Abstract
Two spectral editing techniques for simultaneously detecting glutathione (GSH) and lactate (Lac) in the human brain at 3T are described and evaluated. These methods, ‘sMEGA’ and ‘DEW’, were optimized to detect GSH and Lac simultaneously at 3T, using density-matrix simulations and validation in phantoms. Simulations to test for co-edited metabolites within the detected GSH region of the spectrum were also performed. In vivo data were acquired in the midline parietal region of seven subjects using both methods and compared to conventional MEGA-PRESS acquisitions of GSH and Lac.
Simulations and phantom experiments show that sMEGA and DEW have a high editing efficiency for both GSH and Lac. In the phantom, the editing efficiency of GSH was >88% relative to a conventional GSH MEGA-PRESS acquisition while for Lac, the editing efficiency was >95% relative to a conventional Lac MEGA-PRESS acquisition. Simulations also show that the editing efficiency of both methods is comparable to separate MEGA-PRESS acquisitions of the same metabolites. In addition, simulations and in vivo spectra show that at an echo time of 140 ms there is a partial overlap between Cr and GSH peaks and that NAA/NAAG are sufficiently resolved from GSH. In vivo measurements show that both sMEGA and DEW edit GSH and Lac reliably with the same editing efficiency as conventional MEGA-PRESS acquisitions of the same metabolites with measured GSH integrals of 2.23 ± .51, 2.31 ± .38, 2.38 ± .53 and measured Lac integrals of 1.72 ± .67, 1.55 ± .35, and 1.53 ± .54 for MEGA-PRESS, DEW, and sMEGA respectively. Simultaneous detection of GSH and Lac using sMEGA and DEW is possible at 3T with high editing efficiency.
Keywords: brain, edited magnetic resonance spectroscopy, glutathione, lactate
Graphical Abstract
Two spectral editing techniques for simultaneously detecting glutathione (GSH) and lactate (Lac) in the human brain at 3T are described and evaluated. Simultaneous spectral editing methods (‘sMEGA’ and ‘DEW’) were optimized to detect GSH and Lac simultaneously, simulations, phantom, and in vivo experiments show that sMEGA and DEW have a high GSH and Lac editing efficiency and that the editing efficiency of both methods is comparable to separate MEGA-PRESS acquisitions of the same metabolites.
Introduction
Glutathione (GSH) is the brain’s main antioxidant, functional impairment of which is associated with oxidative stress (1, 2). Lactate (Lac) is an indicator of non-oxidative glycolysis, elevation of which is considered an indicator of metabolic abnormalities or oxygen deficiency (3–5). Both metabolites can be detected non-invasively by in vivo 1H magnetic resonance spectroscopy, and have been implicated in the pathophysiology of various brain pathologies such as schizophrenia (6–9), bipolar disorder (10–13), obsessive-compulsive disorder (14), and chronic fatigue syndrome (15). Detection of either metabolite using MR spectroscopy, however, is not trivial due to their relatively low in vivo concentrations under normal conditions. Thus, they are often detected using spectral editing techniques in order to separate them from the larger, overlapping signals of other more abundant compounds (16).
‘J-difference’ editing using the MEGA-PRESS sequence (17) is currently the most widely used spectral editing technique in vivo. Traditionally, J-difference editing sequences are designed to only detect one molecule at a time. However, if different molecules have coupled spins that have similar chemical shifts and which lie within the bandwidth of the editing pulse, it is possible to simultaneously edit more than one molecule. A well known example of this is the co-editing of glutamate and glutamine (Glx’) in experiments designed to primarily edit GABA, since the coupled Glx resonances at around 2.1 ppm are usually also affected by the GABA editing pulse applied at 1.9 ppm (18). Another approach for editing two compounds, dubbed ‘Double Editing With (DEW) MEGA-PRESS’ (19), alternates editing pulse frequencies between the targeted resonances of two molecules in the acquisitions traditionally considered as ‘ON’ and ‘OFF’, resulting in signals from both molecules being present in the difference spectrum, but with opposite polarity. DEW was originally demonstrated for glutathione and ascorbate (the ‘oxidant profile’) but can also be adopted for other combinations of compounds, such as glutathione and lactate.
Here, these two approaches (i.e. simultaneous editing with less selective editing pulses): MEGA-PRESS with sinc pulses referred to as sinc-MEGA (sMEGA) and DEW with more selective editing pulses are developed and compared for the simultaneous detection of glutathione and lactate. In addition to optimizing editing pulse characteristics, it is also important to consider what TE value is best for detecting both compounds (if each compound optimally edits at a different TE), and also how unwanted co-edited molecules may vary with TE. The two methods are compared in terms of sensitivity, co-editing of overlapping compounds, as well as to conventional single-metabolite MEGA-PRESS acquisitions in vivo.
