Abstract
Extracellular histones are cationic damage-associated molecular pattern molecules capable of directly inducing cellular injury via charge-mediated interactions with plasma membranes. Accordingly, histones released into the plasma during critical illness are known to contribute to the onset and propagation of lung injury. Vascular injury (with consequent degradation of the endothelial glycocalyx) simultaneously releases anionic heparan sulfate fragments (hexa- to octasaccharides in size) into the plasma. It is unknown whether this endogenous release of heparan sulfate fragments modulates charge-dependent histone cytotoxicity, or if exogenous heparan sulfate fragments could therapeutically attenuate histone-induced lung injury. Using isothermic calorimetry, we found that extracellular histones only bind to heparan sulfate fragments ≥ 10 saccharides in size, suggesting that glycocalyx-derived heparan sulfate hexa/octasaccharides are incapable of intercepting/neutralizing circulating histones. However, we found that even heparan sulfate fragments incapable of histone binding (e.g. tetrasaccharides) attenuated histone-induced lung injury in vivo, suggesting a direct, size-independent protective effect of heparan sulfate. We found that histones had no effect on human neutrophils ex vivo but exerted toll-like receptor-independent cytotoxicity on human pulmonary microvascular endothelial cells in vitro. This cytotoxicity could be prevented by either the addition of negatively-charged (i.e. highly-sulfated) heparan sulfate tetrasaccharides (incapable of binding histones) or decasaccharides (capable of binding histones). Taken together, our findings suggest that heparan sulfate oligosaccharides may directly exert pulmonary endothelial-protective effects that attenuate histone-mediated lung injury.
Keywords: glycosaminoglycans, glycocalyx, isothermic calorimetry, neutrophil, endothelium
Introduction
Circulating histones are increasingly recognized as major contributors to organ injury during critical illnesses such as sepsis and trauma (1). Typically sequestered within nuclei as an epigenetically-active component of chromatin, histones may be released into the extracellular space as the consequence of cell necrosis or neutrophil extracellular trap (NET) formation. These extracellular, circulating histones initiate a variety of injurious responses, ranging from damage-associated molecular pattern signaling (mediated via pattern receptors such as toll-like receptor 4, TLR4) to direct cytotoxic effects (mediated in part by the impact of highly-positively-charged histone residues on cell membranes) (1). These injurious effects are particularly apparent in the lung (2, 3); as such, circulating histones are believed to be a major contributor to the onset and propagation of acute lung injury (4).
In accordance with the charge-mediated nature of histone cytotoxicity, the direct injurious effects of extracellular histones can be negated by the exogenous administration of highly-sulfated (and accordingly highly negatively-charged) glycans, such as polysialic acid (5) or heparin (2, 6). Critical illnesses such as sepsis are similarly characterized by the endogenous release of heparan sulfate (HS, a highly negatively-charged glycosaminoglycan) into the plasma during endothelial glycocalyx degradation (7). We have previously identified that plasma HS fragments may persist in humans for up to 5 days after ICU admission for respiratory failure (8). These circulating fragments are highly sulfated (similar to heparin, a highly-sulfated HS (9)) and are typically larger than 6 saccharides in length (degree of polymerization 6, dp6), structural characteristics indicative of an ability to bind positively-charged residues of soluble growth factors (10). We hypothesized that circulating HS would similarly intercept circulating histones and neutralize their cytotoxic effect, thereby serving as an endogenous defense against histone-induced lung injury.
Methods
Materials
To model highly-sulfated circulating HS fragments, we purchased heparin (a highly-sulfated HS) and selectively desulfated heparin oligosaccharides of various sizes from Iduron (Manchester, UK), as previously described (10). Calf thymus histones (CTH, a mixture of histones H1, H2A, H2B, H3, and H4), histone H1, and histone H3 were purchased from Sigma-Aldrich (St. Louis, MO). H4 histone was purchased from Cayman Chemical (Ann Arbor, MI). Primary human lung microvascular endothelial cells were purchased from Lonza (Walkersville, MD). HPMEC-ST1.6R cells, a human pulmonary microvascular endothelial cell line, were generously provided by Dr. Ronald Unger (University of Mainz), the originator of the cell line (11). We have previously demonstrated the relevance of this transformed cell line to primary lung endothelial cells (10). The TLR4 inhibitor TAK-242 was purchased from Calbiochem/EMD Millipore (Billerica, MA); the TLR2 inhibitor CU CPT 22 was purchased from Tocris (Bristol, UK).
Animals
8–10 week-old C57BL/6 male mice were purchased from Jackson Laboratories (Bar Harbor, ME) and allowed to acclimate to our animal facility for 1 week prior to use. Mice were housed in a climate-controlled room at 25 °C with 12 h day:night cycle. All animal protocols were conducted in accordance with National Institutes of Health guidelines and approved by the University of Colorado Institutional Animal Care and Use Committee. All animal experiments were performed in accordance with the ARRIVE guidelines (12). Mice were blindly randomized to treatment groups. All animals were included for analysis; statistical outliers were not excluded. Experimental replicates were accrued over at least 2 separate days (to minimize temporal confounding); each day included corresponding experimental groups and controls.
