Abstract
A germline JAK2V617I point mutation results in hereditary thrombocytosis and shares some phenotypic features with myeloproliferative neoplasm, a hematologic malignancy associated with a somatically acquired JAK2V617F mutation. We established a mouse transduction-transplantation model of JAK2V617I that recapitulated the phenotype of humans with germline JAK2V617I. We directly compared the phenotype of JAK2V617I mice with JAK2V617F mice. The JAK2V617I mice displayed increased marrow cellularity with expanded myeloid progenitor and megakaryocyte populations but this phenotype was less severe than in JAK2V617F mice. JAK2V617I resulted in cytokine hyper-responsiveness without constitutive activation in the absence of ligand whereas JAK2V617F resulted in constitutive activation. This may explain why JAK2V617I produces a mild myeloproliferative phenotype in the mouse model as well as in humans with germline JAK2V617I mutations.
INTRODUCTION
A single gain-of-function somatic point mutation in the Janus kinase 2 (JAK2) gene is present in the majority of patients with Philadelphia-negative myeloproliferative neoplasm (MPN)1–5. JAK2 is a cytoplasmic tyrosine kinase which is critical in intracellular signaling by cytokine receptors such as erythropoietin (Epo), thrombopoietin (Tpo), interleukin-3 (IL-3), granulocyte colony stimulating factor (G-CSF) and granulocyte macrophage colony stimulating factor (GM-CSF). The JAK2V617F mutation results in a constitutively active JAK2, with continual activation of downstream intracellular signaling cascades6–8. Although there is a familial predisposition to acquire MPN9,10 the JAK2V617F mutation is somatically acquired in a hematopoietic stem cell. However, a germline JAK2V617I mutation has recently been identified in a family with hereditary thrombocytosis11,12. The ability of JAK2V617I to confer cytokine independence had previously been shown in Ba/F3 cells randomly mutated at position 617 of JAK213. Like acquired MPN, family members with germline JAK2V617I have thrombocytosis and megakaryocytic hyperplasia in the marrow with increased risk of thrombosis. But unlike acquired MPN, individuals with the JAK2V617I germline mutation do not develop a fibrotic bone marrow, splenomegaly, or transform to acute leukemia. Why germline JAK2V617I recapitulates some aspects but not others of the MPN phenotype in humans is unclear. To delineate the differences between JAK2V617F and JAK2V617I we compared the phenotype of mice with hematopoietic cells expressing JAK2V617F or JAK2V617I.
METHODS
Bone Marrow Transplantation
C57B/6 mice were purchased from Jackson Labs. Retroviral infection and transplantation was performed as previously described14. All mouse work was performed with approval from the Oregon Health & Science University and UC Irvine Institutional Animal Care and Use Committee.
Standard flow cytometry
The following antibodies were used for identification of mature cell populations: CD41 PE (MWReg30, BD Biosciences), CD42d (1C2, Biolegend) CD11b APC (M1/70, BD Biosciences), Gr-1 PerCPCy5.5 (RB6-8C5, ebioscience), TER119 APC (TER119, BioLegend). For hematopoietic progenitor populations the following antibodies were used: APC lineage (Lin) markers (CD3, KT31.1; CD4, GK1.5; CD8, 53–6.7; B220, 6B2; Mac-1, M1/70; Gr-1, 8C5; and TER119, all from BD Biosciences), c-kit APC-Cy7 (2B8, BD Biosciences), CD34 PE (RAM34, BD Biosciences), CD16/32 PE-Cy7 (2.4G2, BD Biosciences), and Sca-1 Pacific Blue (D7, BioLegend). LKS, CMP, GMP, and MEP are defined as in15. Cells were analyzed using an Aria III flow cytometer (BD Biosciences). Data was analyzed using FlowJo software (Treestar).
Phosflow
Cells were stimulated with 0.1, 0.2, or 1 ng/ml of GM-CSF for 15 min at 37°C. Following stimulation, cells were fixed with paraformaldehyde and permeabilized in methanol. Samples were stained with PE- pSTAT3 (pY694) and A647- pSTAT5 (pY701) (BD Biosciences) along with cell surface markers. Cytobank.org was used to analyze data.
