Abstract
The aim of this study was to inspect more closely the microscopic and molecular sympathetic ganglia histology in thromboangiitis obliterans (TAO). The paraffin block and frozen RNAlater-treated tissue of the lumbar sympathetic ganglia of 19 TAO Caucasian male patients were evaluated. The gene expression of cluster of differentiation 4 (CD4) and cluster of differentiation 8 (CD8) markers in the frozen RNAlater-treated sympathetic ganglia tissues were evaluated by real-time polymerase chain reaction. Unexpectedly, lymphocyte infiltration was observed in all of the histological sections, ranging from scattered to moderate lymphocyte infiltration. In seven patients, five of them underwent below-knee amputation, neutrophil infiltration was observed in addition to lymphocyte infiltration. The gene expression of the CD8 marker in all of the samples with the expression of CD4 markers in only four tissue samples was demonstrated. The expression of CD8 in comparison to CD4 was approximately 4.37-fold changes using Pfaffle method. It appears that inflammation of the sympathetic ganglia plays a role in the pathophysiology of TAO and its outcome. Sympathetic ganglia inflammation may be responsible for general vasoconstriction, vascular inflammation, and the increased risk of thrombotic events, by activating the platelets. The dominant infiltration of T cytotoxic lymphocytes and neutrophils in sympathetic ganglia may probably support the idea of possible intracellular infectious pathogen trigger for TAO and consequently infiltration of pathogen-specific T cells into the sympathetic ganglia in TAO.
Keywords: thromboangiitis obliterans, Buerger's disease, sympathetic ganglia, inflammation, sympathectomy
Thromboangiitis obliterans (TAO) is a segmental thrombotic occlusive peripheral vascular disease with unknown etiology, which usually occurs in young male smokers from low socioeconomic classes and leads to multiple amputations and disability. 1 The progress and prognosis of TAO has a very close relationship with smoking, and approximately half of the patients will undergo amputations (e.g., toe, finger, or limbs) in the absence of smoking cessation. 1 2 Allodynia, circadian rhythmic burning pain which interrupts sleeping, and severe pain during rest, are common manifestations of TAO. 1 3 Prostaglandin I2 or prostaglandin E1 analogs have been the usual treatments for pain management and reducing the risk of major amputation. 4 In addition, sympathectomy has been an option for pain management of TAO patients in recent decades. 4
During the evaluation of the arteriography of TAO patients, we noticed that in the patients who had one limb involvement, the diameter of the involved artery was less than the noninvolved artery from its origin, and its appearance was similar to a general vasoconstriction ( Fig. 1 ). This discovery led us to look more closely at the sympathetic ganglia histology and gene expression in TAO.
Fig. 1.
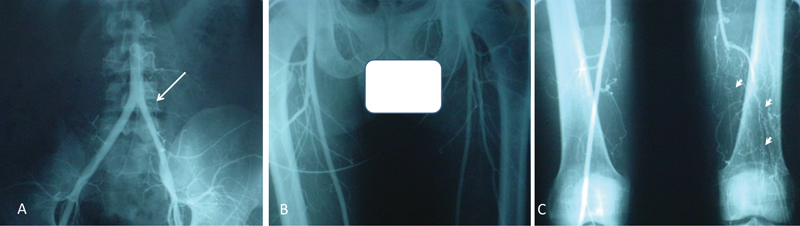
The arteriography of a patient with diagnosis of Buerger's disease. ( A, B ) The diameter of the involved artery is less than the noninvolved artery from its origin and it looks like a general vasoconstriction. ( C ) Corkscrew collaterals.
Materials and Methods
The paraffin block and frozen RNAlater-treated tissue of the lumbar sympathetic ganglia (L2 and L3) of 19 Caucasian male patients with a diagnosis of TAO, based on Olin's criteria 5 including (1) patients' age at disease onset < 45 years; (2) history of tobacco use; (3) distal limb ischemia; (4) exclusion of autoimmune diseases, hypercoagulable states, and diabetes mellitus; (5) exclusion of a proximal source of embolization, and (6) arteriographic findings between 2010 and 2012, were enrolled in this study. All patients signed a broad consent form for the banking of biological samples, including blood, serum, plasma, and tissue, for further use in biomolecular research (the ethical code for banking was 900133 and the ethical code for evaluating gene expression on the banked samples was 950002). The main complaints of the patients were ischemic rest pain, nonhealing ulcer, or focal gangrene.
All samples had a pathology report to confirm that the obtained tissue was sympathetic ganglia. However, new histological sections were obtained from the banked paraffin blocks and rechecked by an expert pathologist. Hematoxylin and eosin stain was used for evaluation of the histological sections of the sympathetic ganglia.
As the facilities for immunohistochemistry (IHC) for cluster of differentiation 4 (CD4) and cluster of differentiation 8 (CD8) markers of T lymphocytes were not available in our laboratory, we evaluated the gene expression of CD4 and CD8 markers in the sympathetic ganglia, which were banked in the freezer at −80 in RNAlater solution by real-time polymerase chain reaction (PCR), TaqMan method to found the ratio of T cytotoxics to T helpers in the sympathetic tissues infiltrated with lymphocytes. The RNA of RNAlater-treated tissues of lumbar sympathetic ganglia were extracted using the QIAGEN RNeasy Mini Kit (Cat. No. 74104, Hilden, Germany) and the cDNA was synthesized using the RevertAid First Strand cDNA kit (Thermo Scientific, K1622, Lithuania). Ribosomal protein large P0 ( RPLP0 ) was considered the housekeeping gene for normalizing the gene expression. Two peripheral blood mononuclear cells of two healthy people were designated the positive controls for CD4 and CD8 gene expression, respectively. The Pfaffl method or the delta–delta Ct method (ΔΔCT) with efficiency correction was used to calculate the ratio of gene expression of CD4 and CD8 as the markers of T helper and cytotoxic lymphocytes. 6 7 Unfortunately, there was no sympathetic ganglia resection from healthy people because the potential side effects rendered it unethical. Only two patients who underwent sympathetic ganglia resection due to hyperhidrosis were included as the negative control group in this study. The sequences of the primers for CD4, CD8, and RPLP0 were borrowed from the PrimerBank of Harvard University, and the probes related to the paired primers were designed by Beacon Designer 7.0. The sequences of the studied genes are summarized in Table 1 .
Table 1. Sequences of the forward and reverse primers and probes for evaluating gene expression of CD4 and CD8 markers and RPLP0 by semiquantitative real-time PCR.
| CD4 | CD8 | RPLP0 | |
|---|---|---|---|
| Primer forward | 5-CGGACCAGATGAATGTAG-3 | 5-AGACCCCTGCATACATAAAGGT-3 | 5-CAGATTGGCTACCCAACTGTT-3 |
| Primer reverse | 5-GAGGACGGTGTGATTAAG-3 | 5-CGCTGTCTCAGCCAGTAGAT-3 | 5-GGAAGGTGTAATCCGTCTCCAC-3 |
| Probe | FAM-CCTCCTGTTCGCCTCCTCTAC-TAMARA | FAM-AGCCTCGCAGGACAGCATCACCATCTT-TAMARA | FAM-TCAACGGGTACAAACGAGTCCTGGCCT-TAMARA |
Abbreviations: CD4, cluster of differentiation 4; CD8, cluster of differentiation 8; PCR, polymerase chain reaction; RPLP0, ribosomal protein large P0.
Results
In total, histological sections from 19 paraffin blocks of sympathetic ganglia belonging to TAO patients were analyzed. In addition, CD4 and CD8 gene expression of the 19 sympathetic ganglia tissues treated with RNAlater solution were evaluated using real-time PCR.
All of the enrolled histological slides belonged to Caucasian male patients with a TAO diagnosis. The mean age of the patients at the time of sympathectomy was 40.9 ± 5.1 years. The chief complaint of 55.5% of them was gangrene, 27.8% nonhealing ulcer, and 16.7% burning pain. About 29.4% of the patients underwent below-knee amputation, and 23.5% had toe amputations up to 1 month after sympathectomy. However, improvement was observed in 47.1% of the patients.
Unexpectedly, lymphocyte infiltration was observed in all of the histological sections, ranging from scattered to moderate lymphocyte infiltration ( Fig. 2A ). In four patients, lymphocyte aggregation was observed ( Fig. 2B ).
Fig. 2.
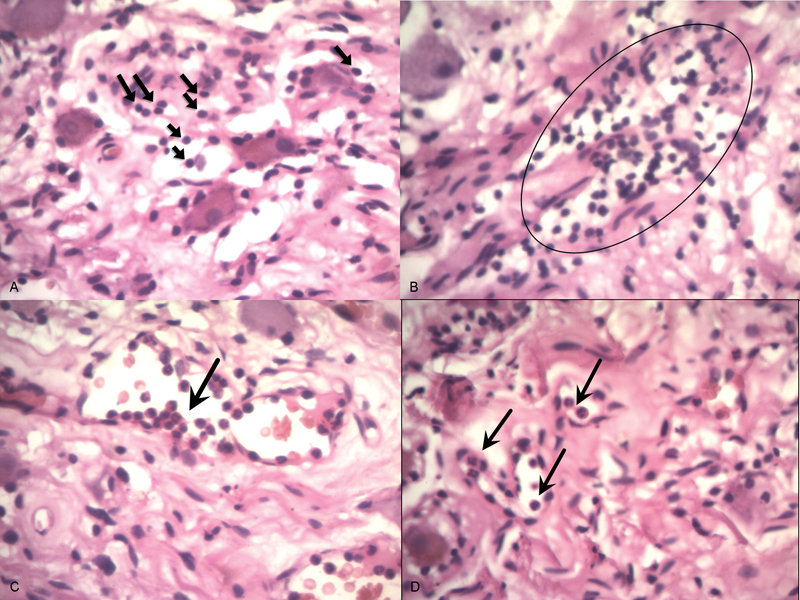
The hematoxylin and eosin stain of sympathetic ganglia histological section (Patient No. 6). ( A ) Lymphocyte infiltration in sympathetic ganglia, ( B ) lymphocyte aggregation, ( C ) neutrophil chemotaxis inside sympathetic ganglia, and ( D ) neutrophil infiltration besides lymphocyte infiltration.
In seven patients, neutrophil infiltration was observed in addition to lymphocyte infiltration, five of them underwent below-knee amputation ( Fig. 2C, D ). However, in two patients with neutrophil infiltration, improvement of ulcer healing and pain score after sympathectomy was observed.
The results of relative quantification of real-time PCR demonstrated gene expression of the CD8 marker in all samples with gene expression of CD4 markers in only four tissue samples. The efficiency of PCR reaction for CD8 was 0.89, for CD4 was 0.884, and for RPLP0 was 0.96.
The expression of CD8 in comparison to CD4 for the four samples was ∼4.37-fold changes using Pfaffle method.
 |
 |
Ratio of CD8/CD4 = 4.37-fold changes.
All of the data related to demographics, chief complaints, histopathological, and real-time findings are summarized in Table 2 .
Table 2. Summarized data related to chief complaint, disease outcome after sympathectomy, histopathological, and the real-time findings of the sympathetic ganglia of 19 patients with Buerger's disease.
| Patient | Chief complaint | Outcome after sympathectomy | Histological findings | CD8 CT | CD4 CT | RPLP0 CT | ||
|---|---|---|---|---|---|---|---|---|
| N | L | L agg | ||||||
| 1 | Nonhealing ulcer | Below-knee amputation | + | Mild | − | 32.83 | Under threshold | 20.71 |
| 2 | Gangrene | Toe amputation | − | Moderate | − | 29.38 | Under threshold | 21.74 |
| 3 | Gangrene | Toe amputation | − | Mild | − | 32.27 | Under threshold | 24.80 |
| 4 | Burning pain | Improvement | − | Scattered | − | 41.03 | Under threshold | 25.66 |
| 5 | Nonhealing ulcer | Toe amputation | − | Mild | + | 24.56 | Under threshold | 25.07 |
| 6 | Gangrene | Below-knee amputation | + | Mild | + | 26.39 | 42.42 | 24.59 |
| 7 | Gangrene | Improvement | − | Mild | − | 33.3 | Under threshold | 25.98 |
| 8 | Nonhealing ulcer | Improvement | − | Moderate | − | 33.98 | Under threshold | 33.32 |
| 9 | Gangrene | Below-knee amputation | + | Mild | − | 32.73 | Under threshold | 23.49 |
| 10 | Gangrene | Below-knee amputation | + | Mild | + | 30.57 | 40.65 | 25.91 |
| 11 | Burning pain | Improvement | − | Mild | − | 37.05 | Under threshold | 25.66 |
| 12 | Gangrene | Below-knee amputation | + | Mild | + | 30.71 | 40.59 | 23.92 |
| 13 | Gangrene | Improvement | + | Moderate | − | 32.26 | Under threshold | 23.44 |
| 14 | Gangrene | Improvement | + | Scattered | − | 38.72 | Under threshold | 26.51 |
| 15 | Gangrene | Toe amputation | − | Mild | − | 30.52 | Under threshold | 30.47 |
| 16 | Burning pain | Improvement | − | Moderate | − | 29.08 | Under threshold | 24.7 |
| 17 | Gangrene | Improvement | − | Mild | − | 32.95 | Under threshold | 24.79 |
| 18 | Nonhealing ulcer | Toe amputation | − | Moderate | − | 32.36 | Under threshold | 25.8 |
| 19 | Nonhealing ulcer | Improvement | − | Mild | − | 33.24 | 39.97 | 26.51 |
Abbreviations: CD4, cluster of differentiation 4; CD8, cluster of differentiation 8; CT, computed tomography; L, lymphocyte; L agg, lymphocyte aggregation; N, neutrophil; RPLP0, ribosomal protein large P0.
According to chi-square test, there was a significant correlation between neutrophil infiltration of the sympathetic ganglia and below-knee amputation ( p < 0.001, Pearson's chi-square test: 17.0). There was also a significant correlation between lymphoid aggregation in the sympathetic ganglia and adverse outcome of the patients ( p = 0.01, Pearson's chi-square test: 8). No significant correlation between the grade of lymphocyte infiltration and the disease outcome was observed ( p = 0.56). There were also no correlations between the clinical manifestation of TAO and histopathological findings of the sympathetic ganglia.
Discussion
Although more than a decade has passed since the first description of TAO, the etiology of the disease is still unknown. 1 While the close relationship between smoking and the progress and outcome of the disease is well defined, the main trigger for developing TAO is not known. 1
Because of the unknown etiology of TAO, there is still no precise protocol for treatment of TAO patients. However, for many years, sympathectomy has been one of the inexpensive treatment options for TAO without serious side effects. 8 The most concerning side effect of lumbar sympathectomy is sexual dysfunction, which has been reported in 24% of patients who underwent bilateral lumbar sympathectomy, and mainly consists of ejaculation disturbances. 9 However, no sexual dysfunction has been reported for unilateral lumbar sympathectomy. 10 11 It has been demonstrated that sympathectomy can provide short-term pain relief by interrupting the sympathetic-nociceptive pathway. 4 It can also improve ulcer healing in TAO patients with complaints of nonhealing ulcers. 12 It has further been implied that the success rate of stem cell therapy in limbs with a previous history of sympathectomy was significantly better than in those patients without a history of sympathectomy. 13 Improvement after sympathectomy was also observed in ∼47% of our patients at their 1-month follow-up. However, the long-term benefit of sympathectomy for TAO is not yet known. It appears that there is not any significant difference in the amputation rate in TAO patients with and without sympathectomy, according to a study conducted on 23 TAO patients. 2 However, it has been demonstrated that sympathectomy can make the amputation as distal as possible in TAO patients, due to improved wound healing. 14
Recently, several studies on infectious pathogens as the likely main trigger of TAO have been conducted. 15 However, even if we are prepared to accept the role of infectious pathogens in the pathophysiology of TAO, we still do not know if infection individually induces the disease or if the infection induces a kind of autoimmunity. 16
In this study, we unexpectedly found lymphocyte infiltration in all of the samples. Besides, neutrophil infiltration was also observed in five patients who underwent below-knee amputation short after sympathectomy. Since the sympathetic ganglia are far from the site of the ischemia and vascular lesions, the existence of inflammation in sympathetic ganglia could be explained by several reasons.
First of all, the microvasculature around sympathetic ganglia may be involved in the process of TAO and the inflammatory cells of the vessels around the sympathetic ganglia extend to the ganglia just like the neural inflammation in the peripheral neurovascular bundle.
Second, the sympathetic ganglia inflammation may be secondary to chronic irritation of the peripheral nerves during ischemia or peripheral vascular lesions. According to a recent study on rat models, chronic irritation of the peripheral nerves can induce T CD4 + lymphocyte infiltration in the sympathetic ganglia. 17 However, in this study, we found that the major infiltrated lymphocyte in the sympathetic ganglia was T cytotoxic (T CD8 + ) not T CD4 + .
Third, the dominant infiltration of T cytotoxic (CD8 + ) lymphocytes and neutrophils in the sympathetic ganglia potentially supports the idea of a possible intracellular infectious pathogen trigger of TAO and, consequently, the infiltration of pathogen-specific T cells into the sympathetic ganglia of TAO. The presence of T helper lymphocytes in four samples of this study might be due to the recruitment of T helpers (CD4 + ), which is secondary to the existing inflammation and likely neuronal damage because of the neuronal protection and functional recovery role of T helpers in the nervous system. 18 19 However, four samples constitute a very low sample size for a conclusion on the possible role of the existing T helpers in the sympathetic ganglia.
Besides the etiology of sympathetic ganglia inflammation in TAO, this pathology may be the responsible of a general vasoconstriction and even vascular inflammation—so-called neurogenic inflammation—and may increase the risk of thrombotic events by activating the platelets. 20 21
Limitations of the Study
As resection of the sympathetic ganglia in healthy people is not ethical and is uncommon in other peripheral vascular diseases or diseases with an indication of sympathectomy (e.g., hyperhidrosis), we only had two patients with hyperhidrosis as the negative control. However, besides our evaluation of the sympathetic ganglia of hyperhidrosis cases, no lymphocyte infiltration was reported according to the studies on the sympathetic ganglia of patients suffering from nondiabetic atherosclerosis obliterans, diabetic foot, and hyperhydrosis. 22 23
Another limitation of our study was the lack of facilities for IHC for CD4 and CD8, the lack of IHC antibodies for CD4 and CD8, and the lack of a technical expert to conduct reliable IHC. We thus substituted “real-time PCR,” which is an acceptable method for identifying lymphocyte types. 24
Conclusion
It appears from this study that inflammation of the sympathetic ganglia plays a role in the pathophysiology of TAO and its outcome. Further studies should focus on resolving the infection in TAO and its possible influence on disease outcome, even in patients who continue smoking.
Funding
The study for real-time PCR on frozen tissues was supported by Mashhad University of Medical Sciences (Research code: 950002).
Footnotes
Conflict of Interest The authors declare that there is no conflict of interest regarding the current article.
References
- 1.Fazeli B, Ravari H. Mechanisms of thrombosis, available treatments and management challenges presented by thromboangiitis obliterans. Curr Med Chem. 2015;22(16):1992–2001. doi: 10.2174/0929867322666150429112111. [DOI] [PubMed] [Google Scholar]
- 2.Olin J W, Shih A. Thromboangiitis obliterans (Buerger's disease) Curr Opin Rheumatol. 2006;18(01):18–24. doi: 10.1097/01.bor.0000198000.58073.aa. [DOI] [PubMed] [Google Scholar]
- 3.Fazeli B. Need for changes in clinical criteria for diagnosing Buerger's disease. Vascular. 2013;21(02):117–118. doi: 10.1177/1708538113479537. [DOI] [PubMed] [Google Scholar]
- 4.Klein-Weigel P F, Richter J G. Thromboangiitis obliterans (Buerger's disease) Vasa. 2014;43(05):337–346. doi: 10.1024/0301-1526/a000371. [DOI] [PubMed] [Google Scholar]
- 5.Olin J W. Thromboangiitis obliterans (Buerger's disease) N Engl J Med. 2000;343(12):864–869. doi: 10.1056/NEJM200009213431207. [DOI] [PubMed] [Google Scholar]
- 6.Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(04):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 7.Spencer M J, Montecino-Rodriguez E, Dorshkind K, Tidball J G. Helper (CD4(+)) and cytotoxic (CD8(+)) T cells promote the pathology of dystrophin-deficient muscle. Clin Immunol. 2001;98(02):235–243. doi: 10.1006/clim.2000.4966. [DOI] [PubMed] [Google Scholar]
- 8.Nesargikar P N, Ajit M K, Eyers P S, Nichols B J, Chester J F. Lumbar chemical sympathectomy in peripheral vascular disease: does it still have a role? Int J Surg. 2009;7(02):145–149. doi: 10.1016/j.ijsu.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Quayle J B. Sexual function after bilateral lumbar sympathectomy and aorto-iliac by-pass surgery. J Cardiovasc Surg (Torino) 1980;21(02):215–218. [PubMed] [Google Scholar]
- 10.Singh S, Kaur S, Wilson P. Early experience with endoscopic lumbar sympathectomy for plantar hyperhidrosis. Asian J Endosc Surg. 2016;9(02):128–134. doi: 10.1111/ases.12275. [DOI] [PubMed] [Google Scholar]
- 11.Nicolas C, Grosdidier G, Granel F, Barbaud A, Schmutz J L. Endoscopic sympathectomy for palmar and plantar hyperhidrosis: results in 107 patients [in French] Ann Dermatol Venereol. 2000;127(12):1057–1063. [PubMed] [Google Scholar]
- 12.Chander J, Singh L, Lal P, Jain A, Lal P, Ramteke V K. Retroperitoneoscopic lumbar sympathectomy for Buerger's disease: a novel technique. JSLS. 2004;8(03):291–296. [PMC free article] [PubMed] [Google Scholar]
- 13.Lee K B, Kang E S, Kim A K et al. Stem cell therapy in patients with thromboangiitis obliterans: assessment of the long-term clinical outcome and analysis of the prognostic factors. Int J Stem Cells. 2011;4(02):88–98. doi: 10.15283/ijsc.2011.4.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishima Y. Arterial insufficiency of the upper extremity with special reference to Takayasu's arteritis and Buerger's disease. J Cardiovasc Surg (Torino) 1982;23(02):105–108. [PubMed] [Google Scholar]
- 15.Fazeli B, Rezaee S A. A review on thromboangiitis obliterans pathophysiology: thrombosis and angiitis, which is to blame? Vascular. 2011;19(03):141–153. doi: 10.1258/vasc.2010.ra0045. [DOI] [PubMed] [Google Scholar]
- 16.Ercolini A M, Miller S D. The role of infections in autoimmune disease. Clin Exp Immunol. 2009;155(01):1–15. doi: 10.1111/j.1365-2249.2008.03834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLachlan E M, Hu P. Inflammation in dorsal root ganglia after peripheral nerve injury: effects of the sympathetic innervation. Auton Neurosci. 2014;182:108–117. doi: 10.1016/j.autneu.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Bradl M, Bauer J, Flügel A, Wekerle H, Lassmann H. Complementary contribution of CD4 and CD8 T lymphocytes to T-cell infiltration of the intact and the degenerative spinal cord. Am J Pathol. 2005;166(05):1441–1450. doi: 10.1016/S0002-9440(10)62361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fehervari Z. Healing the CNS. Nat Immunol. 2015;16(03):228. [Google Scholar]
- 20.Grippo A J, Scotti M A. Stress and neuroinflammation. Mod Trends Pharmacopsychiatry. 2013;28:20–32. doi: 10.1159/000343965. [DOI] [PubMed] [Google Scholar]
- 21.Ozdemir O, Soylu M, Alyan O et al. Association between mean platelet volume and autonomic nervous system functions: increased mean platelet volume reflects sympathetic overactivity. Exp Clin Cardiol. 2004;9(04):243–247. [PMC free article] [PubMed] [Google Scholar]
- 22.Kott I, Urca I, Sandbank U. Lumbar sympathetic ganglia in atherosclerotic patients, diabetic and nondiabetic. A comparative morphological and ultrastructural study. Arch Surg. 1974;109(06):787–792. doi: 10.1001/archsurg.1974.01360060057015. [DOI] [PubMed] [Google Scholar]
- 23.de Oliveira F R, Moura N B, Jr, de Campos J R et al. Morphometric analysis of thoracic ganglion neurons in subjects with and without primary palmar hyperhidrosis. Ann Vasc Surg. 2014;28(04):1023–1029. doi: 10.1016/j.avsg.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Mocellin S, Provenzano M, Rossi C R, Pilati P, Nitti D, Lise M.Use of quantitative real-time PCR to determine immune cell density and cytokine gene profile in the tumor microenvironment J Immunol Methods 2003280(1–2):1–11. [DOI] [PubMed] [Google Scholar]


