Abstract
AIM
To evaluate the utility of fecal calprotectin (FC) in predicting relapse and endoscopic activity during follow-up in an inflammatory bowel disease (IBD) cohort.
METHODS
All FC measurements that were obtained during a 3-year period from patients with inflammatory bowel disease in clinical remission were identified. Data regarding the short-term (6 mo) course of the disease were extracted from the medical files. Exclusion criteria were defined as: (1) An established flare of the disease at the time of FC measurement, (2) Loss to follow up within 6 mo from baseline FC measurement, and, (3) Insufficient data on file. Statistical analysis was performed to evaluate whether baseline FC measurement could predict the short term clinical relapse and/or the presence of mucosal healing.
RESULTS
We included 149 [Crohn’s disease (CD) = 113, Ulcerative colitis (UC) = 36, male = 77] IBD patients in our study. Within the determined 6-month period post-FC measurement, 47 (31.5%) had a disease flare. Among 76 patients who underwent endoscopy, 39 (51.3%) had mucosal healing. Baseline FC concentrations were significantly higher in those who had clinical relapse compared to those who remained in remission during follow up (481.0 μg/g, 286.0-600.0 vs 89.0, 36.0-180.8, P < 0.001). The significant predictive value of baseline median with IQR FC for clinical relapse was confirmed by multivariate Cox analysis [HR for 100μg/g: 1.75 (95%CI: 1.28-2.39), P = 0.001]. Furthermore, lower FC baseline values significantly correlated to the presence of mucosal healing in endoscopy (69.0 μg/g, 30.0-128.0 vs 481.0, 278.0-600.0, in those with mucosal inflammation, median with IQR, P < 0.001). We were able to extract cut-off values for FC concentration with a high sensitivity and specificity for predicting clinical relapse (261 μg/g with AUC = 0.901, sensitivity 87.2%, specificity 85.3%, P < 0.001) or mucosal healing (174 μg/g with AUC = 0.956, sensitivity 91.9%, specificity 87.2%, P < 0.001). FC was better than CRP in predicting either outcome; nevertheless, having a pathological CRP (> 5 mg/L) in addition to the cut-offs for FC, significantly enhanced the specificity for predicting clinical relapse (95.1% from 85.3%) or endoscopic activity (100% from 87.2%).
CONCLUSION
Serial FC measurements may be useful in monitoring IBD patients in remission, as FC appears to be a reliable predictor of short-term relapse and endoscopic activity.
Keywords: Fecal calprotectin, Biomarker, Inflammatory bowel disease, Mucosal healing, Clinical outcome, Relapse, Ulcerative colitis, Crohn’s disease
Core tip: Fecal calprotectin (FC) is a novel biomarker aiming to facilitate the assessment of inflammatory bowel disease (IBD) activity as its expression is driven by the presence of intestinal inflammation. Our present retrospective study provides evidence that FC measurement during clinical remission may be helpful in identifying early those cases with a higher risk of recurrence. Moreover, lower FC values seem to correlate with endoscopic quiescence of the disease. Thus, FC monitoring may be effective in recognizing distinct subgroups of IBD patients offering the opportunity to the clinician to tailor their management accordingly in order to achieve optimal disease control.
INTRODUCTION
Inflammatory bowel disease (IBD) encompasses two major clinical phenotypes, ulcerative colitis (UC) and Crohn’s disease (CD). Both present with chronic inflammation of the intestines that tends to fluctuate over time, with periods of remission alternating with flares of variable severity. Avoidance of clinical relapse and long-term maintenance of remission is of critical importance in IBD, as failure to control inflammatory activity is associated not only with impaired quality of life of patients but also worse long-term outcomes, including increased potential for colonic carcinogenesis[1,2].
The vital importance of maintaining longitudinal clinical remission emphasizes the need to accurately monitor inflammatory activity in IBD and predict imminent disease flares. Such knowledge would, then, allow for modifications in the treatment strategy, in order to avoid recurrence of overt inflammation. This approach is in line with the recent reappraisal of therapeutic goals in IBD with the introduction of the “treat-to-target” principle[3]. According to the latter concept, clinical remission should be paired with biological and endoscopic evidence of mucosal inflammatory inactivity in IBD patients. Biological inactivity may be indicated by the absence of markers of inflammation in the peripheral blood (ESR, CRP) or feces (calprotectin), whereas mucosal healing is the most accepted endoscopic target. Periodical check for such markers during clinical remission would help to identify those patients in higher risk for recurrence and in need for therapy adjustments. In fact, mucosal healing has emerged as a reliable prognostic marker for disease course[4], as it is associated with lower 2-year probability for clinical flare in both CD and UC[5]. Nevertheless, repeated colonoscopies are needed to continuously screen patients in remission for the presence of subclinical mucosal inflammation. This may create patient discomfort, increase the risk of procedure-related adverse events, and apply a significant financial burden on the health system. Therefore, discovery of non-invasive biomarkers with high correlation to the presence of mucosal healing would constitute a cost-effective substitute to repeated endoscopies.