Isothermic calorimetry
Isothermal titration calorimetry (ITC) was used to directly assess binding between heparin oligosaccharides and histones. ITC experiments were performed with a MicroCal- ITC200 system (GE Healthcare, Life Science, USA) in buffer containing 20 mM HEPES (pH 7.3) and 100 mM NaCl. All solutions were thermo-stated and degassed before loading to the instrument. In each individual experiment, 2.4 µl aliquots of heparin oligosaccharide (400µM) was injected into the cell (350 µl) containing histone solution (68.2 µM) through the computer-controlled microsyringe at an interval of 3 minutes. Heats of the reaction were determined by integration of the observed peaks. The experimental data were fitted to a theoretical titration curve using the software supplied by MicroCal. A standard one-site model was used with ΔH (enthalpy change, in kcal/mol), Ka (association constant, in M−1), and N (number of binding sites per monomer) as the variables.
Histone-induced lung injury
Mice were anesthetized with 3% isoflurane, and CTH (25 mg/kg in 100 µl saline) or saline was injected via tail vein. At the time of CTH injection, HS oligosaccharides (dp4, dp10) or saline control was injected (50 µl) via tail vein or retroorbital injection. Injections were repeated 6, 12, and 18 h after CTH. At 24 h, mice were terminally anesthetized and killed via direct cardiac puncture. The right hilum was ligated with 2:0 silk suture, and the right lung was harvested immediately. The right lower lobe was immediately weighed (wet weight), placed in a 60° C drying oven×24 h, and then re-weighed (dry weight). The ratio of wet weight to dry weight was used as a gravimetric measure of lung edema, as previously described (13).
The left lung was lavaged using 1 ml of cold saline, and bronchoalveolar (BAL) fluid was collected (with equal return in all groups). BAL fluid was centrifuged in 1000 rpm for 5 minutes, as supernatant protein was measured using the Bi-Rad Protein Assay Kit. The pellet was resuspended with 400 µl of PBS. Total leukocyte count was measured via a hemocytometer. For neutrophil measurements, 150 µl of the resuspended solution was spun to a slide with Shandon Cytospin 4 (Thermo Fisher, Waltham MA). Hema 3 stain set (Thermo Fisher) was used for Wright-Giemsa stain of the cytoslide. The stained cytoslides were scored by staff who were blinded to the experimental protocol. Total four typical microscopic visual fields were selected randomly for each cytoslide.
Endothelial cell studies
Endothelial cells (either primary human lung microvascular cells (HPMVEC) or the HPMEC-ST1.6R human pulmonary microvascular endothelial cell line (11)) were seeded in a 96-well plate and grown to 90% confluence. For measurement of cell lysis, endothelial cells (primary or cell line) were incubated with CTH at various duration/concentrations in the presence or absence of HS oligosaccharides or TLR inhibitors. Cell death was measured by LDH (Lactate Dehydrogenase) release, using a non-Radioactive Cytotoxicity assay kits (Promega, Madison WI) read by with Glomax Multimode Plate Reader (Promega) in 595 nm. For measurement of TNF-α expression, HPMEC-ST1.6R cells were treated with CTH (50 µg/ml) or lipopolysaccharide (LPS, 10 µg/ml) for two hours in the presence or absence of dp4 or dp10 oligosaccharides. For MLKL (Mixed Lineage Kinase-domain Like) phosphorylation analysis, HPMEC-ST1.6R cells were challenged by CTH (50 µg/ml) for six hours. For VE-Cadherin internalization analysis, primary HPMVEC-lung cells were challenged by CTH (50 µg/ml) for six hours in the presence or absence of dp4 or dp10 (50µM).
Western blotting
Protein concentration was determined with the Bio-Rad Protein Assay Kit. For VE-Cadherin analysis, cytoplasm and membrane were separated with Cell Fractionation Kit (Cell Signaling Technology, Danvers MA). Equal amount of proteins (20 µg) were mixed with loading buffer and subjected to electrophoresis. Separated proteins were transferred onto PVDF membranes. The membranes were then probed with anti-MLKL (58–70) antibody (1:500, Sigma-Aldrich), anti-MLKL (phosphor S358) antibody (1:2000, Abcam, Cambridge UK), anti-Cadherin-5 antibody (1:500, BD Transduction Laboratories, San Jose CA), anti-Sodium Potassium ATPase antibody (1:100000, Abcam) and anti-β-actin antibody (1:2000, Cell Signaling Technology) overnight at 4°C.
Real time-PCR
We performed quantitative PCR using ICAM-1 (Hs 00164932, TaqMan Gene Expression) and TNF-α (Hs 01113624, TaqMan Gene Expression) primers. The reverse transcription reactions were carried out using a Cells-to-CT™ kit (Invitrogen) with 7300 Real Time PCR System (AB Applied Biosystems). PCR conditions included initial denaturation at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 1 min. We calculated relative abundance of mRNA expression in each sample as 2−ΔΔCt.
Human neutrophil studies
Neutrophils were obtained from healthy volunteers donating blood to National Jewish Health (Denver, CO), using the plasma Percoll density centrifugation method as previously described (14). All studies were approved by the National Jewish Health Institutional Review Board (approval number HS1285), and written, informed consent was obtained from all participants prior to blood donation. The study was conducted in accordance with the Declaration of Helsinki. Neutrophils were seeded in a 96-well plate (1X106/well). For conditions in which neutrophils were treated with LPS, neutrophils were pre-treated with 100ng/ml LPS for 1 hour before stimulation with 20 or 200 µg/ml CTH, 100nM fMLP (N-Formylmethionyl-leucyl-phenylalanine) as positive control, or 100nM fMLP plus 20 units/ml superoxide dismutase as negative control. Superoxide Anion Assay Kit (Sigma-Aldrich) was used to measure the luminescence intensity produced by the neutrophils for 4 hours after stimulation with Glomax Multimode Plate Reader (Promega).
