Abstract
Intentional weight loss is an important treatment option for overweight persons with type 2 diabetes mellitus (DM), but the effects on long term fracture risk are not known. The purpose of this Look AHEAD analysis was to evaluate whether long term intentional weight loss would increase fracture risk in overweight or obese persons with DM. Look AHEAD is a multicenter, randomized clinical trial. Recruitment began in August 2001 and follow-up continued for a median of 11.3 years at 16 academic centers. 5145 persons aged 45 – 76 with DM were randomized to either an intensive lifestyle intervention (ILI) with reduced calorie consumption and increased physical activity designed to achieve and maintain ≥7% weight loss or to diabetes support and education intervention (DSE). Incident fractures were ascertained every 6 months by self-report and confirmed with central adjudication of medical records.
The baseline mean age of participants was 59 years, 60% were women, 63% were Caucasian, and the mean BMI was 36 kg/m2. Weight loss over the intervention period (median 9.6 years) was 6.0% in ILI and 3.5% in DSE. 731 participants had a confirmed incident fracture (358 in DSE v. 373 in ILI). There were no statistically significant differences in incident total or hip fracture rates between the ILI and DSE groups. However, compared to the DSE group, the ILI group had a statistically significant 39% increased risk of a frailty fracture (HR = 1.39, 95% CI 1.02, 1.89).
An intensive lifestyle intervention resulting in long term weight loss in overweight/obese adults with DM was not associated with an overall increased risk of incident fracture but may be associated with an increased risk of frailty fracture. When intentional weight loss is planned, consideration of bone preservation and fracture prevention is warranted.
Keywords: Fracture, intentional weight loss, type 2 diabetes, clinical trial
Introduction
Osteoporotic fractures are a prevalent, serious condition that can result in substantial morbidity and increased mortality.1–3 Known risk factors associated with the development of osteoporosis and fractures include older age, family history, female gender, previous history of fracture, history of falls, and lower body mass index (BMI).4–6 Further, in older adults, type 2 diabetes is associated with increased fracture risk compared to persons without diabetes.7–11 Moreover, weight loss has also been shown to be an important risk factor for bone loss and fracture in older adults,12–16 but it is also a cornerstone of treatment in overweight and obese persons who have type 2 diabetes. However, the net effect of intentional weight loss with increased physical activity over extended periods of time on fracture risk in older persons with diabetes is not well understood. To date, there are no large randomized trials of weight loss that also have data on fractures, the most important outcome in terms of skeletal health.
Therefore, the purpose of this manuscript is to examine whether long term intentional weight loss with increased physical activity would increase fracture risk in overweight/obese older persons with type 2 diabetes in the Look AHEAD randomized clinical trial.
Methods
The Look AHEAD randomized clinical trial involved 16 clinical sites across the US (Clinicaltrials.gov Identifier: NCT00017953). The methods and baseline characteristics of the study population have been published17,18 and the protocol is available (www.lookaheadtrial.org). The primary goal of Look AHEAD was to determine whether randomization to intensive lifestyle intervention (ILI) reduced cardiovascular morbidity and mortality relative to Diabetes Support and Education (DSE) among overweight or obese individuals with type 2 diabetes mellitus. During the first 2 years of the trial, the primary-event rate in the control group was lower than expected.19 Therefore, hospitalization for angina was added to the primary cardiovascular outcome, and planned follow-up was extended to a maximum of 13.5 years. On September 14, 2012, the trial intervention was stopped for futility because there was no difference with regard to the primary cardiovascular endpoints between ILI and DSE.20 The independent DSMB made this recommendation and the NIH concurred.
Intervention curricula for both ILI and DSE were developed centrally and have been described in detail.21 ILI aimed at achieving and maintaining at least a 7% weight loss by focusing on reduced caloric intake and increased physical activity. The program included frequent contact throughout the trial, with both group and individual sessions, a calorie goal of 1200–1800 kcal/day (<30% of calories from fat and >15% from protein), use of meal replacement products, and at least 175 minutes per week of moderate intensity physical activity. The most common type of physical activity was walking. A toolbox of strategies was available for participants having difficulty achieving the weight loss goals.
Look AHEAD was also designed to examine secondary outcomes including fracture. Look AHEAD was approved by local Institutional Review Boards and all participants provided informed consent. Look AHEAD complied with the World Medical Association Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects.
Major eligibility criteria for Look AHEAD included the following: 45–76 years of age; type 2 diabetes; body mass index (BMI; kg/m2) of ≥25.0 (≥27 in individuals taking insulin); hemoglobin A1c (HbA1c) <11%, systolic blood pressure (SBP) <160 mmHg, diastolic blood pressure (DBP) <100 mmHg, and triglycerides <600 mg/dl; ability to complete a valid maximal exercise test suggesting it was safe to exercise; and having a primary care provider. Participants could be using any type of glucose-lowering medication, but the percentage of participants using insulin was limited to <30%.
Randomization occurred from August 2001 through April 2004 with an allocation ratio of 1:1 and stratification by clinical site. At baseline and annual clinic visits, weight and height were measured with a digital scale and a standard wall-mounted stadiometer respectively. Body Mass Index (BMI) in kg/m2 was calculated from the measured weights and heights. Participants brought all prescription medicines to the clinic annually for a medication inventory. Bone-active agents were classified from the medication inventory and are used in these analyses. Bone-negative medications were defined as: loop diuretics, selective serotonin reuptake inhibitors (SSRIs), thyroid hormones, oral steroids such as prednisone, tricyclic antidepressants, and thiazolidinedione (TZDs). Bone-positive medications were defined as: androgens (anabolic steroids), calcium, antacids containing calcium, and antiresorptive agents such as bisphosphonates, calcitonin nasal spray, estrogens, and selective estrogen receptor modulators (SERMs). Questionnaires were used to collect demographic characteristics, medical history, smoking history, and alcohol use. Race categories are self-reported. Blood work was completed after overnight fast and was analyzed by the Central Biochemistry Laboratory (Northwest Lipid Research Laboratories, University of Washington, Seattle, WA) using standardized laboratory procedures.
