Abstract
Objectives
We sought to define the lower and upper limits of cerebral blood flow autoregulation (LLA and ULA) and the optimal blood pressure during cardiopulmonary bypass (CPB). We further sought to identify variables predictive of these autoregulation end-points.
Methods
Cerebral autoregulation was monitored continuously with transcranial Doppler in 614 patients during CPB enrolled in three investigations. A moving Pearson’s correlation coefficient was calculated between CBF velocity and MAP to generate the variable mean velocity index (Mx). Optimal MAP was defined as the MAP with the lowest Mx indicating the best autoregulation. The LLA and the ULA were defined as the MAP at which Mx was increasingly pressure passive (i.e., Mx ≥ 0.4) with declining or rising blood pressure, respectively.
Results
The mean (±SD) LLA, ULA, and optimal MAP were 65±12 mmHg, 84±11 mmHg, and 78±11 mmHg, respectively, after adjusting for study enrollment. In 17% of patients, though, the LLA was above this optimal MAP, while in 29% of patients the ULA was below the population optimal MAP. Variables associated with optimal MAP based on multivariate regression analysis were non-white race (increased 2.7 mmHg; p=0.034), diuretics use (decreased 1.9 mmHg; p=0.049), prior carotid endarterectomy (decreased 5.5 mmHg; p=0.019), and duration of CPB (decreased 1.28 per 60 min of CPB). The product of the duration and magnitude that MAP during CPB was below LLA was associated with risk for stroke (p=0.02).
Conclusions
Real-time monitoring of autoregulation may better allow for individualizing MAP during CPB and for improving patient outcomes.
Central Picture
Range of optimal mean arterial pressures.
INTRODUCTION
Since its introduction on May 6, 1953, by JH Gibbons, Jr, cardiopulmonary bypass (CPB) has allowed surgical teams to improve survival and quality of life for patients with life-threatening cardiovascular disease.1–3 Despite multiple technological and patient management advances in the ensuing 60 years, controversy remains regarding the appropriate mean arterial pressure (MAP) for patients during CPB, with no definition of optimal MAP universally accepted.4 The guiding principle of blood pressure management during CPB is that cerebral blood flow (CBF) autoregulation must remain functional during CPB when α-stat pH management is used.4–6 Consequently, a MAP as low as 50 mmHg, and even transiently lower, is often believed to be acceptable during CPB because CBF will not be compromised.5, 7 Cerebral autoregulation, however, might be impaired by many conditions which are prevalent in patients with coronary artery disease.8, 9 Indeed, neurological complications, including stroke and acute cognitive disorders, affect 1–2% and up to 20–40% of patients, respectively, after cardiac surgery.4, 10 Many such complications might be explained by cerebral hypoperfusion.11–14
Cerebral autoregulation can now be monitored at the bedside by continuously calculating the correlation coefficient between slow-wave (i.e., every 20 seconds to 3 minutes) changes in CBF velocity (measured with transcranial Doppler) and MAP.15–17 Using these methods in patients undergoing CPB, our group has reported that MAP at the lower limit of autoregulation (LLA) is broad (40 to 90 mmHg) and difficult to predict by patient demographics and other preoperative factors.16 We have further found that the magnitude and duration that MAP is below the LLA during CPB is associated with major morbidity and mortality postoperatively.18, 19 However, simply raising MAP targets during CPB may be associated with other adverse postoperative outcomes, as we have found that blood pressure excursions above the upper limit of autoregulation (ULA) during CPB are associated with postoperative delirium.20 Together, these studies suggest that guiding hemodynamics to ensure cerebral perfusion within the autoregulation range may be better for patient outcome than choosing these targets empirically. In our investigations, we have observed that as many as 20% of patients will not have a clear LLA due to a pattern of impaired autoregulation.21 In these situations, though, an optimal MAP is still usually observed at which autoregulation is best. Maintaining cerebral perfusion pressure in the optimal autoregulation range is associated with improved outcomes for patients with traumatic brain injury, supporting the potential clinical role of autoregulation monitoring.22 Our prior investigations did not have an adequate sample size to determine with high precision the range of optimal MAP for patients undergoing cardiac surgery with CPB, and we did not evaluate MAP with respect to ULA.
Despite growing data supporting a role for customized MAP targets based on autoregulation monitoring in patients undergoing cardiac surgery, the latter methods remain a research tool. The goal of this study was to expand on prior investigations of CBF autoregulation in order to provide guidance about optimal MAP to clinicians who care for patients undergoing cardiac surgery. We sought to define the LLA and ULA of CBF autoregulation and optimal blood pressure during CPB. We further sought to identify variables predictive of these autoregulation end-points.
