were individually exposed to 20 miracidia for 6 hours in 24-well culture plates and then returned to aquaria. At 1 and 3 days post-infection (d), snails were collected and stored in TRIzol as described above. We chose not to maintain the field-derived snails for longer intervals post-infection as we did not want them to lose their unique field-associated properties while maintained in laboratory aquaria.
In addition to the Illumina RNA-Seq samples indicated above and mentioned throughout this study, we have RNA-Seq data from B. pfeifferi obtained from two 454 GS FLX (Roche, Basel Switzerland) runs and six Illumina-sequenced B. pfeifferi exposed to molluscicide, all field-derived from Kenya (Table 1). These reads were used to aid assembly of the B. pfeifferi de novo transcriptome and were not included in expression studies.
Table 1. Samples used for the study with total read numbers and the percent of reads mapping to the S. mansoni genome that were filtered prior to de novo assemblies.
| Field-collected samples | Replicate | Abbreviation | Paired-end reads mapping to S. mansoni genome‡ | Paired-End Reads/Sample (post- quality filtering) |
|---|---|---|---|---|
| B. pfeifferi control | 1 | control-R1 | 0.07% | 28,903,992 |
| 2 | control-R2 | 0.08% | 34,318,971 | |
| 3 | control-R3 | 0.04% | 27,557,936 | |
|
B. pfeifferi x S. mansoni 1 day post infection (1d) |
1 | 1d-R1 | 0.1% | 36,450,649 |
| 2 | 1d-R2 | 1.5% | 33,634,117 | |
| 3 | 1d-R3 | 1.9% | 30,932,207 | |
|
B. pfeifferi x S. mansoni 3 days post infection (3d) |
1 | 3d-R1 | 4.1% | 30,648,913 |
| 2 | 3d-R2 | 0.1% | 26,445,297 | |
| 3 | 3d-R3 | 13.2% | 31,159,822 | |
| B. pfeifferi shedding S. mansoni (S) | 1 | shedding-R1 | 3.7% | 32,200,842 |
| 2 | shedding-R2 | 8.2% | 33,570,583 | |
| 3 | shedding-R3 | 0.5% | 27,569,638 | |
| B. pfeifferi control x molluscicide | 1 | * | * | 35,289,769 |
| 2 | * | * | 34,450,509 | |
| 3 | * | * | 25,652,418 | |
| B. pfeifferi shedding S. mansoni x molluscicide | 1 | * | * | 30,587,208 |
| 2 | * | * | 35,071,339 | |
| 3 | * | * | 28,843,961 |
*Samples used in the assembly but expression results not discussed in this paper
‡ See Methods for explanation of S. mansoni read mapping
RNA extraction, library preparation, and sequencing
Individual snails stored in TRIzol were homogenized using plastic pestles (USA Scientific, Ocala FL). For each biological treatment (control, 1d, 3d, and shedding), total RNA was purified separately from three individual snails (each snail a biological replicate) using the TRIzol protocol provided by the manufacturer (Invitrogen, Carlsbad CA). RNA samples were further purified using the PureLink RNA Mini Kit (ThermoFisher Scientific, Waltham MA). Genomic DNA contamination was removed with RNase-free DNase I (New England BioLabs, Ipswich MA) at 37°C for 10 minutes. This combination method based on the two RNA extraction assays had been developed in our lab and proved to produce a high quality of RNA from snail samples [47]. RNA quality and quantity was evaluated on a Bioanalyzer 2100 (Agilent Technologies, Santa Clara CA) and Nanodrop 2000 (ThermoFisher Scientific, Waltham MA).
Complementary DNA (cDNA) synthesis and Illumina Hi-Seq sequencing was performed at the National Center for Genome Resources (NCGR) in Santa Fe, NM. Most liquid handling was performed by a Sciclone G3 Automated Liquid Handling Workstation (Caliper Life Sciences, Hopkinton MA) with Multi TEC Control (INHECO, Martinsried Germany). Synthesis of cDNA and library preparation was prepared using Illumina TruSeq protocol according to the manufacturer’s instructions (Illumina, Carlsbad CA). Complementary DNA libraries were paired-end sequenced (2x 50 base reads) on a HiSeq2000 instrument (Illumina, Carlsbad CA).
Pre-processing of Illumina reads and isolation of B. pfeifferi reads
Sequencing adapters, nucleotides with a Phred quality score <20 within a sliding window of 4bp, and non-complex reads were removed using Trimmomatic v.0.3 [63]. Raw read quality control checks were performed before and after Trimmomatic filtering using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/).
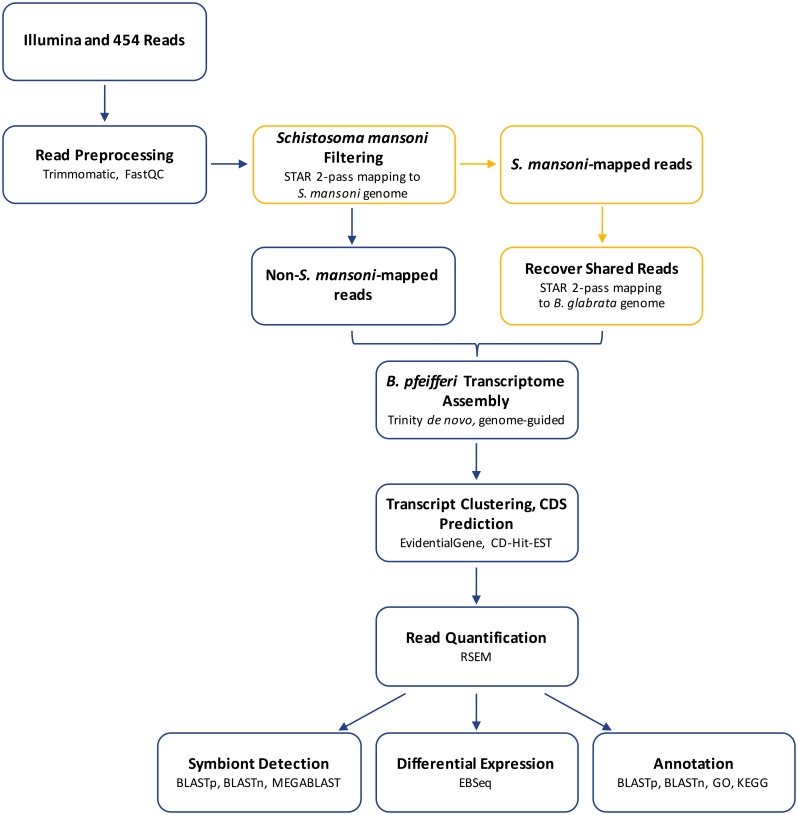
To reduce assembly of chimeric transcripts, we created a novel pipeline to separate reads of related organisms when only one organism has a sequenced genome while also allowing for recovery of shared reads (Fig 1). First, all reads (including control samples) that passed quality filtering were aligned to the S. mansoni genome (GeneDB: S. mansoni v5.0) using STAR v.2.5 2-pass method [64] or Tophat v.2 [65] (see Table 1 for alignment percentages). From examination of the percentage values in Table 1, it may be interpreted that unexposed control actually harbor S. mansoni. However, the reads contributing to the positive percentage values for the controls are ones that we have found to be shared with either B. glabrata or another organism such that they represent a background level of sequence similarity obtained by chance. Although partial mapping of reads may occur, none appear to be expressed S. mansoni transcripts. None of the unexposed control reads mapping to the S. mansoni genome are unequivocally S. mansoni. By contrast, S. mansoni-exposed snails (1d, 3d, shedding) all expressed bona fide S. mansoni genes. Only in 1d, 3d, and shedding snails were transcripts clearly distinctive to S. mansoni found, such as venom allergen proteins (SmVal) (Accessions: AAY43182.1, AAY28955.1, AAZ04924.1, ABO09814.2), tegument allergen-like proteins (Accession: P14202), and cercarial stage-specific proteins (Accession: ABS87642.1), verifying the presence of a S. mansoni infection. This explanation also serves to verify that individual snails (such as 1dR2) with low S. mansoni percentages were indeed infected, such that they could be expected to be responsive to infection. Therefore, relatively low S. mansoni genome mapping, especially for shedding-R3, should not be interpreted that the infection was not successful, but rather as an indication of the transcriptional activity.
Fig 1. Overview of novel bioinformatics pipeline developed to isolate and analyze B. pfeifferi transcriptomic expression from dual RNA-Seq data.
Reads that mapped to S. mansoni were also cross-examined by mapping to the version BglaB1 of the B. glabrata genome (https://www.vectorbase.org/organisms/biomphalaria-glabrata) using STAR. Reads that first mapped to S. mansoni and then also to B. glabrata were determined to be shared reads and added to the reads destined for B. pfeifferi transcriptome de novo assembly.
One issue encountered was to deal with both paired- and single-end reads resulting from initial quality filtering and from discordant or single-mate mapping to the S. mansoni genome. Pseudo-mate reads were created to allow maximum read usage in all stages of analysis (details and script available at https://github.com/lijingbu/RNA-Seq-Tools). This tool, pseudoFastqMate.pl, creates pseudo mate reads for single reads in a fastq file by generating a string of N’s the same length and quality score as its mate read. Reads entirely made up with Ns were ignored during the mapping process and have no impact on the final alignment and read counts.
De novo transcriptome assembly and annotation
Unaligned paired and unpaired reads, determined not to solely belong to S. mansoni, were assembled using Trinity v2.2 RNA-Seq de novo assembler [66,67]. Trinity de novo and B. glabrata genome-guided assemblies were employed to maximize the chances of recovering unique B. pfeifferi transcripts. The de novo assemblies were concatenated and redundancy reduced using the EvidentialGene tr2aacds pipeline [68]. EvidentialGene determines the best set of transcripts based on the coding potential of transcripts generated from multiple assemblies. Only primary transcripts, denoted in EvidentialGene as “okay” and “okalt” were used in further analysis. In silico translation of the transcriptome was done using TransDecoder v3.0 (https://transdecoder.github.io) [65] to extract long open reading frames (ORFs) and identify ORFs with homology to known proteins with blast and pfam searches.
Biomphalaria pfeifferi CDS were annotated based on their closest homologs and predicted functional domains in the following databases and tools: BLASTp with NCBI non-redundant protein database (sequence identity >30%, E-value <10−06), BLASTn with NCBI nucleotide database (sequence identity >70%, E-value < 10−06), Gene Ontology [69], KEGG [70], and InterProScan5 [71]. For query CDS whose top hit was “uncharacterized”, “hypothetical”, or otherwise unknown, the consensus hit (of up to 20 hits that also meet minimum sequence identity and E-value requirements shown above is reported to help elucidate any putative function. Additionally, B. pfeifferi CDS were further scrutinized against molluscan transcripts and proteins identified in the literature.
Identification of non-snail and non-parasite reads
As a consequence of sequencing field-collected specimens, we expected some reads to be of non-B. pfeifferi and non-S. mansoni origin. Screening for the presence of third party symbionts was one of our motivations for investigating field-derived snails in the first place. We performed the de novo assembly pipeline without first removing non-snail or non-schistosome sequences to get a more complete view of the complex environment in which S. mansoni development takes place. CDS coverage, sequence identity, and E-value of BLASTn, BLASTp, and MEGABLAST results were all taken into consideration when determining organism identification. The BLASTn and MEGABLAST against the NCBI nucleotide database had minimum sequence identity of 70% and E-value <10−06 and the BLASTp against the NCBI protein database had a minimum sequence identity of 30% and E-value <10−06. Query coverage (qcov) was also calculated in all BLASTs. When different BLASTs disagreed in their taxonomic assignment, the hit with highest percent query coverage, highest sequence identity, and lowest E-value was chosen, in that order. Although minimum parameters were set, nearly all CDS BLAST hits exceeded these bounds. BLASTp hits tended to have better quality hits because nucleotide sequences from the NCBI nucleotide database often contained non-coding regions that our CDS lack. CDS designated as “undetermined” had hits that did not meet minimum BLAST parameters. CDS that had a non-molluscan BLAST hit but still mapped to the B. glabrata genome (sequence identity >70%, E-value <10−06) were considered “shared” sequences.
Non-B. pfeifferi and non-S. mansoni CDS were categorized into 14 broad taxonomic groups: Mollusca, Amoebozoa, SAR, Viruses, Plantae, Fungi, Bacteria, Rotifera, Platyhelminthes, Arthropoda, Annelida, Nematoda, Chordata, and Miscellaneous. Potential trematode CDS were further filtered to require a minimum of 70% query coverage. Genomes and CDS of specific symbionts of interest (if publicly available) were interrogated using BLASTn (>70% identity, E-value <10−06, query coverage >70%).
Identification of toll-like receptors (TLR) and variable immunoglobulin lectins (VIgLs)
Given that a number of previous studies of Biomphalaria immunobiology have focused on molecules with TLR or immunoglobulin domains, we undertook an analysis of these groups of molecules. Biomphalaria pfeifferi CDS with a BLASTp or BLASTn annotation as a toll-like receptor (TLR), were further screened for toll/interleukin-1 receptor (TIR), leucine-rich repeats (LRR), and transmembrane regions with InterProScan5 and TMHMM (Transmembrane helix prediction based on hidden Markov model) [72]. CDS identified as complete TLRs contained TIR, transmembrane, and LRR domains. Similarly, CDS annotated as a VIgL (FREPs, CREPs, GREPs, and FREDs) were scanned for an immunoglobulin domain and a fibrinogen, C-type lectin, or galectin domain using InterProScan5. For CDS to be identified as a FREP, CREP, or GREP, they had to contain a lectin domain and at least one immunoglobulin domain.
Transcriptome completeness
To estimate the completeness of our B. pfeifferi transcriptome assembly and assess similar transcripts across related species, B. pfeifferi predicted ORFs were compared to other molluscan peptides (the cephalopod Octopus bimaculoides, the oysters Crassostrea gigas and Pinctada fucata, the owl limpet Lottia gigantea, the California sea hare A. californica, as well as two pulmonates: B. glabrata and Radix balthica) using BLASTp (sequence identity >30%, E-value <10−06). ORFs with 100 or more amino acids were extracted from each transcriptome. To maximize sensitivity for retaining ORFs that may have functional significance, predicted ORFs were scanned for homology to known proteins in the Uniref90 database with a subsequent search using PFAM and hmmer3 to identify protein domains.
Differential expression analyses
Properly paired reads not filtered as S. mansoni were mapped to EvidentialGene-generated B. pfeifferi CDS with Bowtie2 [73]. Read abundance was quantified with RSEM (RNA-Seq by expectation maximization) [74]. Pairwise analyses for comparisons between control group and other infected groups were run in EBSeq [75]. Transcripts with a posterior probability of differential expression (PPDE) > = 0.95 were considered significant. With the aim of detecting less abundant transcripts that may still have significant biologically effects (i.e. neuropeptides), we deliberately did not set a minimum read count threshold for detection of DE CDS in EBSeq.
Variation among infected snails with respect to representation of S. mansoni reads, and testing among them for associated differences in host responses
As noted above, field-collected specimens of both snails and schistosomes are naturally more genetically diverse than lab-reared counterparts, so variation in response among infected snails might be expected. In fact, by chance, for each of the time points studied, one of the 3 infected snails examined differed notably from the other two in having fewer normalized S. mansoni read counts (suggestive of less extensive parasite activity and/or more effective host limitation of parasite development). We hypothesized that the snail response is influenced by the extent of S. mansoni representation, as assessed by examining normalized parasite read counts from each infected snail. In addition to doing “3 controls vs. 3 infected” (3v3) comparisons, for each time point we also examined “3 control vs. 2 infected” (3v2) comparisons where the two snails harbored higher S. mansoni read counts to identify CDS whose responses were associated with S. mansoni abundance. We also performed “3 control vs. 1 infected” (3v1) comparisons where the one infected snail was the one with low S. mansoni read counts. The overall DE results include all CDS that were differentially expressed in any of the three comparisons, the results for each comparison being separately singled out and enumerated.
Quantitative PCR validation of differential expression
cDNA was synthesized from 5μg of total RNA from the original samples by the SuperScript II First-Strand Synthesis Kit for RT-PCR (Invitrogen) in a 20μl reaction using random hexamers. Manufacturer directions were followed for the reaction profile. An additional 80μl of molecular grade water was added to the cDNA for a final volume of 100μl. qPCR target primer sequences were generated in Primer3 software [76] and details are shown in S1 Table. We tested probes for single-copy genes only and final selection of qPCR targets were chosen to highlight the variability between replicates. Primer testing verified one product was produced in traditional PCR amplification and in melt curve analyses. RT-qPCR reactions were performed in 20μl reactions according to manufacturer’s directions using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratories, Hercules CA) with 0.5μM primer concentration and 2μl cDNA. Reactions were denatured at 95°C for 2 minutes followed by 40 cycles of 95°C for 5 seconds and annealing/extension and plate read for 30 seconds. Melt curve analysis was performed from 65–95°C at 0.5°C increments for 5 seconds. All biological replicates were run in technical triplicate for each transcript on a Bio-Rad CFX96 system and analyzed with Bio-Rad CFX Manager software.
