Abstract
Synthetic molecular libraries hold great potential to advance the biomaterial development. However, little effort is made to integrate molecules with molecular recognition abilities selected from different libraries into a single biomolecular material. The purpose of this work is to incorporate peptides and nucleic acid aptamers into a porous hydrogel to develop a dual-functional biomaterial. The data show that an anti-integrin peptide can promote the attachment and growth of endothelial cells in a 3D porous poly(ethylene glycol) hydrogel and an antivascular endothelial growth factor aptamer can sequester and release VEGF of high bioactivity. Importantly, the dual-functional porous hydrogel enhances the growth and survival of endothelial cells. This work demonstrates that molecules selected from different synthetic libraries can be integrated into one system for the development of novel biomaterials.
Keywords: Aptamer, endothelial cell, hydrogel, RGD peptide, vascular endothelial growth factor
1. Introduction
A number of synthetic molecular libraries have been recently developed.[1–15] For instance, oligonucleotide libraries have been studied to identify nucleic acid aptamers against a variety of growth factors,[4,6] phage libraries have been designed for selecting a multitude of peptides against cell–surface receptors,[7,16,17] and lipid libraries have been explored to synthesize nanocarriers for intracellular drug delivery.[5,8–10] These libraries hold great potential of enriching the synthesis of biomaterials for various biomedical applications such as drug delivery and regenerative medicine. However, molecules selected from these libraries were mostly studied individually for synthesis of biomaterials. The integration of molecules selected from different libraries into one biomaterial system would advance the diversity and functionality of biomaterials, which has received little attention.
The extracellular matrix (ECM) is a multifunctional material with multiple components, of which soluble growth factors provide cells with biological stimulations and cell adhesion molecules provide cells with biochemical/biophysical cues. These components interact with the cells to determine the cell behavior such as migration, growth, and differentiation.[18] Thus, biomaterials need to possess features to recapitulate the essential functionality of these ECM components to satisfy the needs of the cells when they are developed for biomedical applications such as tissue engineering and regenerative medicine. To fulfill this requirement, it is important to not only select a biocompatible material as the fundamental structural component of the ECM mimic but also functionalize this material with biologically active molecules serving biochemical and biophysical cues.
The ECM mimic studied in this work was a dual-functional poly(ethylene glycol) (PEG) hydrogel that was functionalized with a nucleic acid aptamer and a short peptide. Hydrogels are a crosslinked hydrophilic polymeric network with a large amount of water.[19] Importantly, they possess viscoelastic properties similar to living tissues with good biocompatibility and are highly permeable to the transport of molecules that can be essential nutrients to the cells.[20–22] In particular, PEG hydrogels have received great attention in biomedical applications such as drug delivery and tissue engineering because of their excellent biocompatibility.[23–25] However, pure PEG hydrogels lack biochemical signaling molecules, biophysical cues or both of them for cell regulation.[26] Therefore, great effort has been made in functionalizing PEG hydrogels to meet the requirements of biomedical applications.[27,28]
In this study, an RGD peptide and an anti-VEGF aptamer were used as a model to functionalize the PEG hydrogel. The RGD peptide has been widely studied for cell attachment owing to its ability of binding to integrin receptors.[29–31] The anti-VEGF aptamer is a derivative of an aptamer selected from a chemically modified oligonucleotide library with high affinity of binding VEGF.[4] The peptide and aptamer were chemically incorporated into the PEG hydrogel through free radical polymerization. The functionality of the hydrogel was studied by examining independent and synergistic effect of VEGF release and cell adhesion on endothelial cell growth.
2. Experimental Section
2.1. Materials
Glass slides, sodium hydroxide, acetone, glacial acetic acid, tetramethylethylenediamine (TEMED), ammonium persulfate (APS), sodium bicarbonate, and fetal bovine serum (FBS) were obtained from Fisher Scientific (Pittsburgh, PA). Pluronic F-127, poly(ethylene glycol) diacrylate (PEG700DA, Mn = 700) and 3-(trimethoxysilyl)propyl methacrylate were purchased from Sigma (Saint Louis, MO). Recombinant human vascular endothelial growth factor-165 (VEGF, Mw = 38 200) and its enzyme-linked immunosorbent assay (ELISA) kit were from PeproTech (Rocky Hill, NJ). Modified anti-VEGF aptamer and its complementary oligonucleotide were synthesized by TriLink Biotechnologies (San Diego, CA) and Integrated DNA Technologies (Coralville, IA), respectively. Acrylate-glycine-arginine-glycine-aspartic acid-serine peptide (Acrylate-GRGDS, Mw = 544.0) was synthesized by United Peptides (Herndon, VA). Medium 200 (M200), large vessel endothelial supplement, human umbilical vein endothelial cell (HUVEC), trypsin/EDTA, Geltrex LDEV-Free Reduced Growth Factor Basement Membrane Matrix, and Calcein AM were from Life Technologies (Grand Island, NY). EmbryoMax Ultrapure Water with 0.1% Gelatin was from EMD Millipore (Billerica, MA). CellTiter 96 AQueous One Solution Cell Proliferation (MTS) Assay was from Promega (Madison, WI).