MATERIALS AND METHODS
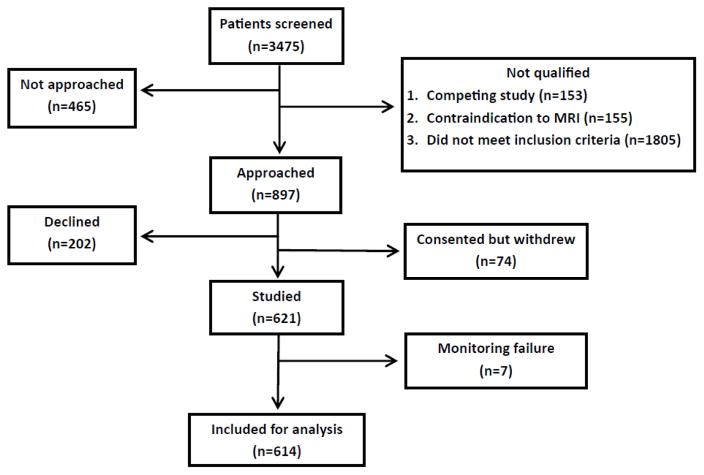
From April 2008 to April 2015, 614 patients undergoing cardiac surgery at The Johns Hopkins Hospital were enrolled in two completed and one on-going prospective trials that evaluated cerebral autoregulation monitoring with transcranial Doppler (TCD) (NCT00981474, NCT00769691).23, 24 A flow diagram of the patients included in this study is shown in in Figure 1. Enrollment in the largest of the three studies was limited to patients at high risk for neurological complications as determined by the Johns Hopkins stroke and encephalopathy risk score.25 Patients undergoing circulatory arrest were excluded. All procedures received the approval of the Institutional Review Board of The Johns Hopkins Medical Institutions, and all patients provided written informed consent. Three hundred forty-six patients included in this analysis were enrolled in a study that included preoperative TCD examination by a trained vascular technologist. As part of that protocol, patients in whom a temporal window for TCD monitoring was not present were excluded from subsequent intraoperative monitoring
Figure 1.
Flow diagram of the patients enrolled in the study and included in this analysis.
Hemodynamic Management and Anesthesia
General anesthesia was induced and maintained with midazolam, fentanyl, and isoflurane, and pancronium or vecuronium was administered for skeletal muscle relaxation. Before CPB, heparin was administered to achieve an activated clotting time > 480 seconds. The CPB flow was non-pulsatile and maintained with a target of 2.0 to 2.4 L/min/m2. Arterial pressure was monitored via a radial artery catheter and was controlled by adjusting CPB flow and the administration of vasoactive medications. The patients were managed with alpha-stat pH management. Normocarbia was ensured with a continuous in-line arterial blood gas monitor that was calibrated hourly. Protamine was administered to reverse the effects of heparin after separation from CPB.
Transcranial Doppler-Based Autoregulation Monitoring
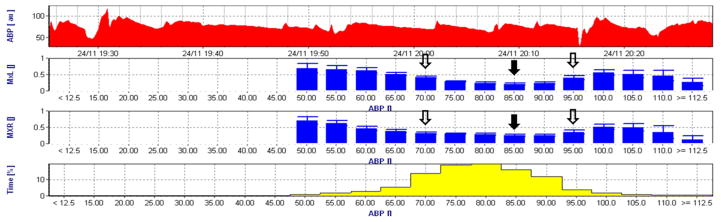
After patients were anesthetized and intubated via the trachea, transcranial Doppler monitoring (DWL, Compumedics DWL, El Paso, TX) of the right and left middle cerebral arteries was initiated by positioning two 2.5-MHz transducers fitted on a headband over the temporal bone. Depth of insonation was varied between 35 and 52 mm until representative spectral CBF flow was identified. Analog arterial pressure and transcranial Doppler signals from the operating room hemodynamic monitor were processed with a data acquisition module (DT9800, Data Translation Inc., Marlboro, MA, USA) and then analyzed by ICM+ software (University of Cambridge, Cambridge, United Kingdom) as described previously.15, 26–28 The signals were filtered as non-overlapping 10-second mean values that were time-integrated, which is equivalent to having a moving average filter with a 10-second time window and resampling at 0.1 Hz, eliminating high-frequency components caused by respiration and pulse waveforms. Additional high-pass filtering was applied with a DC cutoff set at 0.003 Hz. A continuous, moving Pearson’s correlation coefficient between changes in MAP and mean middle cerebral artery blood flow velocity were calculated to render the variable mean velocity index (Mx). Consecutive, average Mx values within a 10-second window were collected as 30 data points to monitor each Mx in a 300-second window. More details of these methods are provided in Supplemntal Table 1. Mx approaches 1 when MAP is outside the limits of autoregulation, indicating pressure-passive CBF. In contrast, Mx approaches 0 or is negative when MAP is within the CBF autoregulation range. Average Mx values obtained during CPB were placed in 5 mmHg MAP bins. The LLA was defined as the MAP at which Mx increased to a value ≥ 0.4 with declining blood pressure. Optimal MAP was defined as that MAP at the lowest Mx indicating the best autoregulation.22 The ULA was defined as the MAP at which Mx increased to ≥0.4 with rising blood pressure.20 A representative autoregulation tracing obtained during CPB is shown in Figure 2. More details about the methods can be found in the Supplemental Material. We separately evaluated the LLA, ULA, and Optimal MAP from the right and left Mx data. For the purpose of data analysis, we took the highest MAP to define the LLA and Optimal MAP and the lowest MAP value for the ULA if there were discrepancies between cerebral hemispheres. We believe that this would be clinically the most applicable method when this type of monitoring becomes widespread. That is, the target MAP would be that which best ensures perfusion to the most compromised hemisphere while trying to avoid MAP > ULA.