Results
Transcriptome sequencing, assembly, and annotation
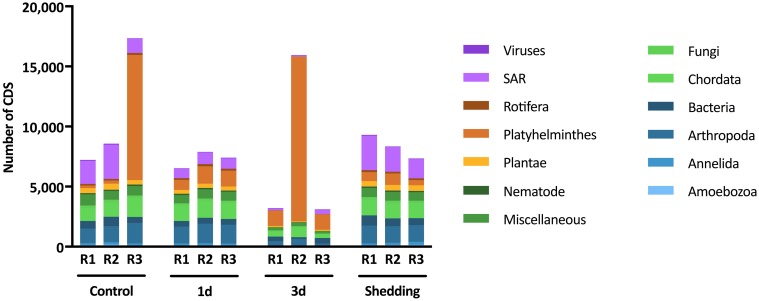
To investigate the gene expression profiles of B. pfeifferi following infection with S. mansoni, we analyzed the transcriptome from Illumina sequencing of infected snails at 1-day (1d), 3-day (3d), and from shedding snails using three biological replicates each (Table 1). The raw and assembled sequence data are available at NCBI under BioProject ID PRJNA383396. The results and statistics describing the B. pfeifferi assembly are summarized in Table 2. Trinity de novo transcript assemblies and additional reads from two 454 runs resulted in 1,856,831 contigs. The EvidentialGene program generated a non-redundant B. pfeifferi transcriptome of 194,344 protein-coding sequences (CDS) that includes isoforms. From nucleotide sequence length histograms, we calculated that more than half of the CDS were between 300–499 nucleotides with 6.7% > = 1500 nucleotides (S1 Fig).
Table 2. Illumina sequencing and B. pfeifferi de novo transcriptome assembly summary metrics.
| Raw Illumina data | |
| Number of paired-end reads sequenced | 563,288,171 |
| Number of reads sequenced | 1,126,576,342 |
| Reads surviving quality filtering and trimming | 1,120,661,048 |
| Reads surviving S. mansoni filtering | 1,048,936,142 |
| Filtered reads used in de novo assemblies | 1,048,936,142 |
| Assembled contigs | 1,805,496 |
| Trinity de novo Illumina | 201,573 |
| Trinity de novo Illumina including shared reads | 225,929 |
| Genome-guided Trinity de novo Illumina | 62,682 |
| Genome-guided Trinity de novo 454 | 71,199 |
| Additional 454 reads | 1,244,113 |
| EvidentialGene clustering | |
| Okay + Okay alternate coding sequences (CDS) | 194,344 |
| % GC | 44.24 |
| N50 | 654 |
| Longest CDS length | 28,302 |
| Median CDS length | 447 |
| Average CDS length | 634.57 |
| Clusters > = 1Kb | 24,802 |
| % positive strand orientation | 53.2% |
| % negative strand orientation | 46.8% |
| TransDecoder-predicted open reading frames (ORFs) | |
| Total predicted ORFs (minimum length = 100 aa) | 166,921 |
| Longest ORF length (aa) | 9,434 |
| Median ORF length (aa) | 157 |
| Average ORF length (aa) | 232.07 |
| Average ORF size of 1,000 longest CDS | 2014.1 |
Five publicly available databases were used to annotate and obtain functional information for the CDS (S1 File; Table 3). The top 20 most common GO assignments are shown in S2 Fig. Six KEGG categories are shown with their constituent classes organized by abundance in S3 Fig. Altogether, 179,030 of 194,344 total (92.1%) CDS were annotated from at least one of the five databases shown in Table 3.
Table 3. CDS and predicted protein annotations using publicly available databases.
| Public Database | Annotation Summary |
|---|---|
| BLASTp x nr | 140,484 CDSs (72.3%) 49,518 unique protein identities |
| BLASTn x nt | 128,028 CDSs (65.9%) 26,708 unique nt identities |
| InterProScan | 137,778 (70.9%) |
| Gene Ontology (GO) | 50,870 CDSs (26.2%) |
| Unique Molecular Function | 3,246 |
| Unique Cellular Component | 1,618 |
| Unique Biological Process | 8,282 |
| KEGG | 145,197 CDSs (74.7%) |
| Unique KEGG orthologous groups | 3,824 |
| Unique KEGG pathways | 387 |
| Unique KEGG classes | 46 |
| Unique KEGG categories | 6 |
| Cellular Processes | 13,845 |
| Environmental Information Processing | 16,093 |
| Genetic Information Processing | 13,722 |
| Human Diseases | 32,748 |
| Metabolism | 41,022 |
| Organismal Systems | 27,767 |
Identification of toll-like receptors (TLRs) and variable immunoglobulin lectins (VIgLs)
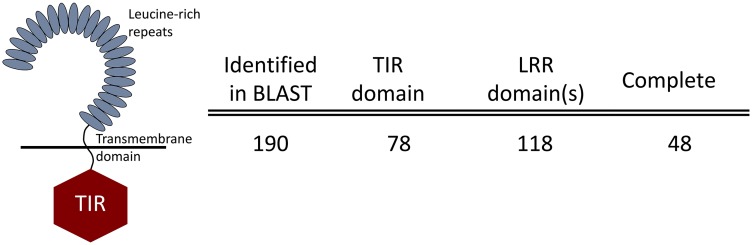
Pattern recognition receptors like TLRs and VIgLs (FREPs, CREPS, and GREPs) are key components of the innate immune response and their involvement in the B. glabrata defense response has been documented [28,77]. The B. glabrata genome contains 56 TLR (toll-like receptor) genes, 27 of which encode complete TLRs [37]. Our B. pfeifferi transcriptome had 190 CDS annotated as a homolog to a B. glabrata TLR (Fig 2). Note that numbers assigned to TLRs in B. glabrata were assigned in the order they were identified and not by homology to vertebrate TLRs. The TLR numbers we refer to for B. pfeifferi match most closely the TLR with the corresponding number from B. glabrata. InterProScan5 analysis revealed 78 of B. pfeifferi TLR CDS contain a TIR (toll/interleukin receptor) domain and 118 have at least one LRR (leucine-rich repeat) domain. In total, we found 48 complete B. pfeifferi TLRs (TIR, transmembrane, LRR domains all present) and 142 partial homologs to B. glabrata TLRs (annotated as a TLR, but not all domains complete and/or confidently identified) in our transcriptional study. Others may certainly exist in the genome of B. pfeifferi.
Fig 2. Identification of the innate immune recognition receptors TLRs in B. pfeifferi.
Partial CDS counts had a BLAST hit against a known TLR but all necessary domains could not be confidently determined by InterProScan5.
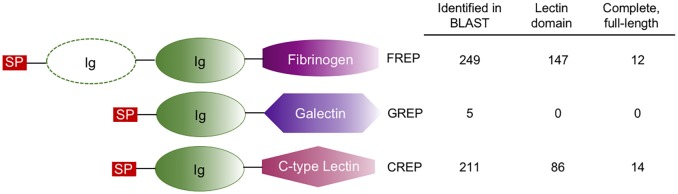
There are 22 FREP genes in the B. glabrata genome [37,77] and all were represented in our B. pfeifferi transcriptome, at least in part. Our BLAST annotations identified 249 B. pfeifferi CDS homologous to B. glabrata FREPs and 12 of these were verified to be full-length FREP homologs (Fig 3). There were no full-length, complete GREPs identified in our transcriptome, but there were 5 CDS with a BLAST annotation homologous to one B. glabrata GREP identified by Dheilly et al. [77] (Fig 3). Four CREPs (C-type lectin protein) have been identified in B. glabrata [77] with 2 of the 14 full-length, complete B. pfeifferi CDS homologous to CREP 1 in B. glabrata (Fig 3).
Fig 3. Identification of the innate immune recognition receptors VIgLs in B. pfeifferi with initial BLAST annotation and then verification of protein domains in InterProScan5.
Sequence homology between related mollusc species
A BLASTp comparison between B. pfeifferi and B. glabrata shows high sequence similarity with 35,150 (95.8%) polypeptides shared between the two species (sequence identity >30% and E-value <1e-06) (Table 4). We found 1,525 B. glabrata polypeptides without homologs in our B. pfeifferi transcriptome. With respect to the 127,626 translated CDS that have homologs to B. glabrata polypeptides, more than half of these have a sequence identity greater than 90% (S4 Fig). To further assess the completeness and to enhance annotation of our B. pfeifferi transcriptome, we searched for homologous polypeptides from genomes of two additional gastropods (Aplysia californica and Lottia gigantea [78]), two bivalves (Pinctada fucata [79]) and Crassostrea gigas [80]), and one cephalopod (Octopus bimaculoides [81]) (Table 4). Shown in S5 Fig is one hypothesis of the phylogeny of molluscs, and mapped onto this are the mollusc genomes that are currently available [82]. Note that the percent identity of homologous sequences follows the general branching pattern. The California sea hare, A. californica, has 88.3% of its polypeptides homologous to B. pfeifferi peptides. The most distantly related mollusc, the California two-spot octopus, O. bimaculoides, is 56.7% homologous at the protein level to B. pfeifferi.
Table 4. Number of polypeptides queried in various molluscs and matches with B. pfeifferi TransDecoder-predicted ORFs.
| Reference | # Reference polypeptides | B. pfeifferi polypeptides matched to reference polypeptides | Download location |
|---|---|---|---|
| Biomphalaria glabrata v1.0 [37] | 36,675 | 127,626 | https://www.ncbi.nlm.nih.gov/genome/annotation_euk/Biomphalaria_glabrata/100/ |
| Aplysia californica v3.0* | 27,591 | 99,884 | http://www.ncbi.nlm.nih.gov/genome/annotation_euk/Aplysia_californica/101/ |
| Lottia gigantea v1.0 [78] | 188,590 | 74,494 | http://genome.jgi.doe.gov/Lotgi1/Lotgi1.download.ftp.html |
| Pinctada fucata v2.0 [79] | 31,477 | 77,341 | http://marinegenomics.oist.jp/pearl/viewer/download?project_id=36 |
| Crassostrea gigas v9 [80] | 45,406 | 80,505 | ftp://ftp.ncbi.nlm.nih.gov/genomes/Crassostrea_gigas/ |
| Octopus bimaculoides v2.0 [81] | 38,585 | 71,395 | http://genome.jgi.doe.gov/pages/dynamicOrganismDownload.jsf?organism=Metazome |
*Genome is publicly available at link provided
Other organismal sequences derived from the de novo assembly
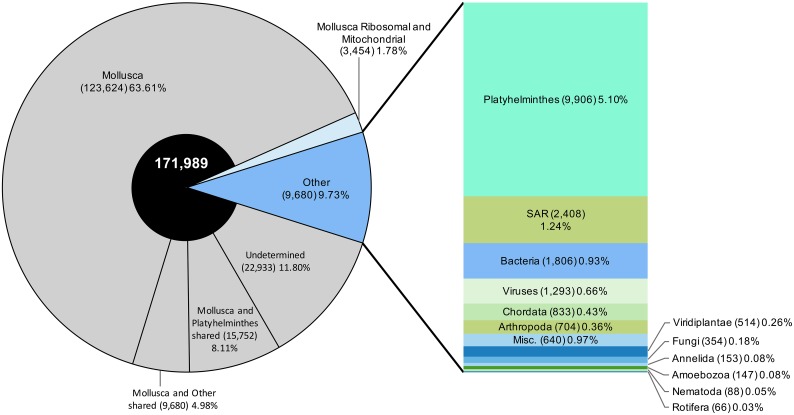
Of the 194,344 CDS assembled post-S. mansoni read filtering, 18,907 (9.73%) of these were determined to be of non-mollusc origin (Fig 4). Some of the non-B. pfeifferi transcripts found were bacteria with most belonging to the genera Escherichia, Mycoplasma, Aeromonas, and Pseudomonas (Fig 5). Among them, a CDS with homology to Neorickettsia sp, a known obligatory symbiont of digenetic trematodes [83], was recovered and has read counts >10 in 2 of our samples that also had relatively high counts of S. mansoni (3d-R3 and shedding-R1) (Table 1; S2 File). In addition, there are three CDS assembled from the infected 454 B. pfeifferi sample that were identified as Paenibacillus spp. and were similar, but not identical, to the snail pathogen Candidatus Paenibacillus glabratella (S2 File) [84].
Fig 4. Identification of all de novo assembled transcripts after S. mansoni read filtering.
Fig 5. Sum of non-B. pfeifferi de novo assembled CDS for each replicate. CDS were counted as present if read count >0.
Among the eukaryotic sequences retrieved from generation of the de novo assembly, there are some familiar snail symbionts listed in S2 and S3 Tables including 1) Chaetogaster annelids, 2) Trichodina ciliates, and 3) Capsaspora owczarzaki [85] and 4) microsporidians [86–89] (see also S2 File and Discussion for further comments).
In addition to prokaryotes and eukaryotes, nearly 1,300 of our assembled CDS were provisionally identified as viruses (Fig 4). Sample Control-R2 had the highest abundance of reads mapping to the viral sequences compared to the other samples, though some putative viral sequences were recovered from all 12 snails examined.
Lastly, even after the initial screening and removal of S. mansoni reads from the nine snails with known S. mansoni infections, some reads remained that were classified as platyhelminth in origin (Fig 4). Two individual snails in particular, control-R3 and 3d-R2, the latter a replicate with low S. mansoni read counts, had many platyhelminth reads (Fig 5). We sequenced a 28S rRNA gene from cDNA of control-R3 using digenean-specific primers [90] to determine if other digeneans were present in our sample. The resulting 28S sequence was identified as belonging to the genus Ribeiroia, members of which are known to occur in East Africa and to infect Biomphalaria [91]. Most of the platyhelminth CDS present in this sample were identified as “hypothetical” but CDS with the highest read abundance are involved in membrane transport and cell structural functions. For 3d-R2, cox1 mitochondrial gene primers amplified an amphistome sequence that groups phylogenetically with an amphistome species (provisionally Calicophoron sukari) that uses B. pfeifferi from East Africa as a first intermediate host [92]. Like control-R3, CDS with the highest read abundance in 3d-R2 were membrane associated and structural with the addition of several myoglobins and surface glycoprotein CDS.
Variation among infected snails with respect to the representation of S. mansoni reads, and associated responses
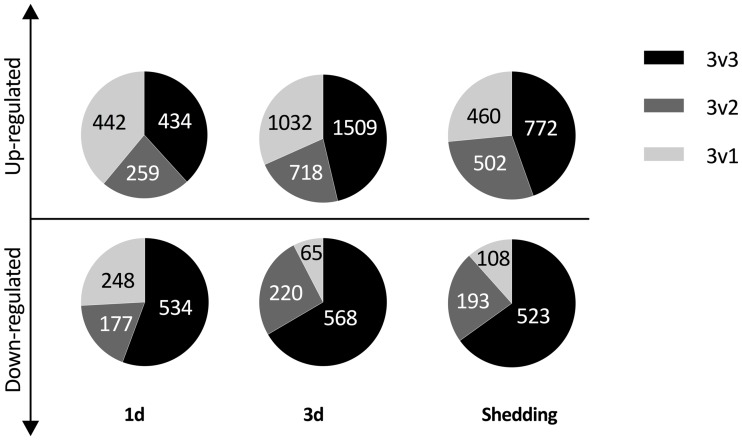
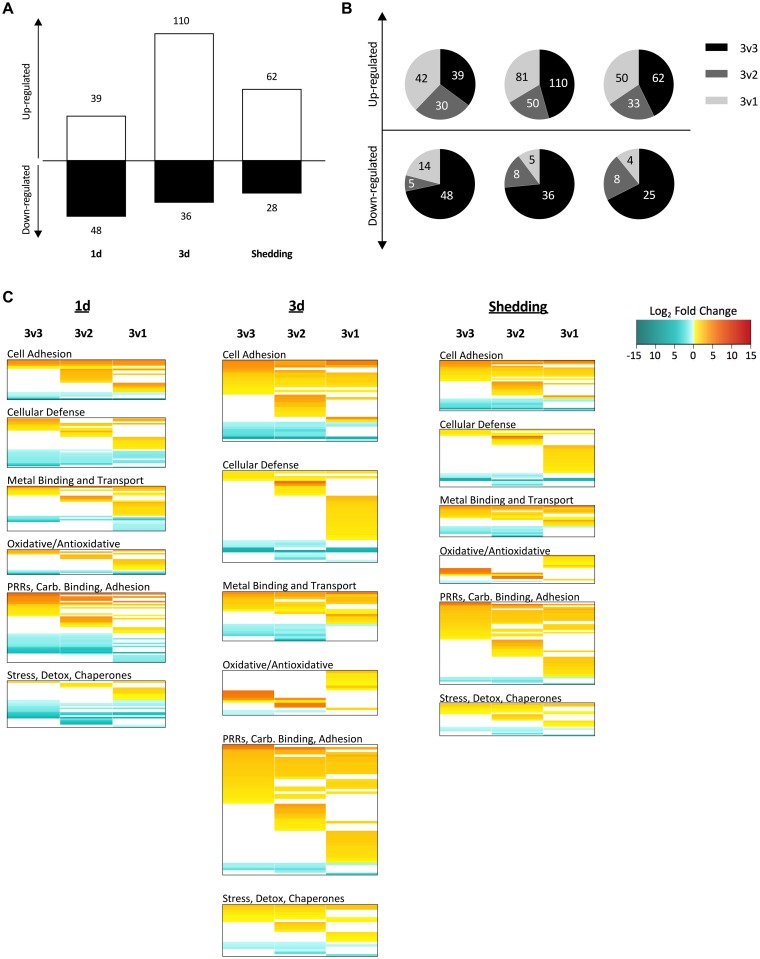
The extent of representation of S. mansoni in the dual transcriptome as measured by read counts is variable among the three replicates for each development time sampled in shedding snails (Table 1). Normalized read abundance of S. mansoni housekeeping genes remained consistently high across all samples, eliminating the possibility that S. mansoni read count variability was due to sampling effects. Because of this inherent variability, we performed additional DE comparisons to the traditional 3 control v 3 experimental (3v3) replicates isolating either the two snails that contained higher S. mansoni read counts (3v2 analysis) or the one snail with the fewest S. mansoni read counts of each triplicate time point (3v1). With respect to the overall response patterns of snails that yielded either high or low numbers of S. mansoni reads, in most cases, for both up- and down-regulated CDS, the majority of significantly differentially expressed CDS fell into the 3v3 comparison category (Fig 6), indicative of uniformity of response across infected snails. For up-regulated features, there were also substantial additional numbers of significant CDS in the 3v2 or 3v1 infected categories, with the latter being greater in 2 of 3 cases. By contrast, for the down-regulated features, at 1d, the snails with high or low S. mansoni read counts did not as clearly differentiate from one another, but the snails with low read counts for S. mansoni (3v1) clearly showed an additional allotment of down-regulated features. For the other two time points, the snails with high and low S. mansoni read counts did separate from one another, and especially noteworthy is the relatively small proportion of down-regulated features in the 3v1 comparisons.
Fig 6. Pie charts of unique CDS found to be differentially expressed in 3v3, 3v2, and 3v1 EBSeq analyses.
B. pfeifferi CDS responsive during S. mansoni infection
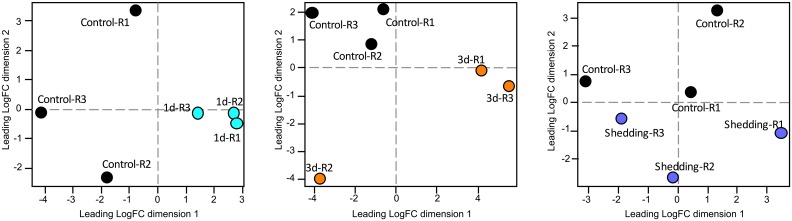
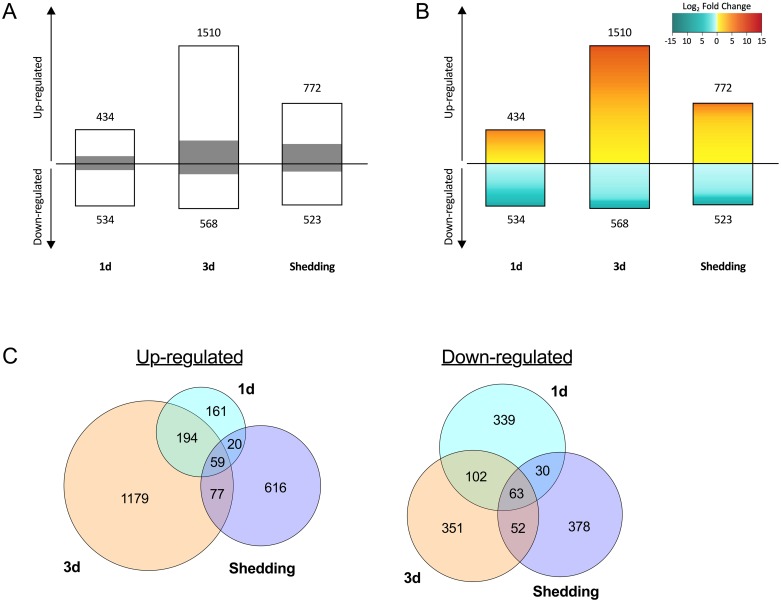
S3 File provides a summary of all CDS retrieved in the DE analysis, S4 File summarizes those general, reproduction or immune system features that were most differentially expressed, and Tables 5 and 6 distill CDS (see Discussion also) that we feel are most worthy of further functional study in B. pfeifferi. Multidimensional scaling (MDS) plots show that for each of the three groups of infected snails, overall transcript expression of the experimental groups is distinct from the control groups (Fig 7). At 1d, snails showed a slight preponderance of down-regulated over up-regulated CDS, but in both 3d and shedding snails, the opposite trend was observed (Fig 8A and 8B). Overall, the most transcriptional activity was in the 3d snails. All three groups of infected snails (1d, 3d, shedding) showed distinct transcriptional profiles, suggesting the snail response is different at each time point (Fig 8C). Generally, each of the three groups has more unique responsive CDS than they do in common with one another. As anticipated, 1d and 3d snails have more shared transcripts both up- and down-regulated than either do with the shedding snails.
Table 5. Highly up- or down-regulated B. pfeifferi CDS whose response may be required for maintaining a patent S. mansoni infection.
| B. pfeifferi CDS | Annotation | Log2FC 3v3 | Log2FC 3v2 | Log2FC 3v1 |
|---|---|---|---|---|
| evgTRINITY_DN89401_c6_g1_i1 | GD13313-like | 9.14 | 9.58 | 6.75 |
| evgTRINITY_DN19832_c0_g1_i1 | deleted in malignant brain tumors 1 protein-like | 6.65 | 7.09 | 4.48 |
| evgTRINITY_DN95353_c0_g1_i1 | collagen alpha-3(VI) chain-like | 6.41 | 6.80 | |
| evglcl|G0WVJSS02FGR88 | cAMP-dependent prot kinase catalytic subunit-like | 6.17 | 6.60 | 4.15 |
| evgTRINITY_DN84392_c3_g1_i1 | galactocerebrosidase-like | 5.41 | 5.53 | 5.14 |
| evgTRINITY_DN104940_c0_g1_i1 | cAMP-dependent prot kinase catalytic subunit-like | 5.32 | 5.79 | 3.06 |
| evgTRINITY_DN84179_c0_g1_i1 | uncharacterized transporter slc-17.2-like | 5.31 | 5.21 | 5.20 |
| evgTRINITY_DN16840_c0_g1_i1 | papilin-like | 5.05 | 5.55 | |
| evgTRINITY_GG_14665_c0_g1_i2 | ctenidin-3-like | -5.43 | -4.89 | |
| evgTRINITY_DN92655_c9_g2_i1 | deoxyribonuclease-1-like | -4.84 | -4.63 | |
| evgTRINITY_DN68720_c0_g1_i1 | testisin-like | -4.69 | -4.18 |
Table 6. Highlights of general, reproductive, and immune responses of B. pfeifferi in response to S. mansoni infection.
| One day post-infection (1d) | |||
| General | Reproductive | Immune | |
| UP-REGULATED | |||
| Overall | phospholipase A2s | Na and Cl dependent glycine transporter 2-like | dermatopontins |
| endoglucanases | neuropeptide Y receptor type 5-like | ficolin-like proteins | |
| Proteases and protease inhibitors | DBH-like monooxygenase protein 1 | macrophage man rec 1-like isoform X1 | |
| Guanine nucleotide-binding protein-like 3 | C-type lectin -6 member A-like | ||
| Translationally-controlled tumor protein | acidic mammalian chitinase-like | ||
| chitinase-3-like protein 1-like | |||
| hemocytin | |||
| laccase-15-like | |||
| laccase-1-like | |||
| tyrosinase-1-like | |||
| Two snails with higher S. mansoni read counts | FMRF-amide receptor-like | Cu, Zn superoxide dismutase | |
| Tyrosinase-like protein tyr-1 | GTPase IMAP family members 4 and 7 | ||
| beta 1,3 glucan-bind protein-like precursor | |||
| complement C1q-like protein | |||
| fibrinogen-related protein 2 (FREP2) | |||
| One snail with least S. mansoni read counts | ATP synthase FO6 | macrophage expressed gene-1 | |
| spermine oxidase | |||
| glutathione-S-transferase | |||
| laccase-2-like | |||
| DOWN-REGULATED | |||
| Overall | glyceraldehyde-3-phosphate dehydrogenase | ovipostatin 2 | FREP12 and its precursors |
| respiratory pigment hemoglobin | tyramine/dopamine β-hydroxylase-like | toll-like receptor 8 | |
| insulin-like peptide 7 –modestly down | FMRF-amide isoform X2 –modestly down | cytidine deaminase | |
| pedal peptide 2 | PTSP-like molecule | zinc metalloproteinase /disintegrin-like | |
| Na dependent nutrient aa transporter1-like | pheromone Alb-1 | ||
| enterin | type 1 serotonin receptor | ||
| FMRF-amide isoform X2 | schistosomin | ||
| cytidine deaminase | |||
| soma ferritins | |||
| COMPLEX (MIXED RESPONSES) | |||
| collagens | |||
| acidic mammalian chitinase-like proteins | |||
| cytochrome c oxidases | |||
| Mucins | |||
| Cytochrome P450 family members | |||
| Multidrug resistance proteins | |||
| Some heat shock proteins | |||
| Three day post-infection (3d) | |||
| General | Reproductive | Immune | |
| UP-REGULATED | |||
| Overall | phospholipase A2s | Na and Cl dependent glycine transporter 2-like | GTPase IMAPs- complex, but mostly up |
| endoglucanases | temptin-like | beta-1,3-glucan binding proteins | |
| Proteases and protease inhibitors | kynurenine 3-monooxygenase-like | complement C1q-like proteins | |
| 17-beta hydroxysteroid dehydrogenase type 6 | probable serine carboxypeptidases (1–5) | ||
| betaine homocysteine-methyltransferase 1-like | glutathione S-transferases | ||
| translationally controlled tumor protein homolog | laccase-2-like | ||
| hemoglobin type 1 | |||
| Two snails with higher S. mansoni read counts | insulin-related peptide-3-like | Tyrosinase-like protein tyr-1 | dermatopontins |
| cytochrome b | Na- and Cl-dependent taurine transporter-like | ficolins | |
| serine proteases alpha and beta | dopamine receptor 2-like | Cu-Zn superoxide dismutases | |
| ADP, ATP carrier-like protein | C-type lectin domain family 6, A-like | ||
| heparinase-like isoform X! | chitinase-3-like protein | ||
| serpin B6-like | chitotriosidase-1-like | ||
| aplysianin-like proteins | |||
| FREP2, FREP5 | |||
| macrophage-expressed gene 1 protein-like | |||
| laccase-15-like | |||
| tyrosinase-1-like | |||
| One snail with least S. mansoni read counts | profilin | ovipostatin 6 | hemocytin |
| cathepsin B and L1-like | yolk ferritin precursor | hemagglutinin/amoebocyte aggreg factor-like X1 | |
| neuroglobinase-like | DBH-like monooxygenase protein 1 | G-type lysozyme | |
| chymotrypsin-like elastase family member | sialate–O-acetylesterase-like protein | ||
| histone transcription factor | peroxidase-like protein | ||
| fibrinogen-like protein A | |||
| FREP 7 | |||
| peptidoglycan-recognition proteins SC2-like | |||
| LRR-containing 15-like, toll-like receptor 13 | |||
| DOWN-REGULATED | |||
| Overall | glyceraldehyde-3-phosphate dehydrogenase | FMRF-amide-like isoform X2 –modestly down | caveolin-1-like |
| aryl hydrocarbon recep nucl translocator-like | tyramine/dopamine β-hydroxylase-like | disintegrin/metalloprot containing prot t17-like | |
| FMRF-amide isoform X2 –modestly down | FREP12 precursors | ||
| PTSP-like molecule | LRR contain G-prot coupled rec 5-like | ||
| pheromone Alb-1 | alpha-crystalline B chain-like | ||
| type 1 serotonin receptor | toll-like receptors 4 and 8 | ||
| schistosomin | cytidine deaminase | ||
| Two snails with higher S. mansoni read counts | tyramine/dopamine β-hydroxylase-like | ||
| COMPLEX (MIXED RESPONSES) | |||
| ornithine decarboxylase | |||
| actins | |||
| collagens | |||
| tubulins | |||
| mucins | |||
| cytochrome P450 members- mostly up | |||
| multidrug resistance proteins | |||
| heat shock proteins | |||
| thioredoxins | |||
| annexins | |||
| putative copper-containing amine oxidases | |||
| soma ferritins | |||
| Shedding | |||
| General | Reproductive | Immune | |
| UP-REGULATED | |||
| Overall | FMRF-amide receptor-like—modestly up | dopamine beta hydroxylase-like | pcrotocadherein Fat 3 or 4-like |
| small cardioactive peptides | FMRF-amide receptor-like | ADAM family mig-17-like | |
| phospholipases A2s | ovipostatin 5 | zinc metalloproteinase nas 13- & 14-like | |
| arginase-1-like isoform X2 | DBH-like monooxygenase protein | ficolins | |
| reverse transcriptase | |||
| protease inhibitors BPTI Kunitz-domain class | |||
| ubiquitin ISG15 | |||
| Angiopoietin–1 receptor | |||
| Angiopoietin-related 2-like | |||
| mucins—complex but most are up | |||
| soma ferritins | |||
| Two snails with higher S. mansoni read counts | endonuclease G mitochondrial-like | yolk ferritin-like and snail yolk ferritin molecules | aplysianin-A-like |
| zinc carboxypeptide A 1-like | Neuropeptide Y receptor type 5-like | mammal ependymin-related prot 1- like | |
| serpinB3-like protease inhibitor | zinc carboxypeptidase A1-like | ||
| cystatin protease inhibitor | beta-1,3-glucan binding protein-like | ||
| putative amine-oxidases (copper containing) | FREP 2, 7 and 14 | ||
| One snail with least S. mansoni read counts | multiple epidermal growth factor-like domains | macrophage-expressed gene | |
| serine/threonine-protein kinase mos-like | C-type lectin -6 member A-like | ||
| chitinase-3-like-protein | |||
| LRR and Ig domain containing protein | |||
| toll-like receptor 3 | |||
| DOWN-REGULATED | |||
| Overall | insulin-like gr fact protein acid labile subunit | ovipostatin 2 | toll-like receptor 7 |
| pedal peptide 2 | dopamine beta-hydroxylase-like | galectin-6 | |
| profilin-like isoform X1 | probable serine carboxypeptidase CPVL | ||
| neuroglobin-like | |||
| calreticulin-like | |||
| tyrosinase tyr-3 | |||
| Two snails with higher S. mansoni read counts | hemoglobin | dihydropyrimidinase | |
| collagen-related | macrophage man recep1-like protein | ||
| tyrosinase-3-like | |||
| One snail with least S. mansoni read counts | tyrosinase-1-like | ||
| COMPLEX (MIXED RESPONSES) | |||
| collagens, mixed but mostly down | |||
| cathepsins | |||
| tubulins | |||
| cytochromes | |||
| ankyrins | |||
| Rho GTPase-activity protein 1-like | |||
| cytochrome P450 family members | |||
| multidrug resistance proteins | |||
| glutathione-S-transferases | |||
| dermatopontins | |||
| GTPase IMAP family members | |||
| thioredoxins—but mostly up | |||
Fig 7. Multidimensional scaling (MDS) plots of pairwise comparisons of control versus 1d, 3d, and shedding replicates used for differential expression analyses.
Fig 8. Biomphalaria pfeifferi differential expression profiles in 1d, 3d, and shedding snails.
(A) Overall expression profiles for up- and down-regulated B. pfeifferi CDS in the 3v3 DE analysis with proportions shown for CDS with annotation known (white) and without annotation (gray) from one of the 5 databases searched (Table 3). Numbers by bars refer to numbers of up- and down-regulated features. (B) Heat map of differentially expressed B. pfeifferi CDS. (C) Up- and down-regulated B. pfeifferi CDS shared between 1d, 3d, and shedding snail groups in the 3v3 DE analysis are shown.
It should also be noted that 59 CDS were up-regulated, and 63 CDS down-regulated in common to all three groups of infected snails (Fig 8C). Those up-regulated across time points include hemocytin, CD209 antigen-like, DBH-like monooxygenase, and a fibrinolytic enzyme. Some ubiquitously down-regulated features include neural cell adhesion molecule 1-like, a TNF receptor, peroxiredoxin 5, F-box/LRR repeat protein 4-like, the cytoprotective hypoxia up-regulated protein 1-like that is triggered by oxygen deprivation and oxidative stress, glutathione-S-transferase omega-1-like, type 1 serotonin receptor 5HT-1Hel, a feeding circuit activating peptide that induces feeding behavior [93], and TLR 7.
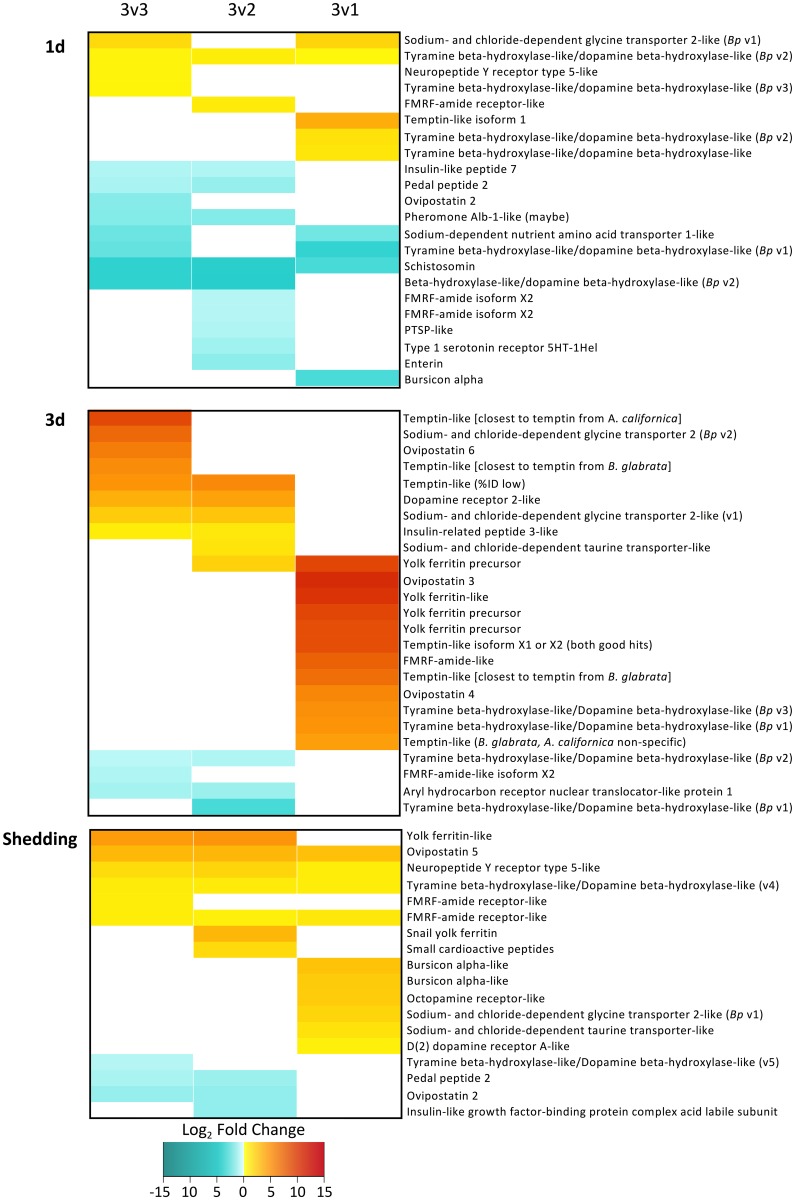
In addition to identifying those CDS up- or down-regulated in common to all three groups of infected snails, we also identified CDS not known to be related to reproduction or defense that exhibited the highest fold expression changes in shedding snails. Snail CDS most highly up-regulated may represent molecules essential for the parasite to sustain a patent infection, or conversely, those most strongly down-regulated may otherwise interfere with parasite development in ways we do not presently understand. A selected few, that had an annotation and were consistently expressed compared to controls in each replicate, are shown in Table 5.
With respect to transcripts involved in reproduction and potentially associated with S. mansoni-induced parasitic castration, we identified homologs to more than 100 invertebrate neuropeptides, hormones, pheromones, and polypeptides involved in reproduction, most of which have been identified in Lymnaea stagnalis, the sea hare Aplysia californica, or in B. glabrata (S4 File; Fig 9, and see Discussion). We also searched for over 500 different genes identified from previous publications that are related to immune, defense or stress responses to various pathogens or environmental stressors (S4 File; Fig 10). Each gene of interest has been organized into one of six broad functional groups for ease of interpretation, although it must be noted that many of these genes have multiple roles and could belong in several functional categories. After 1d, the majority of immune, stress and defense features were up-regulated. Noteworthy from Fig 10B is that for snails with low reads counts for S. mansoni (3v1 comparison), proportionately more features were up-regulated than for snails with high S. mansoni read counts. In two out of three comparisons, snails with low read counts for S. mansoni had fewer down-regulated genes than snails with high levels of S. mansoni read counts.
Fig 9. Biomphalaria pfeifferi CDS identified as neuropeptides, hormones, or involved in reproduction that are differentially expressed in 1d, 3d, and shedding snails.
Note that the 3v3 comparison includes all 3 infected snails within a time point, whereas 3v2 includes the two infected snails with the most S. mansoni reads and the 3v1 includes only the infected snail with the fewest S. mansoni reads.
Fig 10. Differential expression of Biomphalaria pfeifferi defense-related CDS in 1d, 3d, and shedding snails.
(A) Defense CDS in the 3v3 DE analysis. (B) Pie charts of proportions of CDS found to be DE in 3v3, 3v2, and 3v1 analyses. (C) Heat maps show expression levels from each of the three DE analyses highlighting the most relevant biological functional groups. Note that the 3v3 comparison includes all 3 infected snails within a time point, whereas 3v2 includes the two infected snails with the most S. mansoni reads and the 3v1 includes only the infected snail with the fewest S. mansoni reads.
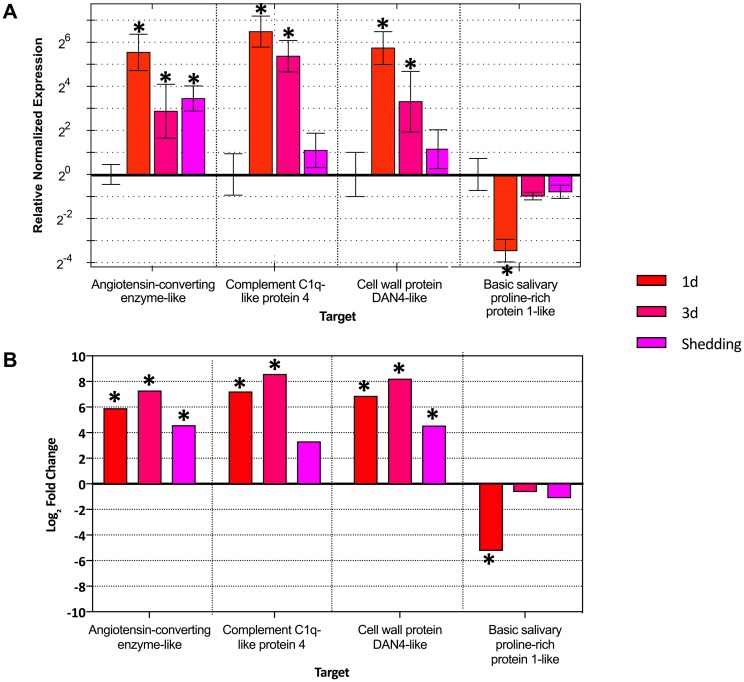
Expression patterns validated by qPCR
Quantitative RT-PCR (qPCR) was used to validate differential expression trends by quantifying mRNA transcripts of four single-copy genes (3 up-regulated and 1 down-regulated) that highlight varying expression patterns in 1d, 3d, and shedding snails. Overall expression patterns are similar between the qPCR and Illumina DE results (Fig 11) with the same variability in DE pattern between replicates echoed in the qPCR. The only difference seen was in the gene DAN4 where the shedding group was not considered significantly DE in the qPCR analysis but was in Illumina analysis.
Fig 11. qPCR results validate Illumina RNA-Seq differential expression results.
(A) Quantitative real-time PCR verifies Illumina trends among biological replicates in 1d, 3d, and shedding samples. (B) Corresponding Illumina DE results for the four genes tested. Asterisks indicate genes that are significantly DE.
Discussion
Considerations regarding the dual-seq dataset and pipeline
This paper represents a novel pipeline for dual RNA-Seq studies where the genome of just one of the interacting partners, the parasite in this case, is available. It also highlights an advantage of using field specimens in RNA-Seq studies to reinforce the notion that individual snails are actually holobionts, and the symbiont species they carry with them may play a role in influencing susceptibility to schistosome infection or in modulating disease transmission. Also, variance among the individual snails within the groups examined presented challenges for traditional bioinformatics analyses but also revealed the heterogeneity that realistically exists among naturally diverse snails and schistosomes as they encounter one another in real-life settings in the field. We must also note that the identity and functional role for many of the CDS remain unknown thus posing rich opportunities for study for the future.
Considerations with respect to compatibility with S. mansoni
The specific B. pfeifferi-S. mansoni system studied here is noteworthy for the high degree of susceptibility shown by the snail to infection [15,16]. Compatibility with S. mansoni is characteristic of B. pfeifferi throughout its range [12]. As a consequence, all snails exposed to S. mansoni or known to be shedding S. mansoni cercariae contained transcripts contributed by S. mansoni. The extent of representation of S. mansoni in the dual transcriptome is variable among the replicates for each time sampled (Table 1). Given the effects of both genetic diversity in S. mansoni [94] and in Biomphalaria snail hosts [34,95] on the rate or extent of S. mansoni development, it is not surprising that field-derived representatives will differ with respect to extent of parasite development and transcriptional activity. Here it should be noted that read counts may not always be fully indicative of S. mansoni biomass in snails as the transcriptional activity of the parasite may vary temporally, both daily [96] and at longer time scales [97], and in response to other stimuli, as noted in the following section regarding symbionts.
Recovered symbiont sequences
Whole snail transcriptome sequencing gave us the opportunity to identify sequences of non-mollusc and non-schistosome origin, including viruses, bacteria and eukaryotes. These sequences provide evidence of symbionts that are found in or on B. pfeifferi and/or S. mansoni. Some of the symbionts identified are surely worthy of further future investigation and may offer potential in application of novel and as yet unforeseen control efforts.
With respect to viruses, in general the array of viruses found in invertebrates has recently been shown to be much more diverse than previously known, including in molluscs [98]. Of the nearly 1,300 of our assembled CDS identified provisionally as viruses, most have homology to Beihai paphia shell viruses, picorna-like viruses, and crawfish viruses. In terms of read abundance, the five most abundant viral CDS we found in B. pfeifferi had the most similarity to the Wenzhou picorna-like virus 33 from the channeled apple snail Pomacea canaliculata, Sanxia picorna-like virus 4 from a freshwater atyid shrimp, Beihai picorna-like virus 47 from a sesarmid crab, bivalve RNA virus G2 a picorna virus from the gills of a bivalve [99], and Beihai hypo-like virus 1 from a razor shell [98]. Picorna viruses have recently been described in both B. glabrata from South America and B. pfeifferi from Oman [100]. Three novel RNA viruses were reported in the B. glabrata genome, the first with similarities to an iflavirus, the second with similarities to a Nora virus or Picornavirales, and the third with similarities to several viruses [37]. Further study is required to confidently designate any of the putative viral sequences recovered as actual infectious entities of snails, or possibly of schistosomes or other digeneans. They might infect other potential hosts like rotifers or diatoms among the symbionts living in B. pfeifferi.
The recovery of a few sequences of the digenean-inhabiting Neorickettsia from two infected snails with relatively high percentages of S. mansoni reads (3d-R3 and shedding-R1) is suggestive of an association. Neorickettsia has been found from non-human schistosomes [101] but further study is needed to document the presence of Neorickettsia in human-infecting schistosomes. For example, the Neorickettsia might be associated with metacercariae of other digeneans that are commonly found encysted in B. pfeifferi from natural habitats.
With respect to eukaryotes, CDS representing the following groups were recovered: 1) Chaetogaster annelids which mostly colonize the external soft surfaces of freshwater snails and are known to ingest digenean miracidia and cercariae [102–105]; 2) Trichodina ciliates known to live on the soft surfaces of snails but with poorly characterized influence on their snail hosts [106]; 3) Capsaspora owczarzaki, a Filasterean amoeba-like symbiont known from Biomphalaria glabrata [107,108]; 4) Microsporidians, not surprising for B. pfeifferi considering microsporidians are known from both Biomphalaria and Bulinus [109]; 5) Perkinsea, an alveolate group of considerable commercial significance in marine bivalves, but with at least two reports suggesting their presence in freshwater habitats as well [110,111]; 6) Rotifers (possibly attached to the shell or ingested) and diatoms (probably ingested) were frequently recovered as well; 7) Four tardigrade CDS were recovered, two from the uninfected control 454-sequenced snail similar to Richtersius coronifer and two from the Illumina de novo assembly similar to Ramazzottius varieornatus. Control-R1 had read counts >10 for the two R. coronifer CDS and 1d-R3 had read counts >10 for a R. varieornatus CDS. It is not unprecedented to find tardigrades associated with snails. Fox and García-Moll [112] identified the tardigrade Echiniscus molluscorum in the feces of land snails from Puerto Rico. Although the tardigrade may have been ingested along with food, the authors did not rule out the possibility that E. molluscorum may be a symbiont of the snail.
It was not surprising that two of our snails yielded several reads mapping to sequences from other digeneans. The first, control-R3, returned sequences consistent with Ribeiroia, representatives of which occur in East Africa and are known to infect Biomphalaria there [91]. It seems most likely this snail had an infection with Ribeiroia sporocysts and/or rediae, though the extent of this infection must have been minimal as the transcriptomics response of this snail was not unusual compared to the other control snails. It may also have been infected with Ribeiroia metacercariae which are most familiarly known to infect amphibians or fish [113,114], but have been recovered and sequence-verified in specimens of Biomphalaria spp. from Kenya (MR Laidemitt, personal communication, April 2017). The other snail, 3d-R2, yielded confirmed amphistome sequences, probably from the commonly recovered species Calicophoron sukari [91], so it may have harbored developing larvae of both S. mansoni and an amphistome, reflective of real-life circumstances in the habitat of origin where this amphistome species is the most common digenean to infect B. pfeifferi [92]. This co-infection may help to explain the relatively low numbers of S. mansoni reads recovered from this snail relative to 3d-R1 and 3d-R3. It has also been noted that B. pfeifferi ingests amphistome metacercariae (A Gleichsner, personal communication, June 2017) which are abundant on the submerged vegetation in the habitat from which the snail was collected, so this may be an alternative explanation for the presence of amphistome reads in 3d-R2. The peculiar nature of infection in this snail further justifies our rationale for including it in the separate analyses (3v1) described in the results.
Some overall highlights of the response of infection
At 1d, snails showed proportionately more down-regulated CDS, possibly reflective of a strong parasite-induced immunomodulatory effect during the establishment phase of infection [54]. For the two additional time points examined, the majority of features in B. pfeifferi were up-regulated (Fig 8; S3 File). This pattern differed from a previous microarray-based expression studies for susceptible B. glabrata for which a predominant trend of down-regulation was noted from 2–32 days post-exposure to S. mansoni [47]. The more comprehensive transcriptional picture resulting from next-gen sequencing provides a different overview of responses following infection with S. mansoni (see also [54]).
Many host CDS responded uniformly across individual snails regardless of the number of S. mansoni reads recovered. However, at 1d and 3d, snails with fewer S. mansoni reads had higher proportions of up-regulated features than did snails with higher numbers of S. mansoni reads. Furthermore, for both 3d and shedding snails, snails with low S. mansoni read counts had smaller proportions of down-regulated features. These patterns are suggestive that up-regulated host responses might limit S. mansoni gene expression and that snails with less parasite gene expression may be less vulnerable to gene down-regulation, but care in interpretation is required as alternative explanations may exist. For example, as noted above, replicate 3d-R2 also contained an amphistome infection. Negative interactions among the two digeneans which are known to occur from experimental studies (MR Laidemitt, personal communication, April 2017) may account for the limited number of S. mansoni reads.
At 1d, up-regulated responses, as exemplified by CDS for phospholipases, endoglucanases, and several proteases and protease inhibitors, were usually less pronounced than at 3d, suggesting it takes a few days to mobilize responses. Notable at 1d were down-regulation of CDS that might lower hemoglobin levels, and influence feeding behavior and heart beat rate. Infected snails exhibited complex mixed responses with respect to mucins, multidrug resistance proteins, glutathione-S-transferases and cytochrome P450 family members. Cytochrome P450s are part of the stress response shown by B. glabrata snails following exposure to molluscicides [49] and to biotic stressors [48]. For heat shock proteins, B. glabrata snails elaborated more complex up-regulated responses following exposure to molluscicides [49] than B. pfeifferi did following exposure to S. mansoni. Complex patterns in stress response gene families were also noted for 3d and shedding snails. It is noteworthy that exposure to S. mansoni, a specific extrinsic biotic stressor, also provokes components of a generalized stress response in B. pfeifferi and B. glabrata [115,116].
Snails with 3 day infections had the highest number of up-regulated CDS. Some of the features down-regulated at 1d were again down at 3d. Additionally, one CDS (aryl hydrocarbon receptor) associated with controlling circadian rhythm [117] was down-regulated. Daily feeding patterns of infected snails [119–121] or patterns of release of cercariae [96] could potentially be influenced by this CDS. Several gene families also showed complex patterns of responses at 3d. Among them were amine oxidases which, as noted by Zhang et al. [48], are involved in oxidation of amine-containing compounds including neurotransmitters, histamines and polyamines [122].
The overall responses of shedding snails were surprising in not being more dramatically altered relative to controls than they were. This is because snails with more advanced schistosome infections (28+ day infections) experience several noteworthy physiological changes, including altered feeding behavior, decreased locomotory activity, increased heartbeat rate [118–121,123] and castration (see section below). From our shedding snails, we noted up-regulated levels of FMRF-amide receptor and small cardioactive peptides that influence heart beat rate. Shedding snails also uniquely showed up-regulated levels of CDS involved in collagen synthesis or epithelial cell and blood vessel formation, processes involved in wound healing [49,123,124], of relevance to a snail experiencing the tissue damage associated with cercarial emergence. Other up-regulated features are indicative of stress. Modestly up-regulated levels of reverse transcriptase are of interest because of previous reports of enhanced RT activity in susceptible B. glabrata exposed to S. mansoni [115].
Down-regulated levels of features potentially helping to explain reduced growth rates [125,126], reduced motility [119,120,127,128] or depleted levels of hemoglobin [129] observed in shedding snails were noted (S3 File). Other down-regulated features of interest were noted including tyrosinase, which is involved in melanin synthesis (see also discussion of reproduction).
Consequences of infection on host reproduction
Snails infected with the proliferating larval stages of digenetic trematodes, including B. pfeifferi infected with S. mansoni, suffer parasitic castration, marked by a sharp or complete reduction in production of eggs [121,125,130]. In B. pfeifferi, egg-laying begins to decline 7–10 days following exposure to S. mansoni and is complete in most snails by 14 days. The time course and extent of castration are influenced by the age of the snail at the time of exposure and by the dose of miracidia received [130,131]. In some cases, a slight increase in egg production compared to unexposed controls can be seen in the pre-shedding period, but this is followed by castration [125,130,131].
Studies of the reproductive physiology of freshwater gastropods have identified a number of peptides and non-peptide mediators (including biogenic monoamines) involved in neuro-endocrine control of reproduction [132,133]. We found evidence for the presence and expression of homologs of over 50 of these neuropeptides in B. pfeifferi (S4 File; Fig 9) and several additional neuropeptide precursors. It has also been noted that in B. glabrata castrated by S. mansoni, repeated exposure to serotonin enabled snails to resume egg-laying [134]. Furthermore, dopamine is present in reduced levels in infected snails, and administration of this catecholamine stimulated the release of secretory proteins from albumen gland cultures of B. glabrata [135] and the related snail Helisoma duryi [136].
Although infections of 1 or 3 days duration are too young to manifest castrating effects, up-regulation of some features with possible inhibitory effect on reproduction were noted at these times. Several features were also down-regulated at 1 day, including ovipostatin 2, a type 1 serotonin receptor (relevant because of serotonin’s ability to stimulate egg-laying), and schistosomin. Schistosomin has been implicated in Lymnaea stagnalis in inhibiting hormones involved in stimulating egg-laying or the albumen gland [137]. A role for schistosomin in reproduction or trematode-mediated castration was not found in B. glabrata infected with S. mansoni [138] and we saw no change in its expression in B. pfeifferi. Kynurenine 3-monooxygenase-like transcripts were up-regulated in all snails with 3 day infections. By degrading tryptophan, this enzyme may limit concentrations of serotonin.
It was of interest to learn if the water-borne pheromones (temptin, enticin, seduction, and attractin) that favor aggregation in Aplysia [139] were expressed in B. pfeifferi, especially given its preference for self-fertilization. We found evidence only for the expression of temptin, which was up-regulated at 3d, but otherwise was not differentially expressed. Likewise, only temptin was isolated in proteins released from B. glabrata [37] and egg-mass proteins [140]. It has been shown to be an attractant for B. glabrata [141].
Our results with shedding snails are most pertinent with respect to parasitic castration. Several reproduction-related neuropeptides, including caudal dorsal cell hormone, and neuropeptides associated with production of egg and egg mass fluids such as snail yolk ferritin (vitellogenin), galactogen synthesis, lipopolysaccharide binding protein/bacterial permeability-increasing proteins (LBP/BPI) or aplysianin/achacin-like protein [140] were not strongly down-regulated as a consequence of infection. Some of the most obvious changes we noted were up-regulated levels of transcripts encoding dopamine beta hydroxylase and especially dopamine beta-hydroxylase–like monooxygenase protein 1, both of which convert dopamine to noradrenaline so their enhanced expression may help to explain the declining levels of dopamine noted in S. mansoni-infected snails [134]. This may in turn help to explain diminished egg production given dopamine’s effect on release of albumen gland proteins. Tyrosinase-1, involved in production of melanin, is down-regulated in shedding snails and this may have the effect of preserving dopamine levels in these snails. At both earlier sampling points, tyrosinase-1 is strongly up-regulated especially in snails with abundant S. mansoni reads, and thus may mark an early phase in initiation of castration by diverting tyrosine to production of melanin as opposed to dopamine. Transcription of enzymes involved in dopamine metabolism are strongly affected in S. mansoni-infected snails. Tyrosinase-1 is also discussed in the next section regarding its potential involvement in defense responses.
There are numerous ovipostatins produced in Biomphalaria (we found 6 different versions in B. pfeifferi), with ovipostatin 5 being the most prominent responder in shedding snails. In L. stagnalis, ovipostatin is passed in seminal fluid from one individual to another during mating and inhibits oviposition in the recipient [132]. Although B. pfeifferi is predominantly a self-fertilizer [20], ovipostatin 5 could potentially down-regulate oviposition in ways not reliant on copulation. Neuropeptide Y inhibits egg-laying in L. stagnalis [142] and though we did not observe up-regulation of this neuropeptide, up-regulated transcripts for neuropeptide Y receptor type 5-like protein in our shedding snails is consistent with a possible enhanced inhibitory effect on reproduction of neuropeptide Y. Strong up-regulation of transcripts for yolk ferritin-like and snail yolk ferritin molecules (vitellogenins) in shedding snails was also observed and is somewhat paradoxical but may suggest they are diverted to the parasite for metabolism since it is known that schistosomes require iron stores for development [143]. Notably, the extent of up-regulation for yolk ferritin-like and snail yolk ferritin, ovipostatin 5, neuropeptide Y receptor type 5-like, and dopamine beta-hydroxylase-like, was the least in the shedding snail expressing the lowest number of normalized S. mansoni reads.
Wang et al. [133] recently used proteomics methods (liquid chromatography tandem mass spectrometry) to examine and identify neuropeptides in central nervous system (CNS) ganglia dissected from B. glabrata, either from control snails or snails at 12 days post infection with S. mansoni. They noted many reproductive neuropeptides, such as egg laying hormone 2, at significantly reduced levels at 12d compared to controls. They also reported an increase in some neuropeptides including FMRFamide, luqin, NKY, and sCAP in infected snail CNS. Based on predicted protein interaction networks, Wang et al. [133] suggested that snail-produced leucine aminopeptidase 2 (LAP2) interacts with several S. mansoni miracidia peptides so may be a key player in regulating parasite-induced changes in host physiology. A homolog to the B. glabrata LAP2 was present in our transcriptome but was not differentially expressed in any sample. When comparing our results to those of Wang et al. [133], it should be noted that our approach was transcriptome-centered, examined different time points post-infection, and was based on whole body extractions of B. pfeifferi, rather than B. glabrata. Our methods may bias against detection of changes in expression of potentially rare neuropeptide transcripts, but cast a wider net for potential downstream effects of castration, so provides a valuable complementary view to the approach taken by Wang et al. [133].
Immune response of B. pfeifferi infected with S. mansoni
At 1d (S4 File; Fig 10), several immune-relevant CDS were up-regulated in all three snails including dermatopontins (frequently noted in B. glabrata studies), ficolins [48], and chitinase attacking enzymes [42,48]. For the two snails with the highest proportions of S. mansoni reads, up-regulated responses were observed for a number of additional immune features. Cu,Zn SOD is of particular interest because previous work has implicated high expression of certain alleles of Cu,Zn SOD with resistance to S. mansoni in the 13-16-R1 strain of B. glabrata, because of Cu,Zn SOD’s involvement in converting superoxide anion to schistosomicidal hydrogen peroxide [144–146]. Our study is in agreement with Hanington et al. [47] who noted up-regulated levels of Cu,Zn superoxide dismutase (SOD) at early time points following exposure of susceptible M line B. glabrata to either S. mansoni or E. paraensei.
Hanington et al. [47] also found both FREP2 and FREP4 to be consistently up-regulated following exposure of M line B. glabrata to S. mansoni or E. paraensei, so much so either might be considered as markers of infection. Although a FREP2 homolog was consistently up-regulated following exposure of B. pfeifferi to infection, a FREP4 homolog was not expressed in B. pfeifferi at any of the time points we examined.
In contrast, among CDS more up-regulated in the 1 day infected snail with a low proportion of S. mansoni reads were macrophage expressed gene-1, known to be up-regulated in both abalone following bacterial infection [147] and from resistant and non-susceptible strains of B. glabrata in early exposure to S. mansoni [148]. Hemocytin was also up-regulated in the 1d snail with low proportion of S. mansoni reads. Hemocytin, a homolog for an insect humoral lectin with a role in hemocyte nodule formation [149], was consistently up-regulated in all S. mansoni-infected snails at all three time points, especially at 3d when it was increased over 8-fold in expression. For both 1 and 3d, hemocytin expression was highest in those snails with fewer S. mansoni reads. FREP3, previously implicated in resistance to S. mansoni in B. glabrata [52], was minimally responsive in this compatible B. pfeifferi system. It was modestly up-regulated only at 1d, in the snail with fewest S. mansoni reads.
Down-regulated immune features at 1d were relatively few but prominent among them were FREP12 and its precursors, toll-like receptor 8 and cytidine deaminase. FREP12 down-regulation has also been noted upon exposure of B. glabrata amebocyte-producing organs to fucoidan, a fucosyl-rich PAMP chosen to mimic the surface of S. mansoni sporocysts [48], and in B. glabrata exposed to S. mansoni [47]. A strain of B. glabrata resistant to S. mansoni exhibits higher levels of a TLR on its immune cells, and exposure to S. mansoni significantly enhances their expression, whereas compatible snails show little response following exposure to infection [31]. Our B. pfeifferi showed no conspicuously up-regulated TLR genes at 1d, and we found no B. pfeifferi TLR with strong homology to the TLR reported by Pila et al. [31], but the relatively strong down-regulation of TLR 8 in this model of compatibility is noteworthy. Although the immune role of cytidine deaminase is not clear, Bouchut et al. [150] associated higher levels of its expression with enhanced resistance to echinostome infections and Ittiprasert et al. [148] observed up-regulation of cytidine deaminase in resistant and non-susceptible B. glabrata in early exposure to S. mansoni. Down-regulation of cytidine deaminase might therefore be associated with lower responsiveness to S. mansoni infections, particularly early in infection (down-regulation also noted at 3d, but not in shedding snails).
The responses of putative immune factors were most extensive in snails at 3d and this is not surprising as this is a critical stage in the early establishment of the parasite. Several CDS mentioned with respect to the 1d response were again noted at 3d. Snails with more S. mansoni reads had high levels of several transcripts including for aplysianin-like proteins and FREP 5. Aplysianin, first described from Aplysia, is an L-amino oxidase that has tumoricidal and bactericidal effects [151], and a distinct aplysianin-like protein exists in egg mass fluids of B. glabrata [140]. Aplysianin-like transcripts were more abundant in echinostome-resistant than susceptible strains of B. glabrata [150]. FREP 5 was shown to be down-regulated in microarray studies of B. glabrata in response to successfully developing S. mansoni or Echinostoma paraensei [47].
The snail with relatively few S. mansoni reads at 3d revealed a different group of up-regulated transcripts, with hemocytin again being prominent. Also notable were distinctive CDS potentially involved in hemocyte aggregation [152], FREP 7, peptidoglycan-recognition proteins SC2-like (PGRPs), and TLR 13. PGRPs are well-known anti-bacterial factors and were found to be up-regulated following exposure of B. glabrata to LPS [153] and to bacteria [53]. Down-regulated features for snails with 3d infections again included cytidine deaminase, FREP12 precursors, and TLR 4 and 8 among others.
Laccases and tyrosinases are two groups of phenoloxidases observed to be responsive in early S. mansoni infection within B. pfeifferi (Table 6; S4 File). Tyrosinase has been isolated from B. glabrata egg masses with a presumptive immunoprotective effect for offspring [140,154]. As mentioned earlier with details of its reproductive consequences, tyrosinase-1 was up-regulated at 1d and 3d. Tyrosinase-1 was down-regulated in the shedding replicate with the least S. mansoni reads and tyrosinase-3 was down-regulated in the two replicates with the most S. mansoni reads. Another type of phenoloxidase, laccase, was shown to have decreased activity in B. glabrata hemolymph beginning at 7 weeks post-infection with S. mansoni [155]. We found laccase-15-like was up-regulated in all three comparisons (3v3, 3v2, 3v1) at both 1d and 3d. Laccase-1-like was up-regulated in all three comparisons at 1d and laccase-2-like was up-regulated in all comparisons at 3d. Laccases were not significantly DE in shedding snails. In B. pfeifferi, the up-regulation of two phenoloxidases, tyrosinase and laccase, at 1d and 3d suggests an increase in the synthesis of early-stage reactions in the melanin pathway, however, further work is needed to determine if melanization is involved in schistosome killing, especially in the B. pfeifferi model characterized by its compatibility.
It is worth noting that members GTPase IMAP family (GIMAP) were found to be up-regulated in 1d and 3d (mostly up-regulated in 3d). The possible role of GIMAPs in immunity has not been realized in protostomes until it was shown that several GIMAPs were up-regulated in the amebocyte organ of B. glabrata following exposure to extrinsic stimuli [48]. This finding was reconfirmed by later work, which demonstrated that GIMAPs not only play a role in immunity, but are highly diverse in the eastern oyster Crassostrea virginica where they were down-regulated following exposure to bacterial infection. GIMAPs may promote hemocyte survival by inhibiting apoptosis [156].
Immune-related responses for shedding snails were surprising for being mostly up-regulated (Fig 10), with only a few features being modestly down-regulated, among them galectin-6. Galectins recognize carbohydrates associated with schistosome surfaces and are implicated as pattern recognition receptors for other pathogens as well [157]. Dihydropyrimidinase and cytidine deaminase, also down-regulated, are additional CDS potentially affecting pyrimidine levels in infected snails. Interestingly, in contrast to 1d and 3d responses, shedding snails did not show up-regulated Cu,Zn SOD levels.
Among those up-regulated features, shedding snails with high levels of S. mansoni reads had distinctly higher responses for aplysianin-A-like, beta-1,3-glucan binding protein-like, and FREPS 2, 7 and 14. By contrast, the snail with a low percentage of S. mansoni reads expressed higher levels of macrophage-expressed gene, chitinase-3-like-protein, a distinct CDS with a leucine rich repeat and immunoglobulin domain, and TLR 3.
Features highlighted in recent genetic linkage studies [32,50,51] including components of the “Guadeloupe Resistance Complex” were sought among B. pfeifferi transcripts. Most did not show strong patterns of up- or down regulation in this compatible species following exposure to S. mansoni, but zinc metalloproteinase/disintegrin-like was down-regulated at 1d and zinc metalloproteinase nas-13- and -14-like showed some up-regulation in shedding snails. Probable serine carboxypeptidases (versions 1–5) revealed a mixed pattern of expression at 3d, but were mostly up-regulated, whereas probable serine carboxypeptidase CPVL was down-regulated in shedding snails. Granulin, a growth factor that drives the proliferation of immune cells was up-regulated at both 1d and 3d [30,33].
