Genes encoding Hv1 voltage‐gated proton (H+) channels were first identified in 2006 (Ramsey et al. 2006; Sasaki et al. 2006), but the mechanism of proton transfer (PT) is still debated. Two basic hypotheses have emerged: (a) explicit ionization of a highly conserved Asp (D112/D1.51) carboxyl group mediates PT by a proton ‘shuttle’ conduction (G SH) mechanism (Musset et al. 2011; Dudev et al. 2015), and (b) protein‐associated water molecules support PT via an ‘aqueous’ conductance (G AQ) that does not require titration of D1.51 but instead utilizes a water wire for Grotthuss‐type PT (Ramsey et al. 2010; Randolph et al. 2016). Here we summarize evidence supporting the latter hypothesis, which is depicted in cartoon form in Fig. 1 A.
Figure 1. Cartoon and atomic models of hydrated central crevice in Hv1.
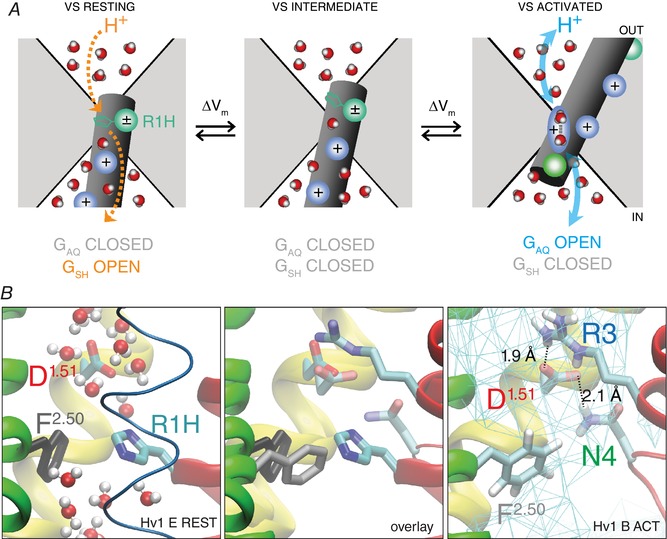
A, cartoon of Hv1 VS domain in resting‐ (left), intermediate‐ (centre) and activated‐state (right) conformations. Solvent‐accessible vestibules extend toward the centre of the membrane from the extracellular (out) and intracellular (in) sides of the protein, creating an hourglass‐shaped central crevice (white area) that is bounded by protein (grey area). The S4 helix is represented as a dark grey cylinder, and the positions of conserved Arg gating charge residues are indicated by shaded blue spheres; the shaded teal sphere (left panel) represents the R205H (R1H) mutation and the side chain is shown in stick representation; the shaded green sphere (right panel) represents N214 (N4.46; N4). Water molecules are schematically shown in coloured space‐filling representation (oxygen, red; hydrogen, white). At large negative potentials (i.e. < −80 mV) the VS adopts a resting‐state conformation (left panel) in which R1 is located midway across the membrane where the electrical field is most highly focused and within the hydrated central crevice; in this location, hydrogen bonds formed between imidazole/imidizolium nitrogen(s) and intra‐ or extracellular water molecules catalyse PT via the G SH mechanism (dashed orange arrows). At intermediate voltages (i.e. −50 to −30 mV), R1H is moved outward to a position where the R1H side chain is no longer positioned to catalyse transmembrane PT via G SH (centre panel). In resting‐ and intermediate‐state conformations, one or more S4 guanidinium+ gating charges block the central crevice, and PT via G AQ is prevented. At more depolarized potentials (i.e. > 0 mV), S4 has moved sufficiently far that waters in the central crevice are accessible to intra‐ and extracellular H+, and Grotthuss‐type PT (blue arrows) in a network of hydrogen‐bonded waters (shaded blue oval schematically represents the Zundel cation, H5O2 +) and G AQ is therefore open (right panel). B, snapshots taken from MD simulations of Hv1 B (Ramsey et al. 2010) activated state (centre and right panels) and Hv1 E (Randolph et al. 2016) R1H resting state (centre and left panels) systems show magnified side views of Hv1 VS domain model structures in isolation and in overlay (MultiSeq STAMP structural alignment, VMD 1.9.3; http://www.ks.uiuc.edu). Helical segments are represented as coloured ribbons (S1, yellow; S2, green; S3, blue; S4, red), except where omitted for clarity (centre and right panels). Side chains of selected residues are shown in stick representation (colour‐coded by atom type: carbon, cyan; nitrogen, blue; oxygen, red; hydrogens, white; H atoms are omitted for clarity in left and centre panels), and indicated by labels (red, D112/D1.51; cyan, R205H/R4.47H/R1H; blue, R211/R4.53/R3; green, N214/N4.56/N4; grey, F150/F2.50); residue numbering is as described previously (Randolph et al. 2016). Water molecules in the Hv1 E system are shown in CPK representation coloured by atom type (red, oxygen; white, hydrogen); waters are omitted for clarity in the centre and right panels. Solid cyan lines (right panel) indicate average water density measured during a 50 ns MD simulation and dashed lines and labels indicate distances between the indicated atoms measured at the end the trajectory, illustrating that D1.51 and R3 engage in multiple close‐range interactions with nearby side chains and water molecules and are unlikely to exclusively form a bidentate pair, as depicted in a previous study (Dudev et al. 2015).
