1. Introduction
Studies focusing on human mucosal-associated invariant T (MAIT) cells have gained considerable momentum in recent years. MAIT cells are found at mucosal barrier sites and their ability to quickly exert effector function indicates a potentially significant role for them in barrier immunity. Changes in MAIT cell frequency and phenotype have been reported in numerous disease settings including acute and chronic infections as well as autoimmune and malignant disorders. Recent reviews provide a comprehensive overview of these clinical studies as well as key aspects of MAIT cell function including antigen recognition, antimicrobial properties and their putative role in the liver[1–11]. Here we provide an overview of the recent human MAIT cell literature to address how a cell that recognizes bacterially-derived antigen and is found in mucosal barrier tissues colonized by commensal bacteria, avoids responding to these commensal-derived antigens while maintaining responsiveness to bacterial infections. We discuss the key role of inflammatory signals in regulating human MAIT cell effector function in this context and review the relationship and the functional properties of human MAIT cells in blood and mucosal tissues. In addition, we highlight gaps in our current knowledge and examine the emerging role of human MAIT cells in controlling barrier immunity tissue homeostasis.
2. Brief Background
MAIT cells are a fairly abundant T-cell subset in humans representing 1–10% of total T-cells in blood and mucosal tissues, and even higher abundance in the liver[12,13]. MAIT cells are characterized by a semi-invariant T cell receptor (TCR) that consists of a conserved TCRα chain[14,15] - TRAV1-2(Vα7.2) and TRAJ33 (Jα33) - paired with a few select Vβ chains[16]. This semi-invariant TCR recognizes antigen in the context of the protein MHC class I - related (MR1)[17]. MR1 appears well conserved across species suggesting an essential role for MAIT cells in the immune response[17–20]. Instead of peptides (presented by MHC I and II) or glycolipids/lipids (presented by CD1), MAIT cells recognize metabolites and thus a different type of antigen compared to any other T cell subset[21]. These metabolites are derived from vitamin B synthesis pathways and additional modifications may occur when presented in the context of MR1 and some, but not all of these MR1 binding metabolites can activate MAIT cells[22–27]. Identification of these antigens allowed the development of MR1 tetramers[22,23], which became available to the broader scientific community through the NIH tetramer core in late 2016. In most studies published so far, MAIT cells have been identified via flow cytometry by co-expression of their invariant TCR α-chain, Vα7.2, along with high expression levels of the C-type lectin CD161 (other names include KLRB1, NKR-P1A). MAIT cells in the blood can also be identified by Vα7.2 expression together with expression of the cytokine receptor IL-18Rα[28,29]. Since conventional T cells can also express Vα7.2 and recent reports suggest that CD161 expression can change in certain scenarios[30,31], using MR1 tetramers is likely the most reliable method to identify MAIT cells. However, it is important to consider that staining with anti-Vα7.2, anti-CD161 antibodies versus the MR1 tetramer shows that these populations are nearly, but not fully congruent in the blood[22,23]. This indicates that some Vα7.2+CD161hi cells are either not MR1-restricted or do not recognize the specific metabolite used to load MR1, which is plausible given evidence for differential antigen recognition by MAIT cells[32,33]. Importantly, recent studies also provide evidence for the existence of Vα7.2− MR1-restricted cells[34,35]. The majority of the currently published literature and literature references in this review are based on the classic definition of MAIT cells (Vα7.2+CD161hi), but it will be necessary to keep these details in mind as the field moves forward and takes advantage of MR1 tetramers.
3. MAIT cell subsets, phenotypes and function in blood
MAIT cells in the blood are typically CD45RO+, CD62Llo, CD122int, CCR7− and quickly acquire effector function when stimulated[12]. The lack of CD62L and CCR7 expression paired with the ability to quickly respond to stimulation is a hallmark of conventional effector memory T-cells[36] and MAIT cells have thus been referred to as effector memory-like[12]. The reason MAIT cells have these characteristics is presumably due to near uniform expression of the transcription factor PLZF[37]. Expression of PLZF is sufficient to induce acquisition of effector memory-like properties in conventional T-cells[38,39] and is required for the innate-like effector function of NKT cells [40,41] and certain gamma delta T-cells[42]. Once activated, MAIT cells isolated from the blood (or stimulated in the presence of other peripheral blood mononuclear cells, PBMCs) secrete IFNγ, TNFα and express the cytolytic molecule granzyme B[12,28,31,43,44]. Overall, it has become clear that there is more heterogeneity in the MAIT cell population than initially appreciated[45]. A small fraction of MAIT cells isolated from the blood can also secrete IL-17 ex vivo following short term stimulation with via CD3/CD28 or PMA and ionomycin[12,46,47]. It is noteworthy that a large fraction of MAIT cells in the blood expresses the transcription factor RORγt[37], which drives T-cells towards IL-17 production[48]. MAIT cells from patients with a loss of function mutation in Stat3 are impaired in their ability to produce IL-17 despite normal RORγt expression indicating a critical role of IL-23R signaling[37]. Additional transcriptions factors expressed in MAIT cells from the blood include Helios, Eomes, and T-bet[49]. How these transcription factors specifically regulate different functional aspects of MAIT cells has yet to be fully elucidated. Finally, MAIT cells in the blood can be divided into different subsets based on CD8 and CD4 co-receptor expression. The relationship of these subsets is unclear, but single cell gene expression analysis from the two major MAIT subsets, CD8+ and CD8− (CD4−) cells, isolated from the blood demonstrated distinct transcriptional differences[44] and their respective frequencies can change independently following infection[46].
4. MAIT cell activation requirements and implications for their function in mucosal tissues
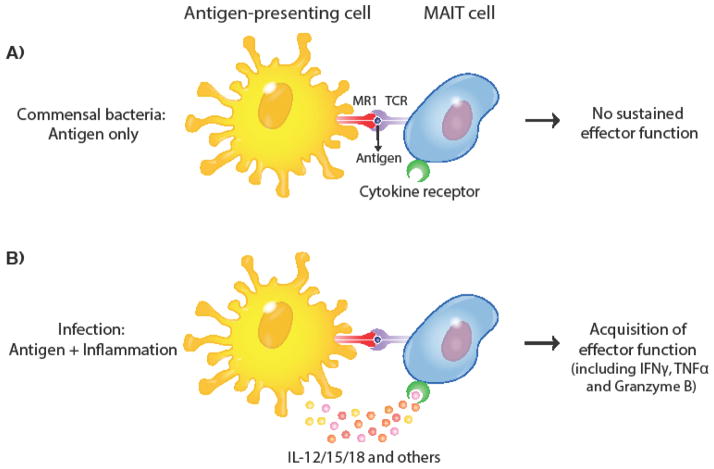
MAIT cells have been identified in human mucosal tissues that are colonized by commensal bacteria including intestines, rectal mucosa and vaginal tissue[12,17,44,50]. Importantly, both commensal and pathogenic species of bacteria have intact riboflavin synthesis pathways and generate metabolites with agonist properties for MAIT cells [51]. Thus, in contrast to conventional T cells[52], MAIT cells cannot use antigen specificity to distinguish commensal from pathogenic bacteria. How can MAIT cells be in close proximity to commensals in tissues without becoming activated given that MR1 is also readily available on antigen-presenting cells[26]? A recent study from our lab demonstrated that once purified, MAIT cells needed more than a TCR signal to acquire sustained effector function and that inflammatory cytokine signals (IL-12/15/18) synergized with the TCR signal to induce potent effector function[44]. In this revised model of MAIT cell activation, encounter with commensal-derived antigen (i.e. a TCR signal only) is not sufficient to activate MAIT cells unless inflammatory cytokines are present (Figure 1), which are typically elicited in the context of a pathogenic infection. The synergistic interaction of cytokine and TCR signal has also been reported in the context of IL-15. IL-15 is a pleiotropic cytokine often produced early during infection by a wide range of cells with potent survival and immunomodulatory effects on T-cells[53]. IL-15 expression within inflamed tissues can provide a co-stimulatory signal to drive memory T-cell effector function[54]. Similar to what has been reported for conventional T cells, Sattler et al showed that addition of IL-15 in the context of suboptimal TCR activation increased MAIT effector function in vitro[55]. While limited antigen availability resulted in minimal IFNγ production by MAIT cells, addition of IL-15 resulted in a synergistic effector response. A comparable effect has been reported for IL-7, which also increases MAIT cell responsiveness[13]. Additional studies are needed to interrogate how MAIT cell function is modulated in various inflammatory environments.
Figure 1. Model of MAIT cell activation.
A functional riboflavin synthesis pathway is found in commensal and pathogenic species, which can lead to MR1-dependent presentation of metabolites to MAIT cells. (A) A TCR signal alone is not sufficient to elicit robust effector function, thus commensal-derived antigen in the absence of additional inflammatory cues is not sufficient to elicit MAIT cell effector function (B) Proinflammatory cytokines including IL-12/15/18 are elicited following infection and synergize with the TCR signal to elicit robust effector function.
How MAIT effector function is eventually turned-off in mucosal tissues, despite the potential continuous antigen exposure from commensals, is unknown. It is important to keep in mind that even healthy mucosal barrier tissues have basal levels of inflammation and additional (MAIT cell intrinsic and extrinsic) control mechanisms are likely in place to help regulate MAIT cell functional properties. Finally, inflammatory cues such as IL-12, IL-15 and IL-18 can be sufficient to activate MAIT cells[56] and do this by directly acting on MAIT cells[44,57] similar to what has been reported for conventional memory T cells[58]. Both conventional memory T cells and MAIT cells can be bystander-activated during viral infections[59–61], but the exact role of MAIT cells during the course of a viral infection is still unclear.
5. MAIT cell subsets, phenotypes and function in mucosal tissues
The tissue microenvironment varies in different anatomical locations and may influence MAIT phenotype and function. Single cell gene expression analysis comparing MAIT cells isolated from blood and rectal mucosa of healthy donors revealed that MAIT cells located in the tissue have increased expression of pro-inflammatory transcripts (but not necessarily protein) such as TNFα and this was more pronounced in the CD8+ MAIT cell subset compared to the CD8− subset[44]. This increase in pro-inflammatory transcripts could allow MAIT cells to respond rapidly in the tissue. Interestingly, expression of these transcripts was not uniformly high all MAIT cells, which displayed a bimodal expression pattern for many of these genes [44] as previously reported in other single cell gene expression analysis datasets[62]. The extent of functional heterogeneity within the MAIT cell population in tissues is still unclear. However, MAIT cells identified in fetal mucosal tissue and the female genital tract (FGT) were shown to have a bias towards IL-17 and IL-22 function compared to MAIT cells in the blood[50,63]. Both of these cytokines have pivotal roles in regulating barrier immunity and tissue homeostasis [64,65] indicating that MAIT cells have a more complex role than just being a guardian against bacterial infections.