Methylcellulose Colony Formation Assays
For Figure 2D 1×106 spleen cells were plated in 1.1ml methylcellulose semi-solid media (M3231, StemCell Technologies) supplemented with 100ng/ml mSCF, 10ng/ml mIL-3 (peprotech) and 3U/ml hEpo (Procrit, Amgen) in triplicate. Colonies were enumerated after 12 days in culture. For Figure 2F GFP+ progenitors (linneg, c-kit+, Sca-1−) were sorted by flow cytometry and plated at a concentration of 1000 cells per 1.1 ml of methylcellulose in triplicate (M3231, StemCell Technologies) supplemented with 100ng/ml mSCF, 10ng/ml mIL-3, 50ng/ml mTPO (peprotech), and 3U/ml hEPO. Plates were examined at 7 days of culture and scored by visual morphology. The morphology of cells in the individual colonies was confirmed by cytospin with Giemsa staining.
Figure 2. JAK2V617I expands myeloid progenitors and megakaryocytes.
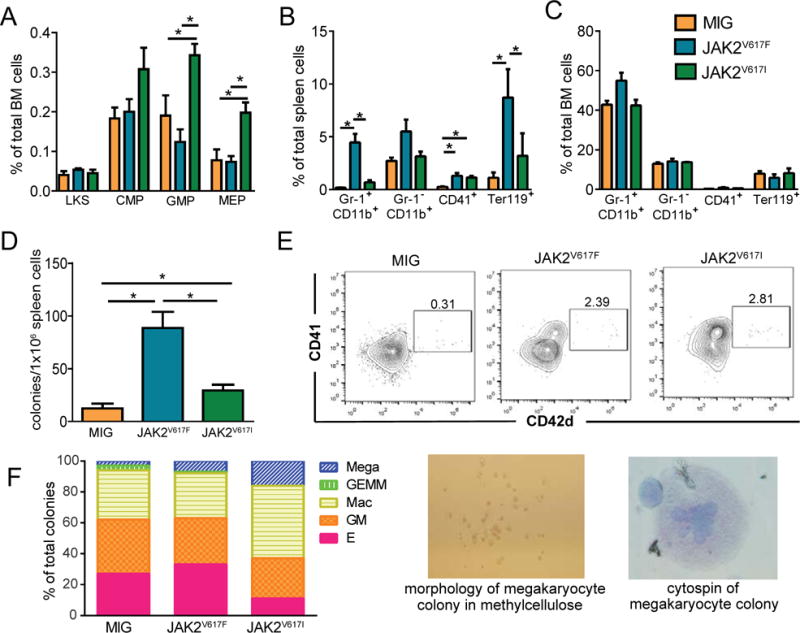
A. Frequency of hematopoietic stem (LKS), common myeloid progenitors (CMP), granulocyte monocyte progenitors (GMP) and megakaryocyte erythroid progenitors (MEP) in the bone marrow of each mouse measured by flow cytometry. B,C. Frequency of granulocytes (Gr-1+ CD11b+), monocytes (Gr-1− CD11b+), megakaryocytic lineage (CD41+) and erythroid lineage cells (Ter119+) in spleen (B) and bone marrow (C) measured by flow cytometry. D. Myeloid colony formation (CFU-GM and CFU-E combined) in methycellulose (M3231, StemCell Technologies) supplemented with mSCF, mIL-3 (peprotech) and hEpo (Procrit, Amgen). E. In vitro differentiation of megakaryocytes (CD41+ CD42d+) from LKS cells on OP9 stromal cell layers (+mTPO, mIL-11, mSCF). F. Relative frequency of myeloid colony types from JAK2V617F, JAK2V617I, and empty vector hematopoietic progenitors. Progenitors were plated in methylcellulose (M3231, StemCell Technologies) supplemented with mSCF, mIL-3, hEpo, and mTPO. After 7 days colonies were scored morphologically and enumerated. Photos of a representative megakaryocyte colony are shown to the right. All bar graphs represent mean (+/-SEM), *denotes p<0.05.