Methods
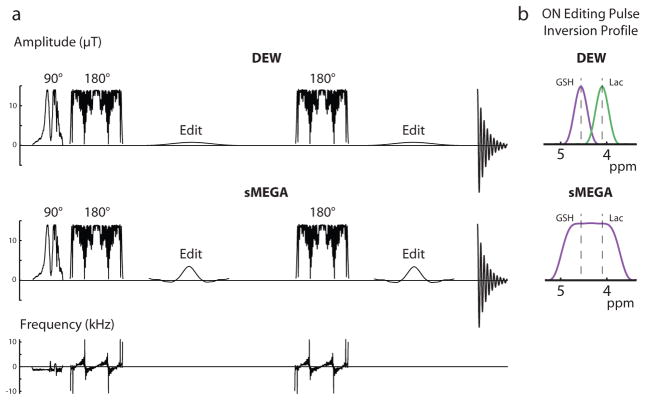
All experiments were performed on a Philips 3T ‘Achieva’ scanner equipped with body coil transmit (B1 = 13.5 μT) and a 32-channel head coil, using a MEGA-PRESS sequence as the starting point for sequence development. The editing sequences used high bandwidth, frequency modulated slice selective refocusing pulses (‘fmref07’, BW = 2.2 kHz) to minimize signal losses associated with chemical shift displacement effects as shown in Figure 1a (3, 20). Thus the chemical shift displacement error (CSDE) between the GSH spins at which the editing pulse is applied at 4.56 ppm and the observed spins at 2.95 ppm is 9.7%. For Lac, the chemical shift displacement error between the spins at which the editing pulse is applied at 4.1 ppm and the observed spins at 1.3 ppm is 16.2%. The basic concept for dual- (or multi-) metabolite editing is that the editing pulses should invert the target coupled resonances of each molecule to be detected; in the case of the sMEGA this was achieved by using a relatively non-selective sinc-derived editing pulse (25 ms duration and a bandwidth 160 Hz and applied at 4.35 ppm in the ON case and at 10 ppm in the OFF case) with a rectangular inversion envelope to edit both GSH (4.56 ppm) and Lac (4.1 ppm) (Figure 1a and b). To adapt the DEW method (19) for simultaneous GSH and Lac detection, Bloch equation simulations were performed to determine the editing pulse frequency selectivity needed so that the 4.56 ppm ON pulse doesn’t significantly invert the 4.1 ppm Lac peak and vice versa. Thus in the case of the DEW method, selective sinc-Gaussian editing pulses (30 ms duration with a bandwidth of 40 Hz) were applied alternatively on Lac (4.1 ppm) and GSH (4.56 ppm) in the ‘ON’ and ‘OFF’ acquisitions respectively (Figure 1a and b). As can be seen from the inversion profiles of the editing pulses shown in manuscript figure 1b, the editing pulses are selective enough to avoid the nearby resonance of the other edited metabolite even in the presence of minor B0 drift. Considering the low bandwidth of these editing pulses, it is estimated that the maximal tolerable B0 drift to maintain at least 90% editing efficiency is 10 Hz. For the conventional MEGA-PRESS acquisitions, 20 ms sinc-Gaussian editing pulses with a bandwidth of 60 Hz were applied at 10 ppm in the OFF acquisition and at 4.1 ppm in the ON acquisition for Lac MEGA-PRESS or at 4.56 ppm in the ON acquisition for GSH MEGA-PRESS.
Figure 1.
Schematic diagram of the three different GSH-Lac editing schemes. (a) The RF pulse sequence of both the DEW method and the sMEGA method. Both methods use high-bandwidth frequency-modulated refocusing pulses but use different editing pulses. (b) The sMEGA method uses an editing pulse with a more rectangular profile to invert both the GSH and Lac spins in the ON sub-acquisition. In the DEW method, more selective editing pulses alternate between ON-GSH or ON-Lac in sub-acquisitions.
For spectral editing of lactate, echo times of 135–144 ms are most commonly used (3, 21, 22) corresponding to TE = 1/J, where J = ~7 Hz (23). There has been some discussion in the literature as to the optimum TE for editing GSH; some publications use relatively short TE (e.g. 68 ms (24), but experiments in phantoms and simulations suggest that maximum J-difference editing is achieved around TE 140–160 ms (20). However experimental studies in vivo have shown a relatively flat dependency of the GSH signal as a function of TE, suggesting that the T2 of GSH may be quite short (e.g. ~90 ms), resulting in signal loss from T2-relaxation tending to offset the effects of J-modulation (20). In the current study, experiments were performed at TE 140 ms for both lactate and GSH.
Spectral Simulations
Density-matrix simulations were performed using ‘FID-A’, a MATLAB-based spectral simulation package (25), with chemical shifts and coupling constants taken from the literature (23, 26, 27). The excitation pulse was assumed to be an ideal 90˚ rotation around the x-axis, but editing and slice-selective refocusing pulses were simulated using actual pulse waveforms. Both methods were simulated for both the GSH and Lac spin systems, and compared to conventional MEGA-PRESS acquisitions of the same metabolites.
To assess the degree that other brain metabolites may co-edit with Lac and GSH, simulations of metabolites with coupled spins that occur within the bandwidth of the editing pulse were also performed. These metabolites included phosphoethanolamine (PE), N-acetyl-aspartate (NAA), N-acetyl aspartyl glutamate (NAAG), creatine (Cr) and aspartate (Asp). Particular attention was paid to NAA and NAAG (including modeling their TE-dependence, ranging from 110 to 160 ms) since these compounds are known to prominently co-edit with GSH, and have the potential to overlap with the detected GSH resonance at 2.95 ppm. Although traditionally Cr is not thought to edit in J-difference spectra, in fact a small coupling (J ≈ 0.3 Hz) does exist between the Cr CH2 and CH3 groups (26, 27), so a 3.0 ppm Cr peak will appear in the difference spectrum since the editing pulse partially inverts the coupled 3.9 ppm Cr CH2 protons (see below). Simulations for each compound were weighted according to their concentration values in literature (23).
Phantom Experiments
sMEGA, DEW, and MEGA-PRESS experiments were performed in 1L phantoms with 26 mM Lac (pH = 7) and 14 mM GSH (pH = 7.1) in separate samples. Scans were performed with TR/TE = 2s/140 ms; GSH and Lac phantom data were acquired in a (3.5 cm)3 voxel with 128 averages. Prospective frequency correction for B0 field drift during the scan was performed based on the frequency of a non-suppressed water reference scan collected once every 8 averages. (28). GSH and Lac editing efficiencies for both sMEGA and DEW were also calculated as a percentage of separate MEGA-PRESS acquisitions for the two metabolites.
In Vivo Experiments
Seven healthy volunteers (three female, age 29 ± 10 years) gave informed written consent after local Institutional Review Board approval. Dual-edited data was collected from 5 subjects, and conventional GSH MEGA-PRESS data and Lac MEGA-PRESS data were also acquired in 5 subjects, three of which overlapped with the subjects from whom dual-edited data was collected. All were acquired with 320 signal averages using VAPOR water suppression. MEGA-PRESS and sMEGA data were acquired in a (4 cm)3 midline posterior frontoparietal region (Figure 7a) while DEW data were acquired in a (3 cm)3 in the same region. Other parameters were the same as in the phantom experiments. As in the phantom experiments, frequency correction for B0 field drift during the scan was performed based on the frequency of the water-unsuppressed scan acquired every 16 averages (28).
The ‘Gannet’ program (29, 30) was used to frequency-and-phase-correct individual transients, based on the 2.0 ppm N-acetyl peak of NAA, before the difference spectra were calculated. In the dual-edited spectra, a Cr peak at 3.03 ppm was fit with a Lorentzian function since the lineshape of Cr was expected to be singlet-like due the coupling between the Cr CH2 and CH3 groups being relatively small and the GSH peak was fit with a Gaussian at 2.95 ppm due to its doublet-like signals and hence, broader lineshape. In the GSH MEGA-PRESS acquisitions, only a Gaussian was fit to the peak at 2.95 ppm. For fitting of lactate, the region of the spectrum around 1.3 ppm was fit with two Gaussian functions for the macromolecule (MM) resonances at 1.24 and 1.43 ppm (coupled to the 4.23 and 4.30 ppm resonances respectively (31)), and two Lorentzians to fit a doublet for the Lac peak at 1.33 ppm with a 7 Hz splitting. The percentage standard error in the amplitude coefficient of the GSH fits and in the area coefficient of the Lac fits were calculated to determine the uncertainty in the fitting. The GSH and Lac integrals were then normalized by an internal water reference signal obtained from the same localized voxel. To test for differences in GSH and Lac editing between the three different methods, two-tailed paired t-tests were performed with statistical significance defined as p < 0.05. The linewidth of the water peak from the water references in the in vivo acquisitions was also measured to evaluate the amount of B0 inhomogeneity present in each scan.
Results
Simulations and experiments in phantoms (shown in Figure 2) confirm that both the sMEGA and DEW methods successfully edit both GSH and Lac with similar sensitivity. In addition, both simulations and phantom experiments show that both methods edit GSH and Lac with a high editing efficiency of at least 88% for GSH and at least 95% for Lac relative to conventional MEGA-PRESS acquisitions, which is also reflected in the ON and OFF subspectra of all three methods (Supplementary Figure 1). In addition, these methods can be performed without any increases in specific absorption rate values which was 1.32 w/kg for all three methods. While both methods co-edit the same metabolites: PE, Cr, NAA, NAAG, and aspartate, sMEGA co-edits most of these metabolites to a significantly greater degree than the DEW method because of its less selective editing pulse profiles. Of these co-edited metabolites, only the Cr peak overlaps with that of the edited GSH signal (as shown in Figure 3). In sMEGA, this co-edited Cr peak has the same polarity as GSH, but in DEW it has the opposite polarity due to it being co-edited by the Lac editing pulse but not by the GSH editing pulse.
