Abstract
Objective
Cinnamaldehyde (CA) is a food compound that has previously been observed to be protective against obesity and hyperglycemia in mouse models. In this study, we aimed to elucidate the mechanisms behind this protective effect by assessing the cell-autonomous response of primary adipocytes to CA treatment.
Methods
Primary murine adipocytes were treated with CA and thermogenic and metabolic responses were assessed after both acute and chronic treatments. Human adipose stem cells were differentiated and treated with CA to assess whether the CA-mediated signaling is conserved in humans.
Results
CA significantly activated PKA signaling, increased expression levels of thermogenic genes and induced phosphorylation of HSL and PLIN1 in murine primary adipocytes. Inhibition of PKA or p38 MAPK enzymatic activity markedly inhibited the CA-induced thermogenic response. In addition, chronic CA treatment regulates metabolic reprogramming, which was partially diminished in FGF21KO adipocytes. Importantly, both acute and chronic effects of CA were observed in human adipose stem cells isolated from multiple donors of different ethnicities and ages and with a variety of body mass indexes (BMI).
Conclusions
CA activates thermogenic and metabolic responses in mouse and human primary subcutaneous adipocytes in a cell-autonomous manner, giving a mechanistic explanation for the anti-obesity effects of CA observed previously and further supporting its potential metabolic benefits on humans. Given the wide usage of cinnamon in the food industry, the notion that this popular food additive, instead of a drug, may activate thermogenesis, could ultimately lead to therapeutic strategies against obesity that are much better adhered to by participants.
Keywords: Cinnamaldehyde, Obesity, Thermogenesis, Subcutaneous adipocytes
1. Introduction1
The global obesity epidemic calls for effective intervention and treatments. Adipocytes in the subcutaneous fat depots have been reported to provide metabolic benefits, including their capability to be recruited and activated by thermogenic stimuli and thereby potentially improve systemic energy homeostasis [1]. The classical pathway for the activation of thermogenesis in adipocytes is through the activation of PKA and downstream enzymes including p38 MAPK, which in turn leads to the induction of the transcription of key thermogenic genes [2].
Previous studies have shown that cinnamaldehyde (CA), an essential oil found in cinnamon, can reduce diet-induced weight gain and improve glucose homeostasis when given to mice as a supplement in food [3, 4]. Interestingly, cinnamon consumption has also been reported to correlate with lower levels of fasting blood glucose in human studies [5]. However, the molecular mechanisms behind these effects have not yet been elucidated. Here, we show that CA directly acts on subcutaneous adipocytes to activate thermogenesis and PKA signaling and that chronic CA treatment induces thermogenic and metabolic reprogramming in these cells. Importantly, the beneficial effects of CA treatment that we observe in murine adipocytes are conserved in human subcutaneous fat cells, suggesting that CA may potentially be used to counteract obesity and improve systemic metabolism in humans.
2. Materials and Methods
2.1. Cell Culture
Primary preadipocytes were isolated from the inguinal fat depot of mice and differentiated as previously described [6]. Human adipose-derived stem cells (hASCs) were isolated from lipoaspirates from the subcutaneous adipose tissue of donors undergoing voluntary surgery (a generous gift from Dr. Jeffrey M. Gimble, Tulane University, New Orleans, Louisiana, USA).
Donor demographics are included in Supplemental Table 1. All specimens were collected and handled under the protocols reviewed and approved by the Western Institutional Review Board (Puyallup, WA) and the University of Michigan Medical School Institutional Review Board (IRBMED). For further isolation, culture, and differentiation information, see the supplemental materials and methods.
2.2. RNA Isolation and RT-qPCR Analysis
RNA was extracted from cells and tissue using TRI Reagent (Sigma, Saint Louis, MO, USA) according to the manufacturer’s instructions. RNA was reverse transcribed to cDNA using M-MLV (Invitrogen, Carlsbad, CA, USA) and qPCR was performed using SYBR Green (Applied Biosystems, Woolston Warrington, UK) on a 7900HT Fast Real-Time PCR system (Applied Biosystems). Fold change was determined using the ΔΔCt method with samples normalized to the reference gene 36B4 (RPLP0). A list of qPCR primers used in this study is included in Supplemental Table 2.
2.3. Western Blot Analysis
Cells were lysed with RIPA buffer supplemented with protease inhibitor cocktail (Roche, Basel, Switzerland) and phosphatase inhibitors. Protein extracts were run on 10% SDS-PAGE gels and transferred to nitrocellulose membranes. Blots were exposed using ECL (Amersham, Pittsburgh, PA, USA). For antibody information, see the supplemental materials and methods.
