Abstract
Aicardi–Goutières syndrome (AGS) is a rare disorder with in utero or postnatal onset of encephalopathy and progressive neurological deterioration. The seven genetic subtypes of AGS are associated with abnormal type I interferon-mediated innate immune response. Most patients with AGS present with progressive microcephaly, spasticity, and cognitive impairment. Some, especially those with type 2 (AGS2), manifest milder phenotypes, reduced childhood mortality, and relative preservation of physical and cognitive abilities. In this report, we describe two siblings (sister and brother), diagnosed with AGS2 in their second decade, who exhibited static encephalopathy since 1 year of age, with spastic quadriplegia and anarthria but preserved intellect. Both were homozygous for the common pathogenic RNASEH2B allele (c.529G>A, p.Ala177Thr). Rather than manifesting calcifications and leukoencephalopathy, both had increased iron signal in the basal ganglia. Our report broadens the clinical and imaging spectrum of AGS2 and emphasizes the importance of including AGS2 in the differential diagnosis of idiopathic spastic cerebral palsy.
Aicardi-Goutières syndrome (AGS) is a rare genetic autoinflammatory disorder affecting the brain and skin.1 Patients typically exhibit either in utero manifestations resembling an acquired infection,2 or postnatal involvement in infancy, characterized by subacute encephalopathy and loss of acquired skills.3 Children with AGS commonly have fevers in the absence of known infection, cerebrospinal (CSF) pleocytosis, and elevated CSF interferon (IFN)-α levels. They develop encephalopathy with irritability, persistent crying, abnormal tone, posturing, and an exaggerated startle response. Over time, severe neurological dysfunction emerges as progressive microcephaly, spasticity, dystonia, and cognitive impairment. Commonly associated symptoms include seizures, glaucoma, and chilblain lesions.2,3 Most patients with AGS have abnormalities on imaging, including white matter abnormalities and brain calcifications primarily affecting the basal ganglia.4,5
There are seven known genetic subtypes of AGS (Table SI, online supporting material) caused by mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR1, or IFIH1; each gene is involved in normal RNA/DNA intracellular metabolism. Abnormalities in the function of these genes induce inappropriate type I IFN-mediated innate immune responses, with complex downstream effects, including direct neurotoxicity and neurodegeneration.6
Some patients with AGS exhibit later onset of symptoms, and lack abnormalities on brain imaging or CSF.2,7–10 Numerous patients with RNASEH2B mutations and AGS type 2 (AGS2) have a milder phenotype with lower childhood mortality and better preservation of function.3,9 We describe two siblings with static encephalopathy emerging during the first year of life; they were diagnosed with AGS2 at the age of 18 years and 13 years respectively. Both had spastic quadriplegia, anarthria, preserved cognitive function, absent leukoencephalopathy or brain calcifications but evidence of excess iron accumulation in the globus pallidus, an unreported feature of AGS. The siblings were homozygous for the c.529G>A mutation in RNASEH2B, a known disease-causing allele with a relatively high frequency in the general population. Clinicians should consider AGS in older individuals with unexplained static encephalopathies, even in the absence of neuroimaging characteristic of AGS.
CASE REPORT
The two siblings were enrolled in the National Institutes of Health Undiagnosed Diseases Program under protocol 76-HG-0238, ‘Diagnosis and Treatment of Patients with Inborn Errors of Metabolism or Other Genetic Disorders’, approved by the Institutional Review Board of the National Human Genome Research Institute. Assent and informed, written consents were obtained from patients and parents.
Patient 1 was an 18-year-old female with spastic quadriplegia and anarthria. She was the first child of healthy, unrelated white parents, and was born at term weighing 3572g following an uncomplicated pregnancy. The patient achieved normal developmental milestones until 9 months, sitting and standing independently. At 10 months she experienced developmental arrest and onset of neurological abnormalities, including low axial tone, distal spasticity, and an exaggerated startle response. She lost her ability to sit or stand and never walked or developed expressive language. At 10 years, she had her first unprovoked seizure, but achieved good seizure control on therapy. She experienced pernio (chilblains) at age 16 controlled with nifedipine. For over a decade, she had communicative abilities restricted to limited facial, head, and extraocular movements. As a young adolescent, she was equipped with a communication board and a laser pointer on the bill of a baseball cap. This approach was later augmented by human–computer interface by which head movements translated into cursor movements using a computer camera sensing light on a reflective sticker applied to her forehead; this provided access to regular high-school course work and social media.
