Abstract
AIM
To evaluate the incidence of anastomotic strictures after intestinal resection in Crohn’s disease (CD), demonstrate long-term efficacy and safety of endoscopic balloon dilation (EBD) in CD strictures and its impact on the diagnosis of subclinical postoperative endoscopic recurrence.
METHODS
Retrospective single tertiary center study based on prospectively collected data between 2010 and 2015 including anastomotic and non-anastomotic strictures.
RESULTS
29% of 162 CD patients included developed an anastomotic stricture. 43 patients with anastomotic strictures and 37 with non-anastomotic strictures underwent EBD; technical success was 97.7% and 100%, respectively, however, 63% and 41% needed repeat dilation during the 4.4-year follow-up. Longer periods between surgery and index colonoscopy and higher lactoferrin levels were associated with the presence of stricture after surgery. Calprotectin levels > 83.35 μg/g and current or past history of smoking were associated with a shorter time until need for dilation (HR = 3.877, 95%CI: 1.480-10.152 and HR = 3.041, 95%CI: 1.213-7.627). Anastomotic strictures had a greater need for repeat dilation (63% vs 41%, P = 0.047). No differences were found between asymptomatic and symptomatic cohorts. Disease recurrence diagnosis was only possible after EBD in a third of patients.
CONCLUSION
EBD is an effective and safe alternative to surgery, with a good short and long-term outcome, postponing or even avoiding further surgery. EBD may allow to diagnose disease recurrence in patients with no clinical signs/biomarkers of disease activity.
Keywords: Crohn’s disease, Endoscopic recurrence, Anastomotic strictures, Non-anastomotic strictures, Endoscopic balloon dilation
Core tip: This study evaluated the incidence of anastomotic strictures after intestinal resection in Crohn’s disease (CD), the long-term efficacy and safety of endoscopic balloon dilation (EBD) in CD strictures and its impact on the diagnosis of subclinical postoperative endoscopic recurrence. Almost one-third of CD patients developed an anastomotic stricture after ileocecal resection/right hemicolectomy. EBD was an effective and safe alternative to surgery, with a good short and long-term outcome, postponing or even avoiding further surgery. EBD also allowed to diagnose disease recurrence in patients with no clinical signs/biomarkers of disease activity. Longer intervals after surgery and higher lactoferrin levels were associated with anastomotic strictures; time until dilation was lower in patients with calprotectin levels > 83.35 μg/g and current/past history of smoking.
INTRODUCTION
Strictures in Crohn’s disease (CD) develop during the course of the disease or as the presenting feature[1]. Up to 50% of CD patients undergo surgical resection within the first 10 years of diagnosis[2]. Disease recurrence often occurs at or above the anastomosis due to ongoing inflammatory activity[3,4]. This can result in luminal narrowing, and strictures (non-anastomotic and anastomotic), with up to 70% of patients requiring additional resection[5], though is unpredictable[6].
Medical therapy for stricture management is limited due to the fibrotic nature. Management includes surgical resection and stricturoplasty but with a high rate of recurrence and need for reoperation[7]. Increasing evidence supports endoscopic balloon dilation (EBD) as a safe and effective alternative to surgery, particularly for ileocecal and anastomotic strictures[8,9]. Technical and clinical success rates (resolution of obstructive symptoms) are seen in 73%-100% and 64%-70%, respectively, with a major adverse event (AE) rate of 2%-6.4%[1,10-12]. Balloon diameters of 25 mm are believed to increase risk of AEs[13]. During long-term follow-up patients needing surgery at 1, 3 and 5 years varies form 13%-17%, 28%-42%, and 36%-42% respectively. Strictures recur following dilation, and re-dilation may be required in up to 20% and 50% by 1 and 5 years, respectively[1,14,15]. The best results following dilation are obtained when stricture length is < 4 cm, and for anastomotic strictures when compared to de novo strictures[1,12,16].
Anastomotic strictures may represent disease recurrence, but data is limited and contradictory as to whether escalation of medical therapy following dilation may prevent the need for repeat dilation or surgery[10,12]. Other factors such as smoking status and disease activity status at the time of dilation may affect outcome of stricture dilation[12], though many studies are limited by short follow-up durations and small cohorts.
We sought to evaluate anastomotic stricture development after intestinal resection in CD and demonstrate long-term efficacy and safety of EBD in CD anastomotic and de novo strictures in a large referral centre cohort and determine the impact of dilation on the diagnosis of subclinical postoperative endoscopic recurrence.
MATERIALS AND METHODS
Retrospective single tertiary center study based on prospectively collected data from a clinical database created for this purpose. Patients were treated from March 2010 to February 2015 including CD patients who had undergone ileocecal resection/right hemicolectomy. All patients were followed at our Inflammatory Bowel Disease (IBD) outpatient clinic and referred for endoscopic evaluation. Inclusion criteria were definitive diagnosis of CD established by clinical, radiographic, endoscopic, and histological criteria and previous surgery and surgical pathology. Exclusion criteria were previous EBD, age < 18 years, stricture length > 6 cm and fistulae or deep ulceration of the strictured segment.
Clinical disease activity was assessed on the day of endoscopic examination, using the Harvey Bradshaw Index (HBI). Clinically inactive disease was defined as a HBI < 5. Postoperative disease activity of the neoterminal ileum was evaluated according to the Modified Rutgeerts’ score[17]. Indication for EBD was to evaluate endoscopic recurrence or symptom/biomarker-driven.
CD patients with non-anastomotic strictures who underwent EBD during the study period were included as a control group (Figure 1).
Figure 1.
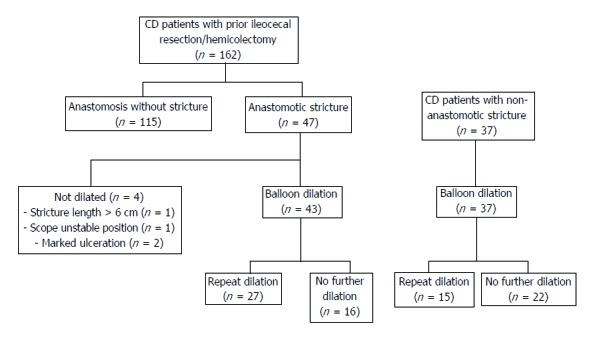
Flowchart with study design.