2.2. Methods
2.2.1. Preparation of Hydrogel Films
Glass was used as a physical support of the hydrogel films. Glass squares (6 × 6 mm2) were silanized using the procedure as published in the literature.[32,33] Briefly, glass squares were sonicated in acetone for 15 min, rinsed with deionized water, sonicated in 1 M NaOH for 15 min, and rinsed with deionized water again. After dried, the glass squares were immersed in a silanization solution (96.1% ethanol, 1.0% 3-(trimethoxysilyl)propyl methacrylate, 0.3% acetic acid, and 2.6% H2O) for 10 min. After silanization, the glass squares were rinsed with ethanol and dried before the preparation of hydrogel films. Prepolymer solutions were comprised of 2% (w/v) Pluronic F-127, 5% (v/v) acetic acid, 2% (v/v) TEMED, 3.3% (w/v) APS, 20% (v/v) PEG700DA, and varied concentration of RGD peptide (0 × 10−3, 0.5 × 10−3, 5 × 10−3, or 10 × 10−3 M). Right after the preparation of this prepolymer solution, 2 μL of the solution was quickly added onto a nonsilanized glass slide. A 6 × 6 mm2 silanized glass square was gently set onto the reaction solution for the formation of a hydrogel film with the dimensions of 6 × 6 mm2. After the film formed, the glass square with the attached hydrogel film was carefully separated from the glass slide manually and was thoroughly washed with gentle agitation in deionized water, in 70% ethanol, and in sterile deionized water to remove unreacted molecules and sterilize the hydrogels.
2.2.2. Preparation of 3D Porous Hydrogel Scaffolds
Porous hydrogels were synthesized using a free radical polymerization reaction coupled with a gas foaming reaction according to an established protocol with minor modifications.[34] In brief, prepolymer solutions (5 μL) as prepared in Section 2.2.1 with 0 × 10−6, 0.5 × 10−6, 2.5 × 10−6, 5 × 10−6, or 25 × 10−6 M anti-VEGF aptamer were added into a cylindrical mold with 0.004 g NaHCO3 to initiate the formation of the gas foam and the hydrogel. After the hydrogels cured, the hydrogels were thoroughly washed with gentle agitation in deionized water, 70% ethanol, and in sterile deionized water to remove unreacted molecules and sterilize the hydrogels. To characterize the porous structure of hydrogels, hydrogels were lyophilized, sputter-coated and imaged with a scanning electron microscope (NanoSEM630, FEI, USA). The anti-VEGF aptamer used to functionalize hydrogels was denoted as Apt(f). The numbers in the brackets of Apt(f2.5) and Apt(f5) show the molar ratios of aptamer to VEGF. The control of Apt(f) is a scrambled nucleic acid sequence, which was denoted as Apt(c).
2.2.3. VEGF Loading and Release
Lyophilized VEGF was reconstituted by dissolving it in M200 with 0.5% Bovine Serum Albumin (BSA). To load VEGF into porous hydrogels, the hydrogels were first blotted with sterilized tissue paper to dehydrate the hydrogels. Next, 25 μL of 0.2 × 10−6 M VEGF solution was aseptically added to the hydrogels and the hydrogels were incubated at 4 °C for 24 h. The VEGF-loaded hydrogels were used for the examination of VEGF retention. Hydrogels were incubated in 1 mL release medium (M200 supplemented with 0.5% BSA) at 37 °C. At predetermined time points, the supernatant was aseptically collected and replaced with 1 mL of fresh release medium. The collected supernatant was stored at −20 °C until VEGF quantification.
2.2.4. VEGF Quantification
VEGF concentration in the supernatant was determined using a recombinant human VEGF165 ELISA kit according to the instructions provided by the manufacturer (PeproTech; Rocky Hill, NJ). To ensure that the VEGF concentration fell within the detectable range of the assay, supernatant samples were diluted prior to analysis. The absorbance of each sample was measured using an Infinite M200 Pro microplate reader (Tecan; Grödig, Austria) at 405 nm and was referenced by subtracting the absorbance at the reference wavelength (650 nm).
2.2.5. HUVEC Culture
Flasks for HUVEC culture were first coated with 5 mL of 0.1% gelatin solution. HUVECs were seeded and expanded in M200 supplemented with the large vessel endothelial supplement at 37 °C in 5% CO2/95% air. HUVECs were harvested at 80% confluency using a trypsin/EDTA solution. After the removal of trypsin, the cells were plated in gelatin-coated flasks or used to seed hydrogels. HUVECs within passage 8 were used for all experiments.
2.2.6. Tube Formation Assay
80 μL of the Geltrex solution was added to each well in a 48-well cell culture plate and then incubated at 37 °C for 30 min to solidify the solution. VEGF was collected from the hydrogels at day 14 using a triggering method as published before[35] and the amount of VEGF was quantified with a VEGF ELISA kit. HUVECs at 80% confluency were trypsinized and resuspended at 2 × 105 cells per mL in basal M200 supplemented with 0.5% FBS. 200 μL of suspended HUVECs was added to the Geltrex-coated wells and the cells were allowed to attach for 30 min. Then, the media were replaced with M200 containing 10 ng mL−1 VEGF collected from the hydrogels. A stock VEGF solution of the same concentration was used as control. The cells were incubated at 37 °C in 5% CO2/95% air for 6 h. After incubation, HUVECs were stained with 2 μg mL−1 Calcein AM for 30 min and the cell images were captured under a fluorescence microscope (Olympus IX73, Center Valley, PA). The total length of tube structure was quantified using ImageJ.
2.2.7. Culture of HUVECs with Hydrogels
To study cell attachment and proliferation on 2D hydrogels, hydrogel films were transferred into a 48-well cell culture plate and 104 cells were seeded in each well. At predetermined time points, optical images were captured under an optical microscope (IX73, Olympus; Center Valley, PA).
