Abstract
Key points
The volume‐regulated anion channel (VRAC) is a swelling‐activated chloride channel that is permeable to inorganic anions and a variety of small organic molecules.
VRAC is formed via heteromerization of LRRC8 proteins, among which LRRC8A is essential, while LRRC8B/C/D/E serve as exchangeable complementary partners.
We used an RNAi approach and radiotracer assays to explore which LRRC8 isoforms contribute to swelling‐activated release of diverse organic osmolytes in rat astrocytes.
Efflux of uncharged osmolytes (myo‐inositol and taurine) was suppressed by deletion of LRRC8A or LRRC8D, but not by deletion of LRRC8C+LRRC8E.
Conversely, release of charged osmolytes (d‐aspartate) was strongly reduced by deletion of LRRC8A or LRRC8C+LRRC8E, but largely unaffected by downregulation of LRRC8D.
Our findings point to the existence of multiple heteromeric VRACs in the same cell type: LRRC8A/D‐containing heteromers appear to dominate release of uncharged osmolytes, while LRRC8A/C/E, with the additional contribution of LRRC8D, creates a conduit for movement of charged molecules.
Abstract
The volume‐regulated anion channel (VRAC) is the ubiquitously expressed vertebrate Cl−/anion channel that is composed of proteins belonging to the LRRC8 family and activated by cell swelling. In the brain, VRAC contributes to physiological and pathological release of a variety of small organic molecules, including the amino acid neurotransmitters glutamate, aspartate and taurine. In the present work, we explored the role of all five LRRC8 family members in the release of organic osmolytes from primary rat astrocytes. Expression of LRRC8 proteins was modified using an RNAi approach, and amino acid fluxes via VRAC were quantified by radiotracer assays in cells challenged with hypoosmotic medium (30% reduction in osmolarity). Consistent with our prior work, knockdown of LRRC8A potently and equally suppressed the release of radiolabelled d‐[14C]aspartate and [3H]taurine. Among other LRRC8 subunits, downregulation of LRRC8D strongly inhibited release of the uncharged osmolytes [3H]taurine and myo‐[3H]inositol, without major impact on the simultaneously measured efflux of the charged d‐[14C]aspartate. In contrast, the release of d‐[14C]aspartate was preferentially sensitive to deletion of LRRC8C+LRRC8E, but unaffected by downregulation of LRRC8D. Finally, siRNA knockdown of LRRC8C+LRRC8D strongly inhibited the release of all osmolytes. Overall, our findings suggest the existence of at least two distinct heteromeric VRACs in astroglial cells. The LRRC8A/D‐containing permeability pathway appears to dominate the release of uncharged osmolytes, while an alternative channel (or channels) is composed of LRRC8A/C/D/E and responsible for the loss of charged molecules.
Keywords: anion channel, cell volume regulation, organic osmolyte release, volume‐regulated anion channel
Key points
The volume‐regulated anion channel (VRAC) is a swelling‐activated chloride channel that is permeable to inorganic anions and a variety of small organic molecules.
VRAC is formed via heteromerization of LRRC8 proteins, among which LRRC8A is essential, while LRRC8B/C/D/E serve as exchangeable complementary partners.
We used an RNAi approach and radiotracer assays to explore which LRRC8 isoforms contribute to swelling‐activated release of diverse organic osmolytes in rat astrocytes.
Efflux of uncharged osmolytes (myo‐inositol and taurine) was suppressed by deletion of LRRC8A or LRRC8D, but not by deletion of LRRC8C+LRRC8E.
Conversely, release of charged osmolytes (d‐aspartate) was strongly reduced by deletion of LRRC8A or LRRC8C+LRRC8E, but largely unaffected by downregulation of LRRC8D.
Our findings point to the existence of multiple heteromeric VRACs in the same cell type: LRRC8A/D‐containing heteromers appear to dominate release of uncharged osmolytes, while LRRC8A/C/E, with the additional contribution of LRRC8D, creates a conduit for movement of charged molecules.
Abbreviations
- GABA
γ‐aminobutyric acid
- LRRC8
leucine‐rich repeat containing 8
- RVD
regulatory volume decrease
- VRAC
volume‐regulated anion channel
Introduction
The volume‐regulated anion channel (VRAC) is the physiologically essential anion/osmolyte release pathway that allows animal cells to regulate their volume when they encounter osmotic swelling. The principal role of VRAC is an ‘emergency’ release of Cl− and other inorganic anions, such as HCO3 −, which accompanies and facilitates the loss of intracellular K+ and osmotically obligated water (Nilius et al. 1997; Okada, 1997; Lang et al. 1998; Mongin & Orlov, 2001; Hoffmann et al. 2009). Since their initial discovery in lymphocytes and epithelial cells, swelling‐activated Cl− currents have been identified in nearly every vertebrate cell type studied, but their molecular identity remained unknown (Cahalan & Lewis, 1988; Hazama & Okada, 1988; Strange et al. 1996; Nilius et al. 1997; Okada, 1997; Pedersen et al. 2016). Two recent seminal studies independently discovered that VRAC is formed by proteins belonging to the family of leucine‐rich repeat containing 8 (LRRC8) (Qiu et al. 2014; Voss et al. 2014). Among the five homologous LRRC8 proteins, LRRC8A (or SWELL1) appears to be essential for channel function but must heteromerize with at least one additional isoform, LRRC8B–E (Qiu et al. 2014; Voss et al. 2014; Stauber, 2015; Jentsch, 2016). Based on the pannexin homology, it has been predicted that VRAC is a hexameric channel with one central pore; however, octameric assembly is also possible based on the recent work involving native gel electrophoresis (Abascal & Zardoya, 2012; Syeda et al. 2016).
In addition to inorganic anions, VRAC, or the similar volume‐sensitive anion channel, is thought to be permeable to a variety of small organic molecules. These include the negatively charged excitatory amino acids and monocarboxylates (aspartate, glutamate and lactate), weakly charged amino acid zwitterions (such as alanine, glutamine, glycine and taurine), and uncharged polyols (e.g. myo‐inositol and sorbitol) (Strange et al. 1996; Kirk, 1997; Nilius et al. 1997; Wehner et al. 2003; Pedersen et al. 2016). The main evidence for VRAC‐mediated transport of organic osmolytes was derived from electrophysiological studies measuring currents carried by organic anions. The electrophysiological findings were corroborated by numerous reports on the shared pharmacological profile of VRAC and a VRAC‐like pathway(s) transporting organic osmolytes (Jackson & Strange, 1993; Boese et al. 1996; Strange et al. 1996; Kirk, 1997; Nilius et al. 1997; Wehner et al. 2003; Abdullaev et al. 2006). The quantitative contribution of organic osmolyte fluxes to regulatory volume decrease (RVD) varies from strong to negligible, depending on the cell type and cytosolic abundance of organic osmolyte species (Kirk, 1997; Kinne, 1998; Wehner et al. 2003). Yet, regardless of the role in cell volume regulation, release of organic molecules has major physiological and pathological significance because many of the released osmolytes possess signalling properties or represent important intracellular metabolites (Kirk, 1997; Mongin, 2016). In the brain, several of the major organic osmolytes, notably glutamate and taurine, act as neurotransmitters and, therefore, the physiological significance of VRAC in this tissue is disproportionately high (Mongin, 2016).
