Abstract
Background and purpose
Cerebral microbleed (CMB) location (deep versus strictly lobar) may elucidate underlying pathology with deep CMBs being more associated with hypertensive vascular disease, and lobar CMBs being more associated with cerebral amyloid angiopathy (CAA). The objective of this study was to determine whether neuroimaging signs of vascular disease and Alzheimer’s pathology are associated with different types of CMBs.
Methods
Among 1,677 non-demented ARIC participants (mean age=76 ± 5 years, 40% male, 26% black) with 3T MRI scans at the fifth exam (2011–13), we fit multinomial logistic-regression models to quantify relations of brain volumes (Alzheimer’s disease (AD) signature regions, total gray matter, frontal gray matter, and white matter hyperintensities (WMH) volumes), infarct frequencies (lacunar, non-lacunar, and total), and APOE (number of ε4 alleles) with CMB location (none, deep/mixed, or strictly lobar CMBs). Models were weighted for the sample-selection scheme and adjusted for age, sex, education, hypertension, ever smoking status, diabetes, race-site membership, and estimated intracranial volume (brain volume models only).
Results
Deep/mixed and strictly lobar CMBs had prevalences of 8% and 16%, respectively. Larger WMH burden, greater total infarct frequency, smaller frontal volumes (in females only), and smaller total gray matter volume were associated with greater risk of both deep and lobar CMBs relative to no CMBs. Greater WMH volume was also associated with greater risks of deep relative to lobar CMBs. Higher lacunar and non-lacunar infarct frequencies were associated with higher risks of deep CMBs, whereas smaller AD signature region volume and APOE ε4 homozygosity were associated with greater risks of lobar CMBs.
Conclusion
CMBs are a common vascular pathology in the elderly. Markers of hypertensive small-vessel disease may contribute to deep CMBs while CAA may drive development of lobar CMB.
Keywords: Cerebral Microbleeds, Magnetic Resonance Imaging, ARIC Study
Introduction
Cerebral microbleeds (CMBs) are represented by small areas of hemosiderin deposition (hemosiderin), detected on brain magnetic resonance imaging (MRI), which are found in approximately 23% of the cognitively normal population over age sixty.1 Cerebral microbleeds predict future risk of hemorrhagic2 and ischemic stroke.3 They are associated with increased cardiovascular mortality.4, 5 CMB location (deep versus strictly lobar) may be indicative of underlying pathology with deep CMBs being more associated with hypertensive vascular disease and lobar CMBs being more associated with cerebral amyloid angiopathy(CAA).6 In patients with AD or vascular dementia, those with lobar only CMBs have a higher amyloid burden than those with mixed lobar and deep CMBs or deep only CMBs.7 The frequency and neuroimaging correlates of CMBs in a large biracial, non-demented population require further investigation.
Using data from the Atherosclerosis Risk in Communities (ARIC) Neurocognitive Study cohort, our goal was to test the hypothesis that lobar-only CMBs are associated with an Alzheimer’s disease (AD) pattern of atrophy and APOE while deep or mixed-deep and lobar CMBS are associated MRI markers of vascular hypertensive disease (infarcts and white-matter hyperintensity (WMH) and with frontal lobe volume in nondemented individuals.
Methods
Participants
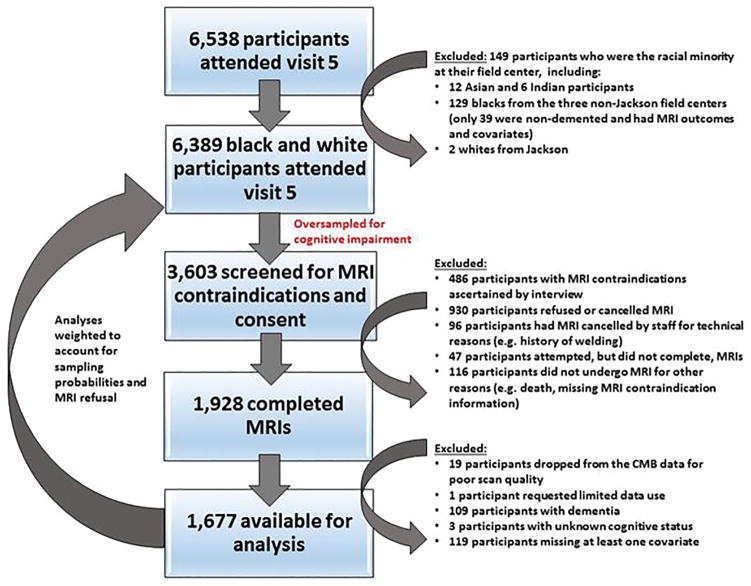
ARIC is a prospective epidemiologic study that began with baseline examination of cardiovascular risk factors in men and women aged 45 to 64 years between 1987 and 1989 who represented 4 US communities (Washington County, MD; Forsyth County, NC; Jackson, MS; and suburban Minneapolis, MN). Between June 2011 and August 2013, ARIC conducted a fifth examination.8 Of 10,749 original ARIC cohort members alive at the start of the fifth examination, 6538 (aged 66–90 years) took part. A subset of fifth visit participants were considered for MRI scans, including: 1) All individuals with cognitive impairment, defined by low Mini-Mental State Examination [MMSE] scores (<19 for blacks and <21 for whites) or a low age-, race-, and education-adjusted z-score on at least one of five cognitive domains (failure on the clock reading test for visuospatial domain or z-scores below −1.5 for memory, language, executive function, or attention domains) accompanied by cognitive decline between visits (below the 10th percentile for change in the delayed word recall, digit symbol substitution, or word fluency test or below the 20th percentile for change on two or more of these tests); 2) all participants with a prior brain MRI; and 3) an age- and field center-stratified random sample of cognitively normal individuals. From this subset, 1,928 participants were free of MRI contraindications, gave informed consent, and completed the MRI scan. We excluded participants from this analysis because of poor scan quality, missing covariate values, limits on data use, dementia (assessment and adjudication detailed in 8), or unknown cognitive status, yielding an analysis sample size of 1,677 participants. See Figure 1 for details. Institutional Review Boards of each ARIC center approved the protocol, and all participants provided written informed consent.
