Abstract
Background
Brown Adipose Tissue (BAT) is associated with higher energy expenditure and lower adiposity in adults. However, the relationship between BAT composition and adiposity in early life is unknown. The objective of this study was to test the hypothesis that brown fat composition at birth is prospectively associated with adiposity gain during the first six months of postnatal life.
Methods
N=35 healthy infants were followed prospectively from intrauterine life and birth through 6 months age. Dixon MRI scans were conducted during the neonatal period to characterize supraclavicular BAT composition. Dual-energy X-ray absorptiometry to assess total body composition was performed within the first month of life and at 6 months age.
Results
After adjusting for potential confounding factors, a more brown-like composition (smaller fat fraction) of the supraclavicular BAT depot was associated with a smaller increase in percent body fat over the first six months of postnatal life.
Conclusions
A more brown-like BAT composition at birth appears to be protective against excess adiposity gain in early life. Newborn BAT tissue may constitute a target for prevention strategies against the subsequent development of obesity.
Introduction
The severity and magnitude of health and other problems conferred by obesity/adiposity and metabolic dysfunction is well-recognized (1). It is also well established that once an individual becomes obese, it is difficult to lose weight, and even more difficult to sustain weight loss (2,3). For these reasons, it is important to gain a better understanding of the early/developmental origins of individual differences in the propensity for weight and fat mass gain, in order to predict obesity (or, more precisely, excess adiposity) risk, and to develop strategies for primary prevention before an individual becomes overweight or obese, or secondary interventions to increase the likelihood of a more sustained response to weight-loss strategies (3,4).
At the individual level, obesity results when energy intake exceeds energy expenditure. Thus, any effective treatment for obesity must alter total energy balance by either decreasing energy intake and/or increasing energy expenditure. In contrast to white adipose tissue (WAT) which functions to store fat, brown adipose tissue (BAT) is a specialized tissue that expends energy via lipid metabolism and thermogenesis pathways, facilitated by its uncoupling protein UCP-1 (5). The presence of substantial depots of BAT in newborns and its crucial, life-preserving role in the early postnatal period in thermoregulation has long been recognized (6,7). Several independent studies indicate that BAT persists into older childhood and adult life and remains physiologically relevant, as evidenced by its positive association with resting energy expenditure (8), skeletal muscle volume (9), and glucose regulation (10) as well as its negative association with BMI (8, 11, 12), total body fat (8,11), and visceral fat (11,13), thereby supporting a protective role of BAT against obesity.
Very little is known about the potential protective effects of BAT characteristics in early life on obesity/adiposity in subsequent periods of life, in part likely due to the lack of noninvasive, validated methods to assess BAT mass and BAT activity in newborns and infants. We recently reported on a non-invasive method to segment and quantify BAT composition in infants using MRI (14). In contrast to the uni-locular adipocyte composition of WAT, BAT has multi-locular adipocytes and is mitochondria and capillary dense. Therefore, the ratio of water to fat is greater in BAT compared with WAT, making it ideally suited for chemical shift imaging (CSI). Fat protons resonate at a frequency of 3.5 ppm higher than water, therefore BAT can be differentiated from WAT spectroscopically (15). CSI takes advantage of the frequency difference, or chemical shift, by observing the contributions of differing phase on the overall signal contribution (16,17). This method has now been validated in rodents (18) and through postmortem histological verification of BAT deposits in a 3-month old infant (19). Fat signal fraction (FF, the ratio of fat-based signal to the sum of combined water- and fat-based signal) mapping has more recently been used to characterize the depletion of fat stores in BAT depots of hypothermic newborns (20) and to demonstrate BAT composition differences between obese and normal weight children (21,22). To our knowledge, normative values of FF in the first year of life have been limited to our previously mentioned work (14) and one cross-sectional report of 12 infants (1–171 days old) (21).
Thus, the goal of the current study was to characterize BAT at birth in a population of healthy human newborns, and to examine the association between newborn BAT characteristics and body composition over the first 6 months of life (as measured using Dual X-ray Absorptiometry (DXA)). Numerous studies provide evidence that child size and growth velocity particularly during the early period of infancy (first months of postnatal life) is among the strongest predictors of childhood obesity. Increased size or rapid weight or fat gain during this early postnatal growth phase is associated with increased risk for childhood (and adult) obesity (23–26) as well as with other related outcomes including metabolic risk (27) hypertension, and asthma in later life (28,29).
