Abstract
Kv11.1 (hERG) is a voltage-gated potassium channel that shows very slow ionic current activation kinetics, and an unusual underlying biphasic gating charge movement with fast and slow components that differ greatly in time course. The structural basis and role of the fast component of gating charge (Qfast) is unclear, and its relationship to the slow activation of hERG channels is not understood. In this study we have used the cut-open oocyte voltage-clamp technique to investigate the relationship of fast gating charge movement-to-residue interactions between D411 at the bottom of the S1, and lower S4 domain charged and uncharged residues. Neutralization of D411 or K538 and V535A prevented Qfast and greatly accelerated overall charge movement. Voltage-clamp fluorometry showed a loss of a fast component of S4 fluorescence in D411N, V535A, and K538Q upon depolarization, whereas [2-(trimethyl ammonium) ethyl] methanethiosulfonate chloride modification of I521C in the outer S4 was enhanced at more negative potentials and at earlier times in these same mutants. A functional interaction between these regions during activation was suggested by ΔΔGo values >4.2 kJ/mol obtained from double mutant cycle analysis. The data indicate that interactions of S1 residue D411 with lower S4 residues stabilizes early closed states of the channel, and that disruption of these interactions results in both faster rates of activation gating and an elimination of the fast component of gating charge movement and of fluorescence. We propose that the Qfast charge movement during activation accompanies transitions through early closed states of the hERG activation pathway, and that the weak voltage dependence of these transitions limits the overall activation rate of hERG channels. Disruption of the D411-S4 interactions destabilizes these early closed states, leaving hERG channels able to activate at a rate similar to conventional potassium channels.
Introduction
The human ether-à-go-go-related gene encodes the α-subunit of the Kv11.1 voltage-gated potassium channel (1), also referred to as “hERG”. Kv11.1 is best known for its role in the cardiac action potential where it underlies the rapid delayed rectifier current, IKr, which brings an end to cardiac systole (2, 3). Disruption of Kv11.1 channel function, through either drug block or genetic mutation, can result in long QT syndrome (2, 4) (disruption to Kv11.1 is type 2), the consequences of which can include torsades de pointes and sudden death (5, 6).
hERG displays voltage-dependent gating behavior that deviates strongly from the more canonical voltage-gated K channels (4, 7, 8) in that the rates of activation and deactivation are slower than those of other Kv channel α-subunits by approximately an order of magnitude (2, 7, 9, 10, 11). The slow activation and deactivation is paired with a fast and voltage-dependent inactivation (12, 13) and tailors the hERG channel to its particular role during the cardiac action potential (14).
hERG shares the general structure of a voltage-gated potassium channel, that of a tetrameric protein with four six-transmembrane segment subunits that come together to delineate a central ion-conducting pore region (15, 16, 17, 18). The first four transmembrane segments make up the voltage-sensing domain (VSD) of the channel, with the fourth transmembrane segment containing a high concentration of positively charged residues that initiate conformational changes in the protein upon alterations in membrane potential (19). Due to poor conservation of alignment between Shaker and hERG, it is difficult to directly compare hERG with Shaker, in which most gating biophysics has historically been done. The distribution of charged residues in the hERG VSD is different in two aspects. First, although it is still suggested that the outer three residues (K525, R528, and R531) transfer most of the gating charge upon depolarization (20), overall, the S4 segment of hERG has one more gating charge than most Kv channels, and these charges are not evenly distributed as hERG has two continuous positive residues (R537 and K538) at the cytoplasmic edge of S4 (Fig. 1 A). Second, hERG has three extra acidic residues (D411, D460, and D509) in S1 and S2, in addition to the widely conserved acidic residues in other Kv channels (D456, D466, and D501). Charge neutralization studies of S4 in hERG indicate that K525 and K538 are involved in stabilizing the closed state or destabilizing the open state (21, 22, 23, 24, 25). Further, from double mutant cycle analysis, it is suggested that K525 and K538 stabilize closed states via interaction with the acid charges on D456 and D411, respectively (25). Thus, it has been suggested that the relatively slower activation of hERG S4 than Shaker channels could be influenced by interactions of these extra acidic residues and positive charges in S4 (25, 26). Additional causes for slowed channel activation may also include factors such as a delayed coupling of the voltage-sensing domain movement to the pore domain that occurs much later in the activation pathway (8, 9). Many of the above studies have relied on ionic current measurements as indirect indicators of activation gating, whereas hERG gating currents themselves, which are direct measures of net charge displacement during VSD movement, have been less commonly recorded—which is at least partly due to the difficulty of recording them. However, it is known that hERG gating currents have radically different properties from gating currents recorded from other Kv channels. Piper et al. (8) described two readily separable components of charge movement in hERG: Qfast and Qslow. Qfast is a poorly understood fast movement of a small amount of gating charge (<5% of the total charge moved) that has an extremely broad voltage-dependent activation (8, 9, 10, 22). Qslow, which comprises >90% of the total gating charge movement, is a much larger but slower-moving component often lasting hundreds of milliseconds, whose more voltage-dependent charge-voltage (Q-V, QV) relationship is hyperpolarized to that of the conductance-voltage (G-V, GV) relationship (8, 9). Whereas the significance of Qslow is fairly well understood, that of Qfast largely remains to be determined.
Figure 1.
hERG gating charge movement is biphasic. (A) Lateral view is given of an open-state hERG subunit (5VA1). Residue D411 in the S1 segment (orange) may interact with charged residues (highlighted in cyan) at the bottom of the S4 (pink). Residue I521, at the top of the S4, is highlighted in green and the presumed gating charge transfer center, F463, is highlighted in red. (B) WT hERG gating (blue) and ionic currents (black) were recorded during a depolarization to +30 mV from a holding potential of −110 mV. (Inset) Given here is a magnification of the fast component of hERG gating charge movement. In the inset, the gating current (blue) is distinguishable from the capacitive current (black) well within the first millisecond of a change in potential. (C) Given here is the voltage-dependence of activation from a QV 1.5-ms protocol and a QV 300-ms protocol; for the QV 1.5 -ms protocol, V0.5 = 34.1 ± 3.5 mV, z = 0.79 ± 0.03 e (n = 3), and for the QV 300-ms protocol, V0.5 = −22.0 ± 1.6 mV, z = 1.8 ± 0.1 e (n = 5). To see this figure in color, go online.