All procedures were performed in an outpatient setting under propofol sedation, with CO2 insufflation, by one of two endoscopists (SL and ERP). Polyethylene glycol based bowel preparation was administered the day before colonoscopy. EBD was performed for strictures that would not allow passage with a colonoscope, regardless of patients’ symptoms. Dilations were performed endoscopically with a through-the-scope balloon (Boston Scientific, Marlborough, MA), of 10-18 mm diameter and lengths of 55 mm. The balloon was filled with diluted contrast, with diameter of the balloon chosen according to endoscopist discretion. Inflation pressure was maintained for 2 min.
Technical success was defined as the ability to pass the colonoscope through the stricture into the neoileum following dilation. Clinical success was defined as improvement of obstructive symptoms (in symptomatic patients). Major AEs were defined as major bleeding (requiring surgery, blood transfusion or hospital admission) and perforation. Minor, self-limited bleeding was not considered an AE. All patients who underwent dilation were endoscopically reevaluated 6-12 mo later. Long-term efficacy was defined as avoidance of surgical resection or repeat dilation after the initial dilation. Patients were followed until stricture resection, last clinic follow-up, or censor date of March, 2017. Escalation of medical therapy was defined as initiation of a thiopurine or anti-TNF within 6 mo of first dilation, as determined by global physician assessment.
All patients gave informed written consent to participate in the study that was approved by the Ethics Committee of our Institution.
Statistical analysis
Categorical variables were described through absolute and relative frequencies. Continuous variables were described as median, minimum and maximum and dichotomized for analysis using the best cut-off on ROC analysis. Hypotheses were tested about the distribution of continuous variables with non-normal distribution, by using the nonparametric Mann-Whitney. The Chi-squared test and Fisher’s exact test were used for differences in proportions of patients experiencing a given outcome. Univariate and multivariate analysis by logistic regression was used to explore the correlation between predictor variables and need of dilation after surgery, as well as need to repeat dilation. To identify independent predictors of need of dilation after surgery, as well as need to repeat dilation, all significant variables evaluated in the univariate analysis were included. Kaplan-Meier survival analysis with log rank statistics was used to assess event-free survival, and Cox conditional proportional hazards regression analysis was used to time-free survival. The results are shown as odds ratio (OR) and hazards ratio (HR) with 95% confidence intervals (CI). All the reported P values were two-sided, and P values of < 0.05 were considered statistically significant. All data were arranged, processed and analyzed with SPSS® v.24.0 data (Statistical Package for Social Sciences).
RESULTS
Population
A total a 162 CD patients (52.5% males, n = 85) who had undergone ileocecal resection/right hemicolectomy were included; the mean age was 42.6 years (SD ± 13.4 years). Baseline demographic characteristics are listed in Table 1. The median follow-up period since colonoscopy index was 4.4 years (1.3-6.8), with a median disease duration of 17.1 years (3.3-52.1). Median time between surgery and index colonoscopy was 7.7 years (range 0.3-37.6 years).
Table 1.
Baseline characteristics of crohn’s disease patients with prior ileocecal resection/right hemicolectomy
| Characteristic | CD (n = 162) |
| Female/male (n) | 77/85 |
| Disease duration in years (median; min-max) | 17.1 (3.3-52.1) |
| Follow-up time in years (median; min-max) | 4.4 (1.3-6.8) |
| Time between surgery and index colonoscopy in years (median; min-max) | 7.7 (0.3-37.6) |
| Age at index colonoscopy (mean; standard deviation) | 42.6 (± 13.4) |
| Montreal classification (n; %) | |
| Age at diagnosis | |
| A1 | 25 (15.5) |
| A2 | 116 (72) |
| A3 | 20 (12.4) |
| Disease location | |
| L1 | 89 (54.9) |
| L2 | 9 (5.6) |
| L3 | 55 (34) |
| L1-4 | 7 (4.3) |
| L3-4 | 2 (1.2) |
| Behavior | |
| B1 | 6 (3.7) |
| B2 | 77 (47.5) |
| B3 | 79 (48.8) |
| Perianal disease | 38 (23.5) |
| Smoking habits (n; %) | |
| Non-smoker | 86 (55.1) |
| Ex-smoker | 34 (21.8) |
| Current smoker | 36 (23.1) |
| Medication at index colonoscopy | |
| Thiopurines | 111 (68.5) |
| Anti-TNFα | 59 (36.4) |
TNFα: Tumor necrosis factor α.
At the time of index colonoscopy, 82% of patients (n = 133) were receiving CD medication: 68.5% (n = 111) thiopurines and 36.4% (n = 59) anti-TNF medication; only 23% of the patients (n = 37) were on combination therapy. Seventeen percent of the patients (n = 27) had obstructive symptoms, with a median HBI of 1 (0-9). Median labs were: haemoglobin 13.5 g/dL (9.6-18.3), albumin 42 g/dL (24.2-352), C-reactive protein 2.8 mg/L (0.1-105.3), median lactoferrin 4.7 μg/g (0.4-216) and median calprotectin 68.5 μg/g (0.5-2051).
Anastomotic strictures
Twenty-nine percent of patients (n = 47) had an anastomotic stricture (17 symptomatic), with 4 also having a non-anastomotic stricture; dilation wasn’t performed in 4 patients: 1 due to stricture length > 6 cm, 2 due to marked ulceration and 1 due to unstable scope position.
The presence of stricture after surgery was associated with presence of obstructive symptoms (37% vs 9%, OR = 6.3, 95%CI: 2.5-14.9, P < 0.001), no medication with thiopurines (74.8% vs 53.2%, OR = 2.6, 95%CI: 1.3-5.3, P = 0.007), longer duration between surgery and index colonoscopy [132 mo (9-439) vs 71 mo (3-415), P = 0.001], older age [47 years (16-72) vs 39 years (18-77), P = 0.031] and lower C-reactive protein levels [2.65 mg/L (0.3-21.9) vs 2.85 mg/L (0.1-105.3), P = 0.045].