2.4. Imaging
Oil red O staining was described in the supplemental materials and methods. For immunofluorescence, primary cells were seeded onto collagen-coated coverslips and differentiated as described above. Differentiated cells were probed using p-PLIN1 Ser517 antibody (Vala Sciences, San Diego, CA, USA), anti-rabbit Alexa 594 antibody (Abcam, Cambridge, MA, USA), and BODIPY (Invitrogen), and imaged using an Olympus FV300 confocal laser-scanning microscope (Melville, NY, USA).
2.5. Statistical Analysis
Statistical analysis was performed using GraphPad Prism 6. Data are expressed as mean ± SEM. Statistical significance was determined using an unpaired two-tailed Student’s t-test for two-group comparisons (*p < 0.05, **p < 0.01, ***p < 0.001). A one-way analysis of variance (ANOVA) was applied for multiple group comparisons involving one independent variable. The data presented is representative of 2–4 independent experiments.
3. Results
3.1. CA activates thermogenesis through PKA signaling in mouse subcutaneous fat cells
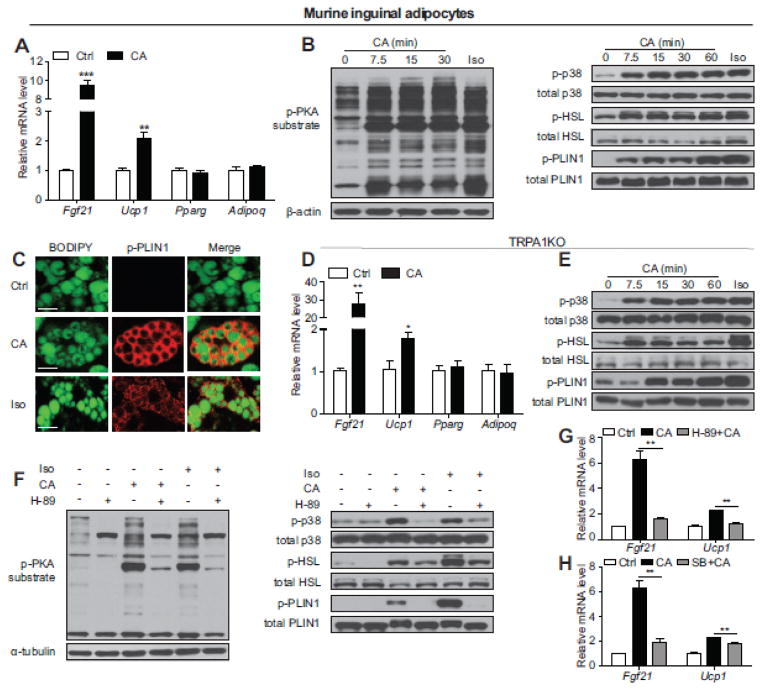
While the beneficial effects of CA on systemic metabolism have recently been observed [3, 4, 7], the underlying molecular basis of these effects remains largely unknown. To explore the possibility that CA influences whole body metabolism through direct action on adipocytes, we treated fully differentiated adipocytes isolated from the murine inguinal subcutaneous fat depot with CA. Subcutaneous fat plays a unique role in maintaining energy homeostasis, demonstrating profound “browning” upon cold exposure and mediating protective metabolic functions during obesity [8]. Acute CA treatments induce expression of thermogenic markers including Fgf21 and Ucp1 without altering expression of general adipogenic markers such as Pparg and Adipoq in these primary subcutaneous adipocytes (Fig. 1A, Supplemental Fig. 1 and 2A). The induction of thermogenesis in response to acute stimuli, such as treatment with β-adrenergic agonist isoproterenol (Iso), is mainly mediated through the activation of PKA and subsequent phosphorylation of p38 MAPK [2]. Indeed, we observed a robust phosphorylation of PKA substrates and p38 MAPK by CA at a comparable level to Iso in inguinal adipocytes (Fig. 1B). In addition to p38, PKA dependent phosphorylation of hormone-sensitive lipase (HSL) and lipid droplet-associated protein perilipin 1 (PLIN1) are also activated by CA in inguinal adipocytes (Fig. 1B and C). CA has been reported to be an agonist for transient receptor potential cation channel, member A1 (TRPA1) [4]. Emerging evidence suggests that signaling through the transient receptor potential (TRP) channels may cell-autonomously mediate adipocyte function [6, 9]. Interestingly, this CA-induced thermogenic response is TRPA1-independent since a similar response was observed in TRPA1KO inguinal adipocytes (Fig 1D and E, Supplemental Fig. 3).