In this study, we have investigated the hypothesis that Qfast in hERG reflects dynamic charge displacement arising from S4 and/or S1 as the bonds between the lower charged residues across these two subunits are broken and channel activation is initiated. We build on prior ionic current work, described above, that D411-K538 interactions stabilize hERG closed states and provide a limiting barrier to activation speed. Through the use of the cut-open Vaseline gap (COVG) voltage-clamp technique, we show that mutations to both D411 and several residues at the bottom of the S4 segment can change the qualitative nature of hERG gating currents by eliminating Qfast. Fluorescence and [2-(trimethyl ammonium) ethyl] methanethiosulfonate chloride (MTSET) studies support the loss of a displacement step in S4 in constructs that lack the Qfast component and show an acceleration of overall charge movement. In a double mutant cycle-gating current analysis of D411 with the residues of the bottom of the S4, we find evidence of a functional interaction of D411 with S4 residue V535 and suggest that the energy required to disrupt early closed state interactions made by D411 both slows the overall gating charge movement and stabilizes early closed states of the channel.
Materials and Methods
Molecular biology
The hERG plasmid was subcloned into the pBluescript SK+ expression vector. All mutations were generated using the QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA) and were confirmed with DNA sequencing by Macrogen (Rockville, MD). For RNA transcription, cDNA was linearized with NotI and cRNA was synthesized from this linearized cDNA using the mMessage mMachine T7 Ultra Transcription Kit (Ambion, Austin TX). A wild-type (WT) hERG or extracellular cysteine-less (WT C-less) background construct (hERG C445V:C449V), and/or additional E519C or I521C constructs, was used for all gating current and fluorescence experiments. The hERG C445V:C449V:I521C was generally used as a control in S1 and S4 mutant experiments and is denoted as “I521C” throughout the article, and was used exclusively for data shown in Fig. 3 and following, except in Fig. 6, where “I521C” was replaced by “E519C”, as noted below. The S1 and S4 mutants were made in this background, except L539A and D540A, which were made in the WT C-less background. I521C was used in the [2-(Trimethyl ammonium) ethyl] Methanethiosulfonate Chloride (MTSET; Toronto Research Chemicals, Toronto, Ontario, Canada) modification experiments, and E519C as the acceptor in tetramethyl-rhodamine-5-maleimide (TMRM; Invitrogen, Carlsbad, CA) fluorescence experiments. The general gating properties of the WT, C-less mutant and C-less I521C controls are quite similar and described in Fig. S1 (ionic currents) and Fig. 2 (gating currents). The C-less I521C mutant exposed to various thiol-modifying reagents has previously been characterized (27).
Figure 3.
D411N, V535A, K538Q, and D540A mutants have reduced Qfast during activation. (A) Initial 50-ms gating current traces of I521C and V535A are given during a depolarization to +30 mV from a holding potential of −110 mV. Qfast was defined as charge moving within 2 ms of a change in potential and peaks above the slower charge movement. (B) Gating currents from 50 ms depolarizations to potentials are given between −60 mV and +30 mV from a holding potential of −110 mV for I521C, D411N, V535A, A536V, R537Q, K538Q, L539A, D540A, and R541A constructs. Scale bars represent 10 ms and 10 nA (y axis). (C) Relationships of Qtotal and Qfast to voltage during 300-ms depolarizations to potentials are shown from −110 mV to +30 mV in 10-mV steps from a holding potential of −110mV. (D) Qfast relative to Qtotal given for all constructs. D411N, V535A, K538Q, and D540A all show significantly reduced amounts of Qfast at potentials >−10 mV, ∗p < 0.05. To see this figure in color, go online.
Figure 6.
Loss of fast gating charge component also reflected in TMRM fluorescence quenching. (A) VCF representative traces shown during depolarizing pulses to −30 mV from a holding potential of −110 mV for E519C, D411N, V535A, R537A, K538Q, and D540A mutants. Quenching of the fluorescent signal was fit with either a single or double exponential fit. (B) Given here is the proportion of fluorescent signal that reflects a fast quenching event. Fast and slow time constants, as well as proportions, are shown in Table S2. (Inset) Shown here is a fluorescence signal from a V535A mutant in response to a −30 mV depolarization from a holding potential of −110 mV. The fluorescence quenching has been fit with a single exponential shown in red. To see this figure in color, go online.
Figure 2.
Mutation of residues D411, V535, K538, and D540 alter both fast and slow components of hERG gating charge movement. (A) Gating currents of mutant channels were recorded during 24-ms depolarizing voltage pulses from −110 mV to +30 mV in 20-mV steps. Pulses were applied every 10 s. Scale bars represent 10 ms along the x axis and 50 nA along the y axis. (B) Charge-voltage relationships from WT, WT C-Less, I521C, D411N, V535A, A536V, R537Q, K538Q, K538R, L539A, and R541A constructs given during 300-ms depolarizing voltage pulses from −110 mV to +30 mV in 10 mV steps. QV fit parameters and n values for these constructs are found in Table S1. The D540A charge-voltage relationship is from 100-ms depolarizing voltage pulses from −110 mV to +30 mV in 10-mV steps. The holding potential used in all experiments was −110 mV. To see this figure in color, go online.
Xenopus oocyte preparation and expression
Experiments carried out in this study were approved by the University of British Columbia Animal Care Committee. Xenopus laevis oocytes were obtained and defolliculated as described previously (9). Selected stage V–VI oocytes were injected using a microdispenser (Drummond Scientific, Broomall, PA) with 50–100 ng of cRNA and incubated for 24–72 h before experiments (9, 27).
COVG recording
Gating current recordings were performed as described previously (9). A more detailed description of this technique is provided in the Supporting Material.
Voltage-clamp fluorometry
Fluorometry was performed simultaneously with a two-electrode voltage clamp as we have described previously (11). A more detailed description of this technique is provided in the Supporting Material.
Data analysis
The kinetics of hERG gating charge movement were measured from off-gating currents (IgOFF) after membrane repolarization, the resulting gating charge (QOFF) data plotted against the duration of depolarization. Curves were fit with a single exponential equation of the form y = y0 + A e−t/τ, where y is the normalized response, A is the amplitude, and τ is the time constant. QV relationships were obtained by integrating the first 150 ms of QOFF as a function of the depolarizing pulse voltage. The median voltage of activation, Vm, was calculated by determining the area between the QV curve and the ordinate axis using the trapezoid method as Vm = , where Qi and Vi are the ith point along the QV curve (28).
Fluorescence quenching responses were fit with either single or double exponential functions of the form y = y0 + A1 e−t/τ1 + A2 e−t/τ2, where A1 and A2 are the amplitudes of each component of the fit, and τ1 and τ2 are the accompanying time constants.