To study cell growth in 3D porous hydrogels, the hydrogels were dehydrated by gently blotting the hydrogels with sterilized tissue paper and then placed into a 48-well plate. 25 μL of cell suspension (5 × 105 cells) was dropped onto each hydrogel and the cells were allowed to be adsorbed into the hydrogel for 30 min. Then the hydrogel was incubated in 500 μL of M200 supplemented with 2% of large vessel endothelial supplement. After cultured for a predetermined period of time, the cells in the hydrogels were stained with the Live/Dead Kit according to the protocol provided from the manufacturer. The fluorescence images of cells in the hydrogels were acquired under a laser scanning confocal microscope (Olympus FV1000; Center Valley, PA) equipped with an argon laser (excitation = 488 nm). The green fluorescence intensity of the bulk hydrogel was quantified by the Maestro In Vivo Imaging System (CRI, Woburn, MA) with the exposure time of 150 ms.
The effect of sustained VEGF release on cells was studied by culturing HUVECs in 3D hydrogels loaded with VEGF. The molar ratio of aptamer to VEGF of 2.5:1 was used in the Apt(f) group based on the release data. RGD functionalized hydrogels and hydrogels functionalized with both RGD and the anti-VEGF aptamer (RGD/Apt(f)) were synthesized as described in Section 2.2.2. After VEGF loading to the RGD hydrogels and RGD/Apt(f) hydrogels, the materials were denoted as RGD/VEGF and RGD/VEGF/Apt(f) hydrogels, respectively. Cells seeding and imaging were carried out using the same methods as mentioned above except with the exposure time of 20 ms.
2.2.8. Cell Viability Assay
The viability of HUVECs was measured by quantifying the reduction of the tetrazolium compound 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) into a colored formazan product. Briefly, the tetrazolium compound was first diluted with the complete HUVEC culture medium. The hydrogel samples were then incubated in 350 μL diluted tetrazolium solution for 4 h at 37 °C in a humidified environment with 5% CO2. The absorbance of the reduced solution was read at 490 nm and was referenced by subtracting the absorbance at the reference wavelength (650 nm).
2.2.9. Labeling Aptamers in the Porous Hydrogel
After the growth of HUVECs in the porous hydrogels for 1 and 7 d, the hydrogels were incubated in cold Dulbecco’s Phosphate-buffered Saline (DPBS) and then soaked into 70% ethanol overnight at 4 °C. The hydrogels were subsequently washed with (DPBS) and stained with 500 μL of 1 × 10−6 M complementary sequence labeled with fluorescein (FAM). Free complementary sequences were removed by washing with Phosphate-buffered Saline (PBS). The fluorescence intensity was quantified by Maestro In Vivo Imaging System (CRI, Woburn, MA). The fluorescence intensities of the hydrogels collected at day 1 and day 7 were normalized to that at day 0.
2.2.10. Statistical Analysis
Quantitative data are presented as the mean ± standard error of the mean. Prism v5.0 (GraphPad Software Inc., La Jolla, CA) was used for statistical analysis. One-way analysis of variance (ANOVA) followed by the Bonferroni post-test or two-way ANOVA followed by the Bonferroni post-test was performed for the comparison of multiple groups. Mann Whitney U test was used for comparison between two groups. The result is considered statistically different if p < 0.05.
3. Results and Discussion
3.1. Enhanced Endothelial Cell Attachment in RGD-Functionalized Porous PEG Hydrogels
Cell attachment to the PEG hydrogel was studied both on the hydrogel film and in the 3D hydrogel environment. The examination of cell attachment on the film was used to determine the minimal RGD concentration for the functionalization of the 3D porous hydrogel.
Few HUVECs were observed on the film without RGD (Figure 1). The cell density was ≈2 cells per mm2 hydrogel. This result is consistent with the data reported in the literature showing that PEG is resistant to cell adhesion.[36] By contrast, cell attachment to the PEG hydrogel could be significantly enhanced after the incorporation of RGD into the hydrogel. When the RGD concentration in the hydrogel was increased from 0 × 10−3 to 0.5 × 10−3 M, the density of cells increased from 2 to 7 cells per mm2. This increase of cell attachment reached 19 cells per mm2 when the concentration of RGD was increased to 5 × 10−3 M. The degree of cell attachment did not increase when the concentration of RGD was increased from 5 × 10−3 to 10 × 10−3 M. The results also showed that the cells could attach to the film and exhibit normal elongated endothelial morphology at 5 × 10−3 M of RGD and beyond whereas the cells did not exhibit their normal elongated morphology when the RGD concentration was lower than 5 × 10−3 M (Figure 1A).
Figure 1.
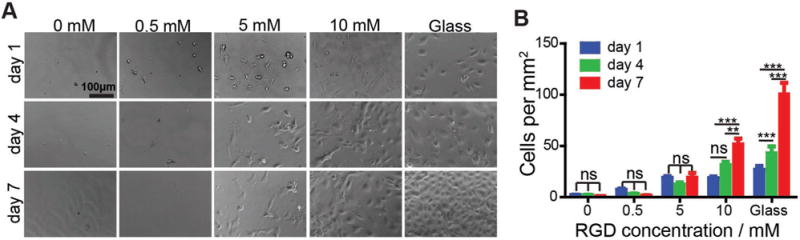
HUVECs adhesion and proliferation on 2D PEG hydrogel film functionalized with RGD peptide. A) Representative images of HUVECs on the PEG hydrogel functionalized with RGD. B) Quantification of HUVECs on the PEG hydrogels. ns: not significant; **, p < 0.01; ***, p < 0.001.
