Abstract
Purpose of Review
The various lumbar interbody fusion (IBF) techniques and the evidence for their use in the treatment of degenerative lumbar spondylolisthesis (DLS) are described in this review.
Recent Findings
The existing evidence is mixed regarding the indications for and utility of IBF in DLS, but its use in the setting of pre-operative instability is most strongly supported. Anterior (ALIF), lateral (LLIF), posterior (PLIF), transforaminal (TLIF), and axial (AxiaLIF) lumbar IBF approaches have been described. While the current data are limited, TLIF may be a better option than PLIF in DLS due the increased operative morbidity and peri-operative complications observed with the latter. LLIF also appears superior to PLIF in light of improved radiologic outcomes, fewer intra-operative complications, and potentially greater improvements in disability. The data comparing LLIF to TLIF are less conclusive. No studies specifically comparing ALIF or AxiaLIF to other IBF techniques could be identified.
Summary
Instability may be the strongest indication for IBF in DLS. When IBF is employed, the authors’ preferred technique is TLIF with posterior segmental spinal instrumentation. Further research is needed.
Keywords: Degenerative lumbar spondylolisthesis, Interbody fusion, Anterior, Posterior, Transforaminal, Lateral, Axial
Introduction
Degenerative lumbar spondylolisthesis (DLS) is the subluxation of one vertebra relative to an adjacent vertebra in the absence of a defect in the neural arch. It is associated with facet joint degeneration and is therefore typically observed in individuals over the age of 50. Affected individuals may suffer from spinal stenosis with resulting back and leg pain [1]. While isolated decompression can be considered in the treatment of select cases of symptomatic DLS [2], decompression with fusion is more frequently performed out of concern that isolated decompression will further destabilize the spine, permitting additional slip progression [3, 4]. The benefit of arthrodesis has been demonstrated in the existing literature, but the optimal method for achieving fusion remains poorly defined [5–7].
Interbody fusion (IBF), a relatively new set of techniques, has become increasingly popular in the treatment of DLS, with utilization rates climbing from 14% in 1999 to 37% in 2011 [3]. However, controversy exists regarding the specific indications for and benefits of IBF overall as well as on the relative merits of the various interbody approaches [8••, 9, 10••]. This report discusses the indications for IBF, explores and compares the various IBF techniques, and presents the authors’ decision-making algorithm for operative management of lumbar DLS.
Indications for Interbody Fusion
Interbody fusion offers a number of potential benefits. It bolsters the biomechanical stability of a construct by stabilizing the anterior column. This can prove especially important in patients with DLS in which the anterior column is unstable (e.g., high-grade spondylolisthesis, unstable slips, degenerative scoliosis, and retained disc height) [11–14]. The insertion of interbody devices can also improve sagittal alignment and restore disc and foraminal height, providing indirect decompression in instances of foraminal and canal stenosis and aiding in spondylolisthesis reduction [12, 15]. From a biological standpoint, it increases the surface area available for fusion and has been shown to enhance fusion rates [8••, 16]. This can be particularly useful in revision procedures when the posterolateral fusion bed may be insufficient, and the surgeon needs to increase the chances of successful arthrodesis [12]. Interbody fusion can be utilized in either a stand-alone fashion or in conjunction with traditional posterior spinal fusion [17–19, 20•].
Despite the above, interbody techniques have not consistently enhanced outcomes in the existing literature [21]. A recent systematic review examining the role of IBF in the setting of DLS found that the majority of available studies identified no radiographic or clinical outcome difference between IBF and other surgical treatments [22]. The increased rigidity conferred by the addition of an interbody device may contribute to elevated rates of adjacent segment degeneration (ASD) and compression fracture [23–26]. However, conflicting data indicate that IBF may instead protect against ASD by generating more segmental lordosis [27].
Both a 2005 Cochrane Review and a 2016 North American Spine Society (NASS) evidence-based clinical guideline were unable to draw conclusions regarding the overall merit of IBF in the surgical treatment of DLS [9, 10••]. In 2014, the American Association of Neurological Surgeons/Congress of Neurological Surgeons (AANS/CNS) Joint Section on Disorders of the Spine and Peripheral Nerves released guidelines including a Grade B recommendation that interbody techniques are associated with increased fusion rates and fewer reoperations (but not necessarily improved radiographic or clinical outcomes) in patients with DLS and pre-operative instability [8••].
In a recent survey, spine surgeons indicated that the need for foraminal decompression and a desire to improve fusion rates were top reasons to include IBF in the operative treatment of DLS, while disc collapse and the absence of radiographic or clinical instability were reasons to avoid it [4].
Overview of Interbody Fusion
Anterior lumbar interbody fusion (ALIF), first described in 1932, has the longest history of use among interbody fusion techniques (Table 1) [18, 28]. This approach involves a paramedian anterior approach through the abdominal musculature and fascia, into the retroperitoneal space, and along the psoas to reach the anterior aspect of the vertebral bodies. Because of the proximity to the abdominal viscera and iliac vessels, orthopedic surgeons may opt to perform this approach with the aid of a vascular access surgeon [29, 30]. ALIF can provide excellent exposure, allowing for insertion of a graft with a large footprint, without compromising the posterior osseous, muscular, and ligamentous structures when performed as a stand-alone procedure [28, 31]. However, due to the course of the major vessels, only the L4–5 and L5–S1 disc spaces are typically accessible via this approach [32]. The L4–5 disc space is closer to the aortic bifurcation and confluence of the common iliac veins and, depending on individual patient vascular anatomy, may be more difficult or even impossible to safely access anteriorly. This is an especially relevant consideration in the setting of DLS, which most commonly affects the L4–5 level [1, 33]. Notable complications of ALIF include retrograde ejaculation, vascular and visceral injury, as well as damage to the sympathetic chain [31, 32]. Surgeons should be particularly cautious when performing ALIF in the presence of DLS because spondylolisthesis may distort and scar normal vascular anatomy, and the procedure has been associated with an increased risk of major vascular injury and greater intra-operative blood loss in the setting of spondylolisthesis [30, 34].
Table 1.
Summary of lumbar interbody fusion techniques
| Technique | Approach | Typical levels | Notable complications | Advantages | Disadvantages |
|---|---|---|---|---|---|
| ALIF | Paramedian anterior incision, through retroperitoneal space, to the anterior aspect of the vertebral bodies. | L4–S1 | Vascular and visceral injury, damage to sympathetic chain, retrograde ejaculation. | Allows for insertion of graft with large footprint. Posterior structures left intact when performed in isolation. | Approach may require aide of access surgeon. L4–5 level may not be accessible. Vascular anatomy may be distorted or scarred in spondylolisthesis. ALL disrupted. Two approaches necessary if desire 360° fusion. |
| PLIF | Posterior approach. Interbody devices inserted bilaterally via laminectomy defect while neural elements retracted to either side. | Any | Dural tears and neural injuries. Potential posterior destabilization. | Approach familiar to spine surgeons. Can achieve 360° fusion via a single approach. | Posterior osseous and soft tissue structures disrupted. Relatively high complication rate due to retraction of neural elements. |
| TLIF | Posterior approach. Single interbody device inserted to midline via unilateral laminotomy and partial facetectomy. | Any | Potential posterior destabilization. | Less neural retraction than PLIF, leading to fewer dural tears and neural injuries. | Posterior osseous and soft tissue structures disrupted. Access may be difficult, especially in the setting of spondylolisthesis. |
| LLIF | Lateral incision, