Abstract
Autism Spectrum Disorder (ASD) is phenotypically and etiologically heterogeneous, with evidence for genetic and environmental contributions to disease risk. Research has focused on the prenatal period as a time where environmental exposures are likely to influence risk for ASD. Epidemiological studies have shown significant associations between prenatal exposure to maternal immune activation (MIA), caused by infections and fever, and ASD. However, due to differences in study design and exposure measurements no consistent patterns have emerged revealing specific times or type of MIA exposure that are most important to ASD risk. No prior studies have examined prenatal MIA exposure and ASD risk in an under-represented minority population of African ancestry. To overcome these limitations, we estimated the association between prenatal exposure to fever and maternal infections and ASD in a prospective birth cohort of an understudied minority population in a city in the United States. No association was found between prenatal exposure to genitourinary infections or flu and the risk of ASD in a nested sample of 116 ASD cases and 988 typically developing controls in crude or adjusted analyses. Prenatal exposure to fever was associated with increased ASD risk (aOR = 2.02 [1.04 – 3.92]) after adjustment for educational attainment, marital status, race, child sex, maternal age, birth year, gestational age and maternal smoking. This effect may be specific to fever during the third trimester (aOR 2.70 [1.00 – 7.29]). Our findings provide a focus for future research efforts and ASD prevention strategies across diverse populations.
Lay summary
We looked at whether activation of the immune system during pregnancy increases the chance a child will develop ASD. We examined 116 children with ASD and 988 children without ASD that came from a predominantly low income, urban, minority population. We found that having the flu or genitourinary tract infections during pregnancy is not related to the child being diagnosed with ASD. However, we did find children were at increased risk for ASD when their mothers had a fever during pregnancy.
Keywords: Autism spectrum disorder (ASD), environmental exposure, maternal exposure, fever, epidemiology, risk factors, minority health
Introduction
Autism spectrum disorder (ASD) is characterized by deficits in social interaction or communication and repetitive behavior or stereotypical interests. ASD is increasingly common, with a prevalence of 1 in 68 children (1 in 42 boys and 1 in 189 girls) as of 2012 (Christensen et al. 2016). ASD is phenotypically and etiologically heterogeneous, with evidence for both genetic and environmental contributions to disease risk (Hallmayer et al. 2011, Persico et al. 2013, Sandin et al. 2014). Converging evidence points to the prenatal period as a time where environmental exposures are most likely to influence ASD risk (Rodier et al. 1996, Rice et al. 2000, Schlotz et al. 2009, Barouki et al. 2012, Marques et al. 2013, Lyall et al. 2014, Stoner et al. 2014). Identification of modifiable ASD risk factors can lead to preventative intervention strategies that may reduce overall ASD burden. Studies to examine a broad range of environmental risk factors for ASD during this critical time window are now emerging.
There is a growing body of evidence suggesting prenatal exposure to maternal immune activation (MIA) and/or systemic inflammation increases the risk of many different types of neurodevelopmental disorders (NDD), including autism. Animal models of MIA have shown behavioral, hormonal, and neuropathologic differences among prenatally exposed offspring relative to their unexposed counterparts (Malkova et al. 2012, Miller et al. 2013, Schwartzer et al. 2013, Bauman et al. 2014, Machado et al. 2015). In addition, MIA-associated differences in immune function (Hsiao et al. 2012), hormone and neurotransmitter levels (Miller et al. 2013, Ohkawara et al. 2015), neuronal and whole brain growth (Shi et al. 2009, Le Belle et al. 2014, Straley et al. 2014), as well as microglial neurodevelopmental regulatory patterns (Miller et al. 2013, Matcovitch-Natan et al. 2016) have been found in animal models and are consistent with observations in humans with ASD.
Human studies have identified associations between prenatal exposure to maternal infection and ASD risk. Two European registry-based population studies and one US HMO-based case-control study have identified associations between maternal infection, bacterial or viral, during pregnancy and increased ASD risk in her offspring (Atladottir et al. 2010, Lee et al. 2015), with the highest elevated risk among women with multiple hospitalizations for infections or those with bacterial infections (Zerbo et al. 2015). One case-control study in Taiwan using medical record data found an elevated risk for ASD after genital or bacterial infections (Fang et al. 2015). In addition, a study that assessed maternal exposure to infection by self-report, rather than medical records, showed potential risk effects for flu (Atladottir et al. 2012), although a recent HMO-based cohort analysis did not observe such an association (Zerbo et al. 2017). A meta-analysis of 15 studies found an increase in ASD risk after any type of maternal infection (Jiang et al. 2016).
Fewer studies have examined the potential impact of fever specifically, rather than infection broadly, on ASD risk. One study found that prolonged febrile episodes were associated with increased risk (Atladottir et al. 2012). A retrospective case-control study based on maternal self-report showed association between fever during pregnancy and increased ASD risk (Zerbo et al. 2013). That study further showed that risk was attenuated in mothers who took anti-pyretic medications to control their fever, but remained elevated in mothers who did not (Zerbo et al. 2013). A prospective study in Norway also found increased risk for ASD after prenatal fever exposure, as well as evidence of a dose-response relationship, with risk rising with multiple maternal fevers (Hornig et al. 2017). Fever exposure has also been shown to adversely influence developing fetal health more generally (Dreier et al. 2014). Infection and fever are common during pregnancy and could have a major impact on disease burden; the prevalence of self-reported infection during pregnancy in the US has been reported to be as high as 63.6%, and 20.5% for fever (Collier et al. 2009).
While previous findings suggest prenatal MIA is an important environmental risk factor for ASD, there are limitations in the literature that need to be addressed. First, the Zerbo et al. case-control study relied on retrospective, rather than prospective, self-reported exposure assessment in its estimate of the relationship between infection or fever exposure and ASD (Zerbo et al. 2013). This study design is vulnerable to differential exposure misclassification due to recall bias. Second, although extracting maternal infection exposure from electronic medical records avoids the limitations associated with retrospective exposure assessment after ASD diagnosis, there are infections or fevers that may never be brought to medical attention or documented. Study designs that rely on exposure data solely from EMR may thus be looking at only severe infections. Furthermore, no EMR-based definition of exposure has assessed the relationship between maternal fever and ASD risk. Finally, four of the prior studies were based on European samples (in Denmark, Sweden, and Norway; Atladottir et al. 2010, Atladottir et al. 2012, Lee et al. 2015, Hornig et al. 2017), and 2 studies were based in California and included predominantly White and Hispanic individuals (>68%) as well as a small proportion of individuals reporting their race as Asian, Black, or Other (Zerbo et al. 2013, Zerbo et al. 2015). Another EMR-based case-control study performed in Taiwan used records from the National Health Insurance Program and thus was comprised entirely of Asian individuals (Fang et al. 2015). While steps have been taken to increase the diversity of the population in which we estimate the association between maternal infection or fever and ASD, no study has tested this question in a predominantly Black American population. This is of critical importance in the United States and expanding research of ASD risk factors into multiple diverse populations is a clear priority for understanding etiology. Here, we overcome previous limitations by performing a prospective analysis of self-reported prenatal exposure to MIA and later ASD risk in an under-represented minority birth cohort in the US.
