Abstract
Early life stress may precipitate psychopathology, at least in part, by influencing amygdala function. Converging evidence across species suggests that links between childhood stress and amygdala function may be dependent upon hypothalamic-pituitary-adrenal (HPA) axis function. Using data from college-attending non-Hispanic European-Americans (n=308) who completed the Duke Neurogenetics Study, we examined whether early life stress (ELS) and HPA axis genetic variation interact to predict threat-related amygdala function as well as psychopathology symptoms. A biologically-informed multilocus profile score (BIMPS) captured HPA axis genetic variation (FKBP5 rs1360780, CRHR1 rs110402; NR3C2 rs5522/rs4635799) previously associated with its function (higher BIMPS are reflective of higher HPA axis activity). BOLD fMRI data were acquired while participants completed an emotional face matching task. ELS and depression and anxiety symptoms were measured using the childhood trauma questionnaire and the mood and anxiety symptom questionnaire, respectively. The interaction between HPA axis BIMPS and ELS was associated with right amygdala reactivity to threat-related stimuli, after accounting for multiple testing (empirical-p=0.016). Among individuals with higher BIMPS (i.e., the upper 21.4%), ELS was positively coupled with threat-related amygdala reactivity, which was absent among those with average or low BIMPS. Further, higher BIMPS were associated with greater self-reported anxious arousal, though there was no evidence that amygdala function mediated this relationship. Polygenic variation linked to HPA axis function may moderate the effects of early life stress on threat-related amygdala function and confer risk for anxiety symptomatology. However, what, if any, neural mechanisms may mediate the relationship between HPA axis BIMPS and anxiety symptomatology remains unclear.
Keywords: Amygdala, early life stress, anxiety, depression, gene, HPA, cortisol
1.0 Introduction
It is undeniable that exposure to early life stress (ELS) predicts various forms of psychopathology (Green et al., 2010). However, the biological correlates contributing to this association are unclear. Research across species shows that ELS may, in part, promote psychopathology by influencing amygdala function (Lupien et al., 2009). The amygdala is critical for establishing the emotional significance of stimuli and enacting changes in physiological arousal and behavioral vigilance in response to provocation (Davis and Whalen, 2001). Nearly every form of psychopathology has been linked to abnormal amygdala function; most consistently, increased amygdala reactivity to threat has been found in stress-related disorders (Hariri, 2015; Shackman et al., 2016). ELS also predicts increased threat-related amygdala reactivity and amygdala-dependent behaviors (e.g., startle response, emotional memory, attentional bias toward threat; (Lupien et al., 2009; Tottenham and Sheridan, 2009), suggesting that ELS-related differences in amygdala reactivity and related behavior are a promising mechanism through which ELS increases psychopathology risk.
Stress- and psychopathology-related differences in amygdala function may be partially driven by associated hypothalamic-pituitary-adrenal (HPA) axis dysfunction (de Kloet et al., 2005). Indeed, amygdala reactivity is positively correlated with endogenous cortisol and pharmacologic agonism of the HPA axis potentiates amygdala reactivity in mouse and man (Bogdan et al., 2016). Collectively, these data suggest that variability in amygdala function may be related to ELS exposure, with independent converging lines of evidence suggesting that HPA axis function, which is highly heritable, (Federenko et al., 2004) temporally stable (Marquez et al., 2005), and disrupted by early life stress exposure (Essex et al., 2011) may be a key factor underlying this relationship. Lastly, multiple genetic association studies have identified polymorphisms coding for HPA axis-related proteins that are associated with functional consequences in the HPA axis (Table 1; Supplementary Material; (Derijk et al., 2008)) and predict stress-related psychopathology (Heim and Binder, 2012), as well as differences in amygdala function (Bogdan et al., 2016). These findings suggest that vulnerability to the effects of ELS may depend on genetically-conferred differences in HPA axis function.
Table 1.
Selected HPA Axis Polymorphisms, Associations with HPA Axis Function and Psychopathology, and Coding Scheme for HPA Axis Biologically-Informed Multilocus Profile Scores (BIMPS)
| ne | SNP | Associations | Genotype (N) | Score | Conferring differences in |
|---|---|---|---|---|---|
| CRHR1 | rs110402 | G allele: increased cortisol response to the DEX/CRH test in severely maltreated individuals (Tyrka et al., 2009), especially in men (Heim et al., 2009), greater peak cortisol response to a psychosocial stress test and significant association of genotype x trait anxiety interaction with higher baseline cortisol levels (Mahon et al., 2013). | AA (73) | 0- Low | |
| AG (173) | 1- Med | ||||
| GG (78) | 2- High | ||||
|
| |||||
| NR3C2 | rs4635799/ rs5522 | TC and CT haplotypes: lower mineralocorticoid promoter activity and increased salivary and plasma cortisol, plasma ACTH, and heart rate in response to a psychosocial stress test (van Leeuwen et al., 2011; van Leeuwen et al., 2010); TT and TC were associated with nominally lowered AUC cortisol compared to CT (Klok et al., 2011b). | TT/TT (63) | 0- Low | |
| TC/TT (36) | 1- Med | ||||
| TT/CT (115) | 1- Med | ||||
| TC/TC (3) | 2- High | ||||
| TC/CT (27) | 2- High | ||||
| CT/CT (64) | 2- High | ||||
|
| |||||
| FKBP5 | rs1360780 | T allele: increased FKBP5 expression leading to impaired negative feedback in the system due to decreased GR- cortisol sensitivity (Binder, 2009; Binder et al., 2004); lower AUC cortisol levels (Velders et al., 2011); impaired CORT recovery in response to a psychosocial stress test (Ising et al., 2008); significant interaction with ELS predicted decreases in methylation, thereby increasing FKBP5 responsiveness to activation (Klengel et al., 2013). | CC (165) | 0- Low | |
| CT (133) | 1- Med | ||||
| TT (27) | 2- High | ||||
Using data from the Duke Neurogenetics Study (DNS), which assesses a wide range of behavioral, experiential, and biological phenotypes among young adult college students, the present study examined whether a biologically informed multilocus profile score (BIMPS) reflecting functionally-relevant genetic variation in the HPA axis (Table 1) predicts amygdala function as well as psychopathology symptoms in the context of ELS. Our BIMPS approach aggregates genetic influence within the HPA axis that has previously been associated with HPA axis function, which may be more consistent with the resolution at which BOLD fMRI and behavioral genetics research is conducted than single SNP analyses. Such polygenic approaches are expected to provide more power by including polymorphisms of small effect that may only collectively significantly contribute to variance. Indeed, prior BIMPS approaches suggest that such profiles can significantly predict variability in neural and psychiatric phenotypes when individual polymorphisms do not (Nikolova et al., 2011; Pearson-Fuhrhop et al., 2014; Stice et al., 2012). Further, by using a biologically-informed approach with a priori rationale for including functional variants in the multilocus profile, as opposed to summary statistics reflecting relationships to a particular disorder, our results are more directly interpretable alongside other HPA axis-related research (Bogdan et al., 2016; Nikolova et al., 2011). Consistent with prior work evaluating single polymorphisms (White et al., 2012), we predicted that genetic variation associated with increased HPA axis activity would predict elevated threat-related amygdala reactivity and anxiety and mood symptomatology among those exposed to relatively elevated ELS.