Methods
Participants
Study participants comprised a cohort of children followed prospectively from intrauterine life and birth through infancy at the University of California, Irvine, Development, Health and Disease Research Program in Orange, CA. Pregnant mothers were recruited between 2011–2015 in early gestation from obstetric care provider clinics. Exclusionary criteria for pregnant mothers included multiple pregnancy, uterine or cervical abnormalities, or conditions associated with dysregulated neuroendocrine and immune function such as endocrine, hepatic or renal disorders or corticosteroid medication use. Exclusion criteria for children (newborns) were preterm delivery <34 weeks gestation, perinatal complications associated with neurological consequences (e.g., hypoxia), and congenital, genetic, or neurologic disorders (e.g., fetal alcohol syndrome, Down syndrome or other aneuploidy, fragile X syndrome). All pregnant mothers who met the inclusion/exclusion criteria at the study clinics were approached consecutively. The final sample for the current analyses consisted of 35 infants. 57% (N=20) of the children were girls. The characteristics of the study population are presented in Table 1. All babies included in this study were born to mothers with uncomplicated pregnancies (no major medical conditions) and were healthy at the time of birth. A total of N=3 infants were born between week 34 and 37 (late preterm), and N=4 infants were small-for gestational age (SGA, birthweight < the 10th percentile for gestational age, N=1 overlap between late preterm and SGA). All the study procedures were approved by the UC Irvine Institutional Review Board, and all mothers provided written informed consent.
Table 1.
Maternal and infant characteristics.
| Maternal characteristics | |
|---|---|
| Pre-pregnancy BMI (mean ±SD) | 28.05 ± 7.20 |
| Gestational weight gain (kg, mean ± SD) | 13.42 ± 7.69 |
|
| |
| Infant characteristics | |
|
| |
| Sex (females) | 57% |
| Gestational age at birth (weeks, mean ±SD) | 38.98 ± 1.44 |
| Birth weight (grams, mean ± SD) | 3274.49 ± 561.07 |
| Feeding status (exclusively breastfeed until 6 months age) | 33% |
| Race/ethnicity | |
| Hispanic White | 40% |
| Hispanic other race | 34% |
| Non-Hispanic White | 14% |
| Non-Hispanic other race | 12% |
Magnetic resonance imaging (MRI) to assess brown adipose tissue characteristics
MRI was performed on a Siemens 3T Tim Trio system (VB17 software) using a 12-channel head coil and neck coils. Scans were conducted using a previously validated chemical-shift 3D gradient echo Dixon method (TR=7.47ms, TE1/TE2=2.45/3.675ms, NA=1, BW/pixel=977Hz, FA=10, Matrix=512×320×160, 0.97×0.97×1mm, Scan Time=3min 2s) (14). Regions of interest were defined as the combination of supraclavicular and axillary deposits and segmented using a semi-automatic procedure as previously described in detail (Figure 2) (14). Seed points for active contour segmentation were manually placed in deposits in supraclavicular, and axillary regions. Seed bubbles of 3mm were used to roughly cover these areas while avoiding any subcutaneous WAT partial volume regions. Supraclavicular and axillary ROIs were limited to four seed points per side to ensure consistent definition. The active contour evolution was iterated until the contours were visually covered by label, between 40 and 50 iterations. The supraclavicular/axillary region was chosen as it is a well-documented site for BAT and the union of supraclavicular and axillary deposits has been shown to be the most repeatable region of BAT measurement in human neonates (14). The FF was averaged across all voxels within the individualized bi-lateral masks resulting in a single reported measure for each individual. Higher fat fraction values are believed to reflect a fattier composition (less BAT-like) and have been associated with the obese state in children (21, 22). Furthermore, cooling-reheating protocols have demonstrated decreases in BAT FF as a result of lipid consumption during cold exposure (30).
Figure 2. Example BAT Fat Fraction Images.
