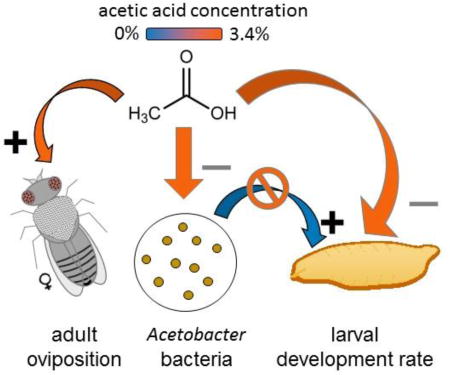
Abstract
Acetic acid is a fermentation product of many microorganisms, including some that inhabit the food and guts of Drosophila. Here, we investigated the effect of dietary acetic acid on oviposition and larval performance of Drosophila. At all concentrations tested (0.34–3.4%), acetic acid promoted egg deposition by mated females in no-choice assays; and females preferred to oviposit on diet with acetic acid relative to acetic acid-free diet. However, acetic acid depressed larval performance, particularly extending the development time of both larvae colonized with the bacterium Acetobacter pomorum and axenic (microbe-free) larvae. The larvae may incur an energetic cost associated with dissipating the high acid load on acetic acid-supplemented diets. This effect was compounded by suppressed population growth of A. pomorum on the 3.4% acetic acid diet, such that the gnotobiotic Drosophila on this diet displayed traits characteristic of axenic Drosophila, specifically reduced developmental rate and elevated lipid content. It is concluded that acetic acid is deleterious to larval Drosophila, and hypothesized that acetic acid may function as a reliable cue for females to oviposit in substrates bearing microbial communities that promote larval nutrition.
Keywords: acetic acid, acid load, Drosophila, fermentation products, gut microbiota, larval development, microbiome
Graphical Abstract
1. Introduction
Insect guts are routinely colonized by microorganisms that promote the health and fitness of the insect host (Engel and Moran, 2013; Graf, 2016). The underlying physiological processes are diverse, but many can be assigned to three broad categories: microbial contribution to insect nutrition (Douglas, 2009), protection against pathogens (Gerardo and Parker, 2014), and production of info-chemicals that modulate insect growth and behavior (Dillon et al., 2000; Shin et al., 2011; Wada-Katsumata et al., 2015). Drosophila is increasingly recognized as a valuable model to study these diverse interactions between insects and their gut microbiota (Broderick and Lemaitre, 2012; Erkosar et al., 2013). This is because Drosophila has unparalleled genetic and genomic resources and, additionally, is an amenable system for microbiome studies. In particular, axenic Drosophila (i.e. microbiologically-sterile insects) can be produced readily (Bakula, 1969; Ridley et al., 2013), and gnotobiotic Drosophila (i.e. with a standardized microbiota) can be generated readily by feeding larval or adult axenic insects with defined microbial taxa (Brummel et al., 2004; Newell et al., 2014; Shin et al., 2011). Various microorganisms contribute to the gut microbiota in Drosophila, including the Acetobacteraceae (e.g. Acetobacter and Gluconobacter species), Lactobacillales (e.g. Lactobacillus, Vagococcus, Enterococcus) and γ-proteobacteria (Enterobacter, Pasteurellaceae) (Brooks et al., 2016; Chandler et al., 2011; Corby-Harris et al., 2007; Cox and Gilmore, 2007; Staubach et al., 2013; Wong et al., 2013).
A major focus of gut microbiome research in insects and other animals is the composition of compounds produced by gut microorganisms and their impact on the physiology of the host. Of key importance are microbial fermentation products, including acetic acid and other short chain fatty acids (SCFAs). In mammals, SCFAs produced by gut microorganisms have multiple physiological effects, including functioning as a respiratory substrate, promoting the integrity of the gut barrier, and influencing feeding rate, as well as acting as epigenetic regulators of gene expression and signal transduction molecules (Kasubuchi et al., 2015; Perry et al., 2016; Rooks and Garrett, 2016; Sampson et al., 2016). SCFA production by gut microorganisms in insects is best understood in wood-feeding termites and some other insects on diets rich in cellulose and other complex polysaccharides, with abundant evidence that microbial SCFAs meet much of the insect carbon and energy requirements (Brune, 2014). However, the production of SCFAs and other fermentation products by gut microorganisms is not restricted to insects feeding on complex carbohydrates, but can also occur in species utilizing sugar-rich diets (Lievens et al., 2015). In particular, the Acetobacteraceae and various Lactobacilli, which are associated with D. melanogaster and other fruit-feeding Drosophila species, are known to release acetic acid as a waste product of fermentative metabolism (Adler et al., 2014; Oude Elferink et al., 2001; Wolfe, 2005).
To date, research on the interaction between microbial fermentation products and Drosophila has been dominated by behavioral studies on the adult fly (Mansourian and Stensmyr, 2015). In particular, acetic acid promotes upwind flight in adults (Becher et al., 2010) and oviposition in mated females (Eisses, 1997; Fluegel, 1981; Joseph et al., 2009) but direct contact with acetic acid is repulsive to adults (Ai et al., 2010; Joseph et al., 2009); these effects are specific to acetic acid, and not driven by other acids tested (Joseph et al., 2009). One possible explanation for these contradictory behavioral responses is that acetic acid is deleterious to adult Drosophila but advantageous for the larvae. This interpretation is consistent with our understanding of the natural history of Drosophila, with female ovipositional choice predicted to be coupled strongly to offspring fitness because the larva, although mobile, is limited to the substrate patch (usually a rotting fruit in nature) chosen by its mother (Reaume and Sokolowski, 2006). These considerations suggest that the larval insect may be the most appropriate life stage for studies on the physiological effects of SCFAs, especially acetic acid, on Drosophila.
Very little is known about the response of Drosophila larvae to acetic acid, beyond the finding that dietary acetic acid promotes insulin signaling and larval development in Drosophila reared on an exceptionally nutrient-poor diet (Shin et al., 2011). In this study, the effect of dietary acetic acid on Drosophila larvae reared on nutritionally-sufficient diet was determined, to illuminate the processes underlying the relationship between oviposition choice and larval performance, and, more broadly, the impact of microbial fermentation products on animal biology.
2. Materials and Methods
2.1 Insects and bacteria
The experimental insects were derived from a culture of Drosophila melanogaster strain Canton S reared at 25°C with 12:12 hour light:dark cycle on food comprising 100 g l−1 glucose (Sigma), 100 g l−1 yeast (inactive; MP Biomedicals),1.2 g l−1 agar (Apex) and preservatives (0.04% phosphoric acid and 0.42% propionic acid [Sigma]). The preservatives were omitted from the food used in experiments. For all experiments, the food was freshly-autoclaved, cooled to ca. 55° C, when acetic acid (Fisher Scientific) was aseptically added, and the food was poured into vials or oviposition chambers (see below) where it solidified at room temperature. The empirically-determined pH of freshly-prepared food was 4.4 units (acetic acid-free); 4.0 units (0.34% acetic acid); 3.7 units (1.7% acetic acid) and 3.2 units (3.4% acetic acid).
