Abstract
Background
Few studies have characterized reference values of normal human skin microanatomy parameters.
Objective
To quantify histologic measurements of epidermal thickness (ET), melanocyte density (MD), hair follicle density (HFD), and eccrine gland density (EGD), as a function of age and anatomic site.
Methods
We searched PubMed, Embase, Web of Science and Cochrane databases for articles published through May 25, 2017. Two reviewers independently screened 2,016 articles; 327 relevant articles and 151 additional articles found via forward or reference citations underwent full-text review by one of four reviewers for relevance, data extraction, and critical appraisal. Weighted averages, meta-analysis, and meta-regression were used in statistical analysis.
Results
Fifty-six articles were included; using all anatomic locations, overall estimates for ET, MD, HFD, and EGD were 99.75 microns (95% CI 83.25–116.25), 955.05 cells/mm2 (95% CI 880.89–1029.21), 1.40 hairs/mm2 (95% CI 0.91–1.89), and 1.28 glands/mm2 (95% CI 0.91–1.64), respectively.
Limitations
There was significant data heterogeneity across studies, possibly due to differences in histological techniques and absence of standardized microanatomy definitions.
Conclusion
We established summary estimates for normal human skin microanatomy parameters.
Keywords: Normal skin, epidermal thickness, melanocyte density, eccrine gland density, hair follicle density, systematic review
Introduction
The microanatomy of human skin is structurally complex and potentially influenced by a multitude of factors, such as age, sex, genetics, skin type, medical comorbidities, and environmental exposures.1–5 Previous studies have reported significant anatomic variation in the morphologic characteristics of the skin;3,6 however, considerable diversity in study design and methodology makes comparison and synthesis of morphologic characteristics difficult. A compilation of the existing evaluations of healthy skin across varied anatomical sites can inform knowledge and the study of site-dependent morphology of cutaneous inflammatory and neoplastic processes and guide interpretation of non-invasive imaging modalities.
The objective of this study was to use meta-analysis techniques to: (1) combine histological measurements of epidermal thickness, stratum corneum thickness, melanocyte density, hair follicle density, and eccrine gland density across anatomic sites, and (2) compare histological characteristics of certain skin microanatomy parameters with respect to age.
Methods
Literature Search
Systematic literature searches were conducted on November 22, 2013 and May 25, 2017 in four databases [MEDLINE (via PubMed), Embase, the Cochrane Library, and Web of Science] for references written in all languages without sex, age, or publication type restrictions. For PubMed, Embase, and Cochrane Library searches, both controlled vocabulary and text words were used in search strategy development. The Web of Science database does not employ a controlled vocabulary, so it was searched using only text words. All search results were combined in a bibliographic management tool (EndNote) and duplicates were eliminated electronically and manually.
The search strategy had two components and both concepts were linked together with the AND operator: (1) anatomic site including head/neck, chest, abdomen, torso, posterior and back, buttocks, genitalia, upper extremities, and lower extremities; (2) microanatomy parameters including epidermal and stratum corneum thickness, melanocyte density, hair follicle density, and sweat/eccrine gland density. For a complete list of MeSH and keyword terms used, please refer to the accompanying PubMed search strategy (Supplemental Information).
Data Abstraction
Two reviewers (M.M. and X.W. or H.X. and Z.W.) independently screened all article titles and abstracts. All identified articles subsequently underwent full-text review by one of four independent reviewers (M.F., X.W., E.C., or Z.W.). Articles were excluded if they lacked relevant microanatomy parameters, were not written in English, measured skin microanatomy in non-healthy participants, or failed to report data numerically. All potentially relevant forward or reference citations underwent full-text review. Data on microanatomy parameter, methodology, technique, subject age, sex, skin type, race and ethnicity, anatomic site, sample size, mean, and standard deviation were systematically extracted from all included articles. Two additional independent reviewers (Z.W. and H.X.) re-examined all articles to exclude studies and extract additional data relevant to analyses.
Skin Microanatomy Parameters and Eligibility Criteria
Multiple different modalities investigating skin microanatomy were found, including ex vivo techniques such as histological sampling as well as in vivo imaging techniques such as reflectance-mode confocal laser microscopy (RCM) and optical coherence topography (OCT). Our original intent was to also include all modalities in our analyses, but given the lack of robust data in our literature search, we restricted analyses to studies with histopathology only.
Epidermal thickness (ET) was defined as distance between top of the stratum corneum to top of the dermis. A wide range of measurement definitions for ET were found in the literature. Many studies used distance between top of the stratum granulosum to top of the dermis. To categorize this measurement, we created an additional parameter called granulosa-epidermal thickness (GET). Stratum corneum thickness (SCT) was defined as the distance between top of the stratum corneum to bottom of the stratum corneum. All thickness parameters were reported in micrometers. Studies with measurements not in metric units (e.g. number of layers) were excluded.
Melanocyte density (MD) was defined as number of melanocytes per mm2. Hair follicle density (HFD) was defined as number of hair follicles per mm2. Eccrine gland density (EGD) was defined as number of eccrine glands per mm2. Studies with measurements not convertible to these units (e.g. number per high power field) were excluded.
All studies with aggregated data from multiple patients that did not include a measure of variance (e.g. standard deviation or standard error) were excluded from analysis. For studies providing individual patient data, the mean and standard deviation of the various parameters were calculated.
Anatomic locations from which skin microanatomy parameters were taken were categorized into six groups: head/neck, upper extremities (including dorsal hand), trunk (including chest, back, abdomen, and axilla), lower extremities (including buttocks and dorsal foot), palms/soles, and genitalia. Since reported age range varied between and within studies, mean patient age for each study was calculated. Mean age was categorized as two categories (0–60 and >60 years) or three categories (0–18, 19–60, and >60 years) according to available data.
Statistical Analysis
Weighted averages for each parameter of interest (ET, GET, SCT, MD, HFD, and EGD) were calculated for all relevant studies and stratified by anatomic location. Weighted averages for ET and melanocyte density were calculated for all relevant studies and stratified by mean age. Meta-analysis was performed using a random-effects model, with each skin microanatomy parameter as an effect size (ES, 95% CI). The I2 statistic was calculated to measure heterogeneity among studies. Forest plots were constructed for each microanatomy parameter. Meta-regression was used to compare skin microanatomy parameters among different anatomic locations as well as age groups. All statistical analyses were carried out using Stata v.14.1 software (Stata Corp., College Station, TX, USA).
Results
Literature Search
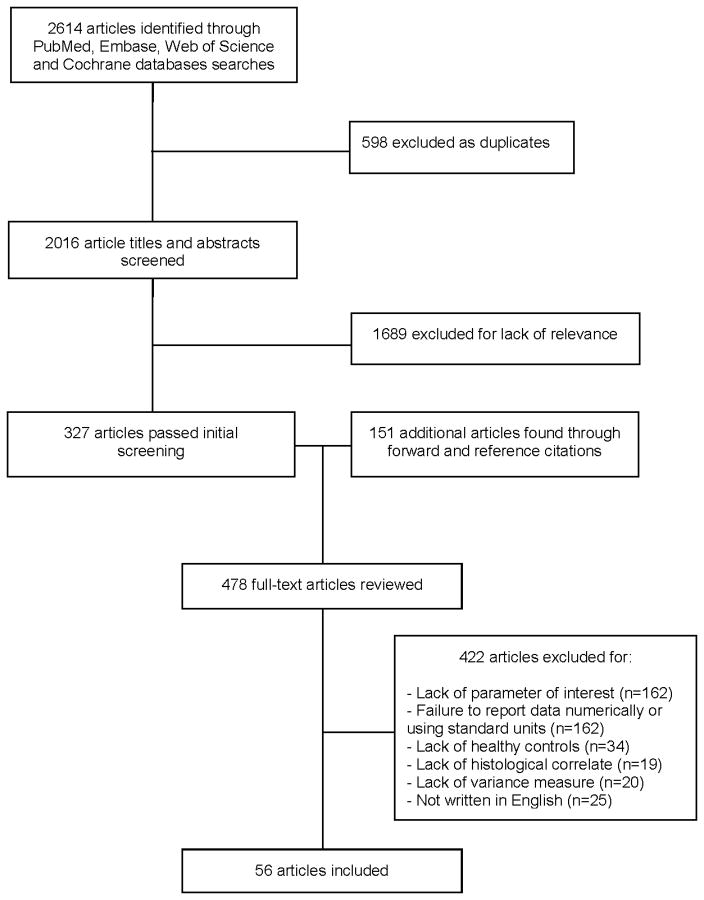
The initial systematic literature search yielded 2,016 unique articles, of which 896 were found in MEDLINE (via Pubmed), 715 in Embase, 348 in Web of Science, and 57 in the Cochrane Library (Figure 1). Of these, a total of 327 articles passed the initial screening based on article title and abstract. Full-text reviews of these 327 articles for relevant forward or reference citations yielded an additional 151 potential articles. Full-text reviews were conducted on a total of 478 articles, of which 422 were excluded, and 56 were included in the final analysis (Supplemental Information).
Figure 1.
Flowchart of studies of skin microanatomy parameters
Epidermal thickness (ET)
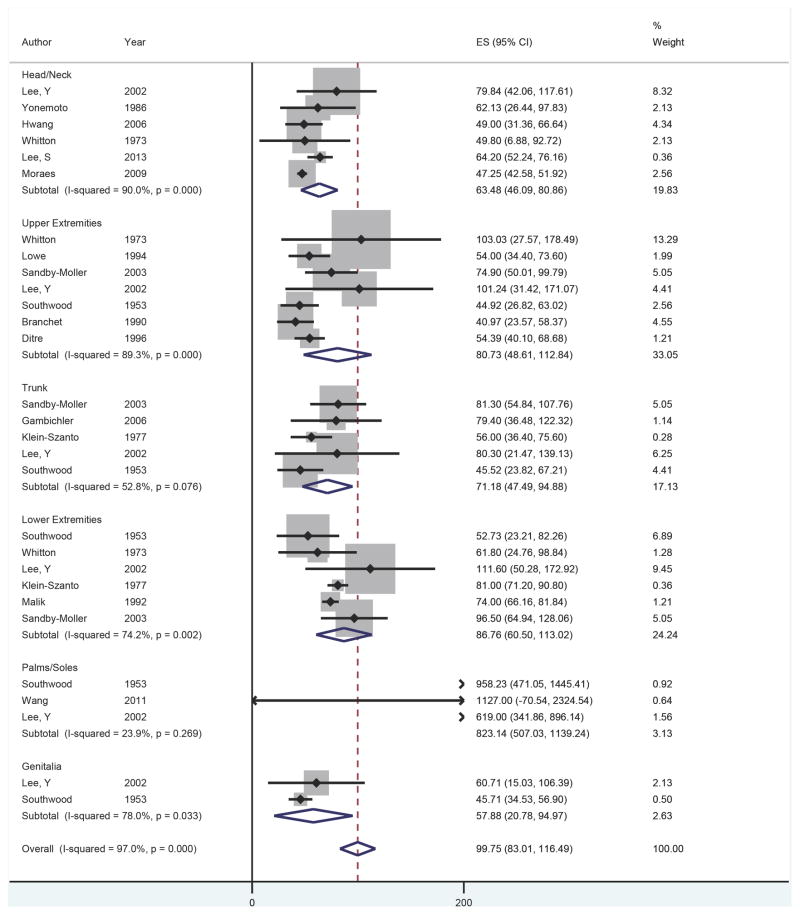
Fifteen studies were included with 29 observations of ET by anatomic location.3,6–19 A forest plot of ET at each anatomic location is presented in Figure 2. The overall ET across all anatomic locations was 99.75 microns (95% CI 83.25–116.25); excluding palms and soles, the overall ET was 76.50 microns (95% CI 62.76–90.04). The palms and soles had the thickest epidermis, followed by the lower extremities, upper extremities, trunk, head/neck, and genitalia. On meta-regression, the palms and soles were significantly thicker compared to the head/neck (β = 669.17, p<0.001). No other significant differences in thickness were observed between anatomic locations.
Figure 2.
