Abstract
AIM
To investigate the efficacy and safety of postoperative adjuvant transcatheter arterial chemoembolization (PA-TACE) in preventing tumor recurrence and improving survival in Barcelona Clinic Liver Cancer (BCLC) early (A) and intermediate (B) stage hepatocellular carcinoma (HCC) patients with microvascular invasion (MVI).
METHODS
A total of 519 BCLC A or B HCC patients treated by liver resection alone or followed by PA-TACE between January 2012 and December 2015 were studied retrospectively. Univariate and multivariate analyses were performed to investigate the risk factors for recurrence-free survival (RFS) and overall survival (OS). Multiple logistic regression was used to identify the clinicopathological characteristics associated with MVI. The rates of RFS and OS were compared among patients with or without MVI treated with liver resection alone or followed by PA-TACE.
RESULTS
Univariate and multivariate analyses demonstrated that serum AFP level > 400 ng/mL, tumor size > 5 cm, tumor capsule invasion, MVI, and major hepatectomy were risk factors for poor OS. Tumor capsule invasion, MVI, tumor size > 5 cm, HBV-DNA copies > 1 x 104 IU/mL, and multinodularity were risk factors for poor RFS. Multiple logistic regression identified serum AFP level > 400 ng/mL, tumor size > 5 cm, and tumor capsule invasion as independent predictors of MVI. Both OS and DFS were significantly improved in patients with MVI who received PA-TACE as compared to those who underwent liver resection alone. Patients without MVI did not show a significant difference in OS and RFS between those treated by liver resection alone or followed by PA-TACE.
CONCLUSION
PA-TACE is a safe adjuvant intervention and can efficiently prevent tumor recurrence and improve the survival of BCLC early- and intermediate-stage HCC patients with MVI.
Keywords: Hepatocellular carcinoma, Microvascular invasion, Postoperative adjuvant transcatheter arterial chemoembolization, Recurrence-free survival, Overall survival
Core tip: Microvascular invasion (MVI) is an independent risk factor attributed to frequent tumor recurrence in Barcelona Clinic Liver Cancer (BCLC) early- and intermediate-stage hepatocellular carcinoma (HCC) patients. Postoperative adjuvant transcatheter arterial chemoembolization (PA-TACE) has been confirmed to be effective in preventing early recurrence and delaying the progression of recurrent tumors, thereby improving the overall survival (OS) of HCC patients with macrovascular invasion. However, whether PA-TACE could provide the survival benefit to HCC patients with MVI remains unclear. The present study showed that PA-TACE is an effective method that can safely prevent tumor recurrence and improve the survival of BCLC early- and intermediate-stage HCC patients with MVI. However, it failed to provide obvious OS or RFS benefits to patients without MVI.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide in males and the second leading cause of cancer-related deaths[1,2], especially in the Asia-Pacific region[3,4]. With advances in surgical techniques, surgical resection has been the most effective curative strategy for early- and intermediate-stage HCC[5,6]; however, the postoperative 5-year recurrence rate is 70%-80%, leading to poor overall survival (OS)[7-9].
Macrovascular invasion and macrovascular thrombosis, such as the invasion of the portal vein, hepatic vein, or hepatic artery, are the hallmarks of HCC[10]. Macrovascular invasion has been well accepted as a major mechanism promoting the growth of residual tumors as well as intrahepatic metastasis, thereby contributing to early recurrence and poor survival in HCC patients after liver resection[11-15]. Recently, microvascular invasion (MVI) has been demonstrated as an independent risk factor associated with early tumor recurrence in patients with single HCC without macrovascular invasion[16-21], which accounts for approximately 15%-60% of HCC patients[22,23]. MVI may promote metastasis by acting as a “seed” to give rise to micro-metastases in the liver parenchyma, and such metastasis is common among patients whose more than five vessels are affected by MVI[24].
Routinely, postoperative adjuvant intervention is recommended for preventing the recurrence in HCC patients with residual tumors after liver resection. Previous studies[13,25-28] confirmed that postoperative adjuvant transcatheter arterial chemoembolization (PA-TACE) prevented early recurrence and delayed the progression of recurrent tumors, thereby improving the OS in HCC patients with macrovascular invasion. However, whether PA-TACE can provide the survival benefit to HCC patients with MVI is yet unclear and necessitates further investigation.
Therefore, the current study investigated the efficacy of PA-TACE in preventing tumor recurrence and improving survival in HCC patients with MVI. The study involved only patients with early- or intermediate-stage HCC based on the Barcelona Clinic Liver Cancer (BCLC) staging system[29], who did not present macrovascular invasion.
MATERIALS AND METHODS
The present study was approved by the Clinical Research Ethics Committee of the Affiliated Tumor Hospital of Guangxi Medical University and performed in compliance with the Helsinki Declaration. Patients were not required to give informed consent to the study as the analysis used anonymous clinical data and individuals cannot be identified based on the data presented. The results of the study were reported according to the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis statement[30].
Patients and study design
All HCC patients diagnosed with BCLC early- or intermediate-stage disease who underwent curative resection at the Affiliated Tumor Hospital of Guangxi Medical University between January 1, 2012 and December 31, 2015 were considered for this retrospective study. Patients who fulfilled the following inclusion criteria were eligible for the study: (1) they initially underwent liver resection with curative intent without any prior treatment for HCC; (2) complete gross resection was achieved, with no residual tumor in the remnant liver as determined by intraoperative visual inspection and a negative resection (R0) margin based on the histological examination; (3) HCC and MVI were confirmed by postoperative histopathology of the surgical samples, and MVI was defined as the microscopic tumor invasion identified in the portal or hepatic vein of the surrounding liver tissue, contiguous to the tumor[31]; (4) no macrovascular invasion was present; (5) no other simultaneous malignancies or distant lymph node metastasis were present; (6) no cardiopulmonary, renal, or cerebral dysfunction was present before liver resection. The patients were excluded if they received treatments (such as TACE and radiotherapy) before liver resection, died of surgical complications or postoperative liver failure, received postoperative sorafenib treatment, or were lost to follow-up within 60 d after discharge.
Univariate and multivariate analyses were used to investigate the risk factors for recurrence-free survival (RFS) and overall survival (OS). Multiple logistic regression was performed to investigate the potential associations between clinicopathological characteristics and MVI. Patients were stratified into four groups depending on whether they had MVI or not and whether they were treated by liver resection alone or with resection followed by PA-TACE. Postoperative RFS and OS rates were compared among the four groups using the Kaplan-Meier method, and significant differences were identified using log-rank analysis.
Treatments
All patients were treated by curative resection, which was defined as no residual tumor and a negative resection (R0) margin based on the histological examination. Surgical procedures were performed as described previously[20,32]. Decisions about liver resection approaches were based on liver function, tumor location, and estimated volume of residual liver according to volume computed tomography (CT). Major hepatectomy was defined as the resection of three or more Couinaud segments, whereas minor hepatectomy as resection of one to two segments[20]. In patients with multiple tumors, the largest lesion was used as the index lesion.
In a subset of patients with MVI, PA-TACE was performed at 1, 3, and 6 mo after hepatectomy. During this procedure, a hepatic arterial catheter was inserted through the femoral artery using the Seldinger technique; the catheter was inserted selectively into the tumor-feeding artery as technically possible. An emulsion of lobaplatin (50 mg), raltitrexed (4 mg), and lipiodol (3-5 mL) was infused into the remnant liver through this catheter. Simultaneously, hepatic angiography and/or CT angiography was carried out to detect any obvious residual tumor in the remnant liver. TACE was conducted on the entire remnant liver.
Follow-up
Serum AFP level and other laboratory tests were routinely monitored. In case of patients treated by curative resection associated with adjuvant TACE, contrast-enhanced dynamic CT or magnetic resonance imaging (MRI) scan was performed before and two weeks after TACE. Moreover, digital subtraction angiography (DSA) was performed concurrently with adjuvant TACE. Recurrent HCC was confirmed when CT or MRI showed stable tumor staining in the pre-contrast phase accompanied by an increase in the serum AFP level. In case of patients who underwent liver resection alone, contrast-enhanced dynamic CT or MRI scans were performed to routinely monitor the condition of the patient since the end of the first month after resection and then at monthly intervals. Recurrent HCC was confirmed by CT or MRI images showing rapid tumor staining in the arterial phase that disappeared in the early venous phase together with an increase in the serum AFP level. DSA was supplemented when tumor lesions were < 1 cm. All patients were followed until death or May 31, 2016.
Statistical analysis
Intergroup differences in continuous variables were assessed for significance using Student’s t-test (normally distributed data) or Mann-Whitney U test (skewed data). Intergroup differences in categorical data were assessed using χ2 or Fisher’s exact test as appropriate. Survival was analyzed using the Kaplan-Meier method, and survival curves were compared using the log-rank test. Uni- and multivariate analyses were carried out using a Cox proportional hazards stepwise model in order to identify independent factors related to OS and RFS. Factors with P < 0.1 in univariate analysis were incorporated into the multivariate analysis. Patient characteristics that independently predicted the MVI were identified using multiple logistic regression. All statistical analyses were performed using SPSS 22.0 (IBM, Chicago, IL, United States). The threshold of significance was defined as P < 0.05, and all P-values were two-tailed.
RESULTS
Between January 2012 and December 2015, 1,110 patients underwent liver resection at the Affiliated Tumor Hospital of Guangxi Medical University. Of these, 103 were excluded as they had other malignancies (n = 34), metastatic carcinoma (n = 14), or recurrent HCC (n = 13) or they had received preoperative TACE treatment (n = 42). The resulting 1007 potentially eligible patients diagnosed with primary liver cancer and treated by liver resection with curative intent for HCC were considered for this retrospective study. Of these patients, 488 were excluded due to the following reasons: 353 had macrovascular invasion (BCLC stage C disease), 120 did not have MVI determined by postoperative histopathological examination, and 15 were lost to follow-up < 2 mo after discharge. Finally, 519 patients who fulfilled the inclusion criteria were enrolled and studied retrospectively.
Clinicopathological characteristics
Based on the postoperative histopathological examination, 260 patients presented MVI; of these, 86 (33.1%) underwent PA-TACE following liver resection; 259 did not have MVI, of these 72 (27.8%) underwent PA-TACE. The remaining patients were treated by liver resection alone (Table 1). Patients with or without MVI who did or did not receive PA-TACE presented similar peri- or postoperative clinicopathological characteristics and surgical resection procedures. Among patients with MVI, those who received PA-TACE showed a significantly lower postoperative recurrence rate than those who underwent resection alone (39.5 vs 58.6%, P = 0.004). The same result was not observed among patients without MVI.
Table 1.
Baseline clinicopathological characteristics of hepatocellular carcinoma patients, stratified by microvascular invasion status and use of postoperative adjuvant transcatheter arterial chemoembolization n (%)
|
No MVI (n = 259) |
MVI (n = 260) |
||||||
| Characteristic | Category | No TACE (n = 187) | TACE (n = 72) | P | No TACE (n = 174) | TACE (n = 86) | P |
| Age (yr) | ≤ 60 | 146 (78.1) | 65 (90.3) | 0.024 | 140 (80.5) | 73 (84.9) | 0.383 |
| > 60 | 41 (21.9) | 7 (9.7) | 34 (19.5) | 13 (15.1) | |||
| Sex | Female | 29 (15.5) | 9 (12.5) | 0.540 | 24 (13.8) | 11 (12.8) | 0.824 |
| Male | 158 (84.5) | 63 (87.5) | 150 (86.2) | 75 (87.2) | |||
| HBsAg | Negative | 19 (10.2) | 6 (8.3) | 0.656 | 18 (10.3) | 14 (16.3) | 0.171 |
| Positive | 168 (89.8) | 66 (91.7) | 156 (89.7) | 72 (83.7) | |||
| Total bilirubin, μmol/L | 11 (3-118.3) | 11.2 (4.9-33.2) | 0.423 | 11.2 (2.8-30.9) | 11.75 (5.1-33.1) | 0.330 | |
| Prothrombin time (s) | 13.10 ± 1.18 | 13.55 ± 1.21 | 0.006 | 13.31 ± 1.38 | 13.31 ± 1.22 | 0.978 | |
| AFP (ng/mL) | ≤ 400 | 128 (68.4) | 44 (61.1) | 0.263 | 89 (51.1) | 49 (57.0) | 0.376 |
| > 400 | 59 (31.6) | 28 (38.9) | 85 (48.9) | 37 (43.0) | |||
| HBV-DNA (IU/mL) | ≤ 104 | 95 (50.8) | 43 (59.7) | 0.197 | 89 (51.1) | 52 (60.5) | 0.156 |
| > 104 | 92 (49.2) | 29 (40.3) | 85 (48.9) | 34 (39.5) | |||
| Pathology data | |||||||
| Size of largest tumor (cm) | ≤ 5 | 121 (64.7) | 38 (52.8) | 0.077 | 76 (43.7) | 41 (47.7) | 0.542 |
| > 5 | 66 (35.3) | 34 (47.2) | 98 (56.3) | 45 (52.3) | |||
| No. of nodules | Single | 155 (82.9) | 62 (86.1) | 0.528 | 137 (78.7) | 73 (84.9) | 0.237 |
| Multiple | 32 (17.1) | 10 (13.9) | 37 (21.3) | 13 (15.1) | |||
| Differentiation | Edmondson 1-2 | 129 (69) | 37 (51.4) | 0.008 | 91 (52.3) | 49 (57) | 0.477 |
| Edmondson 3-4 | 58 (31) | 35 (48.6) | 83 (47.7) | 37 (43) | |||
| Capsule | Complete | 143 (76.5) | 52 (72.2) | 0.478 | 105 (60.3) | 51 (59.3) | 0.872 |
| Incomplete | 44 (23.5) | 20 (27.8) | 69 (39.7) | 35 (40.7) | |||
| Cirrhosis | No | 31 (16.6) | 9 (12.5) | 0.416 | 31 (17.8) | 14 (16.3) | 0.758 |
| Yes | 156 (83.4) | 63 (87.5) | 143 (82.2) | 72 (83.7) | |||
| Surgical data | |||||||
| Type of resection | Minor | 118 (63.1) | 48 (66.7) | 0.592 | 93 (53.4) | 47 (54.7) | 0.855 |
| Major | 69 (36.9) | 24 (33.3) | 81 (46.6) | 39 (45.3) | |||
| Hepatic inflow occlusion | No | 79(42.2) | 25 (34.7) | 0.268 | 53 (30.5) | 32 (37.2) | 0.275 |
| Yes | 108 (57.8) | 47 (65.3) | 121 (69.5) | 54 (62.8) | |||
| Child-Pugh classification | A | 180 (96.3) | 70 (97.2) | 1.000 | 172 (98.9) | 84 (97.7) | 0.601 |
| B | 7 (3.7) | 2 (2.8) | 2 (1.1) | 2 (2.3) | |||
| Hypersplenism | No | 169 (90.4) | 67 (93.1) | 0.497 | 155 (89.1) | 83 (96.5) | 0.043 |
| Yes | 18 (9.6) | 5 (6.9) | 19 (10.9) | 3 (3.5) | |||
| BCLC | 0 | 18 (9.6) | 1 (1.4) | 0.002 | 6 (3.4) | 1 (1.2) | 0.158 |
| A | 151 (80.7) | 54 (75) | 121 (69.5) | 69 (80.2) | |||
| B | 18 (9.6) | 17 (23.6) | 47 (27) | 16 (18.6) | |||
| Recurrence | Yes | 56 (29.9) | 20 (27.8) | 0.731 | 102 (58.6) | 34 (39.5) | 0.004 |
| No | 131 (70.1) | 52 (72.2) | 72 (41.4) | 52 (60.5) | |||
HBsAg: Hepatitis B surface antigen; AFP: Alpha-fetoprotein; BCLC: Barcelona Clinic Liver Cancer; MVI: Microvascular invasion.
Risk factors for poor RFS and OS
Uni- and multivariate analyses demonstrated that serum AFP level > 400 ng/mL, tumor size > 5 cm, incomplete tumor capsule, MVI, and major hepatectomy were risk factors for poor OS, whereas incomplete tumor capsule, MVI, tumor size > 5 cm, HBV-DNA copies > 1 x 104 IU/mL, and multi-nodularity were independent risk factors for poor RFS. Sex, age, HBsAg, serum total bilirubin level, prothrombin time, hypersplenism, Child-Pugh classification B, degree of HCC differentiation, cirrhosis, and hepatic inflow occlusion were not significantly related to OS or RFS (Table 2).
Table 2.