Figure 2.
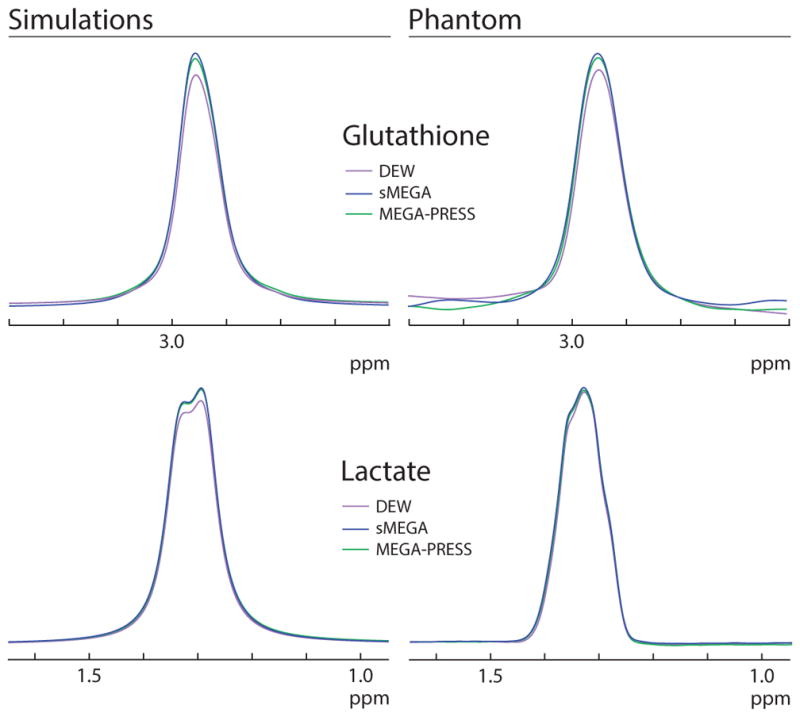
Comparison of the edited GSH and Lac signal in the three methods in both simulations and phantom experiments. For both GSH and Lac, the sMEGA method (blue) performs better than the DEW method (purple). However, both methods maintain a high editing efficiency comparable to that of separate MEGA-PRESS acquisitions of the same metabolites.
Figure 3.
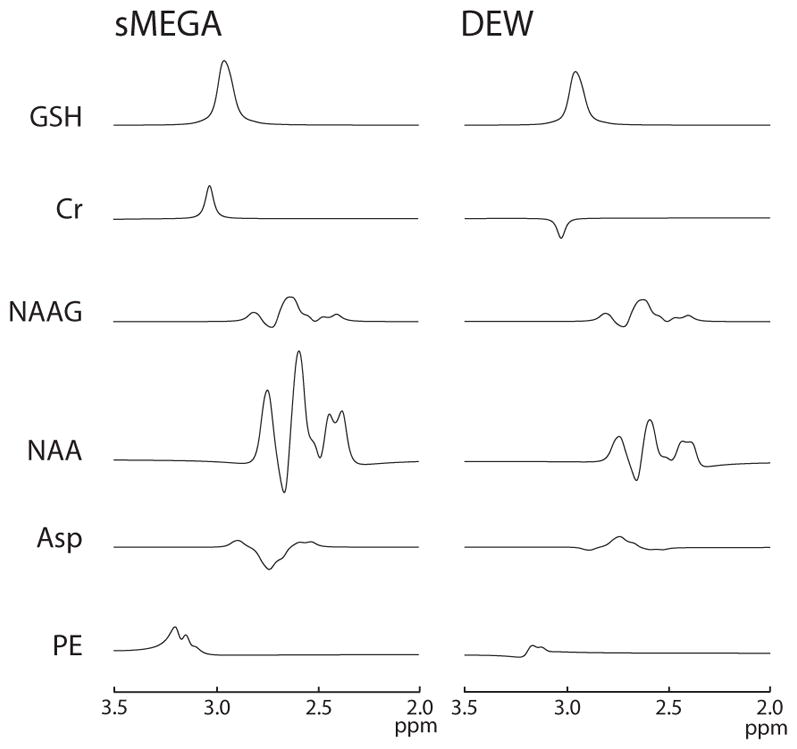
Simulations of co-edited metabolites for the GSH region of the spectrum for sMEGA and DEW: PE, NAA, NAAG, and aspartate (Asp), and Cr are plotted for both methods. sMEGA and DEW co-edit the same metabolites, but with greater intensity in MEGA because of its less selective editing pulses. The co-edited Cr peak has the same sign as the GSH peak in sMEGA, but the opposite polarity in DEW.
