Abstract
Aim
Oropharyngeal administration of colostrum (OAC) has been proposed to provide mother's early milk to very low-birth-weight (VLBW) infants in the first few days of life. The aim of this study was to test the hypothesis that OAC would increase salivary secretory IgA (SsIgA).
Methods
Overall, 30 VLBW infants randomized to receive OAC or sterile water had salivary sampling for SsIgA on the day of life (DOL) 2,7, and 14. The incidence of late-onset sepsis (LOS) and necrotizing enterocolitis (NEC) was determined prospectively. Within and between-group comparisons were made by paired and independent samples t-tests.
Results
Baseline characteristics were similar between groups. SsIgA was higher in OAC versus the control group (p < 0.05) on DOL 7, but not subsequently on DOL 14. There was no difference in LOS or NEC.
Conclusion
OAC increased SsIgA at DOL 7. A large, multicenter trial is needed to determine if OAC decreases LOS or NEC in VLBW infants.
Keywords: colostrum, salivary secretory IgA, VLBW infant, infection
Infection is a leading cause of morbidity and mortality in very low-birth-weight (VLBW) infants < 1,500 g. Increased risk for infection has been linked to multiple characteristics of this population including: lower gestational age, birth weight (BW), the presence of indwelling catheters, and the need for mechanical ventilation.1 Studies have shown a protective effect of breast milk feedings on the incidence of LOS and nosocomial infections in VLBW infants.2,3 Critical illness in the first week of life often delays the introduction of enteral feedings, limiting the immunological benefits provided by breast milk, and placing these infants at higher risk of acquiring infections.
Colostrum, the milk produced in the first few days following birth, contains significant concentrations of immune mediators that provide bactericidal and anti-inflammatory protection.4,5 Recent investigations into the composition of colostrums show an increase in the concentration of protective components with decreasing gestational age at birth as well as a significant decrease in the presence of these factors around 1 week of age.6,7 These trends suggest that immune components in colostrum provide infants with protection against infection.
The presence of an immature gastrointestinal tract and morbidities that decrease gut perfusion prevent many VLBW infants from receiving enteral feeding in the first week of life. These factors coupled with the administration of antibiotics lead to colonization of the intestine with potentially pathogenic bacteria increasing the likelihood of infection.8 Oropharyngeal administration of colostrum (OAC) has been suggested as an alternative method of providing VLBW infants with the potential immune benefits of mother's early milk.9 Unlike orogastric administration which requires swallowing and gastrointestinal absorption, oropharyngeal administration, delivery of 0.2 mLof colostrum to the mucous membranes via a cotton-tipped applicator, involves direct absorption by the mucous membranes of the oropharynx. Studies in animal and human models have shown this route of administration to be a safe and effective means of delivery for cytokines such as interferon α and gamma, IL-2, and granulocyte macrophage colony-stimulating factor with resultant immune stimulation.10-12
The exact mechanism of action of oropharyngeally administered cytokines is unknown. Immune factors present in colostrum likely function through multiple mechanisms to provide immune protection to preterm infants. These factors are absorbed through oral mucosa potentially activating the infant's immune system through the oropharyngeal-associated lymphoid tissue system.13 Several cytokines found in colostrum have been shown to stimulate the differentiation of B lymphocytes suggesting the importance of secretory IgA (sIgA) in providing immunity.14,15 sIgA in colostrum may also be absorbed and work directly to provide immune protection. This is demonstrated by the presence of sIgA in urine and stool of breast milk fed infants.16,17 However, the role of sIgA in providing immunity to VLBW infants is not yet fully understood.
Preliminary evidence suggests that sIgA is an important component of colostrum which may play both direct and indirect roles in providing immune protection to premature infants. To date, research has focused on the immune benefits provided by enteral breast milk. With so many VLBW infants in the neonatal intensive care unit (NICU) receiving limited enteral nutrition during the first week of life, it is important to identify alternative means of delivering a potential immune therapy such as oropharyngeal colostrum. Research from animal and human models supports the use of oropharyngeal administration as a safe and efficacious mode of delivery of immune therapy. If proven efficacious, the practice of OAC in VLBW infants could have far-reaching benefits for these highly fragile babies including lower rates of infection, improved tolerance of enteral feedings, and shorter NICU stays.