Forest plot of epidermal thickness by anatomic location
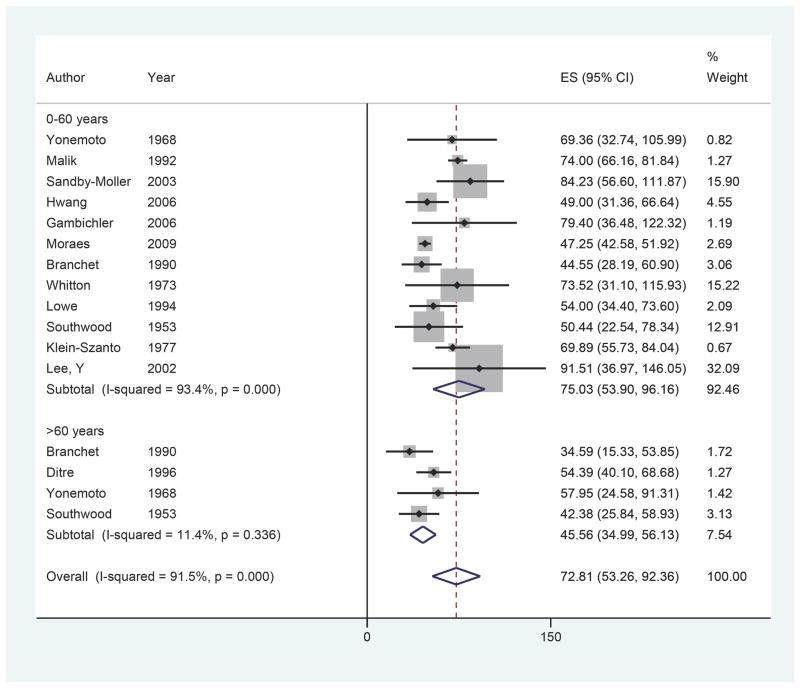
Twelve of 15 studies included age data, yielding 16 observations of ET by mean age (Figure 3).3,5–7,9,10,12–15,17,18 Overall ET was found to be 75.03 microns (95% CI 53.90–96.16) for ages 0–60 compared to 45.56 microns (95% CI 34.99–56.13) for ages 60 and older. While not reaching significance, meta-regression found the ET of the ages > 60 group to be thinner compared to the ages < 60 group (β = −18.75, p=0.089).
Figure 3.
Forest plot of epidermal thickness by age
Results of analyses for GET3,20–36 and SCT3,8,10,35,37–41 by anatomic location are in Supplemental Information.
Melanocyte Density
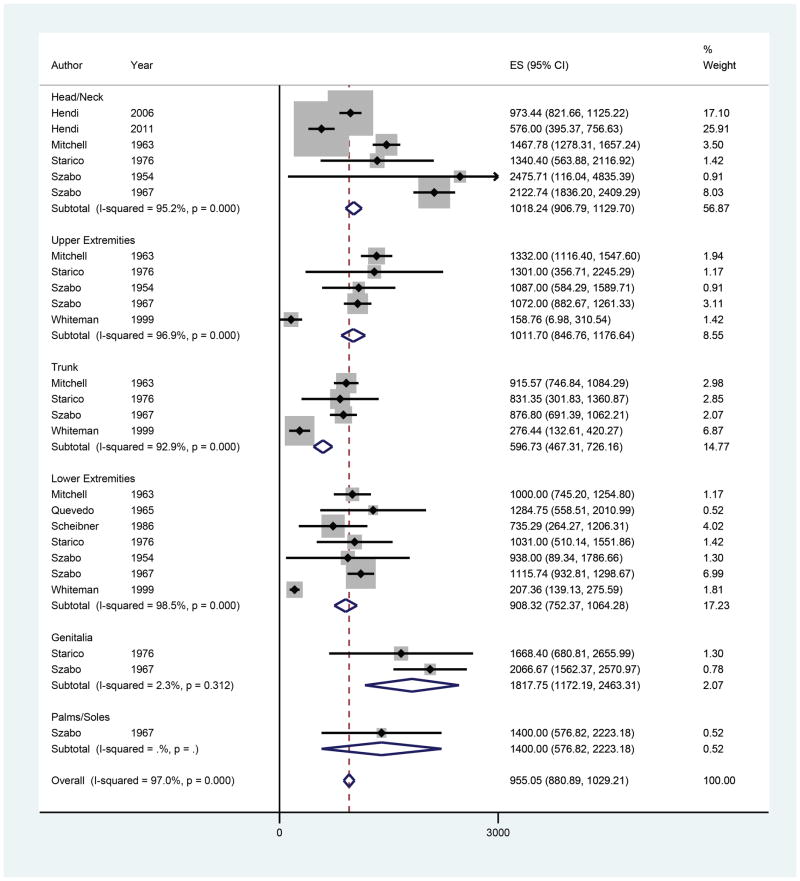
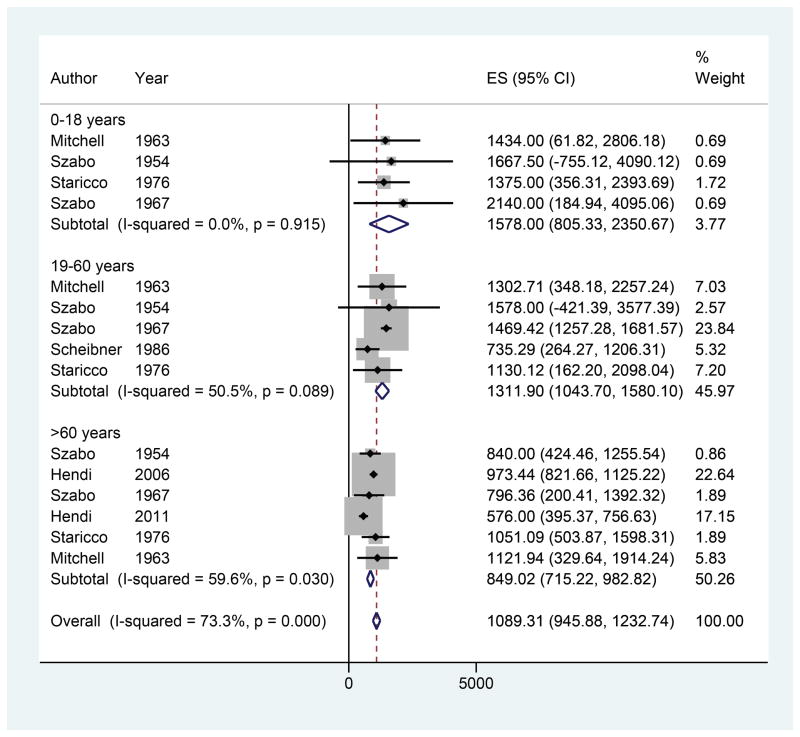
Nine studies were included with 25 observations of MD by anatomic location (Figure 4).4,42–49 The overall estimate of MD was 955.05 cells/mm2 (95% CI 880.89–1029.21). The genitalia had the highest MD, followed by the head/neck, upper extremities, lower extremities, and trunk. There was only one observation of MD for the palms/soles (ES 1400.00, 95% CI 574.82–2223.18). On meta-regression, no anatomic locations exhibited significantly different melanocyte densities compared to the head/neck. In addition, 7 studies included age data yielding 15 observations of MD (Figure 5). Overall MD was found to be 1578.00 cells/mm2 (95% CI 805.33–2350.67) for ages 0–18, 1311.90 cells/mm2 (95% CI 1043.70–1580.10) for ages 19–60, and 849.02 cells/mm2 (95% CI 715.22–982.82) for ages 60 and older. On meta-regression, a trend of decreasing MD was observed with increasing age, but there were no significant differences in MD between the 19–60 age group and >60 age group compared to the 0–18 age group (β = −327.79, p=0.40 and β = −650.56, p=0.11, respectively).
