Abstract
Key points
The parasympathetic nervous system (PNS) is critical for adaptation to environment demands.
Alzheimer's disease (AD), via frontal compensatory processes, may affect PNS regulation, thereby compromising older adults’ capacity for adaptation, and increasing morbidity and mortality risk.
Here we found that AD‐associated neurodegeneration accompanied an overactive anterior cingulate cortex, which in turn resulted in a high level of PNS activity at rest, as well as strong PNS activity withdrawal in response to the mental effort.
This discovery provides the first line of evidence to suggest that AD‐associated neurodegeneration links to altered PNS regulation during mental effort in older adults, and that the compensatory processes accompanying frontal hyperactivation appear to be responsible for these alterations.
Abstract
The parasympathetic nervous system (PNS) is critical for adaptation to environment demands. PNS can reflect an individual's regulatory capacity of frontal brain regions and has been linked to cognitive capacity. Yet, the relationship of PNS function to cognitive decline and abnormal frontal function that characterize preclinical progression toward Alzheimer's disease (AD) is unclear. Here, we aimed to elucidate the relationship between PNS function and AD‐associated neurodegeneration by testing two competing hypotheses involving frontal regions’ activity (neurodegeneration vs. compensation). In 38 older human adults with amnestic mild cognitive impairment (aMCI) or normative cognition, we measured AD‐associated neurodegeneration (AD signature cortical thickness; ADSCT), resting‐state functional magnetic resonance imaging of frontal regions’ spontaneous activation, and an electrocardiography measure of PNS (high frequency heart rate variability; HF‐HRV). HF‐HRV was assessed at rest and during a cognitive task protocol designed to capture HF‐HRV reactivity. Higher HF‐HRV at rest was significantly related to both more severe AD‐associated neurodegeneration (lower ADSCT scores) and worse cognitive ability. Cognitive impairments were also related to greater suppression of HF‐HRV reactivity. High activities of the anterior cingulate cortex significantly mediated relationships between ADSCT and both HF‐HRV at rest and HF‐HRV reactivity. Notably, these relationships were not affected by the clinical phenotype. We show that AD‐associated neurodegeneration is associated with altered PNS regulation and that compensatory processes linked to frontal overactivation might be responsible for those alterations. This finding provides the first line of evidence in a new framework for understanding how early‐stage AD‐associated neurodegeneration affects autonomic regulation.
Keywords: anterior cingulate cortex, heart rate variability, cortical thickness, neurodegeneration, Alzheimer's disease
Key points
The parasympathetic nervous system (PNS) is critical for adaptation to environment demands.
Alzheimer's disease (AD), via frontal compensatory processes, may affect PNS regulation, thereby compromising older adults’ capacity for adaptation, and increasing morbidity and mortality risk.
Here we found that AD‐associated neurodegeneration accompanied an overactive anterior cingulate cortex, which in turn resulted in a high level of PNS activity at rest, as well as strong PNS activity withdrawal in response to the mental effort.
This discovery provides the first line of evidence to suggest that AD‐associated neurodegeneration links to altered PNS regulation during mental effort in older adults, and that the compensatory processes accompanying frontal hyperactivation appear to be responsible for these alterations.
Abbreviations
- ACC
anterior cingulate cortex
- AD
Alzheimer's disease
- ADSCT
Alzheimer's disease signature cortical thickness
- ALFF
amplitude of the low frequency fluctuation
- aMCI
amnestic mild cognitive impairment
- ANS
autonomic nervous system
- BOLD
blood oxygen level dependent
- ECG
electrocardiography
- fMRI
functional magnetic resonance imaging
- HC
healthy control
- HF‐HRV
high frequency heart rate variability
- MNI
Montreal Neurological Institute
- MOCA
Montreal Cognitive Assessment
- PNS
parasympathetic nervous system
Introduction
The autonomic nervous system (ANS), comprising the parasympathetic (PNS) and sympathetic (SNS) branches, is critical for adaptation to environmental demands. While the SNS has long been recognized as the predominant support for ‘fight or flight’ in the face of threat, more recent models emphasize the regulating role of the PNS in supporting flexible responses during adaptation to changing or demanding environments (Thayer et al. 2009). For instance, more active PNS, often indexed by higher high frequency heart rate variability (HF‐HRV), is linked to greater cognitive capacity that can support mental efforts toward adaptation in younger and healthy adults (Hansen et al. 2003). Frontal brain regions (e.g. prefrontal cortex, anterior cingulate cortex (ACC)) play a direct role in regulating PNS. In individuals without cognitive impairment, greater neural efficiency in frontal regions links to higher PNS activity (i.e. higher HF‐HRV). But, in response to cognitive challenges, activation of the frontal regions can lead to PNS withdrawal (i.e. decrease in HF‐HRV) (Thayer et al. 2012). Both aspects reflect the frontal regions’ top‐down regulatory capacity (Thayer et al. 2009). This is of potential relevance for Alzheimer's disease (AD) as frontal regions’ function in AD is compromised by neurodegeneration. Individuals at high risk for AD often have hyperactivation in the frontal regions, likely resulting from compensation for neural deficits of posterior brain regions or insufficient neural efficiency of frontal regions themselves (Li et al. 2014; Lin et al. 2017b). However, despite close links between frontal and PNS function, little is known about whether the early stage of neurodegeneration in AD affects frontal regions’ regulation of PNS. For example, reduced PNS activation during rest is observed in the middle to late stages of AD compared to healthy older adults (Femminella et al. 2014), suggesting altered PNS regulation with more advanced neurodegeneration. However, there have been contradictory findings regarding the level of HF‐HRV in individuals with the earliest clinical phenotype of AD – amnestic mild cognitive impairment (aMCI) (Zulli et al. 2005; Collins et al. 2012; Lin et al. 2014). Because aMCI can be a pathophysiologically heterogeneous group (Jacquemont et al. 2017), these contradictory findings may be due to the examination of HF‐HRV in the context of clinical phenotype instead of AD‐associated neurodegeneration.