Figure 1.
Study Design
Covariate Definitions
Education was self-reported at the first ARIC visit (1987–1989) and categorized as “less than high school,” “high school, GED, or vocational school,” or “some college.” Unless otherwise specified, covariates were defined at visit 5. Blood pressure was categorized as normal (SBP<120 mmHg, DBP<80 mmHg, and no antihypertensive use), prehypertension (120≤ SBP <140 mmHg or 80≤DBP<90 mmHg in absence of antihypertensive use), or hypertension (SBP≥140 mmHg, DBP≥90 mmHg, or antihypertensive use). Diabetes was defined by medication use, a fasting glucose ≥126 mg/dl, or a non-fasting glucose ≥ 200 mg/dl. Smoking status was self-reported; never smoked was defined as anyone reporting never smoking more than 100 cigarettes. Ever smokers included current and former smokers across multiple visits. Race-site indicators for Jackson blacks, suburban Minneapolis whites, and Washington County whites were used, leveraging Forsyth County whites as the reference. Although not used in the regression models due to a lack of statistical significance (p≥0.20 for all global and individual Wald tests), dyslipidemia was defined by cholesterol-lowering medication use, a fasting total cholesterol ≥240 mg/dl, HDL <40 mg/dl (males) or <50 mg/dl (females), or LDL ≥160 mg/dl.
Predictors
Apolipoprotein-E
The apolipoprotein-E (APOE) genotypes were determined via TaqMan assays and the ABI 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). Seven participants did not consent to DNA use and were excluded from all analyses involving APOE.
Imaging
MRI scans were performed at each site on 3 Tesla Siemens scanners. Standardized 3D Magnetization Prepared Rapid Acquisition Gradient-Echo (MPRAGE) and Fluid-attenuated inversion recovery (FLAIR) developed for the multi-center Alzheimer’s Disease Neuroimaging Initiative (ADNI) were used for imaging. 9, 10 The MPRAGE image was used to assess gray matter volumes, and the FLAIR image was used to assess infarcts and quantification of WMH.
FLAIR image assessment
Brain infarcts were assessed visually on FLAIR images by a trained image analyst and confirmed by a radiologist (KK or CRJ) blinded to all clinical information. Lacunar infarcts, small subcortical cavity 3–15mm in diameter with surrounding hyperintensity on FLAIR sequence, were defined according to consensuses recommendations using a cutpoint of 15mm.11 Hyperintensities associated with infarcts are marked and were not included in the total white matter hyperintensity (WMH) volume due to distinct pathophysiologic differences between the two lesions.12 WMH volumes were measured using a semi-automated segmentation algorithm as previously described.13
Structural MRI Analysis
Total intracranial volume (eTIV) estimated from Freesurfer was used.14 Using the Freesurfer atlas15, we pre-specified two regions of interest (ROIs) based on relevance to Alzheimer’s disease or cerebrovascular disease: 1) AD signature ROI: hippocampus, parahippocampal, entorhinal, inferior parietal lobule, precuneus, and cuneus; and 2) frontal ROI: mean cortical volume of regions in the frontal lobe from both right and left hemispheres: rostral/caudal anterior cingulate, rostral/caudal mid-frontal, lateral orbital frontal, medial orbital frontal, paracentral, pars opercularis, pars orbitalis, pars triangularis, precentral, superior frontal, and frontal pole.
Microbleed Outcomes
CMBs were graded using a T2* Gradient Echo (GRE) (TR/TE = 200/20 ms; flip angle = 12°; FOV = 20 cm; in-plane matrix = 256 x 224; phase FOV = 1.00; slice thickness = 3.3 mm. Time is 5 min). CMBs were defined as homogenous hypo-intense lesions ≤10 mm in diameter in the gray or white matter on T2* GRE images as previously described. All CMBs were identified by trained image analysts and secondarily confirmed by a radiologist (KK or CJ) and were categorized as definite or possible with only definite CMBs being used in the analysis. The inter-rater agreement between the two raters on definite versus not-definite CMB was 85% (kappa=68%). 16 Composite maps of CMBs locations across subjects were built by transforming T2* GRE image locations into the T1 image space and applying the discrete cosine transformation to the template space derived from SPM.16 CMBs were categorized as lobar (cortical gray and subcortical or periventricular white matter), deep (deep gray matter: basal ganglia and thalamus; and the white matter of the corpus callosum, internal, external, and extreme capsule), and infratentorial (brain stem and cerebellum). For analysis, definite CMBs were divided into “strictly lobar” microbleeds (participants with ≥1 microbleeds limited to a lobar region) and “deep/mixed microbleeds” (participants with ≥1 microbleeds in a deep or infratentorial region with or without lobar microbleeds) as lobar CMBs in individuals with deep CMBs are similar to those with deep alone.17
Statistical Methods
The WMH volumes were right-skewed and required a logarithmic-base 2 transformation. Lacunar and total infarct frequencies were recoded to collapse values ≥3, while the non-lacunar infarct frequencies were recoded to collapse values ≥2. The distribution of the infarct frequencies and the relationship between infarct frequencies and CMB risks determined these thresholds. Because of the sampling-selection scheme, participants who completed MRI scans were not representative of all participants who completed the fifth visit. Therefore, all statistical tests and models were weighted back to the latter. Weights, equaling the product of the inverse sampling fractions and the inverse probability of completing the examination, were incorporated into survey options in Stata.
