Abstract
Nonmelanoma skin cancer (NMSC) is the most common malignancy in the United States representing a considerable public health burden. Pharmacological suppression of skin photo-carcinogenesis has shown promise in preclinical and clinical studies, but more efficacious photochemopreventive agents are needed. Here, we tested feasibility of harnessing pharmacological disruption of intracellular zinc-homeostasis for photochemoprevention in vitro and in vivo. Employing the zinc-ionophore and FDA-approved microbicidal agent zinc pyrithione (ZnPT), used worldwide in over-the-counter (OTC) topical consumer products, we first demonstrated feasibility of achieving ZnPT-based intracellular Zn2+-overload in cultured malignant keratinocytes (HaCaT-ras II-4; SCC-25) employing membrane-permeable fluorescent probes. Zinc-overload was accompanied by induction of intracellular oxidative stress, associated with mitochondrial superoxide-release as substantiated by MitoSOX-Red™ fluorescence microscopy. ZnPT-induced cell death observable in malignant keratinocytes was preceded by induction of metal (MT2A), proteotoxic (HSPA6, HSPA1A, DDIT3, HMOX1), and genotoxic stress response (GADD45A, XRCC2) gene expression at the mRNA and protein levels. Comet analysis revealed introduction of formamidopyrimidine-DNA-glycosylase (Fpg)-sensitive oxidative DNA lesions. In a photocarcinogenesis model (UV-exposed SKH-1 high-risk mouse skin), topical ZnPT-administration post-UV caused epidermal zinc-overload and stress response gene expression with pronounced blockade of tumorigenesis. Taken together, these data suggest feasibility of repurposing a topical OTC-drug for zinc-directed photochemoprevention of solar UV-induced NMSC.
Graphical abstract
Topical application of zinc pyrithione suppresses carcinogenesis in UV-induced high-risk SKH-1 mice.
INTRODUCTION
Nonmelanoma skin cancer (NMSC) is the most common malignancy in the United States. NMSC incidence is increasing rapidly, presenting a public health burden of considerable magnitude (1–3). Various causative factors involved in NMSC have been identified including solar ultraviolet light (UV) exposure, inflammatory dysregulation, photoimmunosuppression, and human papilloma virus (HPV) infection, and genetic aberrations causing tumor suppressor inactivation and oncogene activation have been involved in malignant progression to NMSC (4–8). High incidence of NMSC combined with poor prognosis associated with metastatic stages of squamous cell carcinoma (SCC) demand the development of novel strategies targeting the disease, particularly at early stages (1–3, 9). Chemoprevention strategies that involve pharmacological suppression of skin photocarcinogenesis have shown promise in preclinical and clinical studies, but more efficacious agents that target tumorigenesis at early stages of the disease are needed (3, 9–18).
The trace metal zinc is an important regulator of skin structure and function, and the essential role of zinc in signaling, gene expression, and enzyme activity determines keratinocyte differentiation and barrier function, Langerhans cell innate and adaptive immune signaling, melanogenesis, and tissue regeneration relevant to sunburn and wound healing (19–23). Cumulative evidence suggests that pharmacological disruption of zinc homeostasis employing nanoparticle- and ionophore-based approaches might represent a valid chemotherapeutic strategy targeting cancer cells (24, 25). Selective lipophilic ionophores facilitate reversible transmembrane transport leading to intracellular zinc overload and dissipation of zinc gradients in cancer cells, and systemic delivery of zinc ionophores has been shown to suppress tumorigenesis in murine xenograft models without causing off-target toxicity (24, 26, 27).
Zinc pyrithione (ZnPT) is a FDA-approved 2:1 coordination complex of the lipophilic ionophore pyrithione (N-hydroxy-2-pyridinethione) and one central zinc cation used worldwide in over-the-counter (OTC) topical antimicrobials and cosmetic consumer products, and anti-dandruff activity of this ionophore if used as a rinse-off product has been attributed to pharmacological disruption of zinc homeostasis in cutaneous commensal fungi (28–31). Our previous research has examined the effects of prolonged topical application of ZnPT, causing zinc overload in keratinocytes of reconstructed human epidermis (29, 30). In addition, we have demonstrated previously that in cultured human primary keratinocytes and reconstructed epidermis ZnPT-induced gene expression is characterized by massive upregulation of metal- (MT2A), heat shock- (HSPA1A, HSPA6), and redox-directed (HMOX1) stress response target genes (29, 30). Importantly, ZnPT-induced cytotoxicity has been observed in various cultured cancer cell lines, and efficacy of ZnPT-based chemotherapeutic intervention has been reported in murine xenograft models of non-cutaneous malignancies (32–34).
In this study, we have tested for the first time feasibility of using ZnPT as a topical zinc ionophore for experimental skin cancer photo-chemoprevention. First, the cytotoxic stress response elicited by this agent was profiled in cultured malignant human keratinocytes, and feasibility of blocking tumorigenic progression was then established in UV-exposed SKH-1 'high risk' mouse skin using a topical ZnPT treatment regimen.
MATERIALS AND METHODS
Chemicals
ZnPT (1-Hydroxypyridine-2-thione zinc salt; CAS Number: 13463-41-7) and pyrithione (2-Mercaptopyridine N-oxide sodium salt; CAS Number: 3811-73-2) were purchased from Sigma Chemical Co (St. Louis, MO, USA). Stock solutions were prepared in DMSO. The cell-permeable pan-caspase inhibitor z-VAD-(OMe)-fmk (CAS 187389-52-2) was obtained from EMD Millipore (Billerica, MA).
Cell culture
The squamous cell carcinoma cell line SCC-25 was obtained by American Type Culture Collection (ATCC) and cultured in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 Hams (DMEM-F12) containing 10 % bovine calf serum (BCS). Malignant tumorigenic c-Harvey-ras-oncogene transfected HaCaT keratinocytes (HaCaT-ras II-4 cells) were provided by G. Timothy Bowden (University of Arizona) and cultured in DMEM containing 10 % BCS (35). Human malignant A375 melanoma cells were obtained from ATCC and cultured in RPMI medium containing 10% BCS (36). All cell lines were maintained in a humidified incubator at 37° C and 5 % CO2.
Transmission electron microscopy
Cells were fixed in situ with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4), post-fixed in 1% osmium tetroxide in cacodylate buffer, washed, scraped and pelleted as described recently (37). Cells were then stained in 2% aqueous uranyl acetate, dehydrated through a graded series (50, 70, 90, and 100%) of ethanol and infiltrated with Spurr’s resin (Sigma, EM0300), then allowed to polymerize overnight at 60 °C. Sections (50 nm) were cut, mounted onto uncoated 150-mesh copper grids, and stained with 2% lead citrate. Sections were examined in a CM12 transmission electron microscope (FEI) operated at 80 kV with digital image collection.
Cell death analysis
Induction of cell death was examined by flow cytometric analysis of annexin-V-FITC/propidium iodide (PI) double stained cells using an apoptosis detection kit following the manufacturer’s instructions (APO-AF, Sigma, St. Louis, MO) as previously published (36, 38).
Intracellular free zinc detection
Intracellular free zinc was detected using the membrane-permeable zinc-specific fluorescent probe zinquin ethyl ester (Enzo Life Sciences International, Inc., Plymouth Meeting, PA, USA) following our published protocol (29, 30). After PBS wash, cells on dish were loaded with zinquin (5 µM) in DPBS followed by incubation in the dark (20 min, 37°C, 5% CO2), multiple washes using DPBS, and fluorescence imaging employing an EVOS FL Auto Cell Fluorescence Imaging system (Thermo Fisher Scientific, Rockford, IL) using the LED light cube for DAPI detection. Additionally, label free phase contrast imaging was acquired to monitor cell morphology. Cell fluorescence of digital microscopy images was quantified using Image J software (NIH; http://rsb.info.nih.gov/ij/download.html). Intracellular zinc was also monitored using the membrane permeable zinc fluorophore FluoZin-3AM (Thermo Fisher; 494 nm excitation, 516 nm emission) according to the manufacturer’s specifications (39). After cells were washed and harvested by trypsinization, cells were loaded with 10 µM FluoZin-3AM in DPBS and incubated for 15 m at 37°C and 5% CO2 in the dark. The cells were centrifuged, washed and resuspended in DPBS. Cells were then treated with ZnPT followed by flow cytometric detection of fluorescence intensity at various time points using a FACS Canto II (BD Biosciences, San Jose, CA).