The purpose of this prospective, randomized, placebo-controlled pilot study was to determine the effect of OAC on salivary sIgA (SsIgA) levels in VLBW infants. We also sought to determine the effect of OAC on rates of late-onset sepsis (LOS) and necrotizing enterocolitis (NEC).
Patients and Methods
Patients
VLBW infants, less than 1,500 g, born at the Penn State Milton S. Hershey Medical Center and admitted to the Penn State Children's Hospital NICU were eligible for the study. The Penn State Hershey Institutional Review Board approved the study protocol, and the trial was registered with Clinicaltrials.gov, number NCT01443091. Fig. 1 depicts patient selection and enrollment. A total of 93 VLBW infants were screened for eligibility. Out of which 63 were excluded as the majority did not meet the inclusion criteria. Eight patients were not approached because there was no one from the study team available to consent the mother. Parental consent was obtained before study enrollment for each infant. This study was not blinded.
Fig. 1.
Flow diagram of study enrollment and subject allocation.
Following informed consent of mothers willing to participate and provide colostrum for their infant, subjects were enrolled between birth and 48 hours of life. Infant subjects were randomized to receive OAC or sterile water (control group). Randomization was performed by the primary investigator using a random numbers table following enrollment. Subjects randomized to treatment received their own mother's colostrum. Twins were randomized to the same group. Inclusion criteria included: BW of less than 1,500 g or 3.3 lb, born at Penn State Milton S. Hershey Medical Center. Exclusion criteria included: infants with major congenital anomalies or chromosomal syndromes incompatible with life, infants of mothers not willing to provide colostrum for their infant in the first week of life, or infants of mothers with known human immunodeficiency virus, hepatitis B, or hepatitis C as these infections may be transmitted through breast milk. All patients enrolled were randomized and received their allocated intervention. There was no loss to follow-up in the study.
Enteral Feeding Protocol
Infants enrolled in this study received enteral feedings with mother's breast milk or formula in accordance with our NICU's feeding protocol and at the discretion of the infant's care team. The enteral feeding protocol for VLBW infants in place during the study period called for 3 to 5 days of trophic or minimal enteral feedings (<20 mL/kg/d) before starting an advance of feeding volume based on BW category. This advance ranged from 10 to 20 mL/kg/day and could be interrupted at the discretion of the care team for signs of feeding intolerance. Total parenteral nutrition (TPN) was continued until infants were receiving an enteral feeding volume equal to 100 mL/kg/d. Enteral feedings were increased to a goal of 150 to 170 mL/kg/d. There was no donor breast milk program in place at the time of the study.
Oral Care Procedure
VLBW infants received oral care with either mother's own colostrum (treatment) or sterile water (control) every 3 hours from day of life (DOL) 2 until DOL 7. The study was not blinded with respect to the application of sterile water or colostrum. For the oral care procedure, 0.2 mL of mother's colostrum or sterile water was applied to the oral mucosa by an intensive care nurse using a cotton-tipped applicator every 3 hours during care times. Nurses caring for infants enrolled in the study were provided with a handout explaining the procedure for administration of colostrum or sterile water and given bedside instruction on the technique for the procedure. Administration of colostrum or sterile water was charted in the electronic medical record and compliance with the procedure was audited by the primary investigator. Adherence to the study protocol was similar for both groups. Apnea and bradycardia events during the procedure as well as the occurrence of aspiration pneumonia were charted according to unit policy, and surveillance for these events continued throughout the study period. The volume of colostrum or sterile water applied to the oropharynx of the subjects was similar to that used in other trials of OAC and is also similar to the volume of sterile water routinely used in NICUs to provide oral care. The small volume is intended to prevent significant swallowing, limiting the risk of aspiration.
Salivary Secretory IgA Analysis
Saliva was collected using precut Whatman grade filter paper strips as previously described by Neu et al in 2007, at three time points (DOL 2, 7, and 14).18 On DOL 2 a salivary sample was collected before the initiation of colostrum or sterile water administration. This sample was collected while the infant was in a rest state immediately before a care time and at least 3 hours after the last enteral feed. Salivary samples were also collected upon completion of the intervention at DOL 7 and 1 week following completion of the intervention at DOL 14. To avoid possible contamination with colostrum, the salivary sample collected on DOL 7 was obtained no less than 4 hours following the final application of colostrum or sterile water.