Methods
Boston Birth Cohort (BBC) Study Description
The Boston Birth Cohort (BBC) is a prospective birth cohort with pregnancy exposure and phenotypic data for mother-child dyads recruited at birth at the Boston Medical Center (BMC) (Wang et al. 2002, Wang et al. 2014). The BBC was initiated in 1998 to investigate environmental and genetic determinants of preterm delivery, with oversampling for preterm birth. Thus, the BBC functions as an enriched risk cohort for ASD, since preterm birth is a known ASD risk factor. Women with a singleton live birth at the BMC are eligible for recruitment, with exclusions for IVF, multiple gestations, chromosomal abnormalities, major birth defects, and preterm deliveries due to maternal trauma. As described in detail in Wang et al. (Wang et al. 2002), participants are contacted 24–72 hours after birth to obtain consent and initiate study enrollment. A subset of BBC children continue to receive postnatal pediatric care at the BMC, which allows us to follow their developmental outcomes.
Unique family-level study IDs for the mother and her child are assigned at the time of BBC enrollment. The field manager controls a log book in which family-level study ID is linked to maternal and offspring BMC hospital ID. This log is used to subsequently match maternal questionnaire data with their child’s EMR data. All BBC data is indexed by the family-level study ID.
The BBC enrolls predominantly urban, low-income minority mothers and their newborns; the diverse population examined in this study is approximately 38% black/African American, 22% Hispanic, 19% Haitian, and 8.5% white (see Table 1). The majority of BBC mothers receive health care through public assistance based insurance programs, e.g. Medicaid or MassHealth.
Table 1.
Characteristics of the ASD case-control study sample from the Boston Birth Cohort (BBC)
| Neurotypical Controls (n=988) | ASD (n=116) | P | |
|---|---|---|---|
| Gravidity, M (SD) | 2.79 (1.79) | 2.85 (1.93) | 0.701 |
| Parity, M (SD) | 1.04 (1.20) | 0.96 (1.22) | 0.491 |
| Maternal agea, M (SD) | 28.25 (6.55) | 30.11 (6.24) | 0.004* |
| Maternal education, n (%) | 0.281 | ||
| Elementary school | 41 (4.3) | 4 (3.5) | |
| Secondary school | 238 (24.7) | 21 (18.6) | |
| High school/GED | 333 (34.6) | 40 (35.4) | |
| Some college | 203 (21.1) | 33 (29.2) | |
| College degree and above | 148 (15.4) | 15 (13.3) | |
| Maternal marital status, n (%) | 0.570 | ||
| Married | 327 (34.0) | 42 (37.5) | |
| Not Married | 661 (66.0) | 74 (62.5) | |
| Race or Ethnicityb, n (%) | 0.472 | ||
| Black | 615 (62.2) | 73 (62.9) | |
| White | 83 (8.4) | 6 (5.2) | |
| Hispanic | 214 (21.7) | 31 (26.7) | |
| Asian | 20 (2.0) | 2 (1.7) | |
| Other | 56 (5.7) | 4 (3.4) | |
| Maternal smokingc, n (%) | 0.179 | ||
| Never | 817 (85.1) | 88 (78.6) | |
| Some | 48 (5.0) | 9 (8.0) | |
| Continuous | 95 (9.9) | 15 (13.4) | |
| Child sex, n (%) | <0.001* | ||
| Female | 585 (59.2) | 31 (26.7) | |
| Male | 403 (40.8) | 85 (73.3) | |
| Gestational age, mean (SD)d | 38.4 (2.7) | 36.5 (4.6) | <0.001* |
| Birth weight, n (%) | 0.072 | ||
| >2500 grams | 763 (78.9) | 81 (71.1) | |
| <2500 grams | 204 (21.1) | 33 (28.9) |
ASD, autism spectrum disorder; BBC, Boston Birth Cohort; M, mean; SD, standard deviation
Maternal age at time of delivery
Black includes self reported Black, African American, Haitian, Cape Verdean, and Caribbean race and ethnicities. Asian includes Asian and Pacific Islander races. The Other category includes individuals with a mixed or other racial background.
Never smokers were defined as mothers with no history of smoking 6 months prior to conception or during pregnancy; some smoking includes mothers that smoked at some point in the window of 6 months prior to conception and delivery but did not smoke throughout that window; continuous is defined as mothers that smoked starting 6 months prior to and throughout pregnancy.
Defined by sonogram
Denotes statistically significant
Outcome Classification
We performed a case-control analysis of children with Autism Spectrum Disorder (ASD) and children with neurotypical development. We used electronic medical record ICD-9-CM diagnosis codes for pediatric inpatient, outpatient, and emergency room visits as documented in the EMR of the Boston Medical Center, between 1 October 2003 and 31 September 2015 (the last date before transition from ICD-9-CM to ICD-10-CM), to define ASD cases and neurotypical controls. Electronic medical records for 121,457 inpatient, outpatient, and emergency room visits to the BMC were available for children enrolled in the Boston Birth Cohort follow-up study. After 2,436 visits contributed by siblings were removed from the dataset, 118,939 records from 2,992 index children remained. On average each child had 39.8 visits to the BMC, with a range of 1 to 463 visits. This indicates considerable contact between children and BMC medical providers. Individuals were classified as an ASD case if their medical records contained any of the following ICD-9-CM codes at least once: 299.00, 299.01, 299.80, 299.81, 299.90, or 299.91. We classified individuals as neurotypical controls if they were never diagnosed with any of the following conditions: ASD, attention deficit hyperactivity disorder (ADHD), intellectual disability (ID), developmental delay (DD), oppositional defiant disorder (ODD), conduct disorder (CD), or congenital anomalies (based on ICD-9-CM codes; see Supplementary Table 1). Sensitivity analyses were performed using a more stringent ASD case definition: to be defined as an ASD case the individual had to have a 299 ACD-9-CM code recorded in their electronic medical record on at least 2 separate occasions. A total of 82 ASD cases met these criteria and were used for sensitivity analyses.