Uni- and multivariate analyses to identify predictors of overall survival or recurrence-free survival in hepatocellular carcinoma patients after potentially curative resection
| Factor |
Univariate |
Multivariate |
||||||
|
OS |
RFS |
OS |
RFS |
|||||
| HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | |
| Sex (male) | 1.487 (0.773-2.864) | 0.235 | 1.354 (0.885-2.071) | 0.163 | ||||
| Age (> 60 yr) | 1.091 (0.627-1.772) | 0.723 | 1.017 (0.723-1.430) | 0.923 | ||||
| HBsAg (+) | 1.275 (0.618-2.630) | 0.511 | 1.365 (0.842-2.214) | 0.207 | ||||
| Total bilirubin (μmol/L) | 0.992 (0.964-1.021) | 0.586 | 0.966 (0.979-1.013) | 0.653 | ||||
| Prothrombin time (s) | 1.071 (0.931-1.232) | 0.340 | 1.064 (0.966-1.173) | 0.210 | ||||
| Child-Pugh classification (B) | 1.423 (0.523-3.875) | 0.490 | 2.074 (1.098-3.915) | 0.024 | ||||
| Hypersplenism (yes) | 0.949 (0.477-1.887) | 0.882 | 1.158 (0.738-1.817) | 0.524 | ||||
| AFP (> 400 ng/mL) | 2.063 (1.379-3.086) | 0.000 | 1.321 (1.007-1.733) | 0.044 | 1.738 (1.152-2.622) | 0.008 | ||
| HBV-DNA (> 1 × 104 IU/mL) | 1.191 (0.798-1.777) | 0.393 | 1.259 (0.962-1.649) | 0.094 | 1.316 (1.002-1.729) | 0.048 | ||
| Tumor size (> 5 cm) | 2.219 (1.456-3.382) | 0.000 | 1.694 (1.291-2.223) | 0.000 | 1.858 (1.213-2.848) | 0.004 | 1.600 (1.216-2.105) | 0.001 |
| Nodule no. (multinodular) | 1.425 (0.885-2.294) | 0.145 | 1.370 (0.985-1.905) | 0.061 | 1.427 (1.024-1.989) | 0.036 | ||
| Differentiation (grades 3-4) | 1.396 (0.934-2.085) | 0.103 | 1.347 (1.027-1.766) | 0.031 | ||||
| Incomplete tumor capsule (yes) | 2.366 (1.582-3.537) | 0.000 | 1.664 (1.266-2.187) | 0.000 | 1.998 (1.329-3.006) | 0.001 | 1.468 (1.114-1.935) | 0.006 |
| MVI (yes) | 3.412 (2.135-5.452) | 0.000 | 2.180 (1.646-2.888) | 0.000 | 2.524 (1.564-4.075) | 0.000 | 1.959 (1.472-2.606) | 0.000 |
| Cirrhosis (yes) | 0.922 (0.522-1.541) | 0.757 | 1.359 (0.918-2.031) | 0.125 | ||||
| Approach of resection (major ) | 1.576 (1.054-2.357) | 0.027 | 1.177 (0.896-1.547) | 0.242 | 1.611 (1.073-2.419) | 0.021 | ||
| Hepatic inflow occlusion (yes) | 0.784 (0.519-1.185) | 0.248 | 0.919 (0.649-1.217) | 0.556 | ||||
OS: Overall survival; RFS: Recurrence-free survival; AFP: Alpha-fetoprotein; MVI: Microvascular invasion.
Multiple logistic regression identified serum AFP level > 400 ng/mL, tumor size > 5 cm, and incomplete tumor capsule as independent predictors of MVI (Table 3).
Table 3.
Logistic regression to identify independent predictors of microvascular invasion in hepatocellular carcinoma patients
| Predictor | HR (95%CI) | P |
| AFP (> 400 ng/mL) | 1.680 (1.167-2.418) | 0.005 |
| Tumor size (> 5 cm) | 1.767 (1.235-2.527) | 0.002 |
| Incomplete tumor capsule (yes) | 1.960 (1.335-2.878) | 0.001 |
AFP: Alpha-fetoprotein.
Efficacy and safety of PA-TACE in the prevention of recurrence and improvement of survival
Among patients with MVI, OS was significantly improved in patients treated by PA-TACE than those treated by liver resection alone (1 year, 92.7% vs 81.3%; 2 years, 86.5% vs 65.7%; 3 years, 73.1% vs 58.8%; 4 years, 67.5% vs 53.9%; P = 0.019; Figure 1B and Table 4). Similarly, RFS was significantly better in patients treated by PA-TACE (median RFS, 37 mo vs 13 mo; RFS at 1 year, 72.1% vs 52.0%; 2 years, 58.3% vs 41.1%; 3 years, 54.9% vs 33.7%; 4 years, 30.9% vs 28.4%; P = 0.002; Figure 1D). Among patients without MVI, OS was similar between those with or without PA-TACE (1 year, 94.2% vs 98.8%; 2 years, 91.9% vs 91.5%; 3 years, 87.6% vs 82.8%; 4 years, 87.6% vs 82.8%; P = 0.872; Figure 1A). Similar results were obtained for RFS (1 year, 83.0% vs 80.7%; 2 years, 71.8% vs 70.1%; 3 years, 61.9% vs 61.2%; 4 years, not available vs 55.9%; P = 0.974; Figure 1C). Since recurrence did not occur in > 50% of patients without MVI, we could not compare median RFS duration between those who did or did not receive PA-TACE.
Figure 1.
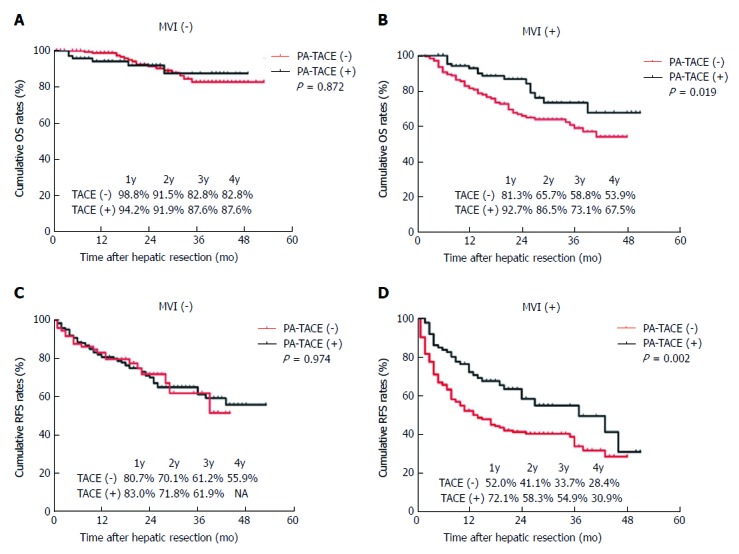
Overall survival and recurrence-free survival curves of hepatocellular carcinoma patients who either underwent liver resection alone or followed by postoperative adjuvant transcatheter arterial chemoembolization. A: OS for HCC/MVI (-) patients (P = 0.872); B: OS for HCC/MVI (+) patients (P = 0.019); C: RFS for HCC/MVI (-) patients (P = 0.974); D: RFS for HCC/MVI (+) patients (P = 0.002). OS: Overall survival; HCC: Hepatocellular carcinoma; MVI: Microvascular invasion; RFS: Recurrence-free survival.