Fluorescence detection of intracellular oxidative stress employing 2’,7’-dichlorodihydrofluorescein diacetate and MitoSOX Red™
Induction of intracellular oxidative stress was analyzed by flow cytometry using 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA) as a non-fluorescent precursor dye following a published standard procedure (38, 40). The production of mitochondrial superoxide was monitored by fluorescence microscopy using the mitochondria-directed superoxide probe MitoSOX Red™ (Thermo Fisher) according to the manufacturer’s protocol. After ZnPT exposure, cells were kept in fresh medium containing MitoSOX Red™ (5 µM) in combination with Hoechst 33342 (Thermo Fisher) as a nuclear counterstain. After a 10 min incubation time, cells were washed with DPBS followed by visualization using an EVOS FL Auto Cell Fluorescence Imaging system using the DAPI/RFP light cube. MitoSOX Red™ fluorescence intensity was then quantified using Image J software.
Mitochondrial transmembrane potential (Δψm)
ZnPT-induced alteration of mitochondrial transmembrane potential was assessed using the potentiometric dye 5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimidazolyl-carbocyanine iodide (JC-1; Sigma, T4069) following our published procedure (38). In brief, cells were trypsinized, washed in PBS, resuspended in 300 µl PBS containing 5 µg/ml JC-1 for 15 min at 37°C and 5% CO2 in the dark, then washed twice in PBS and resuspended in 300 µl PBS. Bivariate analysis was performed by flow cytometry with excitation at 488 nm, and mitochondrial function was assessed as JC-1 green (depolarized mitochondria, detector FL-1) or red (polarized mitochondria, detector FL-2) fluorescence.
Real time RT-PCR expression analysis
Total cellular RNA (3 × 106 cells) was extracted from cells using the RNeasy kit (Qiagen, Valencia, CA, USA) following the manufacturer’s instructions. RNA (200 ng of total RNA in a 50 µl reaction) was reverse transcribed using the TaqMan Reverse Transcription Reagents and 200 ng of total RNA in a 50 µl reaction (Roche Molecular Systems, Branchburg, NJ, USA) following the conditions and specifications described recently (38). Reverse transcription was primed with random hexamers and incubated at 25ºC for 10 min followed by 48ºC for 30 min, 95 ºC for 5 min, and cooling at 4ºC. Each PCR reaction contained 3.75 µL of cDNA, 12.5 µL TaqMan Universal PCR Master Mix (Roche Molecular Systems), 1.25 µL of gene specific primer (Applied Biosystems, Branchburg, NJ): human HSPA6 (Hs00275682_s1), HSPA1A (Hs00359163_s1), HMOX1 (Hs00157965_m1), DDIT3 (Hs00358796_g1), MT2A (Hs02379661_g1), GADD45A (Hs00169255_m1), and ACTB (Hs99999903_m1). Gene-specific product was normalized to ACTB and quantified using the comparative (ΔΔCt) Ct method following the ABI Prism 7000 sequence detection system user manual as described before (16, 36, 38).
Immunoblot analysis
Immunoblot analyses were conducted following the conditions described before (38). The subsequent primary antibodies were used: total eIF2α (9722, Cell Signaling Technologies, Danvers, MA), phospho-eIF2α (9721, Cell Signaling), total p38 (9212, Cell Signaling), phospho-p38 (9211, Cell Signaling), GRP78 (sc-H129, Santa Cruz), ATF-4 (11815, Cell Signaling), XBP-1S (12782, Cell Signaling), HSP70/HSP27 (ADI-SPA-810-F, Enzo), HO-1 (5853, Cell Signaling), XRCC2 (PA5-21279, Life Technologies). Equal protein loading was assessed using anti-ACTB (A4700, Sigma). Horseradish peroxidase- conjugated goat anti-rabbit (111-035035-144) or goat anti-mouse secondary antibody (115-035-146, Jackson Immunological Research) was then employed followed by visualization using enhanced chemiluminescence detection reagents (Thermo Fisher).
Comet assay (alkaline single cell gel electrophoresis)
The alkaline Comet assay was performed according to the manufacturer’s instructions (Trevigen, Gaithersburg, MD) as published recently (38). Cells were then stained with DAPI and visualized with a fluorescence microscope followed by analysis using image J software. At least 100 tail moments for each group were analyzed in order to calculate the mean ± S.D. for each group. The Fpg-FLARE assay for assessment of Fpg-induced strand cleavage at oxidized purine bases was performed according to the manufacturer’s instructions (Trevigen).
Topical ZnPT exposure in the UV-exposed SKH-1 high-risk (tumor-prone) mouse model
Six to eight-week old female SKH-1 Elite™ mice were purchased from Charles River Laboratories (Wilmington, MA) and housed and maintained in accordance with The University of Arizona Animal Care and Use Committee standards under an approved protocol. To generate the standard UV-exposed SKH-1 'high-risk' (tumor-prone) mouse model, SKH-1 mice were subjected to an 18-week UVB regimen [delivering 190 mJ/cm2 per UV exposure (final dose); three times per week; first six weeks of UV exposure: increasing dose regimen (week 1–2: 40 %; week 3–4: 60 %; week 5–6: 80% of final dose per exposure) to allow skin photo-adaptation] (41–43). An irradiation panel of UVB-313 lamps (Q-LAB, Westlake, OH) was used, and the spectral output was quantified using a dosimeter from International Light Inc. (Newburyport, MA), model IL1700, with an SED240 detector for UVB (range 265–310 nm, peak 285 nm) at a distance of 365 mm from the source. At the end of the 18 weeks irradiation period, these mice are tumor free but have a high risk for developing papilloma lesions over the next several weeks. Three weeks after the end of the UV regimen pair-matched 'high risk' mice (6 mice per group) received either topical ZnPT (1 % in 50 µl Vanicream™ (Pharmaceutical Specialties, Inc. Rochester, MN)] or carrier only (Vanicream™), administered three times per week over a three week period, during which papilloma development was monitored. At the end of the topical treatment period (42 days after last UV exposure), final tumor size (tumor burden) and numbers per animal (multiplicity) were determined.
Quantitative analysis of total zinc content in SKH-1 mouse skin using ICP-MS
Topical ZnPT [0.25% and 1% (w/w)] in Vanicream™ (Pharmaceutical Specialties, Inc.) or Vanicream™ only (control) was applied to for 24 h. After 24 h, skin was harvested, rinsed with PBS, dissolved in nitric acid (0.5 mL, 85 °C, 3 h) and prepared for zinc quantification by inductively-coupled plasma mass spectrometry (ICP-MS), following our published procedure (30).
Gene expression analysis by mRNA and protein detection in ZnPT-exposed SKH-1 mouse skin
After short term topical treatment of SKH-1 mice with ZnPT [0.25 % and 1.0 % (w/w); 24 h] in Vanicream™ or Vanicream™ alone, the cream was removed, and tissue was harvested as described earlier (16, 38). Tissue samples were prepared for RNA isolation and RT-PCR profiling following our published procedures (16, 30, 44); mouse Hmox1 (Mm00516005_m1), Hspa1a (Mm01159846_s1), Ddit3 (Mm01135937_g1), and Gadph (Mm99999915_m1) primer/probes were obtained from ABI (Applied Biosystems). Gene-specific product was normalized to Gadph and quantified using the comparative (ΔΔCt) Ct method following the ABI Prism 7000 sequence detection system user manual as described before (16, 36, 38). For immunohistochemical detection of ZnPT-induced cutaneous protein changes after ZnPT treatment (24 h continuous exposure), skin tissues were processed for standard hematoxylin and eosin (H&R) staining and immunohistochemical analysis (16, 38). Immunohistochemistry was performed as previously described, using HO-1 (ADI-SPA-896, Enzo) and Hsp70 (C92F3A-2, Enzo) primary antibodies (16, 30, 38, 44). Primary antibodies were detected employing the Discovery XT Automated Immunostainer technology (Ventana Medical Systems, Tucson, AZ) using a biotinylated streptavidin-horseradish peroxidase and DAB (30,30–diaminobenzidine tetrahydrochloride) system. Digital images were taken by an Olympus BX50 camera (Olympus, Center Valley, PA).