However, the number of the cells did not increase with the time on the film with 5 × 10−3 M of RGD. The number of HUVECs increased with time at 10 × 10−3 M RGD, showing that the cells were able to proliferate on the film functionalized with 10 × 10−3 M RGD. These results demonstrate that the density of cell binding sites in a biomaterial is important in mediating cell attachment and proliferation, which is consistent with the conclusion reported in the previous studies.[37,38] Since 10 × 10−3 M RGD allowed for both attachment and proliferation of HUVECs, this concentration was chosen for the functionalization of the 3D porous PEG hydrogel.
Porous structures of hydrogels are important for cells to migrate and grow during the procedure of tissue regeneration.[39] Moreover, most cells in living organisms grow in 3D microenvironment. Thus, we studied cell growth in the 3D porous RGD-functionalized PEG hydrogel. The porous structures of PEG, PEG functionalized with only RGD, and PEG functionalized with both RGD and the VEGF-binding aptamer (denoted as RGD/Apt(f)) are shown in Figure 2. The images suggest that the hydrogel maintained porous structures after the dual functionalization with RGD and the aptamer.
Figure 2.
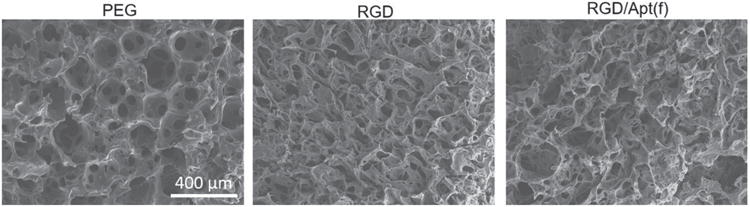
SEM images of porous hydrogels. PEG: 20% PEG hydrogel; RGD: RGD-functionalized 20% PEG hydrogel; RGD/Apt(f): RGD/Aptamer-functionalized 20% PEG hydrogel. The concentrations of RGD and the aptamer were 10 × 10−3 M and 1 × 10−6 M, respectively.
To examine whether HUVECs could attach to the 3D porous PEG hydrogel, the cells were seeded in the PEG hydrogels and stained with Calcein AM for examination under the Maestro In Vivo Imaging System. Little green fluorescence was observed from the nonfunctionalized PEG hydrogel (Figure 3A). By contrast, strong green fluorescence was observed from the RGD-functionalized porous hydrogel. Moreover, the fluorescence intensity at day 7 was greater than those observed at day 1 and 4. It suggests that the cells could survive and grow in the RGD-functionalized 3D porous hydrogel. Scanning laser confocal microscopy was further used to characterize the distribution of the cells in the porous hydrogels (Figure 3B). The microscopic cell observation is consistent with the imaging analysis of the whole hydrogel (Figure 3A), showing that cells could grow inside the RGD-functionalized porous hydrogel (Figure 3B). The confocal microscopy images also showed that the cell distribution in the RGD-functionalized hydrogel was uniform. The MTS result further confirmed that the cells could survive and grow in the RGD-functionalized 3D porous hydrogel.
Figure 3.
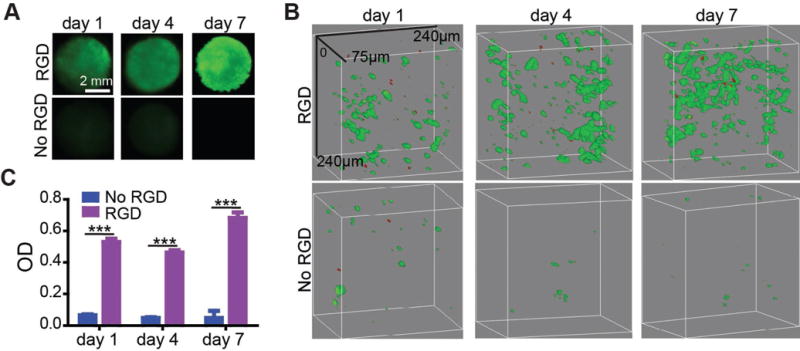
3D culture of HUVECs in RGD-functionalized porous PEG hydrogel. A) Whole hydrogel imaging. HUVECs were stained with Calcein AM. B) Confocal microscopy images of HUVECs in the porous hydrogels. HUVECs were stained with the Live/Dead viability kit. The green color represents live cells and the red color represents dead cells. C) MTS test of HUVEC proliferations. ***, p < 0.001.
RGD, an integrin binding sequence, has two major advantages over large proteins in developing biomaterials for cell adhesion. First, short peptides are simple and cheap to synthesize.[40] Second, peptides can maintain their bioactivity in harsh chemical conjugation conditions that can easily cause protein denaturation.[41] Thus, peptides such as RGD have been widely used to functionalize a variety of hydrogels.[42–46] However, no study has been carried out to demonstrate the synthesis and functions of porous hydrogels functionalized with both peptides and nucleic acid aptamers. After demonstrating the function of the RGD-functionalized PEG hydrogel, we further examined the functions of aptamer-functionalized and aptamer/RGD-functionalized hydrogels.