Table 4.
Survival rates of hepatocellular carcinoma patients in different groups
| Group |
OS rate (%) |
RFS rate (%) |
||||||||
| 1 yr | 2 yr | 3 yr | 4 yr | P | 1 yr | 2 yr | 3 yr | 4 yr | P | |
| MVI (-), TACE (-) | 98.8 | 91.5 | 82.8 | 82.8 | 0.872 | 80.7 | 70.1 | 61.2 | 55.9 | 0.974 |
| MVI (-), TACE (+) | 94.2 | 91.9 | 87.6 | 87.6 | 83.0 | 71.8 | 61.9 | NA | ||
| MVI (+), TACE (-) | 81.3 | 65.7 | 58.8 | 53.9 | 0.019 | 52.0 | 41.1 | 33.7 | 28.4 | 0.002 |
| MVI (+), TACE (+) | 92.7 | 86.5 | 73.1 | 67.5 | 72.1 | 58.3 | 54.9 | 30.9 | ||
OS: Overall survival; RFS: Recurrence-free survival; MVI: Microvascular invasion; TACE: Transcatheter arterial chemoembolization.
Moreover, complications of TACE were summarized in Table 5. Owing to PA-TACE as an adjuvant preventative therapy with a low dose of lipiodol (3-5 mL) and chemotherapeutic drugs lobaplatin (50 mg) and raltitrexed (4 mg), no serious complications were observed in all patients who received PA-TACE in the current study.
Table 5.
Complications and adverse events after postoperative adjuvant transcatheter arterial chemoembolization n (%)
| Complication | Events |
| Nausea and vomiting | 46 (29.11) |
| Fever | 35 (22.2) |
| Pain | 49 (31.0) |
| Alopecia | 5 (3.2) |
| Liver failure | 0 (0.0) |
| Bleeding of esophageal venous plexus | 0 (0.0) |
| Gastrointestinal hemorrhage | 0 (0.0) |
| Heart failure | 0 (0.0) |
| Infection | 0 (0.0) |
| Ectopic embolism syndrome | 0 (0.0) |
| Refractory ascites | 0 (0.0) |
| Pulmonary complication | 2 (1.3) |
| Therapy-related death | 0 (0.0) |
DISCUSSION
With advances in surgical techniques, liver resection remains the most effective curative strategy for HCC[5,6]. Nevertheless, the high incidence of postoperative recurrence usually leads to poor survival[7-9]. Vascular invasion promotes the growth of residual tumors and intrahepatic metastasis, which contributes to early recurrence post liver resection[11-15]. Invisible intra-hepatic micro-metastases likely distribute via the hepatic artery and portal vein system, readily escaping detection during preoperative imaging or intra-operative observation. Recently, MVI has been associated with intrahepatic metastasis after curative resection[33,34]. MVI may promote metastasis by acting as a “seed” to give rise to micro-metastases in the liver parenchyma, and such metastasis is a common occurrence among patients in whom MVI affects more than five vessels[24]. Recent studies revealed that MVI is an independent risk factor for early recurrence in single HCC without macrovascular invasion[16-21,24], which is in agreement with our findings. In the present study, uni- and multivariate analyses showed that MVI is closely related to OS and RFS in HCC patients with early disease.
We conducted multiple logistic regression to identify that serum AFP level > 400 ng/mL, tumor size > 5 cm, and incomplete tumor capsule were closely related to MVI. Our finding that MVI incidence increases with increasing tumor diameter is in agreement with previous reports[35,36]. Nonetheless, tumor size is one of the major prognostic factors in HCC[36], and the aggressive or infiltrative tendency of HCC increases when the tumor diameter is > 3 cm[37,38]. In addition, MVI incidence tends to be high in patients with incomplete tumor capsule, which is associated with an aggressive physiological behavior[39]. Such tumors potentially invade normal tissue containing microvessels, giving rise to MVI[14,40]. A CT scan study showed that the presence of incomplete tumor capsule closely correlated with the absence of MVI. Moreover, the incidence of MVI was high among patients in our cohort with AFP level > 400 ng/mL, which is consistent with previous studies associating elevated AFP level with MVI[35,41].
Postoperative adjuvant intervention is routinely recommended for suppressing the progression of invisible micro-metastatic lesions and preventing recurrence. Normal liver parenchyma receives about 70% of its basic blood supply from the portal venous system, whereas HCC growth, including invisible micro-metastases, depends primarily on the blood supply by the hepatic artery[42-44]. Therefore, lipiodol injection via TACE is speculated to selectively accumulate in the invisible metastatic HCC to block the nutrient vessels when delivered intra-arterially while serving as a carrier of anticancer drugs that allow the sustainable chemotherapeutic killing of microscopic HCC cells[45-47]. Previous studies have shown that PA-TACE can prevent the early recurrence and delay the progression of recurrent tumors, thereby improving OS in HCC patients with macrovascular invasion. However, only a few studies have examined whether PA-TACE can prevent recurrence and improve the survival of HCC patients with MVI.
In the present study, the early-stage HCC patients were stratified according to the MVI status and subgroup analysis was performed. We found that PA-TACE after liver resection significantly prolonged the OS and RFS in patients with MVI. These results were in agreement with a previous study, which showed that PA-TACE at 4 wk after R0 hepatectomy in HCC patients with MVI significantly prolonged the RFS and OS, as well as reduced the rate of early recurrence[20]. On the other hand, in case of patients without MVI in the current study, PA-TACE did not significantly improve OS or RFS. Furthermore, we observed that none of the patients presented serious complications after PA-TACE. These findings suggest that when HCC patients experience postoperative MVI, immediate TACE, as a safe management, may prevent recurrence. However, when MVI is absent, PA-TACE is not strongly recommended for patients with BCLC early- or intermediate-stage HCC. These findings could be utilized for clinical guidance of the treatment and management of early-stage HCC.