In recent years, biomarkers detected in the feces have been proposed as an improved alternative to those measured in the serum for diagnosing residual intestinal inflammation in patients with IBD[6]. The mostly investigated candidate of this category is fecal calprotectin (FC), a calcium and zinc-binding peptide, which originates primarily from neutrophils but also from monocytes[7,8]. It constitutes almost 60% of the total cytosolic protein content in neutrophils and is released upon their activation[9,10]. Thus, the presence of elevated FC is an indicator of mucosal neutrophil infiltration and increased shedding to the intestinal lumen[11]. As these events are typical components of IBD-associated intestinal inflammation, measurement of FC may be useful in this setting[12]. We undertook the present study to evaluate the clinical utility of FC measurement in a large IBD cohort, with a regular follow-up. We report that FC values during remission help to identify those patients with a higher risk of relapse. We also demonstrate that the absence of mucosal healing on follow-up endoscopy correlates strongly with higher FC baseline values. We, further, propose cut-off values for both outcomes (i.e. clinical relapse and ongoing endoscopic activity). Finally, we demonstrate that those cut-offs may be significantly lower in patients with post-surgical recurrence of CD in comparison to surgery-free patients.
MATERIALS AND METHODS
Patient population and study outcomes
In this retrospective study, we recovered from the electronic records of our Hospital all FC measurements that were performed between Jan 2014 and Dec 2016 in patients with IBD, who had a regular follow up at our Department. The main aim of our study was to test whether a baseline FC measurement in IBD patients in clinical remission was predictive of clinical relapse in the following 6 mo. Thus, we excluded from analysis those patients with: (1) An established flare of the disease at the time of FC measurement, (2) Loss to follow up within 6 mo from baseline FC measurement, and (3) Insufficient data on file. Disease relapse was defined as: (1) Significant increase in respective clinical activity indices above accepted cut-offs for remission in CD (Harvey-Bradshaw Index ≥ 5) and UC (Simple Colitis Activity Index ≥ 3) and/or (2) step-up in the patient’s therapeutic regimen, including surgery for intractable disease-related symptoms. A secondary aim of our study was to examine whether the measured FC value could predict the presence or absence of mucosal healing. The latter was defined as an endoscopic Mayo Score of 0 for UC and the absence of mucosal lesions in the colon and terminal ileum for CD, respectively. All endoscopies were performed in our Dpt. by the same experienced endoscopist (GB) thus ensuring uniformity in mucosal healing assessment. For all study participants, demographic, epidemiologic and clinical data were retrieved from the patient files and entered in an SPSS database.
Fecal calprotectin measurement
Fecal samples were collected by patients at home and were brought on the same day to the Biochemical Laboratory of our Hospital. They were stored in deep freeze (-80 °C) and were unfrozen and used for testing at most two weeks after initial storage. For calprotectin measurement in our Hospital a commercially available monoclonal enzyme-linked immunosorbent assay (ELISA) kit (EK-CAL, Bühlmann, Switzerland) was used according to the manufacturer’s instructions. FC levels were expressed as micrograms per gram of feces. The lower limit of detection was at 10 μg/g. Other laboratory markers were routinely done in our Hospital.
Statistical analysis
The SPSS and GraphPad statistical software programs were used for the analysis. Non-parametric tests were used for statistical analysis because of the skewed distribution of FC levels. In particular, comparison between groups was performed by Mann-Whitney (2 groups) or Kruskal-Wallis test (> 2 groups). Values of FC from the same patient were compared by Wilcoxon test. Categorical variables were compared by chi-squared test. Spearman’s r-test was used to assess correlations of FC with other variables.
Univariable and multivariable Cox regression analysis was performed to evaluate whether FC could serve as independent predictor for disease flare. We accepted the hypothesis that the predictive accuracy of FC for disease flare gradually decreases over time so a 6-mo cut-off time-point was tested as described above. Proportional hazard assumptions of other predictive factors were graphically evaluated using log-minus-log plots. The predictive value of FC was adjusted in multivariable Cox regression for all variables achieving a two-sided P < 0.20 in univariable Cox regression. Hazard ratios (HR) were presented per 100 μg/g increment for FC levels.
Receiver operating characteristic (ROC) curve analysis was used to determine specificity, sensitivity and optimal threshold values of FC. The accuracy of FC was evaluated using the area under the curve (AUC) of the ROC and was defined as follows: poor 0.6-0.7; fair 0.7-0.8; good 0.8-0.9; excellent 0.9-1.0. In all cases, an alpha level of < 0.05 was considered to be significant.
RESULTS
Patients
During the 3-year period under review, 300 measurements of FC were sent from IBD patients in our Department. Among those, we identified 208 measurements which were done in 149 (CD = 113, UC = 36, male = 77) IBD patients with a regular follow-up who fulfilled the inclusion criteria. All patients were in clinical remission when FC baseline measurement was performed. Among those, 47 (31.5%) had a clinical relapse within a 6-mo period whereas 102 remained in remission. In addition, 76 patients (CD = 57, UC = 19) underwent endoscopy within the 6-mo period, among whom 39 (CD = 28, UC = 11) demonstrated mucosal healing, as defined in our study protocol.
Increased baseline fecal calprotectin values in patients with inflammatory bowel disease are associated with elevated risk for clinical relapse within 6 mo
Our main aim in the present study was to investigate whether FC measurements during clinical remission could predict which patients were at higher risk for relapse within a 6-mo period. We, therefore, compared the baseline characteristics, including FC values, between patients who had a relapse (n = 47) and those whose disease remained quiescent during the 6-mo period of f-up (n = 102) (Table 1). No differences were observed between the two groups regarding their basic demographic or clinical characteristics. In contrast, our analysis clearly showed that patients who relapsed had significantly higher FC values at baseline (481.0 μg/g, 286.0-600.0 vs 89.0, 36.0-180.8, median with IQR, P < 0.001) (Figure 1). In addition to FC concentration, total WBC count and serum CRP were also statistically different between patients with or without remission within 6 mo (Table 1). FC values significantly correlated with baseline measurements of CRP and WBC (data not shown).