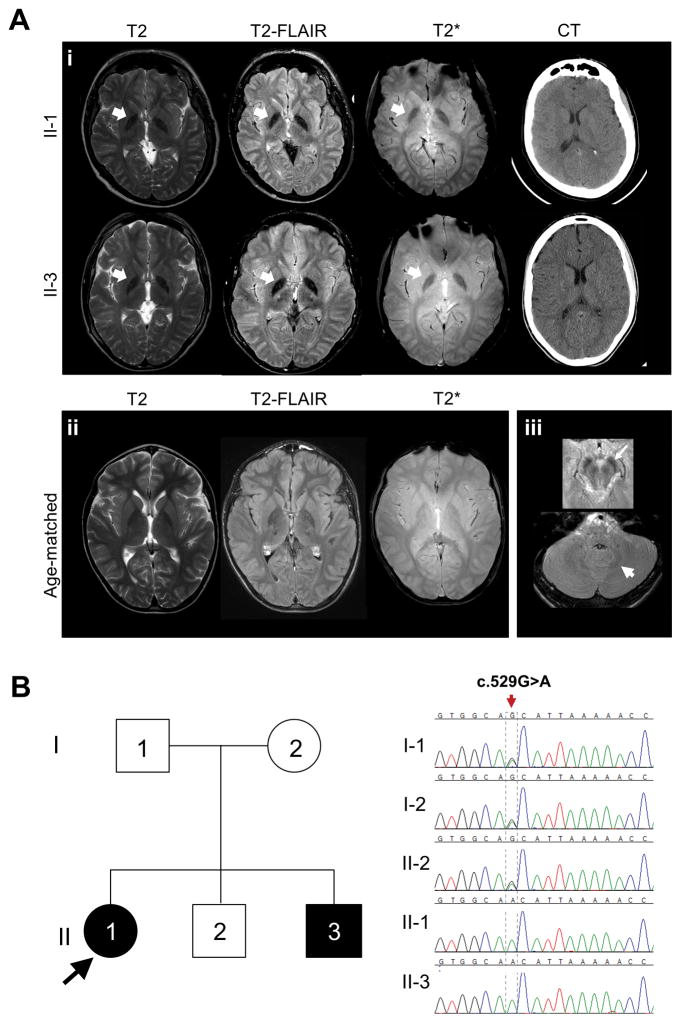
At the time of evaluation, patient 1 was non-ambulatory with profound spasticity and scoliosis. She was alert, socially interactive, and proficient in the use of the computer interface. Eye movements were normal. She was anarthric and unable to generate the simplest vocalizations. She handled secretions and ate a regular diet. Magnetic resonance imaging revealed few non-specific areas of T2 hyperintensity, hypointensity of globus pallidus on T2, T2-fluid-attentuated inversion recovery and T2-* images consistent with iron deposition. A cranial computed tomography scan revealed no evident parenchymal calcifications (Fig. 1a, upper panels). At the age of 19 years, her head circumference was 22.5 inches.
Figure 1.
(a) Neuroimaging results. (i) Magnetic resonance imaging (T2, T2-FLAIR and T2*) and CT of patients 1 (II-1) and 2 (II-3) revealed grossly normal anatomy, nonspecific small white matter lesions, and globus pallidus hypointensity on T2* and T2-FLAIR (arrows), consistent with abnormal metal deposition. Computed tomography scans demonstrated no evident brain parenchymal calcifications. (ii) T2, T2-FLAIR and T2* from an age-matched normal control is shown for comparison. (iii) Axial FLAIR images from patient 2 (II-3) showing red nucleus and substantia nigra, which are iron containing and appear hypointense on FLAIR imaging. Magnetic resonance images were obtained using a 3.0 T Philips Achieva scanner. Acquisition parameters were: T2 (repetition time 5400ms; echo time 100ms; echo train length 26; acquisition time 90s); T2-FLAIR (repetition time 11 000ms; inversion time 2700ms; echo time, 120ms; echo train length 20; acquisition time 90s); and T2* (repetition time 600ms; echo time 15ms; echo train length 5; acquisition time 30s). Age-matched control was scanned on the same scanner with the same acquisition parameters. (b) Family pedigree and molecular data. Affected patients are shown in black. Unaffected family members are shown in white. Chromatogram images show that patients 1 (II-1) and 2 (II-3) are homozygous for the c.529G>A in RNASEH2B, while their father (I-1) mother (I-2), and unaffected brother (II-2) are heterozygous for the mutation. CT, computed tomography; FLAIR, fluid-attenuated inversion recovery.