Figure 4.
Forest plot of melanocyte density by anatomic location
Figure 5.
Forest plot of melanocyte density by age
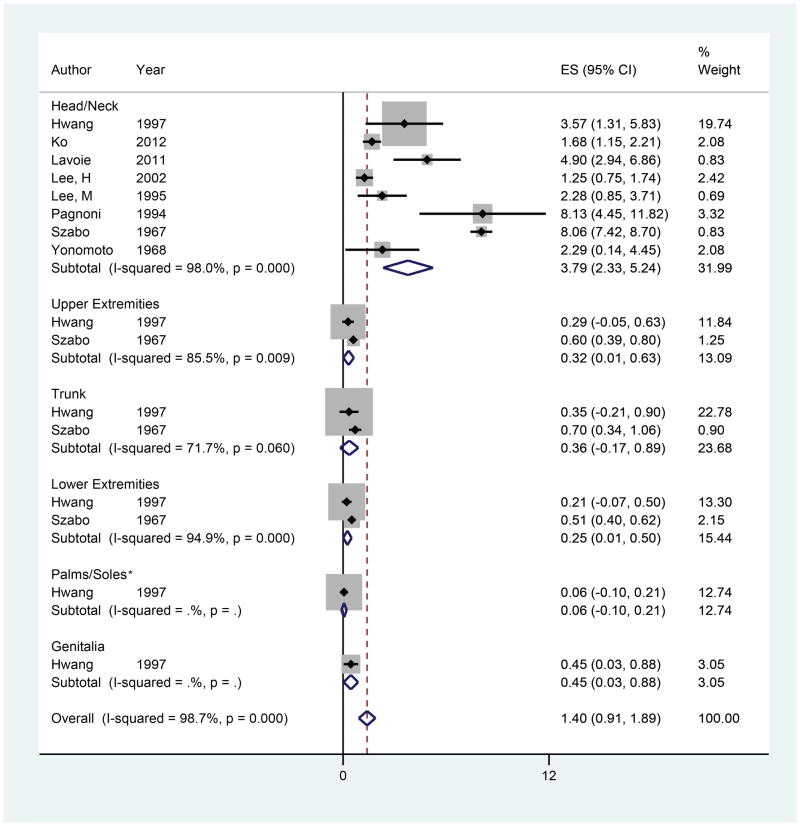
Hair Follicle Density (HFD)
Eight studies were included in the meta-analyses for HFD with 16 observations of HFD by anatomic location (Figure 6).18,48,50–55 The overall estimate of HFD was 1.40 hairs/mm2 (95% CI 0.91–1.89). The head/neck had the highest HFD, followed by the trunk, upper extremities, and lower extremities. There was only one observation of HFD for the genitalia and palms/soles. On meta-regression, the upper extremities, trunk, and lower extremities exhibited a lower HFD compared to the head/neck (β = −2.94, p =0.07; β = −2.86, p=0.08; and β = −3.02, p=0.067, respectively). However, no statistically significant differences in HFD were observed.
Figure 6.
Forest plot of hair follicle density by anatomic location
*Analyses of Palms/Soles include data on dorsal hand, dorsal feet, and fingers.