Genes showing either extraordinary up- or down-regulation following exposure to S. mansoni infection
Unlike B. glabrata for which isolates or inbred lines are known that are resistant to S. mansoni, B. pfeifferi is a species typically discussed in the context of its high compatibility with many S. mansoni isolates. Although particular lineages of B. pfeifferi may certainly come to light that exhibit strong incompatibility, key factors that dictate compatibility might best be sought not among the putative immune factors that characterize the B. glabrata response, but among those genes that exhibit the strongest transcriptional responses, up or down, to S. mansoni exposure (Table 5; S3 File). Strongly up-regulated snail genes may be responsible for encoding proteins essential to S. mansoni development, and those strongly down-regulated may represent parasite-manipulated factors that if left un-manipulated would otherwise discourage parasite development. Certainly such a role has been proposed for schistosomes in altering expression of genes in compatible snails to their advantage [158–160].
Although many B. pfeifferi CDS that were highly altered in their expression are unknowns and thus represent intriguing subjects for future research, some did have homologs in the database and could also represent outstanding future targets for manipulation to discourage S. mansoni development. For example, we note the up-regulation of the protease inhibitor papilin-like and galactocerebrosidase-like. Galactocerebrosidase is an enzyme that removes galactose from galactocerebrocide (a ceramide sphingolipid with a galactose residue) to form a ceramide, an important lipid signaling molecule that has been reported in Crassostrea gigas [161]. A transcript coding for deleted in malignant brain tumor 1 protein-like (DMBT1) was also highly up-regulated in all shedding snails. DMBT1 is a pattern recognition receptor in mammals that belongs to a group of secreted scavenger receptors involved in pathogen binding [162]. However, its role in invertebrate systems needs to be established [159].
In conclusion, provided here is a de novo assembled transcriptional database based on over half a billion paired-end reads for an understudied schistosome vector, B. pfeifferi, one that is probably responsible for transmission of more S. mansoni to people than any other Biomphalaria species. We have deliberately chosen to emphasize the study of field-derived B. pfeifferi and S. mansoni to provide a more realistic view of the context in which they live, and how they interact in the wild, including with third party symbionts. Our approach has revealed that the extent of S. mansoni transcriptional activity varies among snails and this is reflected in different transcriptional responses of the snails, suggestive of diverse trajectories in what is typically a highly compatible host-parasite model. We have highlighted several snail features warranting further study with respect to their roles in potentially supporting or enabling parasite development, that might limit the extent of development, and that might play a role in the diminished egg production typically shown by snails with shedding S. mansoni infections. Another generation of research exploiting the power of techniques like CRISPR-Cas, when it becomes available for snails, will enable further dissection of the functional role of these candidate molecules. A further challenge will then be to determine how the responses of compatible snails, or perhaps of the schistosome parasites within, can be exploited, ideally to prevent or suppress in a highly specific manner the development of schistosome parasites in snails.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(TIF)
(TIF)
(TIF)
(TIF)
Percentages next to organisms show the percent of proteins present in our B. pfeifferi transcriptome predicted by TransDecoder.
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We thank Joseph Kinuthia, Ibrahim Mwangi, and Martin Mutuku for assistance with collection of field samples; Dr. Boris Umylny for comments on the bioinformatics pipeline design; Bethaney Fehrenkamp for qPCR guidance; Dr. Bishoy Hanna for feedback and comments.
The content for this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This paper was published with the approval of the Director of KEMRI.
Data Availability
The raw and assembled sequence data are available at NCBI under BioProject ID PRJNA383396. Additional relevant data are within the paper and its Supporting Information files.
Funding Statement
Technical assistance at the University of New Mexico Molecular Biology Facility was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P30GM110907 (https://www.nigms.nih.gov) and National Institutes of Health CETI COBRE grant P20GM103452 (https://www.nigms.nih.gov). The National Institutes of Health grant R01 AI101438 was the main funding source for this study. Research reported in this publication was supported by the New Mexico Institutional Development Award (IDeA) Network for Biomedical Research Excellence (NM-INBRE) Sequencing and Bioinformatics core at the National Center for Genome Resources (NCGR) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103451. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Schistosomiasis Weekly epidemiological record. 2017. Geneva, Switzerland.
- 2.King CH, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illness 2008;4: 65–79. doi: 10.1177/1742395307084407 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Public health impact of schistosomiasis: disease and mortality. Bulletin of the World Health Organization. 1993;71: 657–662. [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Prevention and control of schistosomiasis and soil-transmitted helminthiasis. World Health Organization technical report series. 2002. Geneva, Switzerland. [PubMed]
- 5.Zhou H, Ohtsuka R, He Y, Yuan L, Yamauchi T, Sleigh AC. Impact of parasitic infections and dietary intake on child growth in the schistosomiasis-endemic Dongting Lake Region, China. American Journal of Tropical Medicine and Hygiene. 2005;72: 534–539. [PubMed] [Google Scholar]
- 6.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. The Lancet. 2004;383: 2253–2264. doi: 10.1016/S0140-6736(13)61949-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King CH, Sturrock RF, Kariuki HC, Hamburger J. Transmission control for schistosomiasis—why it matters now. Trends in Parasitology. 2006;22: 575–582. doi: 10.1016/j.pt.2006.09.006 [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Preventive chemotherapy in human helminthiasis. 2006. Geneva, Switzerland. [Google Scholar]
- 9.Lelo AE, Mburu DN, Magoma GN, Mungai BN, Kihara JH, Mwangi IN, et al. No apparent reduction in schistosome burden or genetic diversity following four years of school-based mass drug ddministration in Mwea, central Kenya, a heavy transmission area. PLoS Neglected Tropical Diseases. 2014;8: e3221–11. doi: 10.1371/journal.pntd.0003221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King CH, Bertsch D. Historical Perspective: Snail Control to Prevent Schistosomiasis. PLoS Neglected Tropical Diseases. 2015;9: e0003657–6. doi: 10.1371/journal.pntd.0003657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sokolow SH, Wood CL, Jones IJ, Swartz SJ, Lopez M, Hsieh MH, et al. Global assessment of schistosomiasis control over the past century shows targeting the snail intermediate host works best. PLoS Neglected Tropical Diseases. 2016;10: e0004794–20. doi: 10.1371/journal.pntd.0004794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frandsen F. Discussion of the relationships between Schistosoma and their intermediate hosts, assessment of the degree of host-parasite compatibility and evaluation of schistosome taxonomy. Z Parasitenkd.1979;58: 275–296. doi: 10.1007/BF00933934 [DOI] [PubMed] [Google Scholar]
- 13.Brown D. Freshwater snails of Africa and their medical importance, Department of Zoology Natural History Museum. London: Taylor & Francis Ltd; 1994. [Google Scholar]
- 14.DeJong RJ, Morgan JA, Paraense WL, Pointier JP, Amarista M, Ayeh-Kumi PFK, et al. Evolutionary relationships and biogeography of Biomphalaria (Gastropoda: Planorbidae) with implications regarding its role as host of the human bloodfluke, Schistosoma mansoni. Molecular Biology and Evolution. 2001;18: 2225–2239. doi: 10.1093/oxfordjournals.molbev.a003769 [DOI] [PubMed] [Google Scholar]
- 15.Mutuku MW, Dweni CK, Mwangi M, Kinuthia JM, Mwangi IN, Maina G, et al. Field-derived Schistosoma mansoni and Biomphalaria pfeifferi in Kenya: A compatible association characterized by lack of strong local adaptation, and presence of some snails able to persistently produce cercariae for over a year. Parasites & Vectors. 2014;7: 485–13. doi: 10.1186/s13071-014-0533-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu L, Zhang S-M, Mutuku MW, Mkoji GM, Loker ES. Relative compatibility of Schistosoma mansoni with Biomphalaria sudanica and B. pfeifferi from Kenya as assessed by PCR amplification of the S. mansoni ND5 gene in conjunction with traditional methods. Parasites & Vectors. 2016: 1–13. doi: 10.1186/s13071-016-1457-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infectious Diseases 6 2006:411–425. doi: 10.1016/S1473-3099(06)70521-7 [DOI] [PubMed] [Google Scholar]
- 18.Altizer S, Ostfeld RS, Johnson PTJ, Kutz S, Harvell CD. Climate change and infectious diseases: From evidence to a predictive framework. Science. 2013;341: 514–519. doi: 10.1126/science.1239401 [DOI] [PubMed] [Google Scholar]
- 19.Stensgaard A-S, Utzinger J, Vounatsou P, Hürlimann E, Schur N, Saarnak CFL, et al. Large-scale determinants of intestinal schistosomiasis and intermediate host snail distribution across Africa: Does climate matter? Acta Tropica. 2013;128: 378–390. doi: 10.1016/j.actatropica.2011.11.010 [DOI] [PubMed] [Google Scholar]
- 20.Charbonnel N, Angers B, Rasatavonjizay R, Brémond P, Debain C, Jarne P. The influence of mating system, demography, parasites and colonization on the population structure of Biomphalaria pfeifferi in Madagascar. Molecular Ecology. 2002;11: 2213–2228. doi: 10.1046/j.1365-294X.2002.01586.x [DOI] [PubMed] [Google Scholar]
- 21.Charbonnel N, Rasatavonjizay R, Sellin E, Brémond P, Jarne P. The influence of genetic factors and population dynamics on the mating system of the hermaphroditic freshwater snail Biomphalaria pfeifferi. Oikos. 2005;108: 283–296. [Google Scholar]
- 22.Campbell G, Noble LR, Rollinson D, Southgate VR, Webster JP, Jones CS. Low genetic diversity in a snail intermediate host (Biomphalaria pfeifferi Krass, 1848) and schistosomiasis transmission in the Senegal River Basin. Molecular Ecology. 2009;19: 241–256. doi: 10.1111/j.1365-294X.2009.04463.x [DOI] [PubMed] [Google Scholar]
- 23.Nguema RM, Langand J, Galinier R, Idris MA, Shaban MA, Al Yafae S, et al. Genetic diversity, fixation and differentiation of the freshwater snail Biomphalaria pfeifferi (Gastropoda, Planorbidae) in arid lands. Genetica. 2013;141: 171–184. doi: 10.1007/s10709-013-9715-8 [DOI] [PubMed] [Google Scholar]
- 24.Eveland LK, Haseeb MA. Laboratory rearing of Biomphalaria glabrata snails and maintenance of larval schistosomes in vivo and in vitro In Biomphalaria snails and larval trematodes. Toledo R. and Fried B, eds. Springer, pp. 33–55. 2011. [Google Scholar]
- 25.Lockyer AE, Jones CS, Noble LR, Rollinson D. Trematodes and snails: an intimate association. Canadian Journal of Zoology. 2004;82: 251–269. doi: 10.1139/z03-215 [Google Scholar]
- 26.Loker ES, Adema CM, Zhang S-M, Kepler TB. Invertebrate immune systems–not homogeneous, not simple, not well understood. Immunological Reviews. 2004;198: 10–24. doi: 10.1111/j.0105-2896.2004.0117.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bayne CJ. Successful parasitism of vector snail Biomphalaria glabrata by the human blood fluke (trematode) Schistosoma mansoni: A 2009 assessment. Molecular & Biochemical Parasitology. 2009;165: 8–18. doi: 10.1016/j.molbiopara.2009.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loker ES. Gastropod immunobiology. Advances in Experimental Medicine and Biology. 2010;708: 17–43. doi: 10.1007/978-1-4419-8059-5_2 [DOI] [PubMed] [Google Scholar]
- 29.Coustau C, Gourbal B, Duval D, Yoshino TP, Adema CM, Mitta G. Advances in gastropod immunity from the study of the interaction between the snail Biomphalaria glabrata and its parasites: A review of research progress over the last decade. Fish and Shellfish Immunology. 2015;46: 5–16. doi: 10.1016/j.fsi.2015.01.036 [DOI] [PubMed] [Google Scholar]
- 30.Pila EA, Gordy MA, Phillips VK, Kabore AL, Rudko SP, Hanington PC. Endogenous growth factor stimulation of hemocyte proliferation induces resistance to Schistosoma mansoni challenge in the snail host. Proceedings of the National Academy of Sciences. 2016;113: 5305–5310. doi: 10.1073/pnas.1521239113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pila EA, Tarrabain M, Kabore AL, Hanington PC. A novel toll-Like receptor (TLR) influences compatibility between the gastropod Biomphalaria glabrata, and the digenean trematode Schistosoma mansoni. PLoS Pathogens. 2016;12: e1005513–23. doi: 10.1371/journal.ppat.1005513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allan ERO, Tennessen JA, Bollmann SR, Hanington PC, Bayne CJ, Blouin MS. Schistosome infectivity in the snail, Biomphalaria glabrata, is partially dependent on the expression of Grctm6, a Guadeloupe Resistance Complex protein. PLoS Neglected Tropical Diseases. 2017;11: e0005362–15. doi: 10.1371/journal.pntd.0005362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitta G, Gourbal B, Grunau C, Knight M, Bridger JM, Théron A. The compatibility between Biomphalaria glabrata snails and Schistosoma mansoni: an increasingly complex puzzle. Advances in Parasitology. 2017;97: 111–145. doi: 10.1016/bs.apar.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 34.Galinier R, Roger E, Moné Y, Duval D, Portet A, Pinaud S, et al. A multistrain approach to studying the mechanisms underlying compatibility in the interaction between Biomphalaria glabrata and Schistosoma mansoni. PLoS Neglected Tropical Diseases. 2017;11: e0005398–25. doi: 10.1371/journal.pntd.0005398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pila EA, Li H, Hambrook JR, Wu X, Hanington PC. Schistosomiasis from a snail’s perspective: advances in snail immunity. Trends in Parasitology. 2017;In press. doi: 10.1016/j.pt.2017.07.006 [DOI] [PubMed] [Google Scholar]
- 36.Portet A, Pinaud S, Tetreau G, Galinier R, Cosseau C, Duval D, et al. Integrated multi-omic analyses in Biomphalaria-Schistosoma dialogue reveal the immunobiological significance of FREP-SmPoMuc interaction. 2017;75:16–27. doi: 10.1016/j.dci.2017.02.025 [DOI] [PubMed] [Google Scholar]
- 37.Adema CM, Hillier LW, Jones CS, Loker ES, Knight M, et al. Whole genome analysis of a schistosomiasis-transmitting freshwater snail. Nature Communications. 2017;8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nowak TS, Woodards AC, Jung Y, Adema CM, Loker ES. Identification of transcripts generated during the response of resistant Biomphalaria glabrata to Schistosoma mansoni infection using suppression subtractive hybridization. Journal of Parasitology. 2004;90: 1034–1040. doi: 10.1645/GE-193R1 [DOI] [PubMed] [Google Scholar]
- 39.Mitta G, Galinier R, Tisseyre P, Allienne J, Girerdchambaz Y, Guillou F, et al. Gene discovery and expression analysis of immune-relevant genes from Biomphalaria glabrata hemocytes. Developmental & Comparative Immunology. 2005;29: 393–407. doi: 10.1016/j.dci.2004.10.002 [DOI] [PubMed] [Google Scholar]
- 40.Guillou F, Mitta G, Galinier R, Coustau C. Identification and expression of gene transcripts generated during an anti-parasitic response in Biomphalaria glabrata. Developmental & Comparative Immunology. 2007;31: 657–671. doi: 10.1016/j.dci.2006.10.001 [DOI] [PubMed] [Google Scholar]