6. MAIT cell trafficking patterns and potential
Most MAIT cells in the blood express CCR6[12,44,46,66], which is implicated in trafficking to mucosal tissues and liver, since its ligand CCL20 is expressed in steady state conditions in organs like gut, lung and liver. Interestingly, CCL20 is typically considered to be the sole ligand for CCR6, but some evidence suggests that beta-defensins may bind to CCR6 as well[67]. In general, T-cell trafficking is guided by tissue-selective adhesion and chemokine receptors that allow cells access to specific tissues including mucosal barrier surfaces[68]. The chemokine receptor expression profile of MAIT cells has not been thoroughly characterized yet though has important implications for homing potential. Reported expression patterns are summarized in Table 1. While expression of CCR6 indicates that MAIT cells can access a wide range of tissues, the lack of CCR7 and CD62L expression suggests that they lack the ability to migrate from blood to lymph nodes via high endothelial venules (HEVs)[69]. Data from lymph node (LN) biopsies of cancer patients and characterization of MAIT cells in fetal tissues suggest that MAIT cells are rare in lymph nodes (LN) [12,63]. The origin of the few MAIT cells found in the lymph node is unclear, since MAIT cells can theoretically also access LNs by leaving tissue and entering LNs via the afferent lymphatics. The signals that control these steps are not nearly as well defined as the steps required for LN entry from blood via HEVs, but two reports provided compelling evidence that CCR7 expression on conventional T-cells greatly enhances their ability to exit peripheral tissues and enter the afferent lymphatics[70,71]. There is currently no direct evidence indicating if MAIT cells leave tissue or if they become tissue-resident akin to conventional tissue-resident memory T-cells[72]. Expression of cytotoxic and regulatory T-cell molecule (CRTAM), which controls residency of T-cells in the gut[73], is also enhanced in MAIT cells isolated from tissues[44], supporting the notion that a resident population is plausible. In addition, MAIT cell frequencies in the blood are diminished in context of various infections and autoimmune diseases, sometimes irreversibly, which could be explained if cells are retained within tissues [1–11].
Table 1.
Chemokine receptor profile for MAIT cells in human blood
| Chemokine Receptor | Fraction of MAIT population* | References |
|---|---|---|
| CCR2 | >80% | [66] |
| CCR5 | >80% | [12,66] |
| CCR6 | >80% | [12,46,66] |
| CCR7 | − | [12] |
| CCR9 | + | [12,63] |
| CXCR3 | <5% | [12,46] |
| CXCR4 | 60–80% | [12,66] |
| CXCR6 | >80% | [12,46,66] |
When frequencies are not reported, + and − simply indicate the overall trend of expression
Conclusion
MAIT cells are located in barrier tissues to perform front-line host defense and have increased pro-inflammatory transcripts within these tissues compared to their blood counterparts highlighting that they are poised to respond rapidly at these sites. We discussed a model (Fig. 1) for MAIT cell activation to explain how these cells control their potent effector function at barrier surfaces. Sustained TCR-mediated responses only occur in the presence of inflammatory signals that are typically indicative of an infection. The need for inflammatory signals to elicit cytotoxic effector function may serve to prevent unwanted responses at these sites to commensal-derived antigen in steady state conditions. Recent studies also suggest a role for MAIT cells in maintaining epithelial integrity and tissue homeostasis via IL-17 and IL-22 production. Additional studies are needed to better understand the full functional potential, the migratory properties and the longevity of MAIT cells in steady state and in disease settings to ultimately facilitate therapeutic targeting.
Highlights.
MAIT cells are located in barrier tissues to perform front---line host defense
MAIT cells in these tissues have increased pro---inflammatory transcripts compared to their blood counterparts and are poised to respond rapidly
A TCR signal is not sufficient to induce sustained MAIT cell effector function, which may prevent MAIT cell responses to commensal antigen in mucosal barrier tissues