The bacterium Acetobacter pomorum DmCS_004, previously isolated from the guts of D. melanogaster (Newell et al., 2014), was used to generate gnotobiotic Drosophila. The bacterial cultures were grown at 30°C in modified MRS medium (mMRS), comprising 12.5 g l−1 vegetable peptone (Becton Dickinson), 7.5 g l−1 yeast extract, 20 g l−1 glucose, 5 g l−1 sodium acetate, 2 g l−1 dipotassium hydrogen phosphate, 2 g l−1 di-ammonium hydrogen citrate, 0.2 g l−1 magnesium sulfate.7H2O, and 0.05 g l−1 manganese sulfate.4H2O (all from Sigma unless stated otherwise). For experiments, the optical density of cultures was determined, the cells were pelleted by centrifugation and resuspended in fresh mMRS at the required final density.
Axenic Drosophila were raised from eggs that had been dechorionated by the method of Newell and Douglas (2014). Briefly, Drosophila eggs deposited overnight were surface-sterilized in three washes of 0.6% hypochlorite solution, rinsed thrice in sterile water and aseptically transferred to sterile 50 ml Falcon tubes, with 40 eggs per tube. To generate insects associated with A. pomorum, 5 × 106 bacterial cells in 50 µl mMRS was added to each tube immediately after introducing the eggs. Sterile technique was used throughout.
2.2 Oviposition assays
The oviposition assays investigated the response of adult female Drosophila to acetic acid. The oviposition cage was an open-ended Perspex cylinder (6 cm high × 5.4 cm diam) placed into the base of a 5.5 cm Petri dish containing 5 ml diet, and plugged at the top with cotton. No-choice oviposition experiments provided the insects with food containing acetic acid at a single concentration, and preference experiments offered a binary choice between food containing and lacking acetic acid. To construct the dishes for preference assays, a 2.5 cm wide segment of acetic acid-supplemented food that had solidified in a 5.5 cm Petri dish was cut aseptically with a sterile scalpel, and transferred to one side of a fresh 5.5 cm Petri dish, an equivalent segment of acetic acid-free food was added to the opposite side of the Petri dish, and the 0.5 cm wide space between the two segments was filled with molten 1.2% agar. All experiments included a choice between two acetic acid-free food segments as negative control.
Oviposition experiments were initiated at 3 hours before the onset of the dark period. Adult Drosophila (3–5 days post-eclosion) were immobilized on ice and 10 females were introduced to each oviposition cage, with 9 replicate cages per treatment. The cages were incubated at 25° C with the 12L:12D photoperiod. For no-choice experiments, the total number of eggs deposited was quantified. For preference experiments, the number of eggs on the test and control segments was determined and the oviposition index of Joseph et al. (2009) was determined: [number of eggs on test food – number of eggs on control food]/total number of eggs.
2.3 Performance and feeding of larval Drosophila
The experiments designed to investigate the impact of acetic acid on larval Drosophila comprised a 2 × 4 factorial design of Drosophila treatment (axenic or associated with A. pomorum) and food (0, 0.34%, 1.7% and 3.4% acetic acid) in 7 ml autoclaved Drosophila food in a sterile 50 ml Falcon tube, with ca. 40 eggs administered per tube. The insects were reared under the routine Drosophila culture conditions. Three experimental designs were adopted. In the first experiment, 15 replicate tubes were monitored for pupae four-times-daily in 4-hours interval after dawn, and the number of individuals that developed to pupae per tube and time to pupation were scored. For the second experiment, the pooled weight of five wandering phase larvae in each of 12 replicate tubes (3–6 samples) was determined to the nearest 0.1 mg with AB204S analytical balance (Mettler Toledo). For the final experiment, 20 feeding final-instar larvae were removed from each of 10 replicate tubes, rinsed free of diet, and then transferred to the test diet comprising 10% glucose, 10% yeast extract, 0.05% erioglaucine disodium salt dye (Blue No. 1, Sigma #861146) and acetic acid at the same concentration as used to rear the insects, solidified with 1.2% agar. After 30 min, the larvae were rinsed free of diet, transferred in groups of 5 individuals to 1.5 ml Eppendorf centrifuge tube (Five larvae each) containing lysing matrix D bulk beads (MP Biomedicals) and 150 sterile water, and homogenized in Fastprep-24 (MP Biomedials). The homogenates were centrifuged at 20,000 × g for 10 min. The absorbance of the supernatant was quantified at 628 nm, and transformed to volume of diet by reference to a calibration curve (0% – 0.005% dye).
2.4 Abundance of A. pomorum in food and flies
The design for these experiments on the effects of acetic acid on the abundance of A. pomorum comprised three replicate experiments, each comprising 16 replicate Falcon tubes containing sterile food inoculated with 5 × 106 cells of A. pomorum; ca. 40 dechorionated Drosophila eggs were introduced aseptically to 8 of the tubes. A. pomorum populations were quantified as number of colony-forming units (CFUs), firstly in the food in all 16 tubes at 7 days after the experiment was set up (6 days post-hatching of the larvae), and secondly in adult flies at 5 days post eclosion for the 8 tubes administered with Drosophila eggs. Diet cores were collected into a 1 ml pipette tip from each vial at day-6 after the larvae hatched and vortexed in 200 µl sterile PBS and 100 µl sterile lysis matrix D beads (MP Biomedicals) for 5 min, following the method of Wong et al. (2015). Adult flies from each vial were anesthetized with CO2, separated by sex to obtain two pools of five individuals for each sex; and each sample was homogenized in 200 µl of sterile PBS and 100 µl of sterile lysis matrix D beads (MP Biomedicals) in a FastPrep-24 instrument (MP Biomedicals) at default speed for 30 s. The samples were diluted with sterile PBS (to 500 µl for food samples and 1 ml for flies), plated in duplicate onto mMRS agar using a WASP-2 spiral plating instrument (Microbiology International) and incubated at 30 °C for 2–3 days. The number of colony forming units (CFUs) was determined using a Protocol 3 colony counter (Microbiology International). The CFUs obtained for duplicate plates were averaged and those values were used for statistical analyses. The negative control plates (without the food or fly inoculum) yielded no microbial growth, and all the colonies on the experimental plates had the predicted morphology (small, tan colonies) for A. pomorum (Newell et al., 2014).
2.5 Lipid content of adult flies
For the analyses of the impact of acetic acid on the lipid content of flies, adult flies at 5 days post-eclosion collected from four tubes that had been set up with ca. 40 dechorionated Drosophila eggs and 5 × 106 bacterial cells of A. pomorum in parallel with each of the three replicate experiments quantifying Acetobacter abundance in adult flies (see above). Flies from each tube were partitioned into three pools of 3–5 individuals for each sex, weighed using a Mettler-Toledo microbalance, and then flash frozen in liquid nitrogen and stored at −80 °C. For quantification of lipid, the samples were homogenized in 125 µl of TET buffer (10 mM Tris pH 8, 1 mM EDTA, 0.1% Triton X-100) and 100 µl of lysis matrix D beads in a FastPrep-24 instrument at default speed for 45 s, and centrifuged for 3 min at 21,000 g. A 50 µl sample of the supernatant was incubated at 72 °C for 30 min and the triglyceride (TAG) content was quantified using the Free Glycerol Reagent (F6428 kit, Sigma), following manufacturer’s instructions, and normalized to fresh weight.
2.6 Statistics
The datasets were analyzed using analysis of variance (ANOVA), following confirmation of normality and homogeneity of variance for either the raw data or, for bacterial CFUs, log-transformed data; and pair-wise comparisons were conducted by Tukey’s post hoc test. The data for larval development time were analyzed in R Software for Statistical Computing, version 2.15.3 using the Survival, coxme, and multcomp packages following the procedure of Newell and Douglas (2014). For the analysis of bacterial abundance and adult weight and TAG content, a mixed effect ANOVA was applied using lme4 (Bates et al., 2015) and car (Fox and Weisberg, 2011) packages, with presence of Drosophila, fly sex and acetic acid concentration as fixed effects, and each replicate experiment and tube, nested within experiment, as random effects. A post-hoc Tukey HSD test was implemented to assess all pairwise comparisons using the lsmeans package (Lenth, 2016) for Tukey’s post hoc testing in R version 3.1.2 (R core team, 2014).
3. Results
3.1 Oviposition response of adult Drosophila to acetic acid
The motivation for this study on the response of Drosophila larvae to acetic acid is the published evidence that the adult fly prefers to deposit eggs on acetic acid-supplemented substrate (see Introduction), suggesting that the larvae may be adapted to acetic acid-rich environments in nature. However, considerable intraspecific variation in ovipositional preferences has been documented in Drosophila (Miller et al., 2011). Therefore, our first experiments tested the ovipositional response to acetic acid of Canton S, the Drosophila strain used in this study.
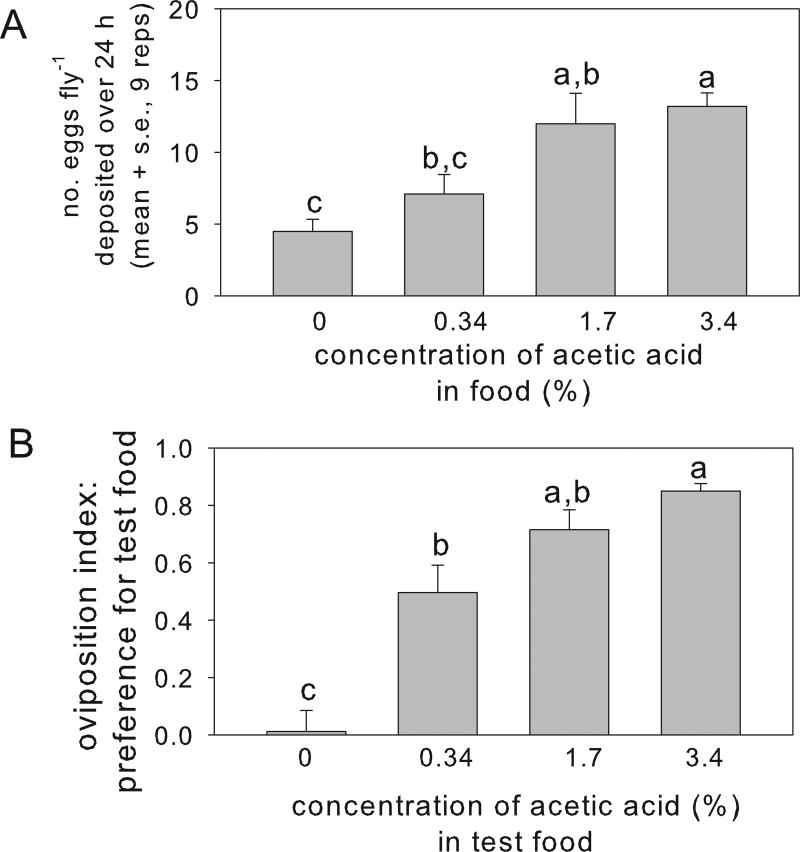
Under no-choice conditions, the number of eggs deposited by the adult females varied significantly with acetic acid content of the oviposition substrate, with three-fold more eggs deposited per fly on substrate containing 3.4% acetic acid than on the acetic acid-free substrate (Fig. 1A). The flies also displayed significant oviposition preference for substrate containing acetic acid over the concentration range tested, relative to acetic acid-free substrate (Fig. 1B). We concluded that the strain of Drosophila used in this study displays a positive ovipositional response to acetic acid, as described in published studies for other Drosophila genotypes (Eisses, 1997; Fluegel, 1981; Joseph et al., 2009).
Fig. 1.
Effect of acetic acid on egg deposition over 24 h by Drosophila. (A) Variation in oviposition with acetic acid concentration in no-choice assay. ANOVA for acetic acid concentration: F3,32 = 8.57, p<0.001). (B) Oviposition preference over 24 h between the control substrate (acetic acid-free food) and test substrate containing acetic acid at three concentrations (the acetic acid-free substrate treatment is the negative control, with mean value that does not differ significantly from zero, p>0.05). ANOVA for oviposition index, calculated as described in Methods section: F3,32 = 27.0, p<0.001). Treatments with different letters are significantly different by Tukey’s post hoc test.
3.2 Impact of acetic acid on the performance of larval Drosophila
We hypothesized that, correlated with its role as oviposition stimulus, acetic acid promotes the fitness of Drosophila larvae. The experimental test of this prediction was conducted with Drosophila maintained under microbiologically-sterile conditions (axenic Drosophila) and colonized with the bacterium A. pomorum (gnotobiotic Drosophila), because previous research suggests that the effects of acetic acid on larval traits can depend on the presence of gut bacteria (Shin et al., 2011). As for other Acetobacteraceae (Wolfe, 2005), the A. pomorum strain used in this study is a net producer of acetic acid when supplied with ethanol, but does not produce acetic acid in ethanol-free medium, as used in these experiments (Kim, unpub data).