RESULTS AND DISCUSSION
JAK2V617I expression induces a mild MPN phenotype
Lethally irradiated C57B/6 mice were transplanted with equal numbers of bone marrow cells expressing JAK2V617F, JAK2V617I, or empty MSCV-IRES-GFP (MIG) vector16. As expected, JAK2V617F mice developed erythrocytosis and leukocytosis. However, JAK2V617I mice had peripheral blood counts similar to empty vector mice (Fig 1A–C). To rule out the possibility that the phenotypic differences we observed in JAK2V617F versus JAK2V617I mice was not simply due to lower expression of JAK2 in JAK2V617I mice, we confirmed equivalent expression of JAK2 in JAK2V617F and JAK2V617I mice by quantitative RT-PCR (data not shown) and Western Blot (Supplemental Figure 1). The lack of thrombocytosis in the JAK2V617I mouse model is not unexpected, as thrombocytosis is not commonly observed in JAK2V617F MPN mouse models. Therefore, we performed a more thorough assessment of the effect of JAK2V617I on hematopoietic progenitors and myeloid cells including megakaryocytes at time of sacrifice.
Figure 1. JAK2V617I causes a mild MPN phenotype.
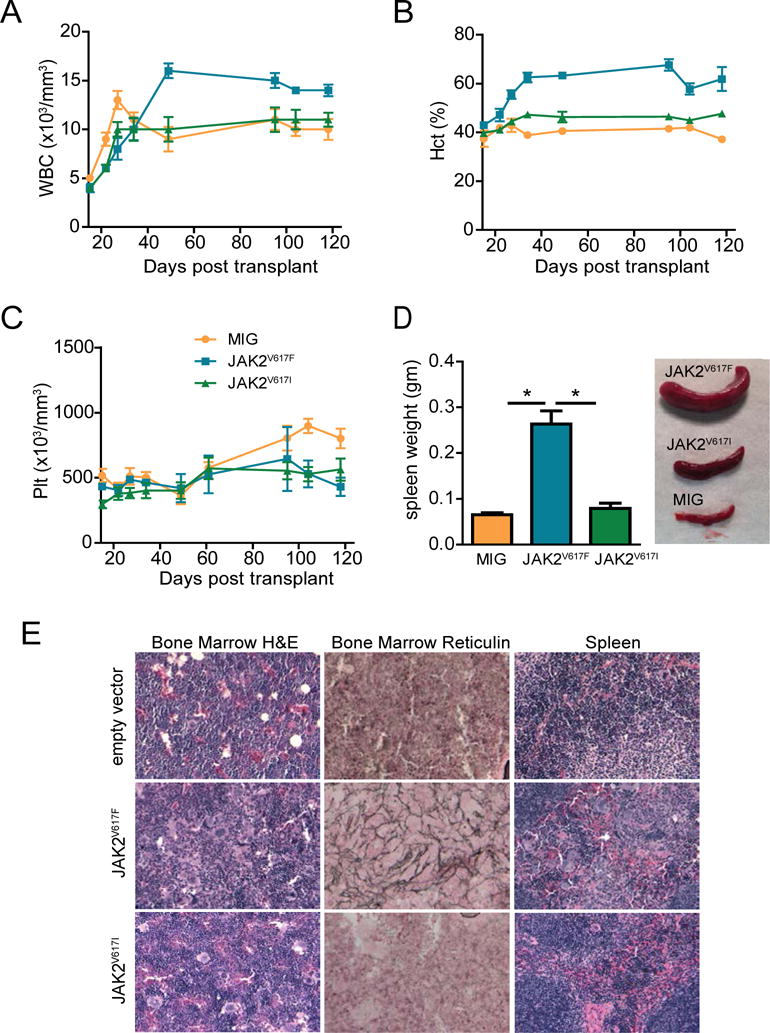
A–C. Peripheral blood was drawn weekly from MIG empty vector, JAK2V617I, and JAK2V617F mice. WBC (A), HCT (B) and platelets (C) were measured using a VetABC hematology analyzer (scil). D. Spleen weight and a representative photo of MIG empty vector, JAK2V617I, and JAK2V617F mice. E. Bone marrow and spleen from each mouse was evaluated by a blinded pathologist. Representative H&E sections of bone marrow, spleen and reticulin stain of bone marrow are shown (20X magnification shown, Leica DC300 camera running IM50 Image Manager software).