The filter papers were then stored in deep freeze (−60°C) before sIgA extraction. Before extraction, collection tubes were removed from the freezer and thawed at room temperature for 10 to 15 minutes. Once thawed, tubes were wiped for condensate and weighed. Postweight values were subtracted from the dry preweight values for each tube before the collection of saliva, and this weight difference was used as a multiplicative coefficient to control for saliva absorption onto the filter paper. SsIgA extraction from filter paper was achieved by using 1,500 μL of 10:1 diluted wash buffer for each tube, followed by placing tubes in tube rotator for 24 hours. Next, tubes were refrozen for 24 hours, then thawed, and vortexed to remix sIgA and buffer. Following remixing, 600 μL of each sample was pipetted into Eppendorf tubes which were weighed before and after this step to determine the volume of saliva. These tubes were then placed in Speed-Vac (Thermo Scientific, Waltham, MA) for 4 to 10 hours to concentrate samples to 120 μL and reweighed for exact sample volume following concentration. On a separate day, samples were thawed to room temperature and then centrifuged at 4,500 rpm for 15 minutes. sIgA levels were then measured by quantitative enzyme-linked immunosorbent assay (ELISA) (ALPCO Immunoassays; Salem, NH) following the protocol provided by the manufacturer. All samples were run in duplicate with sensitivity and precision demonstrated by acceptable intra- and interassay coefficients.
Maternal and infant demographic information was collected from the infant's electronic medical record. We collected data on feeding characteristics such as the timing of first enteral feeding and age at which infants reached full enteral feedings (140 mL/kg/d), and days on parenteral nutrition. To assess the effect of OAC on rates of NEC and nosocomial infection, we collected data related to short-term clinical outcomes such as feeding tolerance, the incidence, and severity of NEC, and nosocomial infection rates. Patients were followed up for these variables until discharge from the NICU. Nosocomial infection was defined as development of suspected or culture-proven LOS, defined as onset after DOL 3. Suspected sepsis was defined as the continuation of antibiotics for greater than 5 days in the face of negative blood cultures. Culture-proven sepsis was defined as antibiotic treatment for greater than 5 days with a positive blood culture. NEC was defined as pneumatosis on abdominal radiograph and staged according to the modified Bell's classification system.
Statistics
Demographic and clinical data were collected prospectively until discharge from the NICU, removed of all identifiers and entered into IBM SPSS Statistics version 21.0 (Armonk, NY). A per-protocol analysis was used in this study. Descriptive statistics (frequencies, means, medians, and scatterplots) were done, and all variables were checked for outliers and normality before analysis. All tests were two-tailed at a 5% significance level. Differences in demographics or outcomes between the control and treatment group infants were determined using independent samples t-tests for continuous variables and Mann–Whitney U tests for nonparametric data. Chi-square tests were used for categorical data. SsIgA levels were natural log transformed before analysis to correct for skewness of the data on tests of normality. For within-group comparisons of SsIgA over time, paired samples t-tests were used to compare related samples. Independent samples t-tests were used for between-group comparisons.
Results
A total of 30 subjects were enrolled in the study. Subjects ranged in BW from 520 to 1,420 g and represented gestational ages between 23 and 32 weeks. All subjects survived to NICU discharge. Analysis of sample characteristics for key variables expected to contribute to study outcomes revealed no significant differences between treatment and control groups (Table 1).
Table 1. Population characteristics.
| Infant characteristics | Colostrum group (N = 17) | Sterile water group (N = 13) | p Value |
|---|---|---|---|
| Gestational age at birtha (wk), M ± SE | 28.4 ± 0.7 | 28.5 ± 0.8 | NS |
| Birth weighta (g), M ± SE | 1,132 ± 64 | 1,079 ± 59 | NS |
| ELBWb, n (%) | 4 (24) | 4 (31) | NS |
| SGAb, n (%) | 2 (12) | 3 (23) | NS |
| AGAb, n (%) | 15 (88) | 10 (77) | NS |
| Male genderb, n (%) | 7 (41) | 8 (62) | NS |
| Cesarean deliveryb, n (%) | 13 (76) | 13 (100) | NS |
| APGAR @ 1 minc, median (IQR) | 4 (3, 7) | 4 (4, 7) | NS |
| APGAR @ 5 minc, median (IQR) | 7 (6, 8) | 8 (6, 9) | NS |
| Invasive mechanical ventilationb, n (%) | 8 (47) | 8 (62) | NS |
| Maternal characteristics | |||
| Agea (y), M ± SE | 26.8 ± 1.6 | 28.0 ± 1.4 | NS |
| Chorioamnionitisb (suspect) | 0 (0) | 3 (23) | NS |
| Maternal antibiotic exposureb | 6 (35) | 7 (54) | NS |
Abbreviations: AGA, appropriate for gestational age; DOL, day of life; IQR, interquartile range; M, mean; NS, not statistically different; SE, standard error; SGA, small for gestational age.