Patient 2 was the 13-year-old brother of patient 1. He was born at term weighing 3856g following an uncomplicated pregnancy. Like his sister, he experienced normal developmental milestones until the age of 9 months, when he lost the ability to sit independently and roll over, and gradually developed spasticity, an exaggerated auditory startle and reflux. At 1 year of age, he was empirically treated with intravenous immunoglobulin and intravenous steroids, with no benefit seen. By 3 years of age, he had not gained additional motor skills. At 15 years of age, brain imaging was similar to that of his sister (Fig. 1b, lower panels), and his head circumference was 22.25 inches. Based on his sister’s experience, communication augmentation strategies were implemented earlier, with improved social and academic achievements.
Patient 1 had undergone ARX sequencing, fragile X studies, comparative genomic hybridization microarray, skin biopsy, mitochondrial enzyme assays, urine organic acids, serum and urine amino acids, muscle biopsy, and spastic paraparesis gene panel, all of which were non-diagnostic. Patient 2’s prior evaluation included normal biogenic amines and rectal biopsy. CSF analyses were normal for both patients. With no diagnosis or evidence of deterioration both patients were considered to have spastic cerebral palsy of unknown cause.
Whole-exome sequencing and single-nucleotide polymorphism mapping (Appendix S1, online supporting information) on the nuclear family (Fig. 1b) showed a biallelic disease-causing mutation in RNASEH2B, i.e. NM_024570.3 (c.529G>A; p.Ala177Thr) in both siblings. No loss of heterozygosity was uncovered over chromosome 13 by close inspection of the single-nucleotide polymorphisms surrounding the causative mutations. The minor allele frequency and prevalence of this mutation appear in Table I. There were no predicted damaging changes in genes for neurodegeneration with brain iron accumulation (PANK2, PLA2G6, C19orf12, CP, FTL and WDR45).
Table I.
Predicted prevalence of homozygotes with c.529G>A variant in RNASEH2B based on ClinSeq and Exome Sequencing Project (ESP) databases
| ClinSeq database | ESP database | ESP database | |
|---|---|---|---|
| Population | Full-study cohort | Full-study cohort | European American |
| Homozygotes with reference allele (n) | 996 | 6477 | 4275 |
| Heterozygotes (n) | 5 | 26 | 25 |
| Homozygotes with c.529G>A mutation (n) | 0 | 0 | 0 |
| Minor allele frequency | 0.0025 | 0.002 | 0.0029 |
| Predicted prevalence of homozygotes with c.529G>A mutation | 1/160 000 | 1/250 230 | 1/118 336 |
DISCUSSION
AGS manifests in infancy with profound motor and cognitive handicaps, mimicking those of an acquired intrauterine infection, including CSF lymphocytosis, elevated cerebral IFN-α, and imaging features of basal ganglia calcification and leukoencephalopathy.4 Our patients presented with a largely isolated and brief monophasic, unexplained neurological regression late in the first year of life, without systemic accompaniments, evolving into static deficits. AGS-consistent features included onset of symptoms in the first year of life, spasticity and, in patient 1, seizures and chilblain lesions later in life, reported in 53% and 43% of AGS patients respectively.3 Their presentations lacked several typical hallmarks of AGS, including characteristic systemic and encephalitic phases, white matter abnormalities, brain calcifications, and CSF abnormalities, although the CSFs were examined late in the course of the disease.