3.2. Aptamer-Functionalized Porous Hydrogels for Strong Sequestration and Controlled Release of Bioactive VEGF
The natural ECM can provide cells with biochemical cues through retaining and releasing growth factors.[47,48] For instance, VEGF can be sequestered by ECM components such as heparin sulfate[49] and fibrinogen,[50] and is released locally to stimulate the growth of endothelial cells.[51] However, synthetic hydrogels without specific biofunctionalization do not have this capability. Thus, it is important to incorporate growth factor-binding ligands into the hydrogel network for retaining and releasing growth factors. This requirement can be satisfied by functionalizing synthetic hydrogels with nucleic acid aptamers. Nucleic acid aptamers are an emerging class of ligands selected from synthetic RNA/DNA libraries with high affinities and specificities.[1,2] Aptamers have been used to functionalize a variety of hydrogels for biomedical applications.[32,34,35,52–55] In this work, the PEG hydrogel was functionalized with an anti-VEGF aptamer to control VEGF release.
The anti-VEGF RNA aptamer (Figure 4A) bearing acrylate was incorporated into the PEG hydrogel via free radical polymerization. A 10-nucleotide spacer was added between the acrylate group and the aptamer to reduce the steric hindrance of the aptamer in binding to VEGF. Without aptamer functionalization, 15% ± 8% of the loaded VEGF was retained within the hydrogel after the incubation of the hydrogel in the release medium for 4 h (Figure 4B). This observation confirms that the porous PEG hydrogel is highly permeable, which is beneficial for cell survival owing to the free transport of oxygen and nutrients but not beneficial for the retention and sustained release of growth factors. By contrast, in the presence of the aptamer, the retention of VEGF could be significantly improved. When the molar ratio of aptamer-to-VEGF was increased from 0 to 0.5:1, the amount of retained VEGF in the hydrogel was increased to 44% ± 4%. At the molar ratio of 5:1, the amount of retained VEGF was further increased to 98% ± 0.7%. After the demonstration of aptamer-mediated VEGF retention, sustained VEGF release from the porous hydrogel was studied. As shown in Figure 4C, the porous PEG hydrogels functionalized with Apt(f) could release VEGF in a sustained manner. The cumulative release of VEGF in the Apt(f2.5) group was 30.24% in one week.
Figure 4.
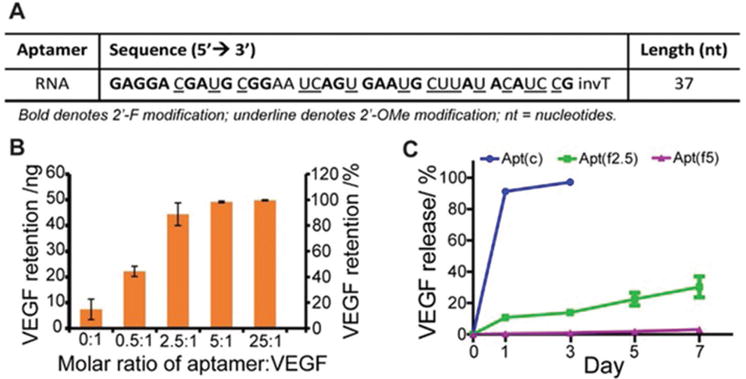
VEGF retention and release. A) The sequence of the anti-VEGF aptamer. B) Effect of the amount of aptamer on VEGF retention. C) Sustained VEGF release from hydrogels. Apt(f): functional aptamer; Apt(c): control aptamer.
As the maintenance of protein bioactivity is important to the function of a protein-releasing biomaterial, we examined whether VEGF in the aptamer-functionalized hydrogel could maintain bioactivity. To do so, the hydrogel was purposely incubated in the release medium for another week after the completion of the controlled release test, and then used for VEGF extraction and collection. The bioactivity of VEGF was examined using the tube formation assay.[56] In the absence of VEGF, the HUVECs remained dispersed (Figure 5). By contrast, in the presence of VEGF collected from the hydrogel, the cells formed spindles and cell networks. Importantly, the total tube length of HUVECs cultured with the VEGF extracted from the hydrogel was 6.1 mm mm−2 whereas that with the stock VEGF was 8.2 mm mm−2. These results showed that VEGF in the aptamer-functionalized porous hydrogel could maintain high bioactivity.
Figure 5.
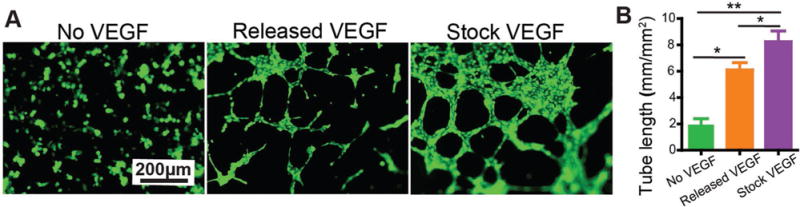
Bioactivity of the released VEGF. A) Tube formation. HUVECs were cultured with M200 containing no VEGF, M200 containing VEGF (10 ng mL−1) collected from the hydrogel or M200 containing stock VEGF (10 ng mL−1). The cells were stained with Calcein AM. B) Quantitative measurement of tube length. **, p < 0.01; *, p < 0.05.
3.3. HUVEC Growth in the Aptamer- and RGD-Functionalized Porous Hydrogel
After the demonstration of the functions of RGD and anti-VEGF aptamer individually, we synthesized the dual-functional hydrogel using both RGD and the aptamer and examined the growth of HUVECs in this porous hydrogel. Since cells can release a variety of molecules that may cause the degradation of the aptamer, it is important to examine the effect of cell presence and growth on the stability of the aptamer. The aptamers in the hydrogels were stained with FAM-labeled complementary sequences to examine their stability (Figure 6A,B). The fluorescence intensity of the RGD/VEGF/Apt(f) group decreased by 8% at day 7. This result suggests that the anti-VEGF aptamer could maintain high stability in the presence of living cells. Indeed, the anti-VEGF aptamer used herein was composed of 2′-F-modified nucleotides and 2′-OMe-modified nucleotides. These modifications have been shown to significantly protect aptamers from degradation in biological fluids.[57] We did not study the biodegradation of the PEG hydrogel or its effect on cell behavior since the hydrogel was synthesized with PEGDA that is not biodegradable. Biodegradable PEG hydrogels can be synthesized by using biodegradable enzyme-sensitive crosslinkers, which will be studied in the future work.