2.0 Methods and Materials
2.1 Participants
Non-Hispanic European/European-American participants (n=325) of the Duke Neurogenetics Study (DNS) with fMRI and genetic data that were processed by January 2014 were available for analyses. Participants provided written informed consent to a study protocol approved by the Duke University institutional review board and were in general good health and free of DNS exclusions, including: 1) medical diagnosis of cancer, stroke, diabetes requiring insulin treatment, chronic kidney or liver disease, or lifetime psychotic symptoms; 2) use of psychotropic, glucocorticoid, or hypolipidemic medication; 3) conditions affecting cerebral blood flow and metabolism (e.g., hypertension); and 4) contraindications to MRI scanning. DSM-IV psychiatric disorders were assessed with the lifetime electronic Mini International Neuropsychiatric Interview (Sheehan et al., 1998) and Structured Clinical Interview for the DSM-IV Axis II (SCID-II (First et al., 1997). DSM-IV diagnosis was not exclusionary as the DNS seeks to establish broad variability in multiple psychiatrically-relevant behavioral phenotypes. Following quality control procedures (Supplementary Material), the final sample consisted of 308 non-Hispanic European/European-American participants (mean ± SD; age=19.70±1.24; 148 males; 63 with DSM-IV Axis I disorder; Table 2). We restricted our sample to non-Hispanic European/European-Americans because the variants included in our profile have been predominantly characterized among samples of European ancestry. European ancestry was determined via self-report and confirmed through ancestrally-informative principal components (see below).
Table 2.
Sample demographics and associations with HPA Axis BIMPS and CTQ
| Variable | N or Mean ± Std Dev | Correlation with BIMPS | Correlation with CTQ |
|---|---|---|---|
| Age | 19.70 ± 1.24 | r= 0.10, p= 0.08 | r=−0.10, p=0.08 |
| Sex (N male) | 148 | r= 0.06, p= 0.29 | r=−0.03, p=0.58 |
| CTQ | 31.18 ± 6.69 | r= −0.04, p= 0.50 | - |
| Any Disorder (covariate used in primary analyses) | 63* | r=0.03, p=0.558 | r=0.018, p=0.752 |
| Agoraphobia without history of Panic Disorder | 6 | r= 0.063, p= 0.27 | r= 0.014, p= 0.81 |
| Alcohol Abuse | 24 | r= 0.000, p= 1.00 | r= −0.036, p= 0.53 |
| Alcohol Dependence | 20 | r= 0.031, p 0.58 | r= 0.025, p=0.66 |
| Anorexia Nervosa | 0 | - | - |
| Bipolar I or II | 3 | r= 0.000, p= 1.00 | r= −0.032, p=0.58 |
| Bipolar NOS (hypomania but no depression) | 3 | r= 0.059, p= 0.30 | r= 0.190, p= 0.001* |
| Bulimia Nervosa | 2 | r= 0.096, p=0.09 | r= −0.056, 0.33 |
| Dysthymia | 0 | - | - |
| Generalized Anxiety Disorder | 8 | r= 0.048, p= 0.40 | r= −0.037, p= 0.51 |
| Major Depressive Disorder | 11 | r= −0.005, p= 0.93 | r= 0.102, p= 0.07# |
| OCD | 5 | r= 0.038, p= 0.51 | r= 0.047, p= 0.42 |
| Panic Disorder (with Agoraphobia) or without | 3 | r= 0.059, p= 0.30 | r= −0.062, p= 0.28 |
| PTSD | 0 | - | - |
| Social Anxiety Disorder | 3 | r= 0.000, p= 1.00 | r= −0.037, p= 0.52 |
| Substance Abuse (cannabis) | 7 | r= −0.013, p=0.82 | r= 0.051, p= 0.37 |
| Substance Dependence (cannabis) | 5 | r= −0.030, p=0.60 | r= 0.073, p= 0.20 |
The Ns of disorders include comorbid presentations, hence the summation of these does not match the total number of individuals with at least one psychiatric disorder.
It is important to note that the significant association observed with bipolar disorder should be interpreted with caution due to the small number of individuals (n=3) with the diagnosis in our sample.
While the association between CTQ and depression was trending in a direction consistent with the literature, it was non-significant. This may be due to the limited number of individuals meeting criteria for MDD in our sample (n=11). Continuous indices of depression and anxiety (e.g., MASQ General Distress Depression and Anhedonic Depression), were consistently and significantly correlated with CTQ (GDD: r=0.243, p=<0.001; AD r=0.334 p = <0.001; GDA: r=0.148, p=0.009; AA: r=0.171, p=0.003).
2.2 Assessment of Early Life Stress and Psychological Symptoms
A battery of self-report questionnaires was used to assess past and current experiences and behavior as well as symptoms of psychopathology (e.g., depression, anxiety). The retrospective Childhood Trauma Questionnaire (CTQ; (Bernstein et al., 2003) was used to assess emotional, physical, and sexual abuse as well as emotional and physical neglect before the age of 17 to measure ELS (Bernstein et al., 2003; Scher et al., 2001). The 77-item Mood and Anxiety Symptom Questionnaire (MASQ; (Watson et al., 1995) was used to measure symptoms common to depression and anxiety (general distress depression, general distress anxiety) as well as anxiety-specific and depression-specific symptoms (anxious arousal, anhedonic depression), providing additional symptom discrimination between depression and anxiety (Watson et al., 1995).
2.3 Genotyping and HPA Axis Biologically Informed Multilocus Profile Score (BIMPS)
DNA was isolated from saliva derived from Oragene DNA self-collection kits (DNA Genotek) customized for 23andMe (www.23andme.com). DNA extraction and genotyping were performed in collaboration with 23andMe by the National Genetics Institute (NGI), a CLIA-certified clinical laboratory and subsidiary of Laboratory Corporation of America. One of two different Illumina arrays, the HumanOmniExpress or HumanOmniExpress-24, which each also had custom content was used to provide genome-wide SNP data. All genotypes used in analyses were directly typed on each array: FKBP5 rs1360780, CRHR1 rs110402, and NR3C2 rs5522 and rs4635799 (call rates >97%; HWE ps > 0.21 within the European American subsample). PHASE (Stephens et al., 2001) was used to generate MR haplotypes (van Leeuwen et al., 2011); in all cases posterior probability was >99%. Eigenstrat was used to generate ancestrally-informative principal components (PCs; (Price et al., 2006) across the entire DNS sample (all ethnicities). K-means cluster plotting and visual inspection of the top 10 components revealed that the top 5 principal components account for divergent ancestral groups within the sample and any outliers (6 SD divergence were excluded as well as those not reporting European ancestry). Subsequently, eigenstrat was again run among individuals reporting European/European-American ancestry to generate components for this specific sample.
An additive biologically-informed multilocus profile score (BIMPS (Nikolova et al., 2011) was constructed using 2 SNPS (FKBP5 rs1360780 and CRHR1 rs110402) and 1 haplotype (NR3C2 rs5522/NR3C2 rs4635799) within HPA axis-related genes that have been functionally associated with differential HPA axis response and recovery (Table 1; for more details, see Supplementary Material). Across all loci, genotypes associated with relatively “high,” “intermediate,” and “low” HPA axis response or feedback impairment, were assigned a score of 2, 1, or 0, respectively, due to evidence of additive associations with HPA axis function for each included polymorphism/haplotype (Table 1). Subsequently scores were summed across loci producing each individuals HPA axis BIMPS score. A higher BIMPS reflects an HPA axis that is overactive and/or has impaired negative feedback. BIMPS ranged from 0–6 and were normally distributed.