Mutant cycle analysis has been assessed by comparing the change in chemical free energy associated with activation, ΔGc = QmaxFVm, where ΔGc is the change in chemical free energy associated with activation, Qmax is the maximum amount of gating charge displacement, F is the Faraday constant (96485 C/mol), and Vm is the median voltage of activation as estimated by the QV curve (28). Qmax was defined as eight equivalent charges, as a past study had suggested this to be the number of charges associated with hERG activation (20). The coupling energy (nonadditivity) of the two mutations was calculated as lΔΔGolcoupling = (ΔGWTo + ΔGM1M2o)–(ΔGM1o + ΔGM2o), where ΔGWTc is the change in chemical free energy associated with activation of the WT channel, ΔGM1M2c is the change in chemical free energy associated with activation of the double mutant construct, and ΔGM1c and ΔGM2c are the chemical free energies associated with activation of the single mutant constructs. The SE value for nonadditivity was calculated as the square root of the sum of the square of SEs of each of the ΔGo values.
Assessment of statistical differences among Vm, time constants, and Afast of channels was done using the software GraphPad Prism (GraphPad Software, La Jolla, CA) to perform one-way analysis of variance with a Dunnett posttest p < 0.05. Assessment of statistical difference in Qfast was done using GraphPad Prism to perform two-way analysis of variance with a Dunnett posttest to compare control with mutant channel values with a significance level set at p < 0.05. Assessment of statistical differences in time constants fit to normalized MTSET modification was done using GraphPad Prism to perform unpaired t-tests with p < 0.05. Assessment of whether ΔΔGo is significantly different than 4.2 kJ/mol was done with GraphPad Prism using a one sample t-test.
Results
Cryo-EM hERG structure shows D411 to fold close to the bottom of S4
One subunit of the open state hERG structure determined by cryo-EM (18) is shown in Fig. 1 A. D411, highlighted in orange, is an acidic residue at the bottom of S1. Prior studies involving D411 have noted that charge reversal and neutralization at this position increases the rate of ionic current activation and hyperpolarizes the GV (25, 26). D411 appears to be in close proximity to the bottom-half of the S4 transmembrane domain, including charged residues highlighted in cyan such as R537, K538, and less so, D540 in the S4-S5 linker. It is unknown how D411 stabilizes the closed state of hERG, although the proximity and likely role in gating of positive charges in the lower S4 segment make them prime candidates for interaction. Gating current measurements are a powerful tool to understand charge movement arising from the S4, and a typical example of gating current at +30 mV illustrating the basic properties of Qfast and Qslow in comparison with the ionic current time course is shown in Fig. 1 B. Clearly, the transient Qfast component of gating current decays very early during ionic current activation, even as the apparent ionic activation time course is slowed by concomitant inactivation. An inset showing Qfast on an expanded time base illustrates that peak gating current (blue) can be separated from clamp transients (black) using the COVG technique. The Qslow component of gating current is sustained and lasts beyond the 300-ms pulse duration used in this protocol.
To measure the voltage dependence of Qfast, WT hERG gating currents were recorded during 1.5-ms depolarizations to potentials ranging from −100 to +160 mV. Integration of the off-gating currents at −110 mV was used to generate the QfastV relationship. The QfastV relationship is shown in Fig. 1 C along with the total QV relationship. These results confirm the broad voltage dependence and shallow slope of this relationship (V0.5 = 34.1 ± 3.5 mV, z = 0.79 ± 0.03 e) first described by Piper et al. (8).
D411 charge neutralization changes the qualitative nature of hERG gating currents
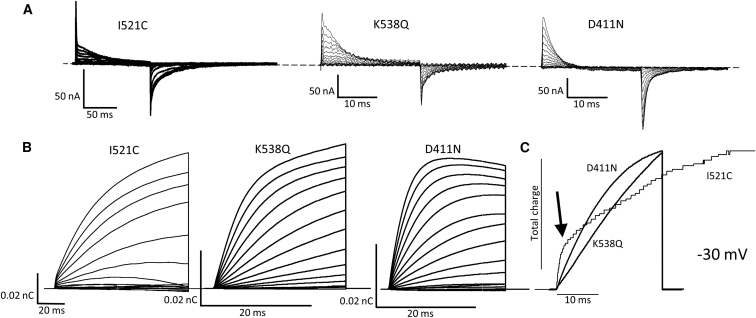
To investigate D411, and residues in the lower part of S4 on the Qfast component’s role in hERG gating, our initial experiments involved comparing the gating currents of WT hERG, I521C, and C-less hERG with a D411 construct mutated to an asparagine to neutralize charge (D411N; Fig. 2 A). Interestingly, the qualitative nature of the on-gating current of the D411N mutant was radically different from I521C. During 24-ms depolarizations to a range of potentials, the I521C record shows a very fast current spike followed by a much slower charge movement throughout the depolarization. Qfast, however, is almost absent in the D411N record, and the Qslow component appears to move much more quickly, with charge movement complete within 20 ms at higher potentials. The elimination of the fast component of gating charge movement in D411N and subsequent increase in the rate at which Qslow activates, prompted us to record gating currents from mutated constructs that might have an electrostatic or steric relationship with D411N (Fig. 1 A).
Some S4 mutations also reduce or abolish Qfast, and speed up Qslow charge components
We performed a short alanine scan of the bottom-half of the S4 and beginning of the S4–S5 linker and recorded voltage-dependent gating currents using the COVG. Expression was poor for R537A and K538A mutants, so we introduced glutamine residues at these positions, which were found to not reduce expression as significantly. Traces of each construct in response to a 24-ms depolarization are shown in Fig. 2 A. Strikingly, V535A, K538Q, and D540A show an obvious reduction in the amplitude of Qfast relative to Qslow compared with I521C, and an overall speeding-up of the bulk of charge movement such that little sustained gating current remains after 24 ms. Constructs A536V, R537Q, L539A, and R541A all retained a similar gating current phenotype to I521C. Total charge-voltage (QV) relationships during 300-ms depolarizations were obtained for WT hERG, I521C, the D411N mutant, and the lower S4 mutants (Fig. 2 B). In the upper panel mutants are depicted that show ∼15 mV shift or less in Vm from WT hERG, whereas the lower panel depicts mutants that show a greater shift (Table S1). A536V, K538Q, and D411N show a significantly more hyperpolarized voltage dependence of charge movement compared to the WT, WT C-less, and I521C constructs. D540A showed an unusual biphasic QV. Mutations at this site had previously been noted to have a similarly shaped GV and the authors also suggested that this channel can reenter an open state at strongly hyperpolarized potentials (29). It is interesting that V535A, which exhibits a loss of Qfast, shows steady-state QV kinetics only 15 mV hyperpolarized from WT hERG, whereas A536V, which exhibits gating currents qualitatively like WT, shows a 20-mV hyperpolarization. The importance of the K538Q mutation is highlighted by the effect on gating currents of the conservative mutation, K538R. Stabilization of the closed state of this construct is evidenced by a 23-mV depolarizing shift in the Vm.