Mice were euthanized at 120 days post-transplant to fully assess the MPN phenotype. Spleen weight of JAK2V617I mice was not statistically different than empty vector mice (Figure 1D), however we found a positive correlation between spleen weight and percentage of GFP-positive cells in the spleen (Supplemental Figure 2). As expected, spleen weight was increased in JAK2V617F mice (Figure 1D). On histologic inspection JAK2V617I and JAK2V617F mice had hypercellular marrows with increased numbers of megakaryocytes. Mild fibrosis was seen in JAK2V617I mice, severe reticulin fibrosis was seen in JAK2V617F mice. The splenic architecture was preserved in JAK2V617I mice, whereas in JAK2V617F mice the splenic architecture was disrupted by invasion of myeloid cells including megakaryocytes (Figure 1E). These data demonstrate that ectopic expression of JAK2V617I in hematopoietic cells induces histologic evidence of MPN but with a milder phenotype as compared to JAK2V617F.
JAK2V617I expands myeloid progenitors and megakaryocytes and mobilizes myeloid progenitors to the spleen
To identify whether JAK2V617I affects the frequency of hematopoietic stem and progenitor cells or expands mature myeloid lineage cells we compared these populations in JAK2V617F, JAK2V617I, and MIG empty vector mice (Figure 2A–C). The bone marrow of JAK2V617I mice had expanded GMP and MEP populations as compared to MIG empty vector and JAK2V617F mice (Figure 2A). Mature granulocyte (Gr-1+CD11b+) and erythroid (Ter119+) populations were expanded in JAK2V617F but not JAK2V617I mice. To determine whether JAK2V617I had mobilized myeloid progenitors to the spleen we compared myeloid colony formation ability from harvested spleen cells of MIG, JAK2V617F, and JAK2V617I mice. We found increased myeloid progenitor activity in the spleens from JAK2V617I and JAK2V617F mice as compared to MIG empty vector (Figure 2D).
Both JAK2V617I and JAK2V617F mice had an increased fraction of megakaryocytes (CD41+) in the spleen as compared to empty vector mice (Figure 2B). To evaluate megakaryopoiesis in more detail we sorted lineageneg, c-kit+, Sca-1+ (LKS) cells ectopically expressing JAK2V617I, JAK2V617F or MIG empty vector onto OP9 stromal cell layers (100 cells/well) in the presence of mouse thrombopoietin (mTPO) (10ng/ml), Stem Cell Factor (mSCF) (50ng/ml), and Interleukin-11 (mIL-11) (10ng/ml) to induce development of megakaryocytes. After 5 days in culture cells were harvested and analyzed by flow cytometry to identify megakaryocytes. Wells seeded with JAK2V617I and JAK2V617F cells had an increased percentage of megakaryocytes (CD41+ CD42d+) as compared to wells seeded with empty vector cells (Figure 2E). We also compared the ability of JAK2V617I, JAK2V617F, and empty vector hematopoietic progenitors to form megakaryocyte colonies in methylcellulose and found a skewing toward megakaryocyte colonies in both JAK2V617I and JAK2V617F as compared empty vector (Figure 2F). Together, these data demonstrate that expression of JAK2V617I drives the expansion of myeloid progenitors and megakaryocytes despite the lack of overt leukocytosis or thrombocytosis in peripheral blood.
JAK2V617I results in cytokine hypersensitivity without constitutive activation
Humans with germline JAK2V617I mutations do not display constitutive activation of the kinase but they do demonstrate cytokine hyper-responsiveness as evidenced by increased phosphorylation of STATs at low concentrations of ligand11. We compared phosphorylated STAT5 in peripheral blood cells taken from JAK2V617I, JAK2V617F, and MIG empty vector mice following stimulation with increasing concentrations of GM-CSF. At all concentrations of GM-CSF tested JAK2V617I and JAK2V617F mice had exaggerated phosphorylation of STAT5 as compared to MIG empty vector mice (Figure 3A). We also measured phospho-STAT3 and STAT5 in unstimulated bone marrow and spleen from each mouse at time of sacrifice, there was no difference between JAK2V617I and MIG empty vector mice. JAK2V617F mice did demonstrate phosphorylation of STAT3 and STAT5 even in the absence of cytokine stimulation, confirming the ability of JAK2V617F but not JAK2V617I to constitutively activate downstream signaling pathways (Figure 3B).