In vivo spectra (as shown in Figure 4) also confirm that the sMEGA sequence edits appreciably more NAA (and NAAG) than DEW. However, there is also an extra co-editing of Cr at 3.02 ppm in the spectra in both methods (as seen in Figure 4). As in the simulations, sMEGA has a co-edited Cr peak that is the same polarity as GSH, but opposite polarity in the DEW method. In addition, there is co-editing of MM2 at 1.24 ppm and MM3 at 1.43 ppm in all three methods that overlaps the Lac peak at 1.31 ppm. After fitting out the co-edited Cr peak and macromolecules, it can be seen in Table 1 that sMEGA and DEW have about equal GSH and Lac editing efficiency and both methods edit GSH and Lac to a similar degree as the conventional MEGA-PRESS acquisition in all subjects. The GSH fitting standard errors were 2.4% ± 0.7% for sMEGA, 0.4% ± 0.01% for MEGA-PRESS, and 1.3% ± 0.4% for DEW while the Lac fitting standard errors were 18.2% ± 4.9% for sMEGA, 16.1% ± 7.4% for MEGA-PRESS, 8.9% ± 3.4% for DEW. Differences in measured metabolite integrals between the three methods were found to be not statistically significant between overlapping subjects and all subjects. In addition, the frequency of the water peak was 4.7 ppm (mean) ± 0.007 ppm (standard deviation) over the different in vivo acquisitions and the total B0 drift over the different in vivo acquisitions was 0.9 Hz (mean) ± 0.4 Hz (standard deviation). Thus, minimal B0 drift was present in the scans and did not affect the editing efficiency of the DEW acquisition. This is also reflected in the in vivo ON and OFF subspectra where it can be seen that the large NAA singlet at 2.0 ppm is aligned in all three methods (supplementary figure 1). In addition, the linewidths of the water peak ranged from ~5 – 8 Hz with a 6.24 Hz (mean) ± 0.97 Hz (standard deviation) indicating that the B0 inhomogeneity was minimal and did not significantly affect the editing efficiency of any of the editing methods.
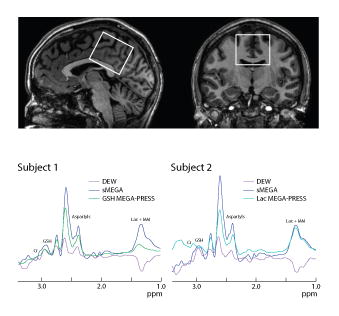
Figure 4.
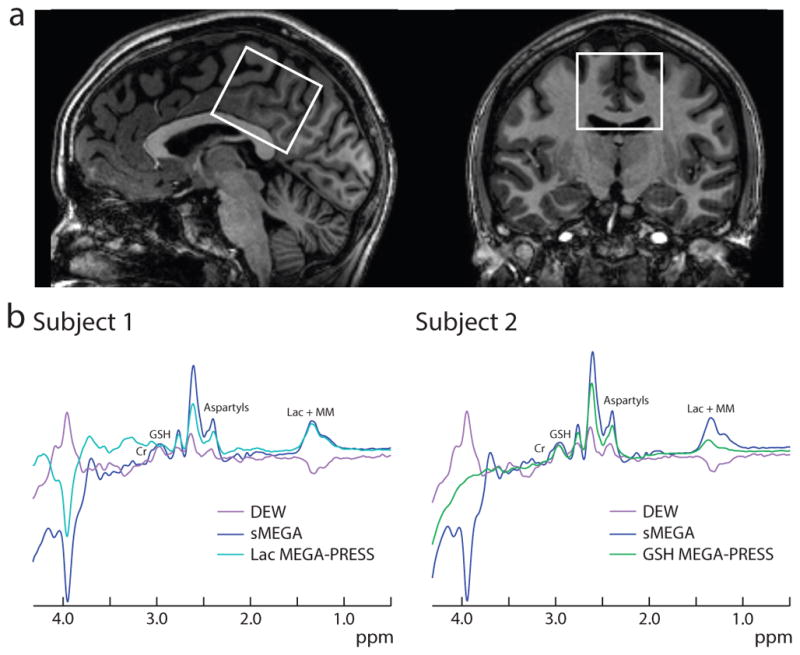
(a) Example voxel placement in a 4 cm × 4 cm × 4 cm midline parietal region overlaid on sagittal and coronal T1-weighted images in one subject. (b) Representative GSH and Lac spectra in two subjects. Note that the GSH peak has the opposite polarity relative to the Lac + MM peak in the DEW spectra and that there is a co-edited Cr peak at 3.02 ppm adjacent to the GSH peak which is inverted relative to GSH in the DEW spectra.
Table 1.