Statistics
Results are presented as means (± SD) of at least three independent experiments. All data sets were analyzed employing one-way analysis of variance (ANOVA) with Tukey’s post hoc test using the Prism 4.0 software. In all bar graphs, means without a common letter differ (p < 0.05). Average tumor burden and multiplicity were compared between treatment groups employing Mann-Whitney nonparametric statistical analysis using the Prism 4.0 software
RESULTS
ZnPT causes intracellular zinc overload in malignant HaCaT-ras II-4 and SCC-25 keratinocytes
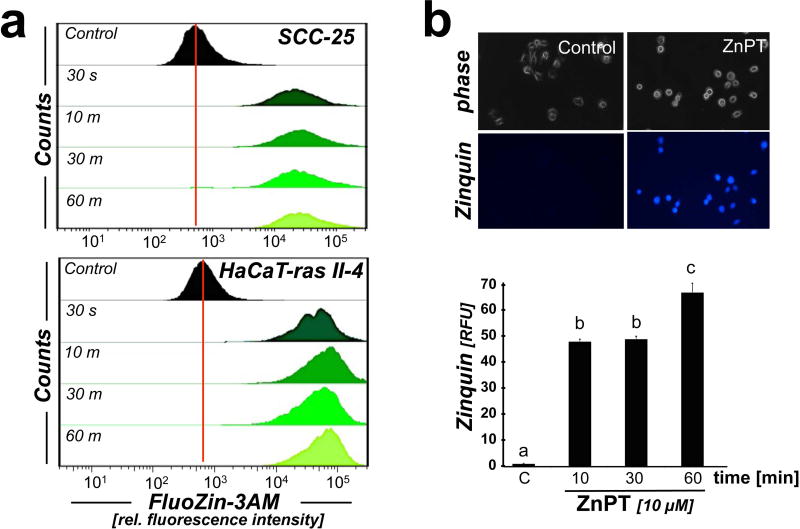
First, in order to test feasibility of pharmacological modulation of intracellular zinc concentrations in malignant HaCaT-ras II-4 (c-Harvey-ras-oncogene transfected HaCaT (35)) and SCC-25 (squamous cell carcinoma) keratinocytes exposed to low micromolar concentrations of ZnPT, the occurrence of intracellular zinc overload was examined employing membrane permeable fluorescent probes (Fig. 1). In both cell lines, flow cytometric detection using the zinc-specific fluorophore FluoZin-3AM revealed the rapid onset of intracellular zinc overload that was detectable within 30 s incubation time and sustained over the 60 min observation time (Fig. 1a). Over the length of the observation period an almost 50-fold increase in FluoZin-3AM fluorescence intensity was observed in SCC-25 cells (Fig 1a, upper panel), and an almost 80-fold increase in FluoZin-3AM fluorescence intensity was observed in HaCaT-ras II-4 cells (Fig. 1 a, lower panel), indicative of a rapid increase in intracellular labile zinc consistent with ZnPT-ionophoric activity.
Figure 1. ZnPT-induced zinc overload in malignant human keratinocytes.
(a) Time course analysis of ZnPT-induced (10 µM) intracellular free zinc elevation, monitored by flow cytometric detection of FluoZin-3AM (n=3; upper panel: SCC-25; lower panel: HaCaT-ras II-4). (b) Time course analysis of ZnPT-induced (10 µM; SCC-25) increase of intracellular free zinc as seen by Zinquin-based fluorescence microscopy (upper panel). Bar graph (lower panel) displays quantitative analysis of Zinquin relative fluorescence intensity (n ≥ 85; mean ± SD).
The occurrence of ZnPT-induced intracellular zinc overload in malignant keratinocytes was also substantiated by independent fluorescence imaging, employing the zinc-specific fluorophore Zinquin in HaCaT-ras II-4 (data not shown) and SCC-25 cells (Fig. 1b). Quantitative image analysis revealed a more than 50-fold enhancement of intracellular fluorescence intensity in ZnPT-exposed over untreated control cells sustained over the length of the observation period. Similar results indicating feasibility of using ZnPT for pharmacological induction of intracellular zinc overload were also obtained in other malignant skin cell lines including human A375 malignant melanoma cells (data not shown).
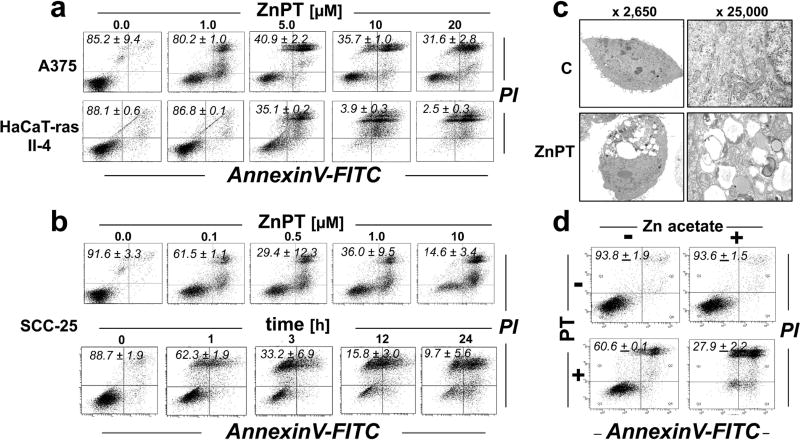
ZnPT causes cytotoxicity in a panel of cultured malignant skin cell lines (HaCaT-ras II-4, SCC-25, A375)
ZnPT-induced cytotoxicity has been documented in various non-cutaneous cancer cell lines including prostate cancer (32), cervical carcinoma (45), acute myeloid leukemia (33), and lung cancer cells (26), but no studies have reported ZnPT activity against skin cancer cell lines. Using a panel of cultured malignant skin cell lines including HaCaT-ras II-4, A375, and SCC-25 cells, we therefore determined the dose response relationship of ZnPT-induced cytotoxicity employing flow cytometric analysis of cell viability (Fig. 2a–b) (35, 36). In all cell lines, significant loss of viability in more than 50% of the cell polulation was observed at low micromolar ZnPT concentrations (≥ 5 µM, 24 h; Fig. 2a–b). SCC-25 cells displayed the most pronounced sensitivity to ZnPT-induced cell death, and significant induction of cell death was observable at concentrations as low as 100 nM (Fig. 2b).
Figure 2. ZnPT-induced cytotoxicity in malignant human skin cell lines.
Dose response of ZnPT-induced cell death. Cells were exposed to ZnPT (≤ 20 µM; 24 h) or left untreated, and viability was assessed by flow cytometry [annexin V-PI staining; numbers in quadrants indicate viable (AV-negative, PI-negative) in percent of total gated cells. (a) A375; HaCaT-ras II-4. (b) SCC-25 cells: ZnPT dose response (upper panel), time course analysis (lower panel; ZnPT; 10 µM). (c) Transmission electron microscopy (fold magnification as indicated) using SCC-25 cells to ZnPT (10 µM; 6 h). (d) SCC-25 cells were exposed to the isolated or combined treatment of zinc acetate (25 µM) and pyrithione (5 µM). Cell viability was then assessed as in panel (a).
Given the high sensitivity of SCC-25 to ZnPT cytotoxicity, we focused our pilot follow up studies on these cells (Fig. 2b–d; Fig. 3–Fig. 4), complemented by data on malignant HaCaT-ras II-4 keratinocytes (Fig. 5). In SCC-25 keratinocytes, time course analysis of ZnPT-induced cell death revealed rapid loss of plasma membrane integrity as indicated by increased PI staining already observable at early time points (1 h), suggesting a non-apoptotic mode of cell death involved in ZnPT-induced cytotoxicity (Fig. 2c). Consistent with a non-apoptotic mode of cell death, no cytoprotection was achieved when ZnPT exposure occurred in the presence of the pan-caspase inhibitor z-VAD-fmk (data not shown), an observation reported by us earlier in primary human keratinocytes (29).
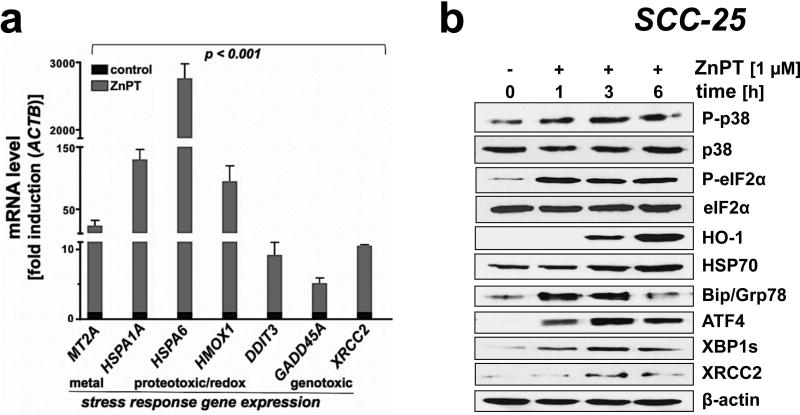
Figure 3. ZnPT-induced stress response target gene expression in malignant SCC-25 keratinocytes.
(a) ZnPT-induced (1 µM; 6 h) upregulation of stress response gene expression monitored at the mRNA level involving redox, heat shock, genotoxic and ER stress [n =3; mean ± SD; means without a common letter differ (ANOVA with Tukey’s post hoc test)]. (b) ZnPT- (1 µM; 1–6 h) modulates the cellular levels of stress response proteins involved in redox, heat shock, genotoxic and ER stress in a time dependent manner; loading control: β-actin.