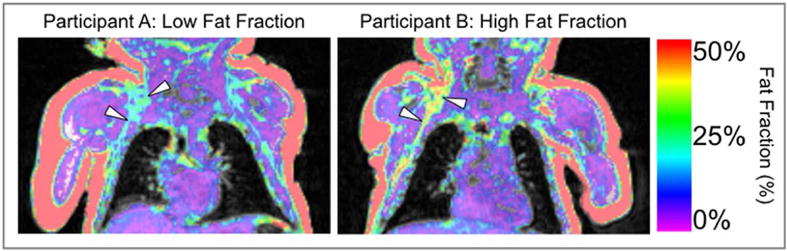
Example participants with relatively low (left) and high fat fraction (right) are shown. The fat fraction (ratio of fat signal to the sum of fat and water) image is displayed on top of an anatomical image (sum of fat and water). Top and bottom white arrows indicate supraclavicular and axillary fat depots respectively.
Infants were, on average, about 1 month old at the time of the MRI scan (26 ± 13 mean (± SD) days). Age at MRI scan and gestational age at birth were not associated with mean BAT FF.
Dual-energy X-ray absorptiometry to assess body composition
A whole body DXA scan was obtained using a Hologic Discovery Scanner (A, QDR 4500 series, Hologic Inc., Bedford, MA) in pediatric scan mode. Calibration using Hologic’s anthropomorphic Spine QC Phantom was performed before each scan. During the scan, infants lay supine while sleeping, wearing only a disposable diaper and swaddled in a light cotton blanket. If the baby moved during the scan, a single repeat was performed once the baby had been pacified. At the time of the newborn DXA assessment, children were, on average, 25.7 days old (mean (± 11.6 (SD) days). The second DXA assessment was conducted, on average, at 6 months age (6.3 ± 0.46 mean (± SD) months). Since older age at the newborn DXA scan but not at the 6 months DXA scan was positively associated with total percent body fat (%BF), we used an age-adjusted newborn measure of %BF in the analyses. Adiposity gain was defined as the difference between the age-adjusted newborn %BF and %BF at six months of age.
Infant feeding
Infant feeding method (breast- or formula feeding) was assessed via maternal interviews at the newborn and 6 months DXA assessment. All babies were exclusively breast fed at the time of the newborn DXA assessment. To control for infant feeding status over the 6 month postnatal period, a variable was computed indexing whether or not the mother continuously breastfed the child until 6 months age.
Maternal anthropometrics, birth outcomes and sociodemographic characteristics
Maternal sociodemographic characteristics (age, education, income, parity, race/ethnicity) were obtained via a standardized structured interview at the first pregnancy visit. Race/ethnicity was determined by self-report using the Office of Management and Budget 1997 Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity (Revision of Statistical Policy Directive No. 15, Race and Ethnic Standards for Federal Statistics and Administrative Reporting). Maternal pre-pregnancy BMI (weight kg/height m2) was computed based on pre-pregnancy weight abstracted from the medical record and maternal height measured at the research laboratory during the first pregnancy visit. Maternal weight at delivery was abstracted from the medical record. Total gestational weight gain (GWG) was calculated by subtracting the pre-pregnancy weight from weight at delivery.
Birth outcomes, including gestational age at birth and newborn birth weight, were abstracted from the medical record.
Statistical analyses
The distributions of continuous variables included in the analyses were tested for normality (Kolmogorov-Smirnov-Test). All continuous variables were normally distributed (p’s > 0.200 for null-hypothesis). Pearson product moment correlations were used to test bivariate associations between mean BAT FF and other variables (maternal pre-pregnancy BMI, weight gain during pregnancy, gestational age at birth, birth weight adjusted for length of gestation, age at scan and infant sex). Differences in BAT FF concentrations between different racial/ethnic groups were tested using a univarite ANOVA. Multiple linear regression was used to quantify the association between BAT FF and change in %BF gain, with and without adjustment for the effects of gestational weight and pre-pregnancy BMI, infant sex, infant feeding status and small-for-gestational age (SGA) status because these factors have been shown to be associated with gain in infant adiposity in previous studies.
Diagnostics were performed to assess the validity of standard linear regression assumptions. No extreme departures from general assumptions were observed, and no observations were removed from the analysis. All statistical analyses were performed using SPSS v21, and the statistical significance level was set at α = 0.05.
Results
Mean BAT FF in neonates was 29.4% ± 4.06 (SD), and ranged from 22.9 – 36.4%, consistent with previous reports of neonatal supraclavicular BAT FF (14, 21). Mean %BF at birth and at 6 months age was 13.8 ± 4.7 (range: 2.2 – 27) and 32.6 ± 9.0 (range: 16.8 – 48.3), respectively. The average change in %BF from 0–6 months age was 18.8 ± 8.5 (range: 5.6 – 37.6).