Figure 2.
Representative graph of cerebral autoregulation monitoring by mean velocity index (Mx) during cardiopulmonary bypass. The top graph represents the time-series of arterial blood pressure (ABP) while the bottom bar-graph the percentage of the time of the recording spent at 5 mmHg bin. Optimal mean arterial pressure (MAP, ABP) for the left and right side of the brain was defined as that MAP with the lowest Mx. Lower limit of cerebral autoregulation (LLA) and upper limit of cerebral autoregulation (ULA) were defined as the MAP at which Mx reached 0.4. In this example, the optimal MAP is 85 mmHg (black arrow) and the LLA and ULA are 70 mmHg and 95 mmHg, respectively (black-outlined arrow).
Sample Size Calculation
The sample size was calculated with an estimation of optimal MAP that was based on CBF autoregulation endpoints collected previously from 225 patients undergoing cardiac surgery.16 In that study average mean MAP at the LLA was 66 mmHg, and the standard deviation (SD) was 12 mmHg. Based on those data, we estimated that a sample size of 553 patients would provide a 1 mmHg margin of error in determining optimal MAP with a 95% confidence interval between 65 and 67 mmHg.
Data Analysis
Complications after surgery were classified based on definitions of the Society of Thoracic Surgery National Cardiac Surgery Database. Continuous variables are summarized as mean and standard deviation (SD). Normality assumption was checked, and non-normally distributed variables were log-transformed. Continuous variables were compared between groups using a t-test or linear regression. Categorical variables were analyzed using the chi-squared test, or with Fisher’s exact test if frequencies were low. Linear regression models were used to examine effects of predictors on continuous outcomes (e.g. LLA, MAP), adjusting for study. Multiple linear regression was used to identify baseline predictors of MAP; the final model was chosen using Akaike Information Criterion (AIC)-based backward selection from among baseline variables in Table 1, keeping demographics (age, gender, ethnicity and smoking history) and study indicator in all models. Stability of the final model was checked using step-wise model selection strategies. Logistic regression models were used for binary outcomes (e.g. CPB complications), adjusting for study. Analyses were performed using and Stata v.14 (Stata Corp, College Station, TX).
Table 1.
Demographics and intraoperative data for the entire cohort and based on enrolled study.
| Variable | Entire Cohort | Study 117 | Study 224 | Study 3 | P-Value |
|---|---|---|---|---|---|
| N=617 | N=183 | N=105 | N=326 | ||
| Age (yr)* | 67.2 ± 10.5 | 64.6 ± 11.3 | 61.2 ± 12.0 | 70.7 ± 8.0 | <0.001 |
| Female gender | 160 (26.1%) | 34 (18.6%) | 29 (27.6%) | 97 (29.8%) | 0.021 |
| White ethnicitya | 520 (85.0%) | 165 (90.2%) | 90 (85.7%) | 265 (81.8%) | 0.039 |
| History of tobacco smoking | 280 (45.6%) | 72 (39.3%) | 45 (42.9%) | 163 (50.0%) | 0.056 |
| COPD | 65 (10.6%) | 18 (9.8%) | 10 (9.5%) | 37 (11.3%) | 0.805 |
| Diabetes | 247 (40.2%) | 53 (29.0%) | 37 (35.2%) | 157 (48.2%) | <0.001 |
| CHF | 106 (17.3%) | 29 (15.8%) | 25 (23.8%) | 52 (16.0%) | 0.15 |