Inflammatory cytokine signals synergize with the TCR signal to induce potent effector function
Footnotes
Conflict of Interest: The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bianchini E, De Biasi S, Simone AM, Ferraro D, Sola P, Cossarizza A, et al. Invariant natural killer T cells and mucosal-associated invariant T cells in multiple sclerosis. Immunol Lett. 2017;183:1–7. doi: 10.1016/j.imlet.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Chandra S, Kronenberg M. Activation and Function of iNKT and MAIT Cells. Elsevier; 2015. pp. 145–201. [DOI] [PubMed] [Google Scholar]
- 3.Bennett MS, Round JL, Leung DT. Innate-like lymphocytes in intestinal infections. Current Opinion in Infectious Diseases. 2015;28:457–463. doi: 10.1097/QCO.0000000000000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gapin L. Check MAIT. J Immunol. 2014;192:4475–4480. doi: 10.4049/jimmunol.1400119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keller AN, Corbett AJ, Wubben JM, McCluskey J, Rossjohn J. MAIT cells and MR1-antigen recognition. Curr Opin Immunol. 2017;46:66–74. doi: 10.1016/j.coi.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Ussher JE, Klenerman P, Willberg CB. Mucosal-Associated Invariant T-Cells: New Players in Anti-Bacterial Immunity. Front Immunol. 2014;5:1907. doi: 10.3389/fimmu.2014.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Napier RJ, Adams EJ, Gold MC, Lewinsohn DM. The Role of Mucosal Associated Invariant T Cells in Antimicrobial Immunity. Front Immunol. 2015;6 doi: 10.3389/fimmu.2015.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salou M, Franciszkiewicz K, Lantz O. MAIT cells in infectious diseases. Curr Opin Immunol. 2017;48:7–14. doi: 10.1016/j.coi.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Wong EB, Ndung’u T, Kasprowicz VO. The role of mucosal-associated invariant T cells in infectious diseases. Immunology. 2017;150:45–54. doi: 10.1111/imm.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Moreira ML, Tsuji M, Corbett AJ, Araújo MSS, Teixeira-Carvalho A, Martins-Filho OA, et al. MAIT-cells: A tailor-made mate in the ancient battle against infectious diseases? Immunol Lett. 2017;187:53–60. doi: 10.1016/j.imlet.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Kurioka A, Walker LJ, Klenerman P, Willberg CB. MAIT cells: new guardians of the liver. Clin Trans Immunol. 2016;5:e98. doi: 10.1038/cti.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dusseaux M, Martin E, Serriari N, Péguillet I, Premel V. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17–secreting T cells. Blood. 2011;117:1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 13.Tang X-Z, Jo J, Tan AT, Sandalova E, Chia A, Tan KC, et al. IL-7 Licenses Activation of Human Liver Intrasinusoidal Mucosal-Associated Invariant T Cells. The Journal of Immunology. 2013;190:3142–3152. doi: 10.4049/jimmunol.1203218. [DOI] [PubMed] [Google Scholar]
- 14.Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H, et al. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. Journal of Experimental Medicine. 1999;189:1907–1921. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porcelli S. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. Journal of Experimental Medicine. 1993;178:1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lepore M, Kalinichenko A, Colone A, Paleja B, Singhal A, Tschumi A, et al. Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRβ repertoire. Nat Commun. 2014;5:ncomms4866. doi: 10.1038/ncomms4866. [DOI] [PubMed] [Google Scholar]
- 17.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 18.Tsukamoto K, Deakin JE, Graves JAM, Hashimoto K. Exceptionally high conservation of the MHC class I-related gene, MR1, among mammals. Immunogenetics. 2012;65:115–124. doi: 10.1007/s00251-012-0666-5. [DOI] [PubMed] [Google Scholar]