There was no significant association between BAT FF and newborn %BF ((unstandardized) β [95% CI] = −0.180 [−0.519 to 0.285], r=0.154, p=0.295). There was a significant association between newborn BAT FF and change in %BF from 0–6 months age. In unadjusted analyses, a lower BAT FF (more “brown fat like” composition) was associated with a smaller change in %BF from 0–6 months (β [95% CI] = 0.893 [0.236 to 1.550], r=0.428, p=0.009, Figure 1), and lower %BF at 6 months age (β [95% CI] = 0.797 [0.048 to 1.503], r=0.348, p=0.037). These effects persisted after adjusting for maternal pre-pregnancy BMI, maternal GWG, infant sex, and SGA and infant feeding status (β [95% CI] = 0.951 [0.293 to 1.610], (partial) r=0.492, p=0.006) for adjusted effect of BAT FF on change in %BF from 0–6 months, and β [95% CI] = 0.974 [0.302 to 1.646], (partial) r=0.489, p=0.006 for adjusted effect of BAT FF on %BF at 6 months). Specifically, in the adjusted model, a one standard deviation unit decrease in newborn BAT FF was associated with an approximately 20% reduction of the average increase in %BF gain from 0–6 months age. Statistical coefficients for the unadjusted and adjusted models are summarized in Table 2.
Figure 1. Newborn BAT FF is Prospectively Associated with 0–6 Month Adiposity Gain.
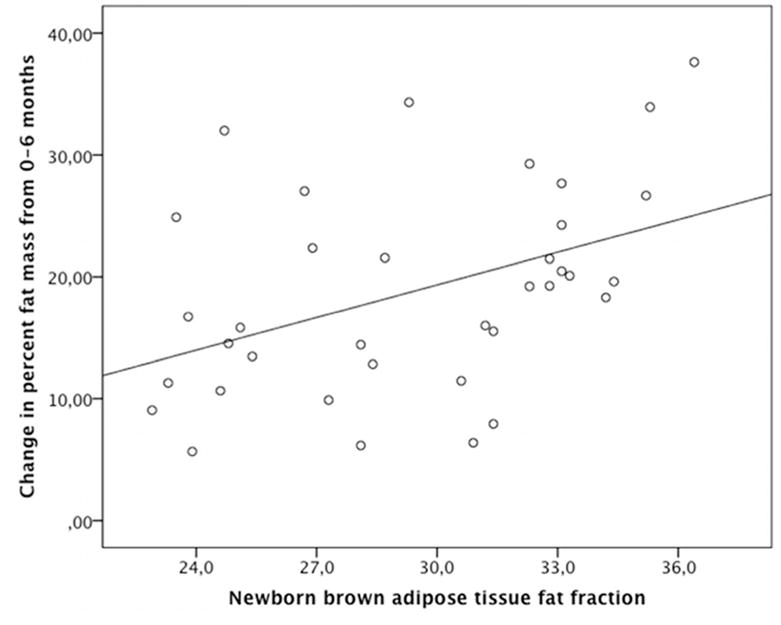
Newborn supraclavicular brown adipose fat fraction (measured by magnetic resonance imaging) and change in percent body fat (measured by Dual-energy X-ray absorptiometry) from 0–6 months age. Higher fat fraction is indicative of a fattier (less BAT-like) composition.
Table 2.
Statistical coefficients of the different models for the effect of BAT FF on infant body composition.
| %BF newborn age β [95% CI] |
%BF at 6 months age β [95% CI] |
change in %BF from 0–6 months age β [95% CI] |
|
|---|---|---|---|
| BAT FF (unadjusted) | −0.180 [−0.519 to 0.285] p=0.295 |
0.893 [0.236 to 1.550] p=0.009 |
0.797 [0.048 to 1.503] p=0.037 |
| BAT FF (adjusted)* | 0.023 [−0.367 to 0.413] p=0.905 |
0.974 [0.302 to 1.646] p=0.006 |
0.951 [0.293 to 1.610] p=0.006 |
adjusted for maternal pre-pregnancy BMI, maternal gestational weight gain, infant sex, small for gestational age and infant feeding status;
%BF: percent body fat; BAT FF: brown adipose tissue fat fraction
Mean BAT FF was not significantly associated with birth weight, birthweight percentile, or weight at 6 month (all r’s < −0.132, p’s > 0.444).