Exposure Definitions
Enrolled BBC mothers were interviewed 24–72 hours after delivery using a standardized postpartum questionnaire to gather information about her pregnancy (Wang et al. 2014). Data on prenatal exposure to flu, fever (excluding intrapartum), and genitourinary tract infections were obtained from self-report based on the questionnaire (for specific wording see Supplementary Table 2). In addition, history of an intrapartum fever (>38C) was abstracted from electronic medical record data by trained study personnel using a standardized form.
For each type of exposure examined—prenatal genitourinary (GU) infection, prenatal flu, maternal fever during pregnancy, and intrapartum maternal fever—we generated a dichotomous categorical variable representing “exposed” or “unexposed.” Children whose mothers responded ‘yes’ to having any vaginal or genital tract or urinary tract infections during this pregnancy (including yeast infections), any fever during this pregnancy, and any flu during this pregnancy were defined as “exposed” for GU, fever, and flu variables, respectively. Similarly, dichotomous trimester-specific variables were derived for flu and fever exposures using trimester-specific information among the subset of mothers that positively responded to having an exposure at any point during pregnancy. For intrapartum fever exposure, individuals were categorized as “exposed” if their mother had an intrapartum temperature > 38C, obtained via abstracted labor and delivery electronic medical records.
Covariate Definitions
Covariates used for adjustment included characteristics of the mother (educational attainment, marital status, race, smoking status, age at delivery) and child (sex, birth year, gestational age at birth according to ultrasound dating, low birth weight). Educational attainment, marital status, race, and smoking prior to and during pregnancy were all self-reported in the postpartum questionnaire. Child sex and birth weight were based on the abstracted medical records. Maternal age at the time of delivery was based on mother’s date of birth recorded in her medical records. We defined gestational age at birth using a combination of the first available ultrasound in the medical records and last menstrual period (Wang et al. 2014).
We defined mothers’ educational attainment as a categorical variable (elementary school, secondary school, high school/GED, some college, or college/postgraduate degree) using the self-report questionnaire data. Marital status was self-reported as married, single, divorced, separated, or widowed; this was dichotomized to “married” or “not married” for our analyses. Race was self-reported by choosing one of the 9 following categories that best reflected the respondent’s background: Black/African American; Asian; Pacific Islander; White; Haitian; Hispanic; Cape Verdean; Other; and Unknown. For our analyses, we then collapsed these responses into five categories to generate the race covariate: (1) Black, (2) White, (3) Hispanic, (4) Asian, and (5) all others. Black includes self-reported Black, African American, Haitian, Cape Verdean, and Caribbean race and ethnicities. White includes all individuals that reported white. Asian includes Asian and Pacific Islander races. The Other category includes all other backgrounds. Maternal smoking was a categorical covariate defined using self-reported data; never smokers were defined as mothers with no history of smoking 6 months prior to conception or during pregnancy. Mothers that smoked at any point in the 6 months prior to conception or at any point during their pregnancy were coded as “some smoking.” Continuous smokers were defined as mothers that smoked 6 months prior to conception and throughout their pregnancy. A dichotomous variable representing low birth weight was generated, with children weighing less than 2500 grams assigned a “1” and children weighing greater than or equal to 2500 grams assigned a “0.”
Analytic dataset
The BBC enrolled a total of 7,939 mother-child pairs at birth at the Boston Medical Center from 1998 to 2013. Postnatal follow-up was restricted to those children who continued to receive pediatric care at the Boston Medical Center. As presented previously, there were no major differences in baseline demographic and clinical characteristics between those with postnatal follow-up data and those lost to follow-up (Li M et al, Pediatrics, 2016).
There were 2,992 children who had postnatal follow up at the Boston Medical Center through 31 September 2015 and were eligible for the present study. ASD cases and neurotypical controls were defined as described above. In the process of identifying neurotypical controls, 1839 children were excluded from final analyses; they were diagnosed with serious conditions such as a congenital anomaly or other developmental disorder or delay, e.g. intellectual disability (Supplementary Table 1). The BBC includes a large number of children without typical neurodevelopment because it oversampled for preterm birth at enrollment, a known risk factor for developmental disabilities. Our final analytic dataset consists of 1153 total children: 120 ASD cases and 1033 neurotypical controls.
We then linked these ASD and neurotypical control children to maternal demographic and questionnaire data on prenatal exposures using a family-level study ID; 4 ASD cases and 45 controls failed to link and were subsequently excluded from the analyses, leaving 988 neurotypical controls and 116 cases. Since there was a low prevalence of missing data in the covariates (ranging from 0% for maternal age and child sex and birth year, to 2.1% for maternal race, and 4.3% for gestational age) and exposures of interest (1.4% missing for genitourinary infections, 2.6% for fever or flu any time during pregnancy, and 8.4% for intrapartum fever), we then removed individuals from further analysis if they were missing data for any covariate included in the final model (maternal education, marital status, race, age, and pregnancy smoking status; child sex, birth year, and gestational age at birth) or exposure of interest (Supplementary Table 3) (Graham 2008). A small number of mothers reported having an exposure to fever (n = 6) or to flu (n = 22) in multiple trimesters (Supplementary Tables 4–6); they were included in our analyses. There were no significant demographic differences between individuals retained for further analysis and those removed due to missing data, although those missing data were enrolled in the study earlier (Supplementary Table 7).
Statistical Analyses
All data cleaning and analysis was performed with R-3.1.3. Summary tables of characteristics of ASD cases and controls, as well as exposed and unexposed, were created with the R package tableone (https://CRAN.R-project.org/package=tableone). Descriptive statistics for categorical variables were obtained with the function chisq.test() with continuity correction, and the function oneway.test() for continuous variables with an assumption of equal variance.
ASD odds ratios for MIA exposures were estimated via unadjusted and adjusted binomial logistic regression using R-3.1.3. For each of the 4 exposures (fever, flu, prenatal GU infection, and intrapartum fever) we performed independent analyses. Our final model was adjusted for socioeconomic status as represented by mothers’ educational attainment, marital status, and race, as well as for maternal age, maternal smoking during pregnancy, child sex, birth year, and gestational age at birth. Beta values from the logistic regression were transformed to obtain odds ratios for association with ASD outcome. A p-value < 0.05 was taken to be evidence for a statistically significant association.