On univariate analysis, the presence of stricture after surgery was associated with longer periods between surgery and index colonoscopy (OR = 1.006, 95%CI: 1.003-1.010, P = 0.001), higher lactoferrin levels (OR = 1.010, 95%CI: 1.000-1.021, P = 0.049), obstructive symptoms (OR = 6.155, 95%CI: 2.546-14.880, P < 0.001), no medication with thiopurines (OR = 2.611, 95%CI: 1.282-5.319, P = 0.008) and older age at index colonoscopy (OR = 1.028, 95%CI: 1.002-1.055, P = 0.033) (Table 2). On multivariate analysis, only longer periods between surgery and index colonoscopy (OR = 1.007, 95%CI: 1.001-1.013, P = 0.027) and higher lactoferrin levels (OR = 1.012, 95%CI: 1.000-1.024, P = 0.043) were associated with the presence of stricture (Table 2).
Table 2.
Univariate and multivariate analysis of risk factors for need for dilation after surgery
| Risk factors |
Univariate analysis |
Multivariate analysis |
||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Significant in univariate and multivariate analysis | ||||||
| Time between surgery and index colonoscopy | 1.006 | 1.003-1.010 | 0.001 | 1.007 | 1.001-1.013 | 0.027 |
| Lactoferrin levels | 1.010 | 1.000-1.021 | 0.049 | 1.012 | 1.000-1.024 | 0.043 |
| Significant in univariate analysis | ||||||
| Subocclusive symptoms | 6.155 | 2.546-14.880 | < 0.001 | - | - | 0.180 |
| No medical treatment with thiopurines | 2.611 | 1.282-5.319 | 0.008 | - | - | 0.500 |
| Age at index colonoscopy | 1.028 | 1.002-1.055 | 0.033 | - | - | 0.932 |
| Not significant in univariate nor multivariate analysis | ||||||
| Smoking status | 1.877 | 0.912-3.861 | 0.087 | |||
| B2 behavior | 1.221 | 0.619-2.408 | 0.565 | |||
| B3 behavior | 0.895 | 0.454-1.767 | 0.750 | |||
| Perianal disease | 1.376 | 0.632-2.996 | 0.421 | |||
| Anti-TNF therapy | 0.893 | 0.443-1.799 | 0.751 | |||
| C-reactive protein levels | 0.975 | 0.935-1.016 | 0.229 | |||
| Calprotectin levels | 1.001 | 1.000-1.002 | 0.125 | |||
| Harvey-Bradshaw index | 1.111 | 0.936-1.318 | 0.229 | |||
| Disease duration | 1.002 | 1.000-1.005 | 0.089 |
OR: Odds ratio; CI: Confidence interval; B1: Non-stenosing and non-penetrating behaviour; B2: Stenosing behaviour; B1: Penetrating behaviour; TNF: Tumor necrosis factor.
In the Cox regression univariate analysis, time until dilation was longer in patients on medication with thiopurines or anti-TNF (HR = 0.476, 95%CI: 0.239-0.946, P = 0.034); in further subgroup analysis, thiopurine treatment was the only medication found to be significantly associated with a longer time to dilation (HR = 0.493, 95%CI: 0.275-0.883, P = 0.017). Obstructive symptoms and calprotectin levels higher than 83.35 μg/g, on the other hand, were associated with a shorter time until dilation (HR = 2.976, 95%CI: 1.622-5.460, P < 0.001 and HR = 3.444, 95%CI: 1.391-8.526, P = 0.008, respectively). There was a trend for current or past history of smoking being associated with a shorter time to dilation in the univariate analysis (HR = 1.752, 95%CI: 0.964-3.185, P = 0.066). In the Cox multivariate analysis, calprotectin levels > 83.35 μg/g and current or past history of smoking were associated with a shorter duration to dilation (HR = 3.877, 95%CI: 1.480-10.152, P = 0.006 and HR = 3.041, 95%CI: 1.213-7.627, P = 0.018) (Figure 2).
Figure 2.
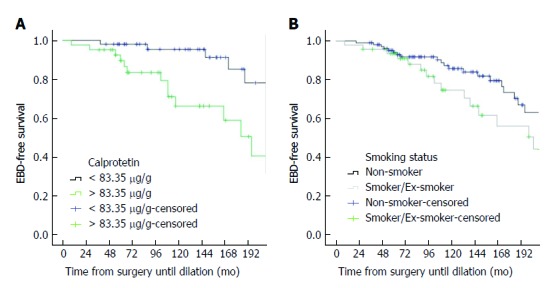
Kaplan-Meier curves showing time from surgery to dilation (in mo) considering calprotectin levels (A) and smoking status (B).
Endoscopic balloon dilation
EBD was performed in 26.5% (n = 43) of patients (Table 3). Technical success was achieved in 97.7% (n = 42). Repeat dilation was required in 62.8% (n = 27) of patients (long-term efficacy: 37.2%), with a total of 85 dilations being performed during the study period [median: 2 (1-5)], with a median balloon dilation of 18mm (range 10-18). Technical success was achieved in 95.3% (n = 81) without major AEs, and only one episode of self-limited bleeding (1.2%). All patients (n = 17) had improvement of obstructive symptoms. Median time to second dilation was 453.5 d (range: 152-1362).
Table 3.