During annual visits and telephone calls every 6 months, staff members who were unaware of study-group assignments (blinded) queried participants about all medical events and hospitalizations including incident fractures. Hospital and other records such as outpatient medical records and x-ray reports were reviewed for potential incident fracture events, with adjudication according to standard criteria by a central review committee of trained physicians who were blinded to study-group assignment. The primary fracture outcome was prespecified as the first occurrence of a fracture. Self-reported fractures of the fingers, toes, face, neck (c-spine), sternum, ribs, and skull were not centrally adjudicated and are not included in the fracture events for this manuscript. Only confirmed centrally adjudicated incident and total fracture events are included in these analyses. As a secondary fracture outcome a frailty fracture endpoint was created a priori and is a composite of the first occurrence of a hip, pelvis, upper arm or shoulder fracture.22 This secondary fracture endpoint was selected because data from the Study of Osteoporotic Fractures (SOF) had previously demonstrated that weight loss was associated with frailty fracture.22 Participants were also asked how the fracture happened, including the following categories: motor vehicle accident/struck by a motor vehicle, fall down stairs, fall from a height, other fall or trip (e.g. while walking or getting out of bed), and other. Beginning in year 8, participants were also asked to self-report the number of falls each year. Fitness was estimated from peak metabolic-equivalent (MET) capacity from performance on a graded exercise treadmill test administered at baseline, year 1, and year 4.23
Statistical analysis
Baseline characteristics were presented as means and standard errors (SE), medians and inter-quartile range (IQR), or frequencies (percentages). Two sample t-tests, Wilcoxon rank-sum test, and chi-square tests were used to compare the baseline characteristics within the two intervention groups. We performed analyses of overall incident fractures, incident frailty fractures, and site specific incident fractures in which we compared time to the first occurrence of fracture, between the two study groups. Participants were censored at the end of follow-up or at time of death. Kaplan-Meier estimates were used to calculate the cumulative proportion of patients who had an event. Cox proportional-hazards regression was used to calculate hazard ratios, 95% confidence intervals, and two-sided p-values for three models. The first model was unadjusted; the second model was adjusted for age and gender; and the third model was adjusted for age, gender, race, baseline BMI, cigarette smoking, alcohol consumption, maximal MET value, self-reported duration of diabetes, bone-active agents (bone positive and negative medications separately), Insulin use from any source, Beck depression score, eGFR, and HbA1c. The stability of the intervention effect on fractures across gender, race, age tertiles, BMI tertiles and bone-active agents was verified using tests of interaction for overall fracture and frailty fractures. Additionally, Cox proportional-hazards regressions were run separately for the two study groups in order to compare time to the first occurrence of fracture between tertiles of percent weight change between baseline and year 1. Proportional hazards assumptions were verified for all models and results were not adjusted for multiple comparisons. Chi-square tests and Wilcoxon rank sum tests were used to determine if a relationship existed between fracture incidence and either fall as the cause of a fracture by randomization arm or the number of falls after year 8 by randomization arm. A p < 0.05 was accepted as statistically significant. No adjustments were made for multiple comparisons. All statistical analyses were completed using SAS 9.4 (SAS Institute, Cary NC) or R Core Team (2014). (R Foundation for Statistical Computing, Vienna, Austria).
Results
Out of the 9,045 who attended a screening visit, 5,145 ethnically diverse overweight and obese subjects with type 2 diabetes mellitus were randomized to either the ILI or DSE arm in the Look AHEAD Trial. (Supplemental Figure 1) At baseline, the participants were on average 59 years old, 60% were women, 63% were Caucasian and the mean BMI was 35.94 kg/m2. There were no statistically significant baseline differences between the ILI and DSE groups including duration of diabetes, smoking status, alcohol intake, physical activity, use of bone-active agents or insulin, Beck depression score, estimate glomerular filtration rate (eGFR), or hemoglobin A1c (HbA1c). (Table 1)
Table 1.
Baseline Characteristics Overall and by Randomization Arm
| Characteristics | Overall | DSE | ILI | P-Value |
|---|---|---|---|---|
| Number of Subjects | 5145 (100) | 2575 (50.05) | 2570 (49.95) | |
| Age (Years) | 58.75 ± 0.1 | 58.9 ± 0.14 | 58.59 ± 0.13 | 0.1 |
| Gender | . | . | . | 0.82 |
| Male | 2082 (40.47) | 1038 (40.31) | 1044 (40.62) | |
| Female | 3063 (59.53) | 1537 (59.69) | 1526 (59.38) | |
| Race/Ethnicity | . | . | . | 0.98 |
| African American | 804 (15.63) | 404 (15.69) | 400 (15.57) | |
| Caucasian | 3252 (63.22) | 1631 (63.34) | 1621 (63.1) | |
| Hispanic | 680 (13.22) | 340 (13.2) | 340 (13.23) | |
| Other | 408 (7.93) | 200 (7.77) | 208 (8.1) | |
| Body weight (kg) | 100.71 ± 0.27 | 100.85 ± 0.37 | 100.56 ± 0.39 | 0.59 |
| Body Mass Index (kg/m^2) | 35.94 ± 0.08 | 36 ± 0.11 | 35.89 ± 0.12 | 0.51 |
| Smoking Status | . | . | . | 0.71 |
| Never | 2576 (50.18) | 1302 (50.7) | 1274 (49.65) | |
| Former | 2331 (45.4) | 1156 (45.02) | 1175 (45.79) | |
| Current | 227 (4.42) | 110 (4.28) | 117 (4.56) | |
| Alcohol Consumption | . | . | . | 0.6 |
| No drinks/week | 3478 (67.82) | 1736 (67.63) | 1742 (68.02) | |
| 1–3 drinks/week | 994 (19.38) | 491 (19.13) | 503 (19.64) | |
| 4+ drinks/week | 656 (12.79) | 340 (13.25) | 316 (12.34) | |
| Physical Activity | . | . | . | |
| Maximal MET value | 7.19 ± 0.03 | 7.18 ± 0.04 | 7.2 ± 0.04 | 0.67 |
| Self-Reported Duration of Diabetes | 5 (2, 10) | 5 (2, 10) | 5 (2, 10) | 0.16 |
| Medications | . | . | . | |
| Bone Positive | 1308 (25.42) | 649 (25.2) | 659 (25.64) | 0.72 |
| Bone Negative | 2378 (46.22) | 1199 (46.56) | 1179 (45.88) | 0.62 |
| Steroids | 6 (0.12) | 2 (0.08) | 4 (0.16) | 0.45 |
| Bisphosphonates | 95 (1.85) | 43 (1.67) | 52 (2.02) | 0.35 |
| TZDs | 1337 (25.99) | 689 (26.76) | 648 (25.21) | 0.21 |
| Insulin Use (from any source) | 980 (19.05) | 500 (19.42) | 480 (18.68) | 0.5 |
| Beck Depression Score | 4 (2, 8) | 4 (2, 8) | 4 (2, 8) | 0.68 |
| eGFR | 94.03 ± 0.32 | 93.68 ± 0.44 | 94.38 ± 0.46 | 0.27 |
| HbA1c | 7.28 ± 0.02 | 7.31 ± 0.02 | 7.25 ± 0.02 | 0.06 |
eGFR = estimated glomerular filtration rate, HbA1c = Hemoglobin A1c. N (%) or Mean ± Standard Error or Median (Q1, Q3). Chi-Square and Students T-test were used, with the exception of Self-Reported Diabetes Duration, the Beck Depression Score which used the Wilcoxon Rank Sum and Steroids which used a Fisher’s Exact Test
Weight loss in the ILI was largest at year 1 (8.6% in the ILI vs. 0.7% in the DSE) but remained significantly greater in ILI throughout the trial. When the study intervention ended (median 9.6 years of follow-up), the mean weight loss from baseline was 6.0% in ILI and 3.5% in DSE. Physical fitness improvement in the ILI was greatest at year 1 but remained significant through year 4 (last time point measured) compared to the DSE group. (Data not shown)
After a median follow-up of 11.3 years, 731 participants had a confirmed incident fracture, with 358 occurring in DSE participants and 373 in ILI participants. The most common fracture types were lower leg/ankle (216), lower arm/wrist (135), and upper arm (humerus)/shoulder/clavicle (131) whereas hip fractures were much less common (46). There were no statistically significant differences in total incident fracture (HR 1.03, 95% CI 0.89–1.19, p = 0.71) or hip fracture rates (HR 1.69, 95%CI 0.93–3.07, p = 0.09) between the ILI and DSE groups. (Table 2 and Figure 1) However, compared to the DSE group, the ILI group had a statistically significant 39% increased risk of a frailty fracture (HR 1.39, 95% CI 1.02–1.89, p = 0.03) which remained statistically significant after adjustment for potential confounders. (Table 2 and Figure 2) In contrast, there was a trend for the ILI group to have fewer lower leg/ankle fractures than the DSE group but the difference did not reach statistical significance (HR 0.80, p = 0.11).