The conclusions of the present study should be noted for several limitations. The present study was retrospective and involved patients from only one medical center, thereby highlighting the need for verification through large, multicenter, preferably prospective trials. Furthermore, PA-TACE drugs and dosages can vary across medical centers. The current cohort may have shown selection bias since we excluded patients whose MVI was not assessed through postoperative histopathology. The current follow-up time was only 4 years; thus, long-term outcomes are yet controversial. Moreover, the AFP levels were not evaluated before and after TACE. Hence, further prospective studies to verify the efficacy of PA-TACE for the prevention of recurrence and improvement of survival in early-stage HCC with MVI remains to be investigated in larger populations.
COMMENTS
Background
Microvascular invasion (MVI) is associated with a high rate of recurrence in patients with hepatocellular carcinoma (HCC). Currently, postoperative adjuvant transcatheter arterial chemoembolization (PA-TACE) is an efficient method for preventing early recurrence and delaying the progression of recurrent tumors, thereby improving the overall survival in HCC patients with macrovascular invasion. However, whether PA-TACE could provide a survival benefit to HCC patients with MVI remains unclear. This study aimed to investigate the efficacy and safety of PA-TACE in the prevention of recurrence and improvement of survival in Barcelona Clinic Liver Cancer (BCLC) early- and intermediate-stage HCC patients with MVI.
Research frontiers
This study aimed to evaluate the efficacy and safety of PA-TACE to prevent recurrence and improve survival in BCLC early- and intermediate-stage HCC patients with or without MVI who were treated by liver resection alone or followed by PA-TACE. The results indicate that PA-TACE is an effective and safe method to prevent the recurrence and improve the survival in BCLC early- and intermediate-stage HCC patients with MVI. Nevertheless, the study failed to provide obvious OS or RFS benefits in patients without MVI.
Innovations and breakthroughs
This study indicates that PA-TACE is an effective and safe approach to prevent recurrence and improve survival in BCLC early- and intermediate-stage HCC patients with MVI. However, obvious OS or RFS benefits in patients without MVI could not be detected.
Applications
This study suggests that PA-TACE is effective and safe for preventing recurrence and improving the survival in patients with BCLC A and B stage HCC with MVI; however, it is not essential when MVI is absent. These findings could be utilized for clinical decision with respect to the treatment and management of BCLC A and B stage HCC.
Terminology
PA-TACE is a minimally invasive procedure performed in interventional radiology to block the blood supply of the tumors by lipiodol and/or drug-loaded microspheres that simultaneously serve as a carrier of anticancer drugs to allow the sustainable chemotherapeutic killing of microscopic HCC cells. PA-TACE was performed at 1, 3, and 6 mo after hepatectomy. During this procedure, a hepatic arterial catheter was inserted through the femoral artery using the Seldinger technique; the catheter was inserted selectively into the tumor-feeding artery as technically plausible. An emulsion of lobaplatin (50 mg), raltitrexed (4 mg), and lipiodol (3-5 mL) was infused into the remnant liver through the catheter. Simultaneously, hepatic angiography and/or computed tomography angiography detected any obvious residual tumor in the remnant liver. TACE was conducted on the entire remnant liver.
Peer-review
This is a retrospective study comprising of 519 HCC patients with BCLC stage A or B disease treated by curative liver resection between 2012 and 2015. Based on the histology, the whole series was divided into 259 cases without and 260 cases with MVI. The study found that PA-TACE is effective and safe for the prevention of recurrence and improvement in the survival of BCLC A and B stage HCC patients with MVI.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Supported by Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, Ministry of Education, No. GKZ201604; Key Project of Guangxi Health and Family Planning Commission, China, No. S201513; and Key Project of Guangxi Science and Technology Department, China, No. Gui Ke AB16380242.
Institutional review board statement: The study was reviewed and approved by the Clinical Research Ethics Committee of Affiliated Tumor Hospital of Guangxi Medical University.
Informed consent statement: Informed consent was not required from the patients for the study as the analysis used anonymous clinical data. Individuals cannot be identified based on the data presented.
Conflict-of-interest statement: The authors declare no conflicts of interest.
Data sharing statement: No additional data are available.
Peer-review started: July 26, 2017
First decision: August 10, 2017
Article in press: September 19, 2017
P- Reviewer: Al-Shamma S, Sergi CM, Tai DI S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Huang Y
Contributor Information
Jia-Zhou Ye, Department of Hepatobiliary Surgery, Affiliated Tumor Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China; Guangxi Liver Cancer Diagnosis and Treatment Engineering and Technology Research Center, Nanning 530021, Guangxi Zhuang Autonomous Region, China; Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, Ministry of Education, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Jun-Ze Chen, Department of General Surgery, The Ninth Affiliated Hospital of Guangxi Medical University, Beihai 536000, Guangxi Zhuang Autonomous Region, China.