Baseline characteristics comparison between anastomotic and non-anastomotic strictures n (%)
| Characteristic | Anastomotic strictures (n = 43) | Non-anastomotic strictures (n = 37) | P value |
| Female/male (n) | 19/24 | 22/15 | 0.173 |
| Disease duration in years (median; min-max) | 19.2 (5.7-52.1) | 16.3 (3.0-45.3) | 0.236 |
| Follow-up since 1st dilation in years (median; min-max) | 4.4 (1.3-6.8) | 2.9 (1.2-6.5) | < 0.001 |
| Time between 1st and 2nd dilation in days (median; min-max) | 453.5 (152-1362) | 368 (157-1705) | 0.796 |
| Age at index colonoscopy (mean; standard deviation) | 44.9 (± 12.2) | 39 (± 12.2) | 0.035 |
| Montreal classification | |||
| Age at diagnosis | 0.368 | ||
| A1 | 6 (14) | 4 (12.9) | |
| A2 | 29 (67.4) | 25 (80.6) | |
| A3 | 8 (18.6) | 2 (6.5) | |
| Disease location | 0.057 | ||
| L1 | 23 (53.5) | 9 (29) | |
| L2 | 1 (2.3) | 4 (12.9) | |
| L3 | 17 (39.5%) | 14 (45.2%) | |
| L1-4 | 2 (4.7%) | 3 (9.7%) | |
| L3-4 | - | 1 (3.2%) | |
| Behavior | |||
| B1 | 1 (2.3) | 4 (12.9) | 0.071 |
| B2 | 22 (51.2) | 19 (61.3) | 0.387 |
| B3 | 20 (46.5) | 8 (25.8) | 0.07 |
| Perianal disease | 12 (27.9) | 9 (29) | 0.916 |
| Smoking habits | 0.635 | ||
| Non-smoker | 22 (52.4) | 11 (44) | |
| Ex-smoker | 8 (19) | 9 (36) | |
| Current smoker | 12 (28.6) | 5 (20) | |
| Medication at index colonoscopy | |||
| Thiopurines | 23 (53.5) | 25 (67.6) | 0.200 |
| Anti-TNFα | 17 (39.5) | 19 (51.4) | 0.289 |
| Obstructive symptoms | 15 (35.7) | 10 (27.8) | 0.454 |
| C-reactive protein | 2.8 (0.3-18.4) | 6.95 (0.2-35.5) | 0.126 |
| Calprotectin | 103.5 (5.9-1356) | 283 (166-321) | 0.789 |
| Lactoferrin | 7.66 (1.05-204.4) | 10.7 (7.1-22.7) | 0.429 |
| Need to repeat dilation | 27 (62.8) | 15 (40.5) | 0.047 |
TNFα: Tumor necrosis factor α.
Endoscopic recurrence, defined as modified Rutgeerts score ≥ i2b was present in 60 patients (37.7%), of which 20 (33%) were diagnosed only after dilation of the anastomotic stricture. Following initial endoscopy and dilation, medical therapy was escalated in 38.5% of patients (n = 15; 3 began thiopurines and 14 began anti-TNF); however, escalation of medical therapy was probably driven by endoscopic recurrence (87%, n = 13) and not by anastomotic stricture presence, as suggested by escalation of therapy in 53% (n = 21) of the patients with endoscopic recurrence and no anastomotic stricture. No agreement was found between endoscopic recurrence and presence of stricture (K = 0.085, P = 0.273). Escalation of medical therapy did not decrease the need for repeat dilation; no other risk factors (gender, age, Montreal classification, perianal disease, smoking habits, previous medical therapy, presence of obstructive symptoms, serum/fecal biomarkers) were found to influence need for repeat dilation. After initial dilation, 4.6% of the patients (n = 2) required anastomotic stricture resection due to worsening or recurrence of symptoms. The median time to progression to surgery was 35 mo (33.4-36.7).
Non-anastomotic strictures
During the study period, a total of 37 CD patients (40.5% males, n = 15) underwent a total of 59 EBD sessions of non-anastomotic strictures (17 in the ileum, 13 in the ileocecal valve, 2 in the ascending colon, 1 in the transverse colon, 3 in the descending colon and 1 in the sigmoid colon) (Table 3). Twenty-seven percent of the patients (n = 10) had obstructive symptoms, with a median HBI of 1 (0-10). Technical success was achieved in all patients (n = 37). Repeat dilation was required in 40.5% (n = 15) of patients (long-term efficacy: 59.5%), with a median balloon dilation of 16.5mm (range 10-18). Technical success and improvement in obstructive symptoms were achieved in all patients without AEs. Following EBD, medical therapy was escalated in 18.9% of patients (n = 7; 4 began thiopurines and 6 began anti-TNF). Median time to second dilation was 368 d (range: 157-1705). Only 1 patient (3%) required surgical resection. No risk factors were found to influence need for repeat dilation.
Anastomotic strictures vs non-anastomotic strictures
Baseline characteristics of patients with anastomotic and non-anastomotic strictures are shown in Table 3. Follow-up since 1st dilation was longer in patients with anastomotic strictures. Patients with anastomotic strictures had a greater recurrence of stenosis (63% vs 41%, P = 0.047). In the univariate analysis, absence of thiopurine medication (OR = 3.1, 95%CI: 1.2-7.9, P = 0.019) and anastomotic strictures (OR = 2.5, 95%CI: 1.01-6.1, P = 0.049) were the only factors found to influence need for repeat dilation.
Asymptomatic and symptomatic cohorts
No statistical significant differences were found between asymptomatic and symptomatic cohorts when comparing baseline disease characteristics, medical therapy at time of dilation, disease activity, EBD procedure, need for further dilations, escalation of medical therapy and need for surgery (Table 4).
Table 4.