Table 1.
Patient characteristics according to clinical course
| Relapse (n = 47) | Remission (n = 102) | P | |
| Age (in yr, mean ± SD, range) | 43.9 ± 17.5 (17-76) | 38.1 ± 14.6 (17-76) | NS |
| Male | 28 (59.6%) | 49 (48.0%) | 0.078 |
| Disease duration (in mo, mean ± SD, range) | 95.1 ± 118.7 (1-560) | 95.3 ± 162.0 (1-1415) | NS |
| Diagnosis | |||
| Crohn’s disease | 38 (80.9%) | 75 (73.5%) | NS |
| Ulcerative Colitis | 9 (19.1%) | 27 (26.5%) | NS |
| Disease location | NS | ||
| Ileal (L1) | 16 | 22 | |
| Colonic (L2) | 4 | 14 | |
| Ileocolonic (L3) | 28 | 29 | |
| Perianal disease (p) | 7 | 11 | |
| Proctitis | 1 | 7 | |
| Left sided | 3 | 8 | |
| Extensive | 5 | 12 | |
| Extraintestinal manifestations | 18 | 35 | NS |
| Fecal calprotectin (in μg/g, median, IQR) | 481 (286-600) | 89 (36-180.8) | < 0.001 |
| CRP (in yr, mean ± SD, range) | 11.3 ± 10.1 (3.0-41.0) | 5.1 ± 5.5 (3.1-42.0) | 0.002 |
| WBC (in thousands, mean ± SD, range) | 8.9 ± 3.3 (3.4-18.2) | 7.5 ± 2.4 (3.8-18.4) | 0.022 |
| Hgb (in g/dL, mean ± SD, range) | 13.2 ± 1.9 (9.7-16.5) | 13.6 ± 1.5 (10.0-16.4) | NS |
| PLT (in thousands, mean ± SD, range) | 277.5 ± 99.5 (117-511) | 256.0 ± 61.4 (103-450) | NS |
Figure 1.

Increased baseline fecal calprotectin values in patients with inflammatory bowel disease are associated with elevated risk for clinical relapse within 6 mo. Patients with disease relapse within 6 mo of follow up have higher FC values at baseline. Column bars represent median values with interquartile range. FC: Fecal calprotectin.
We also observed a dose-response effect of FC concentration on the probability for relapse both at 3- as well as at 6-mo post-measurement. Indeed, as shown in Figure 2, 3.3% and 6.6% patients with a baseline FC concentration of < 250 μg/g relapsed at 3- and 6-mo, respectively, as compared with 25.9% and 63% patients with a baseline value of 250–500 μg/g. Finally, patients with a baseline value greater than 500 μg/g had the highest risk for relapse (40% at 3-mo and 80% at 6-mo, respectively) (P < 0.001 for comparisons at both time points).
Figure 2.
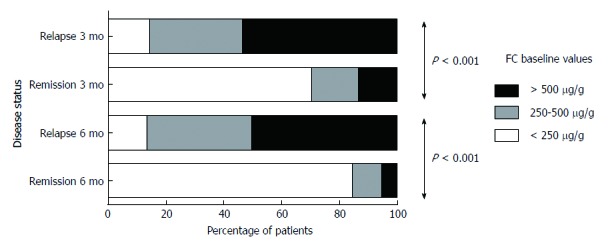
Dose-response effect of fecal calprotectin concentration on the probability for relapse both at 3- as well as at 6-mo post-measurement. Cumulative number of patients with clinical relapse during 6-mo follow-up in relation to baseline concentration of fecal calprotectin. Patients belonging to the groups with higher FC values have an increased probability to relapse within both 3 and 6 mo of follow up. FC: Fecal calprotectin.
To further assess the risk of disease flare according to baseline FC levels, we conducted univariable Cox regression analysis to test baseline variables as predictors for disease relapse. FC levels at baseline were found to be predictive of relapse within 6 mo [HR for 100 μg/g: 1.21 (95%CI: 1.15-1.28), P < 0.001], along with CRP levels and white blood cell count. Moreover, 3 more variables (age, gender and treatment with “aminosalicylates only” at baseline) exhibited a two-sided P < 0.20 in univariable Cox regression and were thus included in the multivariate analysis (Table 2). When conducting multivariate analysis to check for possible interactions, it was shown that FC baseline levels remained a strong independent predictor for disease flare [HR for 100μg/g: 1.75 (95%CI: 1.28-2.39), P = 0.001]. WBC count retained marginal predictive significance in the multivariate analysis, whereas CRP did not (Table 2).
Table 2.
Cox regression analysis: Fecal calprotectin as predictor of disease flare
|
Disease flare |
||||
|
Univariate |
Multivariate |
|||
| HR (95%CI) | P | HR (95%CI) | P | |
| FC (HR per 100 μg/g) | 1.212 (1.147-1.280) | < 0.001 | 1.745 (1.275-2.388) | 0.001 |
| Age (in yr) | 1.018 (0.998-1.037) | 0.055 | 0.973 (0.937-1.011) | NS |
| Gender (male vs female) | 0.577 (0.322-1.033) | 0.064 | 0.643 (0.151-2.749) | NS |
| Disease duration (in mo) | 1.000 (0.998-1.002) | > 0.2 | ||
| Disease (UC vs CD) | 1.373(0.664-2.838) | > 0.2 | ||
| Extraintestinal manifestations (none vs present) | 1.085 (0.856-1.375) | > 0.2 | ||
| Treatment at baseline | 0.915 (0.650-1.287) | > 0.2 | ||
| 5-ASA | 0.408 (0.125-1.331) | 0.137 | 8.805 (0.753-102.913) | NS |
| Immunomodulators (AZA, 6-MP) | 1.193 (0.575-2.474) | > 0.2 | ||
| Anti-TNF | 0.754 (0.391-1.455) | > 0.2 | ||
| Previous Surgery for disease | 1.494 (0.591-3.776) | > 0.2 | ||
| CRP (HR per 1mg/dL) | 1.062 (1.034-1.091) | < 0.001 | 1.010 (0.935-1.093) | NS |
| WBC (HR per 1000) | 1.144 (1.028-1.273) | 0.014 | 1.197 (1.001-1.432) | 0.049 |
| Hgb (HR per 1 g/dL) | 0.887 (0.702-1.121) | > 0.2 | ||
| PLT (HR per 1000) | 1.003 (0.998-1.008) | > 0.2 | ||
FC: Fecal calprotectin; CD: Crohn’s disease; UC: Ulcerative colitis.