To allow a cleaner comparison of exposed and unexposed groups and better take confounding into account, we also conducted a propensity score analysis for exposure to fever. We matched individuals who were exposed to maternal fever any time during their gestation with those who were not exposed to fever by their propensity for exposure to fever, conditional on maternal age, smoking status, race, and educational and marital status, as well as child sex, gestational age at birth, and year of birth, using the R package MatchIt (Ho et al. 2007, Ho et al. 2011); exposed individuals were matched to their nearest unexposed, with a caliper of 0.08 and a case:control ratio of 1:8.
Sensitivity analyses for potential exposure misclassification
To address potential exposure misclassification for prenatal exposure we performed sensitivity analyses using 2 complementary analytic approaches. Both approaches use assumptions about the test’s sensitivity and specificity to reassign false positives and false negatives before calculating a crude corrected OR (Lash et al. 2009). We performed these analyses for prenatal exposure to GU infections, flu, and fever at any time during pregnancy. Because this prenatal exposure information was collected prior to diagnoses, we assume no outcome-based differential misclassification in our models. The first approach we used was a Monte Carlo sensitivity analysis (MCSA). This method utilizes prior probability distributions for the sensitivity and specificity; for each replicate, a value for the sensitivity and specificity are randomly drawn from the distribution and a corrected OR is calculated. We implemented MCSA using the R package episensr (https://CRAN.R-project.org/package=episensr) and the function probsens(). We explored various prior probability distributions of the sensitivity and specificity, including uniform and logit-normal. We set the sensitivity and specificity draws for the cases and controls to be tightly correlated (r = 0.9) and ran 10,000 replicates. Without definitive literature to guide the choice of an informative prior, we chose to model the prior probability of the sensitivity for both cases and controls as a uniform distribution between 0.5 and 1, and the specificity as a uniform distribution between 0.912 and 1; we then obtain a single estimate with an empirical 95% confidence interval for the corrected association between ASD and each prenatal exposure. Because the correction method for exposure misclassification involves subtracting false positive subjects, we are unable to assess for dramatic over-reporting of exposure because it would result in negative cells in the corrected 2×2 table; this sets a lower limit for the specificity we can model in the setting of exposure misclassification.
For our second approach, we first performed a bootstrap resampling of the dataset (10,000 bootstraps) and then conducted a simple sensitivity analysis for misclassification on each bootstrap by reassigning false positives and false negatives and then calculating the average crude corrected OR (Lash et. al 2009). This allows us to explicitly model different ways that bias could affect the sensitivity and specificity of the test for exposure. This was implemented using the episensr functions misclassification() and boot.bias() to empirically derive estimates of the uncertainty in the OR for different models of sensitivity and specificity, including differential and non-differential exposure misclassification.
Results
Sample Description
After defining our analytic dataset, as described in detail in the methods section, we compared maternal and child characteristics in our sample across case and control groups. As expected, we observed significant associations between autism spectrum disorder (ASD) diagnosis and known risk factors, including child male sex, increased maternal age, and lower gestational age in the Boston Birth Cohort (BBC) sample (Table 1). No significant ASD case-control differences were observed for maternal gravidity, parity, education, marital status, race/ethnicity, smoking status, or child birth weight (Table 1). We also observed no difference in the distribution of cases and controls across all study years (Supplementary Figure 1).
Prenatal Exposure to Genitourinary Infection and ASD Risk
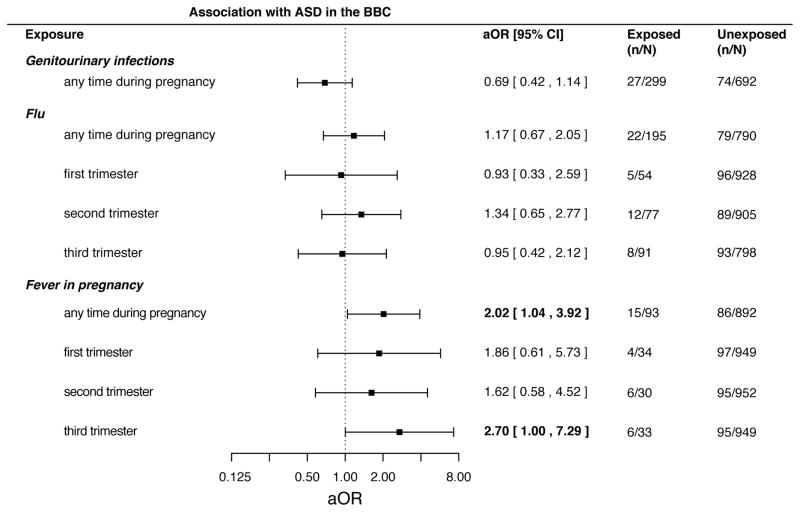
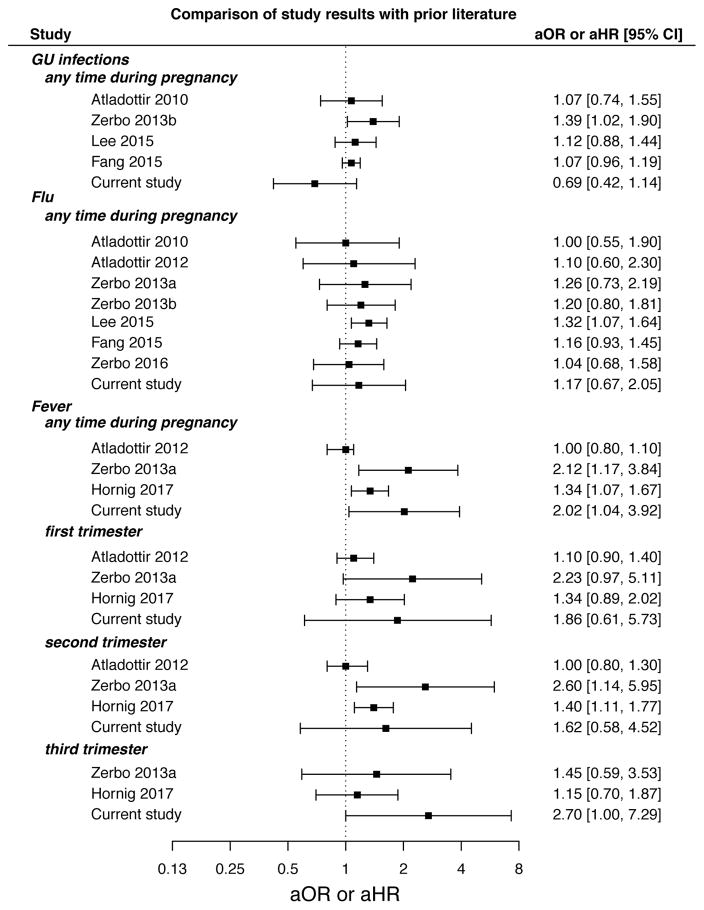
No association was found between self-reported maternal history of genitourinary (GU) infections at any time during pregnancy and risk of ASD development in the offspring in an unadjusted model (OR 0.83 [95% confidence interval 0.52 – 1.32]; Supplementary Table 8). Similarly, no significant association was observed after adjusting for child sex, maternal age, child birth year, maternal smoking status, maternal education, marital status, maternal race, and gestational age (aOR 0.69 [0.42 – 1.14]; Figure 1 and Supplementary Table 8). Our findings in the BBC are consistent with three prior studies (Atladottir 2010, Lee 2015, Fang 2015) that also examined prenatal exposure to GU infections and ASD risk (Figure 2).