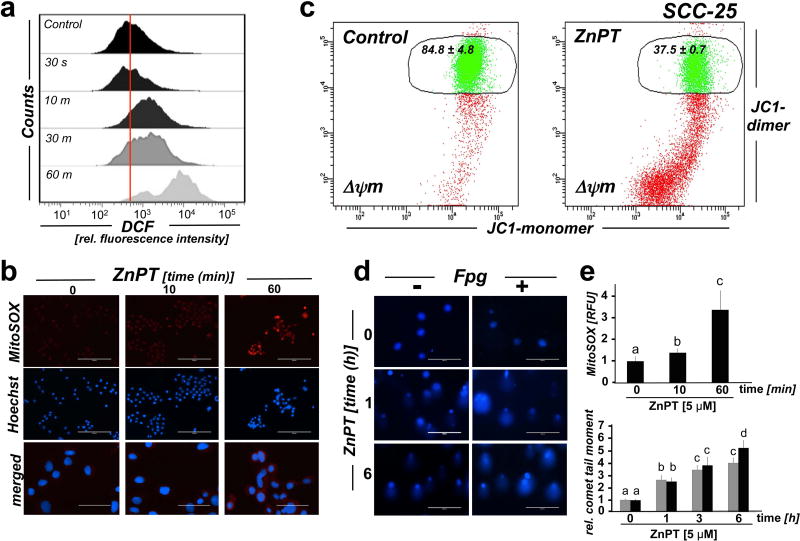
Figure 4. ZnPT-induced mitochondrial redox dysregulation and impairment of genomic integrity in malignant SCC-25 keratinocytes.
Cultured SCC-25 keratinocytes were exposed to ZnPT. (a) Time course analysis of ZnPT (5 µM) induced oxidative stress, monitored by flow cytometric detection of DCF fluorescence intensity. (b) Time course analysis of mitochondrial superoxide production in response to ZnPT (5 µM) exposure using MitoSOX Red™ fluorescence microscopy. (c) Mitochondrial transmembrane depolarization was assessed by flow cytometric analysis of JC-1 stained cells performed upon 1 h continuous exposure to ZnPT [Numbers indicate percentage of cells inside the circle displaying intact Δψm [n=3, mean ± SD (p<0.05)]. (d) Time course analysis of ZnPT-induced (10 µM) impairment of genomic integrity using the comet assay with or without additional Fpg-digest for the visualization of oxidative DNA base damage. (e) Quantitative image analysis. Upper panel: MitoSOX Red™ fluorescence intensity (from panel b); lower panel: Average comet tail moment (from panel d).
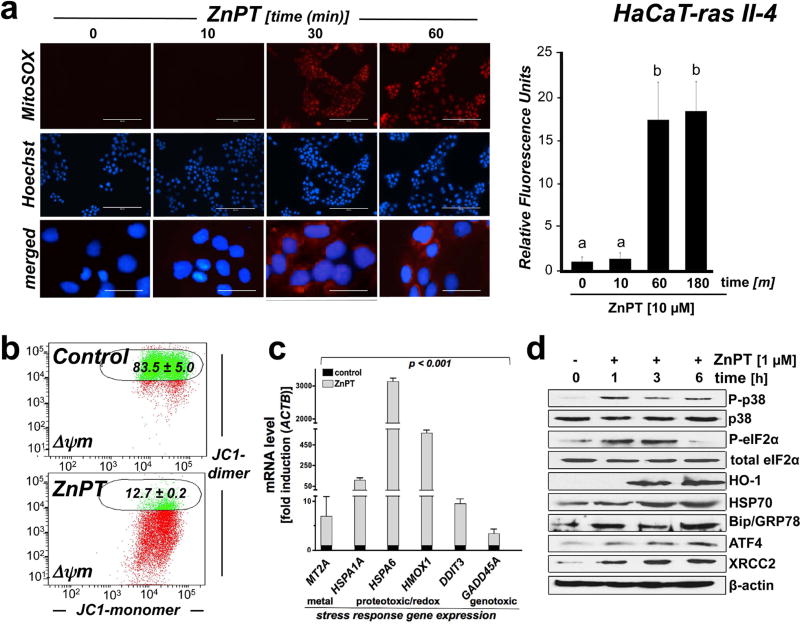
Figure 5. ZnPT-induced mitochondrial redox dysregulation and stress response target gene expression in malignant HaCaT-ras II-4 keratinocytes.
Cultured HaCaT-ras II-4 keratinocytes were exposed to ZnPT. (a) Time course of mitochondrial superoxide production in response to ZnPT (5 µM) as detected by MitoSOX Red™ fluorescence microscopy. Bar graph displays quantitative analysis of relative fluorescence intensity [scale bar: 200 µm (upper panels); 40 µm (overlay)]. (b) Mitochondrial transmembrane depolarization was assessed by JC-1 flow cytometric analysis 1 h after ZnPT (5 µM) treatment; numbers indicate percentage of cells inside the circle displaying intact Δψm [n=3, mean±SD; (p<0.05)]. (c–d) Stress response gene expression in response to ZnPT exposure (1 µM; ≤ 6 h) was determined at the mRNA (fold induction versus ACTB; n =3; mean ± SD) and protein levels using immunoblot analysis (loading control: β-actin).
Transmission electron microscopy (TEM) revealed an early impairment of plasma membrane integrity, combined with extensive vacuolization, nuclear condensation, and ER and mitochondrial disintegration detectable within 6 h exposure time (Fig. 2c). In contrast, cells undergoing isolated exposure to either zinc acetate (25 µM; 24 h) or pyrithione (5 µM; 24 h) remained largely viable, whereas pronounced loss of cell viability resulted from combined exposure to zinc acetate and pyrithione (Fig. 2d), consistent with pyrithione ionophore activity causing intracellular zinc accumulation. A moderate toxicity observed in cells treated with pyrithione only may occur due to interactions with free zinc in medium, undergoing pyrithione-facilitated intracellular accumulation, or disruption of intracellular zinc gradients, a hypothesis to be substantiated by future experiments. Co-treatment with diethylenetriaminepentaacetic acid (DTPA), a cell membrane impermeable zinc chelator, completely protected from ZnPT-induced cell death, consistent with extracellular protective scavenging of zinc ions by the chelator as observed by us before (data not shown) (30). Taken together, these observations strongly suggest that the cytotoxic effects of ZnPT observable in cultured malignant cell lines result from ionophore-dependent zinc overload.
Identification of ZnPT-induced stress response target gene expression in SCC-25 cells
To gain further mechanistic insights on molecular mechanisms underlying ZnPT-induced cytotoxicity elicited in SCC-25 cells, stress response signaling and target gene expression were examined at the mRNA and proteins levels, employing real time RT-PCR and immunoblot analysis, respectively (Fig. 3). Indeed, short term exposure to ZnPT (1 µM; 6 h) caused pronounced transcriptional upregulation of stress response target gene expression comprising (i) the metal stress response gene MT2A (metallothionein 2A; 23-fold), (ii) the heat shock response genes HSPA6 [heat shock protein family A (Hsp70) member 6; 2760-fold] and HSP1A1 [heat shock protein family A (Hsp70) member 1A; 129-fold], and (iii) the oxidative stress response gene HMOX1 (heme oxygenase 1; 94-fold; Fig 3a). Moreover, upregulation of (iv) unfolded protein response (UPR) [DDIT3 (DNA damage-inducible transcript 3; 8-fold)] and (v) genotoxic stress response gene expression [GADD45A (growth arrest and DNA damage inducible alpha; 4-fold); XRCC2 (X-ray repair cross complementing 2; 9-fold)] was observed in response to ZnPT.
Immunoblot analysis confirmed ZnPT-induced gene expression at the protein level (Fig. 3b). Strikingly, within 1 h exposure time, ZnPT stimulated activational phosphorylation (Thr180/Tyr182) of the stress-activated MAPkinase p38 with concomitant inhibitory phosphorylation of eIF2α (eukaryotic translation initiation factor). Furthermore, consistent with early induction of ZnPT-imposed proteotoxic and genotoxic stress responses, pronounced upregulation of protein expression involved in ER stress/UPR signaling (Hsp70, Bip/GRP78, HO-1, GRP78, XBP1s, ATF4) and DNA damage repair by homologous recombination (XRCC2) was detected.