Fig. 1.
Acute CA treatment upregulates thermogenic gene expression and activates PKA signaling in murine subcutaneous fat cells. (A) Real-time qPCR analyses of thermogenic genes (Fgf21 and Ucp1) and adipogenic markers (Pparg and Adipoq) in differentiated murine inguinal adipocytes (n=4) after 400 μM CA treatment for 4 h. It has been reported that CA treatment in 3T3-L1 preadipocytes negatively influences adipogenesis [7]. We observed similar levels of adipogenesis and equal pan-fat marker expression (Pparg and Adipoq) in control and CA treated groups. (B) Representative immunoblots of PKA substrate phosphorylation, p38 MAPK phosphorylation, HSL phosphorylation (Ser660) and PLIN1 phosphorylation (Ser517) in differentiated inguinal adipocytes exposed to 400 μM CA for the indicated time or 10 μM Iso for 10 min as a positive control. (C) Representative immunofluorescence staining of PLIN1 phosphorylation (red) in differentiated inguinal adipocytes treated with 400 μM CA for 1 h or 10 μM Iso for 1 h as a positive control. BODIPY was used to stain lipid droplets (green). Scale bar = 20 μm. (D) Real-time qPCR analyses of thermogenic genes (Fgf21 and Ucp1) and adipogenic markers (Pparg and Adipoq) in differentiated WT or TRPA1KO inguinal adipocytes following vehicle control or 400 μM CA treatment for 4 h (n=6). (E) Representative immunoblots of p38 MAPK phosphorylation, HSL phosphorylation (Ser660) and PLIN1 phosphorylation (Ser517) in differentiated TRPA1KO inguinal adipocytes treated with 400 μM CA for the indicated time or 10 μM Iso for 10 min as a control. (F) Representative immunoblots of phosphorylated PKA substrates, phosphorylated p38 MAPK, phosphorylated HSL (Ser660) and phosphorylated PLIN1 (Ser517) in differentiated inguinal adipocytes treated with 50 μM H-89 for 30 min and then 400 μM CA for 1 h or 10 μM Iso for 10 min as a positive control. (G and H) Real-time qPCR analyses of thermogenic markers (Fgf21, Ucp1) in differentiated inguinal adipocytes treated with 50 μM H-89 (G, n=3) or 10 μM SB203580 (SB, H, n=3) for 1 h and then 400 μM CA for 4 h. All data in A, D, G, and H are presented as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001.
Phosphorylation of PKA substrates, p38 MAPK, HSL and PLIN1 by CA treatment were significantly blocked by PKA inhibitor H-89 in primary inguinal adipocytes (Fig. 1F). Consistently, the induction of Fgf21 and Ucp1 by CA in inguinal adipocytes was substantially suppressed by H-89 and partially reduced by p38 MAPK inhibitor SB203580 (Fig. 1G and H), suggesting that CA-stimulated thermogenesis is dependent on PKA/p38 MAPK signaling. These results were confirmed in immortalized C3H-10T1/2 cells, indicating that the observed response to CA in primary inguinal fat is adipocyte cell-autonomous (Supplemental Fig. 4). Taken together, these data revealed that CA directly stimulates a thermogenic response and PKA activation in murine subcutaneous fat cells.