Independent samples t-test.
Chi-square test.
Mann-Whitney U test.
Measurement of SsIgA over time showed a statistically significant increase at DOL 7 in the colostrum group (n = 17) as compared with the control sterile water group (n = 13) in both within the group and between-group comparisons (Fig. 2). This change was not sustained at DOL 14, 1 week following the intervention. Baseline levels at DOL 2 were similar between groups.
Fig. 2.
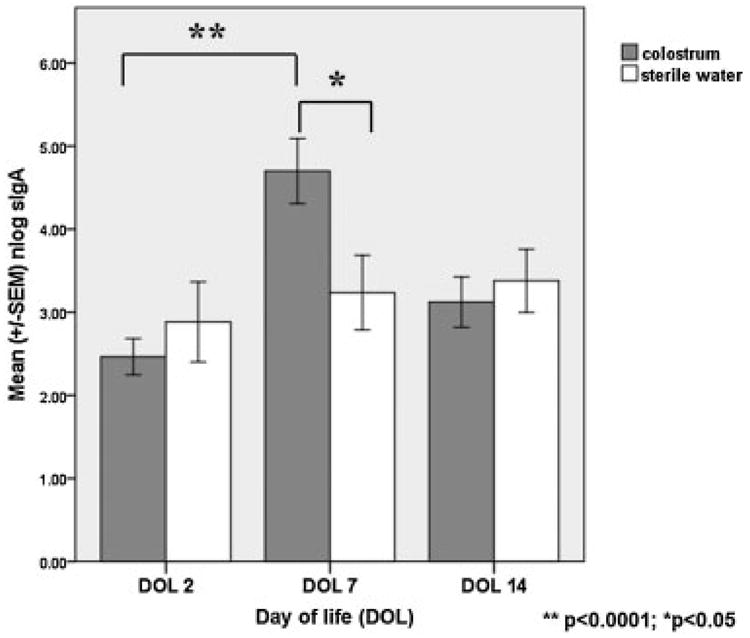
Graphical representation of the differences in salivary secretory IgA (SsIgA) between colostrum-treated versus control infants. Note that SsIgA increased from baseline day 2 to day 7 in treatment group infants (**p < 0.00001, paired sample t-test). Conversely, SsIgA did not increase over time in the control group infants. SsIgA at the day of life 7 was statistically higher in the colostrum-treated infants (*p < 0.05; independent samples t-test).
There were no adverse events reported during this investigation. All subjects survived to discharge from the NICU. Infants were monitored closely for apnea and bradycardia during the intervention period as well as for aspiration pneumonia. There were no apneic, bradycardic events reported with oropharyngeal colostrum or sterile water administration. No subjects were diagnosed with aspiration pneumonia during the intervention period.
Analysis of feeding characteristics: type of enteral feedings, breast milk or formula including amounts of each received, and tolerance of enteral feedings revealed no difference between the treatment and control groups (Table 2). Tolerance of enteral feedings was defined by the number of times feedings were held for longer than 6 hours before attaining full enteral feeding volume. There was no difference between the oropharyngeal colostrum group and the sterile water group in the timing of the onset of enteral feedings or when full enteral feedings were reached. Analysis of our secondary outcomes revealed no significant difference in episodes of suspected sepsis, culture-positive sepsis, or NEC (Table 3).