Baseline disease characteristics, endoscopic balloon dilation procedure, need for further dilations, escalation of medical therapy and need for surgery between asymptomatic and symptomatic cohorts n (%)
| Characteristic | Asymptomatic strictures1 (n = 53) | Symptomatic strictures1 (n = 25) | P value |
| Female/male (n) | 25/28 | 16/9 | 0.165 |
| Disease duration in years (median; min-max) | 17.9 (2.9-45.3) | 19.9 (5.3-52.1) | 0.955 |
| Follow-up since 1st dilation in years (median; min-max) | 3.1 (1.3-6.8) | 3.9 (2.1-6.7) | 0.068 |
| Time between 1st and 2nd dilation in days (median; min-max) | 668 (157-2074) | 877 (152-2242) | 0.790 |
| Age at index colonoscopy (mean ± SD) | 41.7 ± 13.8 | 43 ± 9.3 | 0.425 |
| Montreal classification | |||
| Age at diagnosis | 0.998 | ||
| A1 | 7 (14) | 3 (13.6) | |
| A2 | 36 (72) | 16 (72.7) | |
| A3 | 7 (14) | 3 (13.6) | |
| Disease location | 0.319 | ||
| L1 | 22 (44) | 9 (40.9) | |
| L2 | 5 (10) | - | |
| L3 | 21 (42) | 9 (40.9) | |
| L1-4 | 1 (2) | 4 (18.2) | |
| L3-4 | 1 (2) | - | |
| Behavior | 0.833 | ||
| B1 | 4 (8) | 1 (4.5) | |
| B2 | 28 (56) | 12 (54.5) | |
| B3 | 18 (36) | 9 (40.9) | |
| Perianal disease | 15 (30) | 6 (27.3) | 0.815 |
| Smoking habits | 0.761 | ||
| Non-smoker | 24 (52.2) | 8 (42.1) | |
| Ex-smoker | 8 (17.4) | 4 (21.2) | |
| Current smoker | 14 (30.4) | 7 (36.8) | |
| Previous surgery (ileocecal resection/right hemicolectomy) | 27 (50.9) | 15 (60) | 0.454 |
| Medication at index colonoscopy | |||
| Thiopurines | 33 (62.3) | 13 (52) | 0.390 |
| Anti-TNFα | 21 (39.6) | 14 (56) | 0.175 |
| Combo | 13 (24.5) | 9 (36) | 0.293 |
| C-reactive protein | 6.2 (0.2-35.5) | 3.2 (0.3-19.4) | 0.351 |
| Calprotectin | 107 (18.2-837) | 340 (5.9-1356) | 0.449 |
| Lactoferrin | 7.1 (1.1-102.6) | 37.6 (4.0-204) | 0.071 |
| Therapeutic success | 52 (98.1) | 25 (100%) | 0.377 |
| Balloon diameter (median; min - max) | 18 (10-18) | 18 (13.5-18) | 0.201 |
| Adverse events | 0 (0) | 1 (4) | 0.129 |
| Need to repeat dilation | 27 (50.9) | 15 (60) | 0.454 |
| Number of dilations (median; min - max) | 2 (1-5) | 2 (1-5) | 0.463 |
| Escalation of medical therapy after dilation | 13 (27.7) | 8 (34.8) | 0.541 |
| Need for surgery after dilation | 2 (4) | 1 (4.2) | 0.973 |
Two patients had no information regarding presence of obstructive symptoms (one in each group). TNFα: Tumor necrosis factor α.
DISCUSSION
CD is characterized by chronic, recurrent, transmural inflammation; intestinal strictures are believed to result from partial healing and localized fibrosis[18]. Strictures develop unpredictably after surgery[6]. EBD has emerged as a bridging tool for management of CD strictures with favorable success rates and efficacy[10].
In this study, we found that almost one-third of CD patients develop an anastomotic stricture after ileocecal resection/right hemicolectomy. The mean age at first dilation was 42.6 years, which reflects their etiology as a complication of the surgery. Longer intervals after surgery and higher lactoferrin levels were associated with anastomotic strictures; time until dilation was lower in patients with calprotectin levels > 83.35 μg/g and current/past history of smoking. Previous studies have not studied the development of anastomotic strictures after surgery, however, as a progressive disease, anastomotic strictures will be more likely over time. Both anastomotic and de novo strictures have either inflammatory and/or fibrotic elements. Healing occurs in a defined pattern during bouts of activity and remission, with progression to luminal narrowing leading to stricture formation[17]; on the other hand, elevated serologic markers probably reflect the inflammatory component of the neo terminal ileum instead of the presence of an anastomotic stricture. While there is controversy regarding the effect of smoking on the disease phenotype, literature supports smoking as a factor in complicated disease[19].
Regarding EBD, our technical success was 97.7%, similar to literature (88%-100%)[20] and similar to non-anastomotic strictures (100%), however, this was not associated with a permanent stricture dilation, as 63% of patients with anastomotic strictures and 41% of those with non-anastomotic strictures required additional dilation over a 4.4-year period. The fibrotic pathology of anastomotic strictures may be responsible for the lower response rate of EBD for non-anastomotic strictures. Re-dilation was as technically successful, supporting the evidence that repeated dilations do not reduce the procedural efficacy[20,21]. The rate of major AEs, including bowel perforation and significant bleeding, has been reported to be between 2%-6%[1,8]. AEs have been attributed to balloon size (25 mm diameter), pressure used to dilate and number of dilations per session. Our data confirm safety of EBD for CD strictures. We had no serious AEs, which may be explained by the careful patient selection, fluoroscopy to evaluate stricture characteristics and monitoring, maximum balloon diameter of 18 mm, use of CO2 insufflation, and endoscopists experience.
Long-term outcome following EBD varies. In our study, a long-term efficacy of 37.2% for anastomotic strictures and 59.5% for non-anastomotic strictures was achieved during a follow-up period of 4.4 years. Even though follow-up was longer, results were somewhat inferior to previous studies (52%-69%) when considering only anastomotic strictures. However, EBD in previous studies was symptom-driven, while in ours, EBD was performed for strictures that did not allow passage with a colonoscope, regardless of patients’ symptoms. Besides, EBD delayed time until surgery, with only 3 patients (2 with anastomotic and 1 with non-anastomotic strictures) requiring surgery during follow-up period, suggesting a benefit of EBD. A recent pooled analysis reported a technical success, clinical success, long-term symptomatic and surgical recurrence rates of 89%, 81%, 48% and 29%, respectively[22]; these data are almost exclusively derived from retrospective cohort studies and may somewhat overestimate the actual benefit. In 2013, the European Crohn’s and Colitis Organisation stated that EBD was safe and effective and allowed surgery to be avoided in CD patients with anastomotic strictures[23]. The overall technical success rate in the meta-analysis performed by Hassan et al[8] was 86% (71%-100%), while 41% of patients required repeated EBD allowing an overall long-term clinical efficacy (avoidance of surgery) rate of 58% during a median follow up of 33 mo.