Figure 6.
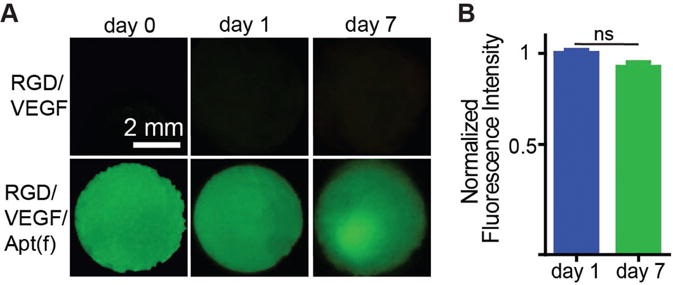
Staining of aptamers in the hydrogel. A) Imaging of the whole hydrogels treated with an FAM-labeled complementary sequence after the hydrogels were incubated in the cell culture media with cells for 0, 1, and 7 d. RGD/VEGF: RGD-functionalized VEGF-loaded hydrogel. RGD/VEGF/Apt(f): RGD/aptamer-functionalized VEGF-loaded hydrogel. B) Fluorescence intensity of the RGD/VEGF/Apt(f) hydrogels at day 1 and 7. The values were normalized to that of day 0. ns: not significant.
To test how the dual functionalization benefits cell growth, HUVECs were seeded in the RGD/aptamer-functionalized VEGF-loaded porous hydrogel and stained with the Calcein AM. The results from both the whole hydrogel imaging and the microscopy imaging demonstrate that the cells could grow in the hydrogels (Figure 7). Importantly, the fluorescence intensity of the cells in the RGD/VEGF/Apt(f) group was twice as much as that of the RGD/VEGF group at day 7 (Figure 7A,B). Moreover, the cell distribution remained uniform during the procedure of cell growth in the dual-functional hydrogel (Figure 7C). These data clearly demonstrate that aptamer-mediated VEGF retention and release were important in promotion of cell growth in the RGD-functionalized porous hydrogels.
Figure 7.
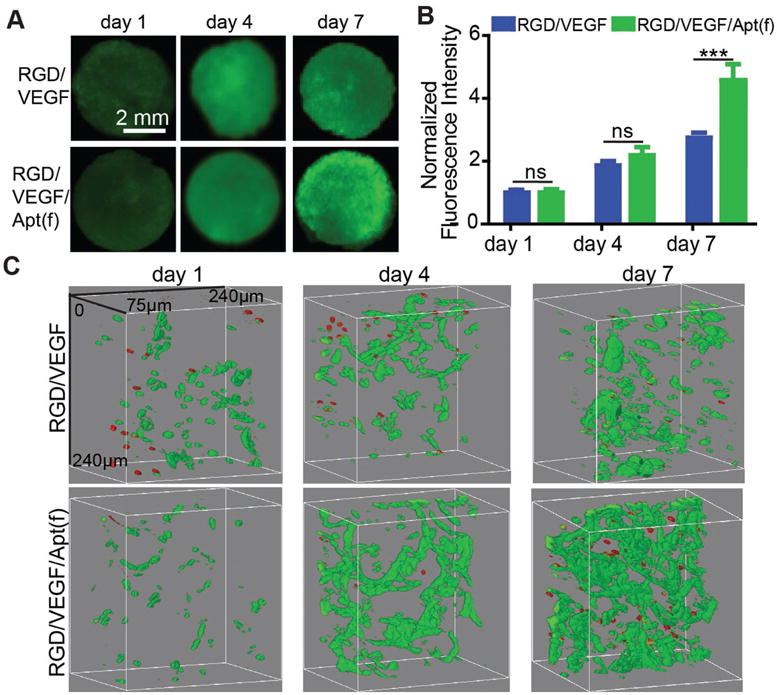
HUVEC growth in the dual-functional 3D porous hydrogels. A) Imaging of the whole hydrogel. RGD/VEGF: RGD-functionalized VEGF-loaded hydrogel. RGD/VEGF/Apt(f): RGD/aptamer-functionalized VEGF-loaded hydrogel. HUVECs were stained with Calcein AM. B) Quantification of fluorescence intensity of the whole hydrogels. C) Confocal microscopy images of the hydrogels. Cells were stained with the Live/Dead staining kit. Green: live cells; red: dead cells. ***: p < 0.001; ns: not significant.
The porous PEG hydrogels were functionalized with two synthetic biomolecules, RGD and the anti-VEGF aptamer. RGD can bind to integrins on the cell surface, functioning as a biophysical cue for the attachment of endothelial cells. The anti-VEGF aptamer can bind to VEGF for retention and sustained release. VEGF is an essential growth factor in the ECM for promotion of angiogenesis that is important to maintain effective transport of oxygen, nutrients, and wastes in tissues. As the data have shown that HUVECs could survive and grow in the dual-functional PEG hydrogel (Figure 7), it is envisioned that this new hydrogel system may find applications in the field of therapeutic angiogenesis.