Poisson and multinomial logistic regression models estimated the effect of each predictor on CMB presence (Yes/No) and location, respectively, while adjusting for age, sex, education, hypertension status, smoking status, diabetes, race-site, and estimated intracranial volume (for models including a brain-volume predictor). We included interaction terms to gauge whether eTIV or predictor effects were modified by age, sex, or race (captured by the Jackson race-site indicator, since all included blacks hailed from that field center); an interaction was deemed significant at the 0.05 level if its global Wald test was less than the Bonferroni corrected threshold (corrected for the total number of experiment-wide interaction tests conducted). Any interaction included in the Poisson model of CMB presence was included in the multinomial logistic model of CMB location and vice versa. We present the effect estimates and 95% confidence intervals for all predictors included in the Poisson and multinomial logistic models but do not perform further adjustments for multiple testing; the associations between many of the predictors and microbleed presence/location were not expected to represent independent statistical tests (particularly for lacunar, non-lacunar, and total infarct frequencies and brain volumes with overlapping regions).
Lastly, we fit Poisson and multinomial logistic models of all neurological predictors simultaneously while adjusting for covariates; we employed a forward selection method, adding neurological predictors in order of significance until the remaining predictors had no appreciable impact (global Wald test p ≥0.20 and individual relative risk [RR] and relative risk ratio [RRR] p-values≥0.20).
Results
The descriptive statistics for the analyzed and excluded participants from the fifth visit are shown in Table 1. White participants from suburban Minneapolis were underrepresented in the analyzed data (24% versus 31% of excluded participants), whereas black participants from Jackson were overrepresented (26% and 20% of the analyzed and excluded participants, respectively). The analysis sample was enriched for mild cognitive impairment (36% compared to 16% in the excluded sample) but contained no demented individuals. Additional cognitive information on the full, analyzed, and excluded samples is detailed in Table SI.
Table 1.
Descriptive Statistics Stratified By Cerebral Microbleed Location
| Characteristic | Comparison of Analyzed Sample to the Excluded Visit 5 Participants | Analyzed Sample Stratified by Cerebral Microbleed Location | |||
|---|---|---|---|---|---|
|
| |||||
| Excluded Sample (N=4,843) | Analyzed Sample (N=1,677) | No CMB (N=1,279) | Deep or Mixed CMB (N=136) | Strictly Lobar CMB (N=262) | |
|
| |||||
| Cognitive Status(N(%)) | |||||
|
| |||||
| Normal | 3671 (77) | 1072 (64) | 835 (65) | 82 (60) | 155 (59) |
| Mild Cognitive Impairment | 766 (16) | 605 (36) | 444 (35) | 54 (40) | 107 (41) |
| Dementia | 342 ( 7) | 0 ( 0) | 0 ( 0) | 0 ( 0) | 0 ( 0) |
|
| |||||
| Covariates | |||||
|
| |||||
| Female (N(%)) | 2829 (58) | 1006 (60) | 785 (61) | 81 (60) | 140 (53) |
|
| |||||
| Age in years (Mean(SD)) | 76 ( 5) | 76 ( 5) | 76 ( 5) | 77 ( 5) | 77 ( 5) |
|
| |||||
| Education (N(%)) | |||||
| Less than High School | 769 (16) | 218 (13) | 156 (12) | 20 (15) | 42 (16) |
| High School, GED, or Vocational School | 2009 (42) | 699 (42) | 531 (42) | 61 (45) | 107 (41) |
| Some College | 2054 (43) | 760 (45) | 592 (46) | 55 (40) | 113 (43) |
|
| |||||
| Race-site (N(%)) | |||||
| Forsyth County blacks | 103 ( 2) | 0 ( 0) | 0 ( 0) | 0 ( 0) | 0 ( 0) |
| Forsyth County whites | 946 (20) | 390 (23) | 288 (23) | 42 (31) | 60 (23) |
| Jackson blacks | 974 (20) | 441 (26) | 322 (25) | 38 (28) | 81 (31) |
| Jackson whites | 2 ( 0) | 0 ( 0) | 0 ( 0) | 0 ( 0) | 0 ( 0) |
| Minneapolis blacks | 10 ( 0) | 0 ( 0) | 0 ( 0) | 0 ( 0) | 0 ( 0) |
| Minneapolis whites | 1489 (31) | 404 (24) | 311 (24) | 27 (20) | 66 (25) |
| Washington County blacks | 16 ( 0) | 0 ( 0) | 0 ( 0) | 0 ( 0) | 0 ( 0) |
| Washington County whites | 1303 (27) | 442 (26) | 358 (28) | 29 (21) | 55 (21) |
|
| |||||
| Diabetes (N(%)) | 1349 (30) | 470 (28) | 353 (28) | 33 (24) | 84 (32) |
|
| |||||
| Hypertension (N(%)) | |||||
| Normal | 502 (11) | 186 (11) | 150 (12) | 12 ( 9) | 24 ( 9) |
| Prehypertensive | 684 (14) | 241 (14) | 184 (14) | 15 (11) | 42 (16) |
| Hypertensive | 3564 (75) | 1250 (75) | 945 (74) | 109 (80) | 196 (75) |
|
| |||||
| Current or Former Smoker (N(%)) | 2595 (61) | 945 (56) | 712 (56) | 82 (60) | 151 (58) |
|
| |||||
| Estimated Total Intracranial Volume in cm3 (Mean(SD)) | - | 1382 (156) | 1379 (157) | 1376 (147) | 1399 (157) |
|