Figure 1.
Forest plot showing adjusted odds ratio (OR) and 95% confidence intervals for the association between prenatal GU infection, flu (overall and trimester-specific), and fever (overall and trimester-specific) and Autism Spectrum Disorder in the Boston Birth Cohort (BBC).
Figure 2.
Forest plot comparing the Boston Birth Cohort results to previously reported results. The plot shows effect estimates and 95% confidence intervals for the association between infection or fever (at any point during pregnancy and by trimester) and autism.
Prenatal Exposure to Flu and ASD Risk
We assessed risk for ASD among children prenatally exposed to flu at any point during gestation or during a specific trimester (trimesters 1, 2, and 3). We observed no association between prenatal flu exposure at any point during gestation and risk of ASD in either unadjusted (OR of 1.14 [95% confidence interval 0.69 – 1.89]; Supplementary Table 8) or adjusted analyses (aOR 1.17 [0.67 – 2.05]; Figure 1 and Supplementary Table 8). Furthermore, neither adjusted nor unadjusted analyses showed an association between ASD risk and prenatal flu exposure specific to any trimester (Figure 1 and Supplementary Table 8). This is consistent with prior studies that reported no association between flu infection at any time during pregnancy and risk of the offspring developing ASD (Figure 2).
Fever during pregnancy and ASD risk
No association between exposure to fever at any time during pregnancy and risk of ASD was found in unadjusted analyses (OR 1.80 [0.99 – 3.27]; Supplementary Table 8). However, a significant association between ASD diagnosis and maternal fever at any time during her pregnancy was found after adjustment for child sex, maternal age, child birth year, maternal smoking status, maternal education, marital status, maternal race, and gestational age (aOR 2.02 [1.04 – 3.92]; see Figure 1 and Supplementary Table 8). We also observed a significant association between maternal fever during the third trimester of pregnancy and child ASD diagnosis (aOR 2.70 [1.00 – 7.29]; Figure 1 and Supplementary Table 8). No association between child ASD status and exposure to maternal fever during the first (aOR 1.86 [0.61 – 5.73]) or second trimesters (aOR 1.62 [0.58 – 4.52]) was found (Figure 1 and Supplementary Table 8).
Despite different covariate choices, our fever associations were consistent in magnitude and direction across all regression models tested (Table 2). However, because we observed differences in the strength of association among the adjusted models we also estimated the fever association using propensity score matching. Eighty-nine individuals exposed to fever were matched to 540 unexposed individuals; good balance on all covariates was achieved after propensity score matching (see Supplementary Table 9 for characteristics of all mother-child pairs by prenatal fever exposure status prior to propensity score matching and Supplementary Figure 2). While propensity score analysis did not show a statistically significant relationship between gestational exposure to maternal fever exposure and ASD, the direction of effect was consistent with our regression models (Table 2).
Table 2.
Comparison of estimated association between ASD and prenatal fever exposure using different analytic models
| Analytic model | Covariates | OR | P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Maternal | Child | ||||||||||
| education | marital status | race | smoking | age | GA | sex | birth year | LBWa | |||
| 1 | 1.80 [0.99 – 3.27] | 0.053 | |||||||||
| 2 | 1.72 [0.93 – 3.19] | 0.085 | |||||||||
| 3 | 1.80 [0.96 – 3.38] | 0.068 | |||||||||
| 4 | 1.86 [0.97 – 3.58] | 0.062 | |||||||||
| 5* | 2.02 [1.04 – 3.92] | 0.037 | |||||||||
| 6 | 1.98 [1.02 – 3.86] | 0.045 | |||||||||
| 7 | 1.95 [1.01 – 3.77] | 0.046 | |||||||||
| 8 | 1.89 [0.995 – 3.59] | 0.051 | |||||||||
| 9 | propensity score matchingb | 1.54 [0.80 – 2.95] | 0.195 | ||||||||
GA, gestational age; LBW, low birth weight
Bold values represent statistically significant ORs
low birth weight is defined as weighing less than 2500 grams at birth
conditional probability of exposure to fever modeled as a function of child sex, maternal age, birth year, maternal smoking, education, marital status, race, gestational age
All main text references to “fully adjusted model” refer to this model
While there is evidence that defining ASD by the presence of one or more diagnosis codes may be the best strategy in some datasets (Dodds et al. 2009), other work has suggested ASD case status be defined as the presence of a 299 ICD-9-CM code in at least 2 instances (Coleman et al. 2015). We thus performed sensitivity analyses using this more stringent ASD case definition. Defining ASD in this manner reduced the number of ASD cases from 101 to 82 while neurotypical controls remained the same (n=884). Logistic regression analyses resulted in a 1.95 [1.03 – 3.68] unadjusted OR for ASD risk after fever exposure at any time during pregnancy; the adjusted OR was 2.11 [1.03 – 4.32]. Thus, using the more stringent ASD case definition did not change the estimated strength, or the significance, of the association between prenatal fever exposure and ASD risk.