For comparison, the conductance-voltage (GV) relationships of WT hERG, I521C, D411N, and S4 mutants are shown in Fig. S1. Here, it is of note that the GV0.5 for both I521C (GV0.5 = −29.4 mV) and D411N (GV0.5 = −20.8 mV) were quite similar, with that of D411N being slightly depolarized to that of I521C (unlike the QV in Fig. 2). The same is true for D540A, but not for mutants V535A and K538Q that have lost Qfast, and whose GV0.5 values are significantly more hyperpolarized relative to the WT and I521C GV0.5 values than their QVm values. These results suggest two things; they are further confirmation of the previously noted poor coupling between the movement of the VSD and the pore domain (9), and also demonstrate that the use of GV relationships to understand the movements of the VSD and gating charge during activation is fraught with problems, likely due to the important steps that occur between VSD activation and actual pore opening. In this study we rely on direct measurements of VSD charge movement and displacement to understand the relationship between S1 and S4 charges during activation of the VSD.
Qfast/Qtotal reduced for mutants D411N, V535A, K538Q, and D540A
Piper et al. (8) defined Qfast as any charge that is moved within the first 2 ms of a depolarization. Here, as a simple way to separate the two components, Qfast was defined as the envelope of current that peaked above the slower moving gating charge in the first 2 ms after a depolarizing stimulus. An example of this region used for integration purposes is shown in Fig. 3 A for I521C and for V535A, which lacks Qfast. Complete sets of gating current tracings between −60 and +30 mV are shown from I521C, D411N, and the S4 mutants in Fig. 3 B and illustrate the clear growth in Qfast in I521C as the potential is increased from −50 mV where it first appears, up to +30 mV. The same is true in other control-like mutants (A536V, R537Q, L359A, and R541A), but not in D411N, V535A, or K538Q, in which Qfast is abolished or greatly reduced. D540A gating currents are more difficult to interpret as overall charge movement is so fast that Qfast and Qslow cannot easily be separated.
The QV relationships for Qfast and total charge (Qtotal) are shown in Fig. 3 C for I521C and D411N, with just the Qfast components for all mutant constructs plotted in Fig. 3 D. The I521C Qfast can be seen to be a small proportion, ∼5% of total charge and shows a relatively linear voltage-dependence, or at least a lack of saturation at +30 mV as described in Fig. 1. At depolarizations of −10 mV and greater, D411N, V535A, K538Q, and D540A were all found to have significantly reduced Qfast components compared to the I521C control. The Qfast component of constructs A536V, R537Q, L539A, and R541A were not found to differ significantly from I521C.
Acceleration of overall charge movement in mutations that abolish Qfast
In contrast to the relatively small change in steady-state properties of overall charge movement that accompanies mutations that abolish the Qfast component of gating charge (Figs. 2 and 3), the non-steady-state kinetics of charge movement are greatly accelerated (Fig. 4). Examples of current and charge movement are shown for the I521C control, K538Q, and D411N, plotted on different time bases (except Fig. 4 C). It can be seen that I521C charge movement is nearly complete during the 100-ms pulses, whereas for the mutants, charge movement appears similar during 24-ms depolarizations. Most interestingly, when charge movement traces from the control and mutant constructs obtained at −30 mV are superimposed in Fig. 4 C, the early step of charge due to Qfast is clearly seen (arrow) before the slower development of charge in I521C channels. In contrast, D411N and K538Q charge envelopes lack a Qfast component and activate monophasically. This is despite the fact that ionic activation in these two mutants is hyperpolarized compared with I521C (Fig. S1).
Figure 4.
Accelerated time course of charge movement in K538Q and D411 mutants, compared with I521C. (A) Gating currents of I521C are given during 100-ms depolarizing voltage pulses from −110 to +30 mV in 20-mV steps and K538 and D411N channels recorded during 24-ms depolarizing voltage pulses from −110 mV to +30 mV in 20-mV steps. Pulses were applied every 10 s. (B) Gating charge envelopes shown for data in (A). Note different timescale for I521C data in (A) and (B). Decaying component of D411N is an artifact of leak subtraction. (C) Overlay of time course of charge development is given at −30 mV for I521C, D411N, and K538Q.
Time constants of activation of gating charge faster for D411N, V535A, K538Q, and D540A
To quantify the faster activation of total gating charge in the mutant channels, we employed an envelope-of-tails protocol to examine gating charge activation. Data in Fig. 5 A show representative traces of I521C and D411N in response to successively longer depolarizations to +40 and 0 mV, respectively, from the holding potential of −110 mV. Shown below, the charge moved during each depolarization was assessed through integration of the first 100–150 ms of gating charge return at a holding potential of −110 mV. The relationship between total charge moved and length of depolarizing stimulus to 0 mV for D411N and +40 mV for I521C was well fit with single exponentials, as shown in Fig. 5 B. The time constant is clearly faster in D411N, even at a more negative potential than I521C and this is borne out over a range of test potentials between −40 and +60 mV (in 20 mV steps), and in select S4 mutants as well as D411N (Fig. 5 C). Some constructs had time constants of gating charge activation that were too slow to be measured at the lower depolarizations. Still, several of the mutant constructs, D411N, V535A, K538Q, and D540A, had significantly faster time constants of activation than the I521C control. A536V was also faster than I521C at some potentials, but when A536V’s hyperpolarized QV relationship is accounted for (see Fig. 2), the faster kinetics were no longer apparent. The conserved mutation, K538R, was slower than I521C at all potentials, again as expected from the position of its QV relationship (Fig. 2 and Table S1).
Figure 5.
Loss of fast gating charge component is correlated with an increased rate of gating charge activation. (A) Representative gating currents from I521C and D411N recordings given during step depolarizations to 0 mV (D411N) and +40 mV (I521C) from a holding potential of −110 mV. The initial pulse duration was 10 ms for all gating current records, and was increased by 10 ms for each successive cycle. The amount of charge moved was measured by integration of the return of the first 150 ms of gating charge during a repolarization to −110 mV. (B) Plot of normalized integrals of gating charge return given versus time of depolarizing steps for I521C at +40 mV and D411N at 0 mV. (C) Time constants of charge activation were calculated through single exponential fits of the Q/Qmax relations. (D) Relationship between relative amount of fast gating charge given during a 300 ms +20 mV depolarization from a holding potential of −110 mV and time constants of charge activation at +20 mV. To see this figure in color, go online.