| |||||
| Predictors of Interest | |||||
|
| |||||
| White Matter Hyperintensity Volume in cm3 (Mean(SD)) | - | 17 (17) | 15 (15) | 27 (23) | 20 (19) |
|
| |||||
| Total AD Signature Region Volume in cm3 (Mean(SD)) | - | 59 ( 7) | 60 ( 7) | 58 ( 7) | 59 ( 7) |
|
| |||||
| Total Gray Volume in cm3 (Mean(SD)) | - | 443 (45) | 444 (45) | 434 (45) | 442 (45) |
|
| |||||
| Frontal Volume in cm3 (Mean(SD))* | 151 (16) | 151 (16) | 148 (16) | 151 (16) | |
| Females | - | 144 (13) | 145 (13) | 141 (12) | 142 (12) |
| Males | - | 160 (15) | 161 (15) | 158 (15) | 161 (15) |
|
| |||||
| Number of Lacunar Infarcts (N(%)) | |||||
| 0 | - | 1390 (83) | 1085 (85) | 101 (74) | 204 (78) |
| 1 | - | 208 (12) | 143 (11) | 23 (17) | 42 (16) |
| 2 | - | 57 ( 3) | 37 ( 3) | 10 ( 7) | 10 ( 4) |
| ≥3 | - | 20 ( 1) | 12 ( 1) | 2 ( 1) | 6 ( 2) |
|
| |||||
| Number of Non-Lacunar Infarcts (N(%)) | |||||
| 0 | - | 1486 (89) | 1156 (91) | 113 (83) | 217 (83) |
| 1 | - | 147 ( 9) | 98 ( 8) | 16 (12) | 33 (13) |
| ≥2 | - | 42 ( 3) | 23 ( 2) | 7 ( 5) | 12 ( 5) |
|
| |||||
| Total Number of Infarcts (N(%)) | |||||
| 0 | - | 1261 (75) | 996 (78) | 88 (65) | 177 (68) |
| 1 | - | 272 (16) | 197 (15) | 27 (20) | 48 (18) |
| 2 | - | 89 ( 5) | 56 ( 4) | 12 ( 9) | 21 ( 8) |
| ≥3 | - | 53 ( 3) | 28 ( 2) | 9 ( 7) | 16 ( 6) |
|
| |||||
| Copies of the APOE ε4 Allele (N(%)) | |||||
| 0 | 3226 (71) | 1160 (72) | 897 (73) | 94 (71) | 169 (67) |
| 1 | 1245 (27) | 416 (26) | 314 (26) | 33 (25) | 69 (27) |
| 2 | 103 ( 2) | 38 ( 2) | 18 ( 1) | 6 ( 5) | 14 ( 6) |
NOTE: SD=Standard deviation. The statistics are the observed frequencies and means;
The frontal volume exhibited significant interactions with sex; thus sex-specific summary statistics are presented along with the overall (combined sex) values.
Among the 1677 non-demented participants analyzed, 24% had CMBs, 16% had strictly lobar CMBs, and 8% had deep or mixed CMBs. The CMB prevalence after accounting for oversampling of cognitive impairment and MRI refusal was similar (22%), with weighted prevalences of 14% and 7% for strictly lobar and deep/mixed CMBs, respectively (see Table SII for weighted summary statistics). The observed prevalences of CMBs in blacks and whites were 27% (9% deep/mixed + 18% strictly lobar) and 23% (8% deep/mixed + 15% strictly lobar), respectively, while the weighted prevalences were 26% (8% deep/mixed + 18% strictly lobar) and 21% (7% deep/mixed + 14% strictly lobar).
Participants with CMBs, regardless of location, had higher prevalence rates of mild cognitive impairment with and without weighting. Summary statistics revealed differences in covariates, particularly sex and hypertension status, across CMB locations. Males were overrepresented in the strictly lobar group. The strictly lobar and deep/mixed groups had the highest proportion of pre-hypertensives and hypertensives, respectively. Simultaneously including all covariates in models of CMB presence and location confirmed associations with sex and hypertension status and established age and race-site effects (see Table SIII). The Jackson site (which recruited blacks only) was not associated with increased risk of CMBs compared to the Forsyth County whites. However, the northern sites (suburban Minneapolis and Washington County which recruited whites) were associated with a lower risk of deep/mixed CMBs compared to the Forsyth County site.
Neuroimaging/APOE
Summary statistics showed that the WMH volume distribution and infarct (lacunar, non-lacunar, and total) frequencies differed by CMB location (see Table 1). Larger WMH volume, greater infarct (lacunar, non-lacunar, and total) frequencies, APOE ε4 homozygosity, smaller AD signature region volume, and smaller total gray volume were associated with an increased prevalence of CMB via single-predictor Poisson regression models adjusted for covariates (see Table 2; single-predictor models without covariate adjustments are shown in Table S IV). The effect of frontal volume on CMB presence differed by sex (interaction p=0.0003); greater frontal volumes were associated with lower risk of CMBs in females and higher risk in males (although the male confidence interval contained one). No other predictors had significant age, sex, or race interactions in the CMB presence or location models. Figures SI and SII display the probabilities of CMB presence across the spectrum of predictor values; the predicted probabilities account for the covariates by averaging across the values in our sample (weighted to the fifth-visit participants). Although the estimated RRs per 1 cm3 change in frontal volume seem similar in the two sexes, the frontal volume by sex interaction is evident in Figure SI.
Table 2.