In addition, we used receiver operating characteristic (ROC) curve analysis to determine potential cut-off FC values for the prediction of short-term clinical relapse. The optimal threshold value for elevated risk of disease flare in our cohort of IBD patients was found to be 261 μg/g with excellent accuracy (AUC = 0.901, sensitivity 87.2%, specificity 85.3%, P < 0.001) (Figure 3A and B).
Figure 3.
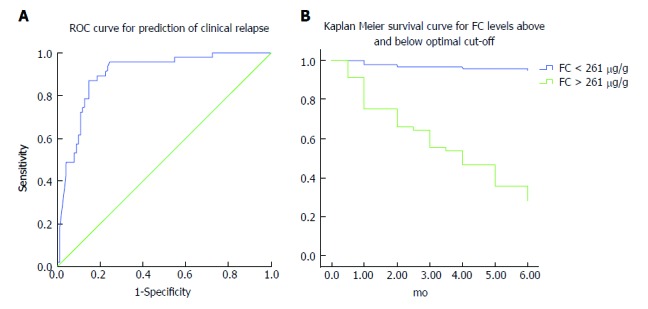
Optimal threshold fecal calprotectin values for predicting elevated risk of disease flare. A: Receiver operating curve analysis: FC baseline levels accurately predict clinical relapse in IBD patients within 6 mo of follow up. B: Disease relapse survival curves (Kaplan-Meier analysis) of patients with FC levels below and above 261 μg/g at baseline. Patients with FC values above this threshold have a substantial risk of disease flare within 6 mo of follow up. FC: Fecal calprotectin; IBD: Inflammatory bowel disease.
To further validate the significance of FC measurements in discriminating patients with clinical relapse, we compared FC concentrations in 23 individual cases where FC was tested in the same patient before and during disease flare. As shown in Figure 4, FC values were significantly increased during flare episodes (592.0, 394.25-600.0 vs 160.5, 98.5-265.8, median with IQR, P < 0.001).
Figure 4.

Fecal calprotectin values significantly increase during flare episodes in the same patient. FC values are significantly increased during flare episodes in comparison to remission in the same patients. FC: Fecal calprotectin.
Taken together, these results demonstrate that FC measurement in remission may be useful in distinguishing which patients are at higher risk of relapse.
Lower baseline fecal calprotectin values correlate with mucosal healing
We, next, sought to determine whether baseline FC measurement was associated with the presence of endoscopic remission or inflammation upon follow-up endoscopy. To answer this question, we studied the subgroup of patients (n = 76) that underwent lower gastrointestinal endoscopy within 6 mo of having a FC measurement (mean period between FC measurement and endoscopy was 4.1 mo) (Figure 5A). It should be noted that FC was not assessed in the majority (> 90%) of patients at the time of endoscopy. In our study, we found that FC values were significantly lower in those patients that had mucosal healing (69.0 μg/g, 30.0-128.0, median with IQR) as compared with those who had inflammation in endoscopy (481.0, 278.0-600.0, P < 0.001). Similarly, to clinical relapse, we, also, used receiver operating characteristic (ROC) curve analysis, to determine cut-off FC values for predicting mucosal inflammation. The optimal value that was derived from our study was found to be 174 μg/g, which offered excellent accuracy for predicting the presence of mucosal healing [sensitivity 91.9%, specificity 87.2%, (AUC = 0.956, P < 0.001)] (Figure 5B).
Figure 5.
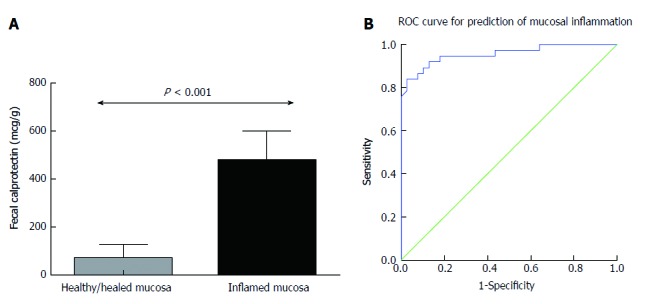
Lower fecal calprotectin baseline values are associated with increased probability of achieving mucosal healing. A: Patients with mucosal healing at endoscopy performed within 6 mo of follow up have lower FC values at baseline. Column bars represent median values with interquartile range. B: Receiver operating curve analysis: FC baseline levels predict accurately the presence of mucosal inflammation in endoscopy performed within 6 mo of FC measurement. FC: Fecal calprotectin.