Figure 3. JAK2V617I results in cytokine hypersensitivity without constitutive activation.
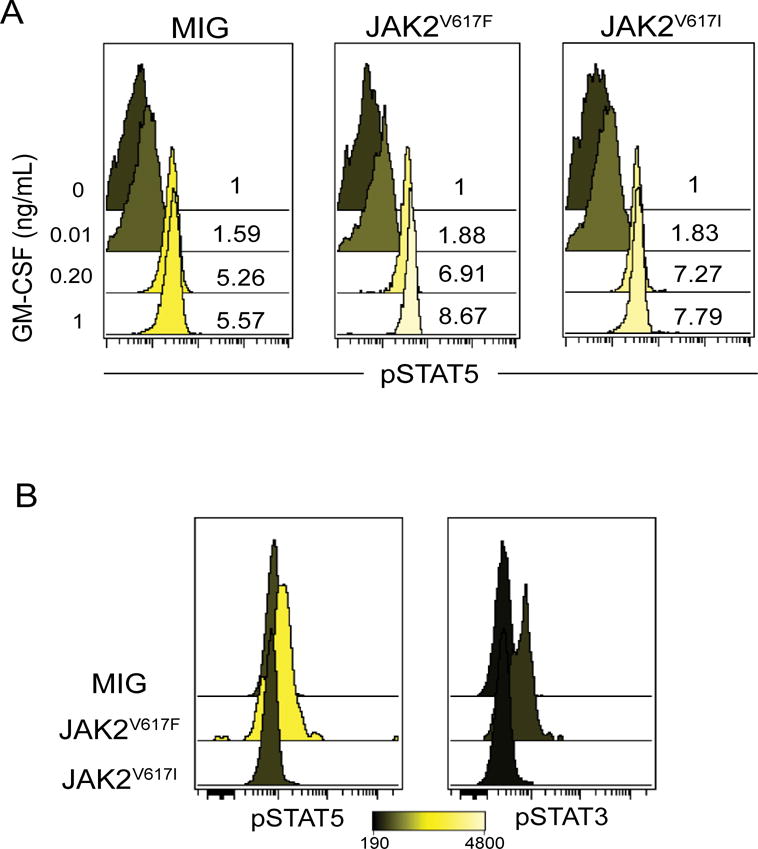
A. Peripheral blood from each group of mice was pooled and stimulated with increasing concentrations of mGM-CSF and then analyzed for phospho-STAT5 (pY694) by flow cytometry. Fold increase in phospho-STAT5 over unstimulated is shown as histogram overlay (gated on GFP+ cells). B. Unstimulated bone marrow from MIG, JAK2V617F and JAK2V617I mice were analyzed for levels of phospho-STAT5 (pY694) and phospho-STAT3 (pY705) using flow cytometry, histogram overlay represents (MFI) of a representative mouse from each group (gated on GFP+ cells).
The JAK2V617I mouse transduction-transplantation model has phenotypic features of acquired MPN such as expansion of megakaryocytes and mobilization of hematopoietic progenitors to the spleen but the phenotype is not as robust as JAK2V617F. It is possible that all cells in the hematopoietic system must express JAK2V617I, as is the case for a germline mutation, in order for JAK2V617I to make a clinically relevant impact in humans. Although the JAK2V617F mutation is somatically acquired in MPN familial clustering is well described7,10. Genome-wide analyses have revealed that JAK2V617F-positive MPN is strongly associated with a specific constitutional JAK2 haplotype (designated 46/1)17–19, suggesting that germline variation is an important contributor to MPN phenotype and predisposition.
Supplementary Material
Acknowledgments
AGF is supported by a Chao Comprehensive Cancer Center Seed Grant, an MPN Challenge Grant, V Foundation Scholar Award, and an American Cancer Society Seed Grant. AA is supported by a National Cancer Institute Career Development Award (4R00CA151670-03), Collins Foundation, Knight Pilot Project and Friends of Doernbecher grants. BJD is a Howard Hughes Medical Institute investigator.