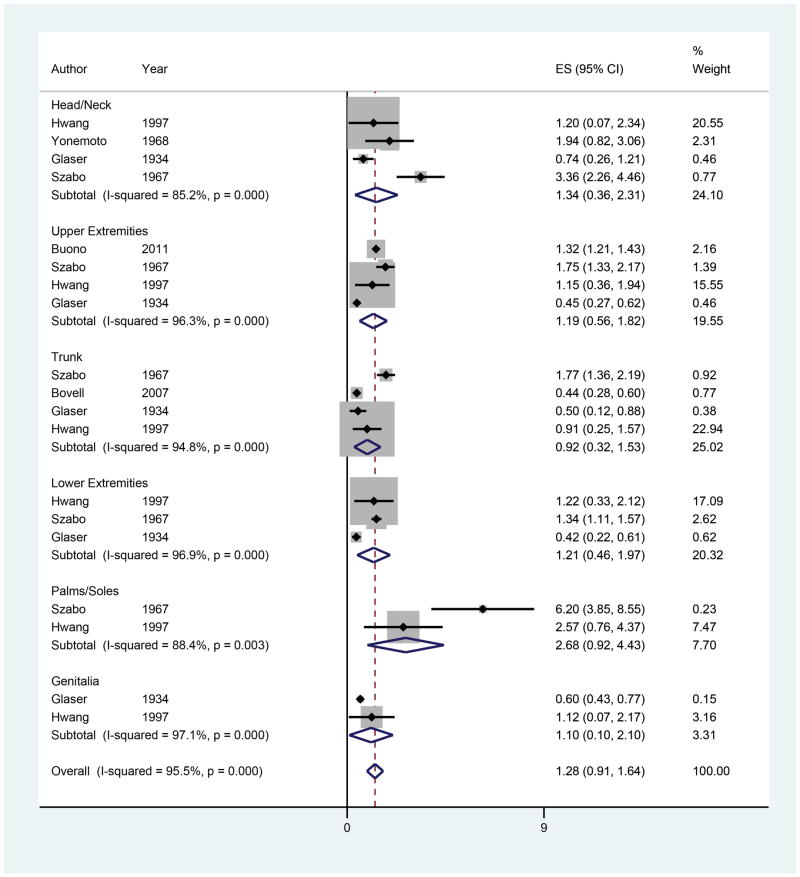
Eccrine Gland Density (EGD)
Six studies were included in the meta-analyses for EGD with 19 observations of EGD by anatomic location (Figure 7).48,50,56–59 The overall estimate of EGD was 1.28 glands/mm2 (95% CI 0.91–1.64). The palms/soles had the highest EGD, followed by the head/neck, lower extremities, upper extremities, genitalia and trunk. On meta-regression, no anatomic locations exhibited significantly different EGD compared to the head/neck.
Figure 7.
Forest plot of eccrine gland density by anatomic location
Discussion
Current knowledge of cutaneous function and skin microanatomy stems largely from investigations into its disease processes.60 Yet, there are few standardized metrics to characterize often-described microanatomy parameters such as ET in healthy skin. Our study aimed to examine and synthesize previous literature and compare histological measurements of various skin microanatomy parameters by age and anatomic site. Establishing such parameters would offer insight for future histopathological correlation studies and could serve to inform the interpretation of in vivo imaging technologies such as RCM and OCT. Moreover, there seems to be limited consensus as to how these parameters should be defined, illustrated by the multitude of definitions of ET in the literature.10,17,61 For example, our review found ET to be reported as: top of the stratum corneum to top of the dermal papillae, top of the stratum corneum to middle of the dermal papillae, and top of the stratum corneum to bottom of the dermal papillae. This lack of standardization led us to define epidermal thickness as top of the stratum corneum to the top of the dermis, therefore including studies using any of the above definitions. The variable definitions highlight a significant challenge in parameter standardization and contribute to the limitations to our study.
The trends and values reported in our study are congruent with our current understanding of skin physiology. For example, the palms/soles were found to have the thickest epidermis and highest EGD, while the head/neck had the highest density of hair follicles. Additionally, studies have consistently shown that the MD of the human skin decreases with advancing age.4,43,49 There was also a degree of consistency with regard to our findings on the various thickness parameters ET, GET and SCT. The overall ET across all anatomic locations (99.75 microns) was found to be comparable to the sum (97.13 microns) of the overall GET (76.60 microns) and SCT (20.53 microns).
There are significant limitations to our study. First, the lack of observations across various anatomic locations (such as the genitalia and palms/soles) for some parameters made it difficult to identify trends with respect to anatomic location and evaluate differences between anatomic locations using meta-regression. The paucity of sample size for meta-analyses can be attributed to many studies not reporting measures of variance and the lack of standardization with regards to units of measurement. As a result, the trends observed for some parameters in our study were not statistically significant. For example, the difference in HFD of the head/neck was an order of magnitude greater than other anatomic locations. However, there were only two studies that investigated the HFD of anatomic locations other than the head/neck, which limited the statistical power of our analyses. Second, our study did not account for the various techniques used to create histological samples. The studies included in our meta-analysis featured a wide range of techniques involving every step of tissue specimen preparation, including: type of histology (surgical vs autopsy), fixation (formalin vs osmium tetroxide), processing (frozen vs paraffin), staining (H&E vs methylene blue), and sectioning (vertical vs horizontal). Additionally, some studies adjusted for specimen shrinkage while others did not, which could result in significant discrepancies in measurements.62,63 We acknowledge the importance of adequately controlling for histological technique, but also recognize the futility of conducting further subgroup analyses owing to the sample size of the current meta-analysis. Third, our analysis included literature from 1934 to 2017, which may have resulted in increased variation between studies attributed to the changes to the preparation and analysis of tissue specimens over the years. This was especially relevant to the determination of MD. DOPA staining, which was more commonly employed in older studies, relies on an enzymatic reaction within melanocytes to produce melanin. However, cross-reactivity with other cells may occur, leading to overestimations in the data. Immunohistochemistry, which stains for specific cell markers on the surface of melanocytes, may therefore be a more accurate enumeration method. Finally, due to variability in the reporting of age ranges between and within studies, we only compared skin microanatomy parameters by mean patient age for each study, which may not be completely representative of how age affects these measurements. All of these factors likely contributed to the substantial heterogeneity of study outcomes in our analyses.
Our study identified and aggregated the findings from previous literature in an attempt to unify the observations of various skin microanatomy parameters. From these results, it is clear that substantial variability exists among the existing studies quantifying these parameters, which is largely attributable to differences in histological methodology, advancement in histological techniques with time, and definition of parameters. The heterogeneity of data found in our study also highlights the need for the creation of metrics to measure healthy skin microanatomy parameters. To better characterize skin pathologies, a consensus on the characteristics of “normal” skin is required. In order to achieve this, we must first define each individual parameter, and then decide on an optimal method for measuring and reporting the parameter. Finally, we should consider the creation of baseline values and ranges of skin microanatomy parameters to which future findings may be compared.
Supplementary Material
Acknowledgments
Statement on Funding: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Abbreviations
- ET
epidermal thickness
- GET
granulosa-epidermal thickness
- SCT
stratum corneum thickness
- MD
melanocyte density
- HFD
hair follicle density
- EGD
eccrine gland density
Footnotes
IRB Review Statement: This study was exempted from IRB review.