After the acute encephalitic phase, which generally lasts from weeks to months, patients with AGS can plateau in their neurological deterioration,2 resulting in static impairment. AGS studies show that IFN activity and CSF pleocytosis are negatively associated with age, suggesting decline in the autoinflammatory process in older patients with AGS. This seems particularly true for patients with AGS2, in which 10 of 32 (31%) patients in a recent study had a normal IFN score at the time of evaluation.2 This suggests that CSF abnormalities may not provide a sensitive diagnostic criterion for AGS, as they might be absent in older patients, patients with less severe disease, or those examined outside the acute encephalitic phase.
Brain imaging findings in AGS generally encompass diffuse brain atrophy, leukoencephalopathy, and/or basal ganglia calcifications; however, rarely, computed tomographay and magnetic resonance imaging could be normal.3,9 The presence of iron deposition in the basal ganglia has not been previously reported in AGS. Iron accumulation, particularly affecting the deep brain nuclei, is characteristic of neurodegeneration with brain iron accumulation disorders, a group of rare progressive monogenic diseases with onset in childhood and adolescence.11 Iron accumulation also occurs in neurodegenerative disorders, including Parkinson and Alzheimer diseases.12 Inflammation, iron deposition and neurodegeneration coexist, but their exact relationships are not well understood.13,14 For example, neuronal death activates gliosis and recruits monocytes that transform to phagocytic macrophages with large pools of labile iron. Macrophages undergo apoptotic death, releasing labile iron to neighboring neurons.13 Excess iron promotes reactive oxygen species causing membrane and DNA breakage, and protein misfolding and aggregation.14 Most patients with AGS are imaged in early childhood, and the lifetime burden of brain iron accumulation might not be evident until later in life. Of note, in this study, the magnitude of signal intensity over the basal ganglia seemed less pronounced on the T2* than in the T2-fluid attenuated inversion recovery images; this is unusual as one would anticipate a ‘blooming’ effect in T2* sequences. While we can merely offer speculations at this time, this finding can further be clarified in the future with the use of other imaging sequences specifically tailored to answer this question. Mutations in RNASEH2B causing AGS2 (Fig. S1, online supporting information), are responsible for approximately a third of all cases of AGS. The single most common RNASEH2B mutation, c.529G>A, was found in 97 of 104 pan-ethnic families with AGS2 (48 homozygotes and 49 compound heterozygotes).3 Although fewer than 200 cases of c.529G>A have been reported, minor allele frequency in large population databases (ExAC and ClinSeq Project) are 0.2% and 0.25% respectively,12,15 with an approximate minor allele frequency of 0.29% for individuals of European descent. These would predict a prevalence of AGS2 due to biallelic c.529G>A mutations alone of 1 in 120 000 in the European population to 1 in 250 000 in the general population.
AGS2 involving the c.529G>A allele is likely under-diagnosed and easily mistaken for spastic cerebral palsy. AGS should be considered in these patients, even in the absence of prototypical brain imaging and CSF findings. Early recognition influences reproductive decisions and may allow for current and future therapeutic interventions targeting the damaging effects of CNS autoinflammation.16
Supplementary Material
Table SI: Seven subtypes of Aicardi–Goutières syndrome.
Appendix S1: Supplementary method.
Ideogram of the human RNASEH2B gene illustrating the various reported disease-causing mutations in the literature. Splicing mutations are shown above the ideogram. Exonic mutations are shown below, with truncating mutations depicted in red, missense mutations in black, and the most common RNASEH2B mutation, the c.529G>A, p.Ala177Thr presented in this report, in blue.
What this paper adds.
We identified two siblings (sister and brother) with atypical Aicardi–Goutières syndrome type 2 due to RNASEH2B mutation. Manifestations included spastic quadriplegia and anarthria but preserved intellect and increased iron signal in the basal ganglia.
RNASEH2B-related Aicardi–Goutières syndrome type 2 can have present with a variable phenotype, including idiopathic spastic cerebral palsy.
Acknowledgments
We are grateful to our patients and their parents. This study was supported by the National Human Genome Research Institute’s Intramural Research Program. We also acknowledge Dr Leslie Biesecker (National Institutes of Health, National Human Genome Research Institute), for his intellectual inputs to this paper. The authors have stated that they had no interests which may be perceived as posing a conflict or bias.