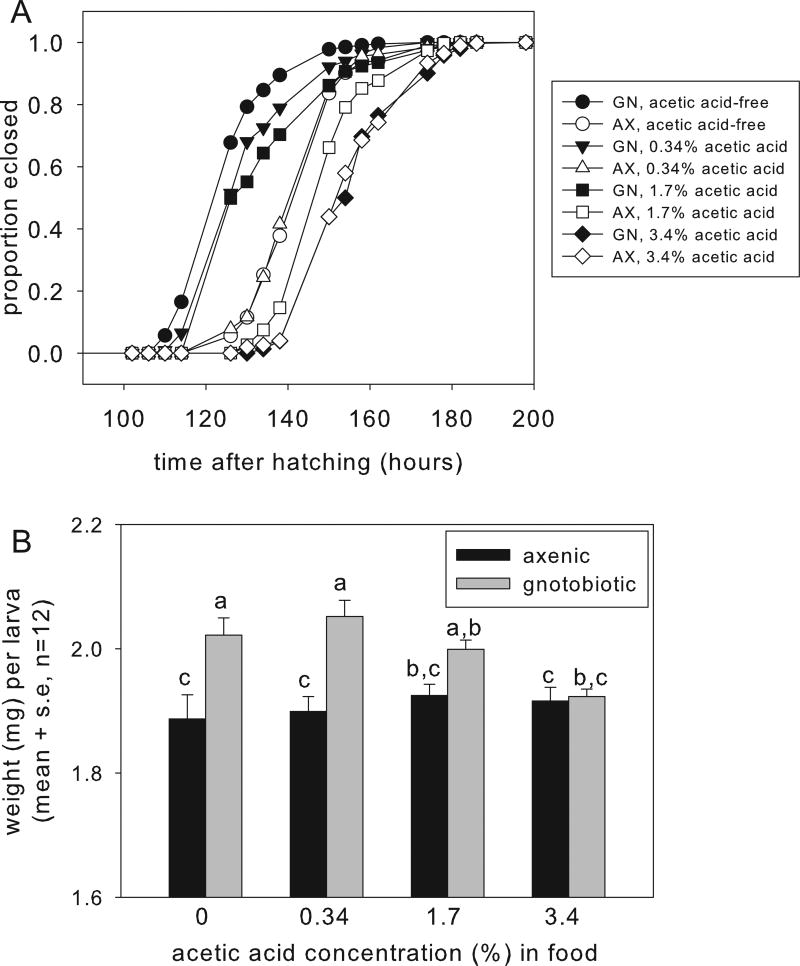
On the acetic acid-free food, the median development time to pupation for the gnotobiotic Drosophila was 126 h. Contrary to our hypothesis that acetic acid promotes larval performance, the development time was significantly extended on each diet of increasing acetic acid concentration (Fig. 2A, with statistics in Table S1). Consistent with previous reports (Newell et al., 2014; Newell and Douglas, 2014), development time of axenic Drosophila on the acetic acid-free diet was extended by 24 h, relative to the gnotobiotic Drosophila bearing A. pomorum. The development time of axenic Drosophila was also extended by dietary acetic acid, with significant effects evident at 1.7% and 3.4% dietary acetic acid (Fig. 2A and Table S1). On the diet with 3.4% acetic acid, larval development time did not differ significantly between gnotobiotic and axenic insects (Table S1).
Fig. 2.
Effect of acetic acid on the performance of gnotobiotic larvae bearing A. pomorum and axenic larvae. A. Development time to pupation with statistical analysis in Table S1. B. Weight of wandering larvae (ANOVA: acetic acid F3,88 = 1.80, p>0.05, bacteria F1,88 = 28.54, p<0.001, interaction F3,88 = 3.65, p=0.016).
As a complementary index of larval performance, we quantified the weight of wandering larvae, i.e. final instar larvae that have transitioned from feeding stage to preparation for pupation (Fig. 2B). The interaction term in the two-way ANOVA of mean weight per larva was statistically significant (see legend to Fig. 2B). Post hoc analysis revealed that the weight of axenic larvae did not vary significantly with acetic acid content of the food, and was significantly lower than the weight of larvae with A. pomorum on foods with 0–0.34% acetic acid, but not at higher acetic acid concentrations. One interpretation of these data that is consistent with the developmental rate data (Fig. 2A) is that the weight gain of gnotobiotic larvae is suppressed at high acetic acid concentrations. However, the duration of the wandering phase in the different treatments was not quantified and we cannot exclude the possibility that, if the wandering phase of gnotobiotic larvae on high acetic acid concentrations was extended, these samples may have included older larvae that were depleted in water or nutrient reserves.
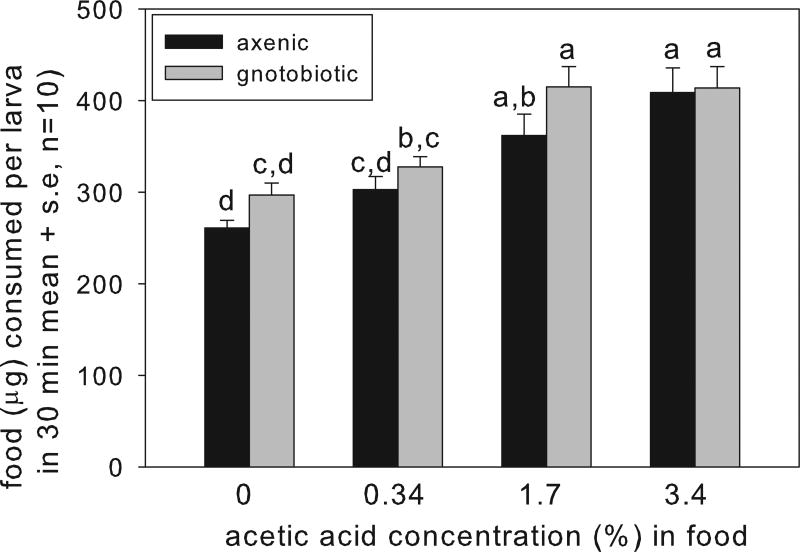
To investigate the basis for the effect of dietary acetic acid on the performance of gnotobiotic larvae, we tested two hypotheses. First, we investigated whether the larval Drosophila on diets containing acetic acid fed slowly, following evidence that Drosophila ingest less food when on diets containing compounds that require energy for detoxification (Mueller and Barter, 2015). The ANOVA revealed that larvae bearing A. pomorum consumed significantly more food than the axenic larvae (although the pair-wise comparisons were not significant on any diet), and, contrary to expectation, food consumption by both larval groups increased significantly with increasing dietary acetic acid content (Fig. 3). These data indicate that the low weight of larvae with A. pomorum on the diet with 3.4% acetic acid could not be attributed to depressed feeding on this diet.
Fig. 3.
Effect of acetic acid supplement of food on food consumption by final-instar larvae (ANOVA: acetic acid F3,72 = 21.52, p=0.01, bacteria F1,72 = 5.01, p=0.028, interaction F3,72 = 0.57, p>0.05).
Our second hypothesis focused particularly on the convergent and poor performance of gnotobiotic and axenic larvae on the diet containing 3.4% acetic acid (Fig. 2). Specifically, we predicted that, at this high concentration, acetic acid suppressed the population of A. pomorum, thereby abrogating A. pomorum-mediated stimulation of larval developmental rate and size. We also quantified key nutritional indices of adult Drosophila, building on evidence that one nutritional index, the lipid content of flies, is inversely related to the abundance of A. pomorum (Newell and Douglas, 2014).