Interestingly when we tried to ascertain the significance of baseline CRP in identifying patients with clinical relapse or mucosal inflammation using ROC curve analysis as described above the results were not as satisfying (AUC = 0.695 for clinical relapse and AUC = 0.599 for mucosal inflammation). Nevertheless, we observed that CRP baseline measurements were useful in improving the specificity of predicting clinical relapse as well as mucosal healing in our study group. In particular, when patients apart from a FC baseline value > 261 μg/g had also CRP higher than normal (> 5 mg/L in our laboratory) clinical relapse could be predicted with a specificity of 95.1%. Similarly, in patients with FC values > 174 μg/g and CRP > 5 mg/dL) identifying the cases with mucosal inflammation could be estimated with a specificity of 100% in our cohort. Thus, CRP measurement may not be very helpful alone in recognizing patients with higher risk for flare. On the other hand, it could be quite useful as a secondary step in reducing false positive cases among those who have already exhibited high FC values, further improving our diagnostic yield of high risk patients.
In our study population, there were also eight patients with CD who developed endoscopic recurrence after right hemicolectomy for L1 disease. We compared this group with 12 surgery-naïve CD patients with ileal disease (L1) who also demonstrated active endoscopic lesions. We found that FC values in the post-surgery group were significantly lower (171.5, 70.8-194.5 vs 581.5, 469.0-600.0, median with IQR, P < 0.001) (Figure 6).
Figure 6.

Endoscopic recurrence in post-surgical patients is associated with considerably lower fecal calprotectin values than in surgery-naïve CD patients with inflammation. FC values are significantly lower in post-surgery CD-L1 patients with inflammation in comparison to surgery-naïve CD-L1 patients. Bars represent median values with interquartile range. FC: Fecal calprotectin; CD: Crohn’s disease.
In all, these results indicate that elevated FC concentrations are indicative of persistent or impending endoscopic inflammation.
DISCUSSION
Several studies have reported strong associations between increased FC values and clinically active CD or UC[13-15], as well as a potential for FC measurement to predict clinical relapses[16-18] and mucosal healing in patients with IBD[19,20]. Nevertheless, the optimal cut-off values and the particular patient groups that may benefit the most from FC monitoring still remain to be defined. This may be the result of diverse disease characteristics between studies and/or technical considerations regarding FC measurement.
In the present study, we provide evidence that, in IBD patients who are in clinical remission, measurement of the concentration of FC may be used as biological marker for the presence of mucosal inflammation. Serial FC concentration measurements may be effectively used to predict ensuing clinical relapses and/or the existence or development of endoscopically active disease.
Our findings strongly support the notion that FC concentration is a highly reliable marker of both ongoing clinical activity as well as imminent disease flare in patients with IBD. First, patients with active disease had, as a group, higher FC values that those in remission. Second, more importantly, when paired FC values were tested in individual patients between clinical remission and disease flare, we always observed a clear increase of FC during the latter. This indicates that, in a single patient, FC values accurately reflect the inflammatory status of the disease. Finally, our main finding is that a random measurement of FC concentration highly correlated with the probability of developing clinically active disease during the following 6 mo. This is in line with previous studies that demonstrated a similar predictive significance of FC concentration[21-23]. In fact, recent evidence supports the notion that FC may be the most promising biomarker in providing an estimate of upcoming flare risk[24-26]. In our study, two additional laboratory parameters, CRP and WBC count, were associated with increased probability for relapse in univariate analysis. Nevertheless, among those three, FC performed better and had the most significant difference between patients with sustained clinical remission and those who relapsed within 6 mo of follow-up. Moreover, FC was the only marker that clearly retained its significance to discriminate the two groups of patients in multivariate analysis. The use of FC as an activity biomarker in IBD holds the additional benefit of being specific for intestinal inflammation and not affected by systemic or extraintestinal inflammation, as is the case with CRP or WBC. In fact, other studies have generated conflicting results regarding the value of CRP for this purpose, with some reporting higher CRP levels in IBD patients who relapsed, but the majority showing no such correlation[17,27-29]. Taken together, these previous studies as well as our own present results point to the fact that serial FC measurements may be the best way to follow-up IBD patients in remission inasmuch they may help clinicians to adjust their therapeutic and diagnostic approaches in individual cases.
In our patient cohort, we were able to define an optimal cut-off FC value of 261 μg/g, which had a strong predictive value for the discrimination of future relapses vs maintenance of remission. Our proposed cut-off is well within the range of FC concentrations that are suitable for predicting a relapse, according to the recent meta-analysis that was reported by Mao et al[24]. Indeed, our sensitivity and specificity values are comparable to those observed by other groups[25,29]. Moreover, we show in our cohort that specificity for assessing our study endpoints, may be further improved by additionally testing for CRP in those with FC values over the cut-off. In our study, we chose to focus on a short period (< 6 mo) of follow-up, post-baseline FC testing. This is in line with recent publications that suggest a stronger correlation between high FC values and early (< 12 mo) rather than late relapse[17,21]. Interestingly, contrary to previous indications that FC may be more useful in predicting relapse in UC than CD[30] and in CD with colon involvement compared to L1 disease[18,27], no such significant differences were observed in our cohort. This, however, may be due to the small number of UC and L1 CD patients in our study.