Because fever and flu may be correlated, as fever is a potential symptom of flu, we sought to further tease apart the relationship between prenatal exposure to fever/flu and ASD. We generated a categorical variable with four levels: neither flu nor fever (reference category; n = 753); fever only (n = 36); flu only (n=137); or both flu and fever (n = 56). We performed a logistic regression with ASD as the outcome and this derived categorical variable as the exposure, with adjustment for additional covariates as in our other models. No exposure category was significantly associated with ASD risk when compared to the reference category: fever (aOR 2.11 [0.75 – 5.94], p = 0.16), flu only (aOR 0.94 [0.47 – 1.90], p = 0.87), or fever and flu (aOR 2.05 [0.90 – 4.65], p = 0.09).
Finally, we compared our findings to three prior studies that examined the association between prenatal exposure to maternal fever and ASD risk. Zerbo et al. and Hornig et al. saw an association between fever and ASD risk at any time during pregnancy, consistent with the current study (Figure 2) (Zerbo 2013a, Hornig 2017). For exposure to maternal fever during pregnancy (prior to 32 weeks) or specifically during trimester 1 or 2, Aladottir et al. did not find an association with ASD risk (Aladottir 2012).
Intrapartum fever is not associated with ASD risk
Because of the association we observed between maternal fever during the third trimester and the risk of ASD in her child, we wanted to examine intrapartum fever exposure as a sensitivity analysis. This variable represents fever in the peripartum period (labor and delivery) only, as opposed to the third trimester. There were three ASD cases out of 50 children who were exposed to intrapartum fever, and 92 ASD cases out of the 886 children who were not exposed. While we are limited by the small number of exposed ASD cases, no association was seen between intrapartum fever and child ASD status in unadjusted (OR 0.52 [0.16 – 1.69]) or adjusted analyses (aOR 0.60 [0.18 – 2.02]; Supplementary Table 8). Because there were only four individuals with both intrapartum fever and fever at any prior point during pregnancy (as measured by questionnaire), we were not able to assess the joint effect.
Effect of potential exposure misclassification
Because our study relies on self-reported exposure data collected shortly after delivery, it is possible that mothers incorrectly recalled or reported their exposure during pregnancy, i.e. there is potential for exposure misclassification. To assess whether our findings were robust to exposure misclassification, we performed a Monte Carlo sensitivity analysis (MCSA). After correcting for systematic exposure misclassification and random error the OR for genitourinary infection was 0.78 [empirical 95% CI 0.43 – 1.38], the OR for flu was 1.21 [0.67 – 2.17], and the OR for fever was 2.68 [1.17 – 9.59]. These results were robust to different choices for the prior probability distribution. Using an alternative, complementary bootstrapping approach to address exposure misclassification, we observed similar results. For example, as shown in Supplementary Table 10, the OR for genitourinary infection was 0.80 [empirical 95% CI 0.46 – 1.44], the OR for flu was 1.19 [0.63 – 2.35], and the OR for fever was 2.77 [1.02 – 8.14] when assuming good test performance (sensitivity and specificity of 0.95). When modeling simultaneous under- and over-reporting (sensitivity 0.6 and specificity 0.90 to 0.92), the OR for genitourinary infection was 0.72 [0.31 – 1.76], the OR for flu was 1.30 [0.48 – 4.00], and the OR for fever was 9.43 [1.47 – 86.28]. Different assumptions about the test characteristics did not substantially alter the estimated associations between ASD risk and genitourinary infection, flu, or fever exposures and were consistent with our main findings.
Discussion
We examined the prospective relationship between prenatal maternal immune activation and Autism Spectrum Disorder (ASD) risk in an understudied, predominantly urban minority population. Our results support previous suggestions of maternal immune activation as a risk factor for ASD, and specifically implicate fever. Our results do not provide evidence for an association between exposure to genitourinary infections or flu during gestation and later diagnosis of ASD. However, we did observe a significant association between prenatal exposure to fever, at any time during pregnancy as well as during the third trimester, and ASD.
This result is consistent with most prior studies showing ASD risk is not related to prenatal exposure to genitourinary infection or flu (Figure 2). Only three prior studies have specifically examined prenatal exposure to fever, at any point in pregnancy or during specific trimesters, and ASD risk, with conflicting results. Our study showing increased ASD risk associated with fever is consistent with Zerbo et al. and Hornig et al. (Figure 2) (Zerbo et al. 2013, Hornig et al. 2017) but not Atladottir et al. (Atladottir et al. 2012). The prenatal exposure definition used by Atladottir et al. included only data through week 32; however, the other studies, including our own, have used fever exposure for the entire pregnancy period (week 1 to birth). Thus, the Atladottir et al. definition did not include a large portion of the third trimester, shown to be significantly associated with ASD in our BBC data (Figures 1 and 2). Although the overall findings between Zerbo et al., Hornig et al., and our study were consistent, these two studies identified a significant association with fever in the second trimester while we observed a significant association with fever in the third trimester. It is possible that this disparity could be due to recall bias and/or exposure misclassification due to differences in study design. Since the BBC exposure data is collected at birth, the recall period for the third trimester is shorter than the first and second trimesters. It is also possible that this could result in greater exposure misclassification in early pregnancy and may influence trimester-specific findings.
Our study sample was derived from an enriched risk cohort, where children were initially recruited with oversampling for preterm birth, a known ASD risk factor. Exposure data was collected at birth, prior to ASD diagnoses, allowing for a relatively short recall time and prospective analysis for ASD. In contrast, Zerbo et al. (Zerbo et al. 2013) used a retrospective case-control design in which the exposure data was ascertained up to 60 months after birth (longer recall) and after ASD diagnosis. Additionally, our study population has a different racial and economic make-up (Table 1) than the population in Zerbo et al., which was approximately 50% white (Zerbo et al. 2013).
Our findings were specific to fever, which could indicate that fever itself contributes to ASD risk. There is evidence that exposure to fever during pregnancy can lead to several different suboptimal developmental outcomes including oral clefts, neural tube defects, and congenital heart defects (Dreier et al. 2014). In support of this, Zerbo et al. (Zerbo et al. 2013) found an attenuation of the fever-ASD association with the use of antipyretics such as acetaminophen. However, rather than fever itself being in the causal ASD pathway, it is also possible that fever is merely acting as a marker of a specific infection that is associated with increased ASD risk but that is not captured by our questionnaire data. The BBC prenatal infection exposure data is ascertained soon after birth with a structured interview and questionnaire designed to ascertain exposures in a reliable manner. Nonetheless, the questionnaire is retrospective and it is possible that mothers remember fever better than infections generally. Finally, it is possible that the pathology of ASD begins during gestational development and leads to increased maternal infections and fever through immunocompromise, although there is little evidence to support this.