AD signature cortical thickness (ADSCT), including atrophy in several posterior brain regions (e.g. bilateral inferior and middle temporal lobes, entorhinal cortex, and fusiform gyrus) has been used as a common index for AD‐associated neurodegeneration (Jack et al. 2015). Deficits in ADSCT are highly correlated to amyloid or tau pathology, disturb brain function, and occur much earlier than the symptomatic preclinical AD phenotype such as aMCI (Jack et al. 2015). In the present study, we enrolled a group of older adults without clinical diagnosis of AD or other types of dementia (healthy control (HC) and older adults with aMCI), and examined two types of relationships: (1) the associations between ADSCT and HF‐HRV at rest and in response to cognitive tasks; (2) the mechanism linking ADSCT with HF‐HRV by focusing on frontal regions’ activity. The frontal regions’ activity was indexed by the amplitude of the low frequency fluctuation (ALFF), measuring the resting‐state regional spontaneous activity with functional magnetic resonance imaging (fMRI) (Hou et al. 2014; Ren et al. 2016). We tested two competing hypotheses that differ in whether the focus is on (A) neurodegeneration per se or (B) compensatory mechanisms that are pronounced in early stages of AD‐associated neurodegeneration. (A) Based on the literature on cognitively normal people (discussed above), greater AD‐associated neurodegeneration or worse cognitive performance would lead to reduced HF‐HRV regardless of the level of frontal regions’ activity. (B) Based on known frontal regions’ compensatory mechanism in early stages of AD‐associated neurodegeneration, greater AD‐associated neurodegeneration would exaggerate the frontal regions’ compensation, which, in turn, would lead to an abnormal increase in HF‐HRV. Note that, in either circumstance, the hypothesized link is with AD‐associated neurodegeneration instead of the clinical phenotype (aMCI vs. HC). In sum, our overarching aim is to test how frontal brain activity interacts with PNS function, and how this differs between neurotypical individuals and those affected by AD‐associated neurodegeneration.
Methods
Ethical approval
The study was approved by the university's research subject review board and all participants provided written informed consent (according to the latest revision of the Declaration of Helsinki).
Participants
Thirty‐eight participants (20 HC and 18 aMCI) completed the study. Participants with aMCI were recruited from university‐affiliated memory clinics using the clinical diagnosis of ‘mild cognitive impairment due to Alzheimer's disease’ (Albert et al. 2011): participants had deficits in memory based on a comprehensive neuropsychological battery but intact basic activities of daily living, in addition to the absence of dementia using NINCDS‐ADRDA criteria per assessment. Participants had to be stable on AD medication (i.e. memantine or cholinesterase inhibitors; 5 participants from aMCI group (27.7%) were in these medications) for 3 months prior to enrolment. Age‐, sex‐ and education‐matched HC participants without self‐reported history of dementia or aMCI were recruited from the community. In addition, all participants were required to be ≥60 years of age, English‐speaking, community‐dwelling, and had adequate visual and auditory acuity for testing, as well as the capacity to give consent based on the research team's assessment. Exclusion criteria included the presence of severe cardiovascular disease (e.g. chronic heart failure), inflammatory (e.g. irritable bowel syndrome), or uncontrollable psychiatric (e.g. major depression) diseases, uncontrollable hypertension, or MRI contraindications (e.g. pacemaker, claustrophobia).
Design and procedure
The present study was cross‐sectional, consisting of two sessions within a 2‐week window. The first session comprised a series of cognitive assessments and interview for demographic and health background. The second session was started in the morning (09.00–11.00 h) in a temperature‐controlled room (20–25°C), and included a 10 min acclimation period with continuous electrocardiography (ECG) assessment followed by a 30 min fMRI (T1 structural imaging and resting‐state fMRI), and then two 10 min cognitive tasks with ECG measured throughout. The tasks included two commonly used computerized executive function tasks: Stroop Color Word and Dual 1‐back task. For the Stroop task, participants were shown a series of coloured words on the screen, and asked to judge the colour of the word regardless of the meaning of the word as quickly and accurately as possible. For the Dual 1‐back task, participants were shown an English letter on the screen, and asked to judge, as quickly and accurately as possible, if the current stimulus matched the letter and position of the previous one. For both tasks, feedback was displayed after the participant responded to an individual trial. Each of the tasks lasted 10 min, and the order of the two tasks was randomized across participants. Instructions and practice were provided before each of the formal tasks. The tasks targeted inhibition and working memory, two executive function domains compromised comparably early in both normal ageing and neurodegeneration (Suchy, 2009). Of note, before coming to the second session appointment, the participants were instructed to avoid caffeine, physical exercise, or taking a β‐blocker in the same morning.