ZnPT induces mitochondrial redox dysregulation and impairment of genomic integrity in malignant SCC-25 keratinocytes
Zinc is an established inducer of mitochondrial oxidative stress causing further zinc release from redox-sensitive intracellular stores including metallothioneins, thereby fueling a vicious cycle thought to underlie zinc overload-induced cellular redox dysregulation (22, 46, 47). In order to explore the occurrence of redox dysregulation downstream of ZnPT-induced zinc overload, we first employed 2’,7’-dichlorofluorescein diacetate (DCF-DA)-based flow cytometric detection of intracellular oxidative stress (Fig. 4a). Indeed, as opposed to an almost immediate occurrence of ZnPT-induced intracellular zinc overload (Fig. 1), a significant increase in DCF fluorescence intensity indicative of oxidative stress occurred only with significant delay, detectable after 10 min continuous exposure with further increase detectable over the 60 min observation time (Fig. 4a). To identify a likely source of intracellular redox dysregulation downstream of zinc overload, we employed live cell fluorescence image analysis using the mitochondria-directed superoxide probe MitoSOX Red™ (Fig. 4b and e). As observed with DCF fluorescence intensity, increased MitoSOX Red™ fluorescence intensity (indicative of mitochondrial superoxide production) was detectable within 10 min exposure time, and flow cytometric analysis of mitochondrial transmembrane potential loss using the potentiometric probe JC-1 further indicated that ZnPT exposure causes mitochondrial disturbance and superoxide production (Fig. 4c).
In addition, following our earlier studies on impairment of genomic integrity by ZnPT exposure (29), the occurrence of ZnPT-induced impairment of genomic integrity and induction of oxidative DNA damage was assessed in malignant keratinocytes using alkaline single cell gel electrophoresis performed with or without formamidopyrimidine DNA-glycosylase (Fpg)-digestion (Fpg-modified comet assay; Fig. 4d–e). Indeed, ZnPT treatment (10 µM) induced genotoxic stress in SCC-25 cells as evident from a pronounced increase in average comet tail moment (2.6-fold over untreated control) observable within 1 h exposure time. Similar results were observed in HaCaT-ras II-4 cells (data not shown). In SCC-25 cells, average comet tail moment determined after 6 h continuous exposure to ZnPT was further elevated by more than five-fold over untreated control, and Fpg-digestion resulted in an additional increase by almost 30 %, an observation suggesting that ZnPT-induced impairment of genomic integrity that may occur through molecular pathways independent of oxidative stress but may be enhanced though zinc overload-associated oxidative stress causing DNA base modification (29).
ZnPT-induced stress responses and target gene expression in malignant HaCaT-ras II-4 keratinocytes
To complement our data obtained in malignant SCC-25 keratinocytes, we also performed an analogous analysis of ZnPT-induced stress responses in malignant HaCaT-ras II-4 epidermal keratinocytes, capable of forming tumors and undergoing metastasis in SCID mice (35). After demonstrating ZnPT-induced zinc-overload and cytotoxicity in these cultured malignant cutaneous keratinocytes (Fig. 1a and Fig. 2a), we also observed the analogous occurrence of mitochondrial superoxide production (as indicated by a more than 15-fold increase in MitoSOX Red™ fluorescence intensity; Fig. 5a) and massive loss of mitochondrial transmembrane potential (Fig. 5b) as observed before with SCC-25 keratinocytes (Fig. 4b–c).
In addition, ZnPT-induced stress response signaling and target gene expression were examined at the mRNA and proteins levels, employing real time RT-PCR and immunoblot analysis, respectively (Fig. 5c–d) as performed before for SCC-25 keratinocytes (Fig. 3). In analogy to our prior data obtained using SCC-25 keratinocytes, we observed that short term exposure to ZnPT (1 µM; 6 h) caused pronounced transcriptional upregulation of stress response target gene expression comprising (i) the metal stress response gene MT2A (6-fold), (ii) the heat shock response genes HSPA6 (3126-fold) and HSP1A1 (106-fold), and (iii) the oxidative stress response gene HMOX1 (560-fold; Fig 5c). Moreover, upregulation of (iv) unfolded protein response (UPR; DDIT3; 9-fold) and (v) genotoxic stress response gene expression (GADD45A 3-fold) was observed in response to ZnPT exposure. Immunoblot analysis confirmed ZnPT-induced gene expression at the protein level (Fig. 5d) as observed before in malignant SCC-25 keratinocytes. Within 1 h exposure time, ZnPT stimulated activational phosphorylation (Thr180/Tyr182) of the stress-activated MAPkinase p38 with concomitant inhibitory phosphorylation of eIF2α. Furthermore, consistent with early induction of ZnPT-imposed proteotoxic and genotoxic stress responses, pronounced upregulation of protein expression involved in ER stress/UPR signaling (Hsp70, Bip/GRP78, HO-1, GRP78, XBP1s, ATF4) and DNA damage repair by homologous recombination (XRCC2) was detected. Taken together, these data indicate that ZnPT exposure elicits a stress response in malignant HaCaT-ras II-4 keratinocytes similar to that observed in SCC-25 keratinocytes.
Topical ZnPT blocks tumorigenic progression in UV-exposed SKH-1 high risk mouse skin
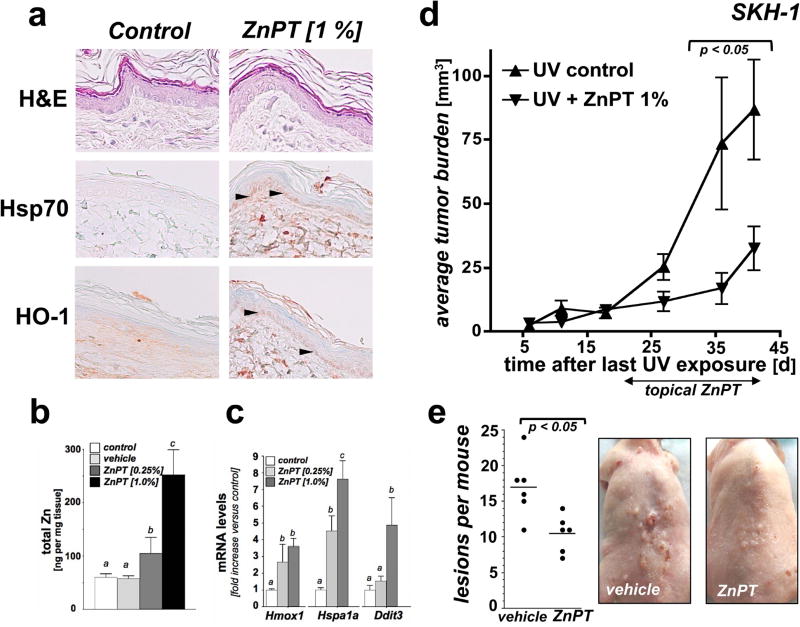
Next, we explored feasibility of using ZnPT delivered in a post-UV topical regimen for the suppression of cutaneous photocarcinogenesis in an established murine model (UV-exposed SKH-1 'high-risk' mouse skin) (Fig. 6) (41, 42). First, following our published data obtained in human reconstructed epidermis we selected topical ZnPT formulations [0.25 and 1% (w/w) in Vanicream™ carrier; 24 continuous exposure] to test feasibility of pharmacological upregulation of cutaneous zinc levels and activation of stress response pathways in murine SKH-1 skin (Fig. 6a–c). We observed that ZnPT (1%) treatment was associated with significant upregulation of stress response gene expression at the protein (HO-1, Hsp70; detectable as increased staining in the basal epidermal layer) and mRNA [Hspa1a (7.6-fold), Hmox1 (3.6-fold), Ddit3 (4.9-fold)] levels as compared to SKH-1 mouse control skin exposed to Vanicream™ alone (Fig. 6a and c). ICP-MS analysis of total skin samples confirmed a treatment-induced increase in zinc content as a function of ZnPT percentage in base formulation [almost two-fold (0.25 % formulation) and four-fold (1 % formulation; Fig. 6b) over carrier only], data that are consistent with our prior observations in ZnPT-exposed human reconstructed epidermis (30). Based on pronounced elevation of cutaneous zinc content and stress response gene expression in ZnPT-exposed versus control skin, we selected the 1% ZnPT formulation for further efficacy testing in a murine solar UV photochemoprevention experiment.
Fig. 6. ZnPT suppresses murine cutaneous photocarcinogenesis in a topical post-UV regimen.