Table 2. TPN and feeding characteristics.
| Infant characteristics | Colostrum group (N = 17) | Sterile water group (N = 13) | p Value |
|---|---|---|---|
| TPN daysa, M ± SE | 22.1 ± 7.0 | 23.6 ± 9.2 | NS |
| Initiation of enteral feeds (DOL)b, M ± SE | 3.9 ± 1.0 | 3.1 ± 0.7 | NS |
| Amount of breast milk on day 7b, % | 71 ±11.3 | 88 ± 8.9 | NS |
| Amount of breast milk on day 14b, % | 78 ± 9.7 | 82 ± 9.9 | NS |
| Attained full feedsa (DOL), M ± SE | 24.2 ± 7.9 | 24.9 ± 9.4 | NS |
| Two or more episodes of withholding feeds > 6 h, n (%)c | 7 (41) | 3 (23) | NS |
Abbreviations: DOL, day of life; M, mean; NS, not statistically different; SE, standard error; TPN, total parenteral nutrition.
Independent samples t-test.
Mann–Whitney U test.
Chi-square test.
Table 3. Between-group comparisons for sepsis and NEC.
| Colostrum group Frequency/% N=17 | Sterile water group Frequency/% N = 13 | p Value | |
|---|---|---|---|
| Suspected sepsis | 0.58 | ||
| None | 7 (41) | 6 (46) | |
| One episode | 6 (35) | 5 (39) | |
| Two or more episodes | 4 (24) | 2 (15) | |
| Culture-positive sepsis | 0.37 | ||
| None | 12 (71) | 10 (77) | |
| One episode | 4 (23) | 3 (23) | |
| Two episodes | 1 (6) | 0 | |
| NEC (stage 2 or 3) | 0.87 | ||
| No | 14 (82) | 11 (85) | |
| Yes | 3 (18) | 2 (15) |
Abbreviation: NEC, necrotizing enterocolitis stage 2 or greater.
Discussion
Earlier pilot trials have shown the administration of oropharyngeal colostrum to extremely low-birth-weight (ELBW) and VLBW infants starting in the first few days of life to be safe and feasible.19–21 Our study findings add to the growing body of evidence supporting the safe use of OAC for VLBW infants. The prior studies by Rodriguez et al and Lee et al included infants born less than 28 weeks gestation whereas our study included infants up to 32 weeks gestation. The infants in our study were also larger, with a mean BW over 1,000 g. This study, therefore, adds to the generalizability of the practice of the administration of oropharyngeal colostrum.
In accordance with previous trials of OAC, the practice seems to be safe and feasible. In our experience, with supportive breastfeeding practices, it is possible to collect sufficient colostrum within the first 48 hours after birth to support oral care with maternal colostrum every 3 hours during the first week of life. Even with larger infants, it was possible to collect sufficient colostrum to support both OAC and enteral feedings if they were clinically indicated. The procedure itself as in prior investigations does not lead to adverse events. Even in intubated patients, there were no adverse events reported such as bradycardia, desaturation, or aspiration.
Our results show that SsIgA levels increased in VLBW infants after receiving OAC for 5 days during the first week of life. This finding supports the findings of similar trials in ELBW infants which reported increases in SsIgA and excretion of urinary sIgA with the administration of oropharyngeal colostrum.19,20 It is plausible that those subjects receiving OAC had absorption of maternal sIgA thereby increasing their production of sIgA. This theory forms the basis for the use of maternal colostrum for immune stimulation or immune therapy in preterm infants.22
Previous research supports the role of immune factors in colostrum in the immune system function of preterm infants. The mechanisms by which this occurs have not been fully elucidated, but cytokines and other immune factors in human milk have been postulated to have both direct and indirect effects on the immune system. The concentrations of immune factors, particularly sIgA, in human milk are inversely proportional to gestational age suggesting a protective benefit to preterm infants. The sIgA in human milk then provides passive immunity by blocking bacterial adherence to mucosal membranes. In the newborn, there is thought to be the little production of sIgA during the first few weeks of age. However, the regulatory mechanisms for induction of sIgA production and activation of an immune function, the gut-associated lymphoid tissue, and oropharyngeal-associated lymphoid tissue, are all present in extremely preterm infants.23 While immature, the immune response in extremely preterm infants has also been shown to be functional with the production of cytokines and immunoglobulins in infected infants.24,25
The protective effect of sIgA, however, may extend beyond the mucosal surface. The stability of the molecule against enzymatic degradation and the excretion of sIgA in the urine of infants receiving oropharyngeal colostrum support the absorption of this molecule from the mucosal surface.19 Urinary and stool excretion of sIgA are also seen in VLBW infants receiving human milk feedings.16,17 Once absorbed, sIgA may have systemic effects on immunity. In vitro studies in mice have shown transepithelial uptake of sIgA and binding to specific dendritic cells in Peyer's patches in the intestine or the gut-associated lymphoid tissue (GALT) leading to the production of anti-inflammatory cytokines and upregulating sIgA-producing B cells.26,27 These findings suggest that maternal sIgA may serve to promote an antiinflammatory or tolerant environment as well as, perhaps, upregulating an infant's sIgA production.