Zi-Hui Li, Department of Hepatobiliary Surgery, Affiliated Tumor Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Tao Bai, Department of Hepatobiliary Surgery, Affiliated Tumor Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China; Guangxi Liver Cancer Diagnosis and Treatment Engineering and Technology Research Center, Nanning 530021, Guangxi Zhuang Autonomous Region, China; Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, Ministry of Education, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Jie Chen, Department of Hepatobiliary Surgery, Affiliated Tumor Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China; Guangxi Liver Cancer Diagnosis and Treatment Engineering and Technology Research Center, Nanning 530021, Guangxi Zhuang Autonomous Region, China; Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, Ministry of Education, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Shao-Liang Zhu, Department of Hepatobiliary Surgery, Affiliated Tumor Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China; Guangxi Liver Cancer Diagnosis and Treatment Engineering and Technology Research Center, Nanning 530021, Guangxi Zhuang Autonomous Region, China; Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, Ministry of Education, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Le-Qun Li, Department of Hepatobiliary Surgery, Affiliated Tumor Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China; Guangxi Liver Cancer Diagnosis and Treatment Engineering and Technology Research Center, Nanning 530021, Guangxi Zhuang Autonomous Region, China; Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, Ministry of Education, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Fei-Xiang Wu, Department of Hepatobiliary Surgery, Affiliated Tumor Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China; Guangxi Liver Cancer Diagnosis and Treatment Engineering and Technology Research Center, Nanning 530021, Guangxi Zhuang Autonomous Region, China; Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, Ministry of Education, Nanning 530021, Guangxi Zhuang Autonomous Region, China. wufeixiang@gxmu.edu.cn.
References
- 1.McGuire S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv Nutr. 2016;7:418–419. doi: 10.3945/an.116.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 4.Gao JD, Shao YF, Xu Y, Ming LH, Wu ZY, Liu GT, Wang XH, Gao WH, Sun YT, Feng XL, et al. Tight association of hepatocellular carcinoma with HBV infection in North China. Hepatobiliary Pancreat Dis Int. 2005;4:46–49. [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 7.Lim KC, Chow PK, Allen JC, Siddiqui FJ, Chan ES, Tan SB. Systematic review of outcomes of liver resection for early hepatocellular carcinoma within the Milan criteria. Br J Surg. 2012;99:1622–1629. doi: 10.1002/bjs.8915. [DOI] [PubMed] [Google Scholar]
- 8.Belghiti J, Panis Y, Farges O, Benhamou JP, Fekete F. Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis. Ann Surg. 1991;214:114–117. doi: 10.1097/00000658-199108000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Association For The Study Of The Liver ; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 13.Kudo M, Kitano M, Sakurai T, Nishida N. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, Nationwide Follow-Up Survey and Clinical Practice Guidelines: The Outstanding Achievements of the Liver Cancer Study Group of Japan. Dig Dis. 2015;33:765–770. doi: 10.1159/000439101. [DOI] [PubMed] [Google Scholar]
- 14.Gouw AS, Balabaud C, Kusano H, Todo S, Ichida T, Kojiro M. Markers for microvascular invasion in hepatocellular carcinoma: where do we stand? Liver Transpl. 2011;17 Suppl 2:S72–S80. doi: 10.1002/lt.22368. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez-Perálvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20:325–339. doi: 10.1245/s10434-012-2513-1. [DOI] [PubMed] [Google Scholar]
- 16.Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, Labow DM, Llovet JM, Schwartz ME. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137:850–855. doi: 10.1053/j.gastro.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sparrelid E, Del Chiaro M. Microvascular Invasion in Hepatitis B Virus-Related Hepatocellular Carcinoma: Another Step Toward Preoperative Evaluation? JAMA Surg. 2016;151:364. doi: 10.1001/jamasurg.2015.4267. [DOI] [PubMed] [Google Scholar]
- 18.Du M, Chen L, Zhao J, Tian F, Zeng H, Tan Y, Sun H, Zhou J, Ji Y. Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer. 2014;14:38. doi: 10.1186/1471-2407-14-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim KC, Chow PK, Allen JC, Chia GS, Lim M, Cheow PC, Chung AY, Ooi LL, Tan SB. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254:108–113. doi: 10.1097/SLA.0b013e31821ad884. [DOI] [PubMed] [Google Scholar]
- 20.Barreto SG, Brooke-Smith M, Dolan P, Wilson TG, Padbury RT, Chen JW. Cirrhosis and microvascular invasion predict outcomes in hepatocellular carcinoma. ANZ J Surg. 2013;83:331–335. doi: 10.1111/j.1445-2197.2012.06196.x. [DOI] [PubMed] [Google Scholar]
- 21.Ünal E, İdilman İS, Akata D, Özmen MN, Karçaaltıncaba M. Microvascular invasion in hepatocellular carcinoma. Diagn Interv Radiol. 2016;22:125–132. doi: 10.5152/dir.2015.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faber W, Stockmann M, Kruschke JE, Denecke T, Bahra M, Seehofer D. Implication of microscopic and macroscopic vascular invasion for liver resection in patients with hepatocellular carcinoma. Dig Surg. 2014;31:204–209. doi: 10.1159/000365257. [DOI] [PubMed] [Google Scholar]
- 23.Sun JJ, Wang K, Zhang CZ, Guo WX, Shi J, Cong WM, Wu MC, Lau WY, Cheng SQ. Postoperative Adjuvant Transcatheter Arterial Chemoembolization After R0 Hepatectomy Improves Outcomes of Patients Who have Hepatocellular Carcinoma with Microvascular Invasion. Ann Surg Oncol. 2016;23:1344–1351. doi: 10.1245/s10434-015-5008-z. [DOI] [PubMed] [Google Scholar]