| Hypertension | 490 (79.8%) | 131 (71.6%) | 76 (72.4%) | 283 (86.8%) | <0.001 |
| Creatinine (g/dL)* | 1.2 ± 1.0 | 1.2 ± 1.2 | 1.5 ± 1.6 | 1.1 ± 0.3 | 0.001 |
| Preoperative eGFR * | 74.4 ± 24.7 | 77.3 ± 25.8 | 73.1 ± 28.9 | 73.2 ± 22.4 | 0.156 |
| Preoperative mean blood pressure (mmHg)* | 93.5 ± 13.3 | 93.7 ± 13.7 | 91.9 ± 14.0 | 93.9 ± 12.9 | 0.39 |
| Preoperative systolic blood pressure (mmHg)* | 136.2 ± 21.2 | 137.1 ± 20.5 | 132.9 ± 22.7 | 136.9 ± 21.0 | 0.198 |
| Preoperative hemoglobin (g/dL)* | 12.5 ± 2.0 | 12.7 ± 2.0 | 12.2 ± 2.2 | 12.5 ± 2.0 | 0.159 |
| Angiotensin-converting enzyme inhibitors-I | 226 (36.8%) | 58 (31.7%) | 37 (35.2%) | 131 (40.2%) | 0.152 |
| Statins | 317 (51.7%) | 116 (63.4%) | 62 (59.0%) | 139 (42.8%) | <0.001 |
| Aspirin | 441 (71.8%) | 119 (65.0%) | 66 (62.9%) | 256 (78.5%) | <0.001 |
| Beta blocker | 379 (61.7%) | 107 (58.5%) | 59 (56.2%) | 213 (65.3%) | 0.137 |
| Ca++ channel blocker | 129 (21.0%) | 26 (14.2%) | 24 (22.9%) | 79 (24.2%) | 0.025 |
| Nitrates | 107 (17.4%) | 28 (15.3%) | 12 (11.4%) | 67 (20.6%) | 0.067 |
| Diuretics | 218 (35.5%) | 42 (23.0%) | 42 (40.0%) | 134 (41.1%) | <0.001 |
| Prior carotid endarterectomy | 23 (3.7%) | 2 (1.1%) | 5 (4.8%) | 16 (4.9%) | 0.078 |
| Prior CVA | 57 (9.3%) | 19 (10.4%) | 5 (4.8%) | 33 (10.1%) | 0.214 |
| Prior TIA | 33 (5.4%) | 10 (5.5%) | 1 (1.0%) | 22 (6.7%) | 0.072 |
| Peripheral vascular disease | 89 (14.5%) | 21 (11.5%) | 10 (.5%) | 58 (17.8%) | 0.043 |
| Coronary artery disease | 467 (76.1%) | 143 (78.1%) | 63 (60.0%) | 261 (80.1%) | <0.001 |
| Cardiopulmonary bypass duration (min)* | 112.8 ± 48.0 | 116.58 ± 43.1 | 104.7 ± 44.5 | 113.4 ± 51.4 | 0.129 |
| Aortic cross clamp duration (min)* | 69.2 ± 32.0 | 72.8 ± 33.6 | 57.1 ± 32.2 | 71.2 ± 30.0 | <0.001 |
| Average mean arterial pressure (mmHg)* | 73.7 ± 8.1 | 72.6 ± 8.5 | 72.0 ± 8.4 | 74.8 ± 7.6 | 0.001 |
| Lowest hemoglobin (g/L)* | 8.2 ± 1.3 | 8.3 ± 1.3 | 8.3 ± 1.4 | 8.1 ± 1.3 | 0.211 |
Data are provided as mean ± SD for continuous variables that were normally distributed. COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; CVA=cerebral vascular accident; TIA, transient ischemic attack.
Non-white race group is predominantly African-Americans, with only 16 patients in Study 3 reporting other race.
RESULTS
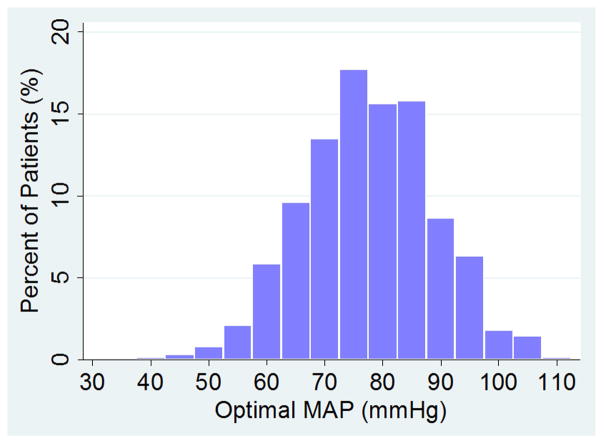
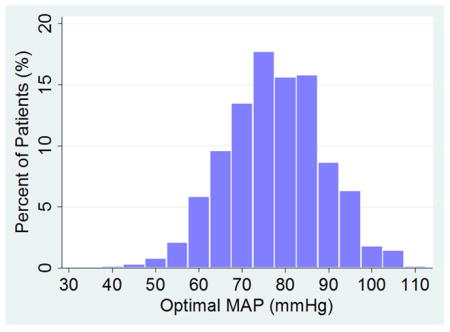
Patient demographics and medical history are listed in Table 1. Predictably, demographic characteristics varied between the three studies. Table 2 presents the autoregulation data measured during CPB. An LLA during CPB was observed in 434 (71%) of 614 patients, and a ULA in 323 (53%) patients, while optimal MAP was observed in all patients. For these patients, the (mean±SD) LLA was 65±12 mmHg and the ULA 84±11 mmHg. The optimal MAP was 78±12 mmHg. The distribution of optimal MAP is shown in Figure 3. A pattern of impaired autoregulation (ie, Mx was >0.4 at all MAP) was observed in 98 (16%) patients. In 82 (13%) other patients, an LLA was not observed which may suggest that the normal fluctuations in MAP that occur during CPB never crossed the autoregulation threshold.
Table 2.