Table 2.
Incident First Fracture by Randomization Assignment
| DSE | ILI | Unadjusted | Adjusted for Age and Gender | Fully Adjusted Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fracture Type | # of Events | Incidence Per 10,000 person-years | # of Events | Incidence Per 10,000 person-years | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| All Fractures | 358 | 139 | 373 | 143 | 1.03 (0.89, 1.19) | 0.71 | 1.04 (0.90, 1.20) | 0.61 | 1.02 (0.88, 1.18) | 0.76 |
| Hand (Not Fingers) | 7 | 3 | 7 | 2 | 0.99 (0.35, 2.82) | 0.98 | 1.01 (0.35, 2.88) | 0.99 | 0.99 (0.35, 2.83) | 0.98 |
| Lower Arm or Wrist | 64 | 23 | 71 | 26 | 1.10 (0.78, 1.54) | 0.59 | 1.11 (0.79, 1.56) | 0.54 | 1.11 (0.79, 1.56) | 0.55 |
| Elbow | 14 | 5 | 17 | 6 | 1.20 (0.59, 2.44) | 0.61 | 1.20 (0.59, 2.44) | 0.61 | 1.23 (0.61, 2.51) | 0.56 |
| Upper Arm (Humerus), Shoulder, or Clavicle | 63 | 23 | 68 | 25 | 1.07 (0.76, 1.51) | 0.70 | 1.09 (0.77, 1.53) | 0.64 | 1.07 (0.75, 1.51) | 0.72 |
| Vertebra (Thoracic or Lumbar) | 26 | 9 | 28 | 10 | 1.06 (0.62, 1.81) | 0.82 | 1.07 (0.63, 1.83) | 0.80 | 1.08 (0.63, 1.86) | 0.77 |
| Tailbone | 3 | 1 | 4 | 1 | 1.31 (0.29, 5.87) | 0.72 | 1.35 (0.30, 6.01) | 0.70 | 1.27 (0.27, 5.92) | 0.76 |
| Pelvis | 4 | 1 | 14 | 5 | 3.47 (1.14, 10.54) | 0.03 | 3.53 (1.16, 10.74) | 0.03 | 3.43 (1.13, 10.42) | 0.03 |
| Hip | 17 | 6 | 29 | 10 | 1.69 (0.93, 3.07) | 0.09 | 1.78 (0.98, 3.25) | 0.06 | 1.63 (0.89, 3.00) | 0.12 |
| Upper Leg (Not Hip) | 5 | 2 | 6 | 2 | 1.18 (0.36, 3.87) | 0.78 | 1.18 (0.36, 3.85) | 0.79 | 1.20 (0.36, 3.94) | 0.77 |
| Knee (Patella) | 25 | 9 | 29 | 10 | 1.15 (0.67, 1.96) | 0.61 | 1.17 (0.68, 1.99) | 0.57 | 1.21 (0.71, 2.09) | 0.48 |
| Lower Leg or Ankle | 119 | 44 | 97 | 35 | 0.80 (0.61, 1.05) | 0.11 | 0.81 (0.62, 1.05) | 0.12 | 0.80 (0.61, 1.04) | 0.10 |
| Foot (Not Toes) | 23 | 8 | 26 | 9 | 1.12 (0.64, 1.96) | 0.69 | 1.11 (0.63, 1.94) | 0.72 | 1.10 (0.63, 1.94) | 0.73 |
| Frailty | 70 | 25 | 98 | 35 | 1.39 (1.02, 1.89) | 0.03 | 1.44 (1.06, 1.96) | 0.02 | 1.39 (1.02, 1.91) | 0.04 |
Frailty fracture is a composite of hip, pelvis, or upper arm/shoulder. Fully adjusted model includes the following variables: Age, Gender, Race, BMI, Smoking Status, Alcohol Consumption, Maximal MET value, Self-Reported Duration of Diabetes, Bone + Meds, Bone - Meds, Insulin Use from Any Source, Beck Depression Score, eGFR, HbA1c. Hazard ratio = HR. Confidence interval = CI.
Figure 1.
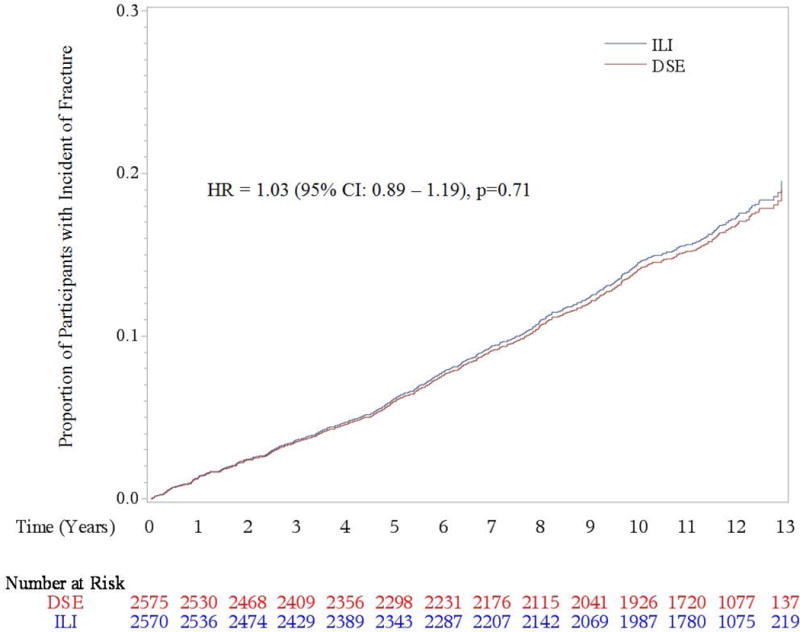
Cumulative Hazards for Incident Total Fracture by Treatment Assignment
Figure 2.