Statistical analyses
All data are reported as means ± standard error. Multiple comparisons were performed by analysis of variance. Multiple comparisons were performed using Dunnett’s test (to control group) or Tukey’s test (to all groups). All results were included for statistical analyses (outliers were not excluded). Statistical significance was defined as p < 0.05.
Results
Structural determinants of histone-HS binding
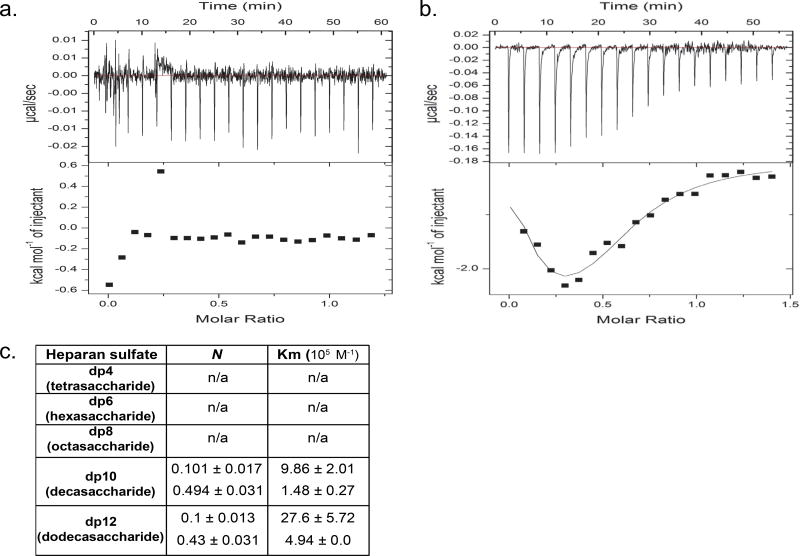
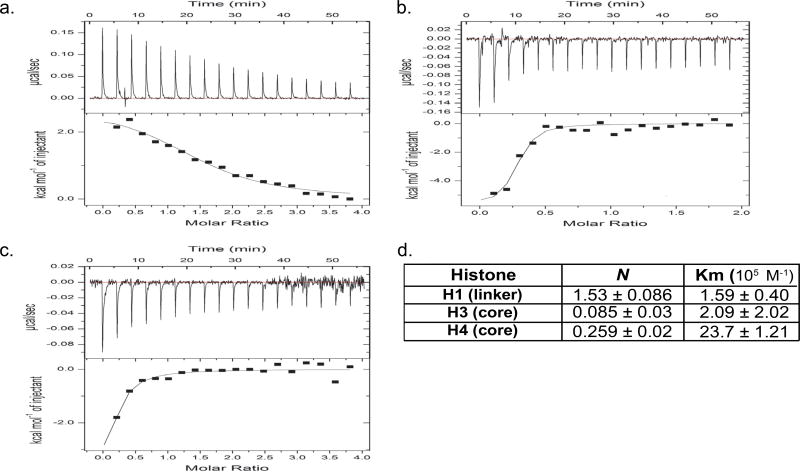
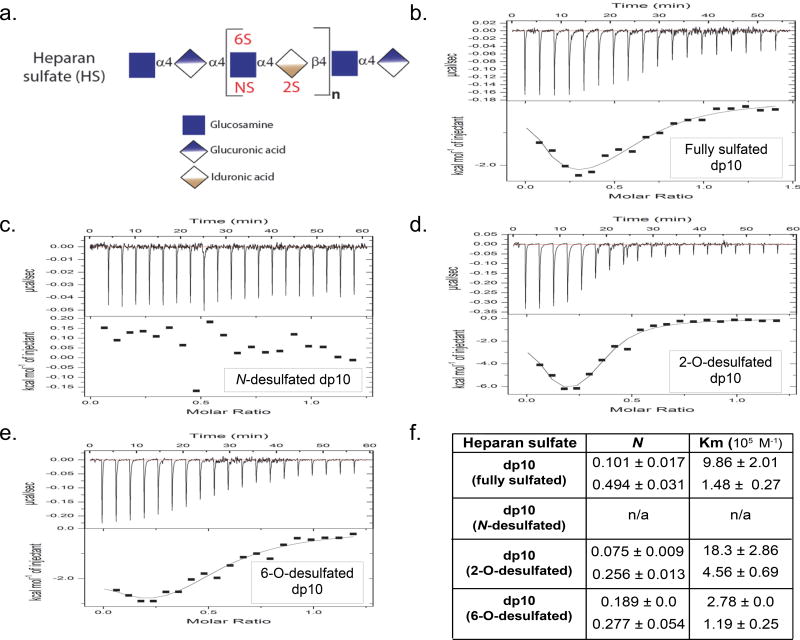
We used isothermic calorimetry (ITC, (15)) to determine the ability of HS oligosaccharides to bind calf thymus histones (CTH), a biologically-relevant 1:2:2:2:2 mixture of H1, H2A, H2B, H3, and H4 histone subtypes previously demonstrated to induce lung injury (3). As circulating HS fragments in sepsis are highly-sulfated, we used heparin (a highly-sulfated HS) oligosaccharides to model septic circulating HS fragments, as previously described (10). As demonstrated by representative isotherms in Fig 1, CTH bound heparin fragments 10 saccharides in length (degree of polymerization 10, dp10) or longer. CTH-dp10 binding demonstrated both exothermic and endothermic reactions (Fig 1b) as well as two binding affinities (Km, Fig 1c), likely reflecting the summation of differential interactions of HS with core (as demonstrated by exothermic H3 and H4 reactions) and linker histones (such as the endothermic H1 reaction) (Fig 2). CTH-dp10 binding required sulfation of HS at the amino position of glucosamine (“N-sulfation”), as dp10 heparin selectively desulfated at the N-position (“N-desulfated” dp10 heparin) demonstrated no HS binding (Fig 3). In contrast, heparin sulfation at other sites (e.g. the 2-O position of iduronic acid or the 6-O position of glucosamine) was dispensable for CTH binding, given the continued ability of selectively 2-O- or 6-O-desulfated heparins to bind CTH. Taken together, these findings suggested that CTH binding (and therefore neutralization) requires N-sulfated HS fragments ≥ 10 saccharides in length. Accordingly, endogenous dp6-dp8 HS fragments observed in sepsis (8), while heavily N-sulfated, would likely be too small to bind and/or neutralize extracellular histones.