Before the discovery of the LRRC8 family, VRAC involvement in the release of various organic osmolytes was questioned (reviewed in Kirk, 1997; Franco, 2003; Shennan, 2008). Several studies provided substantial albeit indirect evidence that inorganic anions and certain organic molecules, such as taurine, may be transported via separate volume‐sensitive pathways (see, for example, Lambert & Hoffmann, 1994; Shennan et al. 1994; Mongin et al. 1999; Stutzin et al. 1999). This idea has been challenged following the identification of LRRC8A. Recent reports provided direct molecular biological evidence for the involvement of LRRC8A in the release of taurine, excitatory amino acids, GABA, glycine, lactate and myo‐inositol (Hyzinski‐Garcia et al. 2014; Qiu et al. 2014; Voss et al. 2014; Gaitan‐Penas et al. 2016; Lutter et al. 2017). Nevertheless, the long‐standing question remains: do LRRC8 proteins form a single, ‘universal’ organic osmolyte channel, or are there multiple swelling‐activated release pathways? The first molecular evidence for the diversity of VRAC channels has been provided by T. J. Jentsch's laboratory, which discovered that LRRC8A–LRRC8D heteromers are specifically responsible for swelling‐activated release of taurine and uptake of the chemotherapy drug cisplatin (Planells‐Cases et al. 2015). The same group further explored the definitive contribution of other LRRC8 subunits to release of diverse organic osmolytes (Lutter et al. 2017). Our present work adds to existing knowledge in two major ways: using RNAi and double‐labelling radiotracer approaches, we shed light on the molecular composition of the native organic osmolyte channels in primary brain cells – astrocytes – and further demonstrate the existence of multiple functionally diverse LRRC8‐containing organic osmolyte channels within a single cell type.
Methods
Ethics statement
All animal procedures in this study were approved by the Institutional Animal Care and Use Committee of the Albany Medical College and strictly conformed to the NIH Guidelines for Care and Use of Laboratory Animals.
Primary astrocyte cultures
Primary rat astrocytes were prepared from newborn or 1‐day‐old Sprague–Dawley rat pups as described in detail elsewhere (Mongin et al. 2011). Briefly, postnatal pups of both sexes were killed by rapid decapitation; their cortices were aseptically dissected from the meninges and hippocampi, and collected into ice‐cold Opti‐MEM. Opti‐MEM and all other cell culture components were from Thermo Fisher Scientific (Waltham, MA, USA), unless otherwise specified. The cortical tissue was minced and transferred into a solution of the recombinant protease TrypLE diluted with Opti‐MEM (1:1, v/v), and additionally supplemented with DNase I (Sigma‐Aldrich, St Louis, MO, USA; 1 mg mL−1). Three enzymatic extractions were carried out at 37°C with continuous slow stirring. The first extraction was discarded and the last two extractions were combined. Brain cells were sedimented by brief centrifugation (1.5 min, ∼1000 g), resuspended in minimum essential medium (MEM) containing 10% heat inactivated horse serum (HIHS), and 50 U mL−1 penicillin and 50 μg mL−1 streptomycin (P/S), and then plated on poly‐d‐lysine‐coated T‐75 culture flasks at a density of 200,000 cells per flask. The primary cultures were grown in MEM plus 10% HIHS, and antibiotics for 2–3 weeks in a humidified atmosphere of 5% CO2, balance air at 37°C. Cell culture medium was changed twice per week. Confluent cultures contained 95–98% astrocytes, as periodically checked by staining with monoclonal antibodies against the astroglial cell marker, glial fibrillary acidic protein (Sigma‐Aldrich, cat. no. G3893).
siRNA transfection of primary astrocytes
Knockdown of VRAC subunits was performed using siRNA constructs from Qiagen (Valencia, CA, USA), as described elsewhere (Hyzinski‐Garcia et al. 2014). Briefly, primary astrocytes grown to 80–90% confluency were transfected using 50 nm siRNA and the Lipofectamine RNAiMAX transfection reagent (Thermo Fisher Scientific) as per the manufacturer's instructions. siRNA/Lipofectamine complexes were prepared in Opti‐MEM, and then added to cells grown in serum‐containing media. After 4‐h incubation, the media were completely replaced with fresh MEM+HIHS+P/S. Changes in mRNA expression levels were determined 48 h post transfection and all functional assays were performed at 96 h. As a negative control, we used the AllStars scrambled siRNA from Qiagen. The catalogue numbers and target sequences for all siRNA species are presented in Table 1.
Table 1.
qPCR primers and siRNA constructs
| Target gene | Catalogue no. for qPCR primers | siRNA name (catalogue no.) | siRNA target sequence |
|---|---|---|---|
| NC | N/A | AllStars NC (SI03650318) | Scrambled (proprietary) |
| LRRC8A | QT01575483 | siLRRC8A_4 (SI01725360) | 5′‐CTCTACCTGAACCGCAACAAA‐3′ |
| LRRC8B | QT00434805 | siLRRC8B_1 (SI01673007) | 5′‐ATGGTCTCACGTCGTCCTATA‐3′ |
| siLRRC8B_2 (SI01673014) | 5′‐CAGATAATCGTCAAAGTCATT‐3′ | ||
| siLRRC8B_4 (SI01673028) | 5′‐CTGGCACAATAACATCGCTTA‐3′ | ||
| LRRC8C | QT01583897 | siLRRC8C_1 (SI01550815) | 5′‐TTGGATGTACTTTACAAGTCA‐3′ |
| siLRRC8C_2 (SI01550822) | 5′‐ACGGCCTGACGTGCCTTTATA‐3′ | ||
| siLRRC8C_4 (SI01550836) | 5′‐TCCCGAGAAGTTCGTGGTTGA‐3′ | ||
| LRRC8D | QT00370111 | siLRRC8D_1 (SI01904287) | 5′‐AGGCATTGAATCAAGACGTAA‐3′ |
| siLRRC8D_2 (SI01904294) | 5′‐CAGCAGTACGTATTTATCAAT‐3′ | ||
| siLRRC8D_4 (SI01904308) | 5′‐TGCCGTCGCCTGCAAATTCAA‐3′ | ||
| LRRC8E | QT01591352 | siLRRC8E_2 (SI01665146) | 5′‐AACGATCAACATATATTTAAG‐3′ |
| siLRRC8E_3 (SI01665153) | 5′‐ATGCCAAGTCCTTATGTTATA‐3′ | ||
| siLRRC8E_4 (SI01665160) | 5′‐CAGCTTAGTTAGTTTCCTATA‐3′ | ||
| GAPDH | QT00199633 | N/A | N/A |
| RPL13a | QT00178675 | N/A | N/A |
| RPS20 | QT00431333 | N/A | N/A |
All validated qPCR primers and RNAi constructs were from Qiagen. N/A, not applicable; NC, negative control.
Measurement of mRNA expression levels using qRT‐PCR
Relative gene expression and efficacy of RNAi gene knockdowns were determined using quantitative RT‐PCR (qRT‐PCR). Primary astrocytes grown in 60 mm Petri dishes were transfected as described above. Forty‐eight hours post‐transfection, mRNA was isolated using the RNAqueous‐4PCR kit (Thermo Fisher Scientific) according to the manufacturer's protocol. The isolated mRNA was immediately converted to cDNA using the iScript cDNA synthesis kit (Bio‐Rad Laboratories, Hercules, CA, USA). Gene expression levels were analysed by quantitative PCR with the SYBR Green master mix (Bio‐Rad) and gene‐specific qPCR primers in a CFX96 Real Time PCR set‐up (Bio‐Rad). The expression levels were normalized within each sample to three housekeeping genes: RPL13a, RPS20 and GAPDH. All primers used are listed in Table 1.