We considered the possibility that being born prematurely or with a low birth weight could be mediators of a relationship between prenatal fever exposure and risk for ASD. However, we found that in our dataset gestational age (as either a continuous or binary variable) was not significantly associated with fever. When we compared analytic models that either included or excluded these factors as covariates (Table 2; e.g. Analytic Model 4 vs 5 for gestational age), the estimated effect size for a fever-ASD association was consistent. Thus, we have treated gestational age as a confounder, rather than a mediator. However, because of the unique characteristics of the enriched-risk BBC cohort, it is possible that this type of relationship is specific to the BBC.
We observed relatively large confidence intervals in our study compared to prior work (Figure 2). This is likely due to the relatively small number of individuals in our study and thus imprecision in these estimates. Because our study relied on the extraction of ASD outcomes from electronic medical records, specifically ICD-9-CM codes, there may be some outcome misclassification. However, we would not expect any outcome misclassification to be differential by exposure status. Additionally, there is significant co-morbidity among the ASD cases in our sample for developmental delay; of our 116 ASD cases, 111 also have a diagnosis for a developmental delay (ICD-9-CM codes between 315.0 and 315.9). Prior research in a similar EMR data set has found this to be true among validated ASD cases (Dodds et al. 2009), and so we believe there is limited outcome misclassification. This conclusion is reinforced by the findings of our outcome misclassification sensitivity analysis, which continued to show a significant relationship between fever and ASD risk after using a more stringent ASD case definition.
We did not perform an adjustment for multiple comparisons. We examined ten total exposures (GU infections; flu, overall and by trimester; fever, overall and by trimester; and intrapartum fever), and the significant association we found between prenatal exposure to fever and ASD risk would not survive a conservative Bonferroni correction. Additional research with larger samples is necessary to clarify the role of prenatal exposure to fever in the development of ASD.
Future studies in urban, low-income, minority populations are needed to replicate our findings. In addition, future work to evaluate potential clinical interventions is needed. One previous study from the Danish national birth cohort did assess the association between anti-pyretic medications and ASD (Liew et al. 2016). Unexpectedly, the results showed an increased risk for ASD with hyperkinetic symptoms and maternal use of acetaminophen during pregnancy (Liew et al. 2016), which is contradictory to results from Zerbo et al. To help resolve these differences in the literature, future studies should collect and evaluate more detailed information on prenatal exposure to fever, including (1) timing during pregnancy, (2) severity, or degree of temperature elevation, (3) duration of fever, and (4) use of anti-pyretics, including timing and dose.
There has been limited prior research on the relationship between prenatal fever exposure and ASD risk, particularly among under represented and minority populations. Our findings expand upon past work, and provide the first evidence supporting prenatal exposure to fever as a risk factor for ASD in an urban, low income, minority population, adding to our knowledge of a highly prevalent modifiable risk factor that may inform public health strategies for primary and secondary prevention.
Supplementary Material
Acknowledgments
Funding source: The Boston Birth Cohort (the parent study) was supported in part by the March of Dimes PERI grants (20-FY02-56, #21-FY07-605), the National Institutes of Health (NIH) grants (R21ES011666, 2R01HD041702, R21HD066471, and R01HD086013); and Maternal and Child Health Bureau (R40MC27443).
Footnotes
Financial disclosure statement: None of the authors have any financial relationship relevant to this article to disclose
Conflict of interest: None of the authors have a conflict of interest pertaining to this work
References
- Atladottir HO, Henriksen TB, Schendel DE, Parner ET. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics. 2012;130(6):e1447–1454. doi: 10.1542/peds.2012-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40(12):1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ. Developmental origins of non-communicable disease: implications for research and public health. Environ Health. 2012;11:42. doi: 10.1186/1476-069X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Iosif AM, Smith SE, Bregere C, Amaral DG, Patterson PH. Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biol Psychiatry. 2014;75(4):332–341. doi: 10.1016/j.biopsych.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DL, Baio J, Van Naarden Braun K, Bilder D, Charles J, Constantino JN, Daniels J, Durkin MS, Fitzgerald RT, Kurzius-Spencer M, Lee LC, Pettygrove S, Robinson C, Schulz E, Wells C, Wingate MS, Zahorodny W, Yeargin-Allsopp M C. Centers for Disease and Prevention. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years--Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill Summ. 2016;65(3):1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman KJ, Lutsky MA, Yau V, Qian Y, Pomichowski ME, Crawford PM, Lynch FL, Madden JM, Owen-Smith A, Pearson JA, Pearson KA, Rusinak D, Quinn VP, Croen LA. Validation of Autism Spectrum Disorder Diagnoses in Large Healthcare Systems with Electronic Medical Records. J Autism Dev Disord. 2015;45(7):1989–96. doi: 10.1007/s10803-015-2358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier SA, Rasmussen SA, Feldkamp ML, Honein MA S. National Birth Defects Prevention. Prevalence of self-reported infection during pregnancy among control mothers in the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2009;85(3):193–201. doi: 10.1002/bdra.20540. [DOI] [PubMed] [Google Scholar]
- Dodds L, Spencer A, Shea S, Fell D, Armson BA, Allen AC, Bryson S. Validity of autism diagnoses using administrative health data. Chronic Dis Can. 2009;29(3):102–107. [PMC free article] [PubMed] [Google Scholar]
- Dreier JW, Andersen AM, Berg-Beckhoff G. Systematic review and meta-analyses: fever in pregnancy and health impacts in the offspring. Pediatrics. 2014;133(3):e674–688. doi: 10.1542/peds.2013-3205. [DOI] [PubMed] [Google Scholar]
- Fang SY, Wang S, Huang N, Yeh HH, Chen CY. Prenatal Infection and Autism Spectrum Disorders in Childhood: A Population-Based Case-Control Study in Taiwan. Paediatr Perinat Epidemiol. 2015;29(4):307–316. doi: 10.1111/ppe.12194. [DOI] [PubMed] [Google Scholar]