Our profile was developed by considering published research examining associations between genetic variation within the canonical HPA axis (i.e., CRHR1, CRHR2, CRHBP, FKBP5, NR3C1, NR3C2) and direct indices of HPA axis function (e.g., cortisol; Table 1). SNPs/haplotypes with at least 2 published studies linking them to measured HPA axis function (i.e., not a disorder associated with HPA axis dysregulation) and with minor allele frequencies greater than 5% were included in analyses. For each gene, we selected one SNP/haplotype for inclusion in the BIMPS to allow for an equal weighting of gene influence and to minimize inflation caused by linkage disequilibrium. If more than one polymorphism within a gene has been associated with HPA axis function, we selected the polymorphism that has been more extensively characterized (e.g., FKBP5 rs1360780) or studied (e.g., CRHR1 rs110402). If two polymorphisms were similarly well-defined, we used the polymorphism included in our microarray (e.g., CRHR1 rs242929 was not included in our microarray, though CRHR1 rs110402 also has one more publication regarding its function). Additionally, if neither the SNP nor a proxy for that SNP were available in our microarray, we were unable to include the SNP in our profile, even if it has been previously associated with indices of HPA axis function (e.g., NR3C1 rs6195 (Kumsta et al., 2007; Wust, 2004) and NR3C1 rs41423247 (Kumsta et al., 2007; van Rossum et al., 2003; Wust, 2004). For NR3C2 no other polymorphisms have been associated with HPA axis function to our knowledge. Further, though CRHBP, CRHR2 and NR3C1 are within the canonical HPA axis, polymorphisms from these genes were not included in the profile because there were; no genetic variants linked to HPA axis function in at least 2 studies (i.e., CRHR2), no association reported within a European/European American sample (i.e., CRHBP; (Binder et al., 2010), or no other genetic variant associated with HPA axis function with a minor allele frequency > 5% (e.g., NR3C1 rs56149945, rs6189) included on our array. By constraining the profile to genetic variation within the canonical HPA axis, rather than genetic variance that may indirectly affect the HPA axis and/or amygdala function (e.g., serotonin-related polymorphisms; (Gotlib et al., 2008), this profile provides an additive index of polygenic variation in the HPA axis associated with its function.
2.4 BOLD fMRI paradigm
Amygdala reactivity to threat-related stimuli was assessed with an emotion face-matching challenge paradigm that consistently elicits robust amygdala reactivity (Ahs et al., 2013). The fMRI paradigm consists of 4 perceptual face-matching blocks interleaved with 5 sensorimotor control shape-matching blocks (i.e., circles, horizontal and vertical ellipses). Each emotion-specific block consists of 6 face-matching trials, in which participants view a trio of faces derived from a standard set (Ekman and Friesen, 1976) of facial affect pictures (expressing angry, fearful, surprised, or neutral emotions), and select which of 2 faces presented on the bottom row of the display matches the target stimulus presented on the top row. In the shape-matching blocks, participants view a trio of geometric shapes and select which of 2 shapes displayed on the bottom matches the target shape presented on top. Each of the 6 face trios is presented for 4 seconds with a variable inter-stimulus interval of 2–6 seconds; total block length is 48 seconds. Each control block consists 6 shape trios are presented for 4 seconds with a fixed inter-stimulus interval of 2 seconds, comprising a total block length of 36 seconds.
BOLD MRI acquisition and preprocessing information may be found in the Supplementary Material. Following preprocessing, linear contrasts employing canonical hemodynamic response functions were used to estimate task-specific (i.e., Faces > Shapes) BOLD responses for each individual. The primary contrast of “Faces > Shapes” was used to assay bilateral amygdala reactivity to cues that are conditioned social signals to threat in the environment. Individual contrast images (i.e., weighted sum of the beta images) were used in second-level random effects models accounting for scan-to-scan and participant-to-participant variability to determine mean contrast-specific responses using one-sample t-tests. A voxel-level statistical threshold of P < 0.05, family wise error (FWE) corrected for multiple comparisons across the bilateral amygdala regions of interest (ROIs), and a cluster-level extent threshold of 10 contiguous voxels was applied to these analyses. The bilateral amygdala ROIs were defined using the AAL template from WFU pickatlas (Whitfield-Gabrieli, 2009).
BOLD parameter estimates from clusters within bilateral amygdala ROIs exhibiting a main effect for the “Faces > Shapes” contrast were extracted using the VOI tool in SPM8 (http://www.fil.ion.ucl.ac.uk/spm) and exported for regression analyses in SPSS (v.18). Extracting parameter estimates from clusters activated by our fMRI paradigm, rather than those specifically correlated with our independent variables of interest, precludes the possibility of any correlation coefficient inflation that may result when an explanatory covariate is used to select a ROI. A recent comparison of various analytic approaches commonly used in the imaging genetics literature recommends this approach (Tong et al., 2016).
2.5 Statistical analysis
To maintain post-quality controlled data variability while limiting the influence of extreme outliers, data points ±3 SDs were winsorized (to 3SDs from the mean; right Faces>Shapes n=1, left Faces>Shapes n=2, and the CTQ (n=2). All data were mean-centered prior to analyses. Further to reduce skew, CTQ scores were log transformed. Prior to the computation of any interaction terms, all continuous values were mean centered. However, raw data values are used for display purposes to aid interpretability. Linear regressions tested whether the BIMPS x ELS interaction predicts amygdala reactivity after accounting for main effects and covariates (i.e., sex and the presence of a DSM-IV disorder, ancestrally informative principal component 1 and 2). Further, consistent with recommendations (Baranger et al., 2016; Keller, 2014), we also included interactions between covariates and variables-of-interest (i.e., ELS x sex; ELS x disorder; ELS x PC1; ELS x PC2; HPA axis BIMPS x sex; HPA axis BIMPS x disorder; HPA axis BIMPS x PC1; HPA axis BIMPS x PC2;) as covariates in our model in an attempt to better account for potential confounds. Analyses of depression and anxiety symptoms were conducted in the same manner with the exception that disorder status and its interactions were removed as a covariate due to concerns of collinearity with the outcome measure (i.e., anxiety and depression symptoms). Post-hoc Johnson-Neyman tests from the PROCESS macro (Hayes, 2013) were used to determine the significant transition points along the moderator of any significant interactions. Holm-Bonferroni sequential FWE correction (Gaetano, 2013) was used to correct for multiple tests of hypothesized neuroimaging phenotypes (2 tests total: i.e., HPA BIMPS x CTQ predicting left and right amygdala reactivity) and FWE-corrected p values are reported. Any findings of potential interest that were not hypothesized (e.g., main effect of HPA axis BIMPS) were corrected for multiple testing correction using false discovery rate (FDR) correction, as we considered such analyses exploratory (Benjamini and Hochberg, 1995; Goeman and Solari, 2014).
2.5.1 Competitive BIMPS Significance Testing
Lastly, to test the specificity of any observed associations, competitive significance testing was performed for any results reaching significance levels following multiple test correction in null hypothesis testing. Three sets of autosomal SNPs were extracted that were matched to the original SNPs on risk allele frequency and independence (MR haplotype was treated as a SNP for convenience; risk allele frequency (RAF) = original SNP RAF ± 0.05; between-SNP r2<0.003). 10,000 random “BIMPS” were then generated by summing the “risk” alleles (based on frequency) for one SNP from each of the three matched sets. Each of these presumably non-associated BIMPS were then tested for interactions with ELS to predict any associations surviving multiple testing correction in null hypothesis testing. Empirical p-values were determined by taking the sum of the number of times a random BIMPS x ELS interaction produced a p-value less than the one obtained in the original analyses, divided by the number of random BIMPS tested (10,000).
3.0 Results
3.1 Demographic Associations
Males met criteria for alcohol abuse more than women, but gender was not associated with any other variables including HPA axis BIMPS and CTQ (Table S1). In the entire sample, CTQ was correlated with the presence of bipolar disorder NOS but not other diagnoses (Table 2).