Modeling Each Predictor Separately While Adjusting for Covariates
| Predictor | CMB (Presence Versus Absence) | CMB Location | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Deep/Mixed Versus No CMB | Strictly Lobar Versus No CMB | Strictly Lobar Versus Deep/Mixed CMB | ||||||
| `
| ||||||||
| RR | 95% CI | RRR | 95% CI | RRR | 95% CI | RRR | 95% CI | |
|
| ||||||||
| Log2(WMH Volume (cm3)) | 1.255 | (1.138, 1.383) | 1.634 | (1.286, 2.076) | 1.232 | (1.065,1.425) | 0.754 | (0.581, 0.978) |
|
| ||||||||
| AD Signature Region Volume (in cm3) | 0.961 | (0.938, 0.984) | 0.956 | (0.901, 1.015) | 0.944 | (0.911,0.978) | 0.987 | (0.925, 1.054) |
|
| ||||||||
| Total Gray Volume (in cm3) | 0.994 | (0.989, 0.998) | 0.990 | (0.980, 0.999) | 0.992 | (0.986, 0.999) | 1.003 | (0.992, 1.014) |
|
| ||||||||
| Number of Lacunar Infarcts | 1.233 | (1.079, 1.409) | 1.629 | (1.212, 2.189) | 1.226 | (0.962, 1.563) | 0.753 | (0.536, 1.057) |
|
| ||||||||
| Number of Non-Lacunar Infarcts | 1.288 | (1.071, 1.549) | 1.649 | (1.065, 2.555) | 1.323 | (0.972, 1.799) | 0.802 | (0.498, 1.291) |
|
| ||||||||
| Total Number of Infarcts | 1.238 | (1.115, 1.376) | 1.595 | (1.262, 2.015) | 1.253 | (1.038, 1.511) | 0.786 | (0.603, 1.024) |
|
| ||||||||
| Copies of the APOE ε4 Allele: | ||||||||
| 0- Reference | ||||||||
| 1 | 0.977 | (0.769, 1.241) | 1.139 | (0.685, 1.892) | 0.903 | (0.629, 1.296) | 0.793 | (0.442, 1.423) |
| 2 | 1.778 | (1.139, 2.773) | 1.962 | (0.673, 5.720) | 2.495 | (1.096, 5.677) | 1.271 | (0.429, 3.767) |
|
| ||||||||
| Predictor Exhibiting Significant Sex Interactions | ||||||||
|
| ||||||||
| Frontal Volume (in cm3) | ||||||||
| Female | 0.976 | (0.963, 0.989) | 0.964 | (0.937, 0.992) | 0.972 | (0.953, 0.991) | 1.008 | (0.977, 1.040) |
| Male | 1.002 | (0.990, 1.015) | 1.001 | (0.973, 1.031) | 1.004 | (0.983, 1.025) | 1.003 | (0.970, 1.037) |
NOTE: RR=relative risk; RRR=relative risk ratio. The Poisson (CMB presence) and multinomial logistic (CMB location) regression models included adjustments for age, sex, education, hypertension, smoking status, diabetes, race-site (used Forsyth as the reference), and estimated intracranial volume. The number of lacunar infarcts and the total number of infarcts were modeled with a single variable that took values 0, 1, 2, or 3 (for ≥3) infarcts. The number of non-lacunar infarcts was modeled with a single variable that took values 0, 1, or 2 (for ≥2) infarcts. Models incorporated weights accounting for the MRI sampling and completion probabilities.
To assess whether these associations were location dependent, we fit multinomial logistic regression models for each neurological predictor adjusted for all covariates. Table 2 shows the relative risk ratios (RRR) from these models, while Figures 2 and S III depict the predictive probabilities for the strictly lobar, deep/mixed, and total CMB outcomes. Smaller frontal volume in females, smaller total gray volume, larger WMH volume, and greater total infarct frequencies were associated with increased probabilities of both strictly lobar and deep/mixed CMBs. The impact of the interaction between frontal volume and sex on each microbleed location is depicted in the last two graphs in Figure S III.
Figure 2.
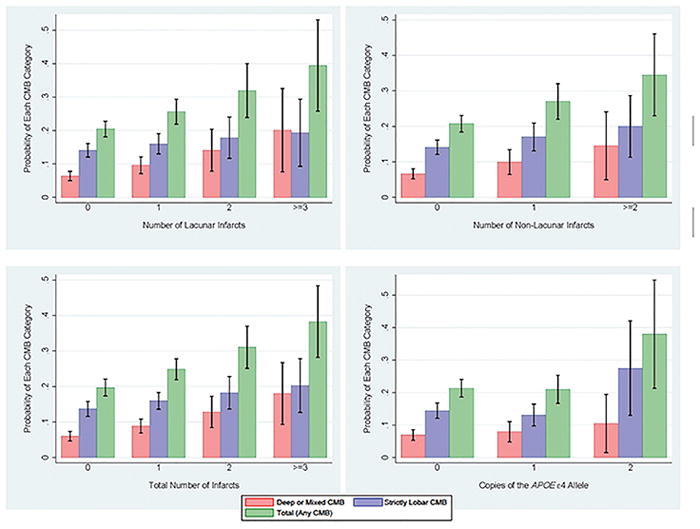
Probability of CMBs by Infarct Counts and APOE Genotype
There were predictors with location-specific effects. Smaller volumes in the AD signature regions (see Figure S III) and APOE ε4 homozygosity (see Figure 2) were associated with increased probabilities of strictly lobar CMBs; although the RRRs of these predictors were similar in the strictly lobar and deep/mixed group, the confidence intervals of the latter contained one and the probability plots indicated negligible effects. Higher lacunar and non-lacunar infarct frequencies were associated with increased probabilities of deep/mixed CMBs. In the model containing all significant predictors together (see Table 3), the total gray volume, lacunar infarcts, and non-lacunar infarcts were dropped. The effect estimates of the remaining predictors were consistent with the single-predictor models. Smaller volumes in the AD-related regions were associated with strictly lobar CMBs after accounting for APOE ε4 status.