Risk factors associated with need for subsequent dilation have been inconsistent. Longer disease duration was associated with a shorter time to repeat dilation[12]; technical success of dilation[8,24,25], length of stricture[8,26], and non-ulcerated stricture[27] were found to be associated with a successful procedure. In our study, the only factors found to influence need for repeat dilation (in the univariate analysis) were absence of thiopurine medications and anastomotic strictures. Escalation of medical therapy did not decrease the need for repeat dilation. Considering therapeutic strategy, Thienpont et al[10] reported no significant effect of systemic medical therapy after dilation on redilation or surgery, while Honzawa et al[28] found that prior use of immunomodulatory drugs improved the clinical outcome of EBD for intestinal strictures in patients with CD. Patients in our study may also have had more severe disease, as demonstrated by the majority receiving immunossupressants at the time of index colonoscopy (immunomodulators: 68.5%; biological therapy: 36.4%), as well as the high rate of repeat dilation. Despite the well-known deleterious association between tobacco use and CD activity, with increased risk of recurrence after surgery and EBD, we did not find any association between smoking and long-term outcome of EBD. Disease activity assessed by serologic markers, endoscopy, and clinical variables such as HBI, disease duration or time between surgery and dilation did not predict the need for repeat dilation, in accordance with previously published data[10,21].
It is controversial whether asymptomatic strictures should be endoscopically treated. Previous studies have found no correlation between patient´s symptoms and clinical scores and endoscopic/radiographic findings after intestinal resection[29-31]. In our study, only 2 patients presented with an HBI > 7 and only 27 patients complained of obstructive symptoms, despite 47 having an anastomotic stricture. Serum biomarkers did not correlate with endoscopic recurrence or presence of strictures, supporting the belief that using only symptoms or C-reactive protein levels to make treatment decisions may increase the risk of disease progression and AEs. Our group believes that dilation of strictures, despite symptoms, has impact on patients’ management and disease course, allowing evaluation of disease activity and therapeutic adjustments. If EBD was not performed, a diagnosis of endoscopic recurrence would not have been possible in 33% of patients, all with normal biomarkers. On the other hand, we did not find any differences between asymptomatic and symptomatic cohorts regarding disease characteristics as well EBD peculiarities.
Limitations of our study include its retrospective nature, being conducted in a tertiary referral center (with referral or selection bias), lack of a control group (medical and surgical therapy), uncertainty of the degree of luminal narrowing caused by inflammation vs fibrosis and escalation of medical treatment biased toward those having active IBD.
In conclusion, smoking habits and longer disease duration after surgery are associated with a higher risk of anastomotic strictures. EBD is a feasible, simple, effective and safe alternative to surgery, with the possibility of being repeated as needed, with excellent symptomatic response, as well as good short-term and long-term outcomes, postponing or avoiding surgery. Considering that a significant number of patients with significant strictures remain asymptomatic with normal biomarkers, and the fact that the disease continues to evolve proximal to the strictures, we believe EBD should be considered for all strictures not transposable by a colonoscope, regardless of the presence or absence of symptoms, in order to adjust treatment in an attempt to alter the natural history of the disease. Thus, EBD is useful not only for symptom resolution but also for evaluating mucosal healing.
ARTICLE HIGHLIGHTS
Research background
Strictures in Crohn’s disease (CD) develop during the course of the disease or as the presenting feature. More than half of CD patients will need surgery within the first 10 years of diagnosis. Medical therapy for stricture management is limited due to the fibrotic nature. Endoscopic balloon dilation (EBD) has been proposed as a safe and effective therapeutic intervention for CD strictures, particularly for ileocecal and anastomotic strictures.
Research motivation
Data on long term efficacy and safety of EBD are limited due to lack of long-term outcome and small cohorts. Up to now there are also some uncertainties regarding the factors associated with long term success rate. Smoking status and disease activity status at the time of dilation may affect outcome of stricture dilation, though many studies are limited by short follow-up durations and small cohorts. Furthermore, as the primary therapeutic goal of CD has shifted from clinical remission to achieving mucosal healing, it may be important to access the mucosa proximal to strictures to evaluate disease recurrence and escalate therapy if needed.
Research objectives
This study aimed to evaluate anastomotic stricture development after intestinal resection in CD and demonstrate long-term efficacy and safety center of EBD in CD anastomotic and de novo strictures in a large referral centre cohort and determine the impact of dilation on the diagnosis of subclinical postoperative endoscopic recurrence.
Research methods
CD patients who had undergone ileocecal resection/right hemicolectomy referred for endoscopic evaluation between March 2010 to February 2015 were included in this study. CD patients with non-anastomotic strictures who underwent EBD during the study period were included as a control group. EBD was performed for strictures that would not allow passage with a colonoscope, regardless of patients’ symptoms. Technical success was defined as the ability to pass the colonoscope through the stricture into the neoileum following dilation. Clinical success was defined as improvement of obstructive symptoms (in symptomatic patients). All patients who underwent dilation were endoscopically reevaluated 6-12 mo later. Long-term efficacy was defined as avoidance of surgical resection or repeat dilation after the initial dilation. Patients were followed until stricture resection, last clinic follow-up, or censor date of March, 2017. Escalation of medical therapy was defined as initiation of a thiopurine or anti-TNF within 6 months of first dilation, as determined by global physician assessment.
All data were prospectively collected in a database created for this purpose. After a 5 year follow up period all data were arranged, processed and analyzed with SPSS® v.24.0 data (Statistical Package for Social Sciences).
Research results
In this study we found that almost one-third of CD patients developed an anastomotic stricture after ileocecal resection/right hemicolectomy. Longer periods between surgery and index colonoscopy and higher lactoferrin levels were associated with the presence of stricture after surgery. Calprotectin levels > 83.35 μg/g and current or past history of smoking were associated with a shorter time until need for dilation (HR = 3.877, 95%CI: 1.480-10.152 and HR = 3.041, 95%CI: 1.213-7.627). Technical success of EBD was 97.7% and 100% for anastomotic and non-anastomotic strictures, respectively, and 63% and 41% of the patients needed repeat dilation during the 4.4-year follow-up. Anastomotic strictures had a greater need for repeat dilation (63% vs 41%, P = 0.047). No differences were found between asymptomatic and symptomatic cohorts. Disease recurrence was diagnosed only after EBD in a third of patients.