Fitted GSH and Lac integrals for all five subjects and all three overlapping subjects for both the sMEGA and DEW methods as well as separate MEGA-PRESS acquisitions of the same metabolites. Both methods maintain a high GSH and Lac editing efficiency, comparable to that of conventional MEGA-PRESS acquisitions.
| All subjects | |||
|---|---|---|---|
| Metabolite | MEGA-PRESS | DEW | sMEGA |
| GSH | 2.23 ± .51 | 2.31 ± .38 | 2.38 ± .53 |
| Lac | 1.72 ± .67 | 1.55 ± .35 | 1.53 ± .54 |
| Overlapping subjects | |||
|---|---|---|---|
| GSH | |||
| Subject | MEGA-PRESS | DEW | sMEGA |
| 1 | 2.66 | 2.67 | 2.30 |
| 23 | 1.442.59 | 1.922.64 | 1.522.78 |
| Lac | |||
| Subject | MEGA-PRESS | DEW | sMEGA |
|
| |||
| 1 | 1.86 | 1.27 | 0.75 |
| 23 | 2.610.75 | 1.701.28 | 1.501.37 |
Discussion
In this article, two methods to simultaneously editing GSH and Lac are presented and compared. Both the sMEGA method and DEW method perform well with a high overall editing efficiency of GSH and Lac comparable to separate acquisitions of each metabolite using conventional MEGA-PRESS. Although both sMEGA and DEW have high editing efficiencies, DEW needs highly selective editing pulses at frequency offsets close to one another (0.46 ppm separation) which makes the robustness of the measurements unlikely to hold in the presence of B0 frequency drift. In this case, the editing pulse for the spins of one metabolite may start to impinge on that of the other resulting in losses of editing efficiency for both metabolites in addition to the usual subtraction artifact issues (28, 32). On the other hand, sMEGA uses less selective editing pulses than DEW, which would make the sequence more robust to B0 field instability. However, the DEW method has less co-edited signals than the sMEGA method. The edited 3.02 ppm Cr signal that partially overlaps with the 2.95 ppm GSH peak is also co-edited to a greater degree in the sMEGA method, but does not appear to impact the quantification of the GSH peak if appropriate spectral fitting is used. The DEW method also has the added benefit of co-editing MM2 to a lesser degree due to partial symmetrical suppression of the 4.3 ppm MM2 resonance from the ON GSH editing pulse placed at 4.56 ppm and the ON Lac editing pulse placed at 4.1 ppm. In the DEW acquisition, the NAA and NAAG aspartyl resonances adjacent to the GSH peak are also co-edited to a lesser degree, thus aiding quantification by reducing spectral overlap between the GSH and aspartyl resonances.
Simultaneous editing of GSH and Lac is possible at 3T due to their similar echo time dependence and editing target frequencies. Compared to separate measurements of each compound individually, simultaneous editing results in a 50% reduction in scan time with essentially the same sensitivity. An echo time of 140 ms results in a high editing efficiency (~95%) of both GSH and Lac (20). This relatively long TE also allows sufficient time for the very selective editing pulses used in DEW and sinc editing pulses used in sMEGA to be played out. Also at this TE, the neighboring NAA and NAAG resonances are well resolved (and in-phase) from the GSH peak, thus facilitating quantification of the GSH peak (supplemental figure 1). In this study, we have shown the feasibility of sMEGA and DEW dual editing techniques. Future studies will be required to compare the reproducibility of the two methods in additional subjects.
One of the relatively surprising results of this study was the identification of a co-edited peak of the Cr CH3 signal at 3.02 ppm, which is traditionally thought be to a singlet, and therefore not to contribute signal intensity to edited spectra. However, recent work has shown that there is a small (~0.3 Hz) long-range J-coupling between the Cr CH2 protons at 3.9 ppm and the CH3 protons, thus giving some co-editing of this signal at long TE. Fortunately it only partially overlaps the edited GSH signal at 2.95 ppm, allowing the two to be separated by appropriate spectral fitting routines.
In conclusion, two methods were compared for the simultaneous editing of GSH and Lac, compounds that have both been implicated in the pathophysiology of a variety of brain pathologies. These methods allow for a 50% reduction in scan time compared to sequential measures of the two metabolites. In healthy subjects, the methods are shown to reliably edit both GSH and Lac with no loss in editing efficiency in comparison to conventional MEGA-PRESS acquisitions of the same metabolites. While the DEW method gives less co-editing of other compounds, the co-edited signals in sMEGA do not hinder quantification of either GSH or Lac in the sMEGA acquisition. Thus, the sMEGA sequence may be preferable especially when B0 field drift is problematic, which may occur when significant gradient heating or cooling is present such, as after gradient-intensive diffusion-weighted MRI and functional MRI scans (33). Regarding field inhomogeneity, since the most selective editing pulse used in this study has a bandwidth of 40 Hz, and the water linewidths measured in this study were less than 10 Hz for all voxels, all the editing methods presented here should be largely unaffected. As MRS acquisitions in different regions of the brain usually have linewidths less than 20 Hz at 3T, B0 field inhomogeneity will generally not have a significant effect on editing efficiency for any of the methods described here.
Supplementary Material
Edited NAA (green) and NAAG (purple) spectra in a phantom as a function of echo time. The NAA and NAAG resonances have the least negative/dispersion components present at TE 130–140 ms.