- 19.Riegert P, Wanner V, Bahram S. Genomics, Isoforms, Expression, and Phylogeny of the MHC Class I-Related MR1 Gene. The Journal of Immunology. 1998;161:4066–4077. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- 20.Boudinot P, Mondot S, Jouneau L, Teyton L, Lefranc M-P, Lantz O. Restricting nonclassical MHC genes coevolve with TRAV genes used by innate-like T cells in mammals. Proc Natl Acad Sci USA. 2016;113:E2983–E2992. doi: 10.1073/pnas.1600674113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491:717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 22.Reantragoon R, Corbett AJ, Sakala IG, Gherardin NA, Furness JB, Chen Z, et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. Journal of Experimental Medicine. 2013;210:2305–2320. doi: 10.1084/jem.20130958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corbett AJ, Eckle SBG, Birkinshaw RW, Liu L, Patel O, Mahony J, et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509:361–365. doi: 10.1038/nature13160. [DOI] [PubMed] [Google Scholar]
- 24.L5pez-Sagaseta J, Dulberger CL, McFedries A, Cushman M, Saghatelian A, Adams EJ. MAIT Recognition of a Stimulatory Bacterial Antigen Bound to MR1. The Journal of Immunology. 2013;191:5268–5277. doi: 10.4049/jimmunol.1301958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491:717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 26.McWilliam HEG, Villadangos JA. How MR1 Presents a Pathogen Metabolic Signature to Mucosal-Associated Invariant T (MAIT) Cells. Trends Immunol. 2017 doi: 10.1016/j.it.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Eckle SBG, Corbett AJ, Keller AN, Chen Z, Godfrey DI, Liu L, et al. Recognition of Vitamin B Precursors and Byproducts by Mucosal Associated Invariant T Cells. J Biol Chem. 2015;290:30204–30211. doi: 10.1074/jbc.R115.685990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Bourhis L, Martin E, Péguillet I, Guihot A, Froux N, Coré M, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nature Immunology. 2010;11:701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 29.Le Bourhis L, Dusseaux M, Bohineust A, Bessoles S, Martin E, Premel V, et al. MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLoS Pathog. 2013;9:e1003681. doi: 10.1371/journal.ppat.1003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leeansyah E, Ganesh A, Quigley MF, Sönnerborg A, Andersson J, Hunt PW, et al. Activation, exhaustion and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood. 2013;121:1124–1135. doi: 10.1182/blood-2012-07-445429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma PK, Wong EB, Napier RJ, Bishai WR, Ndung’u T, Kasprowicz VO, et al. High expression of CD26 accurately identifies human bacteria-reactive MR1-restricted MAIT cells. Immunology. 2015;145:443–453. doi: 10.1111/imm.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gold MC, McLaren JE, Reistetter JA, Smyk-Pearson S, Ladell K, Swarbrick GM, et al. MR1-restricted MAIT cells display ligand discrimination and pathogen selectivity through distinct T cell receptor usage. Journal of Experimental Medicine. 2014;211:1601–1610. doi: 10.1084/jem.20140507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckle SBG, Birkinshaw RW, Kostenko L, Corbett AJ, McWilliam HEG, Reantragoon R, et al. A molecular basis underpinning the T cell receptor heterogeneity of mucosal-associated invariant T cells. The Journal of Experimental Medicine. 2014;211:1585–1600. doi: 10.1084/jem.20140484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lepore M, Kalinichenko A, Calogero S, Kumar P, Paleja B, Schmaler M, et al. Functionally diverse human T cells recognize non-microbial antigens presented by MR1. eLife Sciences. 2017;6:e24476. doi: 10.7554/eLife.24476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gherardin NA, Keller AN, Woolley RE, Le Nours J, Ritchie DS, Neeson PJ, et al. Diversity of T Cells Restricted by the MHC Class I-Related Molecule MR1 Facilitates Differential Antigen Recognition. Immunity. 2016;44:32–45. doi: 10.1016/j.immuni.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 37.Wilson RP, Ives ML, Rao G, Lau A, Payne K, Kobayashi M, et al. STAT3 is a critical cell-intrinsic regulator of human unconventional T cell numbers and function. Journal of Experimental Medicine. 2015;212:855–864. doi: 10.1084/jem.20141992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raberger J, Schebesta A, Sakaguchi S, Boucheron N, Blomberg KEM, Berglöf A, et al. The transcriptional regulator PLZF induces the development of CD44 high memory phenotype T cells. Proc Natl Acad Sci USa. 2008;105:17919–17924. doi: 10.1073/pnas.0805733105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kovalovsky D, Alonzo ES, Uche OU, Eidson M, Nichols KE, Sant’Angelo DB. PLZF Induces the Spontaneous Acquisition of Memory/Effector Functions in T Cells Independently of NKT Cell-Related Signals. The Journal of Immunology. 2010;184:6746–6755. doi: 10.4049/jimmunol.1000776. [DOI] [PubMed] [Google Scholar]
