Abstract
Neointimal hyperplasia is known as a main factor contributing to in-stent restenosis (ISR). Monocytes may play a central role in vessel restenosis process after stent implantation. The aim of this study was to investigate the relationships between the urokinase-type plasminogen activator (PLAU) and vitronectin (Vtn) gene expression levels in peripheral blood mononuclear cell samples isolated from whole blood of 66 patients undergoing coronary artery angiography (22 controls, stenosis < 0.05%; 22 with stent no-restenosis and stenosis < 70%; and 22 with ISR and stenosis > 70%). The Vtn and PLAU gene expression levels were measured by real-time quantitative polymerase chain reaction technique. The age- and gender-independent increases in the expression levels of Vtn (17-fold; p < 0.001) and PLAU (27-fold; p < 0.0001) genes were found in the patients with ISR as compared with the control group. The results suggested that the Vtn and PLAU genes may be involved in the coronary artery ISR.
Keywords: in-stent restenosis, vitronectin, urokinase-type plasminogen activator
One of the current therapeutic approaches in coronary artery stenosis is stent implantation, which is often associated with in-stent restenosis (ISR). 1 2 The restenosis is defined as a renarrowing process with the rates up to 60% in patients undergoing percutaneous transluminal coronary angioplasty (PTCA). 3 4 5 6 The restenosis events include arterial vessel recoil, remodeling, and neointimal hyperplasia. In contrast with neointimal hyperplasia, the arterial vessel recoil and remodeling are usually resolved in new stents. 7 8 Thus, the intensive in-stent neointimal hyperplasia is one of the most important subjects considered to ISR. It is mainly related to vascular smooth muscle cell (VSMC) proliferation and migration. 9
Recent studies suggested that the plasmin activation system plays a crucial role in the improvement of restenosis. Several studies reported that the expression levels of urokinase-type plasminogen activator (PLAU) and plasminogen activator inhibitor-1 (PAI-1) genes relate to the VSMC proliferation and neointima formation. Also, there were the reports on the PAI-1 deficiency to promote the restenosis process. 10 11 12 The studies proposed that arginine–glycine–aspartic acid (RGD) motif on vitronectin (Vtn) protein sequence plays a key role in the cell migration. The Vtn promotes the cell migration by interaction with specific integrin and PLAU receptor (uPAR) (www.hgdb.ir). 13 The uPAR– PLAU complex binds Vtn and accelerates the cell adhesion and migration. 14 15
The main sources of Vtn and PLAU proteins are unclear in the ISR process. Previous studies showed that white blood cells, especially monocytes, increase after stent implantation. Also, the studies suggested that the monocyte accumulation in the stenting site correlates with VSMC proliferation and neointimal growth. 16 Thus, the aim of this study was to investigate the Vtn and PLAU gene expression levels in peripheral blood mononuclear cell (PBMC) samples isolated from patients with the ISR. It may explain the roles of these genes in the VSMC activation.
Methods
Subjects
A total of 66 volunteers undergoing coronary artery angiography participated in the study. All samples were randomly selected from Shahid Rajaee Hospital, Tehran (2015–2016). The subjects were categorized into three groups: 22 healthy subjects (stenosis < 5%) and 44 patients with coronary artery stent implantation (stent no-restenosis [SNR], n = 22; stenosis < 70% with ISR [ISR], n = 22; restenosis > 70%). A medical interview was considered to have no clinical problems (metabolic diseases, myocardial infarction, and stroke). The University Ethics Committee has approved the study, and an informed consent was obtained from all participants.
Sample
The whole-blood samples (10 mL) were collected in ethylenediaminetetraacetic acid vacationers and were transferred into the laboratory using special bags containing cold ice packs.
Peripheral Blood Mononuclear Cell Isolation
The blood sample was diluted with phosphate buffered saline (PBS; 1:1 ratio) and was added into Ficoll solution (3 mL; Sigma-Aldrich). Then, it was gently mixed and was centrifuged for 30 minutes at 400 × g. The PBMC layer was separated from the other layers consisting of red blood cells, granulocytes, and plasma. Afterward, it was washed and centrifuged with PBS (three times, each time for 10 minutes at 200 × g).
Ribonucleic Acid Extraction
Total ribonucleic acid (RNA) was prepared from the PBMC sample (RNA extraction kit, GeneMark, Georgia Institute of Technology, Atlanta, GA) according to the manufacturer's instruction. The RNA concentration was calculated by NanoDrop. The RNA quantity and quality were estimated by OD 260 /OD 280 ratio and gel agarose electrophoresis (2%).