Disclosures: None of the authors have any conflicts of interest to disclose.
References
- 1.Gambichler T, Boms S, Stucker M, et al. Acute skin alterations following ultraviolet radiation investigated by optical coherence tomography and histology. Arch Dermatol Res. 2005;297(5):218–225. doi: 10.1007/s00403-005-0604-6. [DOI] [PubMed] [Google Scholar]
- 2.El-Domyati MM, Ahmad HM, Nagy I, Zahran A. Expression of apoptosis regulatory proteins p53 and Bcl-2 in skin of patients with chronic renal failure on maintenance haemodialysis. Journal of the European Academy of Dermatology and Venereology : JEADV. 2007;21(6):795–801. doi: 10.1111/j.1468-3083.2006.02090.x. [DOI] [PubMed] [Google Scholar]
- 3.Sandby-Moller J, Poulsen T, Wulf HC. Epidermal thickness at different body sites: relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta dermato-venereologica. 2003;83(6):410–413. doi: 10.1080/00015550310015419. [DOI] [PubMed] [Google Scholar]
- 4.Hendi A, Wada DA, Jacobs MA, et al. Melanocytes in nonlesional sun-exposed skin: a multicenter comparative study. Journal of the American Academy of Dermatology. 2011;65(6):1186–1193. doi: 10.1016/j.jaad.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 5.Gambichler T, Matip R, Moussa G, Altmeyer P, Hoffmann K. In vivo data of epidermal thickness evaluated by optical coherence tomography: Effects of age, gender, skin type, and anatomic site. Journal of Dermatological Science. 2006;44(3):145–152. doi: 10.1016/j.jdermsci.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 6.SOUTHWOOD WFW. THE THICKNESS OF THE SKIN. Plastic and Reconstructive Surgery. 1955;15(5):423–429. doi: 10.1097/00006534-195505000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Branchet MC, Boisnic S, Frances C, Robert AM. Skin thickness changes in normal aging skin. Gerontology. 1990;36(1):28–35. doi: 10.1159/000213172. [DOI] [PubMed] [Google Scholar]
- 8.Gambichler T, Boms S, Stucker M, et al. Epidermal thickness assessed by optical coherence tomography and routine histology: preliminary results of method comparison. Journal of the European Academy of Dermatology and Venereology : JEADV. 2006;20(7):791–795. doi: 10.1111/j.1468-3083.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 9.Hwang K, Kim DJ, Hwang SH. Thickness of Korean upper eyelid skin at different levels. The Journal of craniofacial surgery. 2006;17(1):54–56. doi: 10.1097/01.scs.0000188347.06365.a0. [DOI] [PubMed] [Google Scholar]
- 10.Klein-Szanto AJ. Stereologic baseline data of normal human epidermis. The Journal of investigative dermatology. 1977;68(2):73–78. doi: 10.1111/1523-1747.ep12491611. [DOI] [PubMed] [Google Scholar]
- 11.Lee SH, Roh MR, Jung JY, Jee H, Nam KA, Chung KY. Effect of subdermal 1,444-nm pulsed neodymium-doped yttrium aluminum garnet laser on the nasolabial folds and cheek laxity. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al] 2013;39(7):1067–1078. doi: 10.1111/dsu.12183. [DOI] [PubMed] [Google Scholar]
- 12.Lee Y, Hwang K. Skin thickness of Korean adults. Surgical and radiologic anatomy : SRA. 2002;24(3–4):183–189. doi: 10.1007/s00276-002-0034-5. [DOI] [PubMed] [Google Scholar]
- 13.Lowe PM, Woods J, Lewis A, Davies A, Cooper AJ. Topical tretinoin improves the appearance of photo damaged skin. The Australasian journal of dermatology. 1994;35(1):1–9. doi: 10.1111/j.1440-0960.1994.tb01790.x. [DOI] [PubMed] [Google Scholar]
- 14.Malik RA, Metcalfe J, Sharma AK, Day JL, Rayman G. Skin epidermal thickness and vascular density in type 1 diabetes. Diabetic medicine : a journal of the British Diabetic Association. 1992;9(3):263–267. doi: 10.1111/j.1464-5491.1992.tb01773.x. [DOI] [PubMed] [Google Scholar]
- 15.Moraes AB, Haidar MA, Soares JM, Simoes MJ, Baracat EC, Patriarca MT. The effects of topical isoflavones on postmenopausal skin: Double-blind and randomized clinical trial of efficacy. European Journal of Obstetrics Gynecology and Reproductive Biology. 2009;146(2):188–192. doi: 10.1016/j.ejogrb.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Wang YN, Lee K, Ledoux WR. Histomorphological evaluation of diabetic and non-diabetic plantar soft tissue. Foot & ankle international/American Orthopaedic Foot and Ankle Society [and] Swiss Foot and Ankle Society. 2011;32(8):802–810. doi: 10.3113/FAI.2011.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitton JT, Everall JD. The thickness of the epidermis. The British journal of dermatology. 1973;89(5):467–476. doi: 10.1111/j.1365-2133.1973.tb03007.x. [DOI] [PubMed] [Google Scholar]
- 18.Yonemoto Y. Studies on Measurements of the Scalp Tissue of the Japanese Adult (I) Okajimas folia anatomica Japonica. 1968;45:83–97. doi: 10.2535/ofaj1936.45.2-3_83. [DOI] [PubMed] [Google Scholar]
- 19.Ditre CM, Griffin TD, Murphy GF, et al. Effects of alpha-hydroxy acids on photoaged skin: a pilot clinical, histologic, and ultrastructural study. Journal of the American Academy of Dermatology. 1996;34(2 Pt 1):187–195. doi: 10.1016/s0190-9622(96)80110-1. [DOI] [PubMed] [Google Scholar]
- 20.Carlson JA, Ambros R, Malfetano J, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Human pathology. 1998;29(9):932–948. doi: 10.1016/s0046-8177(98)90198-8. [DOI] [PubMed] [Google Scholar]
- 21.Christophers E, Plewig G. Formation of the acrosyringium. Archives of dermatology. 1973;107(3):378–382. [PubMed] [Google Scholar]