Discussion
The current study represents, to the best of our knowledge, the first report in humans linking higher brown fat like composition of newborn supraclavicular fat depot (a primary location of brown fat) with a smaller increase in infant total body fat from birth till 6 months age (a key indicator of increased childhood and adult obesity risk (23–26)). This effect persisted after adjusting for other potential determinants of newborn and infant %BF and change in %BF. The magnitude of the effect translates to an approximately 20% reduction of the average increase in %BF gain from 0–6 months age per standard deviation decrease in newborn BAT FF. We suggest the magnitude of this effect is substantial, particularly for a single factor, considering that in a recent publication (31) a combined set of several factors including race, gestational weight gain, pre-pregnancy BMI, feeding patterns, gestational age at birth and sex accounted for 19% in the variance of adiposity (% body fat) at birth.
Lower BAT FF values are believed to reflect a more BAT-like composition of fat depots and have been associated with lower obesity risk in children (21, 22). The effect observed in the current study may therefore be mediated by the capacity of the more brown-like fat composition to facilitate energy expenditure via lipid metabolism and thermogenesis pathways during the first month of life, thereby reducing white fat deposition from 0–6 months age.
In the current study BAT FF was not associated with newborn %BF. This may be due to the relatively short duration of ex utero thermal exposure at the time of scan (average 26 days after birth) or the relatively limited inter-individual variability in newborn fat stores. While BAT and WAT deposition occurs in utero, non-shivering thermogenesis (that is necessary for stimulating BAT) does not begin until the neonate is born and exposed to the relatively cold ex utero environment. Therefore, the effective exposure time to ex utero conditions, and its consequences on BAT lipid content, are minimal in the newborn period.
The limitations of this study include the use of a dual-echo acquisition rather than a multi echo acquisition and the lack of a measure for BAT activity. This acquisition method lacks the multiple echo points needed for full characterization of the complex lipid profile. In theory, this limits the measurement by having an imperfect water-fat separation within the BAT depots. However, the utility of the dual-echo approach has been demonstrated in previous in vivo human BAT MRI (14, 32, 33), and it has been shown that the use of a dual-echo, versus a multi-echo, acquisition primarily adds a uniform bias relative to MR spectroscopic measurement of hepatic fat fraction (34). Finally, it should be noted that due to the spatial resolution limits of MRI and the interspersed distribution of brown fat cells found in humans, all of the depots measured in this work may contain a partial volume average of brown/beige and white fat cells. The issue of the physiological relevance of BAT FF within these depots warrants further study. First, while the supraclavicular region has demonstrated functional plasticity through positive UCP1 expression in both BAT active and inactive adults (35), our current method is unable to determine whether the neonatal supraclavicular depot exhibits the same physiological properties. Prior evidence suggests a distinct second type of human BAT found in the interscapular region of newborn humans (32). Second, we note that this is a measure of BAT composition and not activity. The FF can then be thought of as either reflective of BAT capacity for future energy expenditure (lower FF is due to greater mitochondrial density) or suggestive of past lipid metabolism (lower FF is due to lipid stores having been utilized) (30). Certainly, these two physiological properties are not uncoupled, and future work is needed in gaining a better understanding of the physiological underpinnings of BAT FF. Future studies also are warranted that incorporate measures of BAT characteristics at 6 months age, as well as concurrent measures of energy expenditure and metabolic function, and also perform longitudinal follow-up to quantify changes in BAT characteristics from birth into infancy, childhood and beyond, along with the concurrent characterization of other infant and child phenotypes of interest in healthy as well as clinical (e.g. small for gestational age) populations.
Our findings may provide the basis for important clinical applications. BAT may provide a pharmacological target in the context of the growing problem of obesity (8, 11, 12, 36, 37). Recent studies suggest that brown-in-white (BRITE) or beige tissue can be induced from WAT through stimulation (38, 39). Thus, potentially altering (optimizing) the initial amount of BAT or BRITE tissue in newborns and its subsequent rate of attrition during infancy and childhood may serve as a primary prevention strategy against the development of obesity and metabolic dysfunction.
Acknowledgments
Statement of Financial Support
This study was supported in part by US PHS (NIH) grants R21 DK-098765, R01 HD-065825, R01 HD-060628, R01 MH-091351, and UCI Institute for Clinical and Translational Science (CTSA grant) UL1 TR000153.