- 41.Lockyer AE, Spinks JN, Walker AJ, Kane RA, Noble LR, Rollinson D, et al. Biomphalaria glabrata transcriptome: Identification of cell-signalling, transcriptional control and immune-related genes from open reading frame expressed sequence tags (ORESTES). Developmental & Comparative Immunology. 2007;31: 763–782. doi: 10.1016/j.dci.2006.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanelt B, Lun C-M, Adema CM. Comparative ORESTES-sampling of transcriptomes of immune-challenged Biomphalaria glabrata snails. Journal of Invertebrate Pathology. 2008;99: 192–203. doi: 10.1016/j.jip.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lockyer AE, Spinks J, Kane RA, Hoffmann KF, Fitzpatrick JM, Rollinson D, et al. Biomphalaria glabrata transcriptome: cDNA microarray profiling identifies resistant- and susceptible-specific gene expression in haemocytes from snail strains exposed to Schistosoma mansoni. BMC Genomics. 2008;9: 634–17. doi: 10.1186/1471-2164-9-634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adema CM, Hanington PC, Lun C-M, Rosenberg GH, Rosenberg GH, Aragon AD, et al. Differential transcriptomic responses of Biomphalaria glabrata (Gastropoda, Mollusca) to bacteria and metazoan parasites, Schistosoma mansoni and Echinostoma paraensei (Digenea, Platyhelminthes). Molecular Immunology. 2010;47: 849–860. doi: 10.1016/j.molimm.2009.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang SM, Adema CM, Kepler TB, Loker ES. Diversification of Ig superfamily genes in an invertebrate. Science. 2004;305: 251–254. doi: 10.1126/science.1088069 [DOI] [PubMed] [Google Scholar]
- 46.Hanington PC, Zhang S-M. The primary role of fibrinogen-related proteins in invertebrates is defense, not coagulation. Journal of Innate Immunology. 2011;3: 17–27. doi: 10.1159/000321882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanington PC, Lun C-M, Adema CM, Loker ES. Time series analysis of the transcriptional responses of Biomphalaria glabrata throughout the course of intramolluscan development of Schistosoma mansoni and Echinostoma paraensei. International Journal for Parasitology. 2010;40: 819–831. doi: 10.1016/j.ijpara.2009.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang S-M, Loker ES, Sullivan JT. Pathogen-associated molecular patterns activate expression of genes involved in cell proliferation, immunity and detoxification in the amebocyte-producing organ of the snail Biomphalaria glabrata. Developmental & Comparative Immunology. 2016;56: 25–36. doi: 10.1016/j.dci.2015.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang S-M, Buddenborg SK, Adema CM, Sullivan JT, Loker ES. Altered gene expression in the schistosome-transmitting snail Biomphalaria glabrata following exposure to niclosamide, the active ingredient in the widely used molluscicide Bayluscide. PLoS Neglected Tropical Diseases. 2015;9: e0004131–21. doi: 10.1371/journal.pntd.0004131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tennessen JA, Bonner KM, Bollmann SR, Johnstun JA, Yeh J-Y, Marine M, et al. Genome-wide scan and test of candidate genes in the snail Biomphalaria glabrata reveal new locus influencing resistance to Schistosoma mansoni. PLoS Neglected Tropical Diseases. 2015;9: e0004077–19. doi: 10.1371/journal.pntd.0004077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tennessen JA, Théron A, Marine M, Yeh J-Y, Rognon A, Blouin MS. Hyperdiverse gene cluster in snail host conveys resistance to human schistosome parasites. PLoS Genetics. 2015;11: e1005067–21. doi: 10.1371/journal.pgen.1005067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanington PC, Forys MA, Loker ES. A somatically diversified defense factor, FREP3, is a determinant of snail resistance to schistosome infection. PLoS Neglected Tropical Diseases. 2012;6: e1591 doi: 10.1371/journal.pntd.0001591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deleury E, Dubreuil G, Elangovan N, Wajnberg E, Reichhart J-M, Gourbal B, et al. Specific versus non-specific immune responses in an invertebrate species evidenced by a comparative de novo sequencing study. PLoS ONE. 2012;7: e32512–15. doi: 10.1371/journal.pone.0032512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pinaud S, Portela J, Duval D, Nowacki FC, Olive M-A, Allienne JF, et al. A shift from cellular to humoral responses contributes to innate immune memory in the vector snail Biomphalaria glabrata. PLoS Pathogens. 2016;12: e1005361–18. doi: 10.1371/journal.ppat.1005361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang H, Zhao QP, Nie P, Jiang MS, Song J. Identification of differentially expressed genes in Oncomelania hupensis chronically infected with Schistosoma japonicum. Experimental Parasitology. 2012;130: 374–383. doi: 10.1016/j.exppara.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 56.Zhao QP, Xiong T, Xu XJ, Jiang MS, Dong HF. De novo transcriptome analysis of Oncomelania hupensis after molluscicide treatment by Next-Generation Sequencing: Implications for Biology and Future Snail Interventions. PLoS ONE. 2015;10: e0118673–16. doi: 10.1371/journal.pone.0118673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berriman M, Haas BJ, LoVerde PT, Wilson RA, Dillon GP, Cerqueira GC, et al. The genome of the blood fluke Schistosoma mansoni. Nature. 2009;460: 352–358. doi: 10.1038/nature08160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verjovski-Almeida S, DeMarco R, Martins EAL, Guimarães PEM, Ojopi EPB, Paquola ACM, et al. Transcriptome analysis of the acoelomate human parasite Schistosoma mansoni. Nature Genetics. 2003;35: 148–157. doi: 10.1038/ng1237 [DOI] [PubMed] [Google Scholar]
- 59.Vermeire JJ, Taft AS, Hoffmann KF, Fitzpatrick JM, Yoshino TP. Schistosoma mansoni: DNA microarray gene expression profiling during the miracidium-to-mother sporocyst transformation. Molecular & Biochemical Parasitology. 2006;147: 39–47. doi: 10.1016/j.molbiopara.2006.01.006 [DOI] [PubMed] [Google Scholar]
- 60.Taft AS, Vermeire JJ, Bernier J, Birkeland SR, Cipriano MJ, Papa AR, et al. Transcriptome analysis of Schistosoma mansoni larval development using serial analysis of gene expression (SAGE). Parasitology. 2009;136: 469–17. doi: 10.1017/S0031182009005733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Horn DJ, Garcia JR, Loker ES, Mitchell KR, Mkoji GM, Adema CM, Takacs-Vesbach CD. Complex intestinal bacterial communities in three species of planorbid snails. Journal of Molluscan Studies. 2012;78: 74–80. doi: 10.1093/mollus/eyr038 [Google Scholar]
- 62.Silva TM, Melo ES, Lopes ACS, Veras DL, Duarte CR, Alves LC, Brayner FA. Characterization of the bacterial microbiota of Biomphalaria glabrata (Say, 1818) (Mollusca: Gastropoda) from Brazil. Letters in Applied Microbiology. 2013;57: 19–25. doi: 10.1111/lam.12068 [DOI] [PubMed] [Google Scholar]
- 63.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30: 2114–2120. doi: 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2012;29: 15–21. doi: 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biology. 2013;14: R36–R36. doi: 10.1186/gb-2013-14-4-r36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotechnology. 2011;29: 644–652. doi: 10.1038/nbt.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nature Protocols. 2013;8: 1494–1512. doi: 10.1038/nprot.2013.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gilbert D. Gene-omes built from mRNA seq not genome DNA. 7th Annual Arthropod Genomics Symposium. Notre Dame. 2013. http://arthropods.eugenes.org/EvidentialGene/about/EvigeneRNA2013poster.pdf and http://globalhealth.nd.edu/7th-annual-arthropod-genomics-symposium/
- 69.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene Ontology: tool for the unification of biology. Nature Genetics. 2000;25: 25–29. doi: 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kanehisa M, Sato Y. KEGG: Kyoto Encycopedia of Genes and Genomes. Nucleic Acid Research. 2000;28: 27–30. doi: 10.1093/nar/28.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jones P, Binns D, Chang HY, Fraser M, Li W, McAnulla C, et al. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30: 1236–1240. doi: 10.1093/bioinformatics/btu031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krogh A, Larsson B, von Heijne G, Sonnhammer ELL. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. Journal of Molecular Biology. 2001;305: 567–580. doi: 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
- 73.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nature Methods. 2012;9: 357–359. doi: 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12: 323–323. doi: 10.1186/1471-2105-12-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leng N, Dawson JA, Thomson JA, Ruotti V, Rissman AI, Smits BMG, et al. EBSeq: an empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics. 2013;29: 1035–1043. doi: 10.1093/bioinformatics/btt087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rozen S, Skaletsky HJ. Primer3. 1998. http://www-genome.wi.mit.edu/genome_software/other/primer3.html. Accessed February 2017.
- 77.Dheilly NM, Duval D, Mouahid G, Emans R, Allienne JF, Galinier R, et al. A family of variable immunoglobulin and lectin domain containing molecules in the snail Biomphalaria glabrata. Developmental & Comparative Immunology. 2015;48: 234–243. doi: 10.1016/j.dci.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simakov O, Marletaz F, Edsinger-Gonzales E, Havlak P, Hellsten U, Kuo D-H, et al. Insights into bilaterian evolution from three spiralian genomes. Nature. 2013;493: 526–531. doi: 10.1038/nature11696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takeuchi T, Kawashima T, Koyanagi R, Gyoja F, Tanaka M, Ikuta T, et al. 2012. Draft genome of the pearl oyster Pinctada fucata: A platform for understanding bivalve biology. DNA Research 19: 117–130 doi: 10.1093/dnares/dss005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang G, Fang X, Guo X, Li L, Luo R, Xu F, et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature. 2012;490: 49–54. doi: 10.1038/nature11413 [DOI] [PubMed] [Google Scholar]
- 81.Albertin CB, Simakov O, Mitros T, Yan Wang Z, Pungor JR, Edsinger-Gonzales E, et al. The octopus genome and the evolution of cephalopod neural and morphological novelties. Nature. 2015;524: 220–224. doi: 10.1038/nature14668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kocot KM, Cannon JT, Todt C, Citarella MR, Kohn AB, Meyer A, et al. Phylogenomics reveals deep molluscan relationships. Nature. 2011;477: 452–456. doi: 10.1038/nature10382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Greiman SE, Vaughan JA, Elmahy R, Adisakwattana P, Van Ha N, Fayton TJ, et al. Real-time PCR detection and phylogenetic relationships of Neorickettsia spp. in digeneans from Egypt, Philippines, Thailand, Vietnam and the United States. Parasitology International. 2017;66: 1003–1007. doi: 10.1016/j.parint.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duval D, Galinier R, Mouahid G, Toulza E, Allienne JF, Portela J, et al. A novel bacterial pathogen of Biomphalaria glabrata: A potential weapon for schistosomiasis control? PLoS Neglected Tropical Diseases. 2015;9: e0003489–20. doi: 10.1371/journal.pntd.0003489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suga H, Chen Z, de Mendoza A, Sebé-Pedrós A, Brown MW, Kramer E, et al. The Capsaspora genome reveals a complex unicellular prehistory of animals. Nature Communications. 2013;4: 2325 doi: 10.1038/ncomms3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cuomo CA, Desjardins CA, Bakowski MA, Golderg J, Ma AT, Becnel JJ, et al. Microsporidian genome analysis reveals evolutionary strategies for obligate intracellular growth. Genome Research. 2012;22: 2478–88. doi: 10.1101/gr.142802.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pombert J-F, Xu J, Smith DR, Heiman D, Young S, Cuomo CA, et al. Complete genome sequence from three genetically distinct strains reveal high intraspecies genetic diversity in the microsporidian Encephalitozoon cuniculi. Eukaryotic Cell. 2013;12: 503–511. doi: 10.1128/EC.00312-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bakowski MA, Priest M, Young S, Cuomo CA, Troemel ER. Genome sequence of the microsporidian species Nematocida sp1 Strain ERTm6 (ATCC PRA-372). Genome Announcements. 2014;2: e00905–14. doi: 10.1128/genomeA.00905-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Desjardins CA, Sanscrainte ND, Goldberg JM, Heiman D, Young S, Zeng Q, et al. Contrasting host-pathogen interactions and genome evolution in two generalist and specialist microsporidian pathogens of mosquitoes. Nature Communications. 2015;6: 7121 doi: 10.1038/ncomms8121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tkach VV, Kudlai O, Kostadinova A. Molecular phylogeny and systematics of the Echinostomatoidea Looss, 1899 (Platyhelminthes: Digenea). International Journal for Parasitology. 2016;46: 171–185. doi: 10.1016/j.ijpara.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 91.Wilson WD, Johnson PT, Sutherland DR, Moné H, Loker ES. A molecular phylogenetic study of the genus Ribeiroia (Digenea): trematodes known to cause limb malformations in amphibians. Journal of Parasitology. 2005;91: 1040–1045. doi: 10.1645/GE-465R.1 [DOI] [PubMed] [Google Scholar]
- 92.Laidemitt MR, Zawadzki ET, Brant SV, Mutuku MW, Mkoji GM, Loker ES. Loads of trematodes: Discovering hidden diversity of paramphistomoids in Kenyan ruminants. Parasitology. 2017;144: 131–147. doi: 10.1017/S0031182016001827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sweedler JV, Li L, Rubakhin SS, Alexeeva V, Dembrow NC, Dowling O, et al. Identification and characterization of the feeding circuit-activating peptides, a novel neuropeptide family of Aplysia. Journal of Neuroscience. 2002;22: 7797–7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Davies CM, Webster JP, Woolhouse MEJ. Trade–offs in the evolution of virulence in an indirectly transmitted macroparasite. Proceeding of the Royal Society B. 2001;268: 251–257. doi: 10.1098/rspb.2000.1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tavalire HF, Blouin MS, Steinauer ML. Genotypic variation in host response to infection affects parasite reproductive rate. International Journal for Parasitology. 2016;46: 123–131. doi: 10.1016/j.ijpara.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 96.Steinauer ML, Hanelt B, Mwangi IN, Maina GM, Agola EL, Kinuthia JM, et al. Introgressive hybridization of human and rodent schistosome parasites in western Kenya. Molecular Ecology. 2008;17: 5062–5074. doi: 10.1111/j.1365-294X.2008.03957.x [DOI] [PubMed] [Google Scholar]
- 97.Theron A. Dynamics of larval populations of Schistosoma mansoni in Biomphalaria glabrata. Annals of Tropical Medicine and Parasitology. 1981;75: 71–77. doi: 10.1080/00034983.1981.11720796 [PubMed] [Google Scholar]
- 98.Shi M, Lin X-D, Tian J-H, Chen L-J, Chen X, Li C-X, et al. Redefining the invertebrate RNA virosphere. Nature. 2016:1–12. doi: 10.1038/nature20167 [DOI] [PubMed] [Google Scholar]
- 99.Rosani U, Gerdol M. A bioinformatics approach reveals seven nearly-complete RNA-virus genomes in bivalve RNA-seq data. Virus Research. 2016:1–10. doi: 10.1016/j.virusres.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 100.Galinier R, Tetreau G, Portet A, Pinaud S, Duval G, Gourbal B. First characterization of viruses from freshwater snails of the genus Biomphalaria, the intermediate host of the parasite Schistosoma mansoni. Acta Tropica. 2017;167: 196–203. doi: 10.1016/j.actatropica.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 101.Park B-K, Kim M-J, Kim E-H, Kim M-S, Na D-G, Chae J-S. Identification of trematode cercariae carrying Neorickettsia risticii in freshwater stream snails. Annals of the New York Academy of Sciences. 2003;990: 239–247. doi: 10.1111/j.1749-6632.2003.tb07371.x [DOI] [PubMed] [Google Scholar]
- 102.Michelson E. The protective action of Chaetogaster limnaei on snails exposed to Schistosoma mansoni. Journal of Parasitology. 1964;50: 441–444. doi: 10.2307/3275851 [PubMed] [Google Scholar]
- 103.Rodgers JK, Sandland GJ, Joyce SR, Minchella DJ. Multi-species interactions among a commensal (Chaetogaster limnaei limnaei), a parasite (Schistosoma mansoni), and an aquatic snail host (Biomphalaria glabrata). Journal of Parasitology, 2005;91: 709–712. doi: 10.1645/GE-421R [DOI] [PubMed] [Google Scholar]
- 104.Ibrahim MM. Population dynamics of Chaetogaster limnaei (Oligochaeta: Naididae) in the field populations of freshwater snails and its implications as a potential regulator of trematode larvae community. Parasitology Research. 2007;101: 25–33. doi: 10.1007/s00436-006-0436-0 [DOI] [PubMed] [Google Scholar]