A further important finding from our study is the association of elevated baseline FC values with endoscopically evident mucosal inflammation during the 6-mo follow-up. This is of particular significance as recent studies have shown that not only the presence of symptoms but also the persistence of subclinical mucosal inflammation may be associated with adverse outcomes in IBD. Such residual inflammation may occur in the absence of clinical activity, while the patient is considered to be in remission[17]. In fact, absence of mucosal healing was predictive of disease relapse, as well as hospitalization and IBD-related surgeries[16]. Thus, the potential of FC as a surrogate marker for this endoscopically defined target is intriguing. Early results have showed promising but not conclusive associations[19,30]. Nevertheless, a recent study by D’Haens et al[20]. Reported that FC levels below 250 μg/g had 94% sensitivity and 62% specificity in predicting mucosal healing in CD patients[20]. In our analysis, we demonstrated a lower optimal threshold value (174 μg/g) which offered comparable sensitivity and improved specificity for assessing the presence of endoscopic inflammation. Notably, upon further analysis, we were able to detect a subgroup of patients with no clinical activity in whom elevated FC levels were predictive of ongoing endoscopically-evident inflammation (data not shown). We are currently contacting a prospective study on the importance of FC on the selection of patients in remission who may be in need for re-classification of inflammatory activity via endoscopy. This may lead to timely therapeutic interventions in high-risk groups and ameliorate the risk for long-term complications. Our results also indicate that FC measurements should always be considered in the appropriate clinical context, as cut-off values may vary in different patient populations. In fact, our findings support the notion that a lower threshold for endoscopy may be needed in CD patients with post-surgery surveillance as endoscopic recurrence was associated with considerably lower FC values than in surgery-naïve CD patients with inflammatory lesions (Figure 6)[31,32].
Our study has important limitations. One such drawback is its retrospective nature as data were collected from the patients’ files. This may have led to collection bias. Furthermore, the number of study participants was relatively small. This did not allow us to separate patients with UC from those with CD. Nevertheless, similar trends were observed between the two populations regarding the utility of FC measurements. On the other hand, among the strengths of our study were the fact that we recruited a well-characterized patient cohort with adequate follow-up, and also the considerable percentage of patients who underwent endoscopy, thus allowing us to better estimate the presence of residual inflammation.
In conclusion, we provide evidence for an important role of FC measurement in the prediction of short-term clinical relapse and endoscopic activity in patients with IBD. Further prospective studies with larger number of patients and separation of UC from CD populations are needed to define the exact role of FC in the diagnostic and treatment algorithms in IBD.
ARTICLE HIGHLIGHTS
Research background
Currently, there is increased need for the discovery of simple to perform, non-invasive biomarkers with high correlation to intestinal inflammatory activity in patients with inflammatory bowel disease (IBD). Fecal calprotectin (FC) measurement has shown promise as a candidate marker for this purpose.
Research motivation
Although the value of measuring FC as an indicator for active inflammation is indisputable, the correlation between FC values and future flare of disease activity has not been fully established. Furthermore, uncertainty still exists regarding the optimal cut-off values of FC for this indication and applicability of its measurement in diverse patient groups.
Research objectives
Our principal aim was to study the clinical significance of measuring FC in IBD patients in clinical remission, both as a biomarker for patients stratification according to their risk for relapse, as well as a surrogate marker of endoscopic mucosal healing.
Research methods
We retrospectively analyzed the electronic medical records of all patients with IBD, with a regular follow up at our department and a FC measurement in a 3-year study period. We specifically focused on patients in stable clinical remission and a medium-term follow-up (at least 6-mo). We then compared two groups of patients: those who remained in remission and those who relapsed (according to pre-defined criteria) during the 6-mo follow-up. A secondary aim of our study was to examine whether the measured FC value could predict the presence or absence of mucosal healing (defined as an endoscopic Mayo Score of 0 for UC and absence of significant mucosal lesions in the colon and terminal ileum for CD, respectively). For all study participants, demographic, epidemiologic and clinical data were retrieved from the patient files and entered in an SPSS database.
Research results
The main findings of our analysis are as follows: First, patients who relapsed within 6-mo from FC measurement had significantly higher baseline FC concentrations than those who remained in remission. Second, patients with mucosal abnormalities in endoscopy (i.e. absence of mucosal healing) had significantly higher FC values as compared to patients that demonstrated endoscopic mucosal healing. Third, we were able to define cut-off values for FC concentrations with high sensitivity and specificity for predicting clinical relapse or endoscopical activity in patients with IBD in clinical remission. Fourth, combining FC with CRP measurements further increases the predictive value for short-term clinical flare. Finally, cut-offs may be significantly lower in patients with post-surgical recurrence of CD in comparison to surgery-free patients.
Research conclusions
Our findings clearly indicate that short-term clinical relapse can be predicted with high accuracy by measuring FC concentration in patients with IBD. Consequently, serial FC measurements may prove to be a very useful tool to monitor subclinical inflammatory activity in IBD patients who are in clinical remission. Furthermore, our results show that a high FC value is a surrogate marker of endoscopically active disease. Given the high importance that mucosal healing has gained in recent years for determining disease outcomes in IBD, FC measurements may become a valuable tool for the selection of those patients who need to have an endoscopy while in clinical remission. Subsequently, treatment modifications may be preemptively implemented in such cases, in order to avoid clinical recurrence of symptoms and the systemic consequences of persisting inflammatory activity.
Footnotes
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Laika General Hospital Ethics Committee and Institutional Review Board.
Informed consent statement: All study participants, provided informed consent for access to data in their patient files.
Conflict-of-interest statement: The authors have no conflict of interest to declare.
Data sharing statement: No additional data are available.
Peer-review started: August 19, 2017
First decision: August 30, 2017
Article in press: September 28, 2017
P- Reviewer: Day AS, Jadallah KA, Sergi CM S- Editor: Wei LJ L- Editor: A E- Editor: Huang Y
Contributor Information
Athanasios Kostas, Academic Department of Gastroenterology, University of Athens Medical School, Laiko General Hospital, Athens 11527, Greece.