- 22.El-Domyati M, Abd-El-Raheem T, Abdel-Wahab H, et al. Fractional versus ablative erbium:yttrium-Aluminum-garnet laser resurfacing for facial rejuvenation: An objective evaluation. Journal of the American Academy of Dermatology. 2013;68(1):103–112. doi: 10.1016/j.jaad.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 23.El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11(5):398–405. doi: 10.1034/j.1600-0625.2002.110502.x. [DOI] [PubMed] [Google Scholar]
- 24.El-Domyati MM, Attia SK, Esmat AM, Ahmad HM, Abdel Wahab HM, Badr BM. Effect of laser resurfacing on p53 expression in photoaged facial skin. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al] 2007;33(6):668–675. doi: 10.1111/j.1524-4725.2007.33141.x. [DOI] [PubMed] [Google Scholar]
- 25.Falstie-Jensen N, Spaun E, Brochner-Mortensen J, Falstie-Jensen S. The influence of epidermal thickness on transcutaneous oxygen pressure measurements in normal persons. Scandinavian journal of clinical and laboratory investigation. 1988;48(6):519–523. doi: 10.3109/00365518809085767. [DOI] [PubMed] [Google Scholar]
- 26.Humphries JD, Parry EJ, Watson RE, Garrod DR, Griffiths CE. All-trans retinoic acid compromises desmosome expression in human epidermis. The British journal of dermatology. 1998;139(4):577–584. doi: 10.1046/j.1365-2133.1998.02451.x. [DOI] [PubMed] [Google Scholar]
- 27.Seite S, Bredoux C, Compan D, et al. Histological evaluation of a topically applied retinol-vitamin C combination. Skin pharmacology and physiology. 2005;18(2):81–87. doi: 10.1159/000083708. [DOI] [PubMed] [Google Scholar]
- 28.Seite S, Colige A, Piquemal-Vivenot P, et al. A full-UV spectrum absorbing daily use cream protects human skin against biological changes occurring in photoaging. Photodermatology, photoimmunology & photomedicine. 2000;16(4):147–155. doi: 10.1034/j.1600-0781.2000.160401.x. [DOI] [PubMed] [Google Scholar]
- 29.Seite S, Moyal D, Richard S, et al. Effects of repeated suberythemal doses of UVA in human skin. European Journal of Dermatology. 1998;7(3):204–209. [Google Scholar]
- 30.Usami S, Okazaki M, Nitta T, Uemura N, Homma T, Akita K. Histological investigation of common insensate flaps obtained from the hand and forearm regions for use in fingertip reconstruction. J Plast Surg Hand Surg. 2017;51(3):182–186. doi: 10.1080/2000656X.2016.1213733. [DOI] [PubMed] [Google Scholar]
- 31.El-Domyati M, Abd-El-Raheem T, Medhat W, Abdel-Wahab H, Al Anwer M. Multiple fractional erbium: yttrium-aluminum-garnet laser sessions for upper facial rejuvenation: clinical and histological implications and expectations. J Cosmet Dermatol. 2014;13(1):30–37. doi: 10.1111/jocd.12079. [DOI] [PubMed] [Google Scholar]
- 32.El-Domyati M, Medhat W, Abdel-Wahab HM, Moftah NH, Nasif GA, Hosam W. Forehead wrinkles: a histological and immunohistochemical evaluation. J Cosmet Dermatol. 2014;13(3):188–194. doi: 10.1111/jocd.12097. [DOI] [PubMed] [Google Scholar]
- 33.El-Domyati M, Barakat M, Awad S, Medhat W, El-Fakahany H, Farag H. Multiple microneedling sessions for minimally invasive facial rejuvenation: an objective assessment. Int J Dermatol. 2015;54(12):1361–1369. doi: 10.1111/ijd.12761. [DOI] [PubMed] [Google Scholar]
- 34.El-Domyati M, Attia SK, El-Sawy AE, et al. The use of Botulinum toxin-a injection for facial wrinkles: a histological and immunohistochemical evaluation. J Cosmet Dermatol. 2015;14(2):140–144. doi: 10.1111/jocd.12144. [DOI] [PubMed] [Google Scholar]
- 35.Uemura N, Okazaki M, Mori H. Anatomical and histological study to determine the border of sole skin. Surg Radiol Anat. 2016;38(7):767–773. doi: 10.1007/s00276-015-1609-2. [DOI] [PubMed] [Google Scholar]
- 36.El-Domyati M, El-Ammawi TS, Medhat W, Moawad O, Mahoney MG, Uitto J. Effects of the Nd:YAG 1320-nm laser on skin rejuvenation: clinical and histological correlations. Journal of cosmetic and laser therapy : official publication of the European Society for Laser Dermatology. 2011;13(3):98–106. doi: 10.3109/14764172.2011.586423. [DOI] [PubMed] [Google Scholar]
- 37.de Fine Olivarius F, Wulf HC, Therkildsen P, Poulsen T, Crosby J, Norval M. Urocanic acid isomers: relation to body site, pigmentation, stratum corneum thickness and photosensitivity. Arch Dermatol Res. 1997;289(9):501–505. doi: 10.1007/s004030050230. [DOI] [PubMed] [Google Scholar]
- 38.Holbrook KA, Odland GF. Regional differences in the thickness (cell layers) of the human stratum corneum: an ultrastructural analysis. The Journal of investigative dermatology. 1974;62(4):415–422. doi: 10.1111/1523-1747.ep12701670. [DOI] [PubMed] [Google Scholar]
- 39.Lock-Andersen J, Therkildsen P, Olivarius FD, et al. Epidermal thickness, skin pigmentation and constitutive photosensitivity. Photodermatology Photoimmunology & Photomedicine. 1997;13(4):153–158. doi: 10.1111/j.1600-0781.1997.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 40.Paes EC, Teepen HJ, Koop WA, Kon M. Perioral wrinkles: histologic differences between men and women. Aesthetic surgery journal/the American Society for Aesthetic Plastic surgery. 2009;29(6):467–472. doi: 10.1016/j.asj.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 41.Scott RC, Corrigan MA, Smith F, Mason H. The influence of skin structure on permeability: an intersite and interspecies comparison with hydrophilic penetrants. The Journal of investigative dermatology. 1991;96(6):921–925. doi: 10.1111/1523-1747.ep12475447. [DOI] [PubMed] [Google Scholar]