- 105.Zimmermann MR, Luth KE, Esch GW. Complex interactions among a nematode parasite (Daubaylia potomaca), a commensalistic annelid (Chaetogaster limnaei limnaei), and trematode parasites in a snail host (Helisoma anceps). Journal of Parasitology. 2011;97: 788–791. doi: 10.1645/GE-2733.1 [DOI] [PubMed] [Google Scholar]
- 106.Hertel LA, Barbosa CS, Santos RAAL, Loker ES. Molecular identification of symbionts from the pulmonate snail Biomphalaria glabrata in Brazil. Journal of Parasitology. 2004;90: 759–763. doi: 10.1645/GE-223R [DOI] [PubMed] [Google Scholar]
- 107.Hertel LA, Bayne CJ, Loker ES. The symbiont Capsaspora owczarzaki, nov. gen. nov. sp, isolated from three strains of the pulmonate snail Biomphalaria glabrata is related to members of the Mesomycetozoea. International Journal for Parasitology. 2002;32: 1183–1191. doi: 10.1016/S0020-7519(02)00066-8 [DOI] [PubMed] [Google Scholar]
- 108.Ruiz-Trillo I, Inagaki Y, Davis LA, Sperstad S, Landfald B, Roger AJ. Capsaspora owczarzaki is an independent opisthokont lineage. Current Biology. 2004;14: R946–7. doi: 10.1016/j.cub.2004.10.037 [DOI] [PubMed] [Google Scholar]
- 109.McClymont EH, Dunn AM, Terry RS, Rollinson D, Littlewood DTJ, Smith JE. Molecular data suggest that microsporidian parasites in freshwater snails are diverse. International Journal for Parasitology. 2005;35: 1071–1078. doi: 10.1016/j.ijpara.2005.05.008 [DOI] [PubMed] [Google Scholar]
- 110.Chambouvet A, Gower DJ, Jirků M, Yabsley MJ, Davis AK, Leonard G, et al. Cryptic infection of a broad taxonomic and geographic diversity of tadpoles by Perkinsea protists. Proceedings of the National Academy of Sciences. 2015;112: E4743–E4751. doi: 10.1073/pnas.1500163112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bråte JBA, Logares R, Berney CED, Ree DK, Klaveness D, Jakobsen KS, Shalchian-Tabrizi K. Freshwater Perkinsea and marine-freshwater colonizations revealed by pyrosequencing and phylogeny of environmental rDNA. The ISME Journal. 2010;4: 1144–1153. doi: 10.1038/ismej.2010.39 [DOI] [PubMed] [Google Scholar]
- 112.Fox I, García-Moll I. Echiniscus molluscorum, new tardigrade from the feces of the land snail, Bulimulus exilis (Gmelin) in Puerto Rico (Tardigrada: Scutechiniscidae). The Journal of Parasitology. 1962;48: 177–181. [Google Scholar]
- 113.Basch PF, Sturrock RF. Life History of Ribeiroia marini (Faust and Hoffman, 1934) comb. n. (Trematoda: Cathaemasiidae). Journal of Parasitology. 1969;55: 1180. [Google Scholar]
- 114.Johnson PTJ, Hoverman JT. Heterogeneous hosts: how variation in host size, behaviour and immunity affects parasite aggregation. Journal of Animal Ecology. 2014;83: 1103–1112. doi: 10.1111/1365-2656.12215 [DOI] [PubMed] [Google Scholar]
- 115.Ittiprasert W, Nene R, Miller A, Raghavan N, Lewis F, Hodgson J, Knight M. Schistosoma mansoni infection of juvenile Biomphalaria glabrata induces a differential stress response between resistant and susceptible snails. Experimental Parasitology. 2009;123: 203–211. doi: 10.1016/j.exppara.2009.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Knight M, Ittiprasert W, Arican-Goktas HD, Bridger JM. Epigenetic modulation, stress and plasticity in susceptibility of the snail host, Biomphalaria glabrata, to Schistosoma mansoni infection. International Journal for Parasitology. 2016;46:389–394. doi: 10.1016/j.ijpara.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 117.Perrigault M, Tran D. Identification of the molecular clockwork of the oyster Crassostrea gigas. PLoS ONE. 2017;12: e0169790–21. doi: 10.1371/journal.pone.0169790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee FO, Cheng TC. Schistosoma mansoni Infection in Biomphalaria glabrata: alterations in heart rate and thermal tolerance in the host. Journal of Invertebrate Pathology. 1971;18: 412–418. doi: 10.1016/0022-2011(71)90047-4 [DOI] [PubMed] [Google Scholar]
- 119.Williams CL, Gilbertson DE. Altered feeding response as a cause for the altered heartbeat rate and locomotor activity of Schistosoma mansoni-infected Biomphalaria glabrata. Journal of Parasitology. 1983;69: 671–676. doi: 10.2307/3281138 [PubMed] [Google Scholar]
- 120.Boissier J, Rivera ER, Moné H. Altered behavior of the snail Biomphalaria glabrata as a result of infection with Schistosoma mansoni. Journal of Parasitology. 2003;89: 429–433. doi: 10.1645/0022-3395(2003)089[0429:ABOTSB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 121.Humphries J. Effects of larval schistosomes on Biomphalaria snails In Biomphalaria snails and larval trematodes. Toledo R. and Fried B, eds. Springer, pp. 103–125. 2011. [Google Scholar]
- 122.Finney J, Moon H-J, Ronnebaum T, Lantz M, Mure M. Human copper-dependent amine oxidases. Archives of Biochemistry and Biophysics. 2014;546: 19–32. doi: 10.1016/j.abb.2013.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee JO, Cheng TC. Increased heart rate in Biomphalaria glabrata parasitized by Schistosoma mansoni. Journal of Invertebrate Pathology. 1970;16: 148–149. [DOI] [PubMed] [Google Scholar]
- 124.Dzik JM. Evolutionary roots of arginase expression and regulation. Frontiers in Immunology. 2014;5: 544 doi: 10.3389/fimmu.2014.00544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sturrock BM. The influence of infection with Schistosoma mansoni on the growth rate and reproduction of Biomphalaria pfeifferi. Annals of Tropical Medicine and Parasitology. 1966;60: 187–97. doi: 10.1080/00034983.1966.11686405 [DOI] [PubMed] [Google Scholar]
- 126.Sturrock RF, Sturrock BM. Observations on some factors affecting growth rate and fecundity of Biomphalaria glabrata (Say). Annals of Tropical Medicine and Parasitology. 1970;64: 349 doi: 10.1080/00034983.1970.11686704 [DOI] [PubMed] [Google Scholar]
- 127.Gérard C. Energy constrain exerted by the parasite Schistosoma mansoni on the locomotion of its snail host, Biomphalaria glabrata. Canadian Journal of Zoology. 1996;74: 594–598. doi: 10.1139/z96-068 [Google Scholar]
- 128.Ibrahim MM. Energy allocation patterns in Biomphalaria alexandrina snails in response to cadmium exposure and Schistosoma mansoni infection. Experimental Parasitology. 2006;112: 31–36. doi: 10.1016/j.exppara.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 129.Lee FO, Cheng TC. Schistosoma mansoni: Alterations in total protein and hemoglobin in the hemolymph of infected Biomphalaria glabrata. Experimental Parasitology. 1972;31: 203–216. [DOI] [PubMed] [Google Scholar]
- 130.Meuleman EA. Host-Parasite Interrelationships Between the Freshwater Pulmonate Biomphalaria pfeifferi and the Trematode Schistosoma mansoni. Netherlands Journal of Zoology. 1971;22: 355–427. doi: 10.1163/002829672X00013 [Google Scholar]
- 131.Ibikounlé M, Mouahid G, Mintsa Nguéma R, Sakiti NG, Kindé-Gasard D, Massougbodji A, Moné H. Life-history traits indicate local adaptation of the schistosome parasite, Schistosoma mansoni, to its snail host, Biomphalaria pfeifferi. Experimental Parasitology. 2012;132: 501–507. doi: 10.1016/j.exppara.2012.09.020 [DOI] [PubMed] [Google Scholar]
- 132.Koene JM. Neuro-endocrine control of reproduction in hermaphroditic freshwater snails: mechanisms and evolution. Frontiers in Behavioral Neuroscience. 2010;4: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang T, Zhao M, Liang D, Bose U, Kaur S, McManus DP, Cummins SF. Changes in the neuropeptide content of Biomphalaria ganglia nervous system following Schistosoma infection. Parasites and Vectors. 2017;10: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Manger P, Li J, Christensen BM, Yoshino TP. Biogenic monoamines in the freshwater snail, Biomphalaria glabrata: influence of infection by the human blood fluke, Schistosoma mansoni. Comparative Biochemistry and Physiology Part A: Physiology. 1996;114: 227–234. doi: 10.1016/0300-9629(95)02131-0 [DOI] [PubMed] [Google Scholar]
- 135.Santhanagopalan V, Yoshino TP. Monoamines and their metabolites in the freshwater snail Biomphalaria glabrata. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology. 2000;125: 469–478. [DOI] [PubMed] [Google Scholar]
- 136.Mukai ST, Kiehn L, Saleuddin AS. Dopamine stimulates snail albumen gland glycoprotein secretion through the activation of a D1-like receptor. Journal of Experimental Biology. 2004;207: 2507–2518. doi: 10.1242/jeb.01052 [DOI] [PubMed] [Google Scholar]
- 137.Dictus WJ, de Jong-Bring M, Boer HH. A neuropeptide (Calfluxin) is involved in the influx of calcium into mitochondria of the albumen gland of the freshwater snail Lymnaea stagnalis. General and Comparative Endocrinology. 1987;65: 439–450. [DOI] [PubMed] [Google Scholar]
- 138.Zhang S-M, Nian H, Wang B, Loker ES, Adema CM. Schistosomin from the snail Biomphalaria glabrata: Expression studies suggest no involvement in trematode-mediated castration. Molecular & Biochemical Parasitology. 2009;165: 79–86. doi: 10.1016/j.molbiopara.2009.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cummins SF, Nichols AE, Amare A, Hummon AB, Sweedle JV, Nagle GT. Characterization of Aplysia enticin and temptin, two novel water-borne protein pheromones that act in concert with attractin to stimulate mate attraction. Journal of Biological Chemistry. 2004;279: 25614–25622. doi: 10.1074/jbc.M313585200 [DOI] [PubMed] [Google Scholar]
- 140.Hathaway JJM, Adema CM, Stout BA, Mobarak CD, Loker ES. Identification of protein components of egg masses indicates parental investment in immunoprotection of offspring by Biomphalaria glabrata (Gastropoda, Mollusca). Developmental & Comparative Immunology. 2010;34: 425–435. doi: 10.1016/j.dci.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pila EA, Peck SJ, Hanington PC. The protein pheromone temptin is an attractant of the gastropod Biomphalaria glabrata. Journal of Comparative Physiology A. 2017:1–12. doi: 10.1007/s00359-017-1198-0 [DOI] [PubMed] [Google Scholar]
- 142.de Jong-Brink M, ter Maat A, Tensen CP. NPY in invertebrates: molecular answers to altered functions during evolution. Peptides. 2001;22: 309–315. doi: 10.1016/S0196-9781(01)00332-1 [DOI] [PubMed] [Google Scholar]
- 143.Jones MK, McManus DP, Sivadorai P, Glanfield A, Moertel L, Belli SI, Gobert GN. Tracking the fate of iron in early development of human blood flukes. The International Journal of Biochemistry and Cell Biology. 2007;39: 1646–1658. doi: 10.1016/j.biocel.2007.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goodall CP, Bender RC, Broderick EJ, Bayne CJ. Constitutive differences in Cu/Zn superoxide dismutase mRNA levels and activity in hemocytes of Biomphalaria glabrata (Mollusca) that are either susceptible or resistant to Schistosoma mansoni (Trematoda). Molecular & Biochemical Parasitology. 2004;137: 321–328. doi: 10.1016/j.molbiopara.2004.06.011 [DOI] [PubMed] [Google Scholar]
- 145.Goodall CP, Bender RC, Brooks JK, Bayne CJ. Biomphalaria glabrata cytosolic copper/zinc superoxide dismutase (SOD1) gene: Association of SOD1 alleles with resistance/susceptibility to Schistosoma mansoni. Molecular & Biochemical Parasitology. 2006;147: 207–210. doi: 10.1016/j.molbiopara.2006.02.009 [DOI] [PubMed] [Google Scholar]
- 146.Blouin MS, Bonner KM, Cooper B, Amarasinghe V, O’Donnell RP, Bayne CJ. Three genes involved in the oxidative burst are closely linked in the genome of the snail, Biomphalaria glabrata. International Journal for Parasitology. 2013;43: 51–55. doi: 10.1016/j.ijpara.2012.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bathige SDNK, Umasuthan N, Whang I, Lim B-S, Won SH, Lee J. Antibacterial activity and immune responses of a molluscan macrophage expressed gene-1 from disk abalone, Haliotis discus discus. Fish and Shellfish Immunology. 2014;39: 263–272. doi: 10.1016/j.fsi.2014.05.012 [DOI] [PubMed] [Google Scholar]
- 148.Ittiprasert W, Miller A, Myers J. Nene V, El-Sayed NM, Knight M. Identification of immediate response genes dominantly expressed in juvenile resistant and susceptible Biomphalaria glabrata snails upon exposure to Schistosoma mansoni. Molecular and Biochemical Parasitology. 2010;169: 27–39. doi: 10.1016/j.molbiopara.2009.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Arai I, Ohta M, Suzuki A, Tanaka S, Yoshizawa Y, Sato R. Immunohistochemical analysis of the role of hemocytin in nodule formation in the larvae of the silkworm, Bombyx mori. Journal of Insect Science. 2013;13: 125–13. doi: 10.1673/031.013.12501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bouchut A, Coustau C, Gourbal B, Mitta G. Compatibility in the Biomphalaria glabrata/Echinostoma caproni model: new candidate genes evidenced by a suppressive subtractive hybridization approach. Parasitology. 2006;134; 575–614. doi: 10.1017/S0031182006001673 [DOI] [PubMed] [Google Scholar]
- 151.Takamatsu N, Shiba T, Muramoto K, Kamiya H. Molecular cloning of the defense factor in the albumen gland of the sea hare Aplysia kurodai. FEBS Letters. 1995;377: 373–376. doi: 10.1016/0014-5793(95)01375-X [DOI] [PubMed] [Google Scholar]
- 152.Fujii N, Minetti T, Casa T, Nakhasi HL, Chen SW, Barbehenn E, et al. Isolation, cDNA cloning and characterization of an 18 kDa hemagglutinin and amebocyte aggregation factor from Lymus polyphemus. Journal of Biological Chemistry. 1992;267: 22452–22459 [PubMed] [Google Scholar]
- 153.Zhang S-M, Zeng Y, Loker ES. Characterization of immune genes from the schistosome host snail Biomphalaria glabrata that encode peptidoglycan recognition proteins and gram-negative bacteria binding protein. Immunogenetics. 2007;59: 883–898. doi: 10.1007/s00251-007-0245-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bai G, Brown JF, Watson C, Yoshino TP. Isolation and characterization of phenoloxidase from egg masses of the gastropod mollusc, Biomphalaria glabrata. Comparative Biochemistry and Physiology—Part B: Biochemistry & Molecular Biology. 1997;118: 463–9. doi: 10.1016/S0305-0491(97)00159-4 [DOI] [PubMed] [Google Scholar]
- 155.Le Clec’h W, Anderson TJ, Chevalier F. Characterization of hemolymph phenoloxidase activity in two Biomphalaria snail species and impact of Schistosoma mansoni infection. Parasites and Vectors. 2016;9 doi: 10.1186/s13071-016-1319-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.McDowell IC, Modak TH, Lane CE, Gomez-Chiarri M. Multi-species protein similarity clustering reveals novel expanded immune gene families in the eastern oyster Crassostrea virginica. Fish and Shellfish Immunology. 2016;53: 13–23. doi: 10.1016/j.fsi.2016.03.157 [DOI] [PubMed] [Google Scholar]
- 157.Yoshino TP, Dinguirard N, Kunert J, Hokke CH. Molecular and functional characterization of a tandem-repeat galectin from the freshwater snail Biomphalaria glabrata, intermediate host of the human blood fluke Schistosoma mansoni. Gene. 2008;411: 46–58. doi: 10.1016/j.gene.2008.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yoshino TP, Bayne CJ. Mimicry of snail host antigens by miracidia and primary sporocysts of Schistosoma mansoni. Parasite Immunology. 1983;5: 317–328. doi: 10.1111/j.1365-3024.1983.tb00747.x [DOI] [PubMed] [Google Scholar]
- 159.Knight M, Arican-Goktas HD, Ittiprasert W, Odoemelam EC, Miller AN, Bridger JM. Schistosomes and snails: a molecular encounter. Frontiers in Genetics. 2014;5: 230 doi: 10.3389/fgene.2014.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Arican-Goktas HD, Ittiprasert W, Bridger JM, Knight M. Differential spatial repositioning of activated genes in Biomphalaria glabrata snails infected with Schistosoma mansoni. PLoS Neglected Tropical Diseases. 2014;8: e3013 doi: 10.1371/journal.pntd.0003013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Timmins-Schiffman E, Roberts S. Characterization of genes involved in ceramide metabolism in the Pacific oyster (Crassostrea gigas). BMC Research Notes. 2012;5: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rosenstiel P, Sina C, End C, Renner M, Lyer S, Till A, et al. Regulation of DMBT1 via NOD2 and TLR4 in intestinal epithelial cells modulates bacterial recognition and invasion. Journal of Immunology. 2007;178(12):8203–11. doi: 10.4049/jimmunol.178.12.8203 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(TIF)
(TIF)
(TIF)
(TIF)
Percentages next to organisms show the percent of proteins present in our B. pfeifferi transcriptome predicted by TransDecoder.
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
The raw and assembled sequence data are available at NCBI under BioProject ID PRJNA383396. Additional relevant data are within the paper and its Supporting Information files.