Research conclusions
EBD is a feasible, simple, effective and safe alternative to surgery, with the possibility of being repeated as needed, with excellent symptomatic response, as well as good short-term and long-term outcomes, postponing or avoiding surgery. Considering that a significant number of patients with significant strictures remain asymptomatic with normal biomarkers, and the fact that the disease continues to evolve proximal to the strictures, we advocate EBD for all strictures regardless of the presence or absence of symptoms, in order to adjust treatment in an attempt to alter the natural history of the disease. Thus, EBD is useful not only for symptom resolution but also for evaluating mucosal healing.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Portugal
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
Institutional review board statement: The study was approved by the Ethics Committee of Centro Hospitalar São João, Porto, Portugal.
Informed consent statement: All patients gave informed written consent to participate in the study.
Conflict-of-interest statement: The authors of this manuscript have no conflict of interest to declare.
Data sharing statement: There is no additional data available.
Peer-review started: August 19, 2017
First decision: August 30, 2017
Article in press: September 19, 2017
P- Reviewer: Dogan UB, Gassler N, Lakatos PL, Shi R S- Editor: Wei LJ L- Editor: A E- Editor: Huang Y
Contributor Information
Susana Lopes, Gastroenterology Department, Faculty of Medicine, Hospital de São João, Porto 4200-319, Portugal.
Eduardo Rodrigues-Pinto, Gastroenterology Department, Faculty of Medicine, Hospital de São João, Porto 4200-319, Portugal.
Patrícia Andrade, Gastroenterology Department, Faculty of Medicine, Hospital de São João, Porto 4200-319, Portugal.
Joana Afonso, Department of Pharmacology and Therapeutics, University of Porto, Porto 4200-319, Portugal.
Todd H Baron, Division of Gastroenterology and Hepatology, University of North Carolina, Chapel Hill, NC 4200, United States.
Fernando Magro, Gastroenterology Department, Faculty of Medicine, Hospital de São João, Porto 4200-319, Portugal; Department of Pharmacology and Therapeutics, University of Porto, Porto 4200-319, Portugal.
Guilherme Macedo, Gastroenterology Department, Faculty of Medicine, Hospital de São João, Porto 4200-319, Portugal. guilhermemacedo59@gmail.com.
References
- 1.Morar PS, Faiz O, Warusavitarne J, Brown S, Cohen R, Hind D, Abercrombie J, Ragunath K, Sanders DS, Arnott I, Wilson G, Bloom S, Arebi N; Crohn’s Stricture Study (CroSS) Group. Systematic review with meta-analysis: endoscopic balloon dilatation for Crohn’s disease strictures. Aliment Pharmacol Ther. 2015;42:1137–1148. doi: 10.1111/apt.13388. [DOI] [PubMed] [Google Scholar]
- 2.Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg. 2000;231:38–45. doi: 10.1097/00000658-200001000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olaison G, Smedh K, Sjödahl R. Natural course of Crohn’s disease after ileocolic resection: endoscopically visualised ileal ulcers preceding symptoms. Gut. 1992;33:331–335. doi: 10.1136/gut.33.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutgeerts P, Geboes K, Vantrappen G, Kerremans R, Coenegrachts JL, Coremans G. Natural history of recurrent Crohn’s disease at the ileocolonic anastomosis after curative surgery. Gut. 1984;25:665–672. doi: 10.1136/gut.25.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landsend E, Johnson E, Johannessen HO, Carlsen E. Long-term outcome after intestinal resection for Crohn’s disease. Scand J Gastroenterol. 2006;41:1204–1208. doi: 10.1080/00365520600731018. [DOI] [PubMed] [Google Scholar]
- 6.Kurer MA, Stamou KM, Wilson TR, Bradford IM, Leveson SH. Early symptomatic recurrence after intestinal resection in Crohn’s disease is unpredictable. Colorectal Dis. 2007;9:567–571. doi: 10.1111/j.1463-1318.2006.01202.x. [DOI] [PubMed] [Google Scholar]
- 7.Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–297. doi: 10.1038/ajg.2009.579. [DOI] [PubMed] [Google Scholar]
- 8.Hassan C, Zullo A, De Francesco V, Ierardi E, Giustini M, Pitidis A, Taggi F, Winn S, Morini S. Systematic review: Endoscopic dilatation in Crohn's disease. Aliment Pharmacol Ther. 2007;26:1457–1464. doi: 10.1111/j.1365-2036.2007.03532.x. [DOI] [PubMed] [Google Scholar]
- 9.Gustavsson A, Magnuson A, Blomberg B, Andersson M, Halfvarson J, Tysk C. Endoscopic dilation is an efficacious and safe treatment of intestinal strictures in Crohn’s disease. Aliment Pharmacol Ther. 2012;36:151–158. doi: 10.1111/j.1365-2036.2012.05146.x. [DOI] [PubMed] [Google Scholar]
- 10.Thienpont C, D’Hoore A, Vermeire S, Demedts I, Bisschops R, Coremans G, Rutgeerts P, Van Assche G. Long-term outcome of endoscopic dilatation in patients with Crohn’s disease is not affected by disease activity or medical therapy. Gut. 2010;59:320–324. doi: 10.1136/gut.2009.180182. [DOI] [PubMed] [Google Scholar]
- 11.Nanda K, Courtney W, Keegan D, Byrne K, Nolan B, O’Donoghue D, Mulcahy H, Doherty G. Prolonged avoidance of repeat surgery with endoscopic balloon dilatation of anastomotic strictures in Crohn’s disease. J Crohns Colitis. 2013;7:474–480. doi: 10.1016/j.crohns.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Ding NS, Yip WM, Choi CH, Saunders B, Thomas-Gibson S, Arebi N, Humphries A, Hart A. Endoscopic Dilatation of Crohn’s Anastomotic Strictures is Effective in the Long Term, and Escalation of Medical Therapy Improves Outcomes in the Biologic Era. J Crohns Colitis. 2016;10:1172–1178. doi: 10.1093/ecco-jcc/jjw072. [DOI] [PubMed] [Google Scholar]