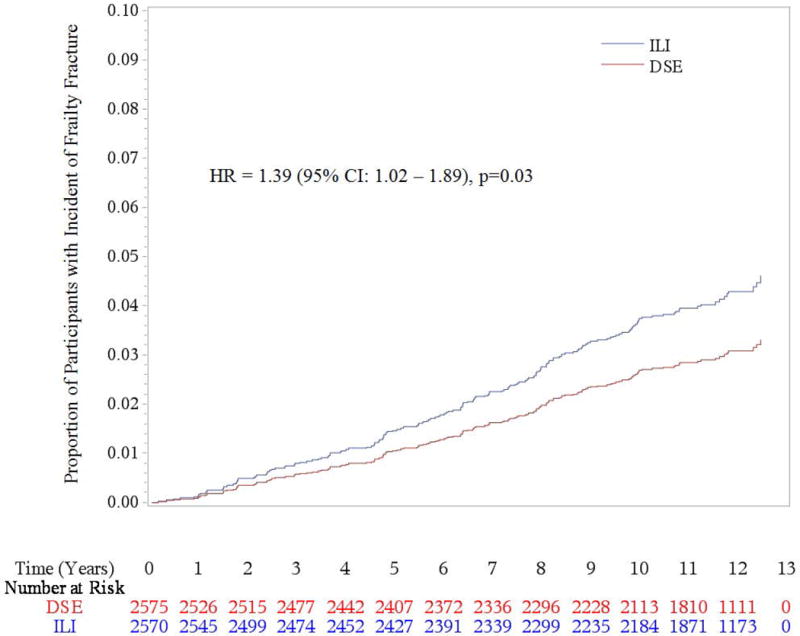
Cumulative Hazard for Incident Frailty Fracture by Treatment Assignment
During the follow-up period, a total of 952 fractures occurred and most fracture events resulted in fracturing a single bone (853 of 952). (See Supplemental Table 2 and Supplemental Table 3) Women in both the ILI and DSE groups were more likely to experience any fracture and frailty fractures compared to men. (See Supplemental Figure 2 and Supplemental Figure 3)
To examine if heterogeneity of effect was present, treatment-subgroup interactions for total fracture and frailty fracture were estimated. Participants who were of younger age at baseline compared to middle and older age (< 56, 56–61, ≥ 61 years old) who were assigned to ILI compared to DSE were at increased risk for fracture (interaction p = 0.042). (Figure 3) A significant U-shaped relationship also existed with baseline fitness with persons in the middle maximal MET tertile group (6.1–≤7.8) who were assigned to ILI compared to DSE being less likely to fracture than those in the lower (<6.1) or higher (≥ 7.8) MET tertile groups (interaction p = 0.03). There was also a trend for persons with a higher baseline BMI (≥ 37.8) compared to lower baseline BMIs (32.6 – 37.7 or < 32.6 kg/m2) who were assigned to ILI compared to DSE to have a higher fracture risk (interaction p = 0.052). No other treatment-subgroup interactions for incident total fracture risk were detected. The same treatment-subgroup interactions were also examined for frailty fracture, but no significant interactions were detected. However, the point estimates of the hazard ratios for all subgroups suggested increased risk of frailty fracture in the ILI group. (See Supplemental Figure 4)
Figure 3.
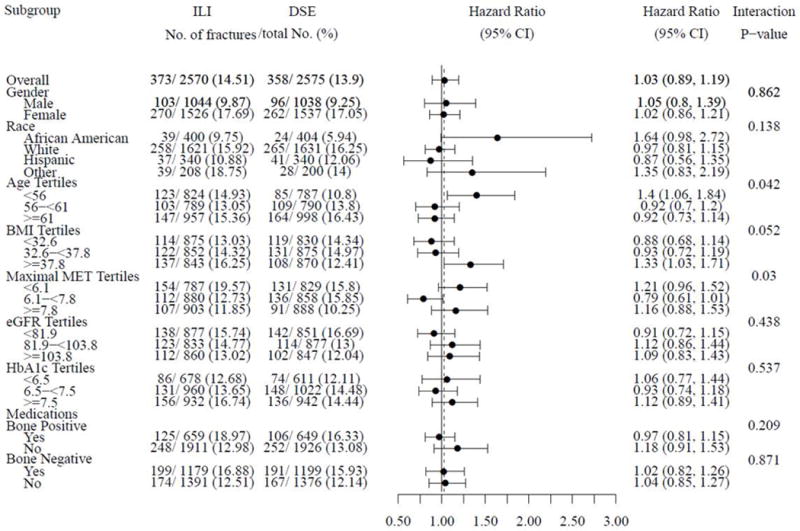
Hazard Ratios (95% CI) of Incident Fractures for Randomization Arm in Subgroups
When asked about falls at annual clinic visits starting at year 8, the DSE group was more likely to report at least two or more falls compared to the ILI group (41.95% in DSE v. 37.49% in ILI, p = 0.0023). Further, the majority of total fractures in both groups were reported to be the result of a fall or trip, but there was no statistically significant difference in total fractures resulting from a fall by treatment assignment (71.2% in DSE v. 73.2% in ILI, p = 0.577). A similar finding of no difference between treatment groups was seen in the frailty fracture outcome resulting from a fall (77.8% in DSE v. 80.6% in ILI, p = 0.703). (See Supplemental Table 1)
Percent weight change in tertiles from baseline to year 1 was examined for the ILI and DSE groups separately. In neither ILI nor DSE was weight change during the first year of the intervention predictive of future fracture risk. (Data not shown).
Discussion
In the Look AHEAD clinical trial in overweight and obese persons with diabetes, an intensive lifestyle intervention that resulted in intentional long term weight loss and improved fitness was not associated with an overall increased risk of total incident fracture. However, incident frailty fracture risk was increased by intentional weight loss despite the increase in physical activity and improved fitness in the intervention group. The increased risk of frailty fracture in the ILI group was also apparent in all subgroups examined. To our knowledge this is the first clinical trial to assess the long term effects of an intentional weight loss intervention on fracture risk in adults with type 2 diabetes.