4. Conclusions
In summary, a dual-functional porous hydrogel has been synthesized using RGD and the anti-VEGF aptamer. The endothelial cells can attach to the dual-functional hydrogel and VEGF can be retained and released in a sustained manner. More importantly, in the presence of both RGD and the aptamer, the endothelial cells can survive and proliferate in the dual-functional porous hydrogel. Therefore, this work has successfully demonstrated that molecules selected from different types of synthetic libraries with the ability of molecular recognition can be integrated into one biomolecular material, which will enrich the development of novel biomaterials. This peptide- and aptamer-functionalized hydrogel system may find broad applications in the fields of 3D cell culture, tissue modeling and regenerative medicine, which will be performed in the future.
Acknowledgments
Research reported in this publication was supported in part by grants of the U.S. National Science Foundation (DMR-1332351) and the National Heart, Lung, Blood Institute of the National Institutes of Health (HL122311). X.W. thanks the Talent Invitation Program of Dalian (TPDL2015) for support. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Nan Zhao, Department of Biomedical Engineering, The Pennsylvania State University, University Park, PA 16802, USA.
Dr. Mark R. Battig, Department of Biomedical Engineering, The Pennsylvania State University, University Park, PA 16802, USA.
Ming Xu, Center for Molecular Immunology and Infectious Disease, Department of Veterinary and Biomedical Sciences, The Pennsylvania State University, University Park, PA 16802, USA.
Prof. Xiuli Wang, College of Basic Medical Science, Dalian Medical University, Dalian 116044, China
Prof. Na Xiong, Center for Molecular Immunology and Infectious Disease, Department of Veterinary and Biomedical Sciences, The Pennsylvania State University, University Park, PA 16802, USA
Prof. Yong Wang, Department of Biomedical Engineering, The Pennsylvania State University, University Park, PA 16802, USA
References
- 1.Colas P, Cohen B, Jessen T, Grishina I. Nature. 1996;380:548. doi: 10.1038/380548a0. [DOI] [PubMed] [Google Scholar]
- 2.Ellington AD, Szostak JW. Nature. 1990;346:818. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 3.Tuerk C, Gold L. Science. 1990;249:505. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 4.Ruckman J, Green LS, Beeson J, Waugh S, Gillette WL, Henninger DD, Claesson-Welsh L, Janjic N. J Biol Chem. 1998;273:20556. doi: 10.1074/jbc.273.32.20556. [DOI] [PubMed] [Google Scholar]
- 5.Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, Bacallado SA, Nguyen DN, Fuller J, Alvarez R. Nat Biotechnol. 2008;26:561. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keefe AD, Pai S, Ellington A. Nat Rev Drug Discovery. 2010;9:537. doi: 10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koivunen E, Wang B, Ruoslahti E. Nat Biotechnol. 1995;13:265. doi: 10.1038/nbt0395-265. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Zahner D, Su Y, Gruen C, Davidson G, Levkin PA. Biomaterials. 2012;33:8160. doi: 10.1016/j.biomaterials.2012.07.044. [DOI] [PubMed] [Google Scholar]
- 9.Wang M, Alberti K, Sun S, Arellano CL, Xu Q. Angew Chem. 2014;126:2937. doi: 10.1002/anie.201311245. [DOI] [PubMed] [Google Scholar]
- 10.Wang M, Zuris JA, Meng F, Rees H, Sun S, Deng P, Han Y, Gao X, Pouli D, Wu Q. Proc Natl Acad Sci USA. 2016;113:2868. doi: 10.1073/pnas.1520244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hook AL, Anderson DG, Langer R, Williams P, Davies MC, Alexander MR. Biomaterials. 2010;31:187. doi: 10.1016/j.biomaterials.2009.09.037. [DOI] [PubMed] [Google Scholar]
- 12.Hook AL, Chang CY, Yang J, Luckett J, Cockayne A, Atkinson S, Mei Y, Bayston R, Irvine DJ, Langer R. Nat Biotechnol. 2012;30:868. doi: 10.1038/nbt.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mei Y, Saha K, Bogatyrev SR, Yang J, Hook AL, Kalcioglu ZI, Cho SW, Mitalipova M, Pyzocha N, Rojas F. Nat Mater. 2010;9:768. doi: 10.1038/nmat2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urquhart AJ, Anderson DG, Taylor M, Alexander MR, Langer R, Davies MC. Adv Mater. 2007;19:2486. [Google Scholar]
- 15.Zonca MR, Yune PS, Heldt CL, Belfort G, Xie Y. Macromol Biosci. 2013;13:177. doi: 10.1002/mabi.201200315. [DOI] [PubMed] [Google Scholar]
- 16.Ivarsson Y, Arnold R, McLaughlin M, Nim S, Joshi R, Ray D, Liu B, Teyra J, Pawson T, Moffat J. Proc Natl Acad Sci USA. 2014;111:2542. doi: 10.1073/pnas.1312296111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun MG, Seo MH, Nim S, Corbi-Verge C, Kim PM. Sci Adv. 2016;2:e1600692. doi: 10.1126/sciadv.1600692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badylak SF, Freytes DO, Gilbert TW. Acta Biomater. 2009;5:1. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman AS. Adv Drug Delivery Rev. 2012;64:18. [Google Scholar]
- 20.Nguyen KT, West JL. Biomaterials. 2002;23:4307. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Wu D, Mutschler MA, Chu CC. Adv Funct Mater. 2012;22:3815. [Google Scholar]