Figure 1. Size-dependence of HS binding to CTH.
(a,b) Representative isotherms of calf thymus histone (CTH, 68.2 µM) binding to highly-sulfated HS oligosaccharides (400 µM). No binding was noted with HS tetrasaccharides (dp4, a); similarly negative isotherms were noted with dp6 and dp8 oligosaccharides. In contrast, fragments ≥ 10 saccharides (dp10, b) in size demonstrated both endothermic (downsloping) and exothermic (upsloping) binding reactions. (c) Isothermic calorimetry quantification of number of binding sites (N) and binding affinity of CTH-HS reactions.
Figure 2. Binding of highly-sulfated HS decasaccharides to different histone subtypes.
(a-c) Representative isotherms of histone H1 (a), H3 (b), and H4 (c, all 20 µM) binding to highly-sulfated dp10 HS (400 µM). Histone H1-HS binding demonstrated an endothermic (downsloping) reaction; in contrast, H3 and H4 binding to HS was exothermic (upsloping). (d) Isothermic calorimetry quantification of number of binding sites (N) and binding affinity of histone-dp10 HS reactions.
Figure 3. Sulfation-dependence of dp10 HS binding to CTH.
a Heparan sulfate, a linear polymer of repeating glucosamine-hexuronic (either glucuronic or iduronic) acid disaccharides, can be sulfated at the 2-O position of iduronic acid ("2S") or the N- or 6-O position of glucosamine ("NS" or "6S"). b-e Representative isotherms of CTH binding to fully sulfated dp10 HS (modeled by heparin, b), N-desulfated dp10 HS (c), 2-O-desulfated dp10 HS (d), and 6-O-desulfated dp10 HS (e, all 20 µM) binding to highly-sulfated dp10 HS (400 µM). f Isothermic calorimetry quantification of number of binding sites (N) and binding affinity of CTH-dp10 HS reactions.
HS oligosaccharides protect against CTH-induced lung injury in vivo
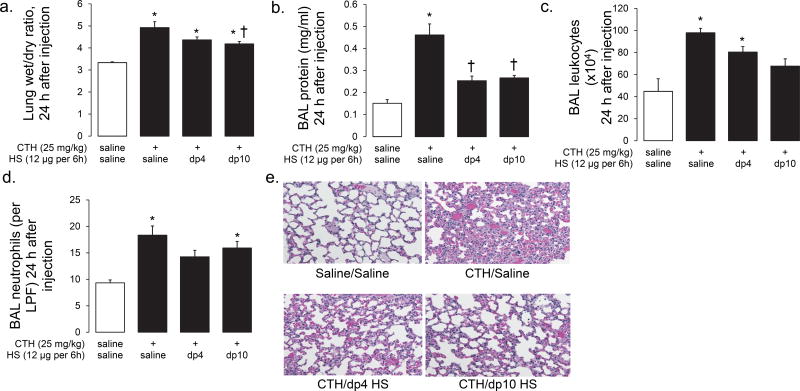
Based upon these ITC experiments, we anticipated that the exogenous administration of sulfated dp10 HS fragments would neutralize circulating CTH and therefore attenuate histone-induced organ injury. Consistent with ITC data, dp10 HS (12 µg administered intravenously every 6 hours, beginning at the injection of intravenous CTH) attenuated CTH-induced lung injury, as demonstrated by decreased lung wet-dry ratio (an index of lung edema, Fig 4a), BAL protein (an index of alveolar protein permeability, Fig 4b), decreased concentrations of BAL leukocytes (indices of alveolar inflammation, Fig 4c,d), and improved lung histology (Fig 4e) 24 h after intravenous CTH. Surprisingly, dp4 oligosaccharides provided similar protective benefit against CTH-induced lung injury (Fig 4a-e), despite the inability to directly bind CTH (Fig 1). Neither dp4 nor dp10 improved CTH-induced mortality (~25% mortality at 24 h in all groups, data not shown). These findings suggested that circulating highly-sulfated HS fragments may have specific lung-protective effects occurring via mechanisms independent of direct CTH binding.
Figure 4. HS oligosaccharides attenuate CTH-induced acute lung injury.