Measures
Imaging data acquisition
A 3T Siemens TrioTIM scanner (Erlangen, Germany) equipped with a 32‐channel receive‐only head coil was used. The session began with a magnetization prepared rapid acquisition gradient echo (MPRAGE) scan (TR/TE = 2530 ms/3.44 ms, TI = 1100 ms, FA = 7, matrix = 256 × 256, resolution 1 mm × 1 mm × 1 mm, slice thickness = 1 mm, 192 slices), which provides high‐resolution T1 structural‐weighted anatomical images for image‐registration purposes. A 2‐D axial fast gradient‐recalled echo pulse sequence was used to generate field maps and correct for field inhomogeneity distortions in echo‐planar imaging sequences. The resting‐state fMRI data were collected using a blood oxygen level‐dependent (BOLD) fMRI protocol with a gradient echo‐planar imaging sequence (TE/TR = 30 ms/2500 ms, FA = 90, slice thickness = 4 mm, matrix = 64 × 64, 4 mm × 4 mm in‐plane resolution, 30 axial slices, 100 volumes).
Cortical thickness
The structural images were processed using FreeSurfer image analysis suite v5.1.0 (http://surfer.nmr.mgh.harvard.edu/). These well‐validated and fully automated procedures have been described previously (Fischl & Dale, 2000). Briefly, all the images were automatically processed with skull stripping, spatial transforms, atlas registration and spherical surface maps. After tessellating the grey–white boundary and locating the grey–pial boundary in FreeSurfer, cortical thickness is calculated as the closest distance between the grey–white matter boundary and the pial mesh at each vertex on the tessellated surface (Fischl & Dale, 2000). Automated cortical parcellation and region of interest (ROI) definition were performed using the Desikan–Killiany Atlas which manually labelled 34 cortical ROIs in each hemisphere (Desikan et al. 2006). Of note, the results of Freesurfer segmentation were examined visually for topological defects, with manual editing performed to correct the defects. ADSCT was defined by averaging cortical thickness of bilateral inferior and middle temporal lobes, entorhinal cortex and fusiform gyrus, a lower value indicating worse AD pathology (Jack et al. 2015) (Fig. 1 A).
Figure 1. AD‐related neurodegeneration and HF‐HRV.
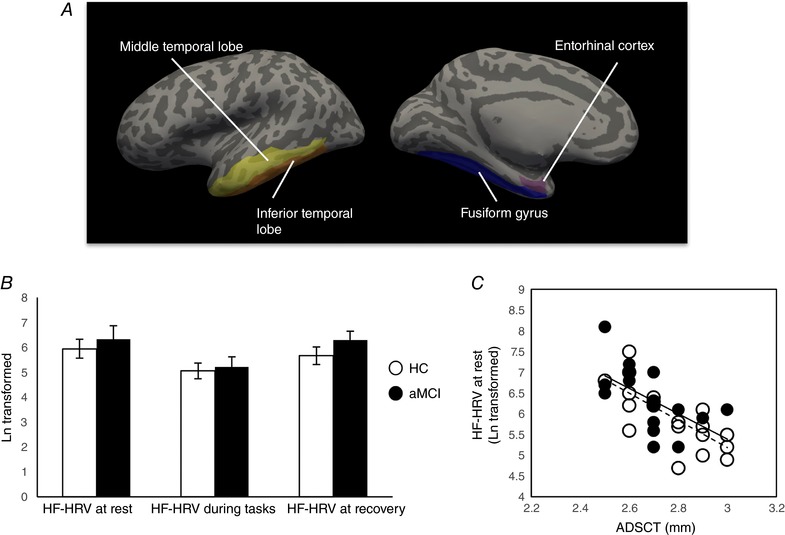
A, AD‐related neurodegeneration was indexed by ADSCT, which was defined by averaging cortical thickness of bilateral inferior and middle temporal lobes, entorhinal cortex and fusiform gyrus. Here, lower value indicated more severe AD pathology. B, mean and SEM of HF‐HRV at rest, during tasks and at recovery by group. There was a significant quadratic pattern for HF‐HRV (i.e. declined from at rest to tasks, and rebounded after recovery) in both groups, but there was no group by status interaction effect. C, there was a significant association between ADSCT and HF‐HRV at rest but not HF‐HRV reactivity (calculated as the difference between HF‐HRV at tasks and rest) controlled for age and sex. The dashed line represents the correlation in HC group, and the continuous line represents the correlation in aMCI group. Similarity is observed relationships for the two groups. [Color figure can be viewed at wileyonlinelibrary.com]
Resting‐state fMRI data preprocessing and analysis
For each participant, the first five volumes of resting‐state fMRI scan were excluded, avoiding the noise related to the equilibrium of the scanner and participants’ adaptation to the scanner. The remaining 95 volumes were slice‐timing and head‐motion corrected, coregistered to their own structure image, and normalized to Montreal Neurological Institute (MNI) standard space, and resampled (3 mm × 3 mm × 3 mm). Data were subsequently smoothed using Gaussian kernel (full width at half maximum (FWHM) 4 mm), and the linear trend, white matter, cerebrospinal fluid and head motion were regressed out using the general linear model. We also removed one participant's data due to the head movement being larger than 2 mm and 2 deg. The preprocessed data were filtered using band pass (0.01–0.08 Hz) to calculate ALFF values. Briefly, the BOLD time courses were converted to the frequency domain using the fast Fourier transform. Then the square root of the power spectrum was calculated, averaged across 0.01–0.08 Hz for each voxel, and defined as the ALFF at the given voxel (Zang et al. 2007). All preprocessing was conducted using the Data Processing Assistant for the resting state‐fMRI package (Chao‐Gan & Yu‐Feng, 2010). For each participant, the ALFF of each voxel was divided by the global mean ALFF value. To determine the frontal regions that were related to HRV at rest and reactivity, separate linear regression was applied to calculate the correlations between ALFF and HF‐HRV at rest, as well as HF‐HRV reactivity, in a voxel‐by‐voxel manner. A threshold of AlphaSim‐corrected P < 0.05 (synthesizing uncorrected individual P < 0.005 and voxel size >22) was applied to all statistical maps (Ledberg et al. 1998).