Spyros I Siakavellas, Academic Department of Gastroenterology, University of Athens Medical School, Laiko General Hospital, Athens 11527, Greece.
Charalambos Kosmidis, Academic Department of Gastroenterology, University of Athens Medical School, Laiko General Hospital, Athens 11527, Greece.
Anna Takou, Biochemistry Department, Laiko General Hospital, Athens 11527, Greece.
Joanna Nikou, Biochemistry Department, Laiko General Hospital, Athens 11527, Greece.
Georgios Maropoulos, Biochemistry Department, Laiko General Hospital, Athens 11527, Greece.
John Vlachogiannakos, Academic Department of Gastroenterology, University of Athens Medical School, Laiko General Hospital, Athens 11527, Greece.
George V Papatheodoridis, Academic Department of Gastroenterology, University of Athens Medical School, Laiko General Hospital, Athens 11527, Greece.
Ioannis Papaconstantinou, 2nd Department of Surgery, University of Athens Medical School, Areteion General Hospital, Athens 11528, Greece.
Giorgos Bamias, Academic Department of Gastroenterology, University of Athens Medical School, Laiko General Hospital, Athens 11527, Greece. gbamias@gmail.com.
References
- 1.Vatn MH. Natural history and complications of IBD. Curr Gastroenterol Rep. 2009;11:481–487. doi: 10.1007/s11894-009-0073-8. [DOI] [PubMed] [Google Scholar]
- 2.Grivennikov SI. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol. 2013;35:229–244. doi: 10.1007/s00281-012-0352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darr U, Khan N. Treat to Target in Inflammatory Bowel Disease: An Updated Review of Literature. Curr Treat Options Gastroenterol. 2017;15:116–125. doi: 10.1007/s11938-017-0130-6. [DOI] [PubMed] [Google Scholar]
- 4.Peyrin-Biroulet L, Ferrante M, Magro F, Campbell S, Franchimont D, Fidder H, Strid H, Ardizzone S, Veereman-Wauters G, Chevaux JB, et al. Results from the 2nd Scientific Workshop of the ECCO. I: Impact of mucosal healing on the course of inflammatory bowel disease. J Crohns Colitis. 2011;5:477–483. doi: 10.1016/j.crohns.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Baert F, Moortgat L, Van Assche G, Caenepeel P, Vergauwe P, De Vos M, Stokkers P, Hommes D, Rutgeerts P, Vermeire S, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010;138:463–468 quiz e10-1. doi: 10.1053/j.gastro.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 6.Tibble JA, Bjarnason I. Fecal calprotectin as an index of intestinal inflammation. Drugs Today (Barc) 2001;37:85–96. doi: 10.1358/dot.2001.37.2.614846. [DOI] [PubMed] [Google Scholar]
- 7.Angriman I, Scarpa M, D’Incà R, Basso D, Ruffolo C, Polese L, Sturniolo GC, D’Amico DF, Plebani M. Enzymes in feces: useful markers of chronic inflammatory bowel disease. Clin Chim Acta. 2007;381:63–68. doi: 10.1016/j.cca.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 8.Bjerke K, Halstensen TS, Jahnsen F, Pulford K, Brandtzaeg P. Distribution of macrophages and granulocytes expressing L1 protein (calprotectin) in human Peyer’s patches compared with normal ileal lamina propria and mesenteric lymph nodes. Gut. 1993;34:1357–1363. doi: 10.1136/gut.34.10.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poullis A, Foster R, Northfield TC, Mendall MA. Review article: faecal markers in the assessment of activity in inflammatory bowel disease. Aliment Pharmacol Ther. 2002;16:675–681. doi: 10.1046/j.1365-2036.2002.01196.x. [DOI] [PubMed] [Google Scholar]
- 10.Aomatsu T, Yoden A, Matsumoto K, Kimura E, Inoue K, Andoh A, Tamai H. Fecal calprotectin is a useful marker for disease activity in pediatric patients with inflammatory bowel disease. Dig Dis Sci. 2011;56:2372–2377. doi: 10.1007/s10620-011-1633-y. [DOI] [PubMed] [Google Scholar]
- 11.Smith LA, Gaya DR. Utility of faecal calprotectin analysis in adult inflammatory bowel disease. World J Gastroenterol. 2012;18:6782–6789. doi: 10.3748/wjg.v18.i46.6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikhtaire S, Shajib MS, Reinisch W, Khan WI. Fecal calprotectin: its scope and utility in the management of inflammatory bowel disease. J Gastroenterol. 2016;51:434–446. doi: 10.1007/s00535-016-1182-4. [DOI] [PubMed] [Google Scholar]
- 13.Xiang JY, Ouyang Q, Li GD, Xiao NP. Clinical value of fecal calprotectin in determining disease activity of ulcerative colitis. World J Gastroenterol. 2008;14:53–57. doi: 10.3748/wjg.14.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denis MA, Reenaers C, Fontaine F, Belaïche J, Louis E. Assessment of endoscopic activity index and biological inflammatory markers in clinically active Crohn’s disease with normal C-reactive protein serum level. Inflamm Bowel Dis. 2007;13:1100–1105. doi: 10.1002/ibd.20178. [DOI] [PubMed] [Google Scholar]