(a) SKH-1 mice (three per group) were treated with either topical ZnPT [0.25 % or 1% (w/w in carrier); 24 h continuous exposure] or vehicle (Vanicream™; control). Paraformaldehyde-fixed, paraffin-embedded 3 µm sections were then analyzed by immunohistochemical detection of Hsp70 and HO-1 with hematoxylin counterstaining. Arrow heads: basal epidermal layer displaying ZnPT-induced Hsp70/HO-1 upregulation. (b) After treatment as specified in (a), total cutaneous zinc content was determined by ICP-MS [Zn (ng/mg tissue)]. (c) After treatment as specified in (a), cutaneous Hmox1 Hspa1a Ddit3 mRNA levels were determined by real time RT-PCR analysis (p < 0.001). (d) After implementation of a chronic UVB exposure regimen [< 190 mJ/cm2; three times per week; 18 weeks] followed by a three week gap period, tumor-prone 'high risk' mice were subjected to topical treatment [1% ZnPT in Vanicream™ versus vehicle only (control), three times per week for 3 weeks; n=6 per group]. ZnPT treatment caused a significant decrease in average tumor burden of UV-exposed papilloma-bearing SKH-1 mice (p < 0.05; assessed as total papilloma volume). (e) Reduction of lesions per mouse as a consequence of ZnPT topical treatment (p < 0.01; Mann-Whitney nonparametric statistical analysis). Representative images taken at the end of the topical treatment regimen are displayed.
Next, following a standard exposure regimen of UVB-induced tumorgenesis, SKH-1 'high risk' mice were generated by subjecting mice to an 18 week UV-exposure regimen followed by a three week gap period, after which topical treatment was initiated in these tumorigenesis-prone 'high risk' mice (42). At the beginning of week 21 (3 weeks after cessation of the chronic UV exposure regimen) 'high risk' mice received either compound in carrier (1% ZnPT in Vanicream™) or carrier only (Vanicream™; three times per week; over a 3 week period). At the end of the treatment period, total tumor burden (tumor area per mouse) and tumor multiplicity (number of lesions per mouse) were compared between treatment groups. At the end of the treatment regimen a significant reduction in average tumor burden and tumor multiplicity was observed in response to ZnPT exposure. In ZnPT-treated versus control SKH-1 'high risk' mice, average tumor burden (mm3) was suppressed by more than 60 % [86.8 ± 19.6 (untreated) versus 32.5 ± 8.6 (ZnPT); p < 0.05] and multiplicity (average number of lesions per mouse) was diminished by almost 40 % [17.0 ± 4.3 (untreated) versus 10.5 ± 2.6 (ZnPT); p < 0.05]. Taken together, these pilot data suggest efficacy of topical ZnPT to suppress UVB-induced tumorigenesis in a post-irradiation treatment regimen.
DISCUSSION
Cutaneous exposure to solar UV radiation is a key causative factor driving increased incidence rates and severity of skin cancer, and in addition to the implementation of behavioral measures that limit excessive, avoidable, or unnecessary solar UV exposure more efficacious molecular strategies for photochemoprevention are needed (2, 3, 11).
Recently, small molecule ionophores that cause cytotoxic disruption of intracellular metal homeostasis have shown promise as an emerging class of chemotherapeutics targeting a variety of malignant cell lines in vitro and in vivo (24). Our previous research has explored the cellular stress response induced in cultured primary epidermal keratinocytes and reconstructs elicited by the FDA-approved topical antimicrobial zinc ionophore ZnPT (29, 30). Based on these earlier findings demonstrating feasibility of ZnPT-induced stress response modulation in keratinocytes, we now demonstrate efficacy of ZnPT-dependent suppression of tumorigenic progression in a SKH-1 mouse model of solar UV-induced skin carcinogenesis.
First, we observed the acute occurrence of pronounced ZnPT-induced zinc overload in malignant human keratinocytes and murine skin (Fig. 1, Fig. 6), data consistent with earlier findings that have demonstrated feasibility of achieving ZnPT-induced cellular zinc overload in various cultured cancer cell lines of non-cutaneous origin employing fluorescence-, ICP-MS-, and AAS (atomic absorption spectrometry)-based analytical methodologies (26, 32, 45). We then confirmed feasibility of achieving ZnPT-induced cell death in malignant keratinocytes eliciting a pronounced metal, proteotoxic, redox, and genotoxic stress response that occurs at early time points of ZnPT exposure, observable in cultured malignant SCC-25 and HaCaT-ras II-4 keratinocytes at low nano- or micromolar concentrations, respectively (Fig. 2–Fig. 5). Importantly, ZnPT-induced cytotoxicity has been demonstrated before in various non-cutaneous cultured cancer cell lines (26, 32, 45). Earlier, we have investigated mechanisms of ZnPT-induced cell death in primary human keratinocytes and epidermal skin reconstructs, and zinc overload-induced cell death has been attributed to cell-type specific diverse pathways including apoptosis, PARP-dependent NAD-depletion, and energy crisis, and necrosis (29, 30, 32, 33, 45). Remarkably, systemic administration of ZnPT has also shown chemotherapeutic efficacy in various murine tumor xenograft models with minimal systemic off target toxicity (26, 33, 34).
Based on this body of novel experimental evidence obtained in vitro combined with the current FDA-approved use of ZnPT as a topical cutaneous therapeutic for antimicrobial dandruff-directed intervention, we then employed an established murine SKH-1 skin model, predisposed to subsequent tumorigenesis after chronic UV-exposure, in order to test feasibility of repurposing this topical therapeutic for cutaneous photochemoprevention. As expected based on our prior studies in human epidermal skin reconstructs (30), topical application of ZnPT in SKH-1 mouse skin caused cutaneous zinc overload (Fig. 6b), and oxidative and proteotoxic stress response gene expression (affecting expression of Hmox1, Hspa1a, Ddit3; Fig. 6a,c). Subsequently, it was observed that a topical ZnPT regimen initiated after termination of the implementation of a chronic tumorigenic UV-exposure regimen caused a pronounced suppression of tumorigenesis in post-UV 'high risk' SKH-1 mouse skin (Fig. 6d,e). Based on these promising prototype data, our current research efforts aim at optimizing ZnPT-dependent suppression of photocarcinogenesis comparing photochemopreventive efficacy between early (-administration during UV-exposure regimen-) and late (-administration after UV exposure is completed as performed in this pilot study-) intervention regimens. Moreover, development of an improved topical delivery system for ZnPT is currently being pursued in our laboratory since the Vanicream carrier, selected in this study based on favorable ZnPT solubility and efficient delivery of ZnPT into the living epidermis as employed by us before, has been associated with controversial concerns regarding the enhancement of tumorigenesis in mouse skin (30, 42, 48). It also remains to be seen if ZnPT-based photochemopreventive intervention as envisioned in this study (that would require an extended cutaneous delivery regimen) is associated with adverse skin reactions and toxicity since current FDA-approved OTC use of ZnPT occurs mostly in rinse-off products such as shampoos, a concern that has been raised earlier given ZnPT-induced effects on primary skin keratinocytes, reconstructed epidermis, and fibroblasts (29, 30, 45).
The role of zinc homeostasis in human carcinogenesis remains poorly understood. Dysregulation of intracellular labile zinc pools by UV-exposure has been observed in skin cell culture (46, 49), and human cutaneous zinc depletion during carcinogenic progression has been reported earlier (50, 51). Cumulative evidence indicates that expression of Zn transporters, zinc ion channels, and zinc-sequestering metallothioneins (i.e. ZnT, ZIP, MT family members) is dysregulated during tumorigenesis, playing a key role in malignant progression, relevant to various malignancies including breast, prostate, and pancreatic cancer (51–53). Interestingly, dietary zinc supplementation has shown efficacy in a murine model of chemical induced cutaneous SCC, and cumulative evidence suggests the efficacy of topical or dietary supplemental zinc administration in skin environmental stress protection, wound healing, and cancer prevention (54–57). In the context of ZnPT-based skin cancer photochemoprevention as reported here for the first time, it should also be mentioned that earlier research has reported ZnPT-induced suppression of UVB-induced murine epidermal hyperplasia, an effect that was attributed to stabilization of HIF-1α, but the obvious role of ZnPT-induced modulation of cellular zinc homeostasis was not addressed experimentally in these studies (58, 59).
Taken together, even though the specific role of zinc dysregulation in photobiological response and carcinogenesis of human skin remains to be explored by future experimentation, the pronounced suppression of UV-induced tumorigenesis, as achieved in this study by topical administration of ZnPT in SKH-1 'high risk' mouse skin after cumulative UV exposure has already occurred, suggests feasibility of repurposing this FDA-approved zinc-directed antimicrobial for topical skin cancer photochemoprevention, potentially benefiting human patient skin that already has accumulated significant skin photodamage.
Acknowledgments
Data generated by the Tissue Acquisition and Cellular/Molecular Analysis Shared Resource (TACMASR), Flow Cytometry Shared Resource Core, and Experimental Mouse Shared Resource (EMSR) were supported by the University of Arizona Cancer Center Support Grant, NIH CA023074. In addition, funding through the following NIH grants contributed to this research: R03CA167580, R03CA212719, ES007091, ES006694.