- 24.Hirokawa F, Hayashi M, Asakuma M, Shimizu T, Inoue Y, Uchiyama K. Risk factors and patterns of early recurrence after curative hepatectomy for hepatocellular carcinoma. Surg Oncol. 2016;25:24–29. doi: 10.1016/j.suronc.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, Watanabe Y, Kojiro M, Sata M. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008;15:1375–1382. doi: 10.1245/s10434-008-9846-9. [DOI] [PubMed] [Google Scholar]
- 26.Kanai T, Hirohashi S, Upton MP, Noguchi M, Kishi K, Makuuchi M, Yamasaki S, Hasegawa H, Takayasu K, Moriyama N. Pathology of small hepatocellular carcinoma. A proposal for a new gross classification. Cancer. 1987;60:810–819. doi: 10.1002/1097-0142(19870815)60:4<810::aid-cncr2820600417>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 27.EDMONDSON HA, STEINER PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 28.Sumie S, Nakashima O, Okuda K, Kuromatsu R, Kawaguchi A, Nakano M, Satani M, Yamada S, Okamura S, Hori M, et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol. 2014;21:1002–1009. doi: 10.1245/s10434-013-3376-9. [DOI] [PubMed] [Google Scholar]
- 29.Zhong C, Guo RP, Li JQ, Shi M, Wei W, Chen MS, Zhang YQ. A randomized controlled trial of hepatectomy with adjuvant transcatheter arterial chemoembolization versus hepatectomy alone for Stage III A hepatocellular carcinoma. J Cancer Res Clin Oncol. 2009;135:1437–1445. doi: 10.1007/s00432-009-0588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin YJ, Lee JW, Lee OH, Chung HJ, Kim YS, Lee JI, Cho SG, Jeon YS, Lee KY, Ahn SI, et al. Transarterial chemoembolization versus surgery/radiofrequency ablation for recurrent hepatocellular carcinoma with or without microvascular invasion. J Gastroenterol Hepatol. 2014;29:1056–1064. doi: 10.1111/jgh.12507. [DOI] [PubMed] [Google Scholar]
- 31.Wang SN, Chuang SC, Lee KT. Efficacy of sorafenib as adjuvant therapy to prevent early recurrence of hepatocellular carcinoma after curative surgery: A pilot study. Hepatol Res. 2014;44:523–531. doi: 10.1111/hepr.12159. [DOI] [PubMed] [Google Scholar]
- 32.Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–1354. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 33.Fan J, Zhou J, Wu ZQ, Qiu SJ, Wang XY, Shi YH, Tang ZY. Efficacy of different treatment strategies for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol. 2005;11:1215–1219. doi: 10.3748/wjg.v11.i8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 35.Nagano Y, Shimada H, Takeda K, Ueda M, Matsuo K, Tanaka K, Endo I, Kunisaki C, Togo S. Predictive factors of microvascular invasion in patients with hepatocellular carcinoma larger than 5 cm. World J Surg. 2008;32:2218–2222. doi: 10.1007/s00268-008-9585-x. [DOI] [PubMed] [Google Scholar]
- 36.Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, Yamaoka Y, Belghiti J, Lauwers GY, Poon RT, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 37.Esnaola NF, Lauwers GY, Mirza NQ, Nagorney DM, Doherty D, Ikai I, Yamaoka Y, Regimbeau JM, Belghiti J, Curley SA, et al. Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J Gastrointest Surg. 2002;6:224–232; discussion 232. doi: 10.1016/s1091-255x(01)00015-4. [DOI] [PubMed] [Google Scholar]
- 38.Jun L, Zhenlin Y, Renyan G, Yizhou W, Xuying W, Feng X, Yong X, Kui W, Jian L, Dong W, et al. Independent factors and predictive score for extrahepatic metastasis of hepatocellular carcinoma following curative hepatectomy. Oncologist. 2012;17:963–969. doi: 10.1634/theoncologist.2011-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu XY, Xi T, Lau WY, Dong H, Xian ZH, Yu H, Zhu Z, Shen F, Wu MC, Cong WM. Pathobiological features of small hepatocellular carcinoma: correlation between tumor size and biological behavior. J Cancer Res Clin Oncol. 2011;137:567–575. doi: 10.1007/s00432-010-0909-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Groeschl RT, Gamblin TC, Turaga KK. Ablation for hepatocellular carcinoma: validating the 3-cm breakpoint. Ann Surg Oncol. 2013;20:3591–3595. doi: 10.1245/s10434-013-3031-5. [DOI] [PubMed] [Google Scholar]
- 41.Chandarana H, Robinson E, Hajdu CH, Drozhinin L, Babb JS, Taouli B. Microvascular invasion in hepatocellular carcinoma: is it predictable with pretransplant MRI? AJR Am J Roentgenol. 2011;196:1083–1089. doi: 10.2214/AJR.10.4720. [DOI] [PubMed] [Google Scholar]
- 42.Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, Giulini SM. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229–235. doi: 10.1097/01.sla.0000197706.21803.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cucchetti A, Piscaglia F, Caturelli E, Benvegnù L, Vivarelli M, Ercolani G, Cescon M, Ravaioli M, Grazi GL, Bolondi L, et al. Comparison of recurrence of hepatocellular carcinoma after resection in patients with cirrhosis to its occurrence in a surveilled cirrhotic population. Ann Surg Oncol. 2009;16:413–422. doi: 10.1245/s10434-008-0232-4. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Li Q, Sun Y, Zheng H, Cui Y, Li H, Zhou H, Hao X. Clinicopathologic features between multicentric occurence and intrahepatic metastasis of multiple hepatocellular carcinomas related to HBV. Surg Oncol. 2009;18:25–30. doi: 10.1016/j.suronc.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Inayoshi J, Ichida T, Sugitani S, Tsuboi Y, Genda T, Honma N, Asakura H. Gross appearance of hepatocellular carcinoma reflects E-cadherin expression and risk of early recurrence after surgical treatment. J Gastroenterol Hepatol. 2003;18:673–677. doi: 10.1046/j.1440-1746.2003.03021.x. [DOI] [PubMed] [Google Scholar]
- 46.Iguchi T, Aishima S, Sanefuji K, Fujita N, Sugimachi K, Gion T, Taketomi A, Shirabe K, Maehara Y, Tsuneyoshi M. Both fibrous capsule formation and extracapsular penetration are powerful predictors of poor survival in human hepatocellular carcinoma: a histological assessment of 365 patients in Japan. Ann Surg Oncol. 2009;16:2539–2546. doi: 10.1245/s10434-009-0453-1. [DOI] [PubMed] [Google Scholar]
- 47.Ng IO, Lai EC, Ng MM, Fan ST. Tumor encapsulation in hepatocellular carcinoma. A pathologic study of 189 cases. Cancer. 1992;70:45–49. doi: 10.1002/1097-0142(19920701)70:1<45::aid-cncr2820700108>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]