Because the BOLD signal may be influenced by cardiac pulses that may confound with HRV examined in the study, we also conducted a secondary control analysis where we used slightly different preprocessing steps, regressing out pulse that was simultanously recorded with the pulse oximetry in the resting‐state fMRI, in addition to white matter, cerebrospinal fluid and head motion. Due to some technical problems (lack of synchronization of physiological and BOLD data, or failure in recording the physiological data), there were only 29 participants (aMCI: 11 and HC: 18, no significant demographic difference from the main sample) in this analysis. As in the main analysis, we identified brain regions within ACC (related to HF‐HRV at rest: ACC1, MNI co‐ordinate: 3, 33, 15, voxel size: 41; and related to HF‐HRV reactivity: ACC2, MNI co‐ordinate: 0, 24, 21, voxel size: 41). Correlations among key variables remained similar, as did indirect effects of ACC for mediating the relationships between ADCST and HF‐HRV at rest (ACC1: B = −5.55, SE = 1.94, Z = −2.86, P = 0.004) or HF‐HRV reactivity controlling for HF‐HRV at rest (ACC2: B = 2.13, SE = 1.17, Z = 1.82, P = 0.068).
HF‐HRV
HF‐HRV was assessed using Biopac electrocardiography of a standard lead‐II electrode configuration. Electrocardiography was continuously monitored at acclimation and during the cognitive tasks in the second session of the study. HRV software (MindWare) was used to process data as described previously (Berntson et al. 1997). Briefly, a series of intervals between consecutive R waves (every 20 s) was preprocessed to remove ectopic beats and artifact. Power spectral analysis was conducted to generate HF‐HRV (0.12–0.40 Hz). HF‐HRV data were expressed in absolute units and then natural log transformed for normalization. HF‐HRV is generally accepted as an index for PNS (Cacioppo et al. 2007). Data at rest and during cognitive tasks (based on the order instead of the specific type of task) were averaged respectively (excluding the first and last minute to avoid noise). HF‐HRV reactivity was then calculated as the discrepancy (HF‐HRV during task – HF‐HRV at rest) with larger positive values indicating greater increases in response to the tasks and larger negative values indicating greater suppression. Of note, in the second session, immediately after the two cognitive tasks we also had 40 min relaxation with ECG applied for the last 13 min. We calculated HF‐HRV during recovery (excluding the first and last minute). In Fig. 1 B, we showed the trajectory of HF‐HRV at rest, during the task, and during recovery by group. The pattern suggests a decline of HF‐HRV during the cognitive tasks, with a return to baseline levels during recovery.
Cognitive assessment
We assessed global cognition, episodic memory and executive function in the first session. Global cognition was assessed using Montreal Cognitive Assessment (MOCA) (Rossetti et al. 2011). Episodic memory was the mean score of standardized values of learning and delayed recall from Rey's Auditory Verbal Learning Test (Estevez‐Gonzalez et al. 2003). Executive function was a composite score using the algorithm provided by the EXAMINER package (Kramer et al. 2014). We used four computerized tasks: 1‐back (different from the cognitively demanding tasks in the second session), dot counting, set shifting and flanker.
Other data analysis
SPSS 22.0 was used for the data analysis. Group comparisons on sample characteristics were conducted using the independent Student's t test and χ2 tests for categorical and continuous variables, respectively. Repeated measures ANOVA was used to examine HF‐HRV across time. Partial Pearson's correlations were conducted for the relationship between HF‐HRV, ADSCT and cognitive assessments controlling for age, sex and group. Mediation models were then estimated to test whether function of frontal regions mediated the effect of the ADSCT on HF‐HRV at rest and in response to tasks (reactivity) (Preacher & Hayes, 2008). Mediation models of HF‐HRV reactivity controlled for resting levels (Benjamin, 1967). Bootstrapping of standard errors (5000 bootstrap draws) was used for indirect effect estimates using the INDIRECT macro from SPSS.
Results
A link between AD‐associated neurodegeneration, HF‐HRV and cognitive function
The aMCI group had significantly worse ADSCT and worse performance on MOCA and episodic memory (but not executive function) than the HC group (Table 1). There were no group differences in HF‐HRV at rest or for task reactivity. Both groups showed significant HF‐HRV decline from rest during the tasks, and with recovery to resting levels following the tasks (quadratic model: HC: F = 8.68, P = 0.008; aMCI: F = 12.33, P = 0.003) without a time by group interaction effect (F = 0.71, P = 0.41) (Fig. 1 B). Although clinically defined groups did not differ on HF‐HRV, as noted in the Introduction, our primary aim was to examine associations between AD‐associated neurodegeneration and HF‐HRV and cognitive function. Indeed, lower ADSCT was significantly related to higher HF‐HRV at rest (Fig. 1 C) and worse cognitive performance; and lower HF‐HRV reactivity (i.e. greater depression in HF‐HRV) was also significantly related to worse cognitive performance (Table 2). These results were unaffected when controlling for group (clinical phenotype).
Table 1.