Intravenous calf thymus histones (CTH, 25 mg/kg) induce lung injury at 24 h, as quantified by increased lung edema (wet/dry ratio, a), alveolar permeability (bronchoalveolar lavage (BAL) protein, b), and alveolar inflammation (BAL leukocyte/neutrophil infiltration (c,d)). These quantitative measures are consistent with qualitative injury apparent on lung histology (e). These indices of injury were attenuated by highly-sulfated HS fragments (dp4 or dp10 heparin oligosaccharides, 12 µg intravenously injected every 6 h). n = 3 - 4 per group. * P < 0.05 compared to saline/saline control; † P < 0.05 compared to CTH/saline.
HS oligosaccharides protect against CTH-induced endothelial activation and cytotoxicity
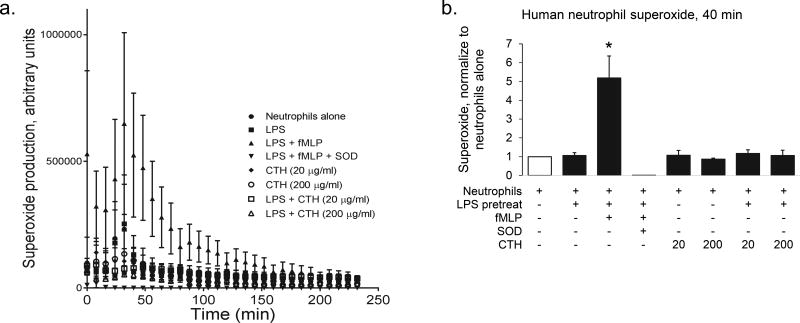
Given in vivo evidence that HS-mediated lung protection does not require direct CTH binding, we sought to determine if HS oligosaccharides have direct action on effector cells contributory to lung injury onset. Using human neutrophils isolated from healthy volunteers, we found that CTH had no effect on neutrophil activation, as quantified by superoxide production (Fig 5).
Figure 5. CTH do not activate primed or unprimed human neutrophils ex vivo.
Neutrophils were isolated from the plasma of healthy humans and treated ex vivo with or without 1 h antecedent LPS 100 ng/ml pretreatment) with fMLP (100 nM, positive control), superoxide dismutase (20 units/ml, negative control), and/or calf thymus histones (CTH, 20 or 200 µg/ml). Superoxide production was measured every 8 min for 4 hours (a); quantification was performed at 40 min (b). n = 3 - 4 biological replicates (each corresponding to a distinct neutrophil collection); * P < 0.05 compared to all other groups.
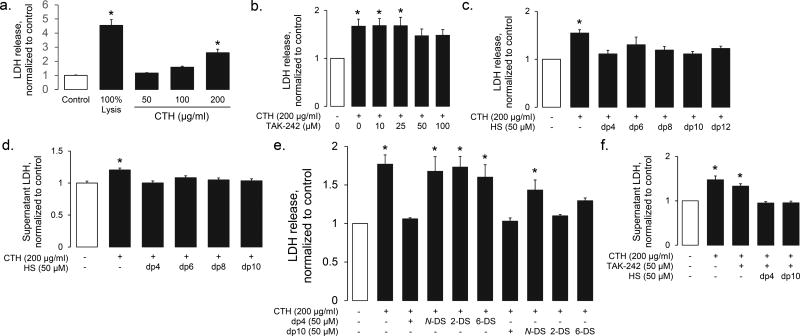
Conversely, CTH had direct effect on human lung microvascular endothelial cells. At high doses (≥ 100 µg/ml), CTH induced lysis of HPMEC-ST1.6R cells, a human lung microvascular endothelial cell line (Fig 6a). CTH-induced endothelial death was minimally attenuated with TLR4 (TAK-242) inhibition (Fig 6b) and not attenuated by TLR2 inhibition (CU CPT22, 0.5 to 100 µM, data not shown), suggesting a direct cytotoxic effect of CTH. In contrast, HS oligosaccharides prevented CTH-induced cytotoxicity in a size-independent (Fig 6c) fashion. These protective effects were similarly observed using primary human lung microvascular endothelial cells, supporting the previously-demonstrated (10) relevance of HPMEC-ST1.6R cells (Fig 6d). The HPMEC-ST1.6R cytoprotective effects of HS were sulfation-dependent (Fig 6e). Interestingly, the observed N-sulfation-dependence of dp10 cytoprotection was identical to the N sulfation-dependence of dp10-CTH binding (Fig 3). In contrast, dp4 HS (which does not bind CTH, Fig 1) required N-, 2-, and 6-sulfation to protect against CTH-induced endothelial cytotoxicity. HS-mediated endothelial protection was additive to TAK242 (Fig 6f), suggesting that any protective effects of circulating HS oligosaccharides occurred independently of TLR4 blockade.
Figure 6. HS oligosaccharides prevent CTH-induced endothelial cytotoxicity.
(a) CTH induce endothelial cell death (as quantified by LDH release from HPMEC-ST1.6R endothelial cells) in a dose-dependent fashion. (b) CTH-induced endothelial (HPMEC-ST1.6R) cytotoxicity is minimally TLR-4 dependent, as demonstrated by only modest protection provided by high-dose TAK-242 (a TLR4 inhibitor). In contrast, HS oligosaccharides (of all sizes) protected against CTH-induced cytotoxicity in HPMEC-ST1.6R cells (c) and primary human lung microvascular endothelial cells (d). (e) The HPMEC-ST1.6R-protective effects of dp4 HS was dependent on N-, 2-O, and 6-O sulfation, while the protective effects of dp10 HS only required N-sulfation. (f) The protective effects of dp4 and dp10 HS were additive to (and therefore likely independent of) high-dose TLR4 inhibition. n > 3 independent replicates for all groups. * P < 0.05 compared to control group.