Optimal mean arterial blood pressure (MAP), the MAP at the lower limit of autoregulation (LLA) and the upper limit of autoregulation (ULA) for the entire cohort and by study. The optimal MAP was defined as the MAP with the lowest mean velocity index (see text). Data are listed as mean±SD or number (%) of patients.
| Entire Cohort N=617 |
Study 117 N=183 |
Study 224 N=105 |
Study 3 N=326 |
P-Value | |
|---|---|---|---|---|---|
| Optimal MAP (mean±SD, mmHg) | 78 ± 11 | 74 ± 12 | 75 ± 10 | 80 ± 11 | <0.001 |
| LLA (mmHg) | 65 ± 12 | 63 ± 11 | 66 ± 13 | 66 ± 12 | 0.024 |
| ULA (mmHg) | 84 ± 11 | 81 ± 10 | 82 ± 9 | 86 ± 12 | 0.002 |
| Patients whose crossed LLA | 434 (71%) | 141 (77%) | 70 (67%) | 223 (68%) | 0.074 |
| Patients whose crossed ULA | 323 (52%) | 102 (56%) | 58 (55%) | 163 (50%) | 0.387 |
Figure 3.
Distribution of optimal mean arterial pressure (MAP) for the entire cohort.
There was no relationship between patient age and the LLA, optimal MAP, or the product of the magnitude and duration for which MAP was below the LLA (ie, the “severity” of MAP below LLA) based on linear regression models. The distribution of optimal MAP is shown in Figure 3. In 17% of patients, the LLA was above this optimal MAP, while in 29% of patients the ULA was below the population optimal MAP. Patients with Mx>0.4 at all MAPs (98 patients) were excluded from the latter analysis because we were unable to determine their LLA or ULA. An individual whose LLA MAP is above that of the group could experience cerebral hypoperfusion if the MAP during CPB is maintained at the group optimal MAP. Conversely, a ULA below the population median value could result in cerebral hyperperfusion if MAP is kept at the group median value. Strokes occurred in 5% of patients after surgery. The product of the magnitude and duration that MAP was less than LLA during CPB was associated with the risk of postoperative stroke (p=0.018).
Predictors of optimal MAP based on multivariate linear regression adjusting for study are listed in Table 3. Non-white race, preoperative use of diuretic drugs, prior carotid endarterectomy, and duration of CPB were independently associated with optimal MAP during CPB. Optimal MAP for was 2.73 mmHg higher (p=0.034) for non-white patients, and 1.9 mmHg lower (p=0.049) for patients receiving diuretics. Optimal MAP was 5.5 mmHg lower (p=0.019) for patients with a prior carotid endarterectomy and 1.28 mmHg lower per 60 min of CPB (p=0.22). No other factors were significantly associated with optimal MAP in the linear regression model. The model explained little of optimal MAP variability, R2=0.1002.
Table 3.
Multiple regression model predicting optimal MAP.
| Variable | Estimate | 95% CI | P-value |
|---|---|---|---|
| Study 1 vs. 3 | −6.03 | (−8.6, −3.9) | <0.001 |
| Study 2 vs. 3 | −4.95 | (−7.5, −2.4) | <0.001 |
| Age | −0.02 | (−0.1, 0.1) | 0.650 |
| Female gender | 0.52 | (−1.5, 2.6) | 0.618 |
| White ethnicity | −2.73 | (−5.3,−0.21) | 0.034 |
| History of tobacco smoking | −0.20 | (−2, 1.6) | 0.823 |
| Hypertension | 1.90 | (−0.39,4.2) | 0.104 |
| Ca++ channel blocker | 1.84 | (−0.34, 4) | 0.098 |
| Diuretics | −1.89 | (−3.8,−0.01) | 0.049 |
| Prior carotid endarterectomy | −5.55 | (−10, −0.9) | 0.019 |
| Cardiopulmonary bypass duration (per 60 min) | −1.28 | (−2.4, −0.19) | 0.022 |
Final model was selected using backward selection based on AIC (Akaike Information Criterion) from baseline predictors listed in Table 1. Demographic variables (age, gender, ethnicity and smoking history) and study were kept in the model. Model R2=0.1002.
DISCUSSION
In this study we found that the MAP (mean±SD) at the LLA during CPB was 65±12 mmHg, ULA was 84±11mmHg, and the optimal MAP 78±11 mmHg. An LLA during CPB was observed in 434 (71%) of 614 patients, and a ULA in 323 (53%) patients, while optimal MAP was observed in all patients. Patient age, history of hypertension, or diabetes were not related to the optimal MAP. Non-white race was predictive of a higher optimal MAP while preoperative diuretic use, prior carotid endarterectomy, and duration of CPB predicted a slightly lower optimal MAP. Overall, the model for predicting optimal MAP explained only a small portion of the variance in this metric.
Blood flow to the brain is kept constant over a range of blood pressures to ensure a steady supply of substrates commensurate with cerebral metabolic demand. Current practices of patient blood pressure management during CPB are based on the concept that global cerebral perfusion should not be compromised as long as MAP is maintained above the LLA. Support for this practice is derived from studies, now more than 2 decades old, which showed that CBF is independent of MAP during CPB to a MAP of 20 to 55 mmHg.4, 5,7,29 In most of those studies, CBF was measured intermittently by 133xenon clearance methods, and the analysis involved pooled or paired comparisons of CBF versus MAP. In contrast, our investigations used continuous measurements of CBF autoregulation throughout CPB and included a larger number of patients.