Complementary Deoxyribonucleic Acid Synthesis
Complementary deoxyribonucleic acid (cDNA) was synthesized with the cDNA Synthesis kit (Prime Script II strand cDNA Synthesis Kit, Takara, Japan) according to the manufacturer's instructions.
Real-Time Quantitative Polymerase Chain Reaction Technique
The Vtn and PLAU gene expression levels were measured by SYBR Green Real-Time qPCR technique (RG-6000 Rotor-Gene, Corbett Research, Sydney, Australia) and were normalized with the actin-β gene. The amplification reaction was performed in a volume (10 μL) containing forward and reverse primers (0.5 μm) and cDNA sample (1 μL). The amplification cycles ( n = 40) were performed at 95°C for 10 seconds and at 59°C for 30 seconds. The primers were designed with the Primer-blast tool ( Table 1 ).
Table 1. Gene primers.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Actin-β | TCCCTGGAGAAGAGCTACG | GTAGTTTCGTGGATGCCACA |
| PLAU | TTGCTCACCACAACGACATT | ATTTTCAGCTGCTCCGGATA |
| Vtn | CCAGACAGCCCCAGTTCATT | GGTGCCATGCCTGAGATGTA |
Abbreviations: PLAU, urokinase-type plasminogen activator; Vtn, vitronectin.
Statistical Analysis
The data were collected using a questionnaire. Statistical analyses were performed using statistical software package (SPSS, version 18.0, SPSS Inc., Chicago, IL). The parametric data were reported as mean ± standard deviation and were evaluated with Student's t -test and analysis of variance test. The nonparametric results were evaluated and compared with Kolmogorov–Smirnov and Kruskal–Wallis tests, respectively. Quality results between the groups were statistically evaluated using chi-square test. p -Value less than 0.05 was considered significant. The gene expression levels were evaluated with 2 −ΔΔct formula.
Results
Study Population
Table 2 shows some demographic and biochemical parameters in the study groups. The lipid profile was not significantly difference between the groups. Furthermore, the groups were controlled on age ( p = 0.205) and gender ( p = 0.751) parameters.
Table 2. Demographic and biochemical parameters in the study population.
| Variable | Control Mean ± SD, % ( n ) |
Stent no-restenosis Mean ± SD, % ( n ) |
In-stent restenosis Mean ± SD, % ( n ) |
p -Value | |
|---|---|---|---|---|---|
| Age (y) | 55.18 ± 12.022 | 60.64 ± 10.144 | 59.36 ± 9.105 | 0.205 | |
| Gender | Male | 63.6% (14) | 72.7% (16) | 72.7% (16) | 0.751 |
| Female | 36.4% (8) | 27.3% (6) | 27.3% (6) | ||
| BMI (kg/m 2 ) | 27.166 ± 4.360 | 27.166 ± 4.42 | 28.38 ± 4.437 | 0.574 | |
| HDL-C (mg/dL) | 42.55 ± 8.101 | 40.98 ± 6.919 | 41.73 ± 6.076 | 0.174 | |
| LDL-C (mg/dL) | 75.15 ± 37.697 | 83.43 ± 28.003 | 63.79 ± 16.525 | 0.108 | |
| TC (mg/dL) | 147.90 ± 46.243 | 156.73 ± 38.388 | 127.68 ± 28.800 | 0.059 | |
| LDL-C/HDL-C | 1.8287 ± 0.93977 | 2.0476 ± 0.65657 | 1.6795 ± 0.38813 | 0.256 | |
| HDL-C/cholesterol | 0.3076 ± 0.09092 | 0.2693 ± 0.05486 | 0.3091 ± 0.06174 | 0.125 | |
| TG (mg/dL) | 120.85 ± 57.793 | 157.27 ± 128.376 | 133.58 ± 81.471 | 0.458 | |
| FBS (mg/dL) | 114.19 ± 33.051 | 113.05 ± 35.170 | 127.61 ± 40.873 | 0.396 | |
| WBC (mg/dL) | 6,885.71 ± 1,673 | 7,365 ± 2,026 | 6,831.58 ± 1,634 | 0.587 | |
| CPK-MB (mg/dL) | 10.96 ± 6.711 | 14.90 ± 12.185 | 16.35 ± 5.683 | 0.295 | |
Abbreviations: BMI, body mass index; CPK-MB, creatinine phosphokinase-myocardial band; FBS, fasting blood sugar; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SD, standard deviation; TC, total cholesterol; TG, triglycerides; WBC, white blood cells.