3.2 Amygdala Function
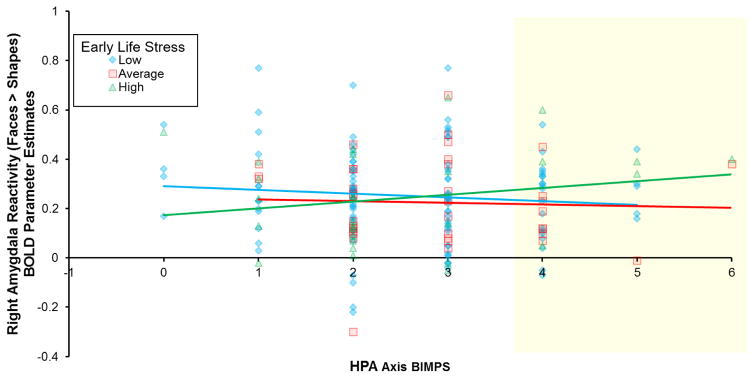
As in prior studies (Ahs et al., 2013), the face matching task reliably elicited bilateral amygdala reactivity (Figure 1). The HPA axis BIMPS x ELS interaction significantly predicted right, but not left, amygdala reactivity (Figure 2; Right: β=0.145, ΔF(1,292)=5.814, ΔR2=0.019, p=0.017, p- FWE=0.034, Table S2; Left: β=0.093, ΔF (1,292)=2.415, ΔR2=0.008, p=0.121, p-FWE=0.121, Table S3).1 Post-hoc Johnson-Neyman tests revealed ELS was only positively coupled with amygdala reactivity among those with relatively high BIMPS (i.e., BIMPS≥4, the upper 21.4% of BIMPS values, all ps < 0.05; Figure 2; alternative plotting is displayed in Figure S1). In addition to surviving multiple testing correction, results also survived competitive BIMPS testing (emp-p=0.016), indicating that the HPA axis BIMPS x ELS interaction predicting right amygdala reactivity to threat is not simply an artifact of summing any 3 random variants of similar frequency. Further, post-hoc tests revealed that both the right basolateral and centromedial amygdala were similarly associated with the HPA axis BIMPS x ELS interaction (Figure S2).
Figure 1.
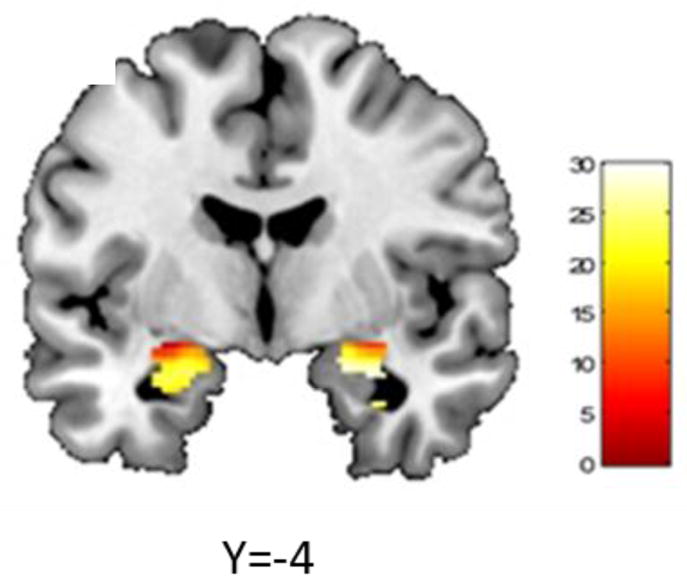
HPA axis BIMPS x ELS associated threat-related right amygdala reactivity across all participants (Y=−4; Right amygdala: max voxel: x= 28, y= −4, z= −20, t = 2.90, PFWE-corr =0.002, 204 voxels; Left amygdala: max voxel: x= −22, y= −6, z= −18, t= 2.85, PFWE-corr = 0.002, 179 voxels).
Figure 2.
An interaction between the hypothalamic-pituitary-adrenal (HPA) axis biologically-informed multilocus profile scores (BIMPS) and early life stress (ELS) predicted right amygdala reactivity. ELS was significantly positively associated with right amygdala reactivity in individuals with high (t(308)=−2.04, p=0.04), but not low (t(308)=−1.53, p=0.13) or average (t(308)=0.55, p=0.58) BIMPS. Regions of significance from the Johnson Neyman tests are denoted in yellow (p<.05). BIMPS and ELS were analyzed as a continuous variable; or ease of visualization, we collapsed the ELS into three groupings: low (25–30), medium (31–37), and high (38–60). For alternative plotting and discussion see Supplemental Figure 1.
Follow-up tests evaluated whether each individual polymorphism or haplotype interacted with ELS to predict amygdala function (Table 3). In contrast to results from our profile, which survived multiple testing correction, we observed nominally significant or trending interactions between ELS and both FKBP5 rs1360780 and NR3C2 haplotype, but these tests did not survive multiple testing correction for the 6 single SNP/haplotype tests (Table 3). The nominally significant and trending FKBP5 and NR3C2 interactions with ELS were driven by positive correlations between amygdala reactivity and childhood adversity among individuals homozygous for FKBP5 T alleles (p=0.10) or NR3C2 haplotypes (p=0.04) associated with elevated cortisol, that was absent in other groups (all other ps > 0.23)
Table 3.
Individual HPA Axis SNPs/Haplotype x CTQ interactions predicting amygdala reactivity
| Left Amygdala | Right Amygdala | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | ΔR2 | ΔF | p | FDR p | B | ΔR2 | ΔF | P | FDR p | |
| FKBP5 x CTQ | 0.022 | <0.001 | 0.144 | 0.705 | 1.00 | 0.097 | 0.009 | 2.666 | 0.104 | 0.21 |
| NR3C2 x CTQ | 0.086 | 0.007 | 1.987 | 0.160 | 0.48 | 0.140 | 0.017 | 5.295 | 0.022 | 0.07 |
| CRHR1 x CTQ | 0.030 | 0.001 | 0.269 | 0.605 | 1.00 | -0.002 | <0.001 | 0.002 | 0.967 | 0.97 |
Note: FDR-corrected p-values represent correction within each SNP across hemispheres. When all are entered simultaneously, all ps > 0.132.
3.3 Psychological Symptoms
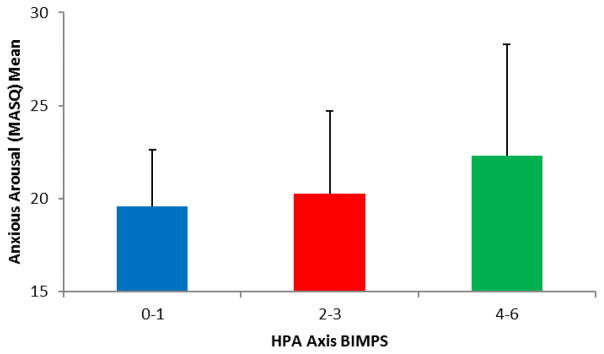
In contrast to our hypotheses, HPA axis BIMPS did not interact with ELS to predict any MASQ subscale (all β<0.041, all ps>0.34). However, in addition to unsurprising positive associations between ELS and all anxiety and depressive subscales of the MASQ (all β > 0.145, all p<0.01), we observed a significant main effect of HPA axis BIMPS on MASQ anxious arousal (β=0.168, ΔF=8.95, ΔR2=0.028, p=0.003, Figure 3; Table S4), but no other MASQ subscale (all β < 0.113, all p>0.054; Tables S5–S8). FDR-correction accounting or 8 tests (i.e., 4 main effects of HPA axis BIMPS and 4 tests of HPA axis BIMPS x ELS interactions for all MASQ scales), showed that this effect survived multiple testing correction (p-FDR=0.02). Further, follow-up competitive BIMPS testing revealed a significant association (emp-p=0.0035). Notably, however, the main effect on anxious arousal was not robust to the inclusion of the HPA axis BIMPS x ELS interaction term (Table S4). No significant associations were observed between psychological symptom scores and amygdala function (R: all rs< 0.044, all ps>0.10; L: all rs<0.068, all ps>0.23).