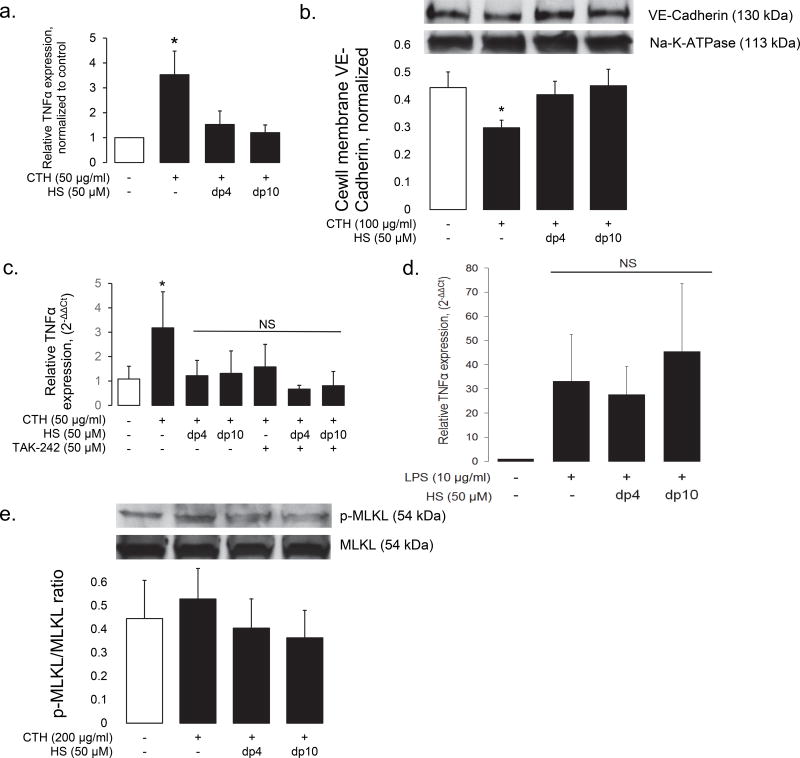
To determine if HS oligosaccharides influenced CTH-induced, pattern receptor-mediated endothelial activation, we treated HPMVEC-1.6R cells with lower (i.e. non-cytotoxic) doses of CTH for 6 hours. 50 µg/ml CTH induced endothelial activation, as demonstrated by induction of endothelial TNFα expression in HPMEC-ST1.6R cells (Fig 7a). The endothelial-activating capabilities of low-dose CTH were confirmed by the loss of membrane VE-Cadherin in CTH-treated primary human lung endothelial cells (Fig 7b). Similar to high-dose CTH-mediated cytotoxicity, low-dose CTH activation of HPMEC-ST1.6R cells was inhibited by the concurrent addition of dp4 or dp10 heparin (Fig 7a). In contrast to cytotoxicity, however, this activation was TLR4-dependent (Fig 7c). Furthermore, the addition of dp4 or dp10 heparin to TAK242-treated cells provided no additional suppression of TNFα expression, suggesting that dp4 and dp10 inhibited low-dose CTH-mediated endothelial activation via a similar mechanisms of TLR4 antagonism. This inhibitory effect is consistent with a previous report suggesting that N-sulfated HS fragments can prevent HMGB1-mediated activation of TLR4 (16). However, highly-sulfated HS oligosaccharides (as modeled by dp4 and dp10 heparin) had no effect on lipopolysaccharide (LPS)-induced TNFα expression (Fig 7d), suggesting that these oligosaccharides are not simply pan-TLR4 inhibitors, but may have specificity for histone- (or HMGB1-) activation of this pattern receptor. Taken together, our findings suggest that HS oligosaccharides have differential effects against high-dose CTH-induced cell necrosis (occurring independently of TLR4, Fig 6) and against lower-dose CTH-induced endothelial cell activation (likely occurring via TLR4 antagonism, Fig 7). Low-dose CTH-mediated endothelial activation did not induce necroptosis (as measured by activation of the effector MLKL, Fig 7e), again suggesting that (high-dose) CTH-induced cytotoxicity occurs via direct action on the endothelial cell membrane.
Figure 7. HS oligosaccharides prevent low-dose CTH-induced endothelial activation.
In contrast to high-dose CTH-induced cytotoxicity, lower doses of CTH induce endothelial cell activation, as quantified in HPMEC-ST1.6R cells by TNFα expression (a) and in primary human lung microvascular endothelial cells by loss of cell-membrane VE-Cadherin (b). (c) Low-dose CTH activation of HPMEC-ST1.6R endothelial cells is TLR-4 dependent, as demonstrated by suppression of TNFα expression by TAK-242. This protective effect is similar to (and not augmented by) dp4 or dp10 HS, suggesting that TAK-242 and HS oligosaccharides inhibit endothelial activation by a shared pathway. (d) Highly-sulfated dp4 and dp10 HS oligosaccharides did not impact LPS-induced TNF-α expression in HPMEC-ST1.6R cells, suggesting that these oligosaccharides do not function as nonspecific TLR4 inhibitors. (e) Low-dose CTH exposure does not activate MLKL, a mediator of necroptosis. MLKL activation is not influenced by HS oligosaccharides. n > 3 independent replicates for all groups. * P < 0.05 compared to control group.