- 15.Schoepfer AM, Beglinger C, Straumann A, Trummler M, Vavricka SR, Bruegger LE, Seibold F. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010;105:162–169. doi: 10.1038/ajg.2009.545. [DOI] [PubMed] [Google Scholar]
- 16.Tibble JA, Bjarnason I. Non-invasive investigation of inflammatory bowel disease. World J Gastroenterol. 2001;7:460–465. doi: 10.3748/wjg.v7.i4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gisbert JP, Bermejo F, Pérez-Calle JL, Taxonera C, Vera I, McNicholl AG, Algaba A, López P, López-Palacios N, Calvo M, et al. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm Bowel Dis. 2009;15:1190–1198. doi: 10.1002/ibd.20933. [DOI] [PubMed] [Google Scholar]
- 18.D’Incà R, Dal Pont E, Di Leo V, Benazzato L, Martinato M, Lamboglia F, Oliva L, Sturniolo GC. Can calprotectin predict relapse risk in inflammatory bowel disease? Am J Gastroenterol. 2008;103:2007–2014. doi: 10.1111/j.1572-0241.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 19.Røseth AG, Aadland E, Grzyb K. Normalization of faecal calprotectin: a predictor of mucosal healing in patients with inflammatory bowel disease. Scand J Gastroenterol. 2004;39:1017–1020. doi: 10.1080/00365520410007971. [DOI] [PubMed] [Google Scholar]
- 20.D’Haens G, Ferrante M, Vermeire S, Baert F, Noman M, Moortgat L, Geens P, Iwens D, Aerden I, Van Assche G, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218–2224. doi: 10.1002/ibd.22917. [DOI] [PubMed] [Google Scholar]
- 21.Zhulina Y, Cao Y, Amcoff K, Carlson M, Tysk C, Halfvarson J. The prognostic significance of faecal calprotectin in patients with inactive inflammatory bowel disease. Aliment Pharmacol Ther. 2016;44:495–504. doi: 10.1111/apt.13731. [DOI] [PubMed] [Google Scholar]
- 22.Lasson A, Simrén M, Stotzer PO, Isaksson S, Ohman L, Strid H. Fecal calprotectin levels predict the clinical course in patients with new onset of ulcerative colitis. Inflamm Bowel Dis. 2013;19:576–581. doi: 10.1097/MIB.0b013e31827e78be. [DOI] [PubMed] [Google Scholar]
- 23.Naismith GD, Smith LA, Barry SJ, Munro JI, Laird S, Rankin K, Morris AJ, Winter JW, Gaya DR. A prospective evaluation of the predictive value of faecal calprotectin in quiescent Crohn’s disease. J Crohns Colitis. 2014;8:1022–1029. doi: 10.1016/j.crohns.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 24.Mao R, Xiao YL, Gao X, Chen BL, He Y, Yang L, Hu PJ, Chen MH. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies. Inflamm Bowel Dis. 2012;18:1894–1899. doi: 10.1002/ibd.22861. [DOI] [PubMed] [Google Scholar]
- 25.Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15–22. doi: 10.1053/gast.2000.8523. [DOI] [PubMed] [Google Scholar]
- 26.Walkiewicz D, Werlin SL, Fish D, Scanlon M, Hanaway P, Kugathasan S. Fecal calprotectin is useful in predicting disease relapse in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:669–673. doi: 10.1002/ibd.20376. [DOI] [PubMed] [Google Scholar]
- 27.García-Sánchez V, Iglesias-Flores E, González R, Gisbert JP, Gallardo-Valverde JM, González-Galilea A, Naranjo-Rodríguez A, de Dios-Vega JF, Muntané J, Gómez-Camacho F. Does fecal calprotectin predict relapse in patients with Crohn’s disease and ulcerative colitis? J Crohns Colitis. 2010;4:144–152. doi: 10.1016/j.crohns.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Kiss LS, Papp M, Lovasz BD, Vegh Z, Golovics PA, Janka E, Varga E, Szathmari M, Lakatos PL. High-sensitivity C-reactive protein for identification of disease phenotype, active disease, and clinical relapses in Crohn’s disease: a marker for patient classification? Inflamm Bowel Dis. 2012;18:1647–1654. doi: 10.1002/ibd.21933. [DOI] [PubMed] [Google Scholar]
- 29.Kallel L, Ayadi I, Matri S, Fekih M, Mahmoud NB, Feki M, Karoui S, Zouari B, Boubaker J, Kaabachi N, et al. Fecal calprotectin is a predictive marker of relapse in Crohn’s disease involving the colon: a prospective study. Eur J Gastroenterol Hepatol. 2010;22:340–345. doi: 10.1097/MEG.0b013e32832bab49. [DOI] [PubMed] [Google Scholar]
- 30.Costa F, Mumolo MG, Ceccarelli L, Bellini M, Romano MR, Sterpi C, Ricchiuti A, Marchi S, Bottai M. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005;54:364–368. doi: 10.1136/gut.2004.043406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orlando A, Modesto I, Castiglione F, Scala L, Scimeca D, Rispo A, Teresi S, Mocciaro F, Criscuoli V, Marrone C, et al. The role of calprotectin in predicting endoscopic post-surgical recurrence in asymptomatic Crohn’s disease: a comparison with ultrasound. Eur Rev Med Pharmacol Sci. 2006;10:17–22. [PubMed] [Google Scholar]
- 32.Scarpa M, D’Incà R, Basso D, Ruffolo C, Polese L, Bertin E, Luise A, Frego M, Plebani M, Sturniolo GC, et al. Fecal lactoferrin and calprotectin after ileocolonic resection for Crohn’s disease. Dis Colon Rectum. 2007;50:861–869. doi: 10.1007/s10350-007-0225-6. [DOI] [PubMed] [Google Scholar]