Demographics and clinical characteristics of aMCI and HC groups
| aMCI (n = 18) | HC (n = 20) | t or χ2 test (P value), d.f. | |
|---|---|---|---|
| Age, mean (SD) | 74.44 (10.60) | 70.95 (9.13) | 1.09 (0.28), 36 |
| Education, mean (SD) | 15.39 (2.87) | 15.75 (2.34) | −0.43 (0.67), 36 |
| Male, n (%) | 8 (44.4) | 8 (40.0) | 0.08 (0.78), 1 |
| Geriatric depression scale, mean (SD) | 3.00 (2.74) | 1.60 (2.01) | 1.81 (0.08), 36 |
| Hypertension, n (%) | 7 (38.9) | 10 (50.0) | 0.47 (0.53), 1 |
| MOCA, mean (SD) | 24.17 (2.55) | 26.10 (2.73) | −2.25 (0.031), 36 |
| Episodic memory, mean (SD) | −0.45 (0.97) | 0.40 (0.72) | −3.07 (0.004), 36 |
| Executive function, mean (SD) | 0.05 (0.69) | 0.39 (0.55) | −1.71 (0.10), 36 |
| HF‐HRV at rest (ms2), mean (SD) | 6.32 (2.29) | 5.95 (1.70) | 0.57 (0.57), 36 |
| HF‐HRV reactivity (ms2), mean (SD) | −1.11 (2.10) | −0.90 (1.72) | −0.34 (0.74), 36 |
| ADSCT (mm3), mean (SD) | 2.67 (0.14) | 2.79 (0.16) | −2.46 (0.019), 36 |
| ALFF in ACC1, mean (SD) | 1.26 (0.48) | 1.09 (0.29) | 1.30 (0.20), 35 |
| ALFF in ACC2, mean (SD) | 1.26 (0.47) | 1.10 (0.37) | 1.17 (0.25), 35 |
Table 2.
Relationships between ADSCT, HF‐HRV and cognition
| Pearson's correlation (P value) | |||||
|---|---|---|---|---|---|
| ADCST | HF‐HRV at rest | HF‐HRV reactivity | ACC1 | ACC2 | |
| ADSCT | |||||
| HF‐HRV at rest | −0.37 (0.025)# | ||||
| HF‐HRV reactivity | 0.27 (0.12) | — | |||
| ACC1 | −0.60 (<0.001)# | 0.70 (<0.001)# | — | ||
| ACC2 | −0.52 (0.001)# | — | −0.74 (<0.001)# | ||
| MOCA | 0.32 (0.059) | −0.38 (0.024)# | 0.39 (0.022)# | −0.48 (0.004)# | −0.44 (0.009)# |
| Episodic memory | 0.25 (0.15) | −0.46 (0.006)# | 0.48 (0.004) | −0.39 (0.019)# | −0.44 (0.008)# |
| Executive function | 0.23 (0.19) | −0.34 (0.049)# | 0.29 (0.087) | −0.43 (0.011)# | −0.38 (0.024)# |
Note: correlations controlled for age and sex.
#Statistical significance remained when also controlling for group and education.
Mediating effect of frontal regions’ function on the relationship between ADSCT and HF‐HRV
Applying the whole brain voxel‐wise analysis of ALFF to HF‐HRV at rest and reactivity, we consistently generated a region located in the ACC in which higher ALFF was significantly related to higher HF‐HRV at rest (ACC1: MNI co‐ordinate: 0, 33, 18, voxel size: 48) and lower HF‐HRV reactivity (ACC2: MNI co‐ordinate: 0, 24, 21, voxel size: 41; Fig. 2 A). Of note, there was also a region generated in the brainstem area. Although ANS is known to be related to brainstem (Femminella et al. 2014), brainstem was not part of our frontal function hypothesis and thus it was not included in the subsequent analyses. There were no group differences in ALFF in either ACC, meaning that the clinical phenotype may not affect frontal activities (Table 1). Controlling for age and sex, lower ALFF in ACC1 and ACC2 was significantly related to higher ADSCT and better cognitive performance. Significant relationships remained when controlling for group (Table 2 and Fig. 2).
Figure 2. Frontal regions’ function and HF‐HRV.
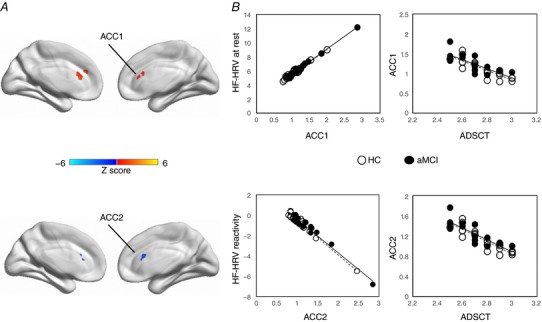
A, with AlphaSim correction at P < 0.05, there was a region located in the anterior cingulate cortex (ACC) in which higher ALFF was significantly related to higher HF‐HRV at rest (ACC1: MNI co‐ordinate: 0, 33, 18, voxel size: 41) and lower HF‐HRV reactivity (ACC2: MNI co‐ordinate: 0, 24, 21, voxel size: 40). B, associations between ACC and HF‐HRV at rest and reactivity controlled for age and sex. The dashed line represents the correlation in HC group, and the continuous line represents the correlation in aMCI group. Similarity is observed relationships for the two groups. [Color figure can be viewed at wileyonlinelibrary.com]
Next, we built a mediation model examining whether ACC mediated the relationship between ADSCT and HF‐HRV (Fig. 3 A). Since we had a relatively small sample size for a well‐controlled mediation analysis, age and sex were not controlled. The results showed that ALFF in ACC1 significantly mediated the association between ADSCT and HF‐HRV at rest (Indirect effect: B = −4.90, SE = 1.69, Z = −2.90, P = 0.004, Fig. 3 B shows all direct effects). Although the correlation between ADSCT and HF‐HRV reactivity did not meet statistical significance (P = 0.12), the coefficient (r = 0.27) suggested a non‐trivial association that warranted testing of the mediational model proposed (Frazier et al. 2004). ALFF in ACC2 also significantly mediated the association between ADSCT and HF‐HRV reactivity, controlling for HF‐HRV at rest (Indirect effect: B = 2.26, SE = 1.08, Z = 2.10, P = 0.036, Fig. 3 C shows all direct effects). When controlling for the group effect, the indirect effects of the two models remained significant.
