Abstract
Purpose of Review
The purpose of this study is to provide an update to the orthopedic field in regard to treatment of the Hill-Sachs lesion and anterior shoulder instability. The review highlights the most current knowledge of epidemiology, clinical evaluation, and surgical methods used to treat Hill-Sachs lesions. It also details the relevant clinical and surgical findings that have been made throughout the literature in the past couple of years.
Recent Findings
The most recent literature covering the Hill-Sachs lesion has focused on the relatively new and unexplored topic of the importance of concomitant injuries while treating a humeral head defect. The glenoid track concept has been clinically validated as a method to predict engagement. 3D-CT has become the “gold standard” for Hill-Sachs imaging; however, it has been noted that 3D-MRI produces results that are not significantly different from CT. Also, it has been found that when the arm is in a position of abduction during the primary injury, there is a higher risk of engagement and subsequent dislocation. Recent studies have demonstrated successful results stemming from purely arthroscopic procedures in treating Hill-Sachs lesions.
Summary
Anterior shoulder instability, specifically the Hill-Sachs lesion, is an area of orthopedic study that is highly active and constantly producing new studies in an attempt of gaining the best outcomes for patients. The past few years have yielded many excellent discoveries, but there is still much more work to be done in order to fully understand the role of the Hill-Sachs lesion in anterior shoulder instability.
Keywords: Hill-Sachs lesion, Anterior shoulder instability, Glenoid track, 3D CT scan, Remplissage procedure, Hill-Sachs reduction procedure
Introduction
A Hill-Sachs lesion is a bony defect of the humeral head that is often linked with recurrent anterior shoulder instability. In fact, it has been proven that the Hill-Sachs lesion is quite common and is demonstrated in 67–93% of anterior dislocations and can reach an incidence rate of 100% in patients with recurrent anterior shoulder instability [1–4]. Hill-Sachs lesion typically occurs with an anteroinferior glenohumeral dislocation event. The dislocation event, as described by Provencher et al. [5], pushes the humeral head anteriorly into contact with the dense anterior glenoid causing a compression fracture along the postsuperolateral aspect of the humeral head. Recurrent dislocations become problematic because the anatomical glenohumeral constraints become increasingly worn down, leading to chronic instability [5]. The lesions vary in depth, width, and orientation, and each different presentation has to be treated in a unique manner. The most common method of determining the Hill-Sachs lesion is the Calandra classification (Tables 1 and 2), which uses arthroscopy to measure the depth of the lesion [6, 7]. Other methods are used in classification, such as radiography described by Rowe et al. [1] and magnetic resonance imaging articulated by Richards et al. [8]
Table 1.
The Calandra classification
| Grade | Description |
|---|---|
| I | Defect in articular surface that does not affect subchondral bone |
| II | Defect includes subchondral bone |
| III | Large defect in the subchondral bone |
Table 2.
Hill-Sachs treatment algorithm
| Lesion involves what % of humeral head articular surface. Informed by XR, CT, MRI, and arthroscopy | ||
|---|---|---|
| < 20% | 20 to 40% | > 40% |
| Rarely of clinical significance | Difficult zone where individual patient factors must be considered | Nearly always clinically significant |
| Non-operative treatment typically effective if symptomatic | Factors to consider: location and orientation of the lesion, glenoid bone loss, engagement, patient demand on joint, patient activity level, and age. | Responsible for recurrent instability |
| Lower end of spectrum: remplissage, reduction | Osteochondral allograft, partial arthroplasty | |
| Higher end: osteochondral allograft, partial arthroplasty | Severe: hemiarthroplasty | |
The Hill-Sachs lesion should not be treated as an isolated injury because other injuries, such as a concomitant glenoid osseous defect or anteriocapsulolabral tear make the situation more complicated [9•]. Widjaja et al. [2] demonstrated this connection in pathology by finding that Hill-Sachs bone loss occurs in conjunction with glenoid bone defect in 62% of patients experiencing anterior shoulder instability. Previously, the humeral head, labrum, and glenoid were often treated and examined independently. It was common methodology to do a classic arthroscopic capsuloligamentous repair while neglecting the bone defect, but the high risk of recurrence proved this approach to be insufficient [10]. Now, with the affirmation of the glenoid track concept by Shaha et al. [9•] and its association with the concept of “engaging” and “non-engaging” lesions presented by Burkhart and de Beer [11•], the understanding of the glenohumeral interaction is rising rapidly. However, despite the increase in understanding, there is still conflict and variation in diagnoses. Kurokawa et al. [12] critiques this common understanding by claiming there needs to be a more precise method for quantifying the severity or depth of a Hill-Sachs lesion. He reported that the prevalence of engaging lesions has been stated to range from 1.5 to 34%, which shows the lack of consistency in grading and evaluation.
Epidemiology
Over 90% of the dislocation events are anteriorly displaced, and a Hill-Sachs lesion can be found in up to 93%. Despite the high incidence of Hill-Sachs lesions, it remains challenging to definitively determine which lesions are causing clinical symptoms and which are incidental. The challenge stems from the fact that there is little consensus on the size of defect that may lead to recurrent instability, in addition to the joint position or movement that could cause an engagement event [4, 13, 14].
The risk factors associated with the Hill-Sachs lesion are directly linked to the likelihood of another anterior dislocation event. In a fairly recent study, Horst et al. [15] found that young age and hyperlaxity of the ligaments surrounding the glenohumeral joint lead to a predisposition for recurrence of dislocation. It has been noted that the problem of hyperlaxity is especially heightened in young patients due to the more limited number of treatment options for a young patients [16]. Horst and his colleagues also found that a larger Hill-Sachs lesion leads to greater risk of recurrent dislocation because of the decreased available contact surface with the glenoid [15]. Neglecting to treat humeral head bone loss is liable to lead to an increased prevalence of anterior shoulder instability.
Clinical Evaluation
History and Physical
The first subjective measure taken with a patient experiencing recurrent shoulder instability should be to have the patient characterize the degree of discomfort, the frequency of dislocation, the presence of neurological problems, and the prior treatments experienced. Lynch et al. [17] describes the importance of inquiring about factors that could increase the risk of recurrence, such as seizures, propensity to fall, or participation in activities that require abduction and external rotation. It is also valuable to make an effort to have the patient describe the positioning of their arm during the traumatic event in order to gain insight into the nature of the injury.
When physically evaluating the shoulder, an important test is the load and shift test (Fig. 1), because it tests the adequacy of the glenoid rim and humeral head by shifting the shoulder anteriorly [17]. If a grinding situation arises, it is due to a Hill-Sachs lesion or a glenoid defect causing rough contact on the articular surface. Another provocative test that proves useful in examining the shoulder is the apprehension test, which focuses on the level of instability in the glenohumeral joint [5]. During the entire physical examination, it is necessary to make continuous comparisons with the contralateral shoulder to determine the severity of the injury.
Fig. 1.
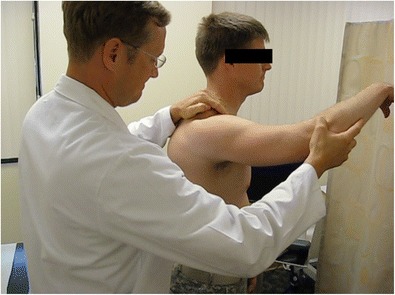
Load and shift test to assess the adequacy of the glenoid by shifting the shoulder anteriorly
Imaging
Multiple different imaging modalities exist to diagnose an osseous humeral defect. These include radiography, computerized tomography (CT), and magnetic resonance imaging (MRI). When using radiography, it is necessary to use the three basic views (AP, axillary, and lateral) and special views (Stryker notch view) to measure Hill-Sachs bone loss [18]. Saliken et al. [19] found that using a Stryker notch view with the arm in internal rotation was reliable and clinically relevant in determining Hill-Sachs defect depth and orientation (Fig. 2). These specialized radiographic views are more accurate than standard radiograph images, but they can be challenging to reproduce due to patient limitations or discomfort [20]. Even with specialized forms of radiographs, it still stands that CT and MRI are superior forms of imaging for a Hill-Sachs defect [21]. This is largely because up to 60% of bony defects can be missed based off looking at radiographs alone [22].
Fig. 2.
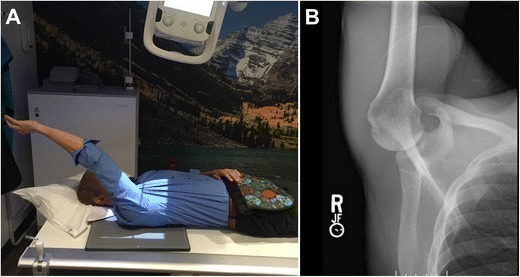
a Patient position for the Stryker notch view. b Radiograph of Stryker notch view of a normal humeral head
CT imaging with 3D osseous reconstruction technology has become the gold standard when determining the severity of Hill-Sachs lesions (Fig. 3a) [21, 23, 24]. Gyftopoulos et al. [25] report that the 3D image allows for an improved conceptualization of the osseous anatomy of the humeral head and therefore is utilized by many orthopedic surgeons. The improved conceptualization leads to 3D-CT being more accurate than 2D imaging (Fig. 3b), and it allows for the most consistent and reproducible measurements of humeral bony defects [26, 27]. When assessing the soft tissue anatomy of patients with glenohumeral instability, MRI has become the superior method [28].
Fig. 3.
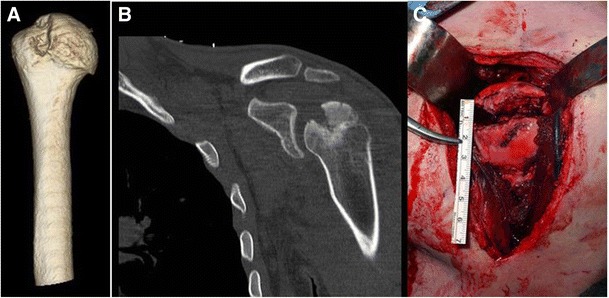
a 3D-CT of Hill-Sachs lesion, the gold standard diagnostic imaging tool currently. b 2D CT scan in the coronal plane demonstrating the Hill-Sachs lesion. c Intraoperative view of a large reverse Hill-Sachs lesion (posterior)
There have been previous studies showing that MRI is capable of quantifying bone loss, but there has been no proof that it should be preferred over 3D-CT [29, 30]. In fact, it has been noted that even with a full array of MRI slices and views, the images are likely to be a poor predictor of bone loss [30]. However, 3D-MR has recently shown potential to be a successful method of assessing glenoid and humeral head bone loss [28]. Stillwater et al. [31•], seeing that there was no up to date study comparing the accuracy of 3D-CT and 3D-MR in patients suffering from glenohumeral instability, developed a study comparing the two imaging techniques in actual patients with glenohumeral instability. The results showed that 3D-CT osseous reconstructions were equivalent to 3D-MR osseous reconstructions, and the measurement differences were found to be statistically insignificant. This study has important implications because it would be beneficial to use 3D-MR in place of CT due to its ability to image without radiation; additionally, patients would only need to get one scan with the MRI instead of obtaining both MRI and CT for preoperative evaluation.
Ultrasonography is another technique that has shown to be useful in quantifying Hill-Sachs lesions [5]. Cicak et al. [32] conducted an intraoperative study and found ultrasonography to be 100% specific and 96% sensitive to the quantification of Hill-Sachs lesions. They also determined the accuracy of their findings to have an overall rate of 97%.
Despite the significant progress that has been made since the early 2000s in our imaging knowledge, there is still a need for further investigation to determine the best imaging modality for quantifying Hill-Sachs lesions [19].
Grading of Hill-Sachs
Defining a Hill-Sachs lesion is a multi-dimensional process because of the requirement for the examination of multiple different factors, such as size and orientation. The typical definition of Hill-Sachs size, although not universally accepted, is a lesion that covers greater than 25% of the humeral head articular surface [33, 34]. Bony defects covering under 25% of the humeral head surface are typically insignificant in an isolated situation. However, depending on concomitant glenoid bone loss and the degree of engagement with the glenoid, even a small Hill-Sachs lesion can become clinically significant [5].
The term for glenoid bone loss in conjunction with a Hill-Sachs lesion is bipolar bone loss, and it is a pathology that must not be overlooked [5]. Ramhamadany and Modi noted that an isolated Hill-Sachs lesion increases the chances of a bipolar defect by a factor of 2.5 to 11 [35]. Arciero et al. [36] addressed the problem of bipolar bone loss in a study by taking a clinically insignificant Hill-Sachs lesion (1.47 cm3) paired with glenoid bone defects ranging from 2 to 6 mm. They found when the clinically insignificant Hill-Sachs lesion was paired with the 2-mm glenoid defect, the result was a 25% reduction of stability. When paired with the 6-mm glenoid defect, they found there to be a 50% reduction of stability. The recent identification of the bipolar phenomenon rendering previously insignificant Hill-Sachs lesion to be significant has become a vital aspect of the classification process.
The Hill-Sachs lesion can also be defined as significant if it is oriented in a position that forces it to engage with the anterior glenoid with the arm position associated with athletic function (i.e., 90° of abduction combined with approximately 90° of external rotation) [5, 10, 37]. Burkhart and de Beer first described the concept of engaging vs. non-engaging Hill-Sachs lesions [11•]. They defined the orientation of an engaging Hill-Sachs lesion to be parallel to the glenoid so the lesion will make contact with the corner of the glenoid. They discovered that a non-engaging lesion passes diagonally across the anterior glenoid, which allows it to make continuous contact with the articular surface, leading to avoidance of the anterior glenoid. It has been noted several times throughout the literature that engagement of a Hill-Sachs lesion is a superior predictor of recurrent stability or failure of arthroscopic surgery compared to predicting outcomes based off size alone [12, 38].
The position of the arm at the initial dislocation event and angle of the Hill-Sachs lesion is another predictor engagement and instability. In one recent study, Di Giacomo et al. [39•] evaluated the correlation between positioning of the arm, angle of Hill-Sachs, and extent of engagement. They found that patients that experienced the dislocation event with their arm in abduction had significantly higher Hill-Sachs angles (32.4° ± 4.7°) than when the arm was in adduction (16.1° ± 2.9°) (Fig. 4a). The higher angle caused from the arm being in abduction resulted in a more slanted lesion with respect to the longitudinal axis of the humerus, which led to a higher degree of engagement and subsequent shoulder instability (Fig. 4b). The lower angle caused from the arm being in adduction resulted in the lesion passing diagonally in a non-parallel fashion across the anterior glenoid, which led to a lower extent of engagement and shoulder instability.