Our finding of increased frailty fracture risk with weight loss is consistent with other reports from the Study of Osteoporotic Fractures that unintentional weight loss is associated with an increase in frailty fractures but not other non-spine fractures.22 In other studies, lower weight and intentional weight loss have also been shown to increase fracture risk.24
The underlying mechanisms leading to the increased frailty fracture risk with weight loss may be multifactorial. These observations of increased frailty fracture risk may be due to bone loss that is associated with weight loss.12,13 Look AHEAD obtained DXA scans on a subgroup of study participants.25,26 During the intentional intensive weight loss phase in year 1, both men and women in the ILI group experienced greater bone loss at the total hip (−1.4% versus −0.4%; p < 0.001) and femoral neck (−1.5% versus −0.8%; p = 0.009) compared to DSE, but change in BMD for the lumbar spine and whole body did not differ between groups.25 Differences between groups were diminished by one-half after 4 years (−0.88% vs. −0.05% per year in ILI and DSE, respectively) but remained significantly different (P < 0.01).26
Increased fracture risk may also be related to loss of lean body mass leading to decreased strength and problems with balance. It is known that intentional weight loss has been associated with loss of lean body mass.15,27 Aging is also associated with changes in body composition including a loss of lean body mass with a concomitant increase in fat mass.28,29 In the Look AHEAD DXA subgroup, lean body mass decreased more in the ILI than DSE between baseline and Year 1 and remained lower through Year 8.30 However, despite these changes in lean body mass, the ILI group increased their physical activity resulting in improved fitness.31 Further in the Look AHEAD trial, the weight loss and improved fitness have been shown to slow the decline in mobility in the ILI group compared to the DSE group.32 Improved fitness and slowing the decline in mobility may also have been responsible for our finding of fewer self-reported falls in the ILI group.
Falls are a leading cause of injury as a person ages and frailty fractures are one of the most serious consequences.33 Persons with diabetes have also been shown to have an increased risk of falling with poor balance and peripheral neuropathy has been suggested in the causal pathway.8 Thus, a mitigating factor for fracture risk may be increasing physical activity and improving fitness/muscle strength in the ILI group. Unfortunately, Look AHEAD does not have data on severity of falls and thus cannot address whether the ILI group experienced more severe falls leading to increase frailty fracture.
Alternate causes of falls in persons with diabetes may be related to diabetes treatment related hypoglycemia. In Look AHEAD, the incidence of self-reported severe hypoglycemia adverse events over the course of the trial was low and not different in the ILI and DSE groups.34 However, the ILI group did experience more hypoglycemic events in the first year of the trial despite pre-specified safety protocols to temporarily adjust insulin, sulfonylureas or glitinide during intensive calorie restriction by study staff.34 After the first year, the ILI and DSE groups were not significantly different regarding hypoglycemic events.34 Thus, it is unlikely that the increased frailty fracture incidence seen throughout the trial in the ILI group can be primarily attributed to this mechanism.
In the Look AHEAD data, there was a trend for a reduction in lower leg/ankle fracture in the ILI group compared to DSE (p = 0.11). Previous studies have shown that increased body weight rather than decreased bone mineral density is a risk factor for ankle fracture, leading investigators to speculate that the higher BMI increases forces applied to the ankle during a fall.35 We speculate that an exacerbating factor for the trend in increased ankle fracture in the DSE group may be their increased BMI compared with ILI.
Our findings suggest that when intentional weight loss is planned, consideration of bone preservation and fracture prevention strategies is warranted. A number of treatments can help to prevent loss of bone including adequate consumption of calcium and vitamin D, load bearing and resistance exercise, smoking cessation and decreasing alcohol consumption.36–39 Further, falls prevention strategies such as strength and balance training or reduction in psychotropic medication use may also help reduce the risk of falls leading to fracture during weight loss.40 In evaluating persons interested in weight loss, use of the WHO Fracture Risk Assessment Tool (FRAX), which allows individual estimation of the ten year probability of fracture in non-osteoporotic persons may be useful when deciding if medication to prevent an osteoporotic fracture is warranted.41–43 However, the FRAX tool does not take into account diabetes status or falls risk which may also increase potential fracture risk.
The strengths of the Look AHEAD clinical trial include randomization to group assignment, high levels of retention, a diverse participant population, and successfully producing long-term intentional weight loss and improvements in physical activity and fitness. Further, fracture outcomes were a preplanned secondary outcome of the trial and the fracture outcomes data resulted from central adjudication after review of medical record data by blinded trained physician adjudicators.
Limitations include only collecting self-reported fall frequency information beginning in year 8 of the trial. Therefore, Look AHEAD cannot estimate fall frequency during the early trial period. Despite not having fall frequency throughout the study, it does not appear that the intensive weight loss intervention was associated with an increased risk of falls later in the trial period that could have explained the increase risk of frailty fracture. Further, the ILI group was no more likely to report their fracture was caused by a fall than the DSE group. Additional limitations include that Look AHEAD fracture ascertainment occurred only every 6 months in both groups and relied on self-report with review of medical records. Thus, under-ascertainment of fractures in both groups is possible especially fractures such vertebral fractures, however this is not likely to be differential by treatment assignment since both groups had the same schedule of ascertainment. Look AHEAD is also limited as the study did not collect information regarding family history of fracture or personal history of fracture before the trial and thus cannot address these characteristics in the data analyses. However, the baseline characteristics were well balanced between the randomized groups thus it is unlikely that this historical data would have been unequally distributed between the randomized groups resulting in potential for confounding.
Conclusion
An intensive lifestyle intervention resulting in long term weight loss in older overweight/obese adults with type 2 diabetes was not associated with an overall increased risk of fracture but may be associated with an increased risk of frailty fracture. When intentional weight loss is planned, consideration of bone preservation and fracture prevention strategies is warranted.
Supplementary Material
Acknowledgments
Look AHEAD Research Group at End of Continuation
Clinical Sites
The Johns Hopkins University Frederick L. Brancati, MD, MHS1
Pennington Biomedical Research Center George A. Bray, MD1
The University of Alabama at Birmingham Cora E. Lewis, MD, MSPH1
Harvard Center
Massachusetts General Hospital. David M. Nathan, MD1
Joslin Diabetes Center: Edward S. Horton, MD1
Beth Israel Deaconess Medical Center: George Blackburn, MD, PhD1
University of Colorado Anschutz Medical Campus James O. Hill, PhD1
Baylor College of Medicine John P. Foreyt, PhD1
The University of Tennessee Health Science Center Karen C. Johnson, MD, MPH1
University of Minnesota Robert W. Jeffery, PhD1
St. Luke’s Roosevelt Hospital Center Xavier Pi-Sunyer, MD1
University of Pennsylvania Thomas A. Wadden, PhD1
University of Pittsburgh John M. Jakicic, PhD1
The Miriam Hospital/Brown Medical School Rena R. Wing, PhD1
The University of Texas Health Science Center at San Antonio Helen P. Hazuda, PhD1
VA Puget Sound Health Care System/University of Washington Steven E. Kahn, MB, ChB1
Southwestern American Indian Center, Phoenix, Arizona and Shiprock, New Mexico William C. Knowler, MD, DrPH1
University of Southern California Anne Peters, MD1
Coordinating Center
Wake Forest University Mark A. Espeland, PhD1
(Additional information about the sites may be found in the supplemental material)
Federal Sponsors
National Institute of Diabetes and Digestive and Kidney Diseases Mary Evans, PhD; Van S. Hubbard, MD, PhD; Susan Z. Yanovski, MD
National Heart, Lung, and Blood Institute Lawton S. Cooper, MD, MPH; Peter Kaufman, PhD, FABMR; Mario Stylianou, PhD
Centers for Disease Control and Prevention Edward W. Gregg, PhD; Ping Zhang, PhD
Funding and Support
Funded by the National Institutes of Health through cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. Additional funding was provided by the National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; NIH Office of Research on Women’s Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (I.H.S.) provided personnel, medical oversight, and use of facilities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the I.H.S. or other funding sources.
Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066); the Harvard Clinical and Translational Science Center (RR025758-04); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); the VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs; and the Frederic C. Bartter General Clinical Research Center (M01RR01346).
The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST® of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche, Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America.
1Principal Investigator
Authors
Karen C. Johnson, MD, MPH, kjohnson@uthsc.edu, Department of Preventive Medicine, University of Tennessee Health Science Center, Memphis, TN
George A. Bray, MD, George.Bray@pbrc.edu, Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, LA
Lawrence J. Cheskin, MD, cheski1@jhmi.edu, Johns Hopkins University, Baltimore, MD
Jeanne M. Clark, MD, MPH, jmclark@jhmi.edu, Johns Hopkins University, Baltimore, MD
Caitlin M. Egan, MS, cegan1@lifespan.org, Weight Control and Diabetes Research Center, Providence, RI
John P. Foreyt, PhD, jforeyt@bcm.tmc.edu, Baylor College of Medicine, Houston, TX
Katelyn R. Garcia, MS, krgarcia@wakehealth.edu, Wake Forest School of Medicine, Wake Forest University, Winston-Salem, NC
Stephen Glasser, MD, sglasser@uab.edu, University of Alabama at Birmingham, Birmingham, ALA
Frank L. Greenway, MD, Frank.Greenway@pbrc.edu, Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, LA
Edward W. Gregg, PhD, edg7@cdc.gov, Centers for Disease Control and Prevention, Atlanta, GA
Helen P. Hazuda, PhD, HAZUDA@uthscsa.edu, The University of Texas Health Science Center at San Antonio, San Antonio, TX
Andrea Hergenroeder, PhD, PT, lockeal@upmc.edu, University of Pittsburgh, Pittsburgh, PA
James O. Hill, PhD, james.hill@ucdenver.edu, University of Colorado Anschutz Medical Campus, Aurora, CO
Edward S. Horton, MD, edward.horton@joslin.harvard.edu, Joslin Diabetes Center, Harvard Medical School, Boston, MA
John M. Jakicic, PhD, jjakicic@pitt.edu, University of Pittsburgh, Pittsburgh, PA
Robert W. Jeffery, PhD, jefferyrw@gmail.com, University of Minnesota, Minneapolis, MN
Steven E. Kahn, MB, ChB, skahn@u.washington.edu, VA Puget Sound Health Care System, University of Washington, Seattle, WA
William C. Knowler, MD, knowler@nih.gov, Southwestern American Indian Center, Phoenix, AZ
Cora E. Lewis, MD, MSPH, celewis@uabmc.edu, University of Alabama at Birmingham, Birmingham, ALA
Marsha Miller, MS RD, marsha.miller@ucdenver.edu, University of Colorado, Aurora, CO
Maria G. Montez, RN, MSHP, CDE, montez@uthscsa.edu, The University of Texas Health Science Center at San Antonio, San Antonio, TX
David M. Nathan, MD, dnathan@partners.org, Massachusetts General Hospital, Harvard University, Boston, MA
Jennifer L. Patricio, MS, jlp2209@cumc.columbia.edu, St. Luke’s Roosevelt Hospital Center, Columbia University, New York, NY
Anne L. Peters, MD, momofmax@mac.com, University of Southern California, Los Angeles, CA
Xavier Pi-Sunyer, MD, MPH, fxp1@columbia.edu, Columbia University Medical Center, New York, NY
Henry J. Pownall, PhD, HJPownall@tmhs.org, Houston Methodist Research Institute, Houston, TX
David Reboussin, PhD, drebouss@wfubmc.edu, Wake Forest School of Medicine, Wake Forest University, Winston-Salem, NC
J. Bruce Redmon, MD, redmo001@umn.edu, University of Minnesota, Minneapolis, MN
Helmut Steinberg, MD, hsteinb1@uthsc.edu, Department of Medicine, University of Tennessee Health Science Center, Memphis, TN
Thomas A. Wadden, PhD, wadden@mail.med.upenn.edu, University of Pennsylvania, Philadelphia, PA
Lynne E. Wagenknecht, DrPH, lwgnkcht@wakehealth.edu, Wake Forest School of Medicine, Winston-Salem, NC
Rena R. Wing, PhD, rwing@lifespan.org, The Miriam Hospital/Brown Medical School, Brown University, Providence, RI
Catherine R. Womack, MD, cwomack@uthsc.edu, Department of Preventive Medicine and Medicine, University of Tennessee Health Science Center, Memphis, TN
Susan Z. Yanovski, MD, sy29f@nih.gov, National Institute of Diabetes and Digestive and Kidney Diseases NIH, Bethesda, MD
Ping Zhang, PhD, paz2@cdc.gov, Centers for Disease Control and Prevention, Atlanta, GA
Ann V. Schwartz, PhD, MPH, aschwartz@psg.ucsf.edu, University of California San Francisco, San Francisco, CA
Disclosure
Karen C. Johnson, MD, MPH – Nothing to disclose
George A. Bray, MD - Nothing to disclose
Lawrence J. Cheskin, MD – reports membership on Medifast, Inc. Scientific Advisory Board
Jeanne M. Clark, MD, MPH – Nothing to disclose
Caitlin M. Egan, MS – Nothing to disclose
John P. Foreyt, PhD - Nothing to disclose
Katelyn R. Garcia, MS - Nothing to disclose
Stephen Glasser, MD - Nothing to disclose
Frank L. Greenway, MD - reports Consultant to Beachbody, Baronova, Basic Research, Eisai Inc. and General Nutrition Corporation. Advisory Board to Zafgen, Endo Pharmaceuticals, Plensat, Novo Nordisk, Nerium, Microbiome Therapeutics and Jenny Craig/Curves. Stock/Stock Options in Plensat, Neothetics and Microbiome Therapeutics.
Edward W. Gregg, PhD - Nothing to disclose
Helen P. Hazuda, PhD – reports grants from National Institute of Diabetes, Digestive, and Kidney Disease (NIDDK) and GE stock owned as part of an IRA.
Andrea Hergenroeder, PhD, PT, - Nothing to disclose
James O. Hill, PhD - Nothing to disclose
Edward S. Horton, MD - Nothing to disclose
John M. Jakicic, PhD - received an honorarium for serving on the Scientific Advisory Board for Weight Watchers International, was the Principal Investigator on a grant to examine the validity of activity monitors awarded to the University of Pittsburgh by Jawbone, Inc., was a co-investigator on a grant award to the University of Pittsburgh by HumanScale, and was a co-investigator on a grant awarded to the University of Pittsburgh by Weight Watchers International.