Radiotracer amino acid release assays
Release rates for the major intracellular organic osmolytes glutamate, taurine and myo‐inositol were quantified using the previously established radiotracer efflux assay (Mongin & Kimelberg, 2005b; Bowens et al. 2013; Hyzinski‐Garcia et al. 2014). We used the non‐metabolizable glutamate analogue d‐[14C]aspartate (55 mCi mmol−1, American Radiolabelled Chemicals, St Louis, MO, USA), [3H]taurine (20 Ci mmol−1, from either American Radiolabelled Chemicals or Perkin Elmer, Waltham, MA, USA), and myo‐[3H]inositol (20.1 Ci mmol−1, Perkin Elmer). Primary astrocytes grown to 80–90% confluency on 18 × 18 mm glass coverslips were transfected with siRNA as described above. Prior to transport experiments, cells were loaded overnight with radiolabelled amino acids by incubating them in cell culture medium containing 0.1–0.2 μCi mL−1 d‐[14C]aspartate and 1–2 μCi mL−1 [3H]taurine or 4–6 μCi mL−1 myo‐[3H]inositol.
To increase experimental throughput, the initial efflux assays were performed in a 12‐well plate format. In this experimental design, cells grown in individual wells were washed from extracellular radiotracers, and then pre‐incubated in Basal isoosmotic medium that has the following composition (in mm): 135 NaCl, 3.8 KCl, 1.2 MgSO4, 1.3 CaCl2, 1.2 KH2PO4, 10 d‐glucose, 10 Hepes (pH 7.4, osmolarity 290 ± 2 mosmol L−1). The Basal medium was then replaced with Na+‐free media, in which Na+ ions were removed by isoosmotic replacement of NaCl with LiCl. This was necessary because astrocytes express very high densities of Na+‐dependent glutamate transporters (GLAST), and in a plate format, re‐accumulate a significant fraction of glutamate released during cell swelling (Schober & Mongin, 2015). Experimental media were replaced and collected three times at 10 min intervals. During the last incubation period, cells were challenged with either isoosmotic (as a control) or hypoosmotic (to induce cell swelling) Na+‐free media. In the hypoosmotic Na+‐free medium, [LiCl] was reduced by 50 mm while concentrations of all other components remained the same (osmolarity ≈ 200 mosmol L−1, ∼30% reduction as compared to isoosmotic conditions). Ten‐minute fractions of isoosmotic or hypoosmotic media were manually collected and analysed for the radiotracer content. The integral release values were normalized to the remaining isotope content in cells, which were lysed using a solution of 2% SDS and 8 mm EDTA, and then compared between isoosmotic versus hypo‐osmotic conditions. 3H and 14C contents in each vial were determined off‐line using a TriCarb 2900 scintillation counter (Perkin Elmer) after adding Ecoscint A scintillation fluid (National Diagnostics, Atlanta, GA, USA).
The majority of osmolyte efflux assays were performed in a Lucite superfusion chamber that has a depression on the bottom to accommodate an 18 × 18 mm glass coverslip and a Teflon screw lid that leaves a ∼200 μm space above the cells. The chamber was continuously perfused with Hepes‐buffered Basal medium or hypoosmotic medium at a flow rate of ∼1.2 mL min−1, in a thermostat incubator at 37°C. The medium in the chamber was exchanged at least 10 times min−1. Because of the fast superfusion rate, d‐[14C]aspartate reuptake was minimal and all experiments were performed in Na+‐containing media. The composition of Basal isoosmotic medium is given above. In the hypoosmotic medium, [NaCl] was reduced by 50 mm while concentrations of all other components remained the same (osmolarity ≈ 200 mosmol L−1, ∼30% reduction). One‐minute superfusate fractions were gathered into scintillation vials using an automated collector. At the end of each experiment, cells were lysed using SDS plus EDTA. The 1‐min fractional release rates were calculated in relation to the total 14C/3H isotope content, which was calculated ‘retrospectively’ using an Excel‐based custom computer program.
Calculations of amino acid net charge
To determine the net charge of the zwitterionic taurine at various pH values, we used a calculation approach based on the Henderson‐Hasselbalch ionization equilibrium equation as originally proposed by Moore (1985). The net charge (Q) of the negative sulfonic and positive amino groups were derived from the following equation:
pKa values of sulfonic and amino groups were accepted as 1.5 and 8.82, respectively. Using these values and calculations, at pH 7.4 only 3.7% of taurine molecules are negatively charged, while at pH 9.8 the fraction of charged molecules reaches ∼91%.
Statistical analysis
All data are presented as means ± SEM. In the majority of cases, statistical differences between experimental groups were determined using one‐way ANOVA followed by the Bonferroni post hoc test for multiple comparisons. In osmolyte release experiments, we separately evaluated (1) maximal release rates in swollen cells, representing amplitude of VRAC response, and (2) the total integral release under hypoosmotic conditions reflecting ‘total’ VRAC activity over a 10 min period in cells which actively regulate their volume. We further evaluated the osmolyte release kinetics using a two‐way ANOVA with Bonferroni post hoc test (only the effects of treatment are presented). Comparisons of normalized expression values were done with Student's one‐sample t test and a Bonferroni post hoc test for multiple comparisons. Prism 5.0 (GraphPad Software, San Diego, CA, USA) was used for statistical analyses. Origin 8.1 (OriginLab, Northhampton, MA, USA) was used for graphing purposes.
Results
Relative expression of LRRC8 subunits and validation of LRRC8 gene‐specific siRNA constructs in primary rat astrocytes
To determine the relative expression of all LRRC8 subunits in astrocytes, we performed qRT‐PCR analysis. Experiments similar in scope have been conducted in our prior study (Hyzinski‐Garcia et al. 2014); however, it was important to re‐test the relative abundance of the LRRC8 isoforms in primary astrocyte cultures which were utilized in the present work. As shown in Fig. 1 A, we found that LRRC8A, B, C and D were expressed at very similar levels (100%, 123%, 75% and 130% for A, B, C and D subunits, respectively, as normalized to LRRC8A). The LRRC8E mRNA levels were significantly lower (∼5%, as compared to LRRC8A).
Figure 1. Relative mRNA expression levels for five members of the LRRC8 protein family and validation of RNAi knockdown of each of the five subunits in primary rat astrocytes.
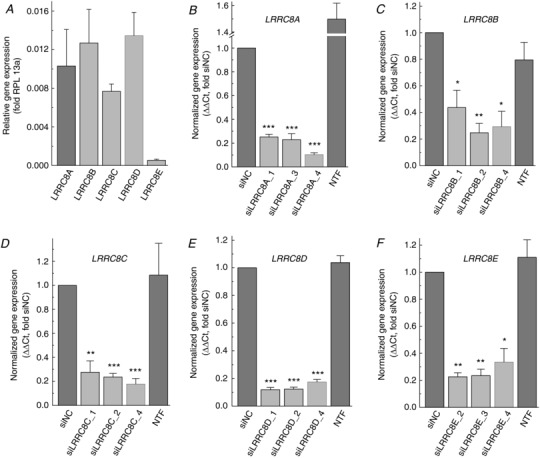
A, quantification of mRNA expression levels for LRRC8A–E using qRT‐PCR. Expression levels were normalized to ribosomal RPL13a within the same samples. Data are the mean values ± SEM of 6 independently prepared astrocytic cultures. B–F, effects of gene‐specific LRRC8 siRNA constructs on mRNA expression levels measured 48 h post‐transfection. LRRC8A knockdown data are derived from Hyzinski‐García et al. (2014) with permission. LRRC8B–E knockdown results are the means ± SEM of 3–4 independent transfections in at least 3 different astrocytic cultures. * P < 0.05, ** P < 0.01, *** P < 0.001 vs. siNC (negative control siRNA). NTF, non‐transfected cells.