G AQ and G SH share the need for hydrogen bonds; one question is whether the PT pathway is constituted purely by water molecules (G AQ) or whether the D1.51 carboxylate is a required component of the pathway (G SH). Early biophysical studies of native voltage‐gated H+ conductances (G vH) provided important insights into H+ channel mechanisms (see DeCoursey, 2003), and the striking similarity to G vH of currents mediated by expressed Hv1 channels (Ramsey et al. 2006; Sasaki et al. 2006; Musset et al. 2008) suggests that they utilize the same PT mechanism. Native G vH is decreased ∼1.9‐fold by deuterium isotope substitution, exhibits a shallow dependence on pHI, and is faster than H3O+ diffusion in water (see DeCoursey, 2003). Together, the data suggest that the hydronium ion (H3O+) is not the permeant species and that the PT mechanism in Hv1 is not identical to the water‐filled gramicidin A channel (DeCoursey, 2003). Importantly, biophysical observations reported prior to the cloning of Hv1 are compatible with a G AQ mechanism in which waters with restricted mobility mediate PT.
The distances and orientations of hydrogen bond donor and acceptor atoms are critical for PT, raising the possibility that PT via a G SH mechanism could be disrupted by mutations that cause even modest changes in protein structure. In the first direct test of the G SH hypothesis, we found that Hv1 channels containing neutralizing mutations at all ionizable residues in the voltage sensor (VS) domain express robust currents (Ramsey et al. 2010); channel function has now been reported in over 50 different mutant constructs (Ramsey et al. 2010; DeCoursey et al. 2016; Randolph et al. 2016). Some mutations produce dramatic shifts in the voltage at which channels open (V THR): for example, V THR ranges from −135 mV in D174N to +135 mV in R205A–R208A (Ramsey et al. 2010). Hv1 is therefore highly tolerant of mutation‐induced structural perturbations, implying that the PT mechanism is either surprisingly malleable or that the architecture of the PT pathway is nearly identical in all mutants. One interpretation of the data is that Hv1 utilizes a G AQ mechanism, and PT in both WT and mutant channels is supported by resident water molecules in the VS central crevice that can adopt multiple, distinct electronic structures, at least some of which are sufficient for PT (Ramsey et al. 2010). A report demonstrating that mutation of D112V (D1.51V) abolishes H+ current but reintroduction of Asp at V116 (V1.55) is sufficient to restore function (Morgan et al. 2013) further demonstrates the inherent plasticity of the PT mechanism in Hv1, and is in good agreement with the G AQ hypothesis.
Current reversal potential (E REV) shifts in solutions of varying anion concentration gradients demonstrate that the normally exquisite selectivity for H+ is eroded in D112 (D1.51) mutant channels, and Cl− is permeant (Musset et al. 2011). Because the dehydration energy for Cl− is large, Cl− permeance strongly argues that the pathway is well hydrated (Hille, 2001). Cl− (and CH3SO3 − or OH−) permeation is therefore diffusive, and fundamentally different from PT. Although D1.51‐mutant channels appear to exhibit a preference for anions, the relative permeance of H+ vs. OH− cannot be discriminated from E REV shifts (Musset et al. 2011). Assuming P H = 0, then D1.51 mutant channels are ∼ 106‐fold selective for Cl− over H+, but if OH− is impermeant (P OH = 0), the mutants are ∼10,000 times selective for H+ over Cl− (Musset et al. 2011). We consider it more likely that D1.51 mutant channels are permeable to both H+ and OH−, and each ion carries a fraction of the total current. R211 (R4.53 or R3) mutations also erode ion selectivity in Hv1 evidently without eliminating PT (Berger & Isacoff, 2011). The effects of mutations in Hv1 can therefore experimentally dissociate effects on PT vs. ion selectivity.