- 13.Saunders BP, Brown GJ, Lemann M, Rutgeerts P. Balloon dilation of ileocolonic strictures in Crohn’s disease. Endoscopy. 2004;36:1001–1007. doi: 10.1055/s-2004-825962. [DOI] [PubMed] [Google Scholar]
- 14.Morini S, Hassan C, Lorenzetti R, Zullo A, Cerro P, Winn S, Giustini M, Taggi F. Long-term outcome of endoscopic pneumatic dilatation in Crohn’s disease. Dig Liver Dis. 2003;35:893–897. doi: 10.1016/j.dld.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Thomas-Gibson S, Brooker JC, Hayward CM, Shah SG, Williams CB, Saunders BP. Colonoscopic balloon dilation of Crohn’s strictures: a review of long-term outcomes. Eur J Gastroenterol Hepatol. 2003;15:485–488. doi: 10.1097/01.meg.0000059110.41030.bc. [DOI] [PubMed] [Google Scholar]
- 16.Lian L, Stocchi L, Remzi FH, Shen B. Comparison of Endoscopic Dilation vs Surgery for Anastomotic Stricture in Patients With Crohn’s Disease Following Ileocolonic Resection. Clin Gastroenterol Hepatol. 2017;15:1226–1231. doi: 10.1016/j.cgh.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 17.Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99:956–963. doi: 10.1016/0016-5085(90)90613-6. [DOI] [PubMed] [Google Scholar]
- 18.Mudter J, Neurath MF. Insight into Crohn’s disease pathomorphology. Abdom Imaging. 2012;37:921–926. doi: 10.1007/s00261-012-9885-3. [DOI] [PubMed] [Google Scholar]
- 19.Lindberg E, Järnerot G, Huitfeldt B. Smoking in Crohn’s disease: effect on localisation and clinical course. Gut. 1992;33:779–782. doi: 10.1136/gut.33.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen M, Shen B. Comparable short- and long-term outcomes of colonoscopic balloon dilation of Crohn’s Disease and benign non-Crohn’s Disease strictures. Inflamm Bowel Dis. 2014;20:1739–1746. doi: 10.1097/MIB.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 21.Atreja A, Aggarwal A, Dwivedi S, Rieder F, Lopez R, Lashner BA, Brzezinski A, Vargo JJ, Shen B. Safety and efficacy of endoscopic dilation for primary and anastomotic Crohn’s disease strictures. J Crohns Colitis. 2014;8:392–400. doi: 10.1016/j.crohns.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Bettenworth D, Gustavsson A, Atreja A, Lopez R, Tysk C, van Assche G, Rieder F. A Pooled Analysis of Efficacy, Safety, and Long-term Outcome of Endoscopic Balloon Dilation Therapy for Patients with Stricturing Crohn’s Disease. Inflamm Bowel Dis. 2017;23:133–142. doi: 10.1097/MIB.0000000000000988. [DOI] [PubMed] [Google Scholar]
- 23.Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, Ferrante M, Götz M, Katsanos KH, Kießlich R, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982–1018. doi: 10.1016/j.crohns.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Scimeca D, Mocciaro F, Cottone M, Montalbano LM, D’Amico G, Olivo M, Orlando R, Orlando A. Efficacy and safety of endoscopic balloon dilation of symptomatic intestinal Crohn’s disease strictures. Dig Liver Dis. 2011;43:121–125. doi: 10.1016/j.dld.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Couckuyt H, Gevers AM, Coremans G, Hiele M, Rutgeerts P. Efficacy and safety of hydrostatic balloon dilatation of ileocolonic Crohn’s strictures: a prospective longterm analysis. Gut. 1995;36:577–580. doi: 10.1136/gut.36.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mueller T, Rieder B, Bechtner G, Pfeiffer A. The response of Crohn’s strictures to endoscopic balloon dilation. Aliment Pharmacol Ther. 2010;31:634–639. doi: 10.1111/j.1365-2036.2009.04225.x. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann JC, Heller F, Faiss S, von Lampe B, Kroesen AJ, Wahnschaffe U, Schulzke JD, Zeitz M, Bojarski C. Through the endoscope balloon dilation of ileocolonic strictures: prognostic factors, complications, and effectiveness. Int J Colorectal Dis. 2008;23:689–696. doi: 10.1007/s00384-008-0461-9. [DOI] [PubMed] [Google Scholar]
- 28.Honzawa Y, Nakase H, Matsuura M, Higuchi H, Toyonaga T, Matsumura K, Yoshino T, Okazaki K, Chiba T. Prior use of immunomodulatory drugs improves the clinical outcome of endoscopic balloon dilation for intestinal stricture in patients with Crohn’s disease. Dig Endosc. 2013;25:535–543. doi: 10.1111/den.12029. [DOI] [PubMed] [Google Scholar]
- 29.Cellier C, Sahmoud T, Froguel E, Adenis A, Belaiche J, Bretagne JF, Florent C, Bouvry M, Mary JY, Modigliani R. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn’s disease. A prospective multicentre study of 121 cases. The Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut. 1994;35:231–235. doi: 10.1136/gut.35.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landi B, Anh TN, Cortot A, Soule JC, Rene E, Gendre JP, Bories P, See A, Metman EH, Florent C. Endoscopic monitoring of Crohn's disease treatment: a prospective, randomized clinical trial. The Groupe d'Etudes Therapeutiques des Affections Inflammatoires Digestives. Gastroenterology. 1992;102:1647–1653. doi: 10.1016/0016-5085(92)91725-j. [DOI] [PubMed] [Google Scholar]
- 31.Regueiro M, Kip KE, Schraut W, Baidoo L, Sepulveda AR, Pesci M, El-Hachem S, Harrison J, Binion D. Crohn’s disease activity index does not correlate with endoscopic recurrence one year after ileocolonic resection. Inflamm Bowel Dis. 2011;17:118–126. doi: 10.1002/ibd.21355. [DOI] [PubMed] [Google Scholar]