Example ON and OFF subspectra for all three methods in simulations, two separate GSH and Lac phantoms, and in vivo experiments. The first column shows the subspectra for both the GSH MEGA-PRESS acquisition and the Lac MEGA-PRESS acquisition. The ON subspectra is light blue for GSH and green for Lac while the OFF subspectra is purple for GSH and yellow for Lac. The second and third columns show the ON (blue) and OFF (orange) subspectra for DEW and sMEGA.
Acknowledgments
This work was supported in part by NIH grants P41 EB015909, R01 EB016089, and T32EB010021.
Abbreviations
- Asp
aspartate
- Cr
creatine
- DEW
Double Editing With
- GABA
gamma-aminobutyric acid
- Glx
glutamate and glutamine
- GSH
glutathione
- Lac
lactate
- MEGA-PRESS
MEscher and GArwood-Point RESolved Spectroscopy
- MM
macromolecule
- NAA
N-acetyl aspartate
- NAAG
N-acetyl aspartyl glutamate
- PE
phosphoethanolamine
- sMEGA
sinc-MEscher and Garwood
- VAPOR
variable power RF
References
- 1.Mytilineou C, Kramer BC, Yabut JA. Glutathione depletion and oxidative stress. Parkinsonism Relat Disord. 2002;8:385–387. doi: 10.1016/s1353-8020(02)00018-4. [DOI] [PubMed] [Google Scholar]
- 2.Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem. 2000;267:4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- 3.Edden RAE, Schär M, Hillis AE, Barker PB. Optimized detection of lactate at high fields using inner volume saturation. Magn Reson Med. 2006;56:912–917. doi: 10.1002/mrm.21030. [DOI] [PubMed] [Google Scholar]
- 4.Alger JR, Frank JA, Bizzi A, et al. Metabolism of human gliomas: assessment with H-1 MR spectroscopy and F-18 fluorodeoxy-glucose PET. Radiology. 1990;177:633–641. doi: 10.1148/radiology.177.3.2243962. [DOI] [PubMed] [Google Scholar]
- 5.Lin DDM, Crawford TO, Barker PB. Proton magnetic resonance spectroscopy in the diagnostic evaluation of suspected mitochondrial disease. AJNR Am J Neuroradiol. 2003;24:33–41. [PMC free article] [PubMed] [Google Scholar]
- 6.Wood SJ, Berger GE, Wellard RM, et al. Medial temporal lobe glutathione concentration in first episode psychosis: a 1H-MRS investigation. Neurobiol Dis. 2009;33:354–357. doi: 10.1016/j.nbd.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Matsuzawa D, Obata T, Shirayama Y, et al. Negative correlation between brain glutathione level and negative symptoms in schizophrenia: a 3T 1H-MRS study. PLoS One. 2008;3:e1944. doi: 10.1371/journal.pone.0001944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beasley CL, Dwork AJ, Rosoklija G, et al. Metabolic abnormalities in fronto-striatal-thalamic white matter tracts in schizophrenia. 2009;109:159–166. doi: 10.1016/j.schres.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das Neves Duarte JM, Kulak A, Gholam-Razaee MM, Cuenod M, Gruetter R, Do KQ. N-acetylcysteine normalizes neurochemical changes in the glutathione-deficient schizophrenia mouse model during development. Biol Psychiatry. 2012;71:1006–1014. doi: 10.1016/j.biopsych.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 10.Dager SR, Friedman SD, Parow A, et al. Brain metabolic alterations in medication-free patients with bipolar disorder. Arch Gen Psychiatry. 2004;61:450–458. doi: 10.1001/archpsyc.61.5.450. [DOI] [PubMed] [Google Scholar]
- 11.Soeiro-de-Souza MG, Pastorello BF, Leite C, da C, Henning A, Moreno RA, Otaduy MCG. Dorsal Anterior Cingulate Lactate and Glutathione Levels in Euthymic Bipolar I Disorder: 1H-MRS Study. Int J Neuropsychopharmacol. 2016;19:pyw032. doi: 10.1093/ijnp/pyw032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosa AR, Singh N, Whitaker E, et al. Altered plasma glutathione levels in bipolar disorder indicates higher oxidative stress; a possible risk factor for illness onset despite normal brain-derived neurotrophic factor (BDNF) levels. Psychol Med. 2014;44:2409–2418. doi: 10.1017/S0033291714000014. [DOI] [PubMed] [Google Scholar]