We propose a unifying hypothesis (Ramsey et al. 2010; Randolph et al. 2016) for selectivity and PT in WT and mutant Hv1 channels that is compatible with all the available experimental data. As reported previously, charged side chains function to repel solution anions (D1.51) or cations (R4.53) from the hydrated VS central crevice, preventing permeation by ions other than H+ (Berger & Isacoff, 2011; Musset et al. 2011; Chamberlin et al. 2015). In the absence of contaminating ions, the hydrated crevice mediates a ‘pure’ H+ current via the water‐based G AQ PT mechanism. D1.51 or R4.53 mutations enhance the diffusive leak of solution ions through the central crevice water network and likely disrupt PT. During intervals between solution ion occupancy, resident waters mediate rapid G AQ‐type PT in both WT and mutant Hv1 channels. Selectivity is determined by the relative rates of PT vs. diffusive ion leak, and is expected to be proportional to the macroscopic permeability ratios. A corollary is that in WT Hv1, solution ions also occasionally leak into the central crevice, and selectivity is therefore not perfect (DeCoursey, 2003). Although there is a paucity of evidence demonstrating permeability to ions other than H+ in WT Hv1, experimental imprecision and variability in whole‐cell seal resistance limit our ability to resolve small E REV shifts, and the magnitude of H+ selectivity remains unknown.
Indirect support for the idea that the central crevice mediates a finite, non‐proton leakage is provided by a widely accepted model of voltage sensor activation. The central crevice forms the permeation pathway for S4 Arg guanidinium group ‘gating charges’, which behave like tethered permeant ions and prevent solution ions from leaking through the VS ‘gating pore’ (Ramsey et al. 2010, Randolph et al. 2016). Resident waters in the VS central crevice may be a general feature of VS domains that facilitate gating charge translocation through the otherwise hydrophobic environment formed by conserved aliphatic and aromatic side chains (Tao et al. 2010; Lacroix et al. 2014; Ramsey et al. 2010; Randolph et al. 2016). Molecular dynamics simulations of VS domain model structures agree that waters are an integral feature of the central crevice (Fig. 1 B), and differences in hydration and hydrogen bond networks may help explain why Hv1 is evidently unique among known VS domains in mediating activated‐state PT (Ramsey et al. 2010; Randolph et al. 2016).
Additional experimental, computational and structural studies of Hv1 and other VS domains are needed to refine atomic and electronic models of gating and PT mechanisms. We lack high resolution open‐channel Hv1 structures (Li et al. 2014; Takeshita et al. 2014) and careful vetting of new models can be painstaking (Randolph et al. 2016), so progress toward understanding PT has been limited. Nonetheless, structural validation is a sine qua non for reliable interpretation of results from computational studies. For example, the inclusion of only one water/hydronium molecule in a simplified Hv1‐like system (Dudev et al. 2015) precludes PT in a water wire a priori. Furthermore, D1.51 interacts with multiple partners in MD simulations that are also absent from the QM study supporting the G SH mechanism (Dudev et al. 2015).
In summary, we currently lack sufficient evidence to unambiguously determine the mechanism of PT in Hv1. Although the G AQ mechanism (Ramsey et al. 2010) is compatible with a wider variety of the available experimental data, additional experimental and computational studies are necessary to discriminate between H+ conduction mechanisms in voltage‐gated proton channels.
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief (250 word) comment. Comments may be submitted up to 6 weeks after publication of the article, at which point the discussion will close and the CrossTalk authors will be invited to submit a ‘LastWord’. Please email your comment, including a title and a declaration of interest, to jphysiol@physoc.org. Comments will be moderated and accepted comments will be published online only as ‘supporting information’ to the original debate articles once discussion has closed.
Additional information
Competing interests
None declared.
Author contributions
Both authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Biographies
Ashley L. Bennett graduated from the University of North Carolina at Wilmington with BS degrees in Chemistry and Biology. At UNCW, she performed molecular dynamics simulations on antimicrobial peptide insertion into POPC membranes under the mentorship of Hee‐Seung Lee, PhD. She is currently a PhD candidate in Physiology and Biophysics at VCU, where her research is focused on Hv1 gating mechanisms.