Footnotes
AUTHOR CONTRIBUTIONS:
SAB, SJM, SBL, and AGF performed experiments, analyzed data, and wrote the manuscript. HYL, TKN, LRR and AA performed experiments, analyzed data, and edited the manuscript. BJD provided research guidance and edited the manuscript.
References
- 1.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005 Mar 19–25;365(9464):1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 2.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005 Apr 28;434(7037):1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 3.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. The New England journal of medicine. 2005 Apr 28;352(17):1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 4.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer cell. 2005 Apr;7(4):387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Zhao R, Xing S, Li Z, et al. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005 Jun 17;280(24):22788–22792. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skoda RC, Duek A, Grisouard J. Pathogenesis of myeloproliferative neoplasms. Experimental hematology. 2015 Aug;43(8):599–608. doi: 10.1016/j.exphem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Rumi E, Passamonti F, Della Porta MG, et al. Familial chronic myeloproliferative disorders: clinical phenotype and evidence of disease anticipation. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25(35):5630–5635. doi: 10.1200/JCO.2007.12.6896. [DOI] [PubMed] [Google Scholar]
- 8.Tapper W, Jones AV, Kralovics R, et al. Genetic variation at MECOM, TERT, JAK2 and HBS1L-MYB predisposes to myeloproliferative neoplasms. Nature communications. 2015;6:6691. doi: 10.1038/ncomms7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones AV, Cross NC. Inherited predisposition to myeloproliferative neoplasms. Therapeutic advances in hematology. 2013 Aug;4(4):237–253. doi: 10.1177/2040620713489144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landgren O, Goldin LR, Kristinsson SY, Helgadottir EA, Samuelsson J, Bjorkholm M. Increased risks of polycythemia vera, essential thrombocythemia, and myelofibrosis among 24,577 first-degree relatives of 11,039 patients with myeloproliferative neoplasms in Sweden. Blood. 2008 Sep 15;112(6):2199–2204. doi: 10.1182/blood-2008-03-143602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mead AJ, Rugless MJ, Jacobsen SE, Schuh A. Germline JAK2 mutation in a family with hereditary thrombocytosis. The New England journal of medicine. 2012 Mar 8;366(10):967–969. doi: 10.1056/NEJMc1200349. [DOI] [PubMed] [Google Scholar]
- 12.Mead AJ, Chowdhury O, Pecquet C, et al. Impact of isolated germline JAK2V617I mutation on human hematopoiesis. Blood. 2013 Mar 27; doi: 10.1182/blood-2012-05-430926. [DOI] [PubMed] [Google Scholar]
- 13.Dusa A, Staerk J, Elliott J, et al. Substitution of pseudokinase domain residue Val-617 by large non-polar amino acids causes activation of JAK2. The Journal of biological chemistry. 2008 May 9;283(19):12941–12948. doi: 10.1074/jbc.M709302200. [DOI] [PubMed] [Google Scholar]
- 14.Bumm TG, Elsea C, Corbin AS, et al. Characterization of murine JAK2V617F-positive myeloproliferative disease. Cancer Res. 2006 Dec 1;66(23):11156–11165. doi: 10.1158/0008-5472.CAN-06-2210. [DOI] [PubMed] [Google Scholar]
- 15.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000 Mar 9;404(6774):193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 16.Fleischman AG, Aichberger KJ, Luty SB, et al. TNFalpha facilitates clonal expansion of JAK2V617F positive cells in myeloproliferative neoplasms. Blood. 2011 Dec 8;118(24):6392–6398. doi: 10.1182/blood-2011-04-348144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilpivaara O, Mukherjee S, Schram AM, et al. A germline JAK2 SNP is associated with predisposition to the development of JAK2(V617F)-positive myeloproliferative neoplasms. Nat Genet. 2009 Apr;41(4):455–459. doi: 10.1038/ng.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones AV, Chase A, Silver RT, et al. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat Genet. 2009 Apr;41(4):446–449. doi: 10.1038/ng.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olcaydu D, Harutyunyan A, Jager R, et al. A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat Genet. 2009 Apr;41(4):450–454. doi: 10.1038/ng.341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