- 13.Chu W-J, Delbello MP, Jarvis KB, et al. Magnetic resonance spectroscopy imaging of lactate in patients with bipolar disorder. 2013;213:230–234. doi: 10.1016/j.pscychresns.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Mintzopoulos D, Gillis TE, Robertson HR, Dalia T, Feng G, Rauch SL, Kaufman MJ. Striatal Magnetic Resonance Spectroscopy Abnormalities in Young Adult Sapap3 Knockout Mice. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:39–48. doi: 10.1016/j.bpsc.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shungu DC, Weiduschat N, Murrough JW, et al. Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR Biomed. 2012;25:1073–1087. doi: 10.1002/nbm.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris AD, Saleh MG, Edden RAE. Edited 1 H magnetic resonance spectroscopy in vivo: Methods and metabolites. 2017;1389:1377–1389. doi: 10.1002/mrm.26619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 18.Snoussi K, Pradhan S, Harris AD, Edden RAE, Barker PB. Optimization of MEGA-PRESS for the simultaneous detection of Glutamate and Glutamine, and GABA. Proceedings of the 23rd Annual Meeting of ISMRM; Toronto, Ontario, Canada. 2015; Abstract 4694. [Google Scholar]
- 19.Terpstra M, Marjanska M, Henry PG, Tkáč I, Gruetter R. Detection of an antioxidant profile in the human brain in vivo via double editing with MEGA-PRESS. Magn Reson Med. 2006;56:1192–1199. doi: 10.1002/mrm.21086. [DOI] [PubMed] [Google Scholar]
- 20.Chan KL, Puts NAJ, Snoussi K, Harris AD, Barker PB, Edden RAE. Echo time optimization for J-difference editing of glutathione at 3T. Magn Reson Med. 2016;504:498–504. doi: 10.1002/mrm.26122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith MA, Koutcher JA, Zakian KL. J-difference lactate editing at 3. 0 Tesla in the presence of strong lipids. J Magn Reson Imaging. 2008;28:1492–1498. doi: 10.1002/jmri.21584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen J, Novotny EJ, Rothman DL. In Vivo Lactate and B-Hydroxybutyrate Editing Using a Pure-Phase Refocusing Pulse Train. Magn Reson Med. 1998;40:783–8. doi: 10.1002/mrm.1910400520. [DOI] [PubMed] [Google Scholar]
- 23.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 24.Terpstra M, Henry P-G, Gruetter R. Measurement of reduced glutathione (GSH) in human brain using LCModel analysis of difference-edited spectra. Magn Reson Med. 2003;50:19–23. doi: 10.1002/mrm.10499. [DOI] [PubMed] [Google Scholar]
- 25.Simpson R, Devenyi GA, Jezzard P, Hennessy TJ, Near J. Advanced processing and simulation of MRS data using the FID appliance (FID-A)-An open source, MATLAB-based toolkit. Magn Reson Med. 2015;33:23–33. doi: 10.1002/mrm.26091. [DOI] [PubMed] [Google Scholar]
- 26.Thomas MA, Yue K, Binesh N, et al. Localized two-dimensional shift correlated MR spectroscopy of human brain. Magn Reson Med. 2001;46:58–67. doi: 10.1002/mrm.1160. [DOI] [PubMed] [Google Scholar]
- 27.Chan KL, Edden RAE, Barker PB. J-difference editing of Creatine in the human brain. Proceedings of the 25th Annual Meeting of ISMRM; Honolulu, Hawai’i, USA. 2017; Abstract 5473. [Google Scholar]
- 28.Edden RAE, Oeltzschner G, Harris AD, et al. Prospective frequency correction for macromolecule-suppressed GABA editing at 3 T. J Magn Reson Imaging. 2016;44:1474–1482. doi: 10.1002/jmri.25304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ. Gannet: a batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging. 2014;1452:1445–1452. doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Near J, Edden R, Evans CJ, Paquin R, Harris A, Jezzard P. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn Reson Med. 2014;50:44–50. doi: 10.1002/mrm.25094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Behar KL, Rothman DL, Spencer DD, Petroff OAC. Analysis of macromolecule resonances in1H NMR spectra of human brain. Magn Reson Med. 1994;32:294–302. doi: 10.1002/mrm.1910320304. [DOI] [PubMed] [Google Scholar]
- 32.Harris AD, Glaubitz B, Near J, et al. Impact of frequency drift on gamma-aminobutyric acid-edited MR spectroscopy. Magn Reson Med. 2014;72:941–948. doi: 10.1002/mrm.25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Sharkawy AEM, Schär M, Bottomley PA, Atalar E. Monitoring and correcting spatio-temporal variations of the MR scanner’s static magnetic field. Magn Reson Mater Physics, Biol Med. 2006;19:223–236. doi: 10.1007/s10334-006-0050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Edited NAA (green) and NAAG (purple) spectra in a phantom as a function of echo time. The NAA and NAAG resonances have the least negative/dispersion components present at TE 130–140 ms.
Example ON and OFF subspectra for all three methods in simulations, two separate GSH and Lac phantoms, and in vivo experiments. The first column shows the subspectra for both the GSH MEGA-PRESS acquisition and the Lac MEGA-PRESS acquisition. The ON subspectra is light blue for GSH and green for Lac while the OFF subspectra is purple for GSH and yellow for Lac. The second and third columns show the ON (blue) and OFF (orange) subspectra for DEW and sMEGA.