Fig. 4.
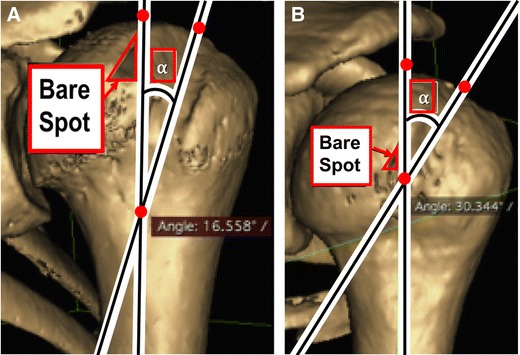
3D computed tomography reconstructions of a left shoulder showing the Hill-Sachs angle, which is the angle between the longitudinal axis of the humeral shaft and the long axis of the Hill-Sachs lesion. a The H-S angle measured 16.5° in a patient who sustained a dislocation with the arm in adduction (ADD) at the time of injury. b The Hill-Sachs angle measured 30.3° in a patient who sustained a dislocation with the arm in abduction (ABD) at the time of injury
Glenoid Track Concept
Engagement directly correlates to the glenoid track concept, which was first described by Yamamoto et al. [40]. The glenoid track concept refers to the area of the glenoid that is covered as the arm shifts during a movement of abduction and external rotation from the inferomedial to the superolateral portion of the humeral head [10]. The area of the glenoid covered during this lateral shift of movement has been noted to be around 83% [5, 10, 12]. If the lesion is said to be off-track, it was thought to be engaging, while an on-track lesion is predicted to be non-engaging. Gyftopoulos et al. [38] set out to find if the on-track off-track method was a valid predictor of engagement. With the help of MRI technology, they found the on-track off-track method to predict engagement accurately in 84.2% of cases, and they found it to have a negative predictive value of 91.1%. The authors concluded with the Fisher test result of p < 0.0001 that the glenoid track concept was a valid test of Hill-Sachs engagement. In a different study, Metzger and his colleagues [41] found that only 15 out of 121 (12.4%) of patients with Hill-Sachs lesions inside the glenoid track experienced engagement. On the other hand, 16 out of the 19 (84.5%) patients with lesions outside of the glenoid track experienced engagement and recurrent shoulder instability.
Shaha et al. [9•] evaluated the glenoid track concept much like Gyftopoulos et al., but they were trying to study the relationship between the glenoid track and arthroscopic Bankart repairs. The results of the study showed them 6 out of the 8 off-track patients (75%) experienced recurrence, while 4 out of the 49 on-track patients (8%) had recurrent dislocations. The negative predictive value was found to be 92%; that is, when the shoulder was on-track, arthroscopic repair rarely failed. In a sub-analysis of patients with non-engaging on-track defects, they determined postoperatively that 89% of the patients had stable shoulders. This study was vital to our preoperative predictive measures because previously there was only the algorithm defined by Giacomo et al. [10]: Glenoid track = 0.83 D − d, where D is the diameter of the inferior glenoid and d is the bone loss. Now, there are valid clinical findings showing the glenoid track can help accurately predict preoperative decisions in regards to preventing recurrent shoulder instability after arthroscopic repair in patients with Hill-Sachs defects.
Despite the recent studies providing data supporting the usage of the glenoid track for treatment of glenohumeral instability, there is still debate to its validity. Schneider et al. [24] conducted a study using 3D CT scan images to measure the variability in glenoid track measurements. In a case study evaluating interobserver reliability, they showed that the observers struggled to come to a consensus as to if the Hill-Sachs defect was on- or off-track, with only a 72% rate of interobserver reliability. They determined the degree of variability in humeral head assessment in relation to the glenoid was too high for the glenoid track measurement to be reliable. Due to the poor interobserver reliability, the authors recommend the avoidance of the glenoid track in preoperative assessment measures.
The clinical relevance of the glenoid track to evaluating shoulder instability is significant, and the progress over recent years has been exciting. However, further investigation is needed to make it the consensus classification method.
Non-surgical Management
Non-surgical management of a Hill-Sachs lesion is warranted when the osseous defect is small (< 20%) or non-engaging with the glenoid. It is also more likely for an anterior dislocation event to call for non-surgical treatment if it is a first time occurrence [42]. Even if a primary dislocation event causes a bony defect in the humeral head, it is common practice for a surgeon to neglect the bony defect and repair other relevant pathology, such as a Bankart lesion repair [5]. However, it is essential to address the Hill-Sachs defect in the setting of an engaging lesion as multiple studies have come out demonstrating the increased recurrence rates of shoulder instability after arthroscopic repair when the Hill-Sachs lesion is not addressed at the time of surgery [6, 12, 17, 34]. Shibayama and Iwaso found that an engaging Hill-Sachs lesion is highly susceptible to recurrence if treated with a typical arthroscopic capsuloligamentous repair with no attention to the osseous defect [6]. Boileau et al. [43] also states that it is not surprising that an untreated Hill-Sachs lesion leads to postoperative recurrent instability because the articular arc deficit still exists and will cause engagement with the anterior glenoid rim, thus resulting in failure of the repair over time.
For patients that are poor surgical candidates (i.e., older age, lower demand, or increased medical issue) with clinically significant lesions, non-surgical treatment is the preferred method to management of the shoulder instability [17]. In non-surgical scenarios, it is vital that the patient is on a rehabilitation program under the supervision of a trained physical therapist. Provencher et al. [5] recommend for therapy to focus on deltoid strength, rotator cuff muscles, and most importantly the scapular stabilizers. It is also common for surgeons to place patients, especially in-season athletes, in a sling or immobilization device in order to promote healing during the acute injury period without surgery.
Surgical Management
Surgical management of a Hill-Sachs lesion is determined based off clinical evaluation and symptoms of instability. The majority of these defects requiring surgery is due to their size (Fig. 5c) and/or engagement, or due to the concomitant injuries that often arise with a Hill-Sachs lesion. There are several different treatment options to address anterior shoulder instability, both open procedures and arthroscopic techniques [10, 17, 44]. In the late 1990s and early 2000s, it was believed that open surgical techniques were superior to arthroscopic techniques in treating the glenohumeral joint [11, 45]. Now, it has been shown in the literature that both open and arthroscopic surgeries are viable options with similar outcomes and failure rates [34]. Harris et al. [46] gathered the outcome results from 26 Bankart repair studies and found no statistical difference between open and arthroscopic approaches.
Fig. 5.
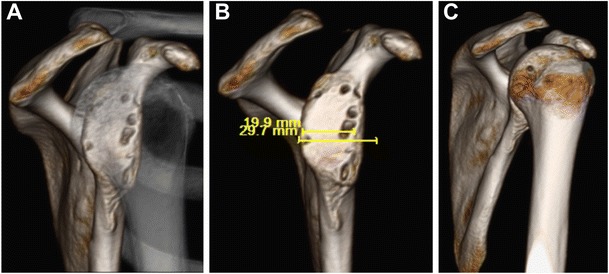
Bipolar bone loss. a En face view of glenoid bone loss through translucent humeral head. b En face view of glenoid with humerus digitally subtracted. c Humeral head Hill-Sachs lesion with glenoid digitally subtracted
It is critical while assessing the surgical merit of a Hill-Sachs lesion to consider the potential bipolar nature of the injury and concomitant injuries (Fig. 5). It is common for a surgeon to treat the primary instability event, whether it be glenoid bone loss or a Bankart lesion. Glenoid bone loss of more than 20% would most likely be addressed with a coracoid transfer (Latarjet procedure) or an iliac crest bone graft or allograft, while an isolated Bankart lesion would be fixed with an arthroscopic Bankart repair [47, 48]. The mindset behind solely addressing the glenoid lesion (soft tissue or bony) is that with the increased articular surface and/or repaired labrum to the rim will increase the glenohumeral stability preventing the Hill-Sachs lesion from causing recurrent instability. Likewise, surgeons will often only fix the Bankart lesion believing that it alone will keep the shoulder stable. It is common practice for a Bankart repair or Latarjet to prevent recurrence, but there are also cases where the humeral head defect is large or engaging and must be addressed to prevent further instability [17, 43, 49]. Rowe et al. [1] first reported in their series that 76% of the failures after anterior shoulder instability surgery had the presence of a Hill-Sachs lesion.
Remplissage
The remplissage technique has become a popular method in managing Hill-Sachs lesions. The term remplissage, originally described by Purchase et al. [50], involves an arthroscopic posterior capsulodesis and infraspinatus tenodesis, with the fixation of the soft tissue to the Hill-Sachs lesion [5]. The operation was coined remplissage because it is a French term for “filling,” and the procedure is the filling of the Hill-Sachs defect with capsule and infraspinatus tendon. The success of the remplissage procedure is a result of its ability to convert the Hill-Sachs lesions from an intraarticular defect to an extraarticular defect, thus lowering the predisposition or humeral head excursion for engagement with the anterior glenoid rim and recurrent subluxations. Koo et al. revised the procedure with the “double pulley” technique, which allows for the sutures to be tied over the infraspinatus tendon instead of through it [51]. This technique has proven to be successful, but in 2016, Alexander et al. [52] further modified the technique. Their technique still utilizes the double pulley technique, but they conducted a percutaneous placement of the two suture anchors in the single skin-and-deltoid incision. The major advantage of this new method is it avoids the need to go into the subacromial space to retrieve or tie sutures.
The remplissage technique is utilized in patients with engaging Hill-Sachs lesions in conjunction with mild glenoid bone loss, and it is most often used concurrently with an arthroscopic or open Bankart repair (Fig. 6) [10]. Longo et al. [53] found that remplissage exhibits the lowest recurrence rates and greatest outcome scores. The range of recurrence rates for a remplissage and concurrent Bankart repair, as documented by many authors, ranges from 0 to 8% [54–56]. Park et al. [57] reported 85% satisfaction rate in patients that had combined arthroscopic Bankart repair and remplissage for a large engaging Hill-Sachs lesion. Many unique methods of performing a remplissage exist, but the common advantage in all of them is the avoidance of the risk factors associated with bone grafts. A disadvantage associated with remplissage is that it usually alters the rotator cuff muscles, thus altering the shoulder anatomy [58]. This can also lead to reduced external rotation or posterior superior shoulder pain, but this topic is still controversial [59].
Fig. 6.
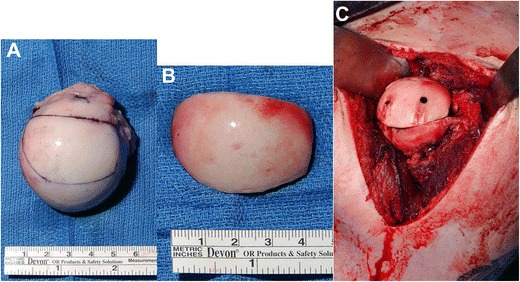
Humeral head allograft. a Graft preparation. b Finished intraoperative product. c Graft placement with screw fixation
In the literature, there has been debate as to whether the remplissage procedure leads to loss in range of motion and stiffness, but it is becoming clearer that there is a correlation between loss of internal-external range of motion and the remplissage. Elkinson et al. [60] conducted a study on eight different cadaveric specimens, where they created Hill-Sachs lesions of 15 and 30%, respectively, and followed with a remplissage. In the specimens with a 15% lesion, there was a significant reduction in internal-external range of motion in adduction (15.1° ± 11.1°, p = 0.039), but not in abduction. In specimens with a 30% lesion, a significant reduction in range of motion was also detected in adduction (14.5° ± 11.3°, p = 0.049) and not abduction. They also determined a significant increase in joint stiffness (p = 0.060) following the remplissage procedure with the 15% defect group. An additional study by Giles et al. [61] found similar results by finding that the remplissage lead to a significant increase in joint stiffness (p ≤ 0.047) when repairing a Hill-Sachs defect. They also found the procedure to cause a reduction in internal-external range of motion in both abduction and adduction. Overall, these studies were able to show the effectiveness of the procedure to restore stability and prevent recurrence, but they were also able to highlight the potential setbacks of the procedure.
Hill-Sachs Reduction
There exist multiple different methods of filling the Hill-Sachs defect or repairing the lesion, but Garcia et al. [62] developed a novel reduction approach to filling a Hill-Sachs defect. In this technique, they used a bone tamp to carefully raise the cortical surface of the humerus in small increments. Once they restored articular congruity, they backfilled the void with Quickset (Arthrex) injectable calcium phosphate bone cement through the lateral tunnel. The study was designed to compare the reduction efficacy to remplissage, and they determined that differences in biomechanical stability are insignificant between the two. However, they did find that the reduction technique allowed for 5° more external rotation. They claim their method to be superior to the remplissage in acute situations because of its ability to preserve local anatomy while yielding similar results.
Stachowicz et al. [63] conducted a similar study with 18 cadaveric humeri. They used a kyphoplasty balloon to reduce the Hill-Sachs lesion and then injected bone cement into the void. They were able to show a 99.3% reduction to the original humeral head volume, further proving reduction to be a successful method. The limitation to this study was that it lacked biomechanical data with clinical relevance, but the study above by Garcia et al. addressed that issue.
The success of the various reduction techniques lies in the fact that it increases the articular arc of the humerus as it rotates on the glenoid, which helps prevent engagement and recurrent instability [5].
Humeral Head Reconstruction
Bone augmentation of the humeral head has been shown to successfully manage large Hill-Sachs lesions with or without concomitant glenoid bone loss (Fig. 6a–c) [64, 65]. Previously, allograft procedures had always been done via an open procedure, which was invasive and could lead to negative results. Miniaci et al. [64] experienced some negative results in a cohort study involving 18 patients that underwent open osteochondral allograft transplantation. They had patients develop osteoarthritis, partial graft collapses, and one patient had a mild subluxation. Despite the difficulties, they were able to achieve a 89% patient return to work rate, which shows the validity of the allograft reconstruction itself (Fig. 7). Another study done by DiPaola and colleagues reaffirmed the effectiveness of an allograft for humeral head reconstruction. They had four patients undergo an open reconstruction procedure with an average postoperative American Shoulder and Elbow Surgeon (ASES) score of 85.3 (range, 61 to 94.5), an average UCLA score of 28.4 (range, 23 to 31), and none of the patients experiencing recurrent instability [66].
Fig. 7.
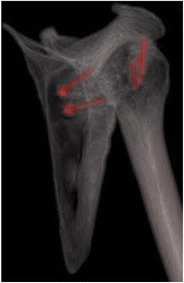
Postoperative imaging showing osteochondral allograft union into the Hill-Sachs lesion
Snir et al. [67] advanced the procedure by developing a purely arthroscopic approach to osteochondral allograft reconstruction of a Hill-Sachs lesion. This was a novel approach, and it was necessary to have a fresh-frozen, side and size-matched osteoarticular humeral head allograft to achieve the best results. Assuming the surgeon gets a close approximation of the size for the allograft plug, this technique restores the native articular surface without compromising the shoulder’s range of motion. Bakshi et al. offered clinical outcomes showing the importance of precise allograft plug approximation. They found a perfect allograft repair has significantly less anterior translation with an anterior load in comparison to an imperfect allograft repair, which is not size-matched [37]. The allograft reconstruction technique has also been shown to restore joint biomechanics better than other procedures (i.e., reduction and remplissage) [33, 61]. The disadvantages of this technique are the technical difficulty of the procedure, graft reabsorption, graft failure, and cyst formation.
Partial Humeral Head Arthroplasty
Partial resurfacing of the humeral head impression fracture with a cobalt-chrome articular component is a relatively new technique that offers advantages in comparison to other osseous defect repair procedures [68]. Previously, this technique had been employed for cases of glenohumeral osteoarthritis, avascular necrosis, and rheumatoid arthritis. However, multiple authors since then have documented the ability of the implants to be used in cases of anterior shoulder instability [69, 70]. The advantages to this technique include the lack of donor site morbidity when compared with autograft, shorter operation time, and no associated graft resorption or hardware removal, and it also allows for the avoidance of disease transmission [71]. Disadvantages include the technical difficulty in obtaining adequate fixation of the implant and an inability to align the surface of the prosthesis with the humeral head articular surface [68]. One study performed recently by Sweet et al. conducted a partial humeral head resurfacing procedure on 20 different shoulders with a mean follow-up of 32.7 months (range, 17–66 months). The results proved the procedure to be highly successful as the ASES score improved from 24.1 to 78.8, and the mean Simple Shoulder Test (SST) score increased from 3.95 to 9.3 [72]. The operation is performed using an open deltopectoral approach to ensure adequate visualization of the humeral head defect. With the assistance of intraoperative and preoperative measurements, an implant of appropriate size is chosen to repair the anatomic defect and secured into place. At the conclusion of the procedure, it is imperative for the stability of the glenohumeral joint to be tested intraoperatively to ensure that no other concomitant injuries need to be addressed [68].
Hemiarthroplasty
Complete humeral head resurfacing, or hemiarthroplasty, has been described as being indicated in older patients with a Hill-Sachs defect > 40% of the articular surface and younger patients with chronic defects and significant cartilage reduction [73]. The number of these procedures being done around the world is increasing, and the amount of known intraoperative complications is relatively small. In a review study, Cowling et al. [74] examined the data from 12,559 primary shoulder hemiarthroplasties, and they found 315 (2.5%) to have complications following the procedure. The complications included humeral head fractures, glenoid fractures, shaft penetration, vascular injuries, and nerve injuries. They also made an important discovery that the superior surgical approach led to a significantly reduced number of complications and intraoperative fractures.
Due to the findings from Cowling and his colleagues [74] being very recent, the standard method of conducting a hemiarthroplasty procedure is a deltopectoral approach that exposes the proximal humerus. In the setting of chronic anterior shoulder instability, it has been documented that increasing the amount of retroversion intraoperatively by 10°–15° may offer enhanced postoperative stability. It is also critical for the surgeon to assess the state of the glenoid and the labrum in order to ensure all concomitant pathologies are intact [68]. This procedure offers a high rate of preventing further recurrence and maintaining a stable shoulder. One meta-analysis study conducted by Aim et al. [75] that included 613 patients who underwent complete humeral head resurfacing found the rate of return to sport to be 80.7% (range, 57.1–97.3%). Ultimately, hemiarthroplasty is continuing to prove itself as a successful surgical repair for shoulders with large and engaging Hill-Sachs defects.
Weber Osteotomy
Another treatment option utilized in the past for the management of Hill-Sachs lesions, specifically engaging lesions in this case, is the Weber Osteotomy. This technique consists of transecting the proximal humerus transversely at the surgical neck and retroverting the humeral head with relative to the humeral shaft, with the objective being to theoretically achieve derotation of the humeral head, thus preventing re-engagement of the lesion. However, this procedure is uncommon given the variability in the derotation achieved and its reportedly high complication rate [76].
Summary
Osseous defects in the glenohumeral joint are issues that can cause severe shoulder instability with high risk of recurrent dislocations. In the recent years, there have been a multitude of studies progressing our knowledge over diagnosis and treatment concerning shoulder instability. Hill-Sachs lesions are unavoidable when talking about anterior shoulder instability because of their high prevalence in patients with recurrent instability. The exact quantification of a clinically significant Hill-Sachs defect is controversial and still not exactly determined. However, with the progress of knowledge about the glenoid track concept and engagement, the diagnosis and preoperative decisions are becoming more accurate. It is also critical that a surgeon conducts a thorough physical examination and makes use of the most appropriate imaging techniques in order to achieve the best diagnosis and accurately assess the size of the Hill-Sachs lesion to dictate management.
In the past several years, it has been found that a Hill-Sachs lesion is rarely an isolated situation. It is important to consider concomitant injuries including bipolar bone loss (glenoid and humeral side) in addition to the Bankart tear. Oftentimes, it is sufficient to solely address the glenoid bone loss by increasing the articular arc to prevent engagement, or to repair the Bankart lesion to decrease the humeral head excursion. However, when the Hill-Sachs lesion is large and engaging, it must be addressed in order to prevent recurrent instability. There are multiple techniques that can be used in combination with a Bankart repair or as primary technique that includes fix or fill in the bony defect using remplissage, reduction of the lesion, humeral head allograft, partial or full humeral head resurfacing, or rotational osteotomy. There are benefits and drawbacks to each technique, and they must all be considered based on the size of the humeral head lesion and for each unique patient situation.
Compliance with Ethical Standards
Conflict of Interest
Jake A. Fox, Anthony Sanchez, and Tyler J. Zajac declare that they have no conflict of interest.
Matthew T. Provencher reports grants from Health South East, grants from Norway, grants from Arthrex, personal fees from Arthrex, personal fees from JRF Ortho, and publishing royalties from Arthrex, outside the submitted work. He also reports the following patents issued: 9226743, 20150164498, 20150150594, and 20110040339.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Management of Anterior Shoulder Instability
This study was conducted at the Steadman Philippon Research Institute, Vail, CO.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. Apparent causes of failure and treatment. J Bone Joint Surg Am. 1984;66(2):159–168. doi: 10.2106/00004623-198466020-00001. [DOI] [PubMed] [Google Scholar]
- 2.Widjaja AB, Tran A, Bailey M, Proper S. Correlation between Bankart and Hill-Sachs lesions in anterior shoulder dislocation. ANZ J Surg. 2006;76(6):436–438. doi: 10.1111/j.1445-2197.2006.03760.x. [DOI] [PubMed] [Google Scholar]
- 3.Yiannakopoulos CK, Mataragas E, Antonogiannakis E. A comparison of the spectrum of intra-articular lesions in acute and chronic anterior shoulder instability. Arthroscopy. 2007;23(9):985–990. doi: 10.1016/j.arthro.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Welsh MF, Willing RT, Giles JW, Athwal GS, Johnson JA. A rigid body model for the assessment of glenohumeral joint mechanics: influence of osseous defects on range of motion and dislocation. J Biomech. 2016;49(4):514–519. doi: 10.1016/j.jbiomech.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Provencher MT, Frank RM, Leclere LE, et al. The Hill-Sachs lesion: diagnosis, classification, and management. J Am Acad Orthop Surg. 2012;20(4):242–252. doi: 10.5435/JAAOS-20-04-242. [DOI] [PubMed] [Google Scholar]
- 6.Shibayama K, Iwaso H. Hill-Sachs lesion classification under arthroscopic findings. J Shoulder Elb Surg. 2017;26(5):888–894. doi: 10.1016/j.jse.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Calandra JJ, Baker CL, Uribe J. The incidence of Hill-Sachs lesions in initial anterior shoulder dislocations. Arthroscopy. 1989;5(4):254–257. [DOI] [PubMed]
- 8.Richards RD, Sartoris DJ, Pathria MN, Resnick D. Hill-Sachs lesion and normal humeral groove: MR imaging features allowing their differentiation. Radiology. 1994;190(3):665–668. doi: 10.1148/radiology.190.3.8115607. [DOI] [PubMed] [Google Scholar]
- 9.Shaha JS, Cook JB, Rowles DJ, Bottoni CR, Shaha SH, Tokish JM. Clinical validation of the glenoid track concept in anterior glenohumeral instability. J Bone Joint Surg Am. 2016;98(22):1918–1923. doi: 10.2106/JBJS.15.01099. [DOI] [PubMed] [Google Scholar]
- 10.Di Giacomo G, Itoi E, Burkhart SS. Evolving concept of bipolar bone loss and the Hill-Sachs lesion: from “engaging/non-engaging” lesion to “on-track/off-track” lesion. Arthroscopy. 2014;30(1):90–98. doi: 10.1016/j.arthro.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677–694. doi: 10.1053/jars.2000.17715. [DOI] [PubMed] [Google Scholar]
- 12.Kurokawa D, Yamamoto N, Nagamoto H, et al. The prevalence of a large Hill-Sachs lesion that needs to be treated. J Shoulder Elb Surg. 2013;22(9):1285–1289. doi: 10.1016/j.jse.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 13.Randelli P, Ragone V, Carminati S, Cabitza P. Risk factors for recurrence after Bankart repair a systematic review. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2129–2138. doi: 10.1007/s00167-012-2140-1. [DOI] [PubMed] [Google Scholar]
- 14.Cirpar M, Gudemez E, Cetik O, Uslu M, Eksioglu F. Quadrilateral space syndrome caused by a humeral osteochondroma: a case report and review of literature. HSS J. 2006;2(2):154–156. doi: 10.1007/s11420-006-9019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horst K, Von Harten R, Weber C, et al. Assessment of coincidence and defect sizes in Bankart and Hill-Sachs lesions after anterior shoulder dislocation: a radiological study. Br J Radiol. 2014;87(1034):20130673. doi: 10.1259/bjr.20130673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dewing CB, Horan MP, Millett PJ. Two-year outcomes of open shoulder anterior capsular reconstruction for instability from severe capsular deficiency. Arthroscopy. 2012;28(1):43–51. doi: 10.1016/j.arthro.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Lynch JR, Clinton JM, Dewing CB, Warme WJ, Matsen FA. Treatment of osseous defects associated with anterior shoulder instability. J Shoulder Elb Surg. 2009;18(2):317–328. doi: 10.1016/j.jse.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 18.HALL RH, ISAAC F, BOOTH CR. Dislocations of the shoulder with special reference to accompanying small fractures. J Bone Joint Surg Am. 1959;41-A(3):489–494. doi: 10.2106/00004623-195941030-00013. [DOI] [PubMed] [Google Scholar]
- 19.Saliken DJ, Bornes TD, Bouliane MJ, Sheps DM, Beaupre LA. Imaging methods for quantifying glenoid and Hill-Sachs bone loss in traumatic instability of the shoulder: a scoping review. BMC Musculoskelet Disord. 2015;16:164. doi: 10.1186/s12891-015-0607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bigliani LU, Newton PM, Steinmann SP, Connor PM, Mcllveen SJ. Glenoid rim lesions associated with recurrent anterior dislocation of the shoulder. Am J Sports Med. 1998;26(1):41–45. doi: 10.1177/03635465980260012301. [DOI] [PubMed] [Google Scholar]
- 21.Bishop JY, Jones GL, Rerko MA, Donaldson C, Group MS 3-D CT is the most reliable imaging modality when quantifying glenoid bone loss. Clin Orthop Relat Res. 2013;471(4):1251–1256. doi: 10.1007/s11999-012-2607-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bushnell BD, Creighton RA, Herring MM. Bony instability of the shoulder. Arthroscopy. 2008;24(9):1061–1073. doi: 10.1016/j.arthro.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Rerko MA, Pan X, Donaldson C, Jones GL, Bishop JY. Comparison of various imaging techniques to quantify glenoid bone loss in shoulder instability. J Shoulder Elb Surg. 2013;22(4):528–534. doi: 10.1016/j.jse.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 24.Schneider AK, Hoy GA, Ek ET, et al. Interobserver and intraobserver variability of glenoid track measurements. J Shoulder Elb Surg. 2017;26(4):573–579. doi: 10.1016/j.jse.2016.09.058. [DOI] [PubMed] [Google Scholar]
- 25.Gyftopoulos S, Beltran LS, Yemin A, et al. Use of 3D MR reconstructions in the evaluation of glenoid bone loss: a clinical study. Skelet Radiol. 2014;43(2):213–218. doi: 10.1007/s00256-013-1774-5. [DOI] [PubMed] [Google Scholar]
- 26.Kwon YW, Powell KA, Yum JK, Brems JJ, Iannotti JP. Use of three-dimensional computed tomography for the analysis of the glenoid anatomy. J Shoulder Elb Surg. 2005;14(1):85–90. doi: 10.1016/j.jse.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Bokor DJ, O'Sullivan MD, Hazan GJ. Variability of measurement of glenoid version on computed tomography scan. J Shoulder Elb Surg. 1999;8(6):595–598. doi: 10.1016/S1058-2746(99)90096-4. [DOI] [PubMed] [Google Scholar]
- 28.Gyftopoulos S, Yemin A, Mulholland T, et al. 3DMR osseous reconstructions of the shoulder using a gradient-echo based two-point Dixon reconstruction: a feasibility study. Skelet Radiol. 2013;42(3):347–352. doi: 10.1007/s00256-012-1489-z. [DOI] [PubMed] [Google Scholar]
- 29.Gyftopoulos S, Hasan S, Bencardino J, et al. Diagnostic accuracy of MRI in the measurement of glenoid bone loss. AJR Am J Roentgenol. 2012;199(4):873–878. doi: 10.2214/AJR.11.7639. [DOI] [PubMed] [Google Scholar]
- 30.Provencher MT, Bhatia S, Ghodadra NS, et al. Recurrent shoulder instability: current concepts for evaluation and management of glenoid bone loss. J Bone Joint Surg Am. 2010;92(Suppl 2):133–151. doi: 10.2106/JBJS.J.00906. [DOI] [PubMed] [Google Scholar]
- 31.Stillwater L, Koenig J, Maycher B, Davidson M. 3D-MR vs. 3D-CT of the shoulder in patients with glenohumeral instability. Skelet Radiol. 2017;46(3):325–331. doi: 10.1007/s00256-016-2559-4. [DOI] [PubMed] [Google Scholar]
- 32.Cicak N, Bilić R, Delimar D. Hill-Sachs lesion in recurrent shoulder dislocation: sonographic detection. J Ultrasound Med. 1998;17(9):557–560. doi: 10.7863/jum.1998.17.9.557. [DOI] [PubMed] [Google Scholar]
- 33.Sekiya JK, Wickwire AC, Stehle JH, Debski RE. Hill-Sachs defects and repair using osteoarticular allograft transplantation: biomechanical analysis using a joint compression model. Am J Sports Med. 2009;37(12):2459–2466. doi: 10.1177/0363546509341576. [DOI] [PubMed] [Google Scholar]
- 34.Garcia GH, Liu JN, Dines DM, Dines JS. Effect of bone loss in anterior shoulder instability. World J Orthop. 2015;6(5):421–433. doi: 10.5312/wjo.v6.i5.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramhamadany E, Modi CS. Current concepts in the management of recurrent anterior gleno-humeral joint instability with bone loss. World J Orthop. 2016;7(6):343–354. doi: 10.5312/wjo.v7.i6.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arciero RA, Parrino A, Bernhardson AS, et al. The effect of a combined glenoid and Hill-Sachs defect on glenohumeral stability: a biomechanical cadaveric study using 3-dimensional modeling of 142 patients. Am J Sports Med. 2015;43(6):1422–1429. doi: 10.1177/0363546515574677. [DOI] [PubMed] [Google Scholar]
- 37.Bakshi NK, Jolly JT, Debski RE, Sekiya JK. Does repair of a Hill-Sachs defect increase stability at the glenohumeral joint? Orthop J Sports Med. 2016;4(5):2325967116645091. doi: 10.1177/2325967116645091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gyftopoulos S, Beltran LS, Bookman J, Rokito A. MRI evaluation of bipolar bone loss using the on-track off-track method: a feasibility study. AJR Am J Roentgenol. 2015;205(4):848–852. doi: 10.2214/AJR.14.14266. [DOI] [PubMed] [Google Scholar]
- 39.Di Giacomo G, Golijanin P, Sanchez G, Provencher MT. Radiographic analysis of the Hill-Sachs lesion in anteroinferior shoulder instability after first-time dislocations. Arthroscopy. 2016;32(8):1509–1514. doi: 10.1016/j.arthro.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto N, Itoi E, Abe H, et al. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elb Surg. 2007;16(5):649–656. doi: 10.1016/j.jse.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Metzger PD, Barlow B, Leonardelli D, Peace W, Solomon DJ, Provencher MT. Clinical application of the “glenoid track” concept for defining humeral head engagement in anterior shoulder instability: a preliminary report. Orthop J Sports Med. 2013;1(2):2325967113496213. doi: 10.1177/2325967113496213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alentorn-Geli E, Álvarez-Díaz P, Doblas J, et al. Return to sports after arthroscopic capsulolabral repair using knotless suture anchors for anterior shoulder instability in soccer players: minimum 5-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2016;24(2):440–446. doi: 10.1007/s00167-015-3895-y. [DOI] [PubMed] [Google Scholar]
- 43.Boileau P, Villalba M, Héry JY, Balg F, Ahrens P, Neyton L. Risk factors for recurrence of shoulder instability after arthroscopic Bankart repair. J Bone Joint Surg Am. 2006;88(8):1755–1763. doi: 10.2106/JBJS.E.00817. [DOI] [PubMed] [Google Scholar]
- 44.Armitage MS, Faber KJ, Drosdowech DS, Litchfield RB, Athwal GS. Humeral head bone defects: remplissage, allograft, and arthroplasty. Orthop Clin North Am. 2010;41(3):417–425. doi: 10.1016/j.ocl.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Kandziora F, Jäger A, Bischof F, Herresthal J, Starker M, Mittlmeier T. Arthroscopic labrum refixation for post-traumatic anterior shoulder instability: suture anchor versus transglenoid fixation technique. Arthroscopy. 2000;16(4):359–366. doi: 10.1016/S0749-8063(00)90079-3. [DOI] [PubMed] [Google Scholar]
- 46.Harris JD, Gupta AK, Mall NA, et al. Long-term outcomes after Bankart shoulder stabilization. Arthroscopy. 2013;29(5):920–933. doi: 10.1016/j.arthro.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 47.Ghodadra N, Gupta A, Romeo AA, et al. Normalization of glenohumeral articular contact pressures after Latarjet or iliac crest bone-grafting. J Bone Joint Surg Am. 2010;92(6):1478–1489. doi: 10.2106/JBJS.I.00220. [DOI] [PubMed] [Google Scholar]
- 48.Burkhart SS, De Beer JF, Barth JR, et al. Results of modified Latarjet reconstruction in patients with anteroinferior instability and significant bone loss. Arthroscopy. 2007;23(10):1033–1041. doi: 10.1016/j.arthro.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Tauber M, Resch H, Forstner R, Raffl M, Schauer J. Reasons for failure after surgical repair of anterior shoulder instability. J Shoulder Elb Surg. 2004;13(3):279–285. doi: 10.1016/j.jse.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 50.Purchase RJ, Wolf EM, Hobgood ER, Pollock ME, Smalley CC. Hill-sachs “remplissage”: an arthroscopic solution for the engaging Hill-Sachs lesion. Arthroscopy. 2008;24(6):723–726. doi: 10.1016/j.arthro.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 51.Koo SS, Burkhart SS, Ochoa E. Arthroscopic double-pulley remplissage technique for engaging Hill-Sachs lesions in anterior shoulder instability repairs. Arthroscopy. 2009;25(11):1343–1348. doi: 10.1016/j.arthro.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 52.Alexander TC, Beicker C, Tokish JM. Arthroscopic remplissage for moderate-size Hill-Sachs lesion. Arthrosc Tech. 2016;5(5):e975–e979. doi: 10.1016/j.eats.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Longo UG, Loppini M, Rizzello G, et al. Remplissage, humeral osteochondral grafts, weber osteotomy, and shoulder arthroplasty for the management of humeral bone defects in shoulder instability: systematic review and quantitative synthesis of the literature. Arthroscopy. 2014;30(12):1650–1666. doi: 10.1016/j.arthro.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 54.Boileau P, O'Shea K, Vargas P, Pinedo M, Old J, Zumstein M. Anatomical and functional results after arthroscopic Hill-Sachs remplissage. J Bone Joint Surg Am. 2012;94(7):618–626. doi: 10.2106/JBJS.K.00101. [DOI] [PubMed] [Google Scholar]
- 55.Buza JA, Iyengar JJ, Anakwenze OA, Ahmad CS, Levine WN. Arthroscopic Hill-Sachs remplissage: a systematic review. J Bone Joint Surg Am. 2014;96(7):549–555. doi: 10.2106/JBJS.L.01760. [DOI] [PubMed] [Google Scholar]
- 56.Wolf EM, Arianjam A. Hill-Sachs remplissage, an arthroscopic solution for the engaging Hill-Sachs lesion: 2- to 10-year follow-up and incidence of recurrence. J Shoulder Elb Surg. 2014;23(6):814–820. doi: 10.1016/j.jse.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 57.Park MJ, Tjoumakaris FP, Garcia G, Patel A, Kelly JD. Arthroscopic remplissage with Bankart repair for the treatment of glenohumeral instability with Hill-Sachs defects. Arthroscopy. 2011;27(9):1187–1194. doi: 10.1016/j.arthro.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 58.Ratner D, Backes J, Tokish JM. Arthroscopic reduction and balloon humeroplasty in the treatment of acute hill-sachs lesions. Arthrosc Tech. 2016;5(6):e1327–e1332. doi: 10.1016/j.eats.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nourissat G, Kilinc AS, Werther JR, Doursounian L. A prospective, comparative, radiological, and clinical study of the influence of the “remplissage” procedure on shoulder range of motion after stabilization by arthroscopic Bankart repair. Am J Sports Med. 2011;39(10):2147–2152. doi: 10.1177/0363546511416315. [DOI] [PubMed] [Google Scholar]
- 60.Elkinson I, Giles JW, Faber KJ, et al. The effect of the remplissage procedure on shoulder stability and range of motion: an in vitro biomechanical assessment. J Bone Joint Surg Am. 2012;94(11):1003–1012. doi: 10.2106/JBJS.J.01956. [DOI] [PubMed] [Google Scholar]
- 61.Giles JW, Elkinson I, Ferreira LM, et al. Moderate to large engaging Hill-Sachs defects: an in vitro biomechanical comparison of the remplissage procedure, allograft humeral head reconstruction, and partial resurfacing arthroplasty. J Shoulder Elb Surg. 2012;21(9):1142–1151. doi: 10.1016/j.jse.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 62.Garcia GH, Degen RM, Bui CNH, McGarry MH, Lee TQ, Dines JS. Biomechanical comparison of acute Hill-Sachs reduction with remplissage to treat complex anterior instability. J Shoulder Elb Surg. 2017;26(6):1088–1096. doi: 10.1016/j.jse.2016.11.050. [DOI] [PubMed] [Google Scholar]
- 63.Stachowicz RZ, Romanowski JR, Wissman R, Kenter K. Percutaneous balloon humeroplasty for Hill-Sachs lesions: a novel technique. J Shoulder Elb Surg. 2013;22(9):e7–13. doi: 10.1016/j.jse.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 64.Miniaci A, Gish MW. Management of anterior glenohumeral instability associated with large Hill-Sachs defects. Tech Should Elbow Surg. 2004;5(3):170–175. doi: 10.1097/01.bte.0000137216.70574.ba. [DOI] [Google Scholar]
- 65.Kropf EJ, Sekiya JK. Osteoarticular allograft transplantation for large humeral head defects in glenohumeral instability. Arthroscopy. 2007;23(3):322.e321–322.e325. doi: 10.1016/j.arthro.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 66.DiPaola MJ, Jazrawi LM, Rokito AS, et al. Management of humeral and glenoid bone loss—associated with glenohumeral instability. Bull NYU Hosp Jt Dis. 2010;68(4):245–250. [PubMed] [Google Scholar]
- 67.Snir N, Wolfson TS, Hamula MJ, Gyftopoulos S, Meislin RJ. Arthroscopic anatomic humeral head reconstruction with osteochondral allograft transplantation for large Hill-Sachs lesions. Arthrosc Tech. 2013;2(3):e289–e293. doi: 10.1016/j.eats.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mascarenhas R, Rusen J, Saltzman BM, et al. Management of humeral and glenoid bone loss in recurrent glenohumeral instability. Adv Orthop. 2014;2014:640952. doi: 10.1155/2014/640952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grondin P, Leith J. Case series: combined large Hill-Sachs and bony Bankart lesions treated by Latarjet and partial humeral head resurfacing: a report of 2 cases. Can J Surg. 2009;52(3):249–254. [PMC free article] [PubMed] [Google Scholar]
- 70.Moros C, Ahmad CS. Partial humeral head resurfacing and Latarjet coracoid transfer for treatment of recurrent anterior glenohumeral instability. Orthopedics. 2009;32(8). 10.3928/01477447-20090624-21.. [DOI] [PubMed]
- 71.Saito H, Itoi E, Minagawa H, Yamamoto N, Tuoheti Y, Seki N. Location of the Hill-Sachs lesion in shoulders with recurrent anterior dislocation. Arch Orthop Trauma Surg. 2009;129(10):1327–1334. doi: 10.1007/s00402-009-0854-4. [DOI] [PubMed] [Google Scholar]
- 72.Sweet SJ, Takara T, Ho L, Tibone JE. Primary partial humeral head resurfacing: outcomes with the HemiCAP implant. Am J Sports Med. 2015;43(3):579–587. doi: 10.1177/0363546514562547. [DOI] [PubMed] [Google Scholar]
- 73.Sahajpal DT, Zuckerman JD. Chronic glenohumeral dislocation. J Am Acad Orthop Surg. 2008;16(7):385–398. doi: 10.5435/00124635-200807000-00004. [DOI] [PubMed] [Google Scholar]
- 74.Cowling PD, Holland P, Kottam L, Baker P, Rangan A. Risk factors associated with intraoperative complications in primary shoulder arthroplasty. Acta Orthop. 2017;7:1–5. 10.1080/17453674.2017.1362155.. [DOI] [PMC free article] [PubMed]
- 75.Aim F, Werthel JD, Deranlot J, Vigan M, Nourissat G. Return to sport after shoulder arthroplasty in recreational athletes: a systematic review and meta-analysis. Am J Sports Med. 2017;1:363546517714449. 10.1177/0363546517714449. [DOI] [PubMed]
- 76.Brooks-Hill AL, Forster BB, van Wyngaarden C, Hawkins R, Regan WD. Weber osteotomy for large Hill-Sachs defects: clinical and CT assessments. Clin Orthop Relat Res. 2013;471(8):2548–2555. doi: 10.1007/s11999-013-3024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