Discussion
The highly-positively charged nature of extracellular histones contributes to their localization and biological function. When incorporated into neutrophil extracellular traps, their cationic nature exerts direct antimicrobial function, mediated by a charge-based destabilization of bacterial cellular membranes. When not constrained within NETs, circulating histones may similarly cause direct cytotoxicity against host cells, initiating and/or propagating multi-system organ injury (1).
Circulating histone cytotoxicity and localization may therefore be influenced by endogenous negatively-charged ligands, including sulfated glycosaminoglycans such as HS. As the pulmonary endothelium is lined by a particularly thick (> 1.5 µm) endothelial glycocalyx enriched in full-length HS, circulating histones are largely sequestered within the lung (2). This sequestration may protectively buffer the systemic effects of low-dose histones endogenously released during illness. However, in the presence of overwhelming concentrations of histones, or when the protective endothelial glycocalyx is degraded during critical illnesses such as sepsis, vascular beds may be subjected to histone-induced endothelial cell death, propagating tissue injury.
While loss of endothelial glycocalyx thickness during critical illness would therefore be anticipated to worsen histone-induced endothelial injury, this harm may be offset by the release of highly-sulfated HS fragments into the circulation during glycocalyx degradation. Intravenous infusion of exogenous, full-length glycosaminoglycans (including heparin (a highly-sulfated HS (2, 6)) or the chondroitin sulfate-rich Inter-alpha inhibitor (17)) attenuated histone-induced organ injury. Whether circulating (endothelial glycocalyx-derived) HS fragments would provide similar protective benefit is uncertain, however, particularly given reports that fragmented HS can propagate inflammation via activation of toll-like receptor signaling (18).
We found that administration of heparin oligosaccharides, mimicking the highly-sulfated circulating HS fragments observed within the blood of septic animals (10) and humans (8), protects the pulmonary endothelium in vitro (Fig 6) and lungs in vivo (Fig 5) from high doses of exogenous histones. To our surprise, this protective effect of HS oligosaccharides occurred independently of the ability to bind circulating histones, with endothelial-protective effects noted after injection of HS oligosaccharides (e.g. dp4) too short to bind to histones (Fig 1). These data indicate that highly-sulfated HS fragments (regardless of size) may directly protect endothelial membrane integrity. This finding is consistent with a previous report demonstrating that pretreatment of pulmonary endothelial cells with heparinase-III (which liberates highly-sulfated HS fragments) attenuates cationic peptide-induced barrier dysfunction, while pretreatment with heparinase-I (which liberates undersulfated fragments) has no effect (19). Furthermore, exogenous highly-sulfated glycosaminoglycans may directly promote homeostatic vascular repair, either via direct incorporation into the reconstituting endothelial glycocalyx (20) or via augmentation of endothelial-reparative growth factor pathways (such as FGF2-FGFR1 signaling (10)).
In addition to directly damaging cell membranes, histones may additionally activate endothelial signaling by serving as a damage associated molecular pattern molecule, sensed by pattern receptors such as TLR4 (1). Indeed, we observed that lower-dose (50 µg/ml) CTH induced endothelial TNFα expression in a TLR4-dependent fashion (Fig 7). Similar to the protective effect observed against high-dose CTH-induced cytotoxicity, HS oligosaccharides suppressed low-dose CTH-induced endothelial TNFα expression. This ability of HS degradation products to suppress TNFα signaling, while seemingly counter to reports identifying HS as an endogenous TLR4 ligand (18), is consistent with reports by our group (21) and others (22, 23) demonstrating anti-inflammatory effects of soluble HS. Interestingly, these protective effects of HS were not observed in LPS-induced (TLR4-mediated) endothelial activation, potentially suggesting ligand specificity of HS as a TLR4 inhibitor.
In summary, our work identifies that soluble, highly-sulfated HS protects against histone-induced lung injury independently of its ability to bind histones. This protection is mediated in part via TLR-4-independent inhibition of histone-induced endothelial cytotoxicity, and in part due to suppression of endothelial inflammatory activation. These findings suggest a protective role of circulating highly-sulfated HS fragments released during endothelial glycocalyx degradation, as well as potential therapeutic approach to prevent endothelial cell injury during critical illness. Future studies are needed to determine the mechanisms by which sulfated glycosaminoglycans can directly stabilize endothelial cells against histone-induced lysis.
Acknowledgments
The authors thank Dr. Ken Malcolm (National Jewish Health) for assistance with human neutrophil studies and Dr. Ronald Unger (University of Mainz) for providing HPMEC-ST1.6R cells.
This work has been supported by National Institutes of Health (NIH) grant R01 HL125371 (to EPS).
Footnotes
The authors declare no conflicts of interest.