To knockdown individual LRRC8 subunits, we tested three commercially available siRNAs per subunit, which targeted distinct regions of each LRRC8 mRNA species (target sequences shown in Table 1). The LRRC8A‐targeting siRNAs were validated in our prior work (Hyzinski‐Garcia et al. 2014), but additionally shown in Fig. 1 B for reference purposes. All of the tested siRNAs reduced LRRC8 subunit expression by 60–90%, with some constructs demonstrating more effective knockdown than others (Fig. 1 B–F). In all the subsequent functional experiments, we utilized the most effective siRNA species (≥ 80% efficacy).
Preliminary screens for the role of LRRC8 proteins in swelling‐activated release of organic osmolytes
To probe for the relative significance of each of the LRRC8A–E subunits in organic osmolyte release from primary rat astrocytes, we initially used simultaneously measured efflux of [3H]taurine and d‐[14C]aspartate in a multi‐well plate format, which allows for a medium‐throughput collection of data. As outlined in Methods, these experiments were performed in Na+‐free hypoosmotic medium to prevent the highly effective reuptake of d‐[14C]aspartate by the Na+‐dependent excitatory amino acid transporter GLAST (Schober & Mongin, 2015). As shown in Fig. 2, the most effective reduction of swelling‐activated release of both [3H]taurine and d‐[14C]aspartate was observed with siRNA targeting LRRC8A (∼70% inhibition). LRRC8B knockdown was completely ineffective in blocking release of both radiotracers. Downregulation of LRRC8C, LRRC8D and LRRC8E produced moderate (∼25–30%) inhibition of d‐[14C]aspartate release (Fig. 2). Efflux of [3H]taurine was impacted in a similar fashion for LRRC8C and LRRC8E. However, targeting LRRC8D uniquely inhibited the release of [3H]taurine significantly more as compared to d‐[14C]aspartate (Fig. 2, 45% vs. 25%, P < 0.001).
Figure 2. Preliminary screening for the effects of LRRC8A–E siRNAs on swelling‐activated d‐[14C]aspartate and [3H]taurine release from primary astrocytes.
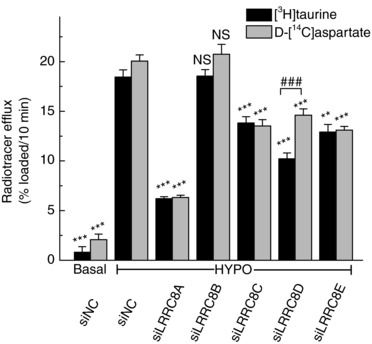
Astrocytes grown on 12‐well plates were transfected with gene‐specific LRRC8 siRNAs. Ninety‐six hours post‐transfection, [3H]taurine and d‐[14C]aspartate release values were measured simultaneously in cells preloaded with both radiotracers. Integral 10‐min release values were quantified for isoosmotic (290 mosmol L−1) or hypoosmotic (200 mosmol L−1) conditions. Na+‐free media was used to prevent d‐[14C]aspartate reuptake (see Methods). Data are the means ± SEM of 6–9 independent experiments per treatment group and 18 controls, performed in at least 2 different astrocyte cultures. ** P < 0.01, *** P < 0.001, vs. negative control siRNA (siNC). ### P < 0.001 [3H]taurine vs. d‐[14C]aspartate release.
RNAi knockdown of LRRC8D potently inhibits swelling‐activated release of taurine and myo‐inositol with no effect on the efflux of glutamate
Our results on the potential unique role of the LRRC8D protein in VRAC channels contributing to release of [3H]taurine resemble the recent findings in HEK293 cells (Planells‐Cases et al. 2015; Lutter et al. 2017). To further explore the specific contribution of the LRRC8D subunit in the release of organic osmolytes, we conducted double‐label radiotracer assays in a Lucite superfusion set‐up, allowing for high temporal resolution collection of data. Rapid medium exchange in the Lucite chamber allowed for the measurement of d‐[14C]aspartate release in hypoosmotic medium with ‘physiological’ Na+ levels because the speed of perfusion minimizes Na+‐dependent reuptake of excitatory amino acids. As shown in Fig. 3 A, LRRC8D knockdown with two independent siRNA constructs equally and significantly inhibited [3H]taurine efflux by ∼50%. In striking contrast, the release of d‐[14C]aspartate was essentially insensitive to LRRC8D knockdown, even though it was measured in the same cells. As a positive control, knockdown of LRRC8A produced a strong and approximately equal inhibition of both [3H]taurine and d‐[14C]aspartate release, again measured in the same cells (compare Fig. 3 A and B).
Figure 3. Effects of the LRRC8A or LRRC8D RNAi knockdowns on swelling‐activated efflux of [3H]taurine (A) and d‐[14C]aspartate (B).
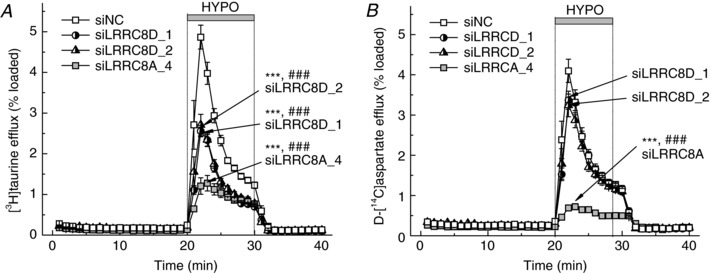
Astrocytic [3H]taurine and d‐[14C]aspartate release kinetics were measured simultaneously in cells preloaded with both radiotracers. Cells were transfected with negative control siRNA (siNC) or gene specific siLRRC8A, siLRRC8D_1, or siLRRC8D_2. For clarity, the results with [3H]taurine and d‐[14C]aspartate are presented in separate panels. Data are the means ± SEM of 6–7 independent experiments per group performed in 3 different astrocyte cultures. *** P < 0.001 maximal release values vs. siNC; ### P < 0.001 integral 10‐min release values vs. siNC.
The insensitivity of d‐[14C]aspartate release to LRRC8D knockdown differed from the data of ‘plate’ release assays, which demonstrated limited, but statistically significant inhibition with the same treatment (Fig. 2). Similar trends were observed for knockdowns of LRRC8C and LRRC8E (see below). These relatively small discrepancies between two assays are likely due to the differences in experimental conditions: ‘plate’ assays were done in Na+‐free media, while the Lucite chamber experiments were performed with Na+‐containing media.
In order to understand the basis for apparent discrimination between [3H]taurine and d‐[14C]aspartate by the LRRC8D‐containing VRAC, we explored the effect of LRRC8D siRNA on the release of the uncharged polyol myo‐[3H]inositol. As seen in Fig. 4, two independent siRNA constructs which targeted LRRC8D nearly completely inhibited swelling‐activated myo‐[3H]inositol efflux. The same degree of inhibition was seen in cells treated with the LRRC8A siRNA (Fig. 4). These data strongly suggest that LRRC8A/LRRC8D heteromers represent the main permeability route for myo‐[3H]inositol.
Figure 4. Effects of LRRC8A or LRRC8D knockdowns on the swelling‐activated release of myo‐[3H]inositol.