The same constructs with an absent or reduced Qfast component of gating charge also had a faster activation rate of total gating charge. Fig. 5 D shows a linear regression of the time constants of charge activation versus Qfast/Qtotal for all constructs at +20 mV, a potential at which all mutants show near-complete charge movement during a 300-ms depolarization. The linear regression showed an R2 value of 0.81 when fit through the means, suggesting a strong correlation between the loss of the fast component of gating charge movement and the speed of activation of total gating charge in the channel.
Fluorescence recordings demonstrate loss of a fast fluorescence component for D411N, V535A, K538Q, and D540A
Several studies of hERG gating have used voltage-clamp fluorometry (VCF) (7, 11, 30) to directly monitor movements of different parts of the VSD, mainly S4. Covalent attachment of a fluorophore to residues in the S3–S4 linker between E518 and I521 has allowed a variety of signals to be obtained that reflect aspects of the activation process. Attachment to E519C has been shown to give rise to a fluorescence signal with a very rapid fast fluorescence quenching and a slower fluorescence quenching component apparent in the first 10–20 ms after a depolarizing stimulus (11), analogous in some ways to the Qfast and Qslow components of gating charge that have been characterized above. After introduction of the mutation E519C to the hERG constructs and covalent attachment of the fluorophores of TMRM to E519C, VCF experiments were performed to test the idea that fast components of fluorescence quenching might track the physical displacement of the VSD underlying the initial Qfast charge movement.
Representative TMRM fluorescence recordings from E519C, D411N, V535A, R537A, K538Q, and D540A constructs in response to a step to −30 mV from a holding potential of −110 mV are shown in Fig. 6 A. In agreement with the prior findings of Es-Salah-Lamoureux et al. (11), the fluorescence quenching showed an initial rapid component followed by a slower maintained quenching component. Of the constructs tested, only R537A remained similar to the E519C control in the relative amount of fast fluorescence quenching and their time constants (Table S2). From the records it can be seen that V535A, D411N, and K538Q retained very little or none of the fast fluorescence quenching. In contrast, D540A showed only a rapid component and no slow fluorescence component. We attribute the D540A result to rapid acceleration of total VSD movement, in agreement with our gating charge data (Figs. 2 and 3 B). The three mutants, D411N, V535A, and D540A, were found to have only a single component of fluorescence quenching after exponential fitting of mean data, whereas K538Q retained a small fast component. Fast and slow constants for the mutants are shown in Table S2 and support the general findings from the gating current recordings described earlier, in that mutants in which the fast quenching was reduced or abolished showed an acceleration of the slow time constant of fluorescence quenching compared with E519C (p < 0.05; Table S2). Mean data for the fast fluorescence component amplitude as a proportion of the total quenching are shown in Fig. 6 B, and all constructs studied, with the exception of R537A, showed a highly significant reduction in the fast fluorescence quenching during depolarization when compared to E519C. These VCF results support the idea that a discrete rapid physical displacement of S4 occurs early in the channel activation process, which is followed by a slower rearrangement of the S4 domains as activation of the VSD continues. The two components of fluorescence correlate temporally with the Qfast and Qslow components of gating charge and their presence or otherwise is accurately predicted from the proportion of Qfast/Qslow in the D411N and S4 mutants.
MTSET modification experiments confirm voltage-dependent S4 movement is displaced to more negative potentials and accelerated in K538Q
A second method to physically track voltage sensor movement is to induce exposure of a Cys-labeled residue and follow the subsequent ion current modification by methane-thiosulfonate reagents. We have previously shown that I521C, close to the top of S4, exhibits clear state-dependent effects upon exposure to MTSET. It shows little response to MTSET at −120 mV but its deactivation kinetics are modified in a voltage-dependent manner upon depolarization (27, 31). The experimental method and tail current modification are illustrated in Fig. 7 A. After control currents were obtained during 2-s pulses to + 40 mV (shaded current trace), cells were held at a range of test potentials (−80 mV in this example) and exposed to MTSET for 10 min, before being washed off. Subsequent voltage pulses revealed channel modification. There was little effect on tail currents from cells held at or more negative than −100 mV, and maximum slowing of deactivation occurring with holding potentials of 0 and +20 mV. The ratio of end amplitude/peak amplitude of the tail current was plotted against the holding potential in Fig. 7 B. The V0.5 for tail current modification in five cells was −51 ± 7 mV in control I521C channels. The effect of MTSET on K538Q channels was quite different. A large degree of modification was already apparent at −100 mV and the overall voltage-dependence of tail current modification was hyperpolarized with a V0.5 of −104 ± 31 mV. The result suggests that in K538Q channels, I521C at the top of S4 is exposed to the extracellular space at −110 mV, whereas in control I521C channels, it remains buried. As well, the hyperpolarization of the V0.5 in K538Q suggests that the energy barrier to voltage-dependent S4 movement is lessened by this mutation.
Figure 7.
Voltage- and time-dependent modification of I521C-K538Q-hERG by MTSET. (A) Given here is an example of MTSET modification of ionic currents. Cell was held at −80 mV and pulsed to +40 mV every 20 s. Tail currents were measured at −110 mV (shaded trace is I521C). Cell was then rested at −80 mV and exposed to 1 mM MTSET for 10 min. After MTSET was removed, currents recorded are shown as example black trace. (B) Cells were held at different holding potentials during MTSET exposure and wash-off. Voltage-dependence of after-effects of MTSET in I521C and K538Q constructs was expressed as ratio of end to peak tail current amplitudes. (C) Cells were held at −120 mV, pulses to 0 mV applied on a 10% duty cycle, 5/50 ms, or less frequently as shown. Presence of MTSET is shown as the shaded region. Holding current before each pulse was measured and shown below in the diary plot. (D) Given here are fitted rate constants of tail current modification at each depolarizing pulse duration in I521C and K538Q at 0 mV. Data from diary plots is as in (C). Fit time constants were 58 ± 15 ms for I521C (0 mV), and 15 ± 6 ms for K538Q (p < 0.05). To see this figure in color, go online.