Monitoring of cerebral autoregulation may provide a more precise approach for determining individualized MAP targets during CPB, but this method requires specialized equipment and technical expertise. Our results suggest that the identified optimal MAP during CPB that we identified will reliably ensure perfusion within the autoregulation range for only 71% to 83% of patients, though. In others, the LLA will be above the identified population optimal MAP or the ULA will below this value.
In our study 16% of patients exhibited a pattern of impaired autoregulation in which Mx was >0.4 at all MAPs. We have previously observed that this pattern is associated with risk for stroke.21 Even in this group, or for those whose MAP does not cross the LLA, an optimal MAP can be identified. Many centers modify empiric MAP targets for CPB based on patient age. In our analysis, however, we found no association between optimal MAP and patient age. In this study we found a significant relationship between the product of the magnitude and duration that MAP was below the LLA and risk for stroke. In a prior study we found a relationship between MAP below the LLA in the intensive care unit after cardiac surgery and release of the brain-specific injury biomarker glial fibrillary acidic protein.30 These data support a role of cerebral hypoperfusion in adverse neurological complications after cardiac surgery. Simply raising MAP targets during CPB, however, could also lead to adverse events. That is, we have found previously that the magnitude and duration that MAP is above the ULA during CPB is associated with postoperative delirium.20 A plausible explanation for this outcome is that cerebral hyperperfusion in the context of heightened systemic inflammation related to cardiac surgery with CPB might result in adverse cerebral events.31
Taken together, our data indicate the potential importance of maintaining MAP at a level that is neither below nor above the limits of autoregulation to optimize perfusion of the brain. In this study we do not report on postoperative delirium or cognitive dysfunction. Moreover, our findings of a link between MAP below the LLA and stroke must be considered as an association. Whether targeting MAP based on autoregulation metrics can lead to lower rates of stroke cannot be necessarily concluded from this study. We are currently conducting a prospectively randomized trial of autoregulation determined MAP versus the standard of care during CPB (NIH R01HL092259). The primary end-point of that ongoing study is a composite of adverse neurological events including stroke, brain MRI determined acute ischemic brain injury, and alterations in cognition from baseline.
As with previous studies, we defined the limits of autoregulation as the MAP at which Mx increased to a value ≥ 0.4. This value associated with sepsis-associated delirium and mortality for patients with traumatic brain injury.22, 34 Nonetheless, this cutoff is admittedly arbitrary and may not reflect the true lower limit, which could be an Mx > 0.3.35 Regardless, any dichotomous definition of the LLA based on Mx fails to consider that vasoactive compensatory responses to blood pressure changes may persist even when MAP is outside the confines of autoregulation. In addition to its retrospective design, our study has several limitations. Cerebral blood vessel reactivity might be affected by body temperature. Body temperature were not included in our analysis as we were unable to acquire this information in our data acquisition scheme. Seven patients were excluded from analysis because of the lack of an identifiable transcranial Doppler insonating window. However, because our sample size was above that required for identifying optimal MAP with precision, it seems unlikely that the exclusion of some patients for that reason led to an unintended bias in our results. Regardless, this finding underscores the limitations of transcranial Doppler-based autoregulation monitoring that may preclude is routine adoption. Methods based on processing of near infrared spectroscopy data hold promise as a clinically more feasible method for widespread cerebral autoregulation monitoring.17–21 Cerebral autoregulation measurements were based on CBF velocity in the middle cerebral arteries. Whether the LLA or optimal MAP thus identified applies to other major cerebral arterial systems is not known. Further, cerebral injury during cardiac surgery is primarily regional and may occur in vascular territories distal to an arterial stenosis or in the presence of small vessel disease12 Autoregulation distal to a flow-limiting stenosis would be expected to be impaired and the CBF pressure dependent. Determining optimal MAP for areas of the brain without stenosis may still provide adequate MAP for proximal vascular territories that provide the “driving pressure” across the stenosed vessel. Finally, the patients in this study were mostly those at high risk for brain injury. Whether our findings are applicable to lower risk patients is not known.
These data suggest that optimal MAP during CPB is 78±11 mmHg but in 17% and 29% of patients the LLA or ULA, respectively, will be outside this range predisposing to unintentional cerebral hypo- or hyper-perfusion. Our finding of a relationship between MAP below the LLA during CPB and stroke supports a role of cerebral hypoperfusion and stroke. Development of clinical cerebral autoregulation monitors should be made a high priority to enable care providers the tools to individualize MAP during CPB.
Supplementary Material
Perspective Statement.
There is no consensus on optimal mean arterial pressure (MAP) during cardiopulmonary bypass (CPB). Cerebral autoregulation monitoring in 614 patients during CPB showed that optimal MAP for patients was 78±11 mmHg. However, this target will not be accurate in 17% to 29% of patients. Real-time monitoring of autoregulation may better ensure perfusion during CPB.