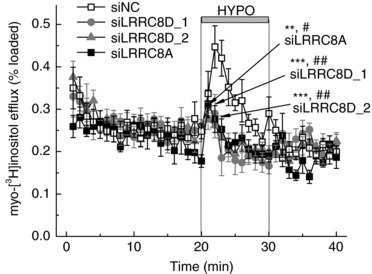
Hypoosmotic medium‐activated myo‐[3H]inositol efflux was measured in cells transfected with negative control siRNA (siNC) or the gene‐specific siLRRC8A, siLRRC8D_1, or siLRRC8D_2. Data are the means ± SEM of 6–8 independent experiments per group performed in 2 astrocyte cultures. ** P < 0.01, *** P < 0.001, maximal release values vs. negative control siRNA (siNC); # P < 0.05, ## P < 0.01 integral 10‐min release values vs. siNC.
Combined siRNA knockdown of LRRC8C and LRRC8E suppresses efflux of glutamate with no impact on release of taurine or myo‐inositol
Based on the data presented here and elsewhere (Planells‐Cases et al. 2015; Lutter et al. 2017), LRRCA/D heteromers are likely responsible for the release of uncharged osmolytes, such as taurine and myo‐inositol. The molecular composition of the release pathway for charged osmolytes including the excitatory amino acids glutamate and aspartate is less clear. To address this gap in knowledge, we measured osmolyte release kinetics in astrocytes treated with siRNAs targeting LRRC8C and LRRC8E. Individual knockdowns of LRRC8C and E produced no significant impact on the efflux of [3H]taurine (Fig. 5 A) or d‐[14C]aspartate (Fig. 5 B). We next performed double knockdown of LRRC8C and LRRC8E, which again produced no effect on the release of [3H]taurine (Fig. 6 A), but in the same cells significantly reduced efflux of d‐[14C]aspartate by ∼40% (Fig. 6 B, P < 0.01). The additive effects of LRRC8C and LRRC8E deletion suggest that these subunits are mutually replaceable and together are responsible for the movement of negatively charged amino acids.
Figure 5. Effects of LRRC8C or LRRC8E knockdown on astrocytic swelling‐activated efflux of [3H]taurine (A) and d‐[14C]aspartate (B).
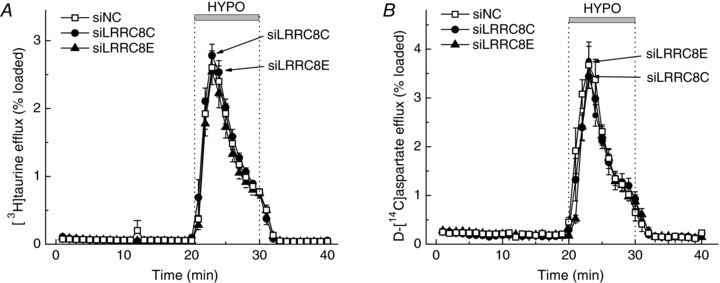
Cells were transfected with negative control siRNA (siNC), or the gene‐specific siLRRC8C or siLRRC8E. [3H]Taurine and d‐[14C]aspartate release rates were measured simultaneously in cells preloaded with both radiotracers. Data are the means ± SEM of 4–5 independent experiments per group performed in 3 different astrocyte cultures.
Figure 6. Effects of the double knockdowns of LRRC8C+E or LRRC8C+D on the swelling‐activated efflux of [3H]taurine (A) and d‐[14C]aspartate (B).
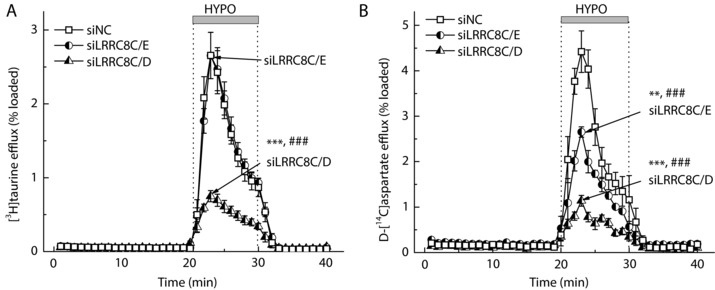
Astrocytes were transfected with negative control siRNA (siNC) or the gene‐specific siLRRC8C plus siLRRC8E, or siLRRC8C plus siLRRC8D. [3H]Taurine and d‐[14C]aspartate release rates were measured simultaneously in cells preloaded with both radiotracers. Data are the means ± SEM of 4–5 independent experiments per group performed in 3 different astrocyte cultures. ** P < 0.01, *** P < 0.001, maximal release values vs. siNC; ### P < 0.001, integral 10‐min release values vs. siNC.
To further verify the unique contribution of LRRC8C and LRRC8E subunits to movement of charged molecules, we measured the effect of LRRC8C and LRRC8E siRNAs on the efflux of the uncharged myo‐[3H]inositol. Similar to the results seen with [3H]taurine (Fig. 6 A), knockdown of LRRC8C or E, individually or in combination, had no effect on the efflux of myo‐[3H]inositol (Fig. 7).
Figure 7. Effects of LRRC8C, LRRC8E or LRRC8C+E knockdowns on the swelling‐activated release of myo‐[3H]inositol.
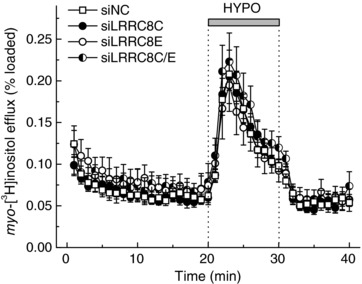
Astrocytes were transfected with the negative control siRNA (siNC) or the gene‐specific siLRRC8C, siLRRC8E, or siLRRC8C+siLRRC8E. Data are the means ± SEM of 6–8 independent experiments per group performed in 2 astrocyte cultures.
LRRC8C/D knockdown potently inhibits the release of both [3H]taurine and d‐[14C]aspartate
To complete our systematic evaluation of the contribution of LRRC8 subunits to the release of organic osmolytes, we additionally performed combined knockdowns of LRRC8C and LRRC8D. Perhaps most surprisingly, we found that simultaneous downregulation of LRRC8C and LRRC8D dramatically inhibited the efflux of both [3H]taurine and d‐[14C]aspartate measured in the same cells (Fig. 6 A and B). The degree of inhibition for the two radiotracers was very similar to the effects of deletion of the essential VRAC subunit LRRC8A (compare Fig. 6 to Fig. 3). Overall, these findings are inconsistent with a simple model suggesting that LRRC8D is present only in the ‘uncharged osmolyte’ channel (for further discussion, see the following sections).
Modification of amino acid charge with pH changes eliminates sensitivity of [3H]taurine release to LRRC8D knockdown
To further explore the idea that charged and uncharged organic osmolytes are released via separate swelling‐activated pathways, we used varying pH conditions to alter the charge on the zwitterionic taurine. This manoeuvre has been extensively used in the past for measuring taurine currents via VRAC (Jackson & Strange, 1993; Boese et al. 1996). At physiological pH 7.4, <4% of taurine is negatively charged, while the bulk of taurine molecules are electroneutral. Changing the pH to 9.8 makes ∼91% of taurine molecules negatively charged (see Methods). At pH 7.4, knockdown of LRRC8D causes a significant reduction in the [3H]taurine efflux rates (Figs 2 and 3, and new data in Fig. 8) by as much as ∼50%. When compared side‐by‐side in the multi‐well plate format, changing pH from 7.4 to pH 9.8 completely eliminated the sensitivity of taurine release to LRRC8D knockdown (Fig. 8 A). To prove that this effect is specific, we additionally downregulated the LRRC8A subunit, and found that [3H]taurine efflux is blunted independently of pH of the assay media (Fig. 8 A). Since alkaline conditions strongly increased [3H]taurine release, we normalized the data to the negative control siRNA‐treated cells at the same pH for a clearer comparison (Fig. 8 B). Overall, the results of these experiments suggest that switching the charge of the taurine molecule from neutral to charged, diverts its movement from the LRRC8A/D containing VRAC heteromers to another permeability pathway that incorporates LRRC8A but does not depend on LRRC8D (see additional discussion below).