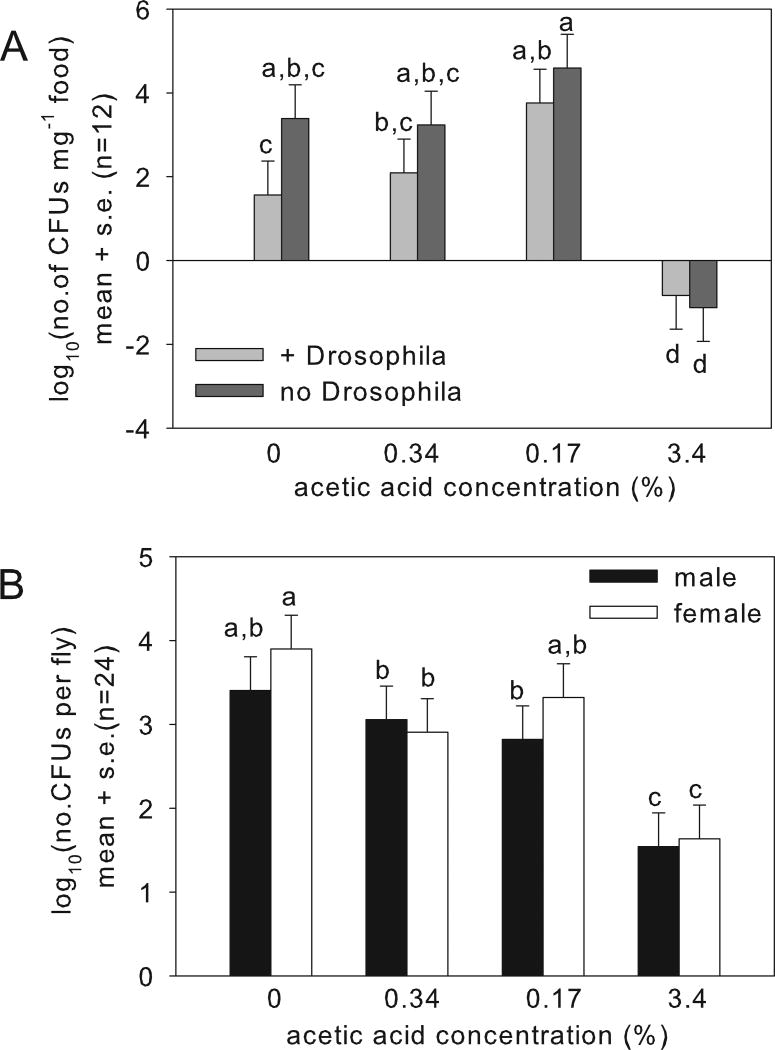
At 6 days after larval hatching, food samples yielded A. pomorum colonies, but the CFU counts varied significantly with acetic acid concentration (Fig. 4A with statistics in legend). Consistent with our prediction, the CFU density was significantly reduced in the tubes with 3.4% acetic acid, relative to lower acetic acid concentrations. A. pomorum abundance was also reduced in tubes containing Drosophila relative to insect-free vials, as reported previously (Wong et al., 2015), but the presence of the insects did not significantly affect the response of the bacteria to acetic acid.
Fig. 4.
Variation in abundance of the bacterium Acetobacter pomorum with acetic acid concentration. A. Abundance in food at day-6 after hatching of the larvae ANOVA: +/no Drosophila F1,86 = 6.20, p=0.015; acetic acid concentration F3,86 = 38.3, p<0.0001, interaction F3,86 = 1.57, p>0.05. B. Abundance in flies at day-5 post eclosion. ANOVA: sex: F1,173 = 3.886, p=0.05; acetic acid: F3,173 = 54.52, p<2e-16; interaction: F3,173 = 1.817, p>0.05.
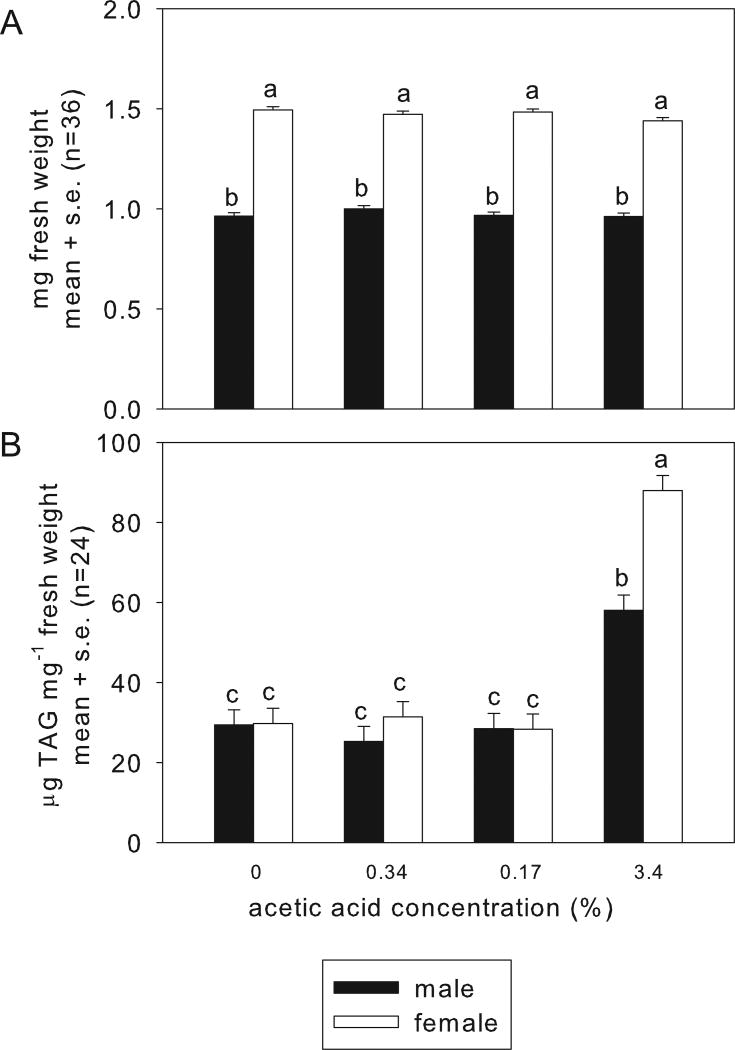
The insects in the vials were harvested as adults, at 5 days post-eclosion. Every adult fly tested bore A. pomorum. The bacterial populations recovered from flies on food without acetic acid and with 0.34% and 1.7% acetic acid comprised 103 – 105 cells; but were significantly reduced, to <100 cells in the flies on 3.4% acetic acid diet (Fig. 4B). Insect weight varied significantly with sex (in Drosophila, males are smaller than females), but not with acetic acid (Fig. 5A). These data indicate that the weight deficit of larvae reared on 3.4% acetic acid (Fig. 2B) did not persist into adulthood, and that acetic acid at the concentrations tested is not generally deleterious for the insects. However, the Drosophila reared on 3.4% acetic acid had significantly elevated lipid content (Fig. 5B), as predicted from their small A. pomorum populations. Taken together, these data indicate that the deleterious effect of high acetic acid levels on the bacterium contributes to the observed traits of the gnotobiotic Drosophila on food containing 3.4% acetic acid.
Figure 5.