Acknowledgments
Source of funding: This work was supported in part by Grant-in-Aid Number 103363[from the Mid-Atlantic Affiliate of the American Heart Association] and grant R01HL092259 [from the National Institutes of Health] to Dr. Hogue.
Abbreviations and Acronyms
- CBF
cerebral blood flow
- CPB
cardiopulmonary bypass
- LLA
lower limit of cerebral autoregulation
- MAP
mean arterial pressure
- Mx
mean velocity index
- ULA
upper limit of autoregulation
Footnotes
Clinical Trial: NCT00981474, NCT00769691
Conflict of Interest: Dr. Hogue receives consulting fees, lecture honorarium, and research funding from Medtronics, Inc, Minneapolis, MN.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Daijiro Hori, Division of Cardiac Surgery, Department of Surgery, The Johns Hopkins University School of Medicine, Baltimore, MD.
Yohei Nomura, Division of Cardiac Surgery, Department of Surgery, The Johns Hopkins University School of Medicine, Baltimore, MD.
Masahiro Ono, Department of Cardiac Surgery, The Texas Heart Institute, Houston, TX.
Brijen Joshi, Department of Anesthesiology and Critical Care Medicine, The Johns Hopkins University School of Medicine, Baltimore, MD.
Kaushik Mandal, Division of Cardiac Surgery, Department of Surgery, The Johns Hopkins University School of Medicine, Baltimore, MD.
Duke Cameron, Division of Cardiac Surgery, Department of Surgery, The Johns Hopkins University School of Medicine, Baltimore, MD.
Masha Kocherginsky, Department of Preventive Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL.
Charles W. Hogue, Department of Anesthesiology and the The Bluhm Cardiovascular Institute, Northwestern University Feinberg School of Medicine, Chicago, IL.
References
- 1.Gibbon JH., Jr Application of a mechanical heart and lung apparatus to cardiac surgery. Minn Med. 1954;37:171–185. passim. [PubMed] [Google Scholar]
- 2.Hannan EL, Wu C, Walford G, et al. Drug-eluting stents vs. coronary-artery bypass grafting in multivessel coronary disease. N Engl J Med. 2008;358:331–341. doi: 10.1056/NEJMoa071804. [DOI] [PubMed] [Google Scholar]
- 3.Abdallah MS, Wang K, Magnuson EA, et al. Quality of life after PCI vs CABG among patients with diabetes and multivessel coronary artery disease: a randomized clinical trial. JAMA. 2013;310:1581–1590. doi: 10.1001/jama.2013.279208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogue CW, Jr, Palin CA, Arrowsmith JE. Cardiopulmonary bypass management and neurologic outcomes: an evidence-based appraisal of current practices. Anesth Analg. 2006;103:21–37. doi: 10.1213/01.ANE.0000220035.82989.79. [DOI] [PubMed] [Google Scholar]
- 5.Murkin JM, Farrar JK, Tweed WA, McKenzie FN, Guiraudon G. Cerebral autoregulation and flow/metabolism coupling during cardiopulmonary bypass: the influence of PaCO2. Anesth Analg. 1987;66:825–832. [PubMed] [Google Scholar]
- 6.Schell RM, Kern FH, Greeley WJ, et al. Cerebral blood flow and metabolism during cardiopulmonary bypass. Anesth Analg. 1993;76:849–865. doi: 10.1213/00000539-199304000-00029. [DOI] [PubMed] [Google Scholar]
- 7.Taylor KM. The hemodynamics of cardiopulmonary bypass. Semin Thorac Cardiovasc Surg. 1990;2:300–312. [PubMed] [Google Scholar]
- 8.Yamamoto M, Meyer JS, Sakai F, Yamaguchi F. Aging and cerebral vasodilator responses to hypercarbia: responses in normal aging and in persons with risk factors for stroke. Arch Neurol. 1980;37:489–496. doi: 10.1001/archneur.1980.00500570037005. [DOI] [PubMed] [Google Scholar]
- 9.Schoof J, Lubahn W, Baeumer M, et al. Impaired cerebral autoregulation distal to carotid stenosis/occlusion is associated with increased risk of stroke at cardiac surgery with cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2007;134:690–696. doi: 10.1016/j.jtcvs.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354:1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 11.Moraca R, Lin E, Holmes JHt, et al. Impaired baseline regional cerebral perfusion in patients referred for coronary artery bypass. J Thorac Cardiovasc Surg. 2006;131:540–546. doi: 10.1016/j.jtcvs.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 12.Gottesman RF, Sherman PM, Grega MA, et al. Watershed strokes after cardiac surgery: diagnosis, etiology, and outcome. Stroke. 2006;37:2306–2311. doi: 10.1161/01.STR.0000236024.68020.3a. [DOI] [PubMed] [Google Scholar]
- 13.Gottesman RF, Hillis AE, Grega MA, et al. Early postoperative cognitive dysfunction and blood pressure during coronary artery bypass graft operation. Arch Neurol. 2007;64:1111–1114. doi: 10.1001/archneur.64.8.noc70028. [DOI] [PubMed] [Google Scholar]