Figure 8. Effect of extracellular pH on the sensitivity of [3H]taurine release to the knockdown of LRRC8A or LRRC8D .
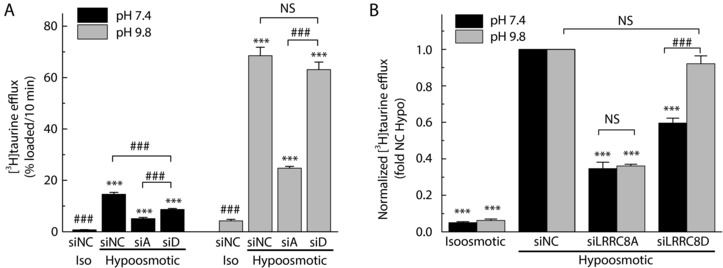
A, 10‐min [3H]taurine release values were determined in cells treated with negative control siRNA (siNC), siLRRC8A or siLRRC8D. Cells were exposed to either isoosmotic (290 mosmol L−1) or hypoosmotic (200 mosmol L−1) Na+‐free media in which pH values were adjusted to 7.4 (black bars), or 9.8 (grey bars). Data are the means ± SEM of 6–9 independent experiments per group performed in 2 different astrocyte cultures. *** P < 0.001, vs. siNC isoosmotic release values under the same pH conditions. ### P < 0.001, vs. hypoosmotic release values in siNC‐treated cells under the same pH conditions. B, the same data as in the left panel were normalized to siNC hypoosmotic release values under matching pH conditions. *** P < 0.001, vs. hypoosmotic release in siNC group; ###P < 0.001, effects of siRNA at pH 7.4 vs. pH 9.8.
Discussion
In the present study, we extensively characterized the contribution of the recently discovered LRRC8 proteins to swelling‐activated organic osmolyte release in cultured primary astrocytes, the cell type that is central to cell volume control in the CNS (see discussion in Mongin, 2016). In addition to the previously established critical role of LRRC8A, we identified (1) preferential dependence of the efflux of uncharged organic osmolytes on the expression levels of LRRC8D, and (2) selective sensitivity of the movement of the charged organic osmolytes on the presence of LRRC8C and LRRC8E. Although further biophysical studies are needed to establish the exact stoichiometry of the LRRC8‐containing glial VRACs, the simplest deduction from our work is that LRRC8A/D heteromers form a conduit for the release of uncharged organic molecules, while a more complex assembly containing LRRC8A, C, D and E creates a separate pathway that preferentially moves negatively charged osmolytes. The hypothetical composition of the two glial VRACs is shown in Fig. 9 and discussed below. These and other findings of the current work demonstrate the co‐existence of at least two functionally distinct LRRC8‐containing heteromeric VRAC channels in rat astrocytes.
Figure 9. Graphical summary of RNAi analysis of VRAC composition in rat astrocytes.
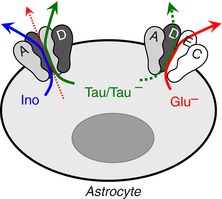
Cell swelling activates at least two populations of heteromeric VRAC channels. LRRC8A/LRRC8D‐containing heteromers dominate the release of the uncharged osmolytes myo‐inositol (Ino) and taurine (Tau) with limited movement of charged molecules (thin dotted line). A separate subset of LRRC8A/C/D/E‐containing heteromers are permeable to anionic glutamate (Gluˉ) and aspartate, and also conduct taurine in its anionic form (Tauˉ). [Color figure can be viewed at wileyonlinelibrary.com]
The existence of the LRRC8A/D‐containing channel for neutral organic osmolytes is firmly supported by our present findings and recently published data. Planells‐Cases et al. (2015) were the first to report that deletion of LRRC8D eliminates swelling‐activated release of [3H]taurine and uptake of the electroneutral chemotherapeutic agent cisplatin in KBM7 and HEK293 cells. Subsequently, a study from the same group recapitulated near complete dependence of [3H]taurine efflux on expression of LRRC8D, and found similar trends for swelling‐activated fluxes of myo‐[3H]inositol and [3H]GABA in HEK293 cells (Lutter et al. 2017). Importantly, the release of all three osmolytes were rescued by heteroexpressing the combination of LRRC8A and LRRC8D subunits in erase‐and‐replace experiments. Therefore, the LRRC8A/D heteromers are sufficient to mediate volume‐dependent release of certain organic molecules. In our hands, LRRC8D knockdown reduced release of [3H]taurine by ∼50% only, but completely inhibited efflux of myo‐[3H]inositol. Importantly, in the same cells, we observed no significant impact on simultaneously measured transport of the negatively charged d‐[14C]aspartate. The acidic amino acids aspartate and glutamate have isoelectric point (pI) values of 2.77 and 3.2, respectively, and are negatively charged at pH 7.4. The polyol myo‐inositol has no group capable of ionization and is therefore completely uncharged. The zwitterionic taurine has a pI value of 5.16, translating to <4% of the molecules carrying negative charge at pH 7.4. When we modified pH to 9.8, which makes ∼91% of taurine molecules negatively charged, [3H]taurine efflux was no longer affected by the deletion of LRRC8D. However, the inhibitory effects of LRRC8A knockdown were equally strong at pH 7.4 and 9.8. These data clearly point to the presence of an alternative native channel for negatively charged molecules, which requires LRRC8A but is not critically dependent on LRRC8D.
The molecular composition of glial VRAC, which accommodates charged organic osmolytes, appears to be complex. By the process of elimination and mRNA expression data, we first tested if LRRC8A/C heteromers mediate the movement of charged molecules. In high resolution efflux assays, deletion of LRRC8C produced no significant impact on the d‐[14C]aspartate release rates. Similarly, deletion of LRRC8E was also ineffective. Only when LRRC8C and LRRC8E siRNAs were combined, d‐[14C]aspartate (but not [3H]taurine) fluxes were strongly inhibited. On the surface, it means that both LRRC8C and LRRC8E determine the selectivity of the LRRC8‐containing VRAC for charged molecules, and that these subunits are mutually replaceable. This idea is consistent, at least in part, with the literature findings that LRRC8A/E, and to a lesser degree, LRRC8A/C heteromers are capable of sustaining d‐[14C]aspartate efflux in HEK293 cells (Lutter et al. 2017). Lutter et al. suggested that LRRC8E is the primary component of the swelling‐activated release pathway for charged molecules. This appears not to be the case in rat astrocytes, perhaps due to the low levels of LRRC8E expression. The most perplexing finding of our present work was the dramatic inhibition of d‐[14C]aspartate efflux by combination of LRRC8C+LRRC8D siRNAs. This synergistic action is inconsistent with the idea that LRRC8D is present only in the ‘electroneutral’ channel, but could be explained by the fact that deletion of LRRC8C and LRRC8D would leave only LRRC8A and E and there is not sufficient LRRC8E to sustain functional channels. Therefore, the simplest explanation for our finding is presented in Fig. 9. We think that in astroglial cells, LRRC8A partners with LRRC8C, LRRC8E and LRRC8D to form a permeability pathway for charged molecules. An alternative explanation for our siRNA results targeting charged osmolyte release would be that d‐[14C]aspartate fluxes are shared between two heteromeric channels composed of LRRC8A/C/E and LRRC8A/D subunits. In this case, combined deletion of LRRC8C and LRRC8D subunits would also strongly impact both release pathways. Yet, this latter hypothesis is poorly compatible with our experimental results, namely negligible sensitivity of the swelling‐activated d‐[14C]aspartate transport to single deletion of either LRRC8C or LRRC8D.