Table 3.
Modeling All Predictors Together While Adjusting for Covariates
| Predictor | CMB (Presence Versus Absence) | CMB Location | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Deep/Mixed Versus No CMB | Strictly Lobar Versus No CMB | Strictly Lobar Versus Deep/Mixed CMB | ||||||
|
| ||||||||
| RR | 95% CI | RRR | 95% CI | RRR | 95% CI | RRR | 95% CI | |
|
| ||||||||
| Log2(WMH Volume (cm3)) | 1.213 | (1.092, 1.348) | 1.545 | (1.187, 2.011) | 1.188 | (1.016, 1.388) | 0.769 | (0.577, 1.024) |
|
| ||||||||
| AD Signature Region Volume (cm3) | 0.964 | (0.936, 0.994) | 0.970 | (0.902, 1.042) | 0.943 | (0.899, 0.988) | 0.972 | (0.897, 1.054) |
|
| ||||||||
| Frontal Volume (cm3) | ||||||||
| Female | 0.990 | (0.975, 1.005) | 0.981 | (0.948, 1.016) | 0.991 | (0.968, 1.014) | 1.009 | (0.971, 1.049) |
| Male | 1.015 | (1.001, 1.030) | 1.017 | (0.986, 1.050) | 1.023 | (0.998, 1.050) | 1.006 | (0.969, 1.044) |
|
| ||||||||
| Total Number of Infarcts | 1.129 | (1.002, 1.272) | 1.401 | (1.059, 1.854) | 1.105 | (0.900,1.356) | 0.788 | (0.578, 1.076) |
|
| ||||||||
| Copies of the APOE ε4 Allele: | ||||||||
| 0- Reference | ||||||||
| 1 | 0.957 | (0.758, 1.208) | 1.111 | (0.658, 1.877) | 0.873 | (0.606, 1.257) | 0.785 | (0.430, 1.435) |
| 2 | 1.621 | (1.076, 2.441) | 1.772 | (0.629, 4.992) | 2.193 | (1.001, 4.808) | 1.238 | (0.417, 3.672) |
NOTE: RR=relative risk; RRR=relative risk ratio. The covariates adjusted for include age, male, education, hypertension, smoking status, diabetes, race-site (used Forsyth as the reference), and estimated intracranial volume. Total gray volume, lacunar infarcts, and non-lacunar infarcts did not impact the model (global Wald p-value≥0.20 and individual RR and RRR had p-values≥0.20) when included with the other predictors. Thus, both were omitted from this model.
Discussion
In a biracial group of non-demented elderly individuals, the presence of CMBs was associated with older age, male sex, hypertension, and cognitive impairment. CMBs were not associated with diabetes, dyslipidemia, or history of smoking. Greater WMH, total infarcts, lacunar infarcts, non-lacunar infarcts, APOE ε4 allele homozygosity, lower total gray-matter volume, and AD signature-region volume were associated with CMBs; frontal volume was associated with reduced CMB presence in women but not men. The imaging associations differed by CMB location implying distinct pathophysiology, as we hypothesized. Lacunar infarcts were associated with deep CMBs, while lower volume in AD signature regions was associated with strictly lobar CMBs. These findings support prior observations that deep CMBs are more associated with hypertensive small-vessel pathologies and strictly lobar CMBs with AD pathologies.7 In fact, the mean WMH was greater in those with deep CMBs compared to strictly lobar CMBs, and APOE ε4 homozygosity was associated with strictly lobar CMBs but not deep CMBs.
Age and hypertension are consistent risk factors for CMBs across population based studies.17–19 Prior studies in predominantly European or European-American populations reported similar frequency.18, 19 In the ARIC study, no significant difference in risk of CMB presence or location were detected by race. Two prior racially and ethnically diverse studies on CMBs revealed no difference in CMB frequency between black, white and Hispanic individuals20, 21. Despite the association of CMBs with imaging markers of small-vessel disease, diabetes was not associated with CMBs in the present study or in the Framingham or Rotterdam studies.17, 18 Similarly, in a prior ARIC study, diabetes was associated with infarcts but not white-matter hyperintensity22 highlighting that small-vessel pathologies have overlapping but distinct mechanisms.
Male sex was associated with CMBs similar to the Framingham study18 and AGES Reykjavik studies23 but differed from the Rotterdam Scan study, where no sex difference was detected.19 The increased frequency of CMBs in men is interesting because WMH, also considered small vessel pathology and associated with CMBs, is greater in both deep and periventricular regions in women compared to men.24, 25 While WMH is associated with CMBs, there is a difference in the sex distribution with CMBs being more common in men and WMH more severe in women. Understanding these sex differences between types of small-vessel pathologies may provide insights into future prevention studies. In a prior ARIC publication, WMH volume was associated with lower frontal ROI volume.26 Therefore, it is not surprising that CMBs are associated with lower frontal ROI volume in women, who tend to have more WMH than men.
In the Rotterdam and the recent Framingham studies, the presence of an APOE ε4 allele (i.e., homozygotes or heterozygotes) was associated with strictly lobar CMBs.17–19 In contrast, in the present study and the AGES Reykjavik study,23 APOE ε4 homozygosity was associated with CMBs while heterozygosity was not. In an autopsy series comparing CMBs identified in the hospital versus the population-based Framingham cohort, lobar CMBs were only associated with significant cerebral amyloid angiopathy in the hospital-based cohort.27 Therefore, additional studies in population- based samples, including amyloid PET and autopsies, are necessary to better understand the mechanisms of lobar CMBs.