Urokinase-Type Plasminogen Activator Gene Expression
The PLAU gene expression level increased in the patients with coronary artery stent implantation. Fig. 1 shows that the PLAU gene expression elevates up to 27-folds in patients with coronary ISR ( p < 0001). Furthermore, the linear and multiple regression analyses showed that the PLAU gene expression levels are not correlated to lipid profile ( Table 3 ) and age/gender parameters ( Table 4 ).
Fig. 1.
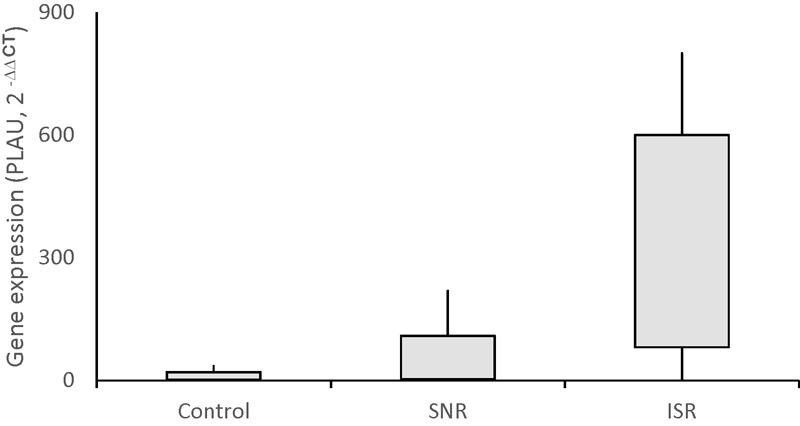
Urokinase-type plasminogen activator gene expression levels in study groups. Control (stenosis < 5%), stent no-restenosis (SNR) < 70%, and in-stent restenosis (ISR) >70%. ISR versus control and SNR; p < 0.0001.
Table 3. Linear regression analyses for PLAU gene regression level.
| Parameter | r -Value | p -Value |
|---|---|---|
| HDL-C/cholesterol | –0.003 | 0.97 |
| LDL-C/cholesterol | 0.022 | 0.86 |
| LDL-C/HDL-C | –0.009 | 0.94 |
| CPK-MB (mg/dL) | 0.323 | 0.067 |
| WBC (count/L) | –0.035 | 0.78 |
| FBS (mg/dL) | 0.033 | 0.80 |
| Hb (mg/dL) | 0.009 | 0.94 |
| TG (mg/dL) | 0.037 | 0.77 |
| HDL-C (mg/dL) | 0.034 | 0.79 |
| Cholesterol (mg/dL) | 0.000 | 0.99 |
| VLDL-C (mg/dL) | –0.017 | 0.90 |
| LDL-C (mg/dL) | 0.006 | 0.96 |
| Age (y) | –0.008 | 0.94 |
Abbreviations: CPK-MB, creatinine phosphokinase-myocardial band; FBS, fasting blood sugar; Hb, hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PLAU, urokinase-type plasminogen activator; TG, triglycerides; VLDL-C, very-low-density lipoprotein cholesterol; WBC, white blood cells.
Table 4. Multiple regression analysis for PLAU.
| Variable | β | SE | t | p- Value |
|---|---|---|---|---|
| Age (y) | –0.007 | 0.206 | –0.049 | 0.96 |
| Gender | 0.285 | 0.831 | 1.077 | 0.133 |
Abbreviation: PLAU, urokinase-type plasminogen activator.
Vitronectin Gene Expression
The Vtn gene expression level increased up to 17-folds in the patients with coronary ISR ( p < 001) ( Fig. 2 ). However, the Vtn gene expression was not correlated to lipid profile ( Table 5 ). Also, the multiple regression analysis did not show an association between Vtn gene expression levels and age/gender parameters ( Table 6 ).
Fig. 2.
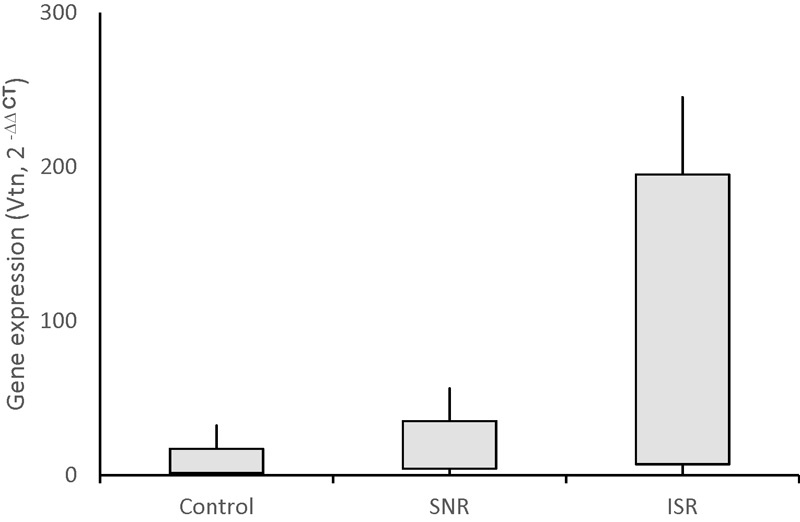
Vitronectin gene expression levels in study groups. Control (stenosis < 5%), stent no-restenosis (SNR) <70%, in-stent restenosis (ISR) >70%. ISR versus control and SNR; p < 0.001.
Table 5. Linear regression analyses for Vtn gene regression level.
| Parameter | r -Value | p- Value |
|---|---|---|
| HDL-C/cholesterol | 0.036 | 0.78 |
| LDL-C /cholesterol | –0.009 | 0.86 |
| LDL-C/HDL-C | –0.036 | 0.78 |
| CPK-MB (mg/dL) | 0.341 | 0.052 |
| WBC (count/L) | –0.028 | 0.83 |
| FBS (mg/dL) | 0.046 | 0.72 |
| Hb (mg/dL) | –0.012 | 0.92 |
| TG (mg/dL) | 0.037 | 0.75 |
| HDL-C (mg/dL) | 0.041 | 0.79 |
| Cholesterol (mg/dL) | –0.026 | 0.84 |
| VLDL-C (mg/dL) | –0.029 | 0.95 |
| LDL-C (mg/dL) | –0.029 | 0.82 |
| Age (y) | –0.21 | 0.89 |
Abbreviations: CPK-MB, creatinine phosphokinase-myocardial band; FBS, fasting blood sugar; Hb, hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides; VLDL-C, very-low-density lipoprotein cholesterol; Vtn, vitronectin; WBC, white blood cells.
Table 6. Multiple regression analysis for Vtn.
| Variable | β | SE | t | p -Value |
|---|---|---|---|---|
| Age (year) | –0.021 | 0.160 | –0.166 | 0.98 |
| Gender | 0.121 | 0.62 | 0.978 | 0. 33 |
Abbreviation: Vtn, vitronectin.
Discussion
The coronary artery restenosis, known as vessel renarrowing, is a serious complication in patients undergoing PTCA. 17 The neointimal hyperplasia is the main cause for vessel renarrowing events. It usually occurs due to the proliferation of VSMCs and their migration into vessel endothelium. 3 4 5 6 In this field, some studies reported a positive correlation between circulating monocytes and neointimal volume in the patients with ISR. It is proposed that some factors secreted from circulating monocytes may stimulate the VSMC proliferation and motility leading to neointimal hyperplasia. 18 19 In normal states, the studies reported that the activated monocytes secrete numerous factors such as cytokines, metalloproteinases, and some compounds to stimulate the VSMCs. A bibliographic search predicted the extracellular monocyte proteins ( n = 71) involved in VSMC signaling pathways. The study proposed that the cytokine–cytokine receptor interaction, focal adhesion, and regulation of actin cytoskeleton pathways may be related to VSMC proliferation and migration. 20 The PLAU is one these proteins to be related to coronary artery restenosis. Some studies showed that the PLAU–uPAR complex is involved in the proteolytic processes following the tissue remodeling and cellular migration. 21 Moreover, it may activate latent growth factors. 22 The studies also reported that the active PLAU catalyzes highly specific the plasminogen into plasmin so that it promotes extracellular matrix degradation and matrix metalloproteinase (MMP) activation. 23 Therefore, the PLAU overexpression is expected to develop the restenosis after coronary artery stent implantation due to the increase of PLAU and uPAR interactions and the improvement of proteolytic events within extracellular space.
On the other hand, uPAR may also interact with other proteins including Vtn. The RGD motif on Vtn is an interactive site to many adhesive macromolecules. 24 PAI-1 binds to the RGD region, thereby antagonizing the function of Vtn. Furthermore, the Vtn mediates the interaction of PLAU with integrins and promotes the cellular proliferation, migration, focal adhesion, and integrin-mediated pathways. 25 More evidences showed that the Vtn promotes the cellular migration by competing for the binding of PAI-1. Our results demonstrated the increase of the Vtn gene expression in the patients with ISR. These findings suggested that upon increasing of PLAU, Vtn, and uPAR interactions on the VSMC membrane, the restenosis process may be developed due to the VSMC migration into vessel endothelium in stenting site. 26 27 Based on the roles of Vtn and PLAU in VSMC motility, we suggested that the PBMCs may be a main source for the secretion of factors that may simulate VSMC proliferation and migration in patients with ISR.
Conclusion
The results suggested that the PBMCs may be a main source for the secretion of PLAU and Vtn. The findings also proposed that the increase of these genes may promote the restenosis process. However, a need for more research may be appropriate.
References
- 1.Yang Z K, Shen Y, Hu J et al. Impact of coronary collateral circulation on angiographic in-stent restenosis in patients with stable coronary artery disease and chronic total occlusion. Int J Cardiol. 2017;227:485–489. doi: 10.1016/j.ijcard.2016.10.117. [DOI] [PubMed] [Google Scholar]
- 2.Fischman D L, Leon M B, Baim D S et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. N Engl J Med. 1994;331(08):496–501. doi: 10.1056/NEJM199408253310802. [DOI] [PubMed] [Google Scholar]
- 3.Indolfi C, Pavia M, Angelillo I F. Drug-eluting stents versus bare metal stents in percutaneous coronary interventions (a meta-analysis) Am J Cardiol. 2005;95(10):1146–1152. doi: 10.1016/j.amjcard.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 4.Denes L, Entz L, Jancsik V. Restenosis and therapy. Int J Vasc Med. 2012;2012:406236. doi: 10.1155/2012/406236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serruys P W, de Jaegere P, Kiemeneij F et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. N Engl J Med. 1994;331(08):489–495. doi: 10.1056/NEJM199408253310801. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann R, Mintz G S. Coronary in-stent restenosis - predictors, treatment and prevention. Eur Heart J. 2000;21(21):1739–1749. doi: 10.1053/euhj.2000.2153. [DOI] [PubMed] [Google Scholar]
- 7.Hong Y J, Jeong M H, Ahn Y et al. Incidence, predictors, and clinical impact of tissue prolapse after stent implantation for saphenous vein graft disease: intravascular ultrasound study. Int J Cardiol. 2013;168(03):3073–3075. doi: 10.1016/j.ijcard.2013.04.090. [DOI] [PubMed] [Google Scholar]
- 8.Thorpe P E. Identification and treatment of restenosis in failing venous stents: the role of intravascular ultrasound. J Vasc Surg Venous Lymphat Disord. 2014;2(01):109–110. doi: 10.1016/j.jvsv.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y L, Liu L Z, He Z H, Ding K H, Xue F. Phenotypic transformation and migration of adventitial cells following angioplasty. Exp Ther Med. 2012;4(01):26–32. doi: 10.3892/etm.2012.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baldwin J F, Sood V, Elfline M A et al. The role of urokinase plasminogen activator and plasmin activator inhibitor-1 on vein wall remodeling in experimental deep vein thrombosis. J Vasc Surg. 2012;56(04):1089–1097. doi: 10.1016/j.jvs.2012.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schäfer K, Schroeter M R, Dellas C et al. Plasminogen activator inhibitor-1 from bone marrow-derived cells suppresses neointimal formation after vascular injury in mice. Arterioscler Thromb Vasc Biol. 2006;26(06):1254–1259. doi: 10.1161/01.ATV.0000215982.14003.b7. [DOI] [PubMed] [Google Scholar]
- 12.Ge J, Shen C, Liang C, Chen L, Qian J, Chen H. Elevated matrix metalloproteinase expression after stent implantation is associated with restenosis. Int J Cardiol. 2006;112(01):85–90. doi: 10.1016/j.ijcard.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Noorabad-Ghahroodi F, Abdi S, Zand A H, Najafi M. HGDB: a web retrieving cardiovascular-associated gene data. Int J Cardiol. 2017;232:117–120. doi: 10.1016/j.ijcard.2017.01.043. [DOI] [PubMed] [Google Scholar]
- 14.Schousboe S L, Egelund R, Kirkegaard T, Preissner K T, Rodenburg K W, Andreasen P A. Vitronectin and substitution of a beta-strand 5A lysine residue potentiate activity-neutralization of PA inhibitor-1 by monoclonal antibodies against alpha-helix F. Thromb Haemost. 2000;83(05):742–751. [PubMed] [Google Scholar]
- 15.Cao D J, Guo Y L, Colman R W. Urokinase-type plasminogen activator receptor is involved in mediating the apoptotic effect of cleaved high molecular weight kininogen in human endothelial cells. Circ Res. 2004;94(09):1227–1234. doi: 10.1161/01.RES.0000126567.75232.46. [DOI] [PubMed] [Google Scholar]
- 16.Egashira K, Zhao Q, Kataoka C et al. Importance of monocyte chemoattractant protein-1 pathway in neointimal hyperplasia after periarterial injury in mice and monkeys. Circ Res. 2002;90(11):1167–1172. doi: 10.1161/01.res.0000020561.03244.7e. [DOI] [PubMed] [Google Scholar]
- 17.Hirose S, Ashikaga T, Hatano Y et al. Treatment of in-stent restenosis with excimer laser coronary angioplasty: benefits over scoring balloon angioplasty alone. Lasers Med Sci. 2016;31(08):1691–1696. doi: 10.1007/s10103-016-2039-z. [DOI] [PubMed] [Google Scholar]
- 18.Fukuda D, Shimada K, Tanaka A, Kawarabayashi T, Yoshiyama M, Yoshikawa J. Circulating monocytes and in-stent neointima after coronary stent implantation. J Am Coll Cardiol. 2004;43(01):18–23. doi: 10.1016/j.jacc.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 19.Moreno P R, Bernardi V H, López-Cuéllar J et al. Macrophage infiltration predicts restenosis after coronary intervention in patients with unstable angina. Circulation. 1996;94(12):3098–3102. doi: 10.1161/01.cir.94.12.3098. [DOI] [PubMed] [Google Scholar]
- 20.Khosravi M, Hoseini-Fard S R, Najafi M.System study of vascular smooth muscle cell (VSMC) activation-related signaling pathways by monocyte and macrophage cellsCurr Signal Transduct Ther2017. 12, 10.2174/1574362412666161118173050
- 21.Fuhrman B. The urokinase system in the pathogenesis of atherosclerosis. Atherosclerosis. 2012;222(01):8–14. doi: 10.1016/j.atherosclerosis.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 22.Linton M F, Babaev V R, Huang J, Linton E F, Tao H, Yancey P G. Macrophage apoptosis and efferocytosis in the pathogenesis of atherosclerosis. Circ J. 2016;80(11):2259–2268. doi: 10.1253/circj.CJ-16-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown S, Meroueh S O, Fridman R, Mobashery S. Quest for selectivity in inhibition of matrix metalloproteinases. Curr Top Med Chem. 2004;4(12):1227–1238. doi: 10.2174/1568026043387854. [DOI] [PubMed] [Google Scholar]
- 24.De Lorenzi V, Sarra Ferraris G M, Madsen J B, Lupia M, Andreasen P A, Sidenius N. Urokinase links plasminogen activation and cell adhesion by cleavage of the RGD motif in vitronectin. EMBO Rep. 2016;17(07):982–998. doi: 10.15252/embr.201541681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hillig T, Engelholm L H, Ingvarsen S et al. A composite role of vitronectin and urokinase in the modulation of cell morphology upon expression of the urokinase receptor. J Biol Chem. 2008;283(22):15217–15223. doi: 10.1074/jbc.C700214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleetwood A J, Achuthan A, Schultz H et al. Urokinase plasminogen activator is a central regulator of macrophage three-dimensional invasion, matrix degradation, and adhesion. J Immunol. 2014;192(08):3540–3547. doi: 10.4049/jimmunol.1302864. [DOI] [PubMed] [Google Scholar]
- 27.Ekmekçi O B, Ekmekçi H.Vitronectin in atherosclerotic disease Clin Chim Acta 2006368(1-2):77–83. [DOI] [PubMed] [Google Scholar]