Effect of dietary acetic acid on adult Drosophila. The insects were associated with Acetobacter pomorum and raised from egg to 5 days p.e. adulthood on the test diets. A. Weight per fly. ANOVA: sex: F1,264.50 = 2557, p<2e-16; acetic acid: F3,264.45 = 2.49, p>0.05; interaction: F3,264.51 = 2.12, p>0.05. B. TAG content of flies. ANOVA: sex: F1,264.11 = 3.886, p<2.2e-16; acetic acid: F3,264.10 = 54.52, p<2.2e-16; interaction: F3,264.11 = 1.817, p<2.2e-16.
4. Discussion
Adult female insects make oviposition decisions based on chemical and often visual and textural cues that are predictive of suitable conditions for egg development and, in many species, also larval growth (Gripenberg et al., 2010). Within this adaptive paradigm, the mismatch between oviposition choices and positional or feeding choices in adult female Drosophila (Joseph et al., 2009; Lihoreau et al., 2016) is interpreted most readily in terms of maternal selection of suitable sites for offspring development. With respect to the role of acetic acid as an oviposition stimulant, however, most consideration has been given to the neurobiological basis of this behavior (Gou et al., 2014; Joseph et al., 2009), and the consequences for offspring performance have not previously been investigated.
A key result of this study is that acetic acid at concentrations that promote oviposition (0.34–3.4%) does not increase offspring fitness by the criteria of larval developmental rate and weight. These indices are appropriate because they are strongly representative of fitness under natural conditions, where larvae develop in a rotting fruit which is both spatially-limited and ephemeral, and individuals that grow and develop fast are at a strong selective advantage (Nunney, 1990; Reaume and Sokolowski, 2006). Our results, therefore, raise questions about both physiological basis of larval response to acetic acid and the selective basis of the adult female ovipositional response to acetic acid. These topics are now considered in turn.
Dietary acetic acid depresses the performance of larval Drosophila, at least for the acetic acid concentrations, insect genotype and dietary conditions used in this study. The development time of Drosophila larvae is known to increase with decreasing pH (Hodge, 2001), and the demonstration in this study of progressive extension in development time with increasing dietary acetic acid concentration in both gnotobiotic and axenic insects can, most reasonably, be attributed to the metabolic burden of acid load. Insects maintain very tight controls over acid-base balance (Harrison, 2001); and pH homeostasis of both cells and extracellular fluids, especially the hemolymph and gut lumen, is energetically-demanding, being mediated predominantly by H+-V-ATPase activity, especially in the gut epithelium and Malpighian tubules (Dow, 2009; Larsen et al., 2014; Overend et al., 2016). At the highest acetic acid concentration tested, this physiological effect was compounded in gnotobiotic insects by the suppression of the bacterium Aceteobacter pomorum, which promotes larval development (Newell et al., 2014; Shin et al., 2011), such that the development time of gnotobiotic and axenic larvae was equivalent. The effect of dietary acetic acid on larval Drosophila in this study differs from Shin et al. (2011), who obtained increased larval development rate and reduced adult lipid content in acetic acid-supplemented medium in the presence of A. pomorum. At least two factors may have contributed to the difference in results: Shin et al. (2011) used a lower concentration (0.2%) of acetic acid than in this study (0.34–3.4%); and a nutrient-poor diet (described, specifically, by Yamada et al. (2015) as nitrogen-deficient), while nutritionally-sufficient diet was used in this study. The effect of the interaction between acetic acid concentrationand dietary nutrient levels on larval performance is an important topic for future study.
The acid load incurred by the Drosophila larvae that ingested acetic acid-supplemented food is compounded by the promotion of feeding rates by dietary acetic acid. The larval feeding response is likely driven by pH, which increases progressively with increasing dietary acetic acid concentration (see methods), and this effect is independent of the microbial status of the insect (the interaction term in the ANOVA is not significant, see legend to Fig. 3). These data build on previous studies focusing primarily on adult Drosophila that have demonstrated increased feeding rate of adult males with decreasing food pH in the range pH 5–9 (Deshpande et al., 2015), but suppressed adult feeding on high acetic acid concentrations, which yields pH values ≤3 units (more acidic than the media used in this study) (Charlu et al., 2013). It is also known that the Drosophila larva can detect bitterness (pH <5 units) by olfactory receptor neurons principally associated with the dorsal organ and gustatory receptors on the terminal organ. The architecture of both chemosensation and neural processing of acidity is also largely established (Gerber and Stocker, 2007; van Giesen et al., 2016).
The finding that acetic acid promotes both oviposition and larval feeding, despite its negative effect on larval fitness in the laboratory, can be interpreted in two alternative ways. The first is that the laboratory conditions adequately capture the oviposition and feeding behavior of the insects under field conditions but yield misleading results for larval fitness, i.e. that acetic acid would enhance larval fitness under more “natural” conditions. In principle, this interpretation could be tested by quantifying the interaction between dietary acetic acid concentration and diet composition, including natural diets of rotting fruit, under field conditions. An alternative interpretation is that the ovipositing female may utilize acetic acid as a cue for environmental factors that are advantageous for the larvae under natural conditions. Although definitive explanations for the selective factors shaping female oviposition decisions must await study of natural Drosophila populations, insight into the likely factors comes from consideration of the source of acetic acid. In the sugar-rich environments inhabited by Drosophila larvae, the principal source of acetic acid is the combined metabolism of yeasts and Acetobacteraceae bacteria. Specifically, many yeasts ferment sugars to ethanol, which is utilized as a respiratory substrate by Acetobacters, with acetic acid as a by-product (Wolfe, 2005). Thus, acetic acid may be a reliable cue for the presence of a mixed microbial community that confers nutritional benefits of B vitamins and protein for rapid larval development (Deshpande et al., 2015; Stamps et al., 2012; Storelli et al., 2011; Wong et al., 2014), as well as the absence of taxa, e.g. mycelial fungi, that are deleterious for Drosophila (Stensmyr et al., 2012). Acetic acid is also released from some bacteria grown in isolation, notably the hetero-fermentative lactobacilli which can produce acetic acid from sugars and other complex organic substrates. The quantitative significance of these bacteria is likely to be relatively small under most conditions. This is because these bacteria are favored in substrates containing complex carbohydrates (e.g. cornmeal diets used in some laboratories) and with neutral or basic pH (Deshpande et al., 2015), and tend to be minor members of the gut microbiota in natural D. melanogaster populations (Staubach et al., 2013; Wong et al., 2013).
These considerations address the response of Drosophila to acetic acid in the specific context of an insect adapted to a microbe-rich diet of rotting fruit. There is, however, a broader context: that animals generally display multiple physiological responses to acetic acid and other fermentation products, with far-reaching consequences for metabolism, immune system function and behavior (see Introduction). This suggests that the specific responses of Drosophila are founded, in an evolutionary sense, on highly conserved physiological circuits that play an important role in maintaining animal health and fitness. Further research on the interactions between Drosophila and microbial fermentation products, including acetic acid, can, in this way, promote our understanding of the processes by which Drosophila is adapted to its specific ecological circumstances and the fundamental mechanisms underlying health.
Supplementary Material
Table S1. Statistical analysis of Drosophila development time. Statistically significant values (p<0.0001) are shown in bold, and non-significant values are in italics.