In addition to hypertension, lacunar strokes and WMH volume were associated with deep CMBs. Similarly, in a cohort of dementia patients with Alzheimer’s dementia or subcortical vascular cognitive impairment, the number of lacunar strokes was associated with having a deep CMB.7 Left ventricular hypertrophy28 was associated with CMBs, supporting the notion that deep CMBs represent a manifestation of hypertensive small-vessel disease.
In the current study, lobar CMBs were associated with atrophy in AD signature regions but not with lacunar stroke. Since AD commonly coexists with cerebral amyloid angiopathy pathology,29 this finding may reflect this strong association. The association between AD signature atrophy and lobar CMBs was not explained by the association of APOE ε4 and CMBs. In a model adjusting for APOE ε4 status, AD signature atrophy remained associated with lobar CMBs.
This study has several limitations. While participants with dementia were excluded from the current analysis, the participants selected in this study were not a random sample of all surviving ARIC participants because those selected for imaging included persons who had been in the prior ARIC MRI study or who had low cognitive scores. While our objective was to characterize the non-demented population, the exclusion of participants with dementia may lead to an underestimation of CMB frequency and weaken the association seen between AD signature region atrophy and CMBs. There were a greater number of participants with strictly lobar than deep/mixed CMBs; thus, the failure to identify significant AD signature-region volume or APOE ε4 homozygosity associations could be due to insufficient power in the latter rather than differences in pathology. On the other hand, the deep/mixed CMB group could be more heterogeneous in etiology due to the individuals with both deep and lobar CMBs; this may mask differences in AD pathology between the groups. All black participants included in this investigation were from the Jackson field center, making it impossible to distinguish the effect of race from the effect of the field center, particularly in tests of interactions. Only 39 blacks from the Forsyth County, suburban Minneapolis, and Washington County field centers were non-demented, gave consent, and had all necessary information (MRI outcomes and covariates), limiting their use in analyses. Lastly, we did not have sufficient numbers of microbleeds to perform race-stratified analyses while adjusting for covariates. Only 38 black participants had deep or mixed microbleeds and 81 had strictly lobar microbleeds. Although we tested the interaction between the Jackson site and each of the predictors, race-stratified analyses may be particularly important for predictors (such as the APOE ε4 allele) which differ in frequency between whites and blacks.
Conclusion
In a biracial study of non-demented participants, the frequency of CMBs remained substantial. AD signature region atrophy and APOE ε4 homozygosity were associated with lobar CMBs while lacunar infarcts were associated with deep CMBs.
Supplementary Material
Acknowledgments
Sources of Funding:
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Neurocognitive data is collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH (NHLBI, NINDS, NIA and NIDCD), and with previous brain MRI examinations funded by R01-HL70825 from the NHLBI.
The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Conflicts of Interest/Disclosures
J. Simino and T. Griswold have no disclosures
J. Graff-Radford receives funds from the Myron and Jane Hanley Career Development Award
K. Kantarci serves on the Data Safety Monitoring Board for Takeda Global Research & Development Center, Inc.; the data monitoring boards of Pfizer and Janssen Alzheimer Immunotherapy; and is funded by the NIH
D. Knopman served on a Data Safety Monitoring Board for Lilly Pharmaceuticals and for Lundbeck Pharmaceuticals and for the DIAN study; served as a consultant to TauRx Pharmaceuticals was an investigator in clinical trials sponsored by Baxter and Elan Pharmaceuticals in the past 2 years; is currently an investigator in a clinical trial sponsored by TauRx; and receives support from the NIH.
C. Jack serves as a consultant for Janssen, Bristol-Meyer-Squibb, General Electric, and Johnson & Johnson; is involved in clinical trials sponsored by Allon and Baxter, Inc.; and receives research support from Pfizer, Inc., and the NIA.
A. Richey Sharrett is funded by the NIH.
M. S. Albert is funded by the NIH.
T. Moseley is funded by the NIH.
P. Vemuri is funded by the NIH.
R. Gottesman is Associate Editor for Neurology.
B. Gwen Windham is an investigator in a clinical trial sponsored by ACADIA Pharmaceuticals, received travel support related to this study, but does not receive financial compensation.