- 42.Hendi A, Brodland DG, Zitelli JA. Melanocytes in long-standing sun-exposed skin: quantitative analysis using the MART-1 immunostain. Archives of dermatology. 2006;142(7):871–876. doi: 10.1001/archderm.142.7.871. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell RE. The Effect of Prolonged Solar Radiation on Melanocytes of the Human Epidermis. The Journal of investigative dermatology. 1963;41:199–212. doi: 10.1038/jid.1963.97. [DOI] [PubMed] [Google Scholar]
- 44.Quevedo WC, Jr, Szabo G, Virks J, Sinesi SJ. Melanocyte populations in UV-irradiated human skin. The Journal of investigative dermatology. 1965;45(4):295–298. doi: 10.1038/jid.1965.131. [DOI] [PubMed] [Google Scholar]
- 45.Scheibner A, Hollis DE, McCarthy WH, Milton GW. Effects of sunlight exposure on Langerhans cells and melanocytes in human epidermis. Photo-dermatology. 1986;3(1):15–25. [PubMed] [Google Scholar]
- 46.Staricco RJ, Pinkus H. Quantitative and Qualitative Data on the Pigment Cells of Adult Human Epidermis1. Journal of Investigative Dermatology. 1957;28(1):33–45. doi: 10.1038/jid.1957.4. [DOI] [PubMed] [Google Scholar]
- 47.Szabo G. The number of melanocytes in human epidermis. British medical journal. 1954;1(4869):1016–1017. doi: 10.1136/bmj.1.4869.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szabo G. The regional anatomy of the human integument with special reference to the distribution of hair follicles, sweat glands and melanocytes. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 1967:447–485. [Google Scholar]
- 49.Whiteman DC, Parsons PG, Green AC. Determinants of melanocyte density in adult human skin. Arch Dermatol Res. 1999;291(9):511–516. doi: 10.1007/s004030050446. [DOI] [PubMed] [Google Scholar]
- 50.Hwang K, Baik SH. Distribution of hairs and sweat glands on the bodies of Korean adults: a morphometric study. Acta anatomica. 1997;158(2):112–120. doi: 10.1159/000147920. [DOI] [PubMed] [Google Scholar]
- 51.Ko JH, Huang YH, Kuo TT. Hair counts from normal scalp biopsy in Taiwan. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al] 2012;38(9):1516–1520. doi: 10.1111/j.1524-4725.2012.02462.x. [DOI] [PubMed] [Google Scholar]
- 52.Lavoie A, Fugere C, Fradette J, et al. Considerations in the choice of a skin donor site for harvesting keratinocytes containing a high proportion of stem cells for culture in vitro. Burns. 2011;37(3):440–447. doi: 10.1016/j.burns.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 53.Lee HJ, Ha SJ, Lee JH, Kim JW, Kim HO, Whiting DA. Hair counts from scalp biopsy specimens in Asians. Journal of the American Academy of Dermatology. 2002;46(2):218–221. doi: 10.1067/mjd.2002.119558. [DOI] [PubMed] [Google Scholar]
- 54.Lee MS, Kossard S, Wilkinson B, Doyle JA. Quantification of hair follicle parameters using computer image analysis: a comparison of androgenetic alopecia with normal scalp biopsies. The Australasian journal of dermatology. 1995;36(3):143–147. doi: 10.1111/j.1440-0960.1995.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 55.Pagnoni A, Kligman AM, el Gammal S, Stoudemayer T. Determination of density of follicles on various regions of the face by cyanoacrylate biopsy: correlation with sebum output. The British journal of dermatology. 1994;131(6):862–865. doi: 10.1111/j.1365-2133.1994.tb08590.x. [DOI] [PubMed] [Google Scholar]
- 56.Bovell DL, Corbett AD, Holmes S, MacDonald A, Harker M. The absence of apoeccrine glands in the human axilla has disease pathogenetic implications, including axillary hyperhidrosis. British Journal of Dermatology. 2007;156(6):1278–1286. doi: 10.1111/j.1365-2133.2007.07917.x. [DOI] [PubMed] [Google Scholar]
- 57.Buono MJ, Tabor B, White A. Localized beta-adrenergic receptor blockade does not affect sweating during exercise. American journal of physiology Regulatory, integrative and comparative physiology. 2011;300(5):R1148–1151. doi: 10.1152/ajpregu.00228.2010. [DOI] [PubMed] [Google Scholar]
- 58.Glaser S. Sweat glands in the Negro and the European. American Journal of Physical Anthropology. 1934;18(3):371–376. [Google Scholar]
- 59.Yonemoto Y. Studies on measurements of the scalp tissue of the Japanese adult (II) Okajimas folia anatomica Japonica. 1968;45(4):143–163. doi: 10.2535/ofaj1936.45.4_143. [DOI] [PubMed] [Google Scholar]
- 60.Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. Vol. 3. Philadelphia: Elsevier Saunders; 2012. [Google Scholar]
- 61.Bergstresser PR, Pariser RJ, Taylor JR. Counting and sizing of epidermal cells in normal human skin. The Journal of investigative dermatology. 1978;70(5):280–284. doi: 10.1111/1523-1747.ep12541516. [DOI] [PubMed] [Google Scholar]
- 62.Dauendorffer JN, Bastuji-Garin S, Guero S, Brousse N, Fraitag S. Shrinkage of skin excision specimens: formalin fixation is not the culprit. Br J Dermatol. 2009;160(4):810–814. doi: 10.1111/j.1365-2133.2008.08994.x. [DOI] [PubMed] [Google Scholar]
- 63.Kerns MJ, Darst MA, Olsen TG, Fenster M, Hall P, Grevey S. Shrinkage of cutaneous specimens: formalin or other factors involved? J Cutan Pathol. 2008;35(12):1093–1096. doi: 10.1111/j.1600-0560.2007.00943.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.