A separate brief discussion ought to be dedicated to the role of LRRC8B, which is abundantly expressed in rat astrocytes (our data) and the forebrain tissue (Roth et al. 2006). Prior studies consistently found that LRRC8A/B heteromers are insufficient to sustain VRAC Cl⁻ currents or organic osmolyte release (Voss et al. 2014; Gaitan‐Penas et al. 2016; Lutter et al. 2017). Yet, incorporation of LRRC8B into complexes of other LRRC8 heteromers may modify the biophysical properties (Lutter et al. 2017). In our multi‐well plate assays, we found no evidence of functional effects of LRRC8B deletion when it was targeted alone (Fig. 2) or in combination with LRRC8C or LRRC8D (data not shown). Therefore, at this stage, LRRC8B appears to be a ‘silent’ partner in formation of astrocytic VRACs, and its presence in organic osmolyte release pathways is uncertain. These negative findings should not discourage future additional LRRC8B work, as this protein has been identified as one of the consistently downregulated transcripts in brain tissue surrounding haemorrhage and lacunar strokes (Rosell et al. 2011).
The existence of separate swelling‐activated release pathways for glutamate/aspartate and taurine has important implications for brain physiology. In the central nervous system, glutamate is the most abundant organic osmolyte, which happens to serve as the main excitatory neurotransmitter (Meldrum, 2000). The aminosulfonic acid taurine is also one of the most abundant osmolytes in brain tissue, and acts as an endogenous agonist of the inhibitory glycine and GABAA receptors (Albrecht & Schousboe, 2005). Traditionally, neurons are considered the main source of neurotransmitter release, but recent work in the field pointed to astrocytes as a source of numerous biologically active molecules (‘gliotransmitters’), including glutamate and taurine (Haydon & Carmignoto, 2006). VRAC is just one among numerous gliotransmitter release routes (Malarkey & Parpura, 2008; Hamilton & Attwell, 2010), but it may be disproportionally important in astrocytes. For example, in the hypothalamus and pituitary, specialized subpopulations of astrocytes contain high levels of taurine and release it in an osmosensitive fashion. Such taurine release modulates the activity of vasopressin‐releasing neurons and, consequently, whole‐body fluid homeostasis (reviewed in Hussy et al. 2000). In the context of neuropathologies, VRAC may be strongly activated due to profound astrocytic swelling in stroke, traumatic brain injury, hyponatremia and epilepsy (reviewed in Mongin & Kimelberg, 2005a; Mongin, 2016). As a consequence of VRAC opening, astrocytes are thought to release vast quantities of glutamate and contribute to tissue damage via excessive activation of glutamate receptors. The present work provides an intriguing possibility of discrete modulation of excitatory (glutamate and aspartate) and inhibitory (taurine and glycine) gliotransmitter release pathways by targeting different subunits of the heteromeric LRRC8 channels.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
A.L.S., C.S.W. and A.A.M. designed the project. A.L.S. and C.S.W. performed experiments. A.L.S., C.S.W. and A.A.M. analysed and interpreted the data. A.L.S., C.S.W. and A.A.M. wrote the paper. All authors approved the final version of the manuscript and agreed to accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This study was supported by a grant from the National Institutes of Health (R01 NS061953) to A.A.M.
Linked articles This article is highlighted by a Perspective by Lambert. To read this Perspective, visit https://doi.org/10.1113/JP275171.
A. L. Schober and C. S. Wilson contributed equally to this work and therefore should be considered co‐first authors.
The results of this study were reported in a preliminary form at the 11th International Congress on Cell Volume Regulation and Fluid Homeostasis. Schober AL & Mongin AA (2016). Evidence for multiple LRRC8‐containing organic osmolyte release pathways in glial cells of the brain. Abstract Book. p. 71. DePaul University, Chicago, IL, August 15–18, 2016.
References
- Abascal F & Zardoya R (2012). LRRC8 proteins share a common ancestor with pannexins, and may form hexameric channels involved in cell‐cell communication. Bioessays 34, 551–560. [DOI] [PubMed] [Google Scholar]
- Abdullaev IF, Rudkouskaya A, Schools GP, Kimelberg HK & Mongin AA (2006). Pharmacological comparison of swelling‐activated excitatory amino acid release and Cl− currents in rat cultured astrocytes. J Physiol 572, 677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht J & Schousboe A (2005). Taurine interaction with neurotransmitter receptors in the CNS: an update. Neurochem Res 30, 1615–1621. [DOI] [PubMed] [Google Scholar]
- Boese SH, Wehner F & Kinne RK (1996). Taurine permeation through swelling‐activated anion conductance in rat IMCD cells in primary culture. Am J Physiol Renal Physiol 271, F498–F507. [DOI] [PubMed] [Google Scholar]
- Bowens NH, Dohare P, Kuo YH & Mongin AA (2013). DCPIB, the proposed selective blocker of volume‐regulated anion channels, inhibits several glutamate transport pathways in glial cells. Mol Pharmacol 83, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan MD & Lewis RS (1988). Role of potassium and chloride channels in volume regulation by T lymphocytes. Soc Gen Physiol Ser 43, 281–301. [PubMed] [Google Scholar]
- Franco R (2003). Osmosensitive taurine release: does taurine share the same efflux pathway with chloride and other amino acid osmolytes? Adv Exp Med Biol 526, 189–196. [PubMed] [Google Scholar]
- Gaitan‐Penas H, Gradogna A, Laparra‐Cuervo L, Solsona C, Fernandez‐Duenas V, Barrallo‐Gimeno A, Ciruela F, Lakadamyali M, Pusch M & Estevez R (2016). Investigation of LRRC8‐mediated volume‐regulated anion currents in Xenopus oocytes. Biophys J 111, 1429–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB & Attwell D (2010). Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci 11, 227–238. [DOI] [PubMed] [Google Scholar]
- Haydon PG & Carmignoto G (2006). Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 86, 1009–1031. [DOI] [PubMed] [Google Scholar]
- Hazama A & Okada Y (1988). Ca2+ sensitivity of volume‐regulatory K+ and Cl− channels in cultured human epithelial cells. J Physiol 402, 687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann EK, Lambert IH & Pedersen SF (2009). Physiology of cell volume regulation in vertebrates. Physiol Rev 89, 193–277. [DOI] [PubMed] [Google Scholar]
- Hussy N, Deleuze C, Desarmenien MG & Moos FC (2000). Osmotic regulation of neuronal activity: a new role for taurine and glial cells in a hypothalamic neuroendocrine structure. Prog Neurobiol 62, 113–134. [DOI] [PubMed] [Google Scholar]