Figure 3. Mediation models examining ACC as a mediator for the relationship between ADSCT and HF‐HRV.
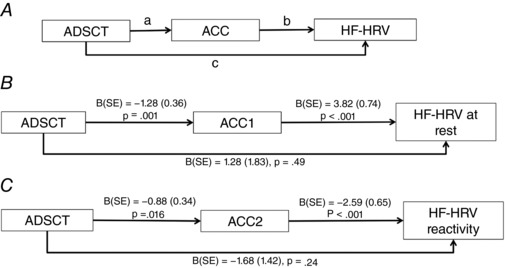
A, conceptual model; B, ALFF in ACC1 significantly mediated the association between ADSCT and HF‐HRV at rest; C, ALFF in ACC2 also significantly mediated the association between ADSCT and HF‐HRV reactivity, controlling for HF‐HRV at rest. Results reported in B and C are direct effects.
Discussion
Here we combined structural MRI, resting‐state fMRI, and a peripheral physiological measure to elucidate the effect of AD‐associated neurodegeneration on PNS. Unlike previous studies of cognitively normal or healthy younger adults where higher HF‐HRV at rest was linked to younger age and better cognitive regulation (e.g. Kemp et al. 2016; Williams et al. 2016), the present study found that more severe AD‐associated neurodegeneration (indexed by lower scores in ADSCT), along with worse cognitive function, was linked to higher HF‐HRV at rest among older adults. Worse cognitive function was also related to larger decline in HF‐HRV, suggesting greater withdrawal of PNS, during the cognitive tasks. Notably, the relationships between ADSCT and HF‐HRV were explained by ACC spontaneous activity level. That is, AD‐associated neurodegeneration accompanied a hyperactive ACC, which, in turn, resulted in a high level of HF‐HRV at rest, as well as stronger HF decline to the tasks. None of these relationships were affected by the clinical phenotype (i.e. HC vs. aMCI). This discovery provides the first line of evidence to suggest that AD‐associated neurodegeneration is associated with altered PNS regulation during mental effort in older adults, and that the compensatory processes accompanying frontal hyperactivation appear to be responsible for these alterations.
The relationship between more severe AD‐associated neurodegeneration and higher HF‐HRV at rest is notable. Studies in healthy adults suggest that lower capacity of frontal regions, including ACC, is associated with reduced HF‐HRV (Thayer et al. 2012). We suggest, however, that the well‐established neural compensatory processes in early AD‐neurodegeneration may help explain these seemingly inconsistent findings. As indicated in the literature, hyperactive ACC often comes from insufficient neural efficiency of frontal regions or the compensatory mechanism for neural loss of posterior regions (Li et al. 2014). Either process can lead to a correspondingly enhanced HF‐HRV at rest or greater suppression of HF‐HRV when engaging in cognitive tasks. It is possible that the positive relationship between HF‐HRV and activity of frontal regions may only be applicable to healthy younger adults when neural efficiency and activity level of frontal regions are in the same direction (McEwen & Gianaros, 2010; Taylor et al. 2010). Given the common phenomenon of compensatory neural mechanisms in ageing and in the early stage of AD‐associated neurodegeneration, simply increasing the frontal activities may not be useful. Instead, enhancing the ‘efficiency’ of the frontal regions may be warranted. Notably, in our recent cognitive training study, cognitive training strengthened such efficiency in older adults with aMCI (Lin et al. 2017a). The intervention approach may provide a viable pathway to determine the causal relationship between neural efficiency and HF‐HRV. After all, adequate PNS function is critical for adaptation to cognitively challenging environments (Thayer et al. 2009). For older adults, including those at risk for AD, strengthening pathways regulating PNS may be efficacious for maintaining functional health in later life.
It is notable that the level of HF‐HRV, ACC activation, and the relationships between ADSCT, ACC activation and HF‐HRV were not differentiated by clinical phenotype. The two groups did differ in ADSCT. This indicates that individuals with aMCI may still have the capacity to rely on frontal regions’ compensation, thus maintaining PNS regardless of AD pathology. Therefore, focusing on the clinical phenotype as a predictor of neuropathological‐related effects in physiological regulation is not sufficient to understand the true effects of neurodegenerative disease. However, regarding the insignificant relationship between clinical phenotype and HF‐HRV, we could not fully exclude the limitation of the small sample size, or lack of power (Quintana, 2017). Also, the participants’ history of attending therapeutic programmes (e.g. physical exercise) that can affect HF‐HRV (Albinet et al. 2010) was not assessed in the present study while physical exercise may be a common activity for older adults with cognitive deficits given its potential for cognitive maintenance. It should also be acknowledged that neurodegeneration is just one of the factors that likely drives pathophysiological heterogeneity in AD risk or aMCI, with others including amyloidosis or tau pathology (Jacquemont et al. 2017). The observed link between neurodegeneration and PNS warrants future exploration of AD‐specific neuropathology and its effect on frontal activation and autonomic regulation.