- 40.Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, et al. The BTB–zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nature Immunology. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, et al. The Transcription Factor PLZF Directs the Effector Program of the NKT Cell Lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreslavsky T, Savage AK, Hobbs R, Gounari F, Bronson R, Pereira P, et al. TCR-inducible PLZF transcription factor required for innate phenotype of a subset of gammadelta T cells with restricted TCR diversity. Proc Natl Acad Sci USa. 2009;106:12453–12458. doi: 10.1073/pnas.0903895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gold MC, Eid T, Smyk-Pearson S, Eberling Y, Swarbrick GM, Langley SM, et al. Human thymic MR1-restricted MAIT cells are innate pathogen-reactive effectors that adapt following thymic egress. Mucosal Immunol. 2013;6:35–44. doi: 10.1038/mi.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slichter CK, McDavid A, Miller HW, Finak G, Seymour BJ, McNevin JP, et al. Distinct activation thresholds of human conventional and innate-like memory T cells. JCI Insight. 2016;1 doi: 10.1172/jci.insight.86292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dias J, Leeansyah E, Sandberg JK. Multiple layers of heterogeneity and subset diversity in human MAIT cell responses to distinct microorganisms and to innate cytokines. Proc Natl Acad Sci USa. 2017;114:E5434–E5443. doi: 10.1073/pnas.1705759114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mpina M, Maurice NJ, Yajima M, Slichter CK, Miller HW, Dutta M, et al. Controlled Human Malaria Infection Leads to Long-Lasting Changes in Innate and Innate-like Lymphocyte Populations. The Journal of Immunology. 2017;199:107–118. doi: 10.4049/jimmunol.1601989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carolan E, Tobin LM, Mangan BA, Corrigan M, Gaoatswe G, Byrne G, et al. Altered Distribution and Increased IL-17 Production by Mucosal-Associated Invariant T Cells in Adult and Childhood Obesity. The Journal of Immunology. 2015;194:5775–5780. doi: 10.4049/jimmunol.1402945. [DOI] [PubMed] [Google Scholar]
- 48.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 49.Leeansyah E, Svärd J, Dias J, Buggert M, Nyström J, Quigley MF, et al. Arming of MAIT Cell Cytolytic Antimicrobial Activity Is Induced by IL-7 and Defective in HIV-1 Infection. PLoS Pathog. 2015;11:e1005072. doi: 10.1371/journal.ppat.1005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gibbs A, Leeansyah E, Introini A, Paquin-Proulx D, Hasselrot K, Andersson E, et al. MAIT cells reside in the female genital mucosa and are biased towards IL-17 and IL-22 production in response to bacterial stimulation. Mucosal Immunol. 2016;10:35–45. doi: 10.1038/mi.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salerno-Goncalves R, Rezwan T, Sztein MB. B Cells Modulate Mucosal Associated Invariant T Cell Immune Responses. Front Immunol. 2014;4 doi: 10.3389/fimmu.2013.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Belkaid Y, Bouladoux N, Hand TW. Effector and memory T cell responses to commensal bacteria. Trends Immunol. 2013;34:299–306. doi: 10.1016/j.it.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perera P-Y, Lichy JH, Waldmann TA, Perera LP. The role of interleukin-15 in inflammation and immune responses to infection: implications for its therapeutic use. Microbes and Infection. 2012;14:247–261. doi: 10.1016/j.micinf.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jabri B, Abadie V. IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction. Nature Reviews Immunology. 2015;15:771–783. doi: 10.1038/nri3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sattler A, Dang-Heine C, Reinke P, Babel N. IL-15 dependent induction of IL-18 secretion as a feedback mechanism controlling human MAIT-cell effector functions. Eur J Immunol. 2015;45:2286–2298. doi: 10.1002/eji.201445313. [DOI] [PubMed] [Google Scholar]