MTSET experiments can also give dynamic information on the movement of the S4 domain according to the method of Rocheleau and Kobertz (32), which allows measurement of the tail current modification rate. The principle here is that cells are repetitively depolarized to a modifying potential for different durations, but for a fixed total time—in this case, 10% of the time, a 10% duty cycle (Fig. 7 C). Cells were held at −120 mV to prevent modification at rest, and pulsed to 0 mV in the I521C control and K538Q mutant during continuous exposure to MTSET. Initially, all cells were pulsed for 5 ms/50 ms, and subsequently for one of a range of pulse durations, from 10–500 ms for a 10% duty cycle (50 ms/0.5 s in Fig. 7 C). Deactivation was rapidly impaired in an exponential manner as shown by the inward change of the holding current shown in the diary plot. Fits to the time course of tail modification for a range of pulse durations from a number of cells are shown in Fig. 7 D. In I521C at 0 mV the maximum modification rate was observed for pulses of ∼200 ms duration, but in K538Q at 0 mV, maximum modification rates were observed for pulses of <100 ms duration with intermediate modification rates seen for 20-, 30-, and 50-ms pulses. Single exponential fits to the modification rates gave time constants for S4 equilibration of 58 ± 15 ms in I521C (0 mV), and 15 ± 6 ms in K538Q (0 mV). The data suggest a more rapid exposure of the S4 during applied depolarization.
Double-mutant cycle analysis reveals functional interaction between D411 and S4 residues V535 and K538
To establish whether a functional interaction was taking place between D411 and the residues at the bottom of the S4 and the beginning of the S4-S5 linker, double mutant constructs were made with D411N and one of the different S4 mutants of interest. Gating currents were obtained from the double mutants during and after 300-ms depolarizations, and charge measurements from integration of the gating currents upon repolarization have been normalized and plotted in Fig. 8. for all single and double mutants. The change in free energy of activation of the double mutant channels was compared to that of the single mutant and I521C channel constructs (see Materials and Methods for more detail). The change in free energy during activation was assessed as ΔGc = QmaxFVm, and Vm and ΔGc values for all constructs are reported in Table S1. Past studies using this type of analysis in voltage-gated ion channels have suggested that double mutants with a ΔΔG0 > 4.2 kJ/mol value indicate a significant interaction between two sites (25, 33). The small size of the hERG gating currents, especially at negative potentials, resulted in significant variation in the calculated median voltages of activation. As the errors seen in Fig. 8 B are large, only the ΔΔG0 value for the double mutant, D411N/V535A, was shown to be significantly greater than 4.2 kJ/mol. However, there is an overall trend toward nonadditivity in double mutants D411N/A536V, D411N/R537Q, and D411N/K538Q compared with D411N/L539A and D411N/R541A. This, along with the proximity of D411 in the S1 to the bottom of the S4, is strongly suggestive of a more general interaction between these two areas. The double mutant construct, D411N-D540A, had an extremely hyperpolarized gating charge movement that could not be accurately measured, and so it was not possible using double mutant cycle analysis to determine whether D540 is also interacting with D411.
Figure 8.
Double mutant cycle analysis reveals functional interaction between D411 on S1 and the S4 residues V535 and K538. (A) Shown here are QV relationships of I521C, V535A, A536V, R537Q, K538Q, L539A, R541A, D411N, D411N/V535A, D411N/A536V, D411N/R537Q, D411N/K538Q, D411N/L539A, and D411N/R541A. QV relationships were obtained from 300-ms activation pulses from a holding potential of −110 mV. The amount of gating charge moved after each sweep was assessed by integrating the first 150 ms of the return of gating charge. Vm values can be found in Table S1. (B) Shown here are ΔΔGc relationships for D411N/V535A, D411N/A536V, D411N/R537Q, D411N/K538Q, D411N/L539A, and D411N/R541A. ΔΔGc was calculated as described in Materials and Methods. Values are presented in Table S1. To see this figure in color, go online.
Discussion
Interactions between S1 and S4 charges regulate activation gating
Determining the basis for the slow activation of hERG has been the subject of numerous studies (20, 22, 23, 24, 25, 26, 30) in which a recurring finding is that the flanking charges of the S4, i.e., K525 and K538, are involved in interactions that stabilize the closed state of the channel (21, 22, 23, 24, 25). K525 has been suggested to interact with F463, D466 (21), and D456 (25) through interactions that are not likely to be electrostatic in nature, as mutations that change voltage dependence are not charge dependent (21, 24). K538 has been suggested to interact with D411 in the closed state (25) through an electrostatic interaction (21). Disruption of any of these interactions has the effect of increasing the rate of activation and hyperpolarizing the voltage dependence of activation, presumably through disrupting the stabilizing influence of early closed state transitions of the channel.
This study investigated interactions between D411 in S1 and the bottom of the S4, as well as the role of this interaction in the fast gating charge movement of hERG. Mutation to sites in both the S4 and S1 (D411N, V535A, K538Q, and D540A) resulted in similar changes to gating, fluorescence, and MTSET phenotypes (Figs. 2, 3, 6, and 7), and increases in activation rates (Figs. 4 and 5), all of which support a previous report of an interaction (25). Additionally, double mutant cycle analysis of gating currents from mutant constructs was used to define S4 interaction partners with D411 (Fig. 8). There was significant nonadditivity between D411 and V535, indicating an interaction between these sites. This interaction of D411 with the bottom of the S4 segment may not be a direct physical interaction between specified residues, but rather suggests that interacting residues both contribute to stabilization of a particular state (33, 34). Although we did not find statistically significant nonadditivity between K538 and D411, this does not necessarily mean that these regions do not interact during the activation process. For example, should K538 and D411 interact only in intermediate states, additivity in our double mutant cycle would be expected (35). As it has been shown that an electrostatic interaction is likely at K538 (21), the S4 and D411 are in close proximity (18), neutralization of either K538 or D411 results in faster time constants of activation and a hyperpolarized QV relationship (Figs. 2 and 5), and that mutation of either K538 or D411 results in similar gating phenotypes (Figs. 2 and 3), an electrostatic interaction between these two residues contributing to a stabilization of a closed state of the channel intermediate along the activation pathway is still likely.
The physical basis of Qfast
Previous studies of hERG gating have also suggested that Qfast results from rapid transitions through early closed states of the channel (8, 10, 11, 31). It was reported to have a QV0.5 that was depolarized with respect to overall charge movement, a shallow voltage dependence and extremely rapid activation and deactivation kinetics, yet constituted <10% of the total charge movement (8). As a result, Qfast appeared at more negative potentials than the majority of the gating charge, but saturated at more positive potentials than Qslow upon depolarization. Although the kinetics of this charge movement have been adequately described, the question remained—what is the structural basis of Qfast? One possibility is that, in the WT channel, extracellular movement of K525 or perhaps intracellularly directed movement of acidic charges of the S1–S3 is the physical basis of Qfast. In the hERG structure (5VA1), the channel is observed in an open state and D466 is observed below the gating charge transfer center (18). This would be in keeping with a downward movement of D466 because it is accessible to extracellular MTSET in the closed state (20). Piper et al. (22) attempted to obtain gating currents from K525A, but found that this mutation did not yield currents. To our knowledge, gating currents of constructs with neutralized acidic S1–S3 residues have not been obtained.