In sum, our data support a new framework suggesting that early stage AD‐associated neurodegeneration is linked to autonomic dysregulation via the involvement of neural inefficiency in frontal regions. A new profile for PNS should be carefully redefined in relation to AD (see Fig. 4 for a proposed preliminary framework). That is, as AD‐associated neurodegeneration initiates, an abnormally active PNS may reflect compensation mechanisms in the frontal regions. However, PNS function eventually worsens as neurodegeneration progresses and starts affecting frontal regions, ultimately decreasing to the levels below normal ageing‐associated PNS activity (Jack et al. 2010; Femminella et al. 2014). Several limitations of this framework need to be acknowledged. First, although we excluded the cardiac pulsation's influence on fMRI to clarify the relationship between fMRI and PNS, other PNS indictors that are less sensitive to the influence of respirations (e.g. root mean square of successive differences) should also be examined. Also, other peripheral haemostatic processes (e.g. immune system, hypothalamic–pituitary–adrenal axis) may interact with the ANS as they are all centrally regulated by the frontal regions (Taylor et al. 2010). A more comprehensive, integrative framework is needed to fully explain the interplay of central and peripheral physiological regulation in AD‐associated neurodegeneration. Second, using a cross‐sectional design, the causal relationships between AD pathology, frontal activation and PNS cannot be confirmed. The next step will be to modify AD pathology or frontal neural efficiency (Lin et al. 2017a), and examine the consequential changes in PNS as discussed above. Third, using voxel‐wise analysis, ACC was the only frontal region associated with HF‐HRV. In the literature, in addition to ACC, striatum and prefrontal cortex play a role in HF‐HRV (Thayer et al. 2012). Region of interest type analysis may be conducted to further confirm the current model with a relatively large sample. Finally, these findings are limited to a cognitively demanding situation, but it is unknown whether the observed alterations in PNS regulation would manifest in response to more metabolically or subjectively demanding situations (e.g. physical, psychological, or social stress) that activate multiple, co‐ordinated systems to support stress adaptation (Segerstrom & Miller, 2004). Contemporary models of ageing underscore the role of stress adaptation in morbidity and mortality outcomes (Epel & Lithgow, 2014). Thus, these findings may have implications for advancing understanding of the role of neurodegeneration in multiple facets of older adults’ function and health.
Figure 4. Conceptual framework of the relationship among PNS, age and AD‐associated neurodegeneration.
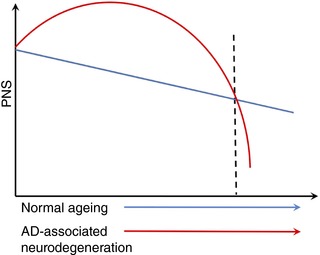
Blue line indicates PNS trajectory in the normal ageing; red line indicates PNS trajectory when AD‐associated neurodegeneration occurs. [Color figure can be viewed at wileyonlinelibrary.com]
Additional information
Competing interests
None declared.
Author contributions
F.L., D.T. and K.L.H. designed and drafted the work; P.R., X.W. and M.A. collected, analysed and interpreted the data, and revised the paper for important intellectual content. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed. The work was conducted at the CogT Lab, University of Rochester.
Funding
The data collection was funded by the Alzheimer's Association New Investigator Grant (NIRG‐14‐317353) to F. Lin, and the manuscript preparation was also funded by NIH R01 grant (NR015452) and R21 grant (AG053193) to F. Lin.
Edited by: Harold Schultz & Diego Contreras
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B & Phelps CH (2011). The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albinet CT, Boucard G, Bouquet CA & Audiffren M (2010). Increased heart rate variability and executive performance after aerobic training in the elderly. Eur J Appl Physiol 109, 617–624. [DOI] [PubMed] [Google Scholar]
- Benjamin LS (1967). Facts and artifacts in using analysis of covariance to “undo” the law of initial values. Psychophysiology 4, 187–206. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH & van der Molen MW (1997). Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology 34, 623–648. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Tassinary LG & Berntson G (2007). Handbook of Psychophysiology. Cambridge University Press. [Google Scholar]
- Chao‐Gan Y & Yu‐Feng Z (2010). DPARSF: a MATLAB toolbox for “Pipeline” data analysis of resting‐state fMRI. Front Syst Neurosci 4, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins O, Dillon S, Finucane C, Lawlor B & Kenny RA (2012). Parasympathetic autonomic dysfunction is common in mild cognitive impairment. Neurobiol Aging 33, 2324–2333. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS & Killiany RJ (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. [DOI] [PubMed] [Google Scholar]
- Epel ES & Lithgow GJ (2014). Stress biology and aging mechanisms: toward understanding the deep connection between adaptation to stress and longevity. J Gerontol A Biol Sci Med Sci 69 (Suppl. 1), S10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez‐Gonzalez A, Kulisevsky J, Boltes A, Otermin P & Garcia‐Sanchez C (2003). Rey verbal learning test is a useful tool for differential diagnosis in the preclinical phase of Alzheimer's disease: comparison with mild cognitive impairment and normal aging. Int J Geriatr Psychiatry 18, 1021–1028. [DOI] [PubMed] [Google Scholar]
- Femminella GD, Rengo G, Komici K, Iacotucci P, Petraglia L, Pagano G, de Lucia C, Canonico V, Bonaduce D, Leosco D & Ferrara N (2014). Autonomic dysfunction in Alzheimer's disease: tools for assessment and review of the literature. J Alzheimers Dis 42, 369–377. [DOI] [PubMed] [Google Scholar]