Also, cytokines in colostrum may upregulate the production of sIgA in preterm infants, thereby stimulating the infant's immune system. These effects are also thought to function at the level of the GALT. Cytokines, particularly IL-6, in mother's milk have been shown to preferentially stimulate the differentiation of B lymphocytes to sIgA-secreting plasma cells.5 Other cytokines implicated in this process include TGF-β and IL-10.
Evidence from these studies suggests a role for maternal colostrum in immune protection through the action of immune factors such as sIgA and cytokines. There is mounting evidence that sIgA may not only exhibit its protective effect through passive mucosal protection but may also be absorbed and stimulate the infant's immune system leading to active immunity. This research also suggests a role for sIgA in the creation of a tolerant, anti-inflammatory environment which may lead to improved tolerance of enteral feedings. Further, in vitro study is needed to delineate these relationships but it may be reasoned that an increase in the amount of sIgA present at the mucosal surface through the practice of OAC may translate into fewer nosocomial infections, NEC, and improved tolerance of feeds.
The increase in SsIgA seen at DOL 7 may have implications for preterm infant immune protection or stimulation. However, clinical advantages of OAC were not seen in our study. In contrast to our findings, a recent prospective randomized trial of oropharyngeal colostrum in preterm infants younger than 28 weeks did find a significant decrease in clinical sepsis in the infants receiving colostrum.19 Additional clinical outcomes related to feeding also were not found to be significant in our study, such as the timing of the start of enteral feedings and time to attain full feedings. Unlike our study, several small clinical investigations have shown positive effects of OAC on enteral feedings. In a prospective cohort study of 15 ELBW infants, OAC was associated with a reduction in the time to attain full feeds.28 Similarly, in a retrospective study investigators found that administration of oropharyngeal colostrum led to an earlier time to start enteral feeds as well as a reduction in the time needed to regain BW.29
Our study has several limitations. This was a pilot trial focused on the feasibility of approach and safety of the intervention technique and as such our sample size was limited. Post-hoc sample size calculations for the primary and secondary outcomes were made to determine the sample size needed for future studies using G*Power version 3.1 statistical software (Universitat Kiel, Germany). At an α of 0.05 and a power of 95%, a future study will need at least 134 patients (67 patients per group) to detect a moderate effect on SsIgA concentration. A similar sample size will be needed for the outcome of suspected sepsis. To test for group differences for NEC with an incidence of 10 to 14%, a sample size of 120 per group would be required allowing for three predictor variables in multivariable logistic regression analysis with an α of 0.05 and power of 95%.
Another possible limitation of the study is the potential effect of breast milk enteral feedings on the increase in SsIgA seen at DOL 7. However, our analysis of feeding characteristics of infants in the study (Table 2) showed no difference in the amount of enteral breast milk between the two groups. Only one subject received any oral feedings at DOL 7, and that subject was in the control group. Also, subjects were receiving very low volumes of enteral feedings at DOL 7 making it improbable that gastroesophageal reflux occurred leading to breast milk in the oropharynx contaminating saliva samples. Finally, careful attention to the timing of saliva sample collection (at least 4 hours from the last application of colostrum) reduces the risk of sample contamination with maternal sIgA. The potential confounding effect of breast milk “contamination” on SsIgA levels seems unlikely given these findings. This potential limitation, however, applies to all clinical studies of this type and would only be possible to control in animal studies fully.
The lack of effect on clinical outcomes may have been impacted by small sample size or the short 5-day duration of OAC in this study. The extent to which a longer duration of treatment (i.e., 14–21 days) may have had on sustained SsIgA levels and reduction in nosocomial infection and/or NEC rates is unknown. In this clinical study, we are not able to determine the mechanism for the increase in SsIgA at DOL 7 in the treatment group. However, we propose the putative mechanism that subjects receiving OAC had higher absorption of maternal sIgA, thereby increasing their sIgA production as compared with control subjects.