- 56.Ussher JE, Bilton M, Attwod E, Shadwell J, Richardson R, de Lara C, et al. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol. 2014;44:195–203. doi: 10.1002/eji.201343509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Finak G, McDavid A, Yajima M, Deng J, Gersuk V, Shalek AK, et al. MAST: a flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biology. 2015;16:1183. doi: 10.1186/s13059-015-0844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chu T, Tyznik AJ, Roepke S, Berkley AM, Woodward-Davis A, Pattacini L, et al. Bystander-Activated Memory CD8 T Cells Control Early Pathogen Load in an Innate-like, NKG2D-Dependent Manner. Cell Reports. 2013;3:701–708. doi: 10.1016/j.celrep.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Odumade OA, Knight JA, Schmeling DO, Masopust D, Balfour HH, Jr, Hogquist KA. Primary Epstein-Barr virus infection does not erode preexisting CD8 +T cell memory in humans. Journal of Experimental Medicine. 2012;209:471–478. doi: 10.1084/jem.20112401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Wilgenburg B, Scherwitzl I, Hutchinson EC, Leng T, Kurioka A, Kulicke C, et al. MAIT cells are activated during human viral infections. Nat Commun. 2016;7:11653. doi: 10.1038/ncomms11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loh L, Wang Z, Sant S, Koutsakos M, Jegaskanda S, Corbett AJ, et al. Human mucosal-associated invariant T cells contribute to antiviral influenza immunity via IL-18-dependent activation. Proc Natl Acad Sci USa. 2016;113:10133–10138. doi: 10.1073/pnas.1610750113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shalek AK, Satija R, Adiconis X, Gertner RS, Gaublomme JT, Raychowdhury R, et al. Single-cell transcriptomics reveals bimodality in expression and splicing in immune cells. Nature. 2013;498:236–240. doi: 10.1038/nature12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leeansyah E, Loh L, Nixon DF, Sandberg JK. Acquisition of innate-like microbial reactivity in mucosal tissues during human fetal MAIT-cell development. Nat Commun. 2014;5:ncomms4143. doi: 10.1038/ncomms4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klatt NR, Estes JD, Sun X, Ortiz AM, Barber JS, Harris LD, et al. Loss of CD103+ DCs and Mucosal IL-17+ and IL-22+ Lymphocytes is Associated with Mucosal Damage in SIV Infection. Mucosal Immunol. 2012;5:646–657. doi: 10.1038/mi.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eyerich K, Dimartino V, Cavani A. IL-17 and IL-22 in immunity: Driving protection and pathology. Eur J Immunol. 2017;47:607–614. doi: 10.1002/eji.201646723. [DOI] [PubMed] [Google Scholar]
- 66.Brozova J, Karlova I, Novak J. Analysis of the Phenotype and Function of the Subpopulations of Mucosal-Associated Invariant T Cells. Scand J Immunol. 2016;84:245–251. doi: 10.1111/sji.12467. [DOI] [PubMed] [Google Scholar]
- 67.Lee AYS, Phan TK, Hulett MD, Körner H. The relationship between CCR6 and its binding partners: Does the CCR6–CCL20 axis have to be extended? Cytokine. 2015;72:97–101. doi: 10.1016/j.cyto.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 68.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 69.Lira SA. A passport into the lymph node. Nature Immunology. 2005;6:866–868. doi: 10.1038/ni0905-866. [DOI] [PubMed] [Google Scholar]
- 70.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nature Immunology. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 71.Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nature Immunology. 2005;6:889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carbone FR. Tissue-Resident Memory T Cells and Fixed Immune Surveillance in Nonlymphoid Organs. The Journal of Immunology. 2015;195:17–22. doi: 10.4049/jimmunol.1500515. [DOI] [PubMed] [Google Scholar]
- 73.Cortez VS, Cervantes-Barragan L, Song C, Gilfillan S, McDonald KG, Tussiwand R, et al. CRTAM controls residency of gut CD4+CD8+ T cells in the steady state and maintenance of gut CD4+ Th17 during parasitic infection. Journal of Experimental Medicine. 2014;211:623–633. doi: 10.1084/jem.20130904. [DOI] [PMC free article] [PubMed] [Google Scholar]