- Hyzinski‐Garcia MC, Rudkouskaya A & Mongin AA (2014). LRRC8A protein is indispensable for swelling‐activated and ATP‐induced release of excitatory amino acids in rat astrocytes. J Physiol 592, 4855–4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PS & Strange K (1993). Volume‐sensitive anion channels mediate swelling‐activated inositol and taurine efflux. Am J Physiol Cell Physiol 265, C1489–C1500. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ (2016). VRACs and other ion channels and transporters in the regulation of cell volume and beyond. Nat Rev Mol Cell Biol 17, 293–307. [DOI] [PubMed] [Google Scholar]
- Kinne RK (1998). Mechanisms of osmolyte release. Contrib Nephrol 123, 34–49. [DOI] [PubMed] [Google Scholar]
- Kirk K (1997). Swelling‐activated organic osmolyte channels. J Membr Biol 158, 1–16. [DOI] [PubMed] [Google Scholar]
- Lambert IH & Hoffmann EK (1994). Cell swelling activates separate taurine and chloride channels in Ehrlich mouse ascites tumor cells. J Membr Biol 142, 289–298. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E & Haussinger D (1998). Functional significance of cell volume regulatory mechanisms. Physiol Rev 78, 247–306. [DOI] [PubMed] [Google Scholar]
- Lutter D, Ullrich F, Lueck JC, Kempa S & Jentsch TJ (2017). Selective transport of neurotransmitters and modulators by distinct volume‐regulated LRRC8 anion channels. J Cell Sci 130, 1122–1133. [DOI] [PubMed] [Google Scholar]
- Malarkey EB & Parpura V (2008). Mechanisms of glutamate release from astrocytes. Neurochem Int 52, 142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum BS (2000). Glutamate as a neurotransmitter in the brain: review of physiology and pathology 2. J Nutr 130, 1007S–1015S. [DOI] [PubMed] [Google Scholar]
- Mongin AA (2016). Volume‐regulated anion channel—a frenemy within the brain. Pflugers Arch 468, 421–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongin AA, Hyzinski‐Garcia MC, Vincent MY & Keller RW Jr (2011). A simple method for measuring intracellular activities of glutamine synthetase and glutaminase in glial cells. Am J Physiol Cell Physiol 301, C814–C822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongin AA & Kimelberg HK (2005a). Astrocytic swelling in neuropathology In Neuroglia, ed. Kettenmann H. & Ransom BR, pp. 550–562. Oxford University Press, Oxford/New York. [Google Scholar]
- Mongin AA & Kimelberg HK (2005b). ATP regulates anion channel‐mediated organic osmolyte release from cultured rat astrocytes via multiple Ca2+‐sensitive mechanisms. Am J Physiol Cell Physiol 288, C204–C213. [DOI] [PubMed] [Google Scholar]
- Mongin AA & Orlov SN (2001). Mechanisms of cell volume regulation and possible nature of the cell volume sensor. Pathophysiology 8, 77–88. [DOI] [PubMed] [Google Scholar]
- Mongin AA, Reddi JM, Charniga C & Kimelberg HK (1999). [3H]Taurine and D‐[3H]aspartate release from astrocyte cultures are differently regulated by tyrosine kinases. Am J Physiol Cell Physiol 276, C1226–C1230. [DOI] [PubMed] [Google Scholar]
- Moore DS (1985). Amino acid and peptide net charges: a simple calculation procedure. Biochem Educ 13, 10–11. [Google Scholar]
- Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V & Droogmans G (1997). Properties of volume‐regulated anion channels in mammalian cells. Prog Biophys Mol Biol 68, 69–119. [DOI] [PubMed] [Google Scholar]
- Okada Y (1997). Volume expansion‐sensing outward‐rectifier Cl− channel: fresh start to the molecular identity and volume sensor. Am J Physiol Cell Physiol 273, C755–C789. [DOI] [PubMed] [Google Scholar]
- Pedersen SF, Okada Y & Nilius B (2016). Biophysics and physiology of the volume‐regulated anion channel (VRAC)/volume‐sensitive outwardly rectifying anion channel (VSOR). Pflugers Arch 468, 371–383. [DOI] [PubMed] [Google Scholar]
- Planells‐Cases R, Lutter D, Guyader C, Gerhards NM, Ullrich F, Elger DA, Kucukosmanoglu A, Xu G, Voss FK, Reincke SM, Stauber T, Blomen VA, Vis DJ, Wessels LF, Brummelkamp TR, Borst P, Rottenberg S & Jentsch TJ (2015). Subunit composition of VRAC channels determines substrate specificity and cellular resistance to Pt‐based anti‐cancer drugs. EMBO J 34, 2993–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP & Patapoutian A (2014). SWELL1, a plasma membrane protein, is an essential component of volume‐regulated anion channel. Cell 157, 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell A, Vilalta A, Garcia‐Berrocoso T, Fernandez‐Cadenas I, Domingues‐Montanari S, Cuadrado E, Delgado P, Ribo M, Martinez‐Saez E, Ortega‐Aznar A & Montaner J (2011). Brain perihematoma genomic profile following spontaneous human intracerebral hemorrhage. PLoS One 6, e16750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RB, Hevezi P, Lee J, Willhite D, Lechner SM, Foster AC & Zlotnik A (2006). Gene expression analyses reveal molecular relationships among 20 regions of the human CNS. Neurogenetics 7, 67–80. [DOI] [PubMed] [Google Scholar]
- Schober AL & Mongin AA (2015). Intracellular levels of glutamate in swollen astrocytes are preserved via neurotransmitter reuptake and de novo synthesis: implications for hyponatremia. J Neurochem 135, 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shennan DB (2008). Swelling‐induced taurine transport: relationship with chloride channels, anion‐exchangers and other swelling‐activated transport pathways. Cell Physiol Biochem 21, 15–28. [DOI] [PubMed] [Google Scholar]
- Shennan DB, McNeillie SA & Curran DE (1994). The effect of a hyposmotic shock on amino acid efflux from lactating rat mammary tissue: stimulation of taurine and glycine efflux via a pathway distinct from anion exchange and volume‐activated anion channels. Exp Physiol 79, 797–808. [DOI] [PubMed] [Google Scholar]
- Stauber T (2015). The volume‐regulated anion channel is formed by LRRC8 heteromers – molecular identification and roles in membrane transport and physiology. Biol Chem 396, 975–990. [DOI] [PubMed] [Google Scholar]
- Strange K, Emma F & Jackson PS (1996). Cellular and molecular physiology of volume‐sensitive anion channels. Am J Physiol Cell Physiol 270, C711–C730. [DOI] [PubMed] [Google Scholar]
- Stutzin A, Torres R, Oporto M, Pacheco P, Eguiguren AL, Cid LP & Sepulveda FV (1999). Separate taurine and chloride efflux pathways activated during regulatory volume decrease. Am J Physiol Cell Physiol 277, C392–C402. [DOI] [PubMed] [Google Scholar]
- Syeda R, Qiu Z, Dubin AE, Murthy SE, Florendo MN, Mason DE, Mathur J, Cahalan SM, Peters EC, Montal M & Patapoutian A (2016). LRRC8 proteins form volume‐regulated anion channels that sense ionic strength. Cell 164, 499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss FK, Ullrich F, Munch J, Lazarow K, Lutter D, Mah N, Andrade‐Navarro MA, von Kries JP, Stauber T & Jentsch TJ (2014). Identification of LRRC8 heteromers as an essential component of the volume‐regulated anion channel VRAC. Science 344, 634–638. [DOI] [PubMed] [Google Scholar]
- Wehner F, Olsen H, Tinel H, Kinne‐Saffran E & Kinne RKH (2003). Cell volume regulation: osmolytes, osmolyte transport, and signal transduction. Rev Physiol Biochem Pharmacol 148, 1–80. [DOI] [PubMed] [Google Scholar]