Our data show that the Qfast component of hERG gating is lost upon disruption of the D411-S4 interaction. This suggests a destabilization of early closed states so that, even at hyperpolarized potentials, channels no longer reside in these early closed states and thus do not transition from them during activation. This is reflected experimentally as an apparent loss of Qfast and an increase in the overall rate of the remaining charge movement. Structurally, we suggest this would involve the charged residues involved in Qfast already being displaced to one side of the gating charge transfer center, no longer passing through it. Our MTSET data (Fig. 7 B) suggest this in showing that in K538Q channels, I521C at the top of the S4 becomes exposed to MTSET at −100 mV, whereas in the control it remains buried. This suggests that due to a destabilization of early closed states in the K538Q mutant, a large hyperpolarizing stimulus is required to return to these states.
An underlying assumption in this study is that Qfast is a charge movement involved in the obligatory activation pathway of hERG. Should this charge be related to a movement of a charged amino acid outside this pathway and uninvolved in channel gating, then it might be expected that return of this charge during repolarization would be observed as a fast, transient current like that of the activation Qfast. However, Qfast is absent upon hyperpolarization after prolonged depolarizations, suggesting that channels traverse a number of slower transitions early during the regular deactivation process, which masks the fast kinetics of Qfast charge return during the last steps of deactivation.
Elimination of Qfast
Whether the changes of charge movement in the mutant channels reflect a loss of Qfast or a merging of Qfast and Qslow as overall charge movement is accelerated in D411N and the S4 mutants is not immediately clear. We have three lines of evidence that suggest that the fast component of gating charge is eliminated and not just obscured in several of our mutant constructs.
First, the time constants of the fast component of gating charge movement and fluorescence quenching, as described in a gating current study by Piper et al. (8), and a fluorescence study by Smith and Yellen (7), are extremely fast (<1 ms), near instantaneous upon depolarization over a wide range of potentials (Fig. 1). In contrast, the time constant of gating charge activation of D411N at 0 mV, for example, is 15 ms (Fig. 5 C), a full order-of-magnitude slower than that of the Qfast component. The Qfast component in I521C moves rapidly even at −30 mV in Fig. 4 C, with the arrow denoting an inflection point as slower charge movement starts. It precedes the D411N and K538Q charge envelopes that show no sign of the rapid early component as they are quite a bit slower, and also monotonic in nature. This is despite the fact that ionic activation in these two mutants is negatively shifted compared to I521C (Fig. S1).
Second, the fluorescence records (Fig. 6) support the gating current data described above. D411N, V535A, and K538Q fluorescence showed a loss of the biphasic signal observed in E519C within the first 10–20 ms during depolarization to −30 mV. Similar to the gating currents, the fast component of the quenching signal was lost upon mutation to D411N, V535A, and K538Q.
Third, it is known that activation of WT hERG gating and ionic currents becomes rate limited at high potentials by a voltage-independent transition (9, 36). The speed of Qfast movement suggests that it is not rate limited by this voltage-independent transition (Fig. 5). As a result of this, past models have suggested that the sequence of the hERG activation pathway is a fast gating transition, followed by a voltage-independent transition, followed by the bulk movement of gating charge (8, 36). In mutants in which the fast component is no longer visibly present, the time constants of gating charge activation at high potentials become faster than has been previously reported for the voltage-independent transition (Fig. 5). In related experiments, MTSET data showed modification of, and therefore extracellular exposure of, the outer S4 I521C residue at more negative potentials, and at more rapid rates in the K538Q mutant than in I521C alone (Fig. 7). We suggest that this is a result of closed states of the channel becoming destabilized, and mutant channels resting further along their activation pathway, after states that would include transitions relevant to Qfast and the voltage-independent transition.
Conclusions
The various types of measurements made here all point to the same conclusions. The D411-S4 interaction stabilizes early closed states of the hERG channel, and transition from these states during channel activation induces a transient Qfast. Mutations to the D411-S4 interaction axis allow the lower S1 and S4 domains to separate and S4 to move outward slightly, exposing I521 at rest. As a result, Qfast no longer appears during activation, and channels can process more rapidly through the remaining activation steps as suggested by the accelerated movement of the main body of gating charge in D411N, K538Q, and V535A (Figs. 2, 3, 4 and 5), and by the faster MTSET modification rates (Fig. 7). The D411-S4 interaction, unique to the ether-à-go-go family of potassium channels, serves as an additional constraint in the hERG activation pathway that stabilizes early closed states of the channel, and underlies the rather slow activation of hERG channels.
Author Contributions
Y.D., D.F., and L.C.M. designed the research. Y.D., Y.W., and L.C.M. performed experiments and analyzed the data. L.C.M. and D.F. wrote the manuscript.
Acknowledgments
This research was supported by the Heart and Stroke Foundation of Canada and Canadian Institutes for Health Research operating grants to D.F. During the course of this work, Y.D. held a postdoctoral fellowship from the Heart and Stroke Foundation, and L.C.M. held a graduate research scholarship from the University of British Columbia.
Editor: Baron Chanda.
Footnotes
Ying Dou and Logan C. Macdonald contributed equally to this work.
Supporting Materials and Methods, one figure, and two tables are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(17)30990-6.