Robert W. Jeffery, PhD - Nothing to disclose
Steven E. Kahn, MB, ChB - Nothing to disclose
William C. Knowler, MD, DrPH - Nothing to disclose
Cora E. Lewis, MD, MSPH - Nothing to disclose
Marsha Miller, MS RD - Nothing to disclose
Maria G. Montez, RN, MSHP, CDE – Nothing to disclose
David M. Nathan, MD – Nothing to disclose
Jennifer L. Patricio, MS - Nothing to disclose
Anne L. Peters, MD – reports consulting/speaking for: Eli Lilly and Company, Boehringer Ingleheim, NovoNordisk, Sanofi, Janssen, Merck, Lexicon
Xavier Pi-Sunyer, MD, MPH - Nothing to disclose
Henry J. Pownall, PhD - Nothing to disclose
David Reboussin, PhD - Nothing to disclose
J. Bruce Redmon, MD - Nothing to disclose
Helmut Steinberg, MD - Nothing to disclose
Thomas A. Wadden, PhD - Nothing to disclose
Lynne E. Wagenknecht, DrPH - Nothing to disclose
Rena R. Wing, PhD - Nothing to disclose
Catherine R. Womack, MD - Nothing to disclose
Susan Z. Yanovski, MD - Nothing to disclose
Ping Zhang, PhD - Nothing to disclose
Ann V. Schwartz, PhD, MPH - Nothing to disclose
Footnotes
Trial Registration: Clinicaltrials.gov Identifier: NCT00017953
Authors roles
Study Design: KCJ, GAB, JMC, JPF, EWG, JOH, SEK, WCK, CEL, XP, DMR, LW, RRW, PZ
Study Conduct: KCJ, LJC, JMC, KME, JPF, EWG, HPH, JOH, SPG, JMJ, RWJ, SEK, WCK, CEL, MM, MGM, DN, AP, JLP, XP, DMR, JBR, ESH, HOS, TAW, LW, RRW, SZY
Data Collection: KCJ, GAB LJC, CME, FLG, HPH, JOH, SEK, WCK, CEL, MM, MGM, DN, AP, JLP, XP, DMR, JBR, RRW, TAW, PZ
Data Analysis: KRG
Data Interpretation: KCJ, KRG, ESH, HPH, JOH, SPG, CEL, AP, XP, AVS, LW
Drafting manuscript: KCJ, KRG, AH, AP
Revising manuscript content: KCJ, GAB, JMC, KRG, EWG, SPG, AH, ESH, JOH, SEK, CEL, AP, XP, JBR, AVS, CRW, TAW, LW, SZY
Approving final version of manuscript: All authors approved the final version
Take responsibility for the integrity of the data analysis: KCJ, KRG, XP, JBR, DMR
Contributor Information
Karen C. Johnson, Email: kjohnson@uthsc.edu, Department of Preventive Medicine, University of Tennessee Health Science Center, Memphis, TN.
Cora E. Lewis, Email: celewis@uabmc.edu, University of Alabama at Birmingham, Birmingham, ALA.
Catherine Womack, Email: cwomack@uthsc.edu, Department of Preventive Medicine and Medicine, University of Tennessee Health Science Center, Memphis, TN.
Katelyn R. Garcia, Email: krgarcia@wakehealth.edu, Wake Forest School of Medicine, Wake Forest University, Winston-Salem, NC.
Lynne Wagenknecht, Email: lwgnkcht@wakehealth.edu, Wake Forest School of Medicine, Wake Forest University, Winston-Salem, NC.
Henry J. Pownall, Email: HJPownall@tmhs.org, Houston Methodist Research Institute, Houston, TX.
Edward S. Horton, Email: edward.horton@joslin.harvard.edu, Joslin Diabetes Center, Harvard Medical School, Boston, MA.
Xavier Pi-Sunyer, Email: fxp1@columbia.edu, Columbia University Medical Center, New York, NY.
Edward W. Gregg, Email: edg7@cdc.gov, Centers for Disease Control and Prevention, Atlanta, GA.
Ann V. Schwartz, Email: aschwartz@psg.ucsf.edu, University of California San Francisco, San Francisco, CA.
Literature cited
- 1.Johnell O, Kanis JA. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos Int. 2004;15(11):897–902. doi: 10.1007/s00198-004-1627-0. [DOI] [PubMed] [Google Scholar]
- 2.Johnell O, Kanis JA, Oden A, et al. Mortality after osteoporotic fractures. Osteoporos Int. 2004;15(1):38–42. doi: 10.1007/s00198-003-1490-4. [DOI] [PubMed] [Google Scholar]
- 3.Tosteson AN, Gabriel SE, Grove MR, Moncur MM, Kneeland TS, Melton LJ., 3rd Impact of hip and vertebral fractures on quality-adjusted life years. Osteoporos Int. 2001;12(12):1042–1049. doi: 10.1007/s001980170015. [DOI] [PubMed] [Google Scholar]
- 4.Kroger H, Tuppurainen M, Honkanen R, Alhava E, Saarikoski S. Bone mineral density and risk factors for osteoporosis–a population-based study of 1600 perimenopausal women. Calcif Tissue Int. 1994;55(1):1–7. doi: 10.1007/BF00310160. [DOI] [PubMed] [Google Scholar]
- 5.Johnell O, Gullberg B, Kanis JA, et al. Risk factors for hip fracture in European women: the MEDOS Study. Mediterranean Osteoporosis Study. J Bone Miner Res. 1995;10(11):1802–1815. doi: 10.1002/jbmr.5650101125. [DOI] [PubMed] [Google Scholar]
- 6.Lewis CE, Ewing SK, Taylor BC, et al. Predictors of non-spine fracture in elderly men: the MrOS study. J Bone Miner Res. 2007;22(2):211–219. doi: 10.1359/jbmr.061017. [DOI] [PubMed] [Google Scholar]
- 7.Bonds DE, Larson JC, Schwartz AV, et al. Risk of fracture in women with type 2 diabetes: the Women’s Health Initiative Observational Study. J Clin Endocrinol Metab. 2006;91(9):3404–3410. doi: 10.1210/jc.2006-0614. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes care. 2002;25(10):1749–1754. doi: 10.2337/diacare.25.10.1749. [DOI] [PubMed] [Google Scholar]
- 9.Ottenbacher KJ, Ostir GV, Peek MK, Goodwin JS, Markides KS. Diabetes mellitus as a risk factor for hip fracture in mexican american older adults. J Gerontol A Biol Sci Med Sci. 2002;57(10):M648–653. doi: 10.1093/gerona/57.10.m648. [DOI] [PubMed] [Google Scholar]
- 10.Strotmeyer ES, Cauley JA, Schwartz AV, et al. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Archives of internal medicine. 2005;165(14):1612–1617. doi: 10.1001/archinte.165.14.1612. [DOI] [PubMed] [Google Scholar]
- 11.Strotmeyer ES. Diabetes and bone health. Diabetes Self Manag. 2008;25(2):11–12. 14. [PubMed] [Google Scholar]
- 12.Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. Journal of the American Geriatrics Society. 2003;51(12):1740–1747. doi: 10.1046/j.1532-5415.2003.51558.x. [DOI] [PubMed] [Google Scholar]
- 13.Riedt CS, Cifuentes M, Stahl T, Chowdhury HA, Schlussel Y, Shapses SA. Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J Bone Miner Res. 2005;20(3):455–463. doi: 10.1359/JBMR.041132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen LB, Kollerup G, Quaade F, Sorensen OH. Bone minerals changes in obese women during a moderate weight loss with and without calcium supplementation. J Bone Miner Res. 2001;16(1):141–147. doi: 10.1359/jbmr.2001.16.1.141. [DOI] [PubMed] [Google Scholar]