Figure 3.
HPA Axis BIMPS predicted elevated anxious arousal (β=0.168, p=0.003). BIMPS were analyzed as a continuous measure but are collapsed into low, average and high groupings for visualization.
4.0 Discussion
This study adopted a biologically-informed multilocus profile (Nikolova et al., 2011) approach to examine whether functionally-relevant genetic variation within the HPA axis interacts with ELS to predict amygdala function as well as symptoms of anxiety and depression. ELS was positively coupled with amygdala function only among those with relatively high BIMPS (Figure 2). BIMPS were further predictive of anxious arousal, though there was no evidence that this was mediated by amygdala function. These data are consistent with research across species suggesting that heightened HPA axis function and stress exposure may influence neural circuitry critical for behavioral vigilance and promote stress-related psychopathology (Kolber and Muglia, 2009). More broadly, these data suggest that carefully curated genetic sets within defined systems based upon a priori functional associations in a BIMPS framework may usefully contribute to our knowledge of individual differences in brain function and behavior (Nikolova et al., 2011); but see also limitations in 4.3).
4.1 Amygdala Function
Independent lines of research suggest that HPA axis function may moderate ELS-related potentiation of threat-related amygdala reactivity. Elevated levels of endogenous cortisol predict increased amygdala reactivity and behavioral responses to threatening stimuli (Tottenham and Sheridan, 2009) and direct pharmacologic stimulation of the HPA axis heightens threat-specific amygdala reactivity (Henckens et al., 2010). Emerging research is also suggestive of a putative pathway through which ELS predicts individual differences in HPA axis-related methylation as well as its function in childhood, which in turn is associated with indices in HPA function and functional connectivity in adulthood (Bogdan et al., 2016). The present results build upon this literature by showing that ELS-related differences in amygdala function may be dependent on functionally-relevant genetic variation within the HPA axis. While our results were statistically significantly only in the right hemisphere, they approached trend levels of significance in the left with similar directional effects. As such, while effects may potentially be stronger in the right hemisphere, which some evidence suggests is more involved in unconscious emotion processing (Gainotti, 2012), our data do not strongly suggest lateralized effects.
Notably, our HPA axis BIMPS interaction with ELS was associated with right amygdala reactivity following multiple testing correction. While 2 components of our profile (FKBP5 rs1360780 and NR3C2 haplotype) showed nominally significant and trend level interactions in directions consistent with prior research (Bogdan et al., 2016; Holz et al., 2014; White et al., 2012), these associations did not survive correction for multiple testing (Table 3). As such, these results provide initial evidence that BIMPS based on a prioi functional associations may capture system-level polygenic architecture and provide greater predictive utility than single SNP analyses (but see also limitations below). Further, employing a BIMPS approach that is constrained to a single system (e.g., the canonical HPA axis) enables us to understand how variation within this system is associated with differential neural functioning alongside other methods (e.g., pharmacologic manipulation) and provides a resolution more consistent with assays of higher order brain function (e.g., system level variation as opposed to single receptor level variation; (Nikolova et al., 2011). Whether amygdala function related to HPA axis genetic variation and ELS go on to predict later psychopathology and related behaviors (e.g., startle) or are mediated by diurnal cortisol levels or challenge-elicited HPA axis reactivity (Binder et al., 2010; Binder et al., 2004; Klok et al., 2011a), (Pagliaccio et al., 2014), requires future research among longitudinal samples at risk for psychopathology that integrate biomarker assessments.
4.2 Psychological Symptoms
Consistent with a wealth of research (Green et al., 2010), ELS predicted symptoms of depression, anhedonic depression, anxiety, and anxious arousal. Anxious arousal was further predicted by HPA axis BIMPS, with increased anxious arousal symptoms endorsed by individuals with higher BIMPS; however, this association did not remain following the introduction of the HPA axis BIMPS x ELS interaction term. Unlike prior research (Bogdan et al., 2016), amygdala function was not associated with anxiety or depressive symptomatology. One possibility for this discrepancy is that our sample is composed of relatively high functioning young adults, with limited clinically significant anxiety (6.8% met DSM-IV criteria for any anxiety-related disorder). Such high functioning may represent a potential resilience to some of the deleterious effects of stress, thereby uncoupling the link between threat-related amygdala reactivity and anxiety typically observed in clinical populations.
4.3 Limitations
It is important to consider the present results in the context of study limitations including cross-sectional study design, lack of endocrine measures, and the limited generalizability of the sample as well as its size. The cross-sectional design prohibits the implementation of longitudinal data analyses within a developmental framework that would allow the concurrent assessment of changes in brain function as well as environmental stress over time. Such research will be particularly important given nonlinear amygdala development (Goddings et al., 2014), differential neural and endocrine associations within children and adults (Hanson et al., 2014), and evidence that the duration of ELS may play important roles in amygdala phenotypes (Mccrory et al., 2011). The lack of endocrine measures prevents the testing of hypothesized models in which genetically-related differences in HPA axis function (e.g., cortisol, stimulated gene expression patterns) mediate the relationship between functional HPA axis variation and amygdala function (Bogdan et al., 2016). The generalizability of our sample is limited because our sample is comprised of relatively high functioning college students. In the context of the present study, it is important to consider this limitation with regard to our assessment of ELS. CTQ total scores in this sample (i.e., M=33.46) are comparable to other community (Scher et al., 2001) and college samples (e.g., UCSD; n=949, M = 35.2 (Wright et al., 2001), but are considerably lower than those typically observed in clinical samples (Schäfer et al., 2007; Watson et al., 2007). However, despite the relatively comparable scores with general population samples, it may be that those who were exposed to elevated ELS in our sample are particularly resilient to its effects. Lastly, our sample is small for a genetic association study, even when considering neural phenotypes. While it has been argued that neural intermediate phenotypes may be associated with larger effects than more distal and heterogeneous collections of behaviors such as psychopathology, emerging research suggests that effect sizes associations between common genetic variation and neural phenotypes are comparable to that of traditional psychiatric diagnoses (Bogdan et al., 2016; Franke et al., 2016).
More broadly, it is important to also consider the benefits and limitations of the large scale study design of the DNS. A clear benefit of this approach is that, unlike smaller single studies designed to explicitly address a single research question, such broad spectrum studies allow for the accumulation of a large well-powered dataset that can be used to address multiple different research questions, including expected small effects of common genetic variation. Further, because many other studies use similar methodology (e.g., HCP; (Barch et al., 2013); Brain Genomics Superstruct Project; (Holmes et al., In press); IMAGEN; (French et al., 2015), results can be readily integrated into prior literature with an increased likelihood that additional data may become available for replication attempts. However, alongside these clear benefits, there are also limitations that must be considered. For instance, such large studies often do not have data that may be useful for a particular research question (e.g., indices of HPA axis function), and, with few exceptions (e.g., Grady Trauma Project; (Binder et al., 2004), often rely on samples of convenience (e.g., university students, volunteers) that limit generalizability. Further, such studies often rely on easily and quickly administered assessments; for example, even though the CTQ is a well-used measure that converges nicely with other assessments of ELS, including interviews, it is relatively limited in its scope of ELS assessment in specific ways (e.g., lacking refined estimates of frequency, severity, and the child’s relationship to the perpetrator). Collaboration with psychiatric epidemiology (e.g., (Carey et al., 2015; Nelson et al., 2016), non-human animal models (e.g., (Dincheva et al., 2015; Wellman et al., 2013), and molecular genetics (e.g., (Arloth et al., 2015)) may prove particularly useful in addressing these limitations in stress-related research going forward (Hariri and Holmes, 2015). Finally, due to multiple research questions being probed by multiple investigators, such large datasets may be subject to a higher risk of false positive and negative associations. Transparency with regard to prior publications, formal procedures for submitting and vetting proposed analyses, and ultimately, replication in independent or federated samples when possible, will help alleviate this concern.