ABBREVIATIONS
- AGS
Aicardi–Goutières syndrome
- AGS2
Aicardi–Goutières syndrome type 2
- IFN
Interferon
References
- 1.Aicardi J, Goutieres F. A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann Neurol. 1984;15:49–54. doi: 10.1002/ana.410150109. [DOI] [PubMed] [Google Scholar]
- 2.Crow YJ, Chase DS, Lowenstein Schmidt J, et al. Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am J Med Genet A. 2015;167A:296–312. doi: 10.1002/ajmg.a.36887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rice G, Patrick T, Parmar R, et al. Clinical and molecular phenotype of Aicardi–Goutieres syndrome. Am J Hum Genet. 2007;81:713–25. doi: 10.1086/521373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali M, Highet LJ, Lacombe D, et al. A second locus for Aicardi-Goutieres syndrome at chromosome 13q14-21. J Med Genet. 2006;43:444–50. doi: 10.1136/jmg.2005.031880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.La Piana R, Uggetti C, Roncarolo F, et al. Neuroradiologic patterns and novel imaging findings in Aicardi-Goutières syndrome. Neurology. 2016;86:28–35. doi: 10.1212/WNL.0000000000002228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fritz-French C, Tyor W. Interferon-α (IFNα) neurotoxicity. Cytokine Growth Factor Rev. 2012;23:7–14. doi: 10.1016/j.cytogfr.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Cuadrado E, Michailidou I, van Bodegraven EJ, et al. Phenotypic variation in Aicardi-Goutières syndrome explained by cell-specific IFN-stimulated gene response and cytokine release. J Immunol. 2015;194:3623–33. doi: 10.4049/jimmunol.1401334. [DOI] [PubMed] [Google Scholar]
- 8.Rice GI, Forte GM, Szynkiewicz M, et al. Assessment of interferon-related biomarkers in Aicardi-Goutières syndrome associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and ADAR: a case-control study. Lancet Neurol. 2013;12:1159–69. doi: 10.1016/S1474-4422(13)70258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crow YJ, Zaki MS, Abdel-Hamid MS, et al. Mutations in ADAR1, IFIH1, and RNASEH2B presenting as spastic paraplegia. Neuropediatrics. 2014;45:386–93. doi: 10.1055/s-0034-1389161. [DOI] [PubMed] [Google Scholar]
- 10.D’Arrigo S, Riva D, Bulgheroni S, et al. Aicardi-Goutières syndrome: description of a late onset case. Dev Med Child Neurol. 2008;50:631–4. doi: 10.1111/j.1469-8749.2008.03033.x. [DOI] [PubMed] [Google Scholar]
- 11.Schneider SA. Neurodegeneration with brain iron accumulation. Curr Neurol Neurosci Rep. 2016;16:9. doi: 10.1007/s11910-015-0608-3. [DOI] [PubMed] [Google Scholar]
- 12.Hagemeier J, Geurts JJ, Zivadinov R. Brain iron accumulation in aging and neurodegenerative disorders. Exp Rev Neurother. 2012;12:1467–80. doi: 10.1586/ern.12.128. [DOI] [PubMed] [Google Scholar]
- 13.Andersen HH, Johnsen KB, Moos T. Iron deposits in the chronically inflamed central nervous system and contributes to neurodegeneration. Cell Mol Life Sci. 2014;71:1607–22. doi: 10.1007/s00018-013-1509-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10(Suppl):S18–25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 15.Biesecker LG, Mullikin JC, Facio FM, et al. The ClinSeq Project: piloting large-scale genome sequencing for research in genomic medicine. Genome research. 2009;19(9):1665–74. doi: 10.1101/gr.092841.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volpi S, Picco P, Caorsi R, Candotti F, Gattorno M. Type I interferonopathies in pediatric rheumatology. Pediatr Rheumatol Online J. 2016;14:35. doi: 10.1186/s12969-016-0094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI: Seven subtypes of Aicardi–Goutières syndrome.
Appendix S1: Supplementary method.
Ideogram of the human RNASEH2B gene illustrating the various reported disease-causing mutations in the literature. Splicing mutations are shown above the ideogram. Exonic mutations are shown below, with truncating mutations depicted in red, missense mutations in black, and the most common RNASEH2B mutation, the c.529G>A, p.Ala177Thr presented in this report, in blue.