This study adds to the growing body of literature demonstrating that OAC increases SsIgA levels in VLBW infants. A larger trial is needed to determine the possible effects on nosocomial infection and NEC in VLBW infants. A multicenter trial is underway to further investigate the effects of oropharyngeal colostrum administration on these outcomes.30 In vitro studies are also necessary to further characterize the effects of specific immune factors such as sIgA on immune stimulation and protection of preterm infants.
Acknowledgments
This study was funded by a Children's Miracle Network Research Grant. Dr. Doheny receives salary support from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health under award number 1R01DK099350. The authors have no conflicts of interest to disclose. We would like to thank Dr. Robert Bonneau and his laboratory in the performance of sample preparation and ELISA analysis and Dr. Fumiyuki Gardner and Ms. Megan Marvin for their help with data collection and statistical analysis. We would also like to thank Barbara Shocker RN MEd IBCLC for her help in subject recruitment as well as the nursing staff at the Penn State Hershey Neonatal Intensive Care Unit for the administration of the study protocol.
References
- 1.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2 Pt 1):285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 2.Furman L, Taylor G, Minich N, Hack M. The effect of maternal milk on neonatal morbidity of very low-birth-weight infants. Arch Pediatr Adolesc Med. 2003;157(01):66–71. doi: 10.1001/archpedi.157.1.66. [DOI] [PubMed] [Google Scholar]
- 3.Schanler RJ, Shulman RJ, Lau C. Feeding strategies for premature infants: beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics. 1999;103(6 Pt 1):1150–1157. doi: 10.1542/peds.103.6.1150. [DOI] [PubMed] [Google Scholar]
- 4.Buescher ES. Anti-inflammatory characteristics of human milk: how, where, why. Adv Exp Med Biol. 2001;501:207–222. doi: 10.1007/978-1-4615-1371-1_27. [DOI] [PubMed] [Google Scholar]
- 5.Garofalo RP, Goldman AS. Cytokines, chemokines, and colony-stimulating factors in human milk: the 1997 update. Biol Neonate. 1998;74(02):134–142. doi: 10.1159/000014019. [DOI] [PubMed] [Google Scholar]
- 6.Araújo ED, Gonçalves AK, Cornetta MdaC, et al. Evaluation of the secretory immunoglobulin A levels in the colostrum and milk of mothers of term and pre-term newborns. Braz J Infect Dis. 2005;9(05):357–362. doi: 10.1590/S1413-86702005000500002. [DOI] [PubMed] [Google Scholar]
- 7.Koenig A, de Albuquerque Diniz EM, Barbosa SF, Vaz FA. Immunologic factors in human milk: the effects of gestational age and pasteurization. J Hum Lact. 2005;21(04):439–443. doi: 10.1177/0890334405280652. [DOI] [PubMed] [Google Scholar]
- 8.Westerbeek EA, van den Berg A, Lafeber HN, Knol J, Fetter WP, van Elburg RM. The intestinal bacterial colonisation in preterm infants: a review of the literature. Clin Nutr. 2006;25(03):361–368. doi: 10.1016/j.clnu.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez NA, Meier PP, Groer MW, Zeller JM. Oropharyngeal administration of colostrum to extremely low birth weight infants: theoretical perspectives. J Perinatol. 2009;29(01):1–7. doi: 10.1038/jp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eid P, Meritet JF, Maury C, Lasfar A, Weill D, Tovey MG. Oromucosal interferon therapy: pharmacokinetics and pharmacodynamics. J Interferon Cytokine Res. 1999;19(02):157–169. doi: 10.1089/107999099314306. [DOI] [PubMed] [Google Scholar]
- 11.Lecciones JA, Abejar NH, Dimaano EE, et al. A pilot double-blind, randomized, and placebo-controlled study of orally administered IFN-alpha-n1 (Ins) in pediatric patients with measles. J Interferon Cytokine Res. 1998;18(09):647–652. doi: 10.1089/jir.1998.18.647. [DOI] [PubMed] [Google Scholar]
- 12.Tovey MG, Meritet JF, Guymarho J, Maury C. Mucosal cytokine therapy: marked antiviral and antitumor activity. J Interferon Cytokine Res. 1999;19(08):911–921. doi: 10.1089/107999099313451. [DOI] [PubMed] [Google Scholar]
- 13.Bocci V, von Bremen K, Corradeschi F, Luzzi E, Paulesu L. What is the role of cytokines in human colostrum? J Biol Regul Homeost Agents. 1991;5(04):121–124. [PubMed] [Google Scholar]