While the BIMPS approach has multiple strengths including a polygenic approach and reliance on prior literature, it is also faced with challenges (Bogdan et al., in press). This approach is reliant upon prior functional associations with particular polymorphisms, which could reasonably only be employed in a handful of systems. Further, BIMPS approaches assume additivity within a given system that neglects probable epistatic interactions and probable downstream regulatory processes (e.g., receptor downregulation). Lastly, there are a plethora of options to construct different profiles which may heighten false positive concerns (e.g., we opted to constrain our profile to the HPA axis, but one could reasonable extend this profile to any variant associated with HPA axis function). Notably, we used criteria to define our profile and previously proposed the profile used in these analyses in a prior publication (Bogdan et al., 2013) (we removed polymorphism rs10482605 because there were not 2 positive studies associating it with cortisol) and did not test other profiles not reported here. As BIMPS research moves forward it will be important to be mindful of the plethora of options it provides to researchers and to attempt replication and extension in the context of the same genetic profile.
4.4 Conclusions
Limitations notwithstanding, the present findings provide novel insight into the relationship between ELS and amygdala function that contributes to a growing literature emphasizing the importance of this pathway in understanding stress-related psychopathology. These results suggest that genetically-conferred differences in HPA axis function may leave individuals vulnerable to ELS-related potentiation of threat-related amygdala reactivity, which may in turn promote future psychopathology in the context of additional stress exposure (Swartz et al., 2015). Future large longitudinal studies collecting a variety of data including brain function and structure as well as psychopathology symptoms and endocrine function across time are needed.
Supplementary Material
Highlights.
HPA axis genetic profile and early life stress interact to predict amygdala function
HPA axis genetic profile is associated with anxiety symptoms
Within system genetic profiles may inform our understanding of psychopathology
Acknowledgments
Funding Sources: The authors are thankful to Duke Neurogenetics Study staff and participants. Christina R. Di Iorio and Lindsay J. Michalski are supported by National Institute of General Medical Sciences [T32 GM081739]. Caitlin E. Carey is supported by National Science Foundation [DGE-1143954]. Dr. Nadia Corral-Frias is supported by National Institute of Mental Health [T32-MH014677]. Dr. Ahmad R. Hariri receives support through National Institute of Drug Abuse [DA033369 and DA031579]. Dr. Ryan Bogdan receives support from the Klingenstein Third Generation Foundation, McDonnell Center for Systems Neuroscience, and National Institute on Aging [R01-AG045231]. There are no conflicts of interest for any of the contributing authors. The Duke Neurogenetics Study is supported by Duke University and National Institute on Drug Abuse grant [DA03369].
Footnotes
Notably, if outliers (n=4; i.e., data points ±3 SDs on CTQ, right, or left amygdala reactivity) are excluded instead of winsorized, results do not differ substantively: Right β=0.142, ΔF(1,288)=2.363, ΔR2=0.041, p=0.019; p-FWE=0.038 Left: β=0.088, ΔF (1,288)=2.178, ΔR2=0.007, p=0.141, p-FWE=0.141. Further, results remain stable to the inclusion of alcohol use scores (AUDIT) and their interactions with predictor variables (β=0.130, ΔF(1,289)=4.482, ΔR2=0.015, p=0.035).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahs F, Davis CF, Gorka AX, Hariri AR. Feature-based representations of emotional facial expressions in the human amygdala. Social cognitive and affective neuroscience. 2013 doi: 10.1093/scan/nst112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arloth J, Bogdan R, Weber P, Frishman G, Menke A, Wagner KV, Schmidt MV, Karbalai N, Darina C, Muller-Myhsok B, Trumbach D, Wurst W, Mehta D, Uhr M, Klengel T, Erhardt A, Drabant EM, Ruepp A, Hariri AR, Binder EB (PGC), MD.DW.G.o.t.PG.C. Genetically determined differences in the immediate transcriptome response to stress predict risk-related brain function and psychiatric disorders. 2015 doi: 10.1016/j.neuron.2015.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranger DA, Ifrah C, Prather AA, Carey CE, Corral-Frias NS, Drabant Conley E, Hariri AR, Bogdan R. PER1 rs3027172 Genotype Interacts with Early Life Stress to Predict Problematic Alcohol Use, but Not Reward-Related Ventral Striatum Activity. Front Psychol. 2016;7:464. doi: 10.3389/fpsyg.2016.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, Glasser MF, Curtiss S, Dixit S, Feldt C, Nolan D, Bryant E, Hartley T, Footer O, Bjork JM, Poldrack R, Smith S, Johansen-Berg H, Snyder AZ, Van Essen DC. Function in the human connectome: task-fMRI and individual differences in behavior. NeuroImage. 2013;80:169–189. doi: 10.1016/j.neuroimage.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S186–195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Binder EB, Owens MJ, Liu W, Deveau TC, Rush aJ, Trivedi MH, Fava M, Bradley B, Ressler KJ, Nemeroff CB. Association of polymorphisms in genes regulating the corticotropin-releasing factor system with antidepressant treatment response. Archives of general psychiatry. 2010;67:369–379. doi: 10.1001/archgenpsychiatry.2010.18. [DOI] [PubMed] [Google Scholar]
- Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, Papiol S, Seaman S, Lucae S, Kohli MA, Nickel T, Kunzel HE, Fuchs B, Majer M, Pfennig A, Kern N, Brunner J, Modell S, Baghai T, Deiml T, Zill P, Bondy B, Rupprecht R, Messer T, Kohnlein O, Dabitz H, Bruckl T, Muller N, Pfister H, Lieb R, Mueller JC, Lohmussaar E, Strom TM, Bettecken T, Meitinger T, Uhr M, Rein T, Holsboer F, Muller-Myhsok B. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Salmeron BJ, Carey CE, Agrawal A, Calhoun VD, Garavan H, Hariri AR, Heinz A, Hill M, Holmes A, Kalin NH, Goldman D. Imaging Genetics and Genomics in Psychiatry: A Critical Review of Progress and Potential. Biol Psychiatry. doi: 10.1016/j.biopsych.2016.12.030. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Hariri AR. Neural embedding of stress reactivity. Nat Neurosci. 2012;15:1605–1607. doi: 10.1038/nn.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Hyde LW, Hariri AR. A neurogenetics approach to understanding individual differences in brain, behavior, and risk for psychopathology. Mol Psychiatry. 2013;18:288–299. doi: 10.1038/mp.2012.35. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Pagliaccio D, Baranger DA, Hariri A. Genetic Moderation of Stress Effects on Corticolimbic Circuitry. Neuropsychopharmacology. 2016;41:275–296. doi: 10.1038/npp.2015.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Williamson DE, Hariri AR. Mineralocorticoid receptor iso/val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. American Journal of Psychiatry. 2012;169:515–522. doi: 10.1176/appi.ajp.2011.11060855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey C, Agrawal A, Zhang B, Conley E, Degenhardt L, Heath A, Li D, Lynskey M, Martin N, Montgomery G, Wang T, Bierut L, Hariri A, Nelson E, Bogdan R. Monoacylglycerol lipase (MGLL) polymorphism rs604300 interacts with childhood adversity to predict cannabis dependence symptoms and amygdala habituation: Evidence from an endocannabinoid system-level analysis. J Abnorm Psychol. 2015;124:860–877. doi: 10.1037/abn0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Derijk RH, van Leeuwen N, Klok MD, Zitman FG. Corticosteroid receptor-gene variants: modulators of the stress-response and implications for mental health. Eur J Pharmacol. 2008;585:492–501. doi: 10.1016/j.ejphar.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Dincheva I, Drysdale A, Hartley C, Johnson D, Jing D, King E, Ra S, Gray J, Yang R, DeGruccio A, Huang C, Cravatt B, Glatt C, Hill M, Casey B, Lee F. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat Commun. 2015;6:6395. doi: 10.1038/ncomms7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Measuring Facial Movement. Environmental Psychology and Nonverbal Behavior. 1976;1:56–75. [Google Scholar]