3.2. CA activates thermogenesis and promotes metabolic reprogramming in human subcutaneous fat cells
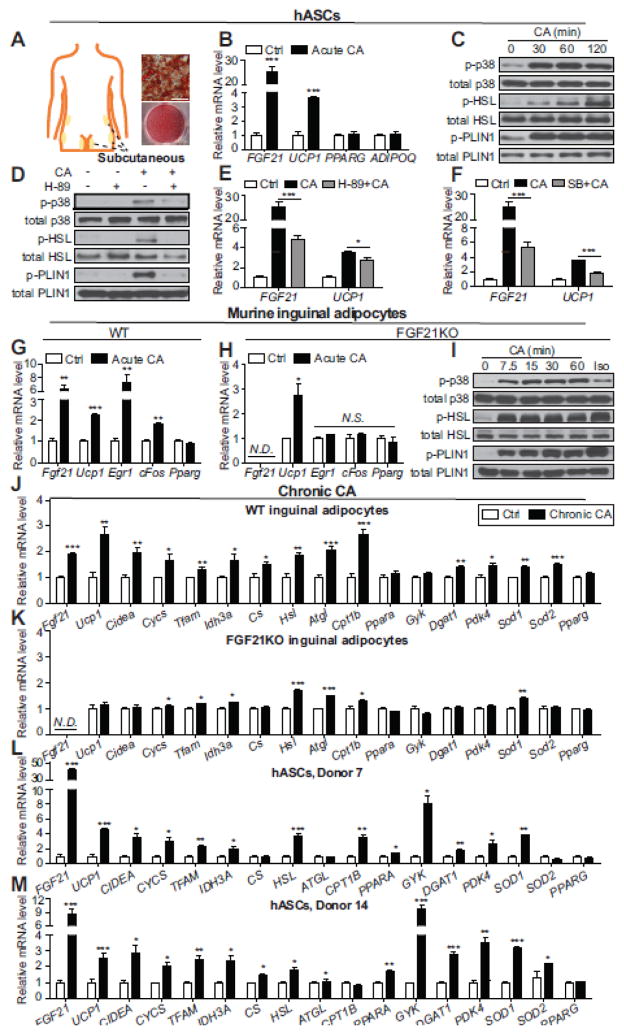
It has been reported that in addition to the supraclavicular area, thermogenic adipocytes also exist in human subcutaneous fat depots [8, 10]. We observed a clear activation of the thermogenic response and PKA signaling in differentiated hASCs isolated from subcutaneous fat depots (Fig. 2A–F, Supplemental Fig. 2B). This phenomenon is consistently observed across multiple human donors of different ethnicities and ages and with varied BMI (Supplemental Table 1 and Supplemental Fig. 5). Previous studies have shown that long-term CA supplementation in food can protect mice from diet-induced obesity and hyperglycemia [3, 7]. In vitro, upon chronic treatment with CA, Fgf21 and Ucp1 were significantly upregulated in both murine inguinal adipocytes and human subcutaneous fat cultures (Supplemental Fig. 6 and Fig. 2J, L, and M). Thermogenic genes (Cidea, Cycs, Tfam) and genes encoding key enzymes in the citric acid cycle (Idh3a, Cs) were upregulated after chronic exposure to CA. Cumulative evidence supports that many aspects of lipid metabolism can be altered in response to cold exposure or β-adrenergic agonist treatment [11, 12]. We observed that chronic CA treatment upregulates the expression of lipolytic genes (Hsl, Atgl), key regulators in fatty acid oxidation (Cpt1b, Ppara), lipogenic genes (Gyk, Dgat1), and Pdk4, an enzyme that enhances lipid metabolism and has recently been reported to be responsible for major metabolic adaptations during white-to-beige conversion of human adipocytes (Fig. 2J, L, and M) [13]. Increased oxidative metabolism in mitochondria is known to be accompanied by increased reactive oxygen species (ROS) production. Chronic CA treatment also upregulated the expression of ROS-detoxifying enzymes including Sod1 and Sod2, which may protect activated thermogenic adipocytes from oxidative damage (Fig. 2J, L, and M). Murine brown adipocytes additionally showed a thermogenic response to both acute and chronic CA treatment, including an increase in oxygen consumption in cells treated with chronic CA (Supplemental figure 7).
Fig. 2.
CA activates a thermogenic response and regulates lipid metabolic gene profiles in human subcutaneous fat cells. (A) Locations of the human subcutaneous fat biopsy sites used in this study and representative Oil Red O staining of differentiated hASCs. Scale bar = 50 μm. (B) Real-time qPCR analyses of thermogenic genes (FGF21 and UCP1) and adipogenic markers (PPARG and ADIPOQ) in differentiated hASCs after 200 μM CA treatment for 4 h (n=4). (C and D) Representative immunoblots of phosphorylated p38 MAPK, phosphorylated HSL (Ser660) and phosphorylated PLIN1 (Ser522) in differentiated hASCs treated with 200 μM CA for the indicated time (C) or 50 μM H-89 for 30 min and then 200 μM CA for 2 h (D). (E and F) Real-time qPCR analyses of thermogenic markers in differentiated hASCs treated with 50 μM H-89 (E, n=3) or 10 μM SB (F, n=3) for 1 h and then 200 μM CA for 4 h. (G and H) Real-time qPCR analyses of thermogenic genes (Fgf21 and Ucp1), FGF21-target genes (Egr1, cFos) and the adipogenic marker Pparg in differentiated inguinal adipocytes of WT mice (G, n=4) or FGF21KO mice (H, n=4) after 400 μM CA treatment for 4 h. (I) Representative immunoblots of phosphorylated p38 MAPK, phosphorylated HSL (Ser660) and phosphorylated PLIN1 (Ser517) in differentiated inguinal adipocytes from FGF21KO mice treated with 400 μM CA for the indicated time. (J–M) Real-time qPCR analyses of thermogenic and metabolic genes in differentiated WT (J, n=4) or FGF21KO (K, n=4) murine inguinal adipocytes after 200 μM CA treatment for 48 h, or differentiated hASCs stimulated with 200 μM CA for 24 h (L and M, n=4). All data in B, E–H, and J–M are presented as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001.