- 14.Field CJ. The immunological components of human milk and their effect on immune development in infants. J Nutr. 2005;135(01):1–4. doi: 10.1093/jn/135.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa J, Sasahara A, Yoshida T, et al. Role of transforming growth factor-beta in breast milk for initiation of IgA production in newborn infants. Early Hum Dev. 2004;77(1-2):67–75. doi: 10.1016/j.earlhumdev.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Goldblum RM, Schanler RJ, Garza C, Goldman AS. Human milk feeding enhances the urinary excretion of immunologic factors in low birth weight infants. Pediatr Res. 1989;25(02):184–188. doi: 10.1203/00006450-198902000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Schanler RJ, Goldblum RM, Garza C, Goldman AS. Enhanced fecal excretion of selected immune factors in very low birth weight infants fed fortified human milk. Pediatr Res. 1986;20(08):711–715. doi: 10.1203/00006450-198608000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Neu M, Goldstein M, Gao D, Laudenslager ML. Salivary cortisol in preterm infants: Validation of a simple method forcollecting saliva for cortisol determination. Early Hum Dev. 2007;83(01):47–54. doi: 10.1016/j.earlhumdev.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Kim HS, Jung YH, et al. Oropharyngeal colostrum administration in extremely premature infants: an RCT. Pediatrics. 2015;135(02):e357–e366. doi: 10.1542/peds.2014-2004. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez NA, Meier PP, Groer MW, Zeller JM, Engstrom JL, Fogg L. A pilot study to determine the safety and feasibility of oropharyngeal administration of own mother's colostrum to extremely low-birth-weight infants. Adv Neonatal Care. 2010;10(04):206–212. doi: 10.1097/ANC.0b013e3181e94133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montgomery D, Baer V, Lambert D, Christensen R. Oropharyngeal administration of colostrum to very low birth weight infants: results of a feasibility trial. Neonatal Intensive Care. 2010;23:27–29. [Google Scholar]
- 22.Gephart SM, Weller M. Colostrum as oral immune therapy to promote neonatal health. Adv Neonatal Care. 2014;14(01):44–51. doi: 10.1097/ANC.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 23.Spencer J, Macdonald TT. Ontogeny of human mucosal immunity In: Macdonald TT, ed Ontogeny of the Immune System of the Gut. Boca Raton, FL: CRC Press; 1990. pp. 23–50. [Google Scholar]
- 24.Stoll BJ, Lee FK, Hale E, et al. Immunoglobulin secretion by the normal and the infected newborn infant. J Pediatr. 1993;122(5 Pt 1):780–786. doi: 10.1016/s0022-3476(06)80026-0. [DOI] [PubMed] [Google Scholar]
- 25.Ng PC, Li K, Wong RP, et al. Proinflammatory and anti-inflammatory cytokine responses in preterm infants with systemic infections. Arch Dis Child Fetal Neonatal Ed. 2003;88(03):F209–F213. doi: 10.1136/fn.88.3.F209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadaoui KA, Corthésy B. Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer's patches with restriction to mucosal compartment. J Immunol. 2007;179(11):7751–7757. doi: 10.4049/jimmunol.179.11.7751. [DOI] [PubMed] [Google Scholar]
- 27.Corthésy B. Role of secretory IgA in infection and maintenance of homeostasis. Autoimmun Rev. 2013;12(06):661–665. doi: 10.1016/j.autrev.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez NA, Groer MW, Zeller JM. A randomized, controlled trial of the oropharyngeal administration of mother's colostrum to extremely low birth weight infants in the first few days of life. Neonatal Intensive Care. 2011;24(04):31–35. [Google Scholar]
- 29.Seigel JK, Smith PB, Ashley PL, et al. Early administration of oropharyngeal colostrum to extremely low birth weight infants. Breastfeed Med. 2013;8(06):491–495. doi: 10.1089/bfm.2013.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez NA, Vento M, Claud EC, Wang CE, Caplan MS. Oropharyngeal administration of mother's colostrum, health outcomes of premature infants: study protocol for a randomized controlled trial. Trials. 2015;16(01):453. doi: 10.1186/s13063-015-0969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]