Highlights.
Acetic acid is a fermentation product of gut bacteria in Drosophila
Acetic acid stimulates egg deposition and ovipositional preference in Drosophila
Acetic acid promotes feeding but prolongs development of larval Drosophila
High acetic acid concentrations suppress populations of gut bacterium Acetobacter
Acetic acid may be a cue for microbial communities that promote Drosophila nutrition
Acknowledgments
This project was supported by a Rawlings Presidential Research Scholarship to G.K., The Dean’s Excellence Diversity Fellowship from Cornell University to J.G.M., Ruth L. Kirschstein National Research Service Award to P.D.N. (grant F32GM099374) from the National Institute of General Medical Sciences (NIGMS), and the NIH grant R01GM095372 to A.E.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler P, Frey LJ, Berger A, Bolten CJ, Hansen CE, Wittmann C. The key to acetate: metabolic fluxes of acetic acid bacteria under cocoa pulp fermentation-simulating conditions. Appl Environ Microbiol. 2014;80:4702–4716. doi: 10.1128/AEM.01048-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai M, Min S, Grosjean Y, Leblanc C, Bell R, Benton R, Suh GS. Acid sensing by the Drosophila olfactory system. Nature. 2010;468:691–695. doi: 10.1038/nature09537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakula M. The persistence of a microbial flora during postembryogenesis of Drosophila melanogaster. J. Invert. Pathol. 1969;14:365–374. doi: 10.1016/0022-2011(69)90163-3. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67:1–48. [Google Scholar]
- Becher PG, Bengtsson M, Hansson BS, Witzgall P. Flying the fly: long-range flight behavior of Drosophila melanogaster to attractive odors. J. Chem. Ecol. 2010;36:599–607. doi: 10.1007/s10886-010-9794-2. [DOI] [PubMed] [Google Scholar]
- Broderick NA, Lemaitre B. Gut-associated microbes of Drosophila melanogaster. Gut Microbes. 2012;3:307–321. doi: 10.4161/gmic.19896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks AW, Kohl KD, Brucker RM, van Opstal EJ, Bordenstein SR. Phylosymbiosis: relationships and functional effects of microbial communities across host evolutionary history. PLoS Biol. 2016;14:e2000225. doi: 10.1371/journal.pbio.2000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummel T, Ching A, Seroude L, Simon AF, Benzer S. Drosophila lifespan enhancement by exogenous bacteria. Proc. Natl Acad. Sci. U S A. 2004;101:12974–12979. doi: 10.1073/pnas.0405207101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune A. Symbiotic digestion of lignocellulose in termite guts. Nat. Rev. Microbiol. 2014;12:168–180. doi: 10.1038/nrmicro3182. [DOI] [PubMed] [Google Scholar]
- Chandler JA, Lang JM, Bhatnagar S, Eisen JA, Kopp A. Bacterial communities of diverse Drosophila species: ecological context of a host-microbe model system. PLoS Genet. 2011;7:e1002272. doi: 10.1371/journal.pgen.1002272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlu S, Wisotsky Z, Medina A, Dahanukar A. Acid sensing by sweet and bitter taste neurons in Drosophila melanogaster. Nat. Commun. 2013;4:2042. doi: 10.1038/ncomms3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corby-Harris V, Pontaroli AC, Shimkets LJ, Bennetzen JL, Habel KE, Promislow DE. Geographical distribution and diversity of bacteria associated with natural populations of Drosophila melanogaster. Appl Environ Microbiol. 2007;73:3470–3479. doi: 10.1128/AEM.02120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CR, Gilmore MS. Native microbial colonization of Drosophila melanogaster and its use as a model of Enterococcus faecalis pathogenesis. Infect Immun. 2007;75:1565–1576. doi: 10.1128/IAI.01496-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande SA, Yamada R, Mak CM, Hunter B, Soto Obando A, Hoxha S, Ja WW. Acidic food pH increases palatability and consumption and extends Drosophila lifespan. J. Nutr. 2015;145:2789–2796. doi: 10.3945/jn.115.222380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon RJ, Vennard CT, Charnley AK. Exploitation of gut bacteria in the locust. Nature. 2000;403:851. doi: 10.1038/35002669. [DOI] [PubMed] [Google Scholar]
- Douglas AE. The microbial dimension in insect nutritional ecology. Funct. Ecol. 2009;23:38–47. [Google Scholar]
- Dow JA. Insights into the Malpighian tubule from functional genomics. J. Exp. Biol. 2009;212:435–445. doi: 10.1242/jeb.024224. [DOI] [PubMed] [Google Scholar]
- Eisses KT. The influence of 2-propanol and acetone on oviposition rate and oviposition site preference for acetic acid and ethanol of Drosophila melanogaster. Behav. Genet. 1997;27:171–180. doi: 10.1023/a:1025697627556. [DOI] [PubMed] [Google Scholar]
- Engel P, Moran NA. The gut microbiota of insects - diversity in structure and function. FEMS Microbiol. Rev. 2013;37:699–735. doi: 10.1111/1574-6976.12025. [DOI] [PubMed] [Google Scholar]
- Erkosar B, Storelli G, Defaye A, Leulier F. Host-intestinal microbiota mutualism: "learning on the fly". Cell Host Microbe. 2013;13:8–14. doi: 10.1016/j.chom.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Fluegel W. Oviposition rhythm in Drosophila melanogaster is influenced by acetic acid. J. Insect Physiol. 1981;27:705–710. [Google Scholar]
- Fox J, Weisberg S. An {R} Companion to Applied Regression. 2. Sage; Thousand Oaks, CA: 2011. [Google Scholar]
- Gerardo NM, Parker BP. Mechanisms of symbiont-conferred protection against natural enemies: an ecological and evolutionary framework. Curr. Opin. Insect Sci. 2014;4:8–14. doi: 10.1016/j.cois.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Gerber B, Stocker RF. The Drosophila larva as a model for studying chemosensation and chemosensory learning: a review. Chem. Senses. 2007;32:65–89. doi: 10.1093/chemse/bjl030. [DOI] [PubMed] [Google Scholar]
- Gou B, Liu Y, Guntur AR, Stern U, Yang CH. Mechanosensitive neurons on the internal reproductive tract contribute to egg-laying-induced acetic acid attraction in Drosophila. Cell Rep. 2014;9:522–530. doi: 10.1016/j.celrep.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J. Lessons from digestive-tract symbioses between bacteria and invertebrates. Annu. Rev. Microbiol. 2016;70:375–393. doi: 10.1146/annurev-micro-091014-104258. [DOI] [PubMed] [Google Scholar]
- Gripenberg S, Mayhew PJ, Parnell M, Roslin T. A meta-analysis of preference-performance relationships in phytophagous insects. Ecol. Lett. 2010;13:383–393. doi: 10.1111/j.1461-0248.2009.01433.x. [DOI] [PubMed] [Google Scholar]
- Harrison JF. Insect acid-base physiology. Annu. Rev. Entomol. 2001;46:221–250. doi: 10.1146/annurev.ento.46.1.221. [DOI] [PubMed] [Google Scholar]