- Graham JW. Missing data analysis: Making it work in the real world. Annual Review of Psychology. 2008;60(6):1–28. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J, Smith K, Lotspeich L, Croen LA, Ozonoff S, Lajonchere C, Grether JK, Risch N. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68(11):1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho DE, Imai K, King G, Stuart EA. Matching as nonparametric preprocessing for reducing model dependance in parametric causal inference. Political Analysis. 2007;15:199–236. [Google Scholar]
- Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. Journal of Statistical Software. 2011;42(8) [Google Scholar]
- Hornig M, Bresnahan MA, Che X, Schultz AF, Ukaigwe JE, Eddy ML, Hirtz D, Gunnes N, Lie KK, Magnus P, Mjaaland S, Reichborn-Kjennerud T, Schjølberg S, Øyen AS, Levin B, Susser ES, Stoltenberg C, Lipkin WI. Prenatal fever and autism risk. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.119. ePub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc Natl Acad Sci U S A. 2012;109(31):12776–12781. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HY, Xu LL, Shao L, Xia RM, Yu ZH, Ling ZX, Yang F, Deng M, Ruan B. Maternal infection during pregnancy and risk of autism spectrum disorders: A systematic review and meta-analysis. Brain Behav Immun. 2016;58:165–172. doi: 10.1016/j.bbi.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Lash TL, Fox MP, Fink AK. Applying Quantitative Bias Analysis to Epidemiologic Data. Springer; 2009. [Google Scholar]
- Le Belle JE, Sperry J, Ngo A, Ghochani Y, Laks DR, Lopez-Aranda M, Silva AJ, Kornblum HI. Maternal inflammation contributes to brain overgrowth and autism-associated behaviors through altered redox signaling in stem and progenitor cells. Stem Cell Reports. 2014;3(5):725–734. doi: 10.1016/j.stemcr.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BK, Magnusson C, Gardner RM, Blomstrom A, Newschaffer CJ, Burstyn I, Karlsson H, Dalman C. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav Immun. 2015;44:100–105. doi: 10.1016/j.bbi.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew Z, Ritz B, Virk J, Olsen J. Maternal use of acetaminophen during pregnancy and risk of autism spectrum disorders in childhood: A Danish national birth cohort study. Autism Res. 2016;9(9):951–958. doi: 10.1002/aur.1591. [DOI] [PubMed] [Google Scholar]
- Lyall K, Schmidt RJ, Hertz-Picciotto I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int J Epidemiol. 2014;43(2):443–464. doi: 10.1093/ije/dyt282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Whitaker AM, Smith SE, Patterson PH, Bauman MD. Maternal immune activation in nonhuman primates alters social attention in juvenile offspring. Biol Psychiatry. 2015;77(9):823–832. doi: 10.1016/j.biopsych.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26(4):607–616. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques AH, O’Connor TG, Roth C, Susser E, Bjorke-Monsen AL. The influence of maternal prenatal and early childhood nutrition and maternal prenatal stress on offspring immune system development and neurodevelopmental disorders. Front Neurosci. 2013;7:120. doi: 10.3389/fnins.2013.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, Sarrazin S, Ben-Yehuda H, David E, Zelada Gonzalez F, Perrin P, Keren-Shaul H, Gury M, Lara-Astaiso D, Thaiss CA, Cohen M, Bahar Halpern K, Baruch K, Deczkowska A, Lorenzo-Vivas E, Itzkovitz S, Elinav E, Sieweke MH, Schwartz M, Amit I. Microglia development follows a stepwise program to regulate brain homeostasis. Science. 2016;353(6301):aad8670. doi: 10.1126/science.aad8670. [DOI] [PubMed] [Google Scholar]
- Miller VM, Zhu Y, Bucher C, McGinnis W, Ryan LK, Siegel A, Zalcman S. Gestational flu exposure induces changes in neurochemicals, affiliative hormones and brainstem inflammation, in addition to autism-like behaviors in mice. Brain Behav Immun. 2013;33:153–163. doi: 10.1016/j.bbi.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Ohkawara T, Katsuyama T, Ida-Eto M, Narita N, Narita M. Maternal viral infection during pregnancy impairs development of fetal serotonergic neurons. Brain Dev. 2015;37(1):88–93. doi: 10.1016/j.braindev.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Persico AM, Napolioni V. Autism genetics. Behav Brain Res. 2013;251:95–112. doi: 10.1016/j.bbr.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J. Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol. 1996;370(2):247–261. doi: 10.1002/(SICI)1096-9861(19960624)370:2<247::AID-CNE8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. JAMA. 2014;311(17):1770–1777. doi: 10.1001/jama.2014.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotz W, Phillips DI. Fetal origins of mental health: evidence and mechanisms. Brain Behav Immun. 2009;23(7):905–916. doi: 10.1016/j.bbi.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Schwartzer JJ, Careaga M, Onore CE, Rushakoff JA, Berman RF, Ashwood P. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transl Psychiatry. 2013;3:e240. doi: 10.1038/tp.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Smith SE, Malkova N, Tse D, Su Y, Patterson PH. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav Immun. 2009;23(1):116–123. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner R, Chow ML, Boyle MP, Sunkin SM, Mouton PR, Roy S, Wynshaw-Boris A, Colamarino SA, Lein ES, Courchesne E. Patches of disorganization in the neocortex of children with autism. N Engl J Med. 2014;370(13):1209–1219. doi: 10.1056/NEJMoa1307491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley ME, Togher KL, Nolan AM, Kenny LC, O’Keeffe GW. LPS alters placental inflammatory and endocrine mediators and inhibits fetal neurite growth in affected offspring during late gestation. Placenta. 2014;35(8):533–538. doi: 10.1016/j.placenta.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Wang G, Divall S, Radovick S, Paige D, Ning Y, Chen Z, Ji Y, Hong X, Walker SO, Caruso D, Pearson C, Wang MC, Zuckerman B, Cheng TL, Wang X. Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA. 2014;311(6):587–596. doi: 10.1001/jama.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, Niu T, Wise PH, Bauchner H, Xu X. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287(2):195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- Zerbo O, Iosif AM, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J Autism Dev Disord. 2013;43(1):25–33. doi: 10.1007/s10803-012-1540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbo O, Qian Y, Yoshida C, Fireman BH, Klein NP, Croen LA. Association Between Influenza Infection and Vaccination During Pregnancy and Risk of Autism Spectrum Disorder. JAMA Pediatr. 2017;171(1):e163609. doi: 10.1001/jamapediatrics.2016.3609. [DOI] [PubMed] [Google Scholar]
- Zerbo O, Qian Y, Yoshida C, Grether JK, Van de Water J, Croen LA. Maternal Infection During Pregnancy and Autism Spectrum Disorders. J Autism Dev Disord. 2015;45(12):4015–4025. doi: 10.1007/s10803-013-2016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.