- 22.Li X, Fu M, Wu J, Zhang C, Deng X, Dhinakar A, Huang W, Qian H, Ge L. Acta Biomater. 2017;51:294. doi: 10.1016/j.actbio.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Zustiak SP, Leach JB. Biomacromolecules. 2010;11:1348. doi: 10.1021/bm100137q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin CC, Anseth KS. Pharm Res. 2009;26:631. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang XH, Li S, Liang L, Xu XD, Zhang XZ, Jiang FG. J Biomater Sci Polym Ed. 2013;24:1185. doi: 10.1080/09205063.2012.745714. [DOI] [PubMed] [Google Scholar]
- 26.Rosales AM, Anseth KS. Nat Rev Mater. 2016;1:15012. doi: 10.1038/natrevmats.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burdick JA, Anseth KS. Biomaterials. 2002;23:4315. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 28.Almany L, Seliktar D. Biomaterials. 2005;26:2467. doi: 10.1016/j.biomaterials.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 29.Ruoslahti E. Annu Rev Cell Dev Biol. 1996;12:697. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 30.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Cell. 1993;73:309. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 31.Hersel U, Dahmen C, Kessler H. Biomaterials. 2003;24:4385. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 32.Gaddes ER, Gydush G, Li S, Chen N, Dong C, Wang Y. Biomacromolecules. 2015;16:1382. doi: 10.1021/acs.biomac.5b00165. [DOI] [PubMed] [Google Scholar]
- 33.Richards E, Li S, Chen N, Battig MR, Wang Y. Biomacromolecules. 2014;15:4561. doi: 10.1021/bm501347s. [DOI] [PubMed] [Google Scholar]
- 34.Battig MR, Huang Y, Chen N, Wang Y. Biomaterials. 2014;35:8040. doi: 10.1016/j.biomaterials.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Battig MR, Chen N, Gaddes ER, Duncan KL, Wang Y. Biomacromolecules. 2016;17:778. doi: 10.1021/acs.biomac.5b01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen N, Zhang Z, Soontornworajit B, Zhou J, Wang Y. Biomaterials. 2012;33:1353. doi: 10.1016/j.biomaterials.2011.10.062. [DOI] [PubMed] [Google Scholar]
- 37.Yang F, Williams CG, Wang D-A, Lee H, Manson PN, Elisseeff J. Biomaterials. 2005;26:5991. doi: 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Tugulu S, Silacci P, Stergiopulos N, Klok HA. Biomaterials. 2007;28:2536. doi: 10.1016/j.biomaterials.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Keskar V, Gandhi M, Gemeinhart EJ, Gemeinhart RA. J Tissue Eng Regener Med. 2009;3:486. doi: 10.1002/term.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams DF. Biomaterials. 2011;32:4195. doi: 10.1016/j.biomaterials.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 41.Bellis SL. Biomaterials. 2011;32:4205. doi: 10.1016/j.biomaterials.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartneck M, Skazik C, Paul NE, Salber J, Klee D, Zwadlo-Klarwasser G. Macromol Biosci. 2014;14:411. doi: 10.1002/mabi.201300362. [DOI] [PubMed] [Google Scholar]
- 43.Hern DL, Hubbell JA. J Biomed Mater Res. 1998;39:266. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 44.Massia SP, Hubbell JA. J Cell Biol. 1991;114:1089. doi: 10.1083/jcb.114.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneider GB, English A, Abraham M, Zaharias R, Stanford C, Keller J. Biomaterials. 2004;25:3023. doi: 10.1016/j.biomaterials.2003.09.084. [DOI] [PubMed] [Google Scholar]
- 46.Wang W, Guo L, Yu Y, Chen Z, Zhou R, Yuan Z. J Biomed Mater Res Part A. 2015;103:1703. doi: 10.1002/jbm.a.35306. [DOI] [PubMed] [Google Scholar]
- 47.Parker A, Clarke JB, Busby WH, Clemmons DR. J Biol Chem. 1996;271:13523. doi: 10.1074/jbc.271.23.13523. [DOI] [PubMed] [Google Scholar]
- 48.Schultz GS, Wysocki A. Wound Repair Regener. 2009;17:153. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 49.Park JE, Keller GA, Ferrara N. Mol Biol Cell. 1993;4:1317. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sahni A, Francis CW. Blood. 2000;96:3772. [PubMed] [Google Scholar]
- 51.Ferrara N. Mol Biol Cell. 2010;21:687. doi: 10.1091/mbc.E09-07-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Battig MR, Soontornworajit B, Wang Y. J Am Chem Soc. 2012;134:12410. doi: 10.1021/ja305238a. [DOI] [PubMed] [Google Scholar]
- 53.Soontornworajit B, Zhou J, Snipes MP, Battig MR, Wang Y. Biomaterials. 2011;32:6839. doi: 10.1016/j.biomaterials.2011.05.074. [DOI] [PubMed] [Google Scholar]
- 54.Li S, Gaddes ER, Chen N, Wang Y. Angew Chem. 2015;127:6055. doi: 10.1002/anie.201500397. [DOI] [PubMed] [Google Scholar]
- 55.Li S, Chen N, Gaddes ER, Zhang X, Dong C, Wang Y. Sci Rep. 2015;5:14297. doi: 10.1038/srep14297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arnaoutova I, George J, Kleinman HK, Benton G. Angiogenesis. 2009;12:267. doi: 10.1007/s10456-009-9146-4. [DOI] [PubMed] [Google Scholar]
- 57.Cummins LL, Owens SR, Risen LM, Lesnik EA, Freier SM, McGee D, Guinosso CJ, Cook PD. Nucleic Acids Res. 1995;23:2019. doi: 10.1093/nar/23.11.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]