Acute CA treatment led to considerable induction of Fgf21, an important metabolic regulator which can be secreted by adipocytes and function in an autocrine or paracrine manner to induce thermogenesis and affect metabolic pathways [14]. In wild type (WT) inguinal adipocytes, CA treatment induced a clear elevation of Egr1 and cFos, known FGF21 target genes (Fig. 2G) [15]. This induction was completely abolished in adipocytes isolated from FGF21KO mice (Fig. 2H, Supplemental Fig. 8), suggesting that FGF21-dependent signaling is activated by CA. The FGF21 knockout did not affect the PKA-mediated acute response to CA treatment in inguinal adipocytes (Fig. 2I). However, metabolic reprogramming upon chronic CA treatment is blunted in the absence of FGF21, suggesting that FGF21 may contribute to certain aspects of the long-term effects of CA on adipocytes through autocrine or paracrine pathways (Fig. 2K).
4. Discussion
Thermogenic adipocytes in subcutaneous fat depots are highly recruitable under certain conditions such as cold exposure [1]. These cells play an important role in maintaining energy homeostasis and improving overall metabolic health [16]. Herein, we show that CA treatment activates thermogenic and metabolic responses in human subcutaneous fat cells derived from multiple donors (Fig. 2 and Supplemental Fig. 5). Recent studies demonstrate that lipolysis and lipogenesis are coupled in adipose tissue during chronic β3-adrenergic receptor stimulation [12]. In cold-exposed mice, genes involved in lipid catabolism and anabolism are both upregulated [11]. Cold-stimulated thermogenic fat activation in humans triggers triglyceride-free fatty acid cycling accompanied with altered metabolic gene profiles [17]. Consistent with these observations, we found that chronic CA treatment leads to a futile metabolic cycle in subcutaneous fat cells which may constitute the key mechanism through which CA consumption leads to metabolic protection. Notably, a recent clinical trial with healthy subjects suggests that acute CA ingestion increases energy expenditure and postprandial fat oxidation, in which the CA dose is judged as “sensorially acceptable” by participants [18]. A pharmacokinetic study revealed that circulating levels of CA were detectable 20 h after oral delivery, indicating that consumption of cinnamon may be a feasible way to activate thermogenesis in subcutaneous fat and ultimately protect against obesity and metabolic disorders [19].
This study investigated the thermogenic and metabolic actions of CA on adipocytes and the mechanisms by which they take place. We found that CA acts through a PKA/p38 MAPK-dependent pathway. Consistently, the phosphorylation of lipolysis- and thermogenesis-related proteins was diminished by treatment with the PKA inhibitor H-89, and thermogenic gene expression was attenuated by treatment with the p38 inhibitor SB203580. We also found that some of the chronic effects of CA are mediated by FGF21, an adipokine that is known to activate thermogenesis in adipocytes [14].
In summary, this study provides evidence that CA induces thermogenesis as well as chronic metabolic remodeling in a fat cell-autonomous manner. Particularly, the thermogenic and metabolic responses of human subcutaneous adipocytes to CA suggest that this may be a promising therapeutic target for obesity.
Supplementary Material
Acknowledgments
Funding and Acknowledgements
This research was supported by the Human Frontier Science Program Young Investigator Grant (RGY0082/2014), the Mallinckrodt grant from the Edward Mallinckrodt Jr. Foundation, grants from the National Institutes of Health (R01DK107583) to JW, (F31DK112625) to MPE, and a postdoctoral fellowship from the American Heart Association to DK (17POST33060001).
Footnotes
Abbreviations: CA: Cinnamaldehyde, hASC: human adipose stem cell, BMI: Body mass index, ROS: Reactive oxygen species
Author Contributions
JJ, MPE, HJ, XQ, JL, and DK performed experiments. JJ, MPE, and JW analyzed data and wrote the manuscript. JW oversaw the study.