- Essex MJ, Shirtcliff EA, Burk LR, Ruttle PL, Klein MH, Slattery MJ, Kalin NH, Armstrong JM. Influence of early life stress on later hypothalamic pituitary adrenal axis functioning and its covariation with mental health symptoms: A study of the allostatic process from childhood into adolescence. Dev Psychopathol. 2011;23:1039–1058. doi: 10.1017/S0954579411000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federenko IS, Nagamine M, Hellhamme DH, Wadhwa PD, Wust S. The heritability of hypothalamus pituitary adrenal axis responses to psychosocial stress is context dependent. Journal of Clinical Endocrinology & Metabolism. 2004;89:6244–6250. doi: 10.1210/jc.2004-0981. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders, (SCID-II) 1997 [Google Scholar]
- Franke B, Stein JL, Ripke S, Anttila V, Hibar DP, van Hulzen KJ, Arias-Vasquez A, Smoller JW, Nichols TE, Neale MC, McIntosh AM, Lee P, McMahon FJ, Meyer-Lindenberg A, Mattheisen M, Andreassen OA, Gruber O, Sachdev PS, Roiz-Santianez R, Saykin AJ, Ehrlich S, Mather KA, Turner JA, Schwarz E, Thalamuthu A, Yao Y, Ho YY, Martin NG, Wright MJ, Enigma C, O'Donovan MC, Thompson PM, Neale BM, Medland SE, Sullivan PF Schizophrenia Working Group of the Psychiatric Genomics C, Psychosis Endophenotypes International C, Wellcome Trust Case Control C. Genetic influences on schizophrenia and subcortical brain volumes: large-scale proof of concept. Nat Neurosci. 2016;19:420–431. doi: 10.1038/nn.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French L, Gray C, Leonard G, Perron M, Pike G, Richer L, Séguin J, Veillette S, Evans C, Artiges E, Banaschewski T, Bokde A, Bromberg U, Bruehl R, Buchel C, Cattrell A, Conrod P, Flor H, Frouin V, Gallinat J, Garavan H, Gowland P, Heinz A, Lemaitre H, Martinot J, Nees F, Orfanos D, Pangelinan M, Poustka L, Rietschel M, Smolka M, Walter H, Whelan R, Timpson N, Schumann G, Smith G, Pausova Z, Paus T. Early Cannabis Use, Polygenic Risk Score for Schizophrenia and Brain Maturation in Adolescence. JAMA Psychiatry. 2015;72:1002–1011. doi: 10.1001/jamapsychiatry.2015.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainotti G. Unconscious processing of emotions and the right hemisphere. Neuropsychologia. 2012;50:205–218. doi: 10.1016/j.neuropsychologia.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Gaetano J. Holm-Bonferroni sequential correction: An EXCEL calculator. 2013 https://www.researchgate.net/publication/236969037_Holm-Bonferroni_Sequential_Correction_An_EXCEL_Calculator.
- Goddings AL, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore SJ. The influence of puberty on subcortical brain development. Neuroimage. 2014;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeman JJ, Solari A. Multiple hypothesis testing in genomics. Stat Med. 2014;33:1946–1978. doi: 10.1002/sim.6082. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, Mclaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood Adversities and Adult Psychiatric Disorders in the National Comorbidity Survey Replication I. Archives of General Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo Aa, Schaefer SM, Rudolph KD, Shirtcliff Ea, Pollak SD, Davidson RJ. Behavioral Problems After Early Life Stress: Contributions of the Hippocampus and Amygdala. Biological psychiatry. 2014:1–9. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Finding translation in stress research. Nat Neurosci. 2015;18:1347–1352. doi: 10.1038/nn.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR. Looking inside the disordered brain: an introduction to the functional neuroanatomy of psychopathology 2015 [Google Scholar]
- Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach 2013 [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol. 2012;233:102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Heim C, Bradley B, Mletzko TC, Deveau TC, Musselman DL, Nemeroff CB, Ressler KJ, Binder EB. Effect of Childhood Trauma on Adult Depression and Neuroendocrine Function: Sex-Specific Moderation by CRH Receptor 1 Gene. Front Behav Neurosci. 2009;3:41. doi: 10.3389/neuro.08.041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJ, van Wingen GA, Joels M, Fernandez G. Time-dependent effects of corticosteroids on human amygdala processing. J Neurosci. 2010;30:12725–12732. doi: 10.1523/JNEUROSCI.3112-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AJ, Hollinshead M, O’Keefe TM, Petrov VI, Fariello GR, Wald LL, Fischl B, Rosen BR, Mair RW, Roffman JL, Smoller JW, Buckner RL. Brain Genomics Superstruct Project initial data release with structural, functional, and behavioral measures. Scientific Data. doi: 10.1038/sdata.2015.31. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz NE, Buchmann AF, Boecker R, Blomeyer D, Baumeister S, Wolf I, Rietschel M, Witt SH, Plichta MM, Meyer-Lindenberg A, Banaschewski T, Brandeis D, Laucht M. Role of FKBP5 in emotion processing: results on amygdala activity, connectivity and volume. Brain structure & function. 2014 doi: 10.1007/s00429-014-0729-5. [DOI] [PubMed] [Google Scholar]
- Ising M, Depping AM, Siebertz A, Lucae S, Unschuld PG, Kloiber S, Horstmann S, Uhr M, Muller-Myhsok B, Holsboer F. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. Eur J Neurosci. 2008;28:389–398. doi: 10.1111/j.1460-9568.2008.06332.x. [DOI] [PubMed] [Google Scholar]
- Keller MC. Gene x environment interaction studies have not properly controlled for potential confounders: the problem and the (simple) solution. Biol Psychiatry. 2014;75:18–24. doi: 10.1016/j.biopsych.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TW, Mercer KB, Mayberg HS, Bradley B, Nemeroff CB, Holsboer F, Heim CM, Ressler KJ, Rein T, Binder EB. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok MD, Giltay EJ, Van der Does AJ, Geleijnse JM, Antypa N, Penninx BW, de Geus EJ, Willemsen G, Boomsma DI, van Leeuwen N, Zitman FG, de Kloet ER, DeRijk RH. A common and functional mineralocorticoid receptor haplotype enhances optimism and protects against depression in females. Transl Psychiatry. 2011a;1:e62. doi: 10.1038/tp.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok MD, Giltay EJ, Van der Does aJW, Geleijnse JM, Antypa N, Penninx BWJH, de Geus EJC, Willemsen G, Boomsma DI, van Leeuwen N, Zitman FG, de Kloet ER, DeRijk RH. A common and functional mineralocorticoid receptor haplotype enhances optimism and protects against depression in females. Translational psychiatry. 2011b;1:e62. doi: 10.1038/tp.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolber BJ, Muglia LJ. Defining brain region-specific glucocorticoid action during stress by conditional gene disruption in mice. Brain research. 2009;1293:85–90. doi: 10.1016/j.brainres.2009.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumsta R, Entringer S, Koper JW, van Rossum EFC, Hellhammer DH, Wüst S. Sex specific associations between common glucocorticoid receptor gene variants and hypothalamus-pituitary-adrenal axis responses to psychosocial stress. Biological psychiatry. 