References
- 1.Wright TI, Spencer JM, Flowers FP. Chemoprevention of nonmelanoma skin cancer. J Am. Acad. Dermatol. 2006;54:933–946. doi: 10.1016/j.jaad.2005.08.062. [DOI] [PubMed] [Google Scholar]
- 2.Guy GP, Machlin SR, Jr, Ekwueme DU, Yabroff KR. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am. J. Prev. Med. 2015;48:183–187. doi: 10.1016/j.amepre.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wondrak GT. Sunscreen-Based Skin Protection Against Solar Insult: Molecular Mechanisms and Opportunities. In: D.a.H. Alberts LM, editor. Fundamentals of Cancer Prevention. Springer Science & Business Media; 2014. pp. 301–320. [Google Scholar]
- 4.Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26:1657–1667. doi: 10.1093/carcin/bgi123. [DOI] [PubMed] [Google Scholar]
- 5.Molho-Pessach V, Lotem M. Viral carcinogenesis in skin cancer. Curr. Probl. Dermatol. 2007;35:39–51. doi: 10.1159/000106409. [DOI] [PubMed] [Google Scholar]
- 6.Emmert S, Schon MP, Haenssle HA. Molecular biology of basal and squamous cell carcinomas. Adv. Exp. Med. Biol. 2014;810:234–252. doi: 10.1007/978-1-4939-0437-2_13. [DOI] [PubMed] [Google Scholar]
- 7.Prasad R, Katiyar SK. Crosstalk Among UV-Induced Inflammatory Mediators, DNA Damage and Epigenetic Regulators Facilitates Suppression of the Immune System. Photochem. Photobiol. 2016 Dec 9; doi: 10.1111/php.12687. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishisgori C. Current concept of photocarcinogenesis. Photochem. Photobiol. Sci. 2015;14:1713–1721. doi: 10.1039/c5pp00185d. [DOI] [PubMed] [Google Scholar]
- 9.Clifford JL, DiGiovanni J. The promise of natural products for blocking early events in skin carcinogenesis. Cancer Prev. Res. (Phila) 2010;3:132–135. doi: 10.1158/1940-6207.CAPR-09-0267. [DOI] [PubMed] [Google Scholar]
- 10.Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat. Rev. Cancer. 2004;4:23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- 11.Wondrak GT. Let the sun shine in: mechanisms and potential for therapeutics in skin photodamage. Curr. Opin. Investig. Drugs. 2007;8:390–400. [PubMed] [Google Scholar]
- 12.Afaq F, Adhami VM, Mukhtar H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutat. Res. 2005;571:153–173. doi: 10.1016/j.mrfmmm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Katiyar SK. UV-induced immune suppression and photocarcinogenesis: chemoprevention by dietary botanical agents. Cancer Lett. 2007;255:1–11. doi: 10.1016/j.canlet.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tao S, Park SL, de la Vega MR, Zhang DD, Wondrak GT. Systemic administration of the apocarotenoid bixin protects skin against solar UV-induced damage through activation of NRF2. Free Radic. Biol. Med. 2015;89:690–700. doi: 10.1016/j.freeradbiomed.2015.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen AC, Martin AJ, Choy B, Fernandez-Penas P, Dalziell RA, McKenzie CA, Scolyer RA, Dhillon HM, Vardy JL, Kricker A, St George G, Chinniah N, Halliday GM, Damian DL. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N. Engl. J. Med. 2015;373:1618–1626. doi: 10.1056/NEJMoa1506197. [DOI] [PubMed] [Google Scholar]
- 16.Janda J, Burkett NB, Blohm-Mangone K, Huang V, Curiel-Lewandrowski C, Alberts DS, Petricoin EF, 3rd, Calvert VS, Einspahr J, Dong Z, Bode AM, Wondrak GT, Dickinson SE. Resatorvid-based Pharmacological Antagonism of Cutaneous TLR4 Blocks UV-induced NF-kappaB and AP-1 Signaling in Keratinocytes and Mouse Skin. Photochem. Photobiol. 2016;92:816–825. doi: 10.1111/php.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyskens FL, Mukhtar H, Jr, Rock CL, Cuzick J, Kensler TW, Yang CS, Ramsey SD, Lippman SM, Alberts DS. Cancer Prevention: Obstacles, Challenges and the Road Ahead. J. Natl. Cancer Inst. 2016;108 doi: 10.1093/jnci/djv309. ppii: djv309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahsan H, Reagan-Shaw S, Eggert DM, Tan TC, Afaq F, Mukhtar H, Ahmad N. Protective effect of sanguinarine on ultraviolet B-mediated damages in SKH-1 hairless mouse skin: implications for prevention of skin cancer. Photochem. Photobiol. 2007;83:986–993. doi: 10.1111/j.1751-1097.2007.00156.x. [DOI] [PubMed] [Google Scholar]
- 19.Borovansky J. Zinc in pigmented cells and structures, interactions and possible roles. Sb Lek. 1994;95:309–320. [PubMed] [Google Scholar]
- 20.Inoue Y, Hasegawa S, Ban S, Yamada T, Date Y, Mizutani H, Nakata S, Tanaka M, Hirashima N. ZIP2 protein, a zinc transporter, is associated with keratinocyte differentiation. J. Biol. Chem. 2014;289:21451–21462. doi: 10.1074/jbc.M114.560821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharir H, Zinger A, Nevo A, Sekler I, Hershfinkel M. Zinc released from injured cells is acting via the Zn2+-sensing receptor, ZnR, to trigger signaling leading to epithelial repair. J. Biol. Chem. 2010;285:26097–26106. doi: 10.1074/jbc.M110.107490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pirev E, Calles C, Schroeder P, Sies H, Kroncke KD. Ultraviolet-A irradiation but not ultraviolet-B or infrared-A irradiation leads to a disturbed zinc homeostasis in cells. Free Radic. Biol. Med. 2008;45:86–91. doi: 10.1016/j.freeradbiomed.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Hojyo S, Fukada T. Roles of Zinc Signaling in the Immune System. J. Immunol. Res. 2016;2016:6762343. doi: 10.1155/2016/6762343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding WQ, Lind SE. Metal ionophores - an emerging class of anticancer drugs. IUBMB Life. 2009;61:1013–1018. doi: 10.1002/iub.253. [DOI] [PubMed] [Google Scholar]
- 25.Hassan HF, Mansour AM, Abo-Youssef AM, Elsadek BE, Messiha BA. Zinc oxide nanoparticles as a novel anticancer approach; in vitro and in vivo evidence. Clin. Exp. Pharmacol. Physiol. 2017;44:235–243. doi: 10.1111/1440-1681.12681. [DOI] [PubMed] [Google Scholar]
- 26.Magda D, Lecane P, Wang Z, Hu W, Thiemann P, Ma X, Dranchak PK, Wang X, Lynch V, Wei W, Csokai V, Hacia JG, Sessler JL. Synthesis and anticancer properties of water-soluble zinc ionophores. Cancer Res. 2008;68:5318–5325. doi: 10.1158/0008-5472.CAN-08-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franklin RB, Zou J, Zheng Y, Naslund MJ, Costello LC. Zinc Ionophore (Clioquinol) Inhibition of Human ZIP1-Deficient Prostate Tumor Growth in the Mouse Ectopic Xenograft Model: A Zinc Approach for the Efficacious Treatment of Prostate Cancer. Int. J. Cancer Clin. Res. 2016;3 doi: 10.23937/2378-3419/3/1/1037. pii. 037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guthery E, Seal LA, Anderson EL. Zinc pyrithione in alcohol-based products for skin antisepsis: persistence of antimicrobial effects. Am. J. Infect. Control. 2005;33:15–22. doi: 10.1016/j.ajic.2004.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamore SD, Cabello CM, Wondrak GT. The topical antimicrobial zinc pyrithione is a heat shock response inducer that causes DNA damage and PARP-dependent energy crisis in human skin cells. Cell Stress Chaperones. 2010;15:309–322. doi: 10.1007/s12192-009-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamore SD, Wondrak GT. Zinc pyrithione impairs zinc homeostasis and upregulates stress response gene expression in reconstructed human epidermis. Biometals. 2011;24:875–890. doi: 10.1007/s10534-011-9441-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz JR. Zinc Pyrithione: A Topical Antimicrobial With Complex Pharmaceutics. J. Drugs Dermatol. 2016;15:140–144. [PubMed] [Google Scholar]