Footnotes
Conflicts of interest statement: The authors have no conflicts of interest to disclose.
Category of Study: Translational
References
- 1.OECD. Obesity and the Economics of Prevention. OECD Publishing; 2010. [Google Scholar]
- 2.Butte NF, Christiansen E, Sorensen TIA. Energy imbalance underlying the development of childhood obesity. Obesity. 2007;15:3056–3066. doi: 10.1038/oby.2007.364. [DOI] [PubMed] [Google Scholar]
- 3.Muhlhausler B, Smith SR. Early-life origins of metabolic dysfunction: role of the adipocyte. Trends Endocrinol Metab. 2009;20(2):51–7. doi: 10.1016/j.tem.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Ong KK. Early determinants of obesity. Endocr Dev. 2010;19:53–61. doi: 10.1159/000316897. [DOI] [PubMed] [Google Scholar]
- 5.Hu HH, Gilsanz V. Developments in the imaging of brown adipose tissue and its associations with muscle, puberty, and health in children. Front Endocrinol (Lausanne) 2011;2:33. doi: 10.3389/fendo.2011.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satterfield MC, Wu G. Brown adipose tissue growth and development: significance and nutritional regulation. Front Biosci. 2011;16:1589–608. doi: 10.2741/3807. [DOI] [PubMed] [Google Scholar]
- 7.Dawkins MJ, Scopes JW. Non-shivering thermogenesis and brown adipose tissue in the human newborn infant. Nature. 1965;206(980):201–2. doi: 10.1038/206201b0. [DOI] [PubMed] [Google Scholar]
- 8.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. The New England journal of medicine. 2009;360(15):1500–8. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 9.Gilsanz V, Chung SA, Jackson H, Dorey FJ, Hu HH. Functional brown adipose tissue is related to muscle volume in children and adolescents. J Pediatr. 2011;158(5):722–6. doi: 10.1016/j.jpeds.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee P, Greenfield JR, Ho KK, Fulham MJ. A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2010;299(4):E601–6. doi: 10.1152/ajpendo.00298.2010. [DOI] [PubMed] [Google Scholar]
- 11.Saito M, Okamatsu-Ogura Y, Matsushita M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58(7):1526–31. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. The New England journal of medicine. 2009;360(15):1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalfant JS, Smith ML, Hu HH, et al. Inverse association between brown adipose tissue activation and white adipose tissue accumulation in successfully treated pediatric malignancy. The American journal of clinical nutrition. 2012;95(5):1144–9. doi: 10.3945/ajcn.111.030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasmussen JM, Entringer S, Nguyen A, et al. Brown adipose tissue quantification in human neonates using water-fat separated MRI. PLoS One. 2013;8(10):e77907. doi: 10.1371/journal.pone.0077907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton G, Smith DL, Jr, Bydder M, Nayak KS, Hu HH. MR properties of brown and white adipose tissues. J Magn Reson Imaging. 2011;34(2):468–73. doi: 10.1002/jmri.22623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glover GH, Schneider E. Three-point Dixon technique for true water/fat decomposition with B0 inhomogeneity correction. Magn Reson Med. 1991;18(2):371–83. doi: 10.1002/mrm.1910180211. [DOI] [PubMed] [Google Scholar]
- 17.Ma J. Dixon techniques for water and fat imaging. J Magn Reson Imaging. 2008;28(3):543–58. doi: 10.1002/jmri.21492. [DOI] [PubMed] [Google Scholar]
- 18.Hu HH, Smith DL, Jr, Nayak KS, Goran MI, Nagy TR. Identification of brown adipose tissue in mice with fat-water IDEAL-MRI. J Magn Reson Imaging. 2010;31(5):1195–202. doi: 10.1002/jmri.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu HH, Tovar JP, Pavlova Z, Smith ML, Gilsanz V. Unequivocal identification of brown adipose tissue in a human infant. J Magn Reson Imaging. 2012;35(4):938–42. doi: 10.1002/jmri.23531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu HH, Wu TW, Yin L, et al. MRI detection of brown adipose tissue with low fat content in newborns with hypothermia. Magn Reson Imaging. 2014;32(2):107–17. doi: 10.1016/j.mri.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu HH, Yin L, Aggabao PC, Perkins TG, Chia JM, Gilsanz V. Comparison of brown and white adipose tissues in infants and children with chemical-shift-encoded water-fat MRI. J Magn Reson Imaging. 2013;38(4):885–96. doi: 10.1002/jmri.24053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng J, Schoeneman SE, Zhang H, et al. MRI characterization of brown adipose tissue in obese and normal-weight children. Pediatric radiology. 2015 doi: 10.1007/s00247-015-3391-z. [DOI] [PubMed] [Google Scholar]
- 23.Koontz MB, Gunzler DD, Presley L, Catalano PM. Longitudinal changes in infant body composition: association with childhood obesity. Pediatric obesity. 2014;9(6):e141–4. doi: 10.1111/ijpo.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331(7522):929. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stettler N, Kumanyika SK, Katz SH, Zemel BS, Stallings VA. Rapid weight gain during infancy and obesity in young adulthood in a cohort of African Americans. The American journal of clinical nutrition. 2003;77(6):1374–8. doi: 10.1093/ajcn/77.6.1374. [DOI] [PubMed] [Google Scholar]
- 26.Druet C, Stettler N, Sharp S, et al. Prediction of childhood obesity by infancy weight gain: an individual-level meta-analysis. Paediatr Perinat Epidemiol. 2012;26(1):19–26. doi: 10.1111/j.1365-3016.2011.01213.x. [DOI] [PubMed] [Google Scholar]
- 27.Ekelund U, Ong KK, Linne Y, et al. Association of weight gain in infancy and early childhood with metabolic risk in young adults. The Journal of clinical endocrinology and metabolism. 2007;92(1):98–103. doi: 10.1210/jc.2006-1071. [DOI] [PubMed] [Google Scholar]
- 28.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18(7):815–31. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 29.Sonnenschein-van der Voort AM, Arends LR, de Jongste JC, et al. Preterm birth, infant weight gain, and childhood asthma risk: a meta-analysis of 147,000 European children. J Allergy Clin Immunol. 2014;133(5):1317–29. doi: 10.1016/j.jaci.2013.12.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundstrom E, Strand R, Johansson L, Bergsten P, Ahlstrom H, Kullberg J. Magnetic resonance imaging cooling-reheating protocol indicates decreased fat fraction via lipid consumption in suspected brown adipose tissue. PLoS One. 2015;10(4):e0126705. doi: 10.1371/journal.pone.0126705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sauder KA, Kaar JL, Starling AP, Ringham BM, Glueck DH, Dabelea D. Predictors of Infant Body Composition at 5 Months of Age: The Healthy Start Study. The Journal of Pediatrics. 2017 Feb 1; doi: 10.1016/j.jpeds.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lidell ME, Betz MJ, Dahlqvist Leinhard O, et al. Evidence for two types of brown adipose tissue in humans. Nat Med. 2013;19(5):631–4. doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]
- 33.Romu T, Elander L, Leinhard OD, et al. Characterization of brown adipose tissue by water-fat separated magnetic resonance imaging. J Magn Reson Imaging. 2015 doi: 10.1002/jmri.24931. [DOI] [PubMed] [Google Scholar]
- 34.Zand KA, Shah A, Heba E, et al. Accuracy of multiecho magnitude-based MRI (M-MRI) for estimation of hepatic proton density fat fraction (PDFF) in children. J Magn Reson Imaging. 2015 doi: 10.1002/jmri.24888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee P, Swarbrick MM, Zhao JT, Ho KK. Inducible brown adipogenesis of supraclavicular fat in adult humans. Endocrinology. 2011 Jul 26;152(10):3597–602. doi: 10.1210/en.2011-1349. [DOI] [PubMed] [Google Scholar]
- 36.Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. The New England journal of medicine. 2009;360(15):1518–25. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 37.Yoneshiro T, Aita S, Matsushita M, et al. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity. 2011;19(9):1755–60. doi: 10.1038/oby.2011.125. [DOI] [PubMed] [Google Scholar]
- 38.van der Lans AA, Hoeks J, Brans B, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest. 2013;123(8):3395–403. doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beranger GE, Karbiener M, Barquissau V, et al. In vitro brown and “brite”/“beige” adipogenesis: human cellular models and molecular aspects. Biochim Biophys Acta. 2013;1831(5):905–14. doi: 10.1016/j.bbalip.2012.11.001. [DOI] [PubMed] [Google Scholar]