References
- 1.Akoudad S, Darweesh SK, Leening MJ, Koudstaal PJ, Hofman A, van der Lugt A, et al. Use of coumarin anticoagulants and cerebral microbleeds in the general population. Stroke. 2014;45:3436–3439. doi: 10.1161/STROKEAHA.114.007112. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke. 2004;35:1415–1420. doi: 10.1161/01.STR.0000126807.69758.0e. [DOI] [PubMed] [Google Scholar]
- 3.Akoudad S, Portegies ML, Koudstaal PJ, Hofman A, van der Lugt A, Ikram MA, et al. Cerebral microbleeds are associated with an increased risk of stroke: The rotterdam study. Circulation. 2015;132:509–516. doi: 10.1161/CIRCULATIONAHA.115.016261. [DOI] [PubMed] [Google Scholar]
- 4.Altmann-Schneider I, Trompet S, de Craen AJ, van Es AC, Jukema JW, Stott DJ, et al. Cerebral microbleeds are predictive of mortality in the elderly. Stroke. 2011;42:638–644. doi: 10.1161/STROKEAHA.110.595611. [DOI] [PubMed] [Google Scholar]
- 5.Benedictus MR, Prins ND, Goos JD, Scheltens P, Barkhof F, van der Flier WM. Microbleeds, mortality, and stroke in alzheimer disease: The mistral study. JAMA Neurol. 2015;72:539–545. doi: 10.1001/jamaneurol.2015.14. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: A guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park JH, Seo SW, Kim C, Kim GH, Noh HJ, Kim ST, et al. Pathogenesis of cerebral microbleeds: In vivo imaging of amyloid and subcortical ischemic small vessel disease in 226 individuals with cognitive impairment. Ann Neurol. 2013;73:584–593. doi: 10.1002/ana.23845. [DOI] [PubMed] [Google Scholar]
- 8.Knopman DS, Gottesman RF, Sharrett AR, Wruck LM, Windham BG, Coker L, et al. Mild cognitive impairment and dementia prevalence: The atherosclerosis risk in communities neurocognitive study (aric-ncs) Alzheimers Dement (Amst) 2016;2:1–11. doi: 10.1016/j.dadm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The alzheimer’s disease neuroimaging initiative (adni): Mri methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantarci K, Weigand SD, Przybelski SA, Preboske GM, Pankratz VS, Vemuri P, et al. Mri and mrs predictors of mild cognitive impairment in a population-based sample. Neurology. 2013;81:126–133. doi: 10.1212/WNL.0b013e31829a3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kantarci K, Knopman DS, Dickson DW, Parisi JE, Whitwell JL, Weigand SD, et al. Alzheimer disease: Postmortem neuropathologic correlates of antemortem 1h mr spectroscopy metabolite measurements. Radiology. 2008;248:210–220. doi: 10.1148/radiol.2481071590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raz L, Jayachandran M, Tosakulwong N, Lesnick TG, Wille SM, Murphy MC, et al. Thrombogenic microvesicles and white matter hyperintensities in postmenopausal women. Neurology. 2013;80:911–918. doi: 10.1212/WNL.0b013e3182840c9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on mri scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Kantarci K, Gunter JL, Tosakulwong N, Weigand SD, Senjem MS, Petersen RC, et al. Focal hemosiderin deposits and beta-amyloid load in the adni cohort. Alzheimers Dement. 2013;9:S116–123. doi: 10.1016/j.jalz.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poels MM, Vernooij MW, Ikram MA, Hofman A, Krestin GP, van der Lugt A, et al. Prevalence and risk factors of cerebral microbleeds: An update of the rotterdam scan study. Stroke. 2010;41:S103–106. doi: 10.1161/STROKEAHA.110.595181. [DOI] [PubMed] [Google Scholar]
- 18.Romero JR, Preis SR, Beiser A, DeCarli C, Viswanathan A, Martinez-Ramirez S, et al. Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the framingham heart study. Stroke. 2014;45:1492–1494. doi: 10.1161/STROKEAHA.114.004130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, et al. Prevalence and risk factors of cerebral microbleeds: The rotterdam scan study. Neurology. 2008;70:1208–1214. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- 20.Caunca MR, Del Brutto V, Gardener H, Shah N, Dequatre-Ponchelle N, Cheung YK, et al. Cerebral microbleeds, vascular risk factors, and magnetic resonance imaging markers: The northern manhattan study. Journal of the American Heart Association. 2016:5. doi: 10.1161/JAHA.116.003477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiegman AF, Meier IB, Schupf N, Manly JJ, Guzman VA, Narkhede A, et al. Cerebral microbleeds in a multiethnic elderly community: Demographic and clinical correlates. Journal of the neurological sciences. 2014;345:125–130. doi: 10.1016/j.jns.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knopman DS, Penman A, Catellier D, Coker L, Shibata DK, Sharrett A, et al. Vascular risk factors and longitudinal changes on brain mri the aric study. Neurology. 2011;76:1879–1885. doi: 10.1212/WNL.0b013e31821d753f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sveinbjornsdottir S, Sigurdsson S, Aspelund T, Kjartansson O, Eiriksdottir G, Valtysdottir B, et al. Cerebral microbleeds in the population based ages-reykjavik study: Prevalence and location. J Neurol Neurosurg Psychiatry. 2008;79:1002–1006. doi: 10.1136/jnnp.2007.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sachdev PS, Parslow R, Wen W, Anstey KJ, Easteal S. Sex differences in the causes and consequences of white matter hyperintensities. Neurobiol Aging. 2009;30:946–956. doi: 10.1016/j.neurobiolaging.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 25.van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam scan study. Stroke. 2008;39:2712–2719. doi: 10.1161/STROKEAHA.107.513176. [DOI] [PubMed] [Google Scholar]
- 26.Knopman DS, Griswold ME, Lirette ST, Gottesman RF, Kantarci K, Sharrett AR, et al. Vascular imaging abnormalities and cognition: Mediation by cortical volume in nondemented individuals: Atherosclerosis risk in communities-neurocognitive study. Stroke. 2015;46:433–440. doi: 10.1161/STROKEAHA.114.007847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez-Ramirez S, Romero JR, Shoamanesh A, McKee AC, Van Etten E, Pontes-Neto O, et al. Diagnostic value of lobar microbleeds in individuals without intracerebral hemorrhage. Alzheimers Dement. 2015;11:1480–1488. doi: 10.1016/j.jalz.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SH, Park JM, Kwon SJ, Kim H, Kim YH, Roh JK, et al. Left ventricular hypertrophy is associated with cerebral microbleeds in hypertensive patients. Neurology. 2004;63:16–21. doi: 10.1212/01.wnl.0000132525.36804.a1. [DOI] [PubMed] [Google Scholar]
- 29.Love S. Contribution of cerebral amyloid angiopathy to alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75:1–4. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.