- Fischl B & Dale AM (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97, 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier PA, Tix AP & Barron KE (2004). Testing moderator and mediator effects in counseling psychology research. J Couns Psychol 51, 115–134. [Google Scholar]
- Hansen AL, Johnsen BH & Thayer JF (2003). Vagal influence on working memory and attention. Int J Psychophysiol 48, 263–274. [DOI] [PubMed] [Google Scholar]
- Hou Y, Wu X, Hallett M, Chan P & Wu T (2014). Frequency‐dependent neural activity in Parkinson's disease. Hum Brain Mapp 35, 5815–5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC & Trojanowski JQ (2010). Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 9, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Wiste HJ, Weigand SD, Knopman DS, Mielke MM, Vemuri P, Lowe V, Senjem ML, Gunter JL, Reyes D, Machulda MM, Roberts R & Petersen RC (2015). Different definitions of neurodegeneration produce similar amyloid/neurodegeneration biomarker group findings. Brain 138, 3747–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont T, De Vico Fallani F, Bertrand A, Epelbaum S, Routier A, Dubois B, Hampel H, Durrleman S & Colliot O; Alzheimer's Disease Neuroimaging Initiative (2017). Amyloidosis and neurodegeneration result in distinct structural connectivity patterns in mild cognitive impairment. Neurobiol Aging 55, 177–189. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Lopez SR, Passos VM, Bittencourt MS, Dantas EM, Mill JG, Ribeiro AL, Thayer JF, Bensenor IM & Lotufo PA (2016). Insulin resistance and carotid intima‐media thickness mediate the association between resting‐state heart rate variability and executive function: A path modelling study. Biol Psychol 117, 216–224. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Mungas D, Possin KL, Rankin KP, Boxer AL, Rosen HJ, Bostrom A, Sinha L, Berhel A & Widmeyer M (2014). NIH EXAMINER: conceptualization and development of an executive function battery. J Int Neuropsychol Soc 20, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledberg A, Akerman S & Roland PE (1998). Estimation of the probabilities of 3D clusters in functional brain images. Neuroimage 8, 113–128. [DOI] [PubMed] [Google Scholar]
- Li X, Cao M, Zhang J, Chen K, Chen Y, Ma C, Fleisher A, He Y & Zhang Z (2014). Structural and functional brain changes in the default mode network in subtypes of amnestic mild cognitive impairment. J Geriatr Psychiatry Neurol 27, 188–198. [DOI] [PubMed] [Google Scholar]
- Lin F, Heffner K, Mapstone M, Chen DG & Porsteisson A (2014). Frequency of mentally stimulating activities modifies the relationship between cardiovascular reactivity and executive function in old age. Am J Geriatr Psychiatry 22, 1210–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Heffner KL, Ren P & Tadin D (2017a). A role of the parasympathetic nervous system in cognitive training. Curr Alzheimer Res 14, 784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Ren P, Lo RY, Chapman BP, Jacobs A, Baran TM, Porsteinsson AP & Foxe JJ; Alzheimer's Disease Neuroimaging Initiative (2017b). Insula and inferior frontal gyrus’ activities protect memory performance against Alzheimer's disease pathology in old age. J Alzheimers Dis 55, 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS & Gianaros PJ (2010). Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci 1186, 190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ & Hayes AF (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 40, 879–891. [DOI] [PubMed] [Google Scholar]
- Quintana DS (2017). Statistical considerations for reporting and planning heart rate variability case‐control studies. Psychophysiology 54, 344–349. [DOI] [PubMed] [Google Scholar]
- Ren P, Lo RY, Chapman BP, Mapstone M, Porsteinsson A & Lin F (2016). Longitudinal alteration of intrinsic brain activity in the striatum in mild cognitive impairment. J Alzheimers Dis 54, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti HC, Lacritz LH, Cullum CM & Weiner MF (2011). Normative data for the Montreal Cognitive Assessment (MoCA) in a population‐based sample. Neurology 77, 1272–1275. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC & Miller GE (2004). Psychological stress and the human immune system: a meta‐analytic study of 30 years of inquiry. Psychol Bull 130, 601–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchy Y (2009). Executive functioning: overview, assessment, and research issues for non‐neuropsychologists. Ann Behav Med 37, 106–116. [DOI] [PubMed] [Google Scholar]
- Taylor AG, Goehler LE, Galper DI, Innes KE & Bourguignon C (2010). Top‐down and bottom‐up mechanisms in mind‐body medicine: development of an integrative framework for psychophysiological research. Explore (NY) 6, 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredrikson M, Sollers JJ 3rd & Wager TD (2012). A meta‐analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev 36, 747–756. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus‐Rose E & Johnsen BH (2009). Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self‐regulation, adaptation, and health. Ann Behav Med 37, 141–153. [DOI] [PubMed] [Google Scholar]
- Williams DP, Thayer JF & Koenig J (2016). Resting cardiac vagal tone predicts intraindividual reaction time variability during an attention task in a sample of young and healthy adults. Psychophysiology 53, 1843–1851. [DOI] [PubMed] [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ & Wang YF (2007). Altered baseline brain activity in children with ADHD revealed by resting‐state functional MRI. Brain Dev 29, 83–91. [DOI] [PubMed] [Google Scholar]
- Zulli R, Nicosia F, Borroni B, Agosti C, Prometti P, Donati P, De Vecchi M, Romanelli G, Grassi V & Padovani A (2005). QT dispersion and heart rate variability abnormalities in Alzheimer's disease and in mild cognitive impairment. J Am Geriatr Soc 53, 2135–2139. [DOI] [PubMed] [Google Scholar]