2007;62:863–869. doi: 10.1016/j.biopsych.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Mahon PB, Zandi PP, Potash JB, Nestadt G, Wand GS. Genetic association of FKBP5 and CRHR1 with cortisol response to acute psychosocial stress in healthy adults. Psychopharmacology (Berl) 2013;227:231–241. doi: 10.1007/s00213-012-2956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez C, Nadal R, Armario A. Responsiveness of the hypothalamic-pituitary-adrenal axis to different novel environments is a consistent individual trait in adult male outbred rats. Psychoneuroendocrinology. 2005;30:179–187. doi: 10.1016/j.psyneuen.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Mccrory E, Brito SAD, Viding E. The impact of childhood maltreatment : a review of neurobiological and genetic factors. 2011;2:1–14. doi: 10.3389/fpsyt.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EC, Agrawal A, Heath AC, Bogdan R, Sherva R, Zhang B, Al-Hasani R, Bruchas MR, Chou YL, Demers CH, Carey CE, Conley ED, Fakira AK, Farrer LA, Goate A, Gordon S, Henders AK, Hesselbrock V, Kapoor M, Lynskey MT, Madden PA, Moron JA, Rice JP, Saccone NL, Schwab SG, Shand FL, Todorov AA, Wallace L, Wang T, Wray NR, Zhou X, Degenhardt L, Martin NG, Hariri AR, Kranzler HR, Gelernter J, Bierut LJ, Clark DJ, Montgomery GW. Evidence of CNIH3 involvement in opioid dependence. Mol Psychiatry. 2016;21:608–614. doi: 10.1038/mp.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova YS, Ferrell RE, Manuck SB, Hariri AR. Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsychopharmacology. 2011;36:1940–1947. doi: 10.1038/npp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D, Luby JL, Bogdan R, Agrawal A, Gaffrey MS, Belden AC, Botteron KN, Harms MP, Barch DM. Stress-system genes and life stress predict cortisol levels and amygdala and hippocampal volumes in children. Neuropsychopharmacology. 2014;39:1245–1253. doi: 10.1038/npp.2013.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson-Fuhrhop KM, Dunn EC, Mortero S, Devan W, Falcone G, Lee P, Holmes A, Hollinshead M, Roffman J, Smoller J, Rosand J, Cramer S. Dopamine genetic risk score predicts depressive symptoms in healthy adults and adults with depression. PLoS ONE. 2014;9:e93772. doi: 10.1371/journal.pone.0093772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A, Patterson N, Plenge R, Weinblatt M, Shadick N, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature genetics2. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Schäfer I, Reininghaus U, Langeland W, Voss A, Zieger N, Haasen C, Karow A. Dissociative symptoms in alcohol-dependent patients: associations with childhood trauma and substance abuse characteristics. Comprehensive Psychiatry. 2007;48:539–545. doi: 10.1016/j.comppsych.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Scher CD, Stein MB, Asmundson GJ, McCreary DR, Forde DR. The childhood trauma questionnaire in a community sample: psychometric properties and normative data. Journal of traumatic stress. 2001;14:843–857. doi: 10.1023/A:1013058625719. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Tromp DP, Stockbridge MD, Kaplan CM, Tillman RM, Fox AS. Dispositional negativity: An integrative psychological and neurobiological perspective. Psychol Bull. 2016;142:1275–1314. doi: 10.1037/bul0000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavas J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Supplem):22–33. [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. American Journal of Human Genetics. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS, Epstein H, Smolen A. Multilocus genetic composite reflecting dopamine signaling capacity predicts reward circuitry responsivity. J Neurosci. 2012;32:10093–10100. doi: 10.1523/JNEUROSCI.1506-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Knodt AR, Spenser RR, Hariri AR. A neural biomarker of psychological vulnerability to future life stress. Neuron. 2015;85:505–511. doi: 10.1016/j.neuron.2014.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Chen Q, Nichols TE, Rasetti R, Callicott JH, Berman KF, Weinberger DR, Mattay VS. Seeking Optimal Region-Of-Interest (ROI) Single-Value Summary Measures for fMRI Studies in Imaging Genetics. PLoS One. 2016;11:e0151391. doi: 10.1371/journal.pone.0151391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: effects on hypothalamic-pituitary-adrenal axis reactivity. Biol Psychiatry. 2009;66:681–685. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen N, Bellingrath S, de Kloet ER, Zitman FG, DeRijk RH, Kudielka BM, Wust S. Human mineralocorticoid receptor (MR) gene haplotypes modulate MR expression and transactivation: implication for the stress response. Psychoneuroendocrinology. 2011;36:699–709. doi: 10.1016/j.psyneuen.2010.10.003. [DOI] [PubMed] [Google Scholar]
- van Leeuwen N, Kumsta R, Entringer S, de Kloet ER, Zitman FG, DeRijk RH, Wüst S. Functional mineralocorticoid receptor (MR) gene variation influences the cortisol awakening response after dexamethasone. Psychoneuroendocrinology. 2010;35:339–349. doi: 10.1016/j.psyneuen.2009.07.006. [DOI] [PubMed] [Google Scholar]
- van Rossum EFC, Koper JW, van den Beld A, Uitterlinden A, Arp P, Ester W, Janssen J, Brinkmann AO, de Jong FH, Grobbee D, Pols H, Lamberts SW. Identification of the BclI polymorphism in the glucocorticoid receptor gene: association with sensitivity to glucocorticoids in vivo and body mass index. Clin Endocrinol (Oxf) 2003;59:585–592. doi: 10.1046/j.1365-2265.2003.01888.x. [DOI] [PubMed] [Google Scholar]
- Velders FP, Kuningas M, Kumari M, Dekker MJ, Uitterlinden AG, Kirschbaum C, Hek K, Hofman A, Verhulst FC, Kivimaki M, Van Duijn CM, Walker BR, Tiemeier H. Genetics of cortisol secretion and depressive symptoms: a candidate gene and genome wide association approach. Psychoneuroendocrinology. 2011;36:1053–1061. doi: 10.1016/j.psyneuen.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark La, Strauss ME, McCormick Ra. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of abnormal psychology. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Watson S, Owen BM, Gallagher P, Hearn AJ, Young AH, Ferrier IN. Family history, early adversity and the hypothalamic-pituitary-adrenal (HPA) axis: Mediation of the vulnerability to mood disorders. Neuropsychiatric disease and treatment. 2007;3:647–653. [PMC free article] [PubMed] [Google Scholar]
- Wellman C, Camp M, Jones V, MacPherson K, Ihne J, Fitzgerald P, Maroun M, Drabant E, Bogdan R, Hariri A, Holmes A. Convergent effects of mouse Pet-1 deletion and human PET-1 variation on amygdala fear and threat processing. Exp Neurol. 2013;250:260–269. doi: 10.1016/j.expneurol.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MG, Bogdan R, Fisher PM, Munoz KE, Williamson DE, Hariri AR. FKBP5 and emotional neglect interact to predict individual differences in amygdala reactivity. Genes Brain Behav. 2012;11:869–878. doi: 10.1111/j.1601-183X.2012.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S. Artifact Detection and QA Manual 2009 [Google Scholar]
- Wright KD, Asmundson GJ, McCreary DR, Scher C, Hami S, Stein MB. Factorial validity of the Childhood Trauma Questionnaire in men and women. Depression and anxiety. 2001;13:179–183. [PubMed] [Google Scholar]
- Wust S. Common Polymorphisms in the Glucocorticoid Receptor Gene Are Associated with Adrenocortical Responses to Psychosocial Stress. Journal of Clinical Endocrinology & Metabolism. 2004;89:565–573. doi: 10.1210/jc.2003-031148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.