- 14.Floyd TF, Harris F, McGarvey M, Detre JA. Recurrence of stroke after cardiac surgery: insight into pathogenesis via diffusion-weighted and continuous arterial spin labeling perfusion magnetic resonance imaging. J Cardiothorac Vasc Anesth. 2007;21:106–109. doi: 10.1053/j.jvca.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Czosnyka M, Brady K, Reinhard M, Smielewski P, Steiner LA. Monitoring of cerebrovascular autoregulation: facts, myths, and missing links. Neurocrit Care. 2009;10:373–386. doi: 10.1007/s12028-008-9175-7. [DOI] [PubMed] [Google Scholar]
- 16.Joshi B, Ono M, Brown C, et al. Predicting the limits of cerebral autoregulation during cardiopulmonary bypass. Anesth Analg. 2012;114:503–510. doi: 10.1213/ANE.0b013e31823d292a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brady K, Joshi B, Zweifel C, et al. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke. 2010;41:1951–1956. doi: 10.1161/STROKEAHA.109.575159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ono M, Arnaoutakis GJ, Fine DM, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med. 2013;41:464–471. doi: 10.1097/CCM.0b013e31826ab3a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ono M, Brady K, Easley RB, et al. Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. J Thorac Cardiovasc Surg. 2014;147:483–489. doi: 10.1016/j.jtcvs.2013.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hori D, Brown C, Ono M, et al. Arterial pressure above the upper cerebral autoregulation limit during cardiopulmonary bypass is associated with postoperative delirium. Br J Anaesth. 2014;113:1009–1017. doi: 10.1093/bja/aeu319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ono M, Joshi B, Brady K, et al. Risks for impaired cerebral autoregulation during cardiopulmonary bypass and postoperative stroke. Br J Anaesth. 2012;109:391–398. doi: 10.1093/bja/aes148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aries MJ, Czosnyka M, Budohoski KP, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012;40:2456–2463. doi: 10.1097/CCM.0b013e3182514eb6. [DOI] [PubMed] [Google Scholar]
- 23.Brady KM, Mytar JO, Lee JK, et al. Monitoring cerebral blood flow pressure autoregulation in pediatric patients during cardiac surgery. Stroke. 2010;41:1957–1962. doi: 10.1161/STROKEAHA.109.575167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ono M, Zheng Y, Joshi B, Sigl JC, Hogue CW. Validation of a stand-alone near-infrared spectroscopy system for monitoring cerebral autoregulation during cardiac surgery. Anesth Analg. 2013;116:198–204. doi: 10.1213/ANE.0b013e318271fb10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKhann GM, Grega MA, Borowicz LM, Jr, Baumgartner WA, Selnes OA. Stroke and encephalopathy after cardiac surgery: an update. Stroke. 2006;37:562–571. doi: 10.1161/01.STR.0000199032.78782.6c. [DOI] [PubMed] [Google Scholar]
- 26.Steiner LA, Czosnyka M, Piechnik SK, et al. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002:733–738. doi: 10.1097/00003246-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Steiner L, Coles J, Johnston A, et al. Assessment of cerebrovascular autoregulation in head-injured patients: a validation study. Stroke. 2003;34:2404–2409. doi: 10.1161/01.STR.0000089014.59668.04. [DOI] [PubMed] [Google Scholar]
- 28.Brady KM, Lee JK, Kibler KK, et al. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke. 2007;38:2818–2825. doi: 10.1161/STROKEAHA.107.485706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Govier AV, Reves JG, McKay RD, et al. Factors and their influence on regional cerebral blood flow during nonpulsatile cardiopulmonary bypass. Ann Thorac Surg. 1984;38:592–600. doi: 10.1016/s0003-4975(10)62316-8. [DOI] [PubMed] [Google Scholar]
- 30.Hori D, Ono M, Rappold TE, et al. Hypotension After Cardiac Operations Based on Autoregulation Monitoring Leads to Brain Cellular Injury. Ann Thorac Surg. 2015 doi: 10.1016/j.athoracsur.2015.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 2003;75:S715–720. doi: 10.1016/s0003-4975(02)04701-x. [DOI] [PubMed] [Google Scholar]
- 32.Rafiq MK, Connolly D, Randall M, Blank C. Cerebral hyperperfusion syndrome. Pract Neurol. 2013 doi: 10.1136/practneurol-2013-000647. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz RB. Hyperperfusion encephalopathies: hypertensive encephalopathy and related conditions. Neurologist. 2002;8:22–34. doi: 10.1097/00127893-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Pfister D, Siegemund M, Dell-Kuster S, et al. Cerebral perfusion in sepsis-associated delirium. Crit Care. 2008;12:R63. doi: 10.1186/cc6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorrentino E, Budohoski KP, Kasprowicz M, et al. Critical thresholds for transcranial Doppler indices of cerebral autoregulation in traumatic brain injury. Neurocrit Care. 2011;14:188–193. doi: 10.1007/s12028-010-9492-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.