- 32.Carraway RE, Dobner PR. Zinc pyrithione induces ERK- and PKC-dependent necrosis distinct from TPEN-induced apoptosis in prostate cancer cells. Biochim. Biophys. Acta. 2012;1823:544–557. doi: 10.1016/j.bbamcr.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Tailler M, Senovilla L, Lainey E, Thepot S, Metivier D, Sebert M, Baud V, Billot K, Fenaux P, Galluzzi L, Boehrer S, Kroemer G, Kepp O. Antineoplastic activity of ouabain and pyrithione zinc in acute myeloid leukemia. Oncogene. 2012;31:3536–3546. doi: 10.1038/onc.2011.521. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava G, Matta A, Fu G, Somasundaram RT, Datti A, Walfish PG, Ralhan R. Anticancer activity of pyrithione zinc in oral cancer cells identified in small molecule screens and xenograft model: Implications for oral cancer therapy. Mol. Oncol. 2015;9:1720–1735. doi: 10.1016/j.molonc.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meade-Tollin LC, Boukamp P, Fusenig NE, Bowen CP, Tsang TC, Bowden GT. Differential expression of matrix metalloproteinases in activated c-ras-Ha-transfected immortalized human keratinocytes. Br. J. Cancer. 1998;77:724–730. doi: 10.1038/bjc.1998.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cabello CM, Lamore SD, Bair WB, 3rd, Qiao S, Azimian S, Lesson JL, Wondrak GT. The redox antimalarial dihydroartemisinin targets human metastatic melanoma cells but not primary melanocytes with induction of NOXA-dependent apoptosis. Invest. New Drugs. 2012;30:1289–1301. doi: 10.1007/s10637-011-9676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiao S, Tao S, Rojo de la Vega M, Park SL, Vonderfecht AA, Jacobs SL, Zhang DD, Wondrak GT. The antimalarial amodiaquine causes autophagic-lysosomal and proliferative blockade sensitizing human melanoma cells to starvation- and chemotherapy-induced cell death. Autophagy. 2013;9:2087–2102. doi: 10.4161/auto.26506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park SL, Justiniano R, Williams JD, Cabello CM, Qiao S, Wondrak GT. The Tryptophan-Derived Endogenous Aryl Hydrocarbon Receptor Ligand 6-Formylindolo[3,2-b]Carbazole Is a Nanomolar UVA Photosensitizer in Epidermal Keratinocytes. J. Invest. Dermatol. 2015;135:1649–1658. doi: 10.1038/jid.2014.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devinney MJ, 2nd, Reynolds IJ, Dineley KE. Simultaneous detection of intracellular free calcium and zinc using fura-2FF and FluoZin-3. Cell Calcium. 2005;37:225–232. doi: 10.1016/j.ceca.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Wondrak GT, Roberts MJ, Jacobson MK, Jacobson EL. 3-hydroxypyridine chromophores are endogenous sensitizers of photooxidative stress in human skin cells. J. Biol. Chem. 2004;279:30009–30020. doi: 10.1074/jbc.M404379200. [DOI] [PubMed] [Google Scholar]
- 41.Dinkova-Kostova AT, Jenkins SN, Fahey JW, Ye L, Wehage SL, Liby KT, Stephenson KK, Wade KL, Talalay P. Protection against UV-light-induced skin carcinogenesis in SKH-1 high-risk mice by sulforaphane-containing broccoli sprout extracts. Cancer Lett. 2006;240:243–252. doi: 10.1016/j.canlet.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Lu YP, Lou YR, Xie JG, Peng Q, Shih WJ, Lin Y, Conney AH. Tumorigenic effect of some commonly used moisturizing creams when applied topically to UVB-pretreated high-risk mice. J. Invest. Dermatol. 2009;129:468–475. doi: 10.1038/jid.2008.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wulff BC, Kusewitt DF, VanBuskirk AM, Thomas-Ahner JM, Duncan FJ, Oberyszyn TM. Sirolimus reduces the incidence and progression of UVB-induced skin cancer in SKH mice even with co-administration of cyclosporine A. J. Invest. Dermatol. 2008;128:2467–2473. doi: 10.1038/jid.2008.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tao S, Rojo de la Vega M, Quijada H, Wondrak GT, Wang T, Garcia JG, Zhang DD. Bixin protects mice against ventilation-induced lung injury in an NRF2-dependent manner. Sci. Rep. 2016;6:18760. doi: 10.1038/srep18760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rudolf E, Cervinka M. Zinc pyrithione induces cellular stress signaling and apoptosis in Hep-2 cervical tumor cells: the role of mitochondria and lysosomes. Biometals. 2010;23:339–354. doi: 10.1007/s10534-010-9302-8. [DOI] [PubMed] [Google Scholar]
- 46.Stork CJ, Martorano LM, Li YV. UVB radiation induces an increase in intracellular zinc in human epidermal keratinocytes. Int. J. Mol. Med. 2010;26:463–469. doi: 10.3892/ijmm_00000486. [DOI] [PubMed] [Google Scholar]
- 47.Sensi SL, Paoletti P, Bush AI, Sekler I. Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 2009;10:780–791. doi: 10.1038/nrn2734. [DOI] [PubMed] [Google Scholar]
- 48.Staeb F, Gerber E, Kolbe L. Comment on "Tumorigenic effect of some commonly used moisturizing creams when applied topically to UVB-pretreated high-risk mice". J. Invest. Dermatol. 2009;129:515–516. doi: 10.1038/jid.2008.398. author reply 517–518. [DOI] [PubMed] [Google Scholar]
- 49.Lutter K, De Spirt S, Kock S, Kroncke KD, Martin HD, Wagener T, Stahl W. 3,3'-Dihydroxyisorenieratene prevents UV-induced formation of reactive oxygen species and the release of protein-bound zinc ions in human skin fibroblasts. Mol. Nutr. Food Res. 2010;54:285–291. doi: 10.1002/mnfr.200900044. [DOI] [PubMed] [Google Scholar]
- 50.Carruthers C, Suntzeff V. Calcium, copper, and zinc in the epidermal carcinogenesis of mouse and man. Cancer Res. 1946;6:296. [PubMed] [Google Scholar]
- 51.Pan Z, Choi S, Ouadid-Ahidouch H, Yang JM, Beattie JH, Korichneva I. Zinc transporters and dysregulated channels in cancers. Front Biosci. (Landmark Ed) 2017;22:623–643. doi: 10.2741/4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kagara N, Tanaka N, Noguchi S, Hirano T. Zinc and its transporter ZIP10 are involved in invasive behavior of breast cancer cells. Cancer Sci. 2007;98:692–697. doi: 10.1111/j.1349-7006.2007.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zamirska A, Matusiak L, Dziegiel P, Szybejko-Machaj G, Szepietowski JC. Expression of metallothioneins in cutaneous squamous cell carcinoma and actinic keratosis. Pathol. Oncol. Res. 2012;18:849–855. doi: 10.1007/s12253-012-9513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henshall SM, Afar DE, Rasiah KK, Horvath LG, Gish K, Caras I, Ramakrishnan V, Wong M, Jeffry U, Kench JG, Quinn DI, Turner JJ, Delprado W, Lee CS, Golovsky D, Brenner PC, O'Neill GF, Kooner R, Stricker PD, Grygiel JJ, Mack DH, Sutherland RL. Expression of the zinc transporter ZnT4 is decreased in the progression from early prostate disease to invasive prostate cancer. Oncogene. 2003;22:6005–6012. doi: 10.1038/sj.onc.1206797. [DOI] [PubMed] [Google Scholar]
- 55.Sharquie KE, Al-Mashhadani SA, Noaimi AA, Hasan AA. Topical zinc sulphate (25%) solution: a new therapy for actinic keratosis. J. Cutan. Aesthet. Surg. 2012;5:53–56. doi: 10.4103/0974-2077.94331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun J, Shen R, Schrock MS, Liu J, Pan X, Quimby D, Zanesi N, Druck T, Fong LY, Huebner K. Reduction in squamous cell carcinomas in mouse skin by dietary zinc supplementation. Cancer Med. 2016;5:2032–2042. doi: 10.1002/cam4.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gupta M, Mahajan VK, Mehta KS, Chauhan PS. Zinc therapy in dermatology: a review. Dermatol. Res. Pract. 2014;2014:709152. doi: 10.1155/2014/709152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho YS, Lee KH, Park JW. Pyrithione-zinc Prevents UVB-induced Epidermal Hyperplasia by Inducing HIF-1alpha. Korean J. Physiol. Pharmacol. 2010;14:91–97. doi: 10.4196/kjpp.2010.14.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim CH, Kim JH, Moon SJ, Chung KC, Hsu CY, Seo JT, Ahn YS. Pyrithione, a zinc ionophore, inhibits NF-kappaB activation. Biochem. Biophys. Res. Commun. 1999;259:505–509. doi: 10.1006/bbrc.1999.0814. [DOI] [PubMed] [Google Scholar]