- 15.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. The New England journal of medicine. 2011;364(13):1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ensrud KE, Lewis CE, Lambert LC, et al. Endogenous sex steroids, weight change and rates of hip bone loss in older men: the MrOS study. Osteoporos Int. 2006;17(9):1329–1336. doi: 10.1007/s00198-006-0088-z. [DOI] [PubMed] [Google Scholar]
- 17.Look AHEAD Research Group. Look AHEAD: Action for Health in Diabetes: Design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Controlled clinical trials. 2003;24:610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 18.Bray G, Gregg E, Haffner S, et al. Baseline characteristics of the randomised cohort from the Look AHEAD (Action for Health in Diabetes) study. Diab Vasc Dis Res. 2006;3(3):202–215. doi: 10.3132/dvdr.2006.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brancati FL, Evans M, Furberg CD, et al. Midcourse correction to a clinical trial when the event rate is underestimated: the Look AHEAD (Action for Health in Diabetes) Study. Clin Trials. 2012;9(1):113–124. doi: 10.1177/1740774511432726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Look ARG, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. The New England journal of medicine. 2013;369(2):145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Look ARG, Wadden TA, West DS, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring, Md. 2006;14(5):737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ensrud KE, Cauley J, Lipschutz R, Cummings SR. Weight change and fractures in older women. Study of Osteoporotic Fractures Research Group. Archives of internal medicine. 1997;157(8):857–863. [PubMed] [Google Scholar]
- 23.Jakicic JM, Jaramillo SA, Balasubramanyam A, et al. Effect of a lifestyle intervention on change in cardiorespiratory fitness in adults with type 2 diabetes: results from the Look AHEAD Study. Int J Obes (Lond) 2009;33(3):305–316. doi: 10.1038/ijo.2008.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langlois JA, Mussolino ME, Visser M, Looker AC, Harris T, Madans J. Weight loss from maximum body weight among middle-aged and older white women and the risk of hip fracture: the NHANES I epidemiologic follow-up study. Osteoporos Int. 2001;12(9):763–768. doi: 10.1007/s001980170053. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz AV, Johnson KC, Kahn SE, et al. Effect of 1 year of an intentional weight loss intervention on bone mineral density in type 2 diabetes: results from the Look AHEAD randomized trial. J Bone Miner Res. 2012;27(3):619–627. doi: 10.1002/jbmr.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipkin EW, Schwartz AV, Anderson AM, et al. The Look AHEAD Trial: bone loss at 4-year follow-up in type 2 diabetes. Diabetes care. 2014;37(10):2822–2829. doi: 10.2337/dc14-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heymsfield SB, Thomas D, Nguyen AM, et al. Voluntary weight loss: systematic review of early phase body composition changes. Obes Rev. 2011;12(5):e348–361. doi: 10.1111/j.1467-789X.2010.00767.x. [DOI] [PubMed] [Google Scholar]
- 28.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 29.Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MA. Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. The American journal of clinical nutrition. 2002;76(2):473–481. doi: 10.1093/ajcn/76.2.473. [DOI] [PubMed] [Google Scholar]
- 30.Pownall HJ, Bray GA, Wagenknecht LE, et al. Changes in body composition over 8 years in a randomized trial of a lifestyle intervention: the look AHEAD study. Obesity (Silver Spring, Md) 2015;23(3):565–572. doi: 10.1002/oby.21005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. The New England journal of medicine. 2013;369(2):145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rejeski WJ, Ip EH, Bertoni AG, et al. Lifestyle change and mobility in obese adults with type 2 diabetes. The New England journal of medicine. 2012;366(13):1209–1217. doi: 10.1056/NEJMoa1110294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz AV, Nevitt MC, Brown BW, Jr, Kelsey JL. Increased falling as a risk factor for fracture among older women: the study of osteoporotic fractures. American journal of epidemiology. 2005;161(2):180–185. doi: 10.1093/aje/kwi023. [DOI] [PubMed] [Google Scholar]
- 34.Look ARG, Greenway FL. Severe hypoglycemia in the Look AHEAD Trial. J Diabetes Complications. 2016;30(5):935–943. doi: 10.1016/j.jdiacomp.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenfield DM, Eastell R. Risk factors for ankle fracture. Osteoporos Int. 2001;12(2):97–103. doi: 10.1007/s001980170140. [DOI] [PubMed] [Google Scholar]
- 36.Marques EA, Mota J, Carvalho J. Exercise effects on bone mineral density in older adults: a meta-analysis of randomized controlled trials. Age (Dordr) 2012;34(6):1493–1515. doi: 10.1007/s11357-011-9311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanis JA, Johansson H, Johnell O, et al. Alcohol intake as a risk factor for fracture. Osteoporos Int. 2005;16(7):737–742. doi: 10.1007/s00198-004-1734-y. [DOI] [PubMed] [Google Scholar]
- 38.Kanis JA, Johnell O, Oden A, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16(2):155–162. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 39.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. The New England journal of medicine. 1997;337(10):670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 40.Kannus P, Sievanen H, Palvanen M, Jarvinen T, Parkkari J. Prevention of falls and consequent injuries in elderly people. Lancet. 2005;366(9500):1885–1893. doi: 10.1016/S0140-6736(05)67604-0. [DOI] [PubMed] [Google Scholar]
- 41.Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int. 2005;16(6):581–589. doi: 10.1007/s00198-004-1780-5. [DOI] [PubMed] [Google Scholar]
- 42.Kanis JA, Johansson H, Oden A, McCloskey EV. Assessment of fracture risk. Eur J Radiol. 2009;71(3):392–397. doi: 10.1016/j.ejrad.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 43.Kanis JA, Oden A, Johansson H, Borgstrom F, Strom O, McCloskey E. FRAX and its applications to clinical practice. Bone. 2009;44(5):734–743. doi: 10.1016/j.bone.2009.01.373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