Ian Scott Ramsey graduated from Occidental College (Los Angeles, CA, USA) and gained industry experience at Syntex Discovery Research (Palo Alto, CA, USA) before earning a PhD in Pharmacology at Vanderbilt University (Nashville, TN, USA) under the mentorship of Louis J. De Felice, PhD. He joined the Department of Physiology and Biophysics at Virginia Commonwealth University after postdoctoral training with David E. Clapham, MD, PhD (HHMI, Harvard Medical School, Children's Hospital, Boston, MA, USA). His current research interests include elucidation of gating and ion permeation mechanisms in Hv1 and TRP channels.
Linked articles This article is part of a CrossTalk debate. Click the links to read the other articles in this debate: https://doi.org/10.1113/JP274495, https://doi.org/10.1113/JP274982 and https://doi.org/10.1113/JP274984.
References
- Berger TK & Isacoff EY (2011). The pore of the voltage‐gated proton channel. Neuron 72, 991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin A, Qiu F, Wang Y, Noskov SY & Larsson HP (2015). Mapping the gating and permeation pathways in the voltage‐gated proton channel Hv1. J Mol Biol 427, 131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey TE (2003). Voltage‐gated proton channels and other proton transfer pathways. Physiol Rev 83, 475–579. [DOI] [PubMed] [Google Scholar]
- DeCoursey TE, Morgan D, Musset B & Cherny VV (2016). Insights into the structure and function of HV1 from a meta‐analysis of mutation studies. J Gen Physiol 148, 97–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudev T, Musset B, Morgan D, Cherny VV, Smith SM, Mazmanian K, DeCoursey TE & Lim C (2015). Selectivity mechanism of the voltage‐gated proton channel, HV1. Sci Rep 5, 10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B (2001). Ion Channels of Excitable Membranes, 3rd edn Sinauer Associates, Sunderland, MA. [Google Scholar]
- Lacroix JJ, Hyde HC, Campos FV & Bezanilla F (2014). Moving gating charges through the gating pore in a Kv channel voltage sensor. Proc Natl Acad Sci USA 111, E1950–E1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wanderling S, Paduch M, Medovoy D, Singharoy A, McGreevy R, Villalba‐Galea CA, Hulse RE, Roux B, Schulten K, Kossiakoff A & Perozo E (2014). Structural mechanism of voltage‐dependent gating in an isolated voltage‐sensing domain. Nat Struct Mol Biol 21, 244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Musset B, Kulleperuma K, Smith SM, Rajan S, Cherny VV, Pomes R & DeCoursey TE (2013). Peregrination of the selectivity filter delineates the pore of the human voltage‐gated proton channel hHv1. J Gen Physiol 142, 625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musset B, Cherny VV, Morgan D, Okamura Y, Ramsey IS, Clapham DE & DeCoursey TE (2008). Detailed comparison of expressed and native voltage‐gated proton channel currents. J Physiol 586, 2477–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musset B, Smith SM, Rajan S, Morgan D, Cherny VV & Decoursey TE (2011). Aspartate 112 is the selectivity filter of the human voltage‐gated proton channel. Nature 480, 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey IS, Mokrab Y, Carvacho I, Sands ZA, Sansom MS & Clapham DE (2010). An aqueous H+ permeation pathway in the voltage‐gated proton channel Hv1. Nat Struct Mol Biol 17, 869–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey IS, Moran MM, Chong JA & Clapham DE (2006). A voltage‐gated proton‐selective channel lacking the pore domain. Nature 440, 1213–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph AL, Mokrab Y, Bennett AL, Sansom MS & Ramsey IS (2016). Proton currents constrain structural models of voltage sensor activation. Elife 5, e18017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Takagi M & Okamura Y (2006). A voltage sensor‐domain protein is a voltage‐gated proton channel. Science 312, 589–592. [DOI] [PubMed] [Google Scholar]
- Takeshita K, Sakata S, Yamashita E, Fujiwara Y, Kawanabe A, Kurokawa T, Okochi Y, Matsuda M, Narita H, Okamura Y & Nakagawa A (2014). X‐ray crystal structure of voltage‐gated proton channel. Nat Struct Mol Biol 21, 352–357. [DOI] [PubMed] [Google Scholar]
- Tao X, Lee A, Limapichat W, Dougherty DA & MacKinnon R (2010). A gating charge transfer center in voltage sensors. Science 328, 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]