References
- 1.Allam R, Kumar SV, Darisipudi MN, Anders HJ. Extracellular histones in tissue injury and inflammation. J Mol Med (Berl) 2014;92(5):465–72. doi: 10.1007/s00109-014-1148-z. [DOI] [PubMed] [Google Scholar]
- 2.Freeman CG, Parish CR, Knox KJ, Blackmore JL, Lobov SA, King DW, Senden TJ, Stephens RW. The accumulation of circulating histones on heparan sulphate in the capillary glycocalyx of the lungs. Biomaterials. 2013;34(22):5670–6. doi: 10.1016/j.biomaterials.2013.03.091. [DOI] [PubMed] [Google Scholar]
- 3.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15(11):1318–21. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Z, Huang Y, Mao P, Zhang J, Li Y. Sepsis and ARDS: The Dark Side of Histones. Mediators Inflamm. 2015;2015:205054. doi: 10.1155/2015/205054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT. Neutrophil Extracellular Traps Directly Induce Epithelial and Endothelial Cell Death: A Predominant Role of Histones. PloS One. 2012;7(2):e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Wen Z, Guan L, Jiang P, Gu T, Zhao J, Lv X, Wen T. Extracellular histones play an inflammatory role in acid aspiration-induced acute respiratory distress syndrome. Anesthesiology. 2015;122(1):127–39. doi: 10.1097/ALN.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 7.Colbert JF, Schmidt EP. Endothelial and Microcirculatory Function and Dysfunction in Sepsis. Clin Chest Med. 2016;37(2):263–75. doi: 10.1016/j.ccm.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt EP, Li G, Li L, Fu L, Yang Y, Overdier KH, Douglas IS, Linhardt RJ. The circulating glycosaminoglycan signature of respiratory failure in critically ill adults. J Biol Chem. 2014;289(12):8194–202. doi: 10.1074/jbc.M113.539452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostrowski SR, Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. J Trauma Acute Care Surg. 2012;73(1):60–6. doi: 10.1097/TA.0b013e31825b5c10. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Haeger SM, Suflita MA, Zhang F, Dailey KL, Colbert JF, Ford JA, Picon MA, Stearman RS, Lin L, et al. Fibroblast Growth Factor Signaling Mediates Pulmonary Endothelial Glycocalyx Reconstitution. Am J Respir Cell Mol Biol. 2017 doi: 10.1165/rcmb.2016-0338OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krump-Konvalinkova V, Bittinger F, Unger RE, Peters K, Lehr HA, Kirkpatrick CJ. Generation of human pulmonary microvascular endothelial cell lines. Lab Invest. 2001;81(12):1717–27. doi: 10.1038/labinvest.3780385. [DOI] [PubMed] [Google Scholar]
- 12.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18(8):1217–23. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haslett C, Guthrie LA, Kopaniak MM, Johnston RB, Jr, Henson PM. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985;119(1):101–10. [PMC free article] [PubMed] [Google Scholar]
- 15.Dutta AK, Rosgen J, Rajarathnam K. Using isothermal titration calorimetry to determine thermodynamic parameters of protein-glycosaminoglycan interactions. Methods Mol Biol. 2015;1229:315–24. doi: 10.1007/978-1-4939-1714-3_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma L, Wu J, Patel V, Sitapara R, Rao NV, Kennedy TP, Mantell LL. Partially-desulfated heparin improves survival in Pseudomonas pneumonia by enhancing bacterial clearance and ameliorating lung injury. J Immunotoxicol. 2014;11(3):260–7. doi: 10.3109/1547691X.2013.839587. [DOI] [PubMed] [Google Scholar]
- 17.Chaaban H, Keshari RS, Silasi-Mansat R, Popescu NI, Mehta-D'Souza P, Lim YP, Lupu F. Inter-alpha inhibitor protein and its associated glycosaminoglycans protect against histone-induced injury. Blood. 2015;125(14):2286–96. doi: 10.1182/blood-2014-06-582759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodall KJ, Poon IK, Phipps S, Hulett MD. Soluble heparan sulfate fragments generated by heparanase trigger the release of pro-inflammatory cytokines through TLR-4. PloS One. 2014;9(10):e109596. doi: 10.1371/journal.pone.0109596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dull RO, Dinavahi R, Schwartz L, Humphries DE, Berry D, Sasisekharan R, Garcia JG. Lung endothelial heparan sulfates mediate cationic peptide-induced barrier dysfunction: a new role for the glycocalyx. Am J Physiol Lung Cell Mol Physiol. 2003;285(5):L986–95. doi: 10.1152/ajplung.00022.2003. [DOI] [PubMed] [Google Scholar]
- 20.Henry CB, Duling BR. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am J Physiol. 1999;277(2 Pt 2):H508–14. doi: 10.1152/ajpheart.1999.277.2.H508. [DOI] [PubMed] [Google Scholar]
- 21.Lygizos MI, Yang Y, Altmann CJ, Okamura K, Hernando AA, Perez MJ, Smith LP, Koyanagi DE, Gandjeva A, Bhargava R, et al. Heparanase mediates renal dysfunction during early sepsis in mice. Physiol Rep. 2013;1(6):e00153. doi: 10.1002/phy2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashida K, Chen Y, Bartlett AH, Park PW. Syndecan-1 is an in vivo suppressor of Gram-positive toxic shock. J Biol Chem. 2008;283(29):19895–903. doi: 10.1074/jbc.M801614200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lider O, Cahalon L, Gilat D, Hershkoviz R, Siegel D, Margalit R, Shoseyov O, Cohen IR. A disaccharide that inhibits tumor necrosis factor alpha is formed from the extracellular matrix by the enzyme heparanase. Proc Natl Acad Sci USA. 1995;92(11):5037–41. doi: 10.1073/pnas.92.11.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]