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosen ED, Spiegelman BM. What We Talk About When We Talk About Fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 3.Camacho S, Michlig S, de Senarclens-Bezencon C, Meylan J, Meystre J, Pezzoli M, et al. Anti-obesity and anti-hyperglycemic effects of cinnamaldehyde via altered ghrelin secretion and functional impact on food intake and gastric emptying. Sci Rep. 2015;5:7919. doi: 10.1038/srep07919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamura Y, Iwasaki Y, Narukawa M, Watanabe T. Ingestion of cinnamaldehyde, a TRPA1 agonist, reduces visceral fats in mice fed a high-fat and high-sucrose diet. J Nutr Sci Vitaminol (Tokyo) 2012;58:9–13. doi: 10.3177/jnsv.58.9. [DOI] [PubMed] [Google Scholar]
- 5.Allen RW, Schwartzman E, Baker WL, Coleman CI, Phung OJ. Cinnamon use in type 2 diabetes: an updated systematic review and meta-analysis. Ann Fam Med. 2013;11:452–9. doi: 10.1370/afm.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye L, Kleiner S, Wu J, Sah R, Gupta RK, Banks AS, et al. TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell. 2012;151:96–110. doi: 10.1016/j.cell.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khare P, Jagtap S, Jain Y, Baboota RK, Mangal P, Boparai RK, et al. Cinnamaldehyde supplementation prevents fasting-induced hyperphagia, lipid accumulation, and inflammation in high-fat diet-fed mice. Biofactors. 2016;42:201–11. doi: 10.1002/biof.1265. [DOI] [PubMed] [Google Scholar]
- 8.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–63. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 9.Ma S, Yu H, Zhao Z, Luo Z, Chen J, Ni Y, et al. Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. J Mol Cell Biol. 2012;4:88–96. doi: 10.1093/jmcb/mjs001. [DOI] [PubMed] [Google Scholar]
- 10.Bartesaghi S, Hallen S, Huang L, Svensson PA, Momo RA, Wallin S, et al. Thermogenic Activity of UCP1 in Human White Fat-Derived Beige Adipocytes. Mol Endocrinol. 2015;29:130–9. doi: 10.1210/me.2014-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labbe SM, Caron A, Bakan I, Laplante M, Carpentier AC, Lecomte R, et al. In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis. FASEB J. 2015;29:2046–58. doi: 10.1096/fj.14-266247. [DOI] [PubMed] [Google Scholar]
- 12.Mottillo EP, Balasubramanian P, Lee YH, Weng C, Kershaw EE, Granneman JG. Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic beta 3-adrenergic receptor activation. J Lipid Res. 2014;55:2276–86. doi: 10.1194/jlr.M050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barquissau V, Beuzelin D, Pisani DF, Beranger GE, Mairal A, Montagner A, et al. White-to-brite conversion in human adipocytes promotes metabolic reprogramming towards fatty acid anabolic and catabolic pathways. Mol Metab. 2016;5:352–65. doi: 10.1016/j.molmet.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–81. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, et al. Obesity Is a Fibroblast Growth Factor 21 (FGF21)-Resistant State. Diabetes. 2010;59:2781–9. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen P, Levy JD, Zhang YY, Frontini A, Kolodin DP, Svensson KJ, et al. Ablation of PRDM16 and Beige Adipose Causes Metabolic Dysfunction and a Subcutaneous to Visceral Fat Switch. Cell. 2014;156:304–16. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chondronikola M, Volpi E, Borsheim E, Porter C, Saraf MK, Annamalai P, et al. Brown Adipose Tissue Activation Is Linked to Distinct Systemic Effects on Lipid Metabolism in Humans. Cell Metabolism. 2016;23:1200–6. doi: 10.1016/j.cmet.2016.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michlig S, Merlini JM, Beaumont M, Ledda M, Tavenard A, Mukherjee R, et al. Effects of TRP channel agonist ingestion on metabolism and autonomic nervous system in a randomized clinical trial of healthy subjects. Sci Rep. 2016;6:20795. doi: 10.1038/srep20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao H, Xie Y, Yang Q, Cao Y, Tu H, Cao W, et al. Pharmacokinetic study of cinnamaldehyde in rats by GC-MS after oral and intravenous administration. J Pharm Biomed Anal. 2014;89:150–7. doi: 10.1016/j.jpba.2013.10.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.