Supporting Material
References
- 1.Warmke J.W., Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc. Natl. Acad. Sci. USA. 1994;91:3438–3442. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanguinetti M.C., Jiang C., Keating M.T. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 3.Trudeau M.C., Warmke J.W., Robertson G.A. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- 4.Sanguinetti M.C., Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–469. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- 5.Hancox J.C., McPate M.J., Zhang Y.H. The hERG potassium channel and hERG screening for drug-induced torsades de pointes. Pharmacol. Ther. 2008;119:118–132. doi: 10.1016/j.pharmthera.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 6.January C.T., Gong Q., Zhou Z. Long QT syndrome: cellular basis and arrhythmia mechanism in LQT2. J. Cardiovasc. Electrophysiol. 2000;11:1413–1418. doi: 10.1046/j.1540-8167.2000.01413.x. [DOI] [PubMed] [Google Scholar]
- 7.Smith P.L., Yellen G. Fast and slow voltage sensor movements in HERG potassium channels. J. Gen. Physiol. 2002;119:275–293. doi: 10.1085/jgp.20028534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piper D.R., Varghese A., Tristani-Firouzi M. Gating currents associated with intramembrane charge displacement in HERG potassium channels. Proc. Natl. Acad. Sci. USA. 2003;100:10534–10539. doi: 10.1073/pnas.1832721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodchild S.J., Macdonald L.C., Fedida D. Sequence of gating charge movement and pore gating in HERG activation and deactivation pathways. Biophys. J. 2015;108:1435–1447. doi: 10.1016/j.bpj.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodchild S.J., Fedida D. Gating charge movement precedes ionic current activation in hERG channels. Channels (Austin) 2014;8:84–89. doi: 10.4161/chan.26775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Es-Salah-Lamoureux Z., Fougere R., Fedida D. Fluorescence-tracking of activation gating in human ERG channels reveals rapid S4 movement and slow pore opening. PLoS One. 2010;5:e10876. doi: 10.1371/journal.pone.0010876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith P.L., Baukrowitz T., Yellen G. The inward rectification mechanism of the HERG cardiac potassium channel. Nature. 1996;379:833–836. doi: 10.1038/379833a0. [DOI] [PubMed] [Google Scholar]
- 13.Spector P.S., Curran M.E., Sanguinetti M.C. Fast inactivation causes rectification of the IKr channel. J. Gen. Physiol. 1996;107:611–619. doi: 10.1085/jgp.107.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandenberg J.I., Perry M.D., Hill A.P. hERG K+ channels: structure, function, and clinical significance. Physiol. Rev. 2012;92:1393–1478. doi: 10.1152/physrev.00036.2011. [DOI] [PubMed] [Google Scholar]
- 15.MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991;350:232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- 16.Whicher J.R., MacKinnon R. Structure of the voltage-gated K+ channel Eag1 reveals an alternative voltage sensing mechanism. Science. 2016;353:664–669. doi: 10.1126/science.aaf8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long S.B., Tao X., MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 18.Wang W., MacKinnon R. Cryo-EM structure of the open human ether-à-go-go-related K+ channel hERG. Cell. 2017;169:422–430 e10. doi: 10.1016/j.cell.2017.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bezanilla F. The voltage sensor in voltage-dependent ion channels. Physiol. Rev. 2000;80:555–592. doi: 10.1152/physrev.2000.80.2.555. [DOI] [PubMed] [Google Scholar]
- 20.Zhang M., Liu J., Tseng G.N. Gating charges in the activation and inactivation processes of the HERG channel. J. Gen. Physiol. 2004;124:703–718. doi: 10.1085/jgp.200409119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng Y.M., Hull C.M., Claydon T.W. Functional interactions of voltage sensor charges with an S2 hydrophobic plug in hERG channels. J. Gen. Physiol. 2013;142:289–303. doi: 10.1085/jgp.201310992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piper D.R., Hinz W.A., Tristani-Firouzi M. Regional specificity of human ether-à -go-go-related gene channel activation and inactivation gating. J. Biol. Chem. 2005;280:7206–7217. doi: 10.1074/jbc.M411042200. [DOI] [PubMed] [Google Scholar]
- 23.Subbiah R.N., Kondo M., Vandenberg J.I. Tryptophan scanning mutagenesis of the HERG K+ channel: the S4 domain is loosely packed and likely to be lipid exposed. J. Physiol. 2005;569:367–379. doi: 10.1113/jphysiol.2005.097386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subbiah R.N., Clarke C.E., Vandenberg J.I. Molecular basis of slow activation of the human ether-à-go-go related gene potassium channel. J. Physiol. 2004;558:417–431. doi: 10.1113/jphysiol.2004.062588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang M., Liu J., Tseng G.N. Interactions between charged residues in the transmembrane segments of the voltage-sensing domain in the hERG channel. J. Membr. Biol. 2005;207:169–181. doi: 10.1007/s00232-005-0812-1. [DOI] [PubMed] [Google Scholar]
- 26.Liu J., Zhang M., Tseng G.N. Negative charges in the transmembrane domains of the HERG K channel are involved in the activation- and deactivation-gating processes. J. Gen. Physiol. 2003;121:599–614. doi: 10.1085/jgp.200308788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dou Y., Goodchild S.J., Fedida D. The neutral, hydrophobic isoleucine at position I521 in the extracellular S4 domain of hERG contributes to channel gating equilibrium. Am. J. Physiol. Cell Physiol. 2013;305:C468–C478. doi: 10.1152/ajpcell.00147.2013. [DOI] [PubMed] [Google Scholar]
- 28.Chowdhury S., Chanda B. Estimating the voltage-dependent free energy change of ion channels using the median voltage for activation. J. Gen. Physiol. 2012;139:3–17. doi: 10.1085/jgp.201110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanguinetti M.C., Xu Q.P. Mutations of the S4-S5 linker alter activation properties of HERG potassium channels expressed in Xenopus oocytes. J. Physiol. 1999;514:667–675. doi: 10.1111/j.1469-7793.1999.667ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Slyke A.C., Rezazadeh S., Claydon T.W. Mutations within the S4-S5 linker alter voltage sensor constraints in hERG K+ channels. Biophys. J. 2010;99:2841–2852. doi: 10.1016/j.bpj.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z., Dou Y., Fedida D. Components of gating charge movement and S4 voltage-sensor exposure during activation of hERG channels. J. Gen. Physiol. 2013;141:431–443. doi: 10.1085/jgp.201210942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rocheleau J.M., Kobertz W.R. KCNE peptides differently affect voltage sensor equilibrium and equilibration rates in KCNQ1 K+ channels. J. Gen. Physiol. 2008;131:59–68. doi: 10.1085/jgp.200709816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yifrach O., MacKinnon R. Energetics of pore opening in a voltage-gated K+ channel. Cell. 2002;111:231–239. doi: 10.1016/s0092-8674(02)01013-9. [DOI] [PubMed] [Google Scholar]
- 34.Horovitz A. Double-mutant cycles: a powerful tool for analyzing protein structure and function. Fold. Des. 1996;1:R121–R126. doi: 10.1016/S1359-0278(96)00056-9. [DOI] [PubMed] [Google Scholar]
- 35.Chowdhury S., Haehnel B.M., Chanda B. A self-consistent approach for determining pairwise interactions that underlie channel activation. J. Gen. Physiol. 2014;144:441–455. doi: 10.1085/jgp.201411184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang S., Liu S., Rasmusson R.L. A quantitative analysis of the activation and inactivation kinetics of HERG expressed in Xenopus oocytes. J. Physiol. 1997;502:45–60. doi: 10.1111/j.1469-7793.1997.045bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.