- Hodge S. The effect of pH and water content of natural resources on the development of Drosophila melanogaster larvae. Dros. Inf. Serv. 2001;84:38–43. [Google Scholar]
- Joseph RM, Devineni AV, King IF, Heberlein U. Oviposition preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila. Proc. Natl Acad. Sci. U S A. 2009;106:11352–11357. doi: 10.1073/pnas.0901419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7:2839–2849. doi: 10.3390/nu7042839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen EH, Deaton LE, Onken H, O'Donnell M, Grosell M, Dantzler WH, Weihrauch D. Osmoregulation and excretion. Compr. Physiol. 2014;4:405–573. doi: 10.1002/cphy.c130004. [DOI] [PubMed] [Google Scholar]
- Lenth RV. Least-Squares Means: The R Package lsmeans. J. Stat. Softw. 2016;69:1–33. [Google Scholar]
- Lievens B, Hallsworth JE, Pozo MI, Belgacem ZB, Stevenson A, Willems KA, Jacquemyn H. Microbiology of sugar-rich environments: diversity, ecology and system constraints. Environ. Microbiol. 2015;17:278–298. doi: 10.1111/1462-2920.12570. [DOI] [PubMed] [Google Scholar]
- Lihoreau M, Poissonnier LA, Isabel G, Dussutour A. Drosophila females trade off good nutrition with high-quality oviposition sites when choosing foods. J. Exp. Biol. 2016;219:2514–2524. doi: 10.1242/jeb.142257. [DOI] [PubMed] [Google Scholar]
- Mansourian S, Stensmyr MC. The chemical ecology of the fly. Curr. Opin. Neurobiol. 2015;34:95–102. doi: 10.1016/j.conb.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Miller PM, Saltz JB, Cochrane VA, Marcinkowski CM, Mobin R, Turner TL. Natural variation in decision-making behavior in Drosophila melanogaster. PLoS One. 2011;6:e16436. doi: 10.1371/journal.pone.0016436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller LD, Barter TT. A model of the evolution of larval feeding rate in Drosophila driven by conflicting energy demands. Genetica. 2015;143:93–100. doi: 10.1007/s10709-015-9818-5. [DOI] [PubMed] [Google Scholar]
- Newell PD, Chaston JM, Wang Y, Winans NJ, Sannino DR, Wong AC, Dobson AJ, Kagle J, Douglas AE. In vivo function and comparative genomic analyses of the Drosophila gut microbiota identify candidate symbiosis factors. Front. Microbiol. 2014;5:576. doi: 10.3389/fmicb.2014.00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell PD, Douglas AE. Interspecies interactions determine the impact of the gut microbiota on nutrient allocation in Drosophila melanogaster. Appl. Environ. Microbiol. 2014;80:788–796. doi: 10.1128/AEM.02742-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunney L. Drosophila on oranges: colonization, competition, and coexistence. Ecology. 1990;71:1904–1915. [Google Scholar]
- Oude Elferink SJ, Krooneman J, Gottschal JC, Spoelstra SF, Faber F, Driehuis F. Anaerobic conversion of lactic acid to acetic acid and 1, 2-propanediol by Lactobacillus buchneri. Appl. Environ. Microbiol. 2001;67:125–132. doi: 10.1128/AEM.67.1.125-132.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overend G, Luo Y, Henderson L, Douglas AE, Davies SA, Dow JA. Molecular mechanism and functional significance of acid generation in the Drosophila midgut. Sci. Rep. 2016;6:27242. doi: 10.1038/srep27242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature. 2016;534:213–217. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. [Google Scholar]
- Reaume CJ, Sokolowski MB. The nature of Drosophila melanogaster. Curr. Biol. 2006;16:R623–628. doi: 10.1016/j.cub.2006.07.042. [DOI] [PubMed] [Google Scholar]
- Ridley EV, Wong AC, Douglas AE. Microbe-dependent and nonspecific effects of procedures to eliminate the resident microbiota from Drosophila melanogaster. Appl. Environ. Microbiol. 2013;79:3209–3214. doi: 10.1128/AEM.00206-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell. 2016;167:1469–1480 e1412. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SC, Kim SH, You H, Kim B, Kim AC, Lee KA, Yoon JH, Ryu JH, Lee WJ. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science. 2011;334:670–674. doi: 10.1126/science.1212782. [DOI] [PubMed] [Google Scholar]
- Stamps JA, Yang LH, Morales VM, Boundy-Mills KL. Drosophila regulate yeast density and increase yeast community similarity in a natural substrate. PLoS One. 2012;7:e42238. doi: 10.1371/journal.pone.0042238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubach F, Baines JF, Kunzel S, Bik EM, Petrov DA. Host species and environmental effects on bacterial communities associated with Drosophila in the laboratory and in the natural environment. PLoS One. 2013;8:e70749. doi: 10.1371/journal.pone.0070749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensmyr MC, Dweck HK, Farhan A, Ibba I, Strutz A, Mukunda L, Linz J, Grabe V, Steck K, Lavista-Llanos S, et al. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell. 2012;151:1345–1357. doi: 10.1016/j.cell.2012.09.046. [DOI] [PubMed] [Google Scholar]
- Storelli G, Defaye A, Erkosar B, Hols P, Royet J, Leulier F. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab. 2011;14:403–414. doi: 10.1016/j.cmet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- van Giesen L, Hernandez-Nunez L, Delasoie-Baranek S, Colombo M, Renaud P, Bruggmann R, Benton R, Samuel AD, Sprecher SG. Multimodal stimulus coding by a gustatory sensory neuron in Drosophila larvae. Nat. Commun. 2016;7:10687. doi: 10.1038/ncomms10687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada-Katsumata A, Zurek L, Nalyanya G, Roelofs WL, Zhang A, Schal C. Gut bacteria mediate aggregation in the German cockroach. Proc. Natl Acad. Sci. U S A. 2015;112:15678–15683. doi: 10.1073/pnas.1504031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe AJ. The acetate switch. Microbiol. Mol. Biol. Rev. 2005;69:12–50. doi: 10.1128/MMBR.69.1.12-50.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AC, Chaston JM, Douglas AE. The inconstant gut microbiota of Drosophila species revealed by 16S rRNA gene analysis. ISME J. 2013;7:1922–1932. doi: 10.1038/ismej.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AC, Dobson AJ, Douglas AE. Gut microbiota dictates the metabolic response of Drosophila to diet. J. Exp. Biol. 2014;217:1894–1901. doi: 10.1242/jeb.101725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AC, Luo Y, Jing X, Franzenburg S, Bost A, Douglas AE. The host as the driver of the microbiota in the gut and external environment of Drosophila melanogaster. Appl. Environ. Microbiol. 2015;81:6232–6240. doi: 10.1128/AEM.01442-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Statistical analysis of Drosophila development time. Statistically significant values (p<0.0001) are shown in bold, and non-significant values are in italics.