Abstract
Depletion of intracellular calcium stores activates store-operated calcium entry across the plasma membrane in many cells. STIM1, the putative calcium sensor in the endoplasmic reticulum, and the calcium release-activated calcium (CRAC) modulator CRACM1 (also known as Orai1) in the plasma membrane have recently been shown to be essential for controlling the store-operated CRAC current (ICRAC)1–4. However, individual overexpression of either protein fails to significantly amplify ICRAC. Here, we show that STIM1 and CRACM1 interact functionally. Overexpression of both proteins greatly potentiates ICRAC, suggesting that STIM1 and CRACM1 mutually limit store-operated currents and that CRACM1 may be the long-sought CRAC channel.
Receptor-mediated release of Ca2+ from intracellular stores induces Ca2+ entry through calcium release-activated calcium (CRAC) channels5–7. Previous studies have identified STIM1 as the potential sensor for endoplasmic reticulum luminal Ca2+ concentration1,8,9. When Ca2+ is depleted from intracellular stores, STIM1 translocates to vesicular structures (punctae) underneath the plasma membrane, where it is hypothesized to activate CRAC channels residing in the plasma membrane. A second protein, CRACM1, has recently been identified as essential for activating CRAC channels3,4. This protein contains four transmembrane domains, is located in the plasma membrane and, therefore, may represent the CRAC channel itself, a subunit of the channel, or a regulatory molecule that couples to the channel. When overexpressed individually, neither STIM1 nor CRACM1 can significantly potentiate ICRAC1–4.
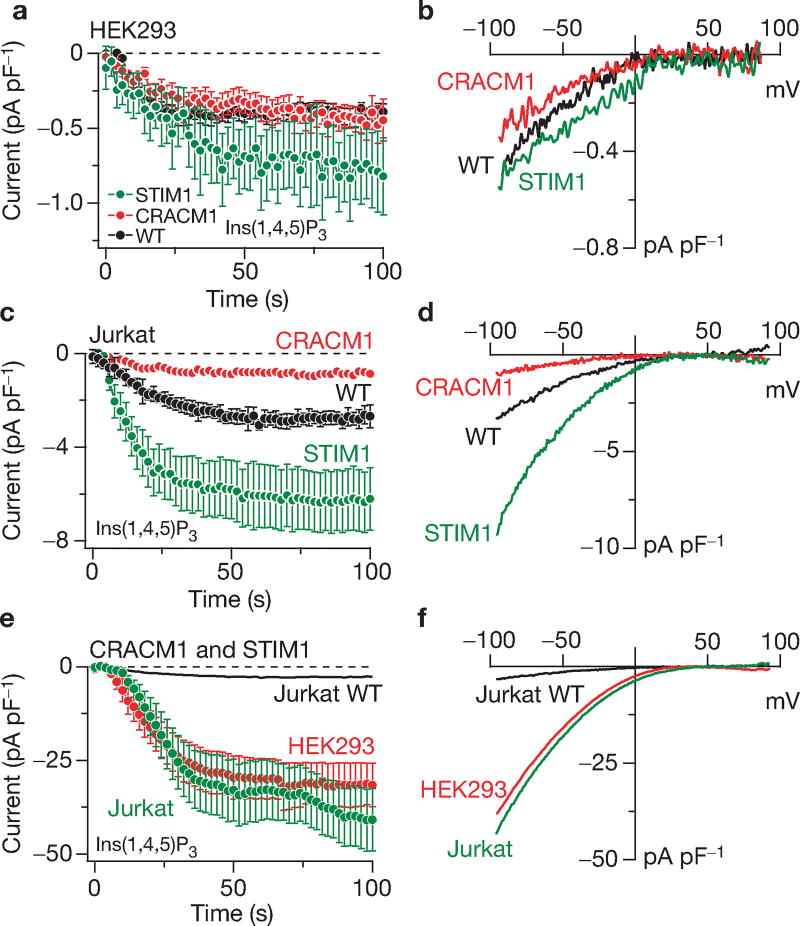
To address the potential interaction of STIM1 and CRACM1, both proteins were overexpressed individually, or in combination, in HEK293 and Jurkat T cells and the CRAC currents were measured in response to Ca2+ store depletion by 20 µM inositol 1,4,5-trisphosphate (Ins(1,4,5)P3). Both cell types normally exhibit native CRAC currents of approximately 0.5 pA pF−1 and approximately 3 pA pF−1, respectively4 (Fig. 1a, c), with typical inwardly rectifying current-voltage (I/V) relationships5,10,11 (Fig. 1b, d). Consistent with previous work1,9, overexpression of STIM1 alone caused a small-to-modest increase in ICRAC in HEK293 and Jurkat cells (Fig. 1a, c). CRACM1 overexpression alone did not affect the CRAC currents induced by store depletion in HEK293 cells (Fig. 1a, b) and caused a small reduction in ICRAC in Jurkat cells (Fig. 1c, d). Unless simply due to a general effect of transfection or variability of ICRAC across preparations, this reduction may be due to some kind of dominant–negative effect. Taken together, the available data on CRACM1 and STIM1 suggest that the individually expressed proteins, although essential for ICRAC manifestation, cannot significantly amplify the current. This would indicate that these proteins are either not sufficient to generate large CRAC currents or that they are stoichiometrically linked and limit each others’ ability to generate CRAC currents above normal. Therefore, we co-overexpressed both proteins in HEK293 cells (see Supplementary Information, Fig. S1) and assessed store-operated currents by patch clamp.
Figure 1.
Individual and combined overexpression of STIM1 and CRACM1. (a) Normalized average time course of Ins(1,4,5)P3-induced (20 µM) ICRAC in HEK293 cells. Currents of individual cells were measured at −80 mV, normalized by their respective cell size, averaged and plotted versus time (± s.e.m.). Cytosolic calcium was clamped to near zero with 10 mM BAPTA. Traces represent native ICRAC in wild-type cells (WT, black circles: n = 10), cells transfected with CRACM1 + GFP (red circles; n = 28) or STIM1 + GFP expressing cells (green circles; n = 13). (b) Average current–voltage (I/V) relationships of ICRAC extracted from representative HEK293 cells at 60 s, representing leak-subtracted currents evoked by 50 ms voltage ramps from −100 to +100 mV, normalized to cell size (pF). Traces represent native ICRAC in wild-type cells (n = 6), cells transfected with CRACM1 + GFP (n = 13) and STIM1 + GFP expressing cells (n = 5). (c) Normalized average time course of Ins(1,4,5)P3-induced (20 µM) ICRAC in Jurkat cells. Currents were analysed as in a (n = 21 for control; n = 11 for CRACM1; n = 12 for STIM1). (d) Averaged I/V traces of ICRAC extracted from representative Jurkat cells at 60 s. Analysis as in b (n = 19 for wild type; n = 7 for CRACM1; n = 12 for STIM1). (e) Normalized average time course of ICRAC in HEK293 or Jurkat cells expressing STIM1 + CRACM1. Analysis as in a (n = 14 for HEK293; n = 17 for Jurkat cells). The time course of ICRAC in wild-type Jurkat cells is included for comparison (same data as in c). (f) Average current–voltage (I/V) data traces of ICRAC extracted from representative HEK293 (red) or Jurkat cells (green) expressing STIM1 + CRACM1. Analysis as in b (n = 14 for HEK293; n = 17 for Jurkat cells). The Jurkat wild-type data trace is plotted for comparison (same data as in d).
The co-overexpression of STIM1 and CRACM1, in both HEK293 and Jurkat cells, is sufficient to generate enormous membrane currents of approximately 30 pA pF−1 on store depletion by Ins(1,4,5)P3 (Fig. 1e). These currents are significantly larger than the corresponding native currents evoked by the same experimental protocol and amount to an approximately 60-fold increase in HEK293 cells and approximately tenfold in Jurkat cells. The currents exhibit a similar time course of activation (Fig. 1e) and the same inwardly rectifying I/V relationship (Fig. 1f), as the well-characterized CRAC current5,10. It should be noted that the average current presented in the graph represents an underestimate, as it excludes cells in which ICRAC increased to well above 50–100 pA pF−1. In these cells, the massive Ca2+ influx presumably saturated the intracellular Ca2+ chelator BAPTA (1,20bis(o-aminophenoxy) ethane-N,N,N',N'-tetraacetic acid), causing the current to inactivate5,10 (see Supplementary Information, Fig. S2).
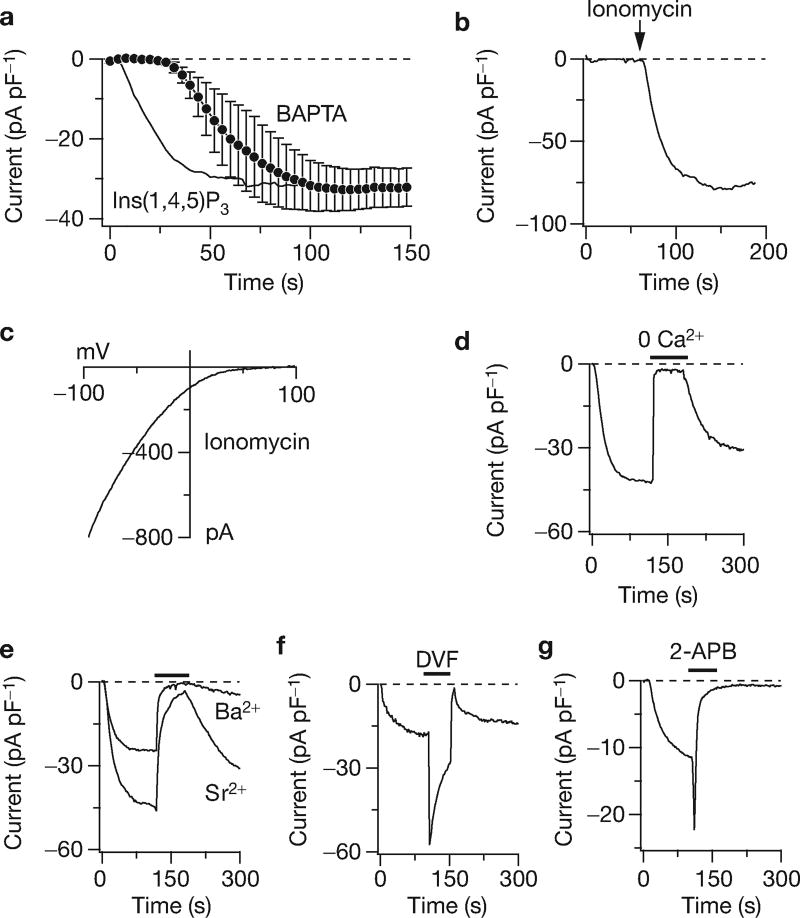
To further characterize these currents, some specific properties that are considered the hallmarks of native CRAC currents were assessed. We established that the current could be activated by other stimuli that cause store depletion. Passive store depletion by 10 mM BAPTA, in the absence of Ins(1,4,5)P3, also recruited CRAC-like currents (Fig. 2a). These developed after a characteristic delay that is likely to represent the time required for Ca2+ to leak out of the stores5. The cells were next perfused with a solution in which the Ca2+ concentration was buffered to approximately 150 nM to avoid store depletion and this did prevent current activation (Fig. 2b). Subsequent application of ionomycin to release Ca2+ from intracellular stores, however, rapidly activated large currents that exhibited the same rectifying I/V as ICRAC (Fig. 2c).
Figure 2.
Co-expression of CRACM1 and STIM1 produces a current with the characteristic features of ICRAC. (a) Normalized average time course of ICRAC in STIM1 + CRACM1 expressing HEK293 cells (black circles; n = 3). Currents of individual cells were measured at −80 mV, normalized by cell size (pF), averaged and plotted versus time (± s.e.m.). Passive store-depletion was induced by clamping cytosolic calcium to near zero using 10 mM BAPTA. For comparison, the solid line reproduces the Ins(1,4,5)P3-induced data shown in Fig. 1e. (b) Time course of a representative HEK293 cell expressing STIM1 + CRACM1 (n = 3), where ICRAC was induced by application of 2 µM ionomycin for 3 s, as indicated by the arrow. Intracellular calcium was clamped to 150 nM with 10 mM BAPTA and 4 mM CaCl2 to prevent passive store-depletion before treatment with ionomycin. (c) I/V data trace of ICRAC extracted from the same cell as in b at 110 s. The voltage protocol was as in Fig. 1b. (d) Representative time course of ICRAC evoked in a HEK293 cell expressing STIM1 + CRACM1 by 20 µM Ins(1,4,5)P3. Extracellular calcium was removed as indicated by the black bar (average inhibition at the end of application = 94% ± 1%, n = 15). (e) Representative HEK293 cell expressing STIM1 + CRACM1 where 10 mM extracellular Ca2+ was replaced with equimolar Ba2+ or Sr2+ during the time indicated by the bar (average inhibition by Ba2+ = 95% ± 1%, n = 14; and by Sr2+ = 81% ± 2%, n = 16). (f) Representative HEK293 cell expressing STIM1 + CRACM1 superfused with a divalent-free (DVF) external solution (n = 3). (g) Application of 50 µM 2-APB initially facilitates and then inhibits Ins(1,4,5)P3-induced ICRAC in a representative HEK293 cell expressing STIM1 + CRACM1 (n = 5).
The current produced by STIM1–CRACM1 overexpression was as specific for Ca2+ ions as ICRAC5,10. Removal of extracellular Ca2+ (nominally Ca2+-free), while retaining normal levels of Mg2+, inhibited the inward currents evoked by store depletion (Fig. 2d). Another specific characteristic of ICRAC is that it can carry Ba2+ and Sr2+ currents, albeit at smaller levels than Ca2+ (refs 5,11,12). Ion substitution experiments in which 10 mM extracellular Ca2+ was replaced by equimolar amounts of Ba2+ and Sr2+ resulted in a strong inhibition of the current and very limited steady-state permeation of these ions compared with Ca2+ (Fig. 2e). ICRAC can also carry monovalent cations, such as Na+, when removing all divalent ions from the extracellular solution and this transiently increases inward currents10. The large currents in STIM1–CRACM1 overexpressing cells showed the same behaviour (Fig. 2f). Whether or not the current exhibited the pharmacological profile of ICRAC, which is known to be enhanced by low concentrations of 2-aminoethoxydiphenylborate (2-APB) and inhibited at higher doses13,14, was also assessed. Treatment with 50 µM 2-APB, after an initial increase as 2-APB concentration built up, completely blocked the current. Although these properties of the large CRAC currents are generally in excellent agreement with those of native ICRAC, it is noteworthy that Sr2+ and Ba2+ currents seem smaller than those described for native ICRAC (refs 5,11,12). This may reflect a genuine, small Ba2+- and Sr2+-permeability of the CRAC channels in STIM1–CRACM1 overexpressing cells, and/or compromised channel function, as a consequence of the removal of extracellular Ca2+ (ref. 15). This difference in divalent permeation may also hint at the possibility of species differences, or that additional proteins — possibly other members of the STIM or CRACM families — may participate in shaping native CRAC currents.
In summary, our data establish that the co-overexpression of STIM1 and CRACM1 greatly amplifies store-operated currents and that these currents possess most of the defining characteristics of ICRAC. This suggests that STIM1 and CRACM1 are entirely sufficient to control the magnitude of the CRAC current. As individual overexpression of either protein fails to augment ICRAC, we conclude that they mutually represent limiting factors for CRAC current manifestation, although in Jurkat cells there may be a surplus of CRACM1 compared with STIM1, as STIM1 overexpression can enhance ICRAC approximately twofold (Fig. 1c). Although more complex interpretations are conceivable, the most parsimonious interpretation of the fact that additional CRACM1 overexpression can amplify ICRAC 10–60-fold (and in some cells well above 100-fold) would be that CRACM1 itself constitutes the CRAC channel.
Supplementary Material
Acknowledgments
We thank M. Bellinger for help with cell culture. This work was supported, in part, by National Institutes of Health (NIH) grants 5-R37-GM053950 (to J.P.K.), R01-AI050200 and R01-NS040927 (to R.P.) and R01-GM065360 (to A.F.).
Footnotes
Note: Supplementary Information (including Methods) is available on the Nature Cell Biology website.
COMPETING FINANCIAL INTERESTS
J.P.K., R.P., A.F. and M.N. are consultants to Synta Pharmaceuticals Corp. (Lexington, MA). J.P.K. and R.P. are members of the scientific advisory board of Synta Pharmaceuticals Corp.
References
- 1.Roos J, et al. J. Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang SL, et al. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feske S, et al. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 4.Vig M, et al. Science. 2006 doi: 10.1126/science.1127883. [DOI] [Google Scholar]
- 5.Hoth M, Penner R. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 6.Parekh AB, Penner R. Physiol. Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 7.Parekh AB, Putney JW., Jr Physiol. Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 8.Liou J, et al. Curr. Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spassova MA, et al. Proc. Natl Acad. Sci. USA. 2006;103:4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoth M, Penner R. J. Physiol. 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zweifach A, Lewis RS. Proc. Natl Acad. Sci. USA. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoth M. Pflugers Arch. 1995;430:315–322. doi: 10.1007/BF00373905. [DOI] [PubMed] [Google Scholar]
- 13.Prakriya M, Lewis RS. J. Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermosura MC, Monteilh-Zoller MK, Scharenberg AM, Penner R, Fleig A. J. Physiol. 2002;539:445–458. doi: 10.1113/jphysiol.2001.013361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zweifach A, Lewis RS. J. Gen. Physiol. 1996;107:597–610. doi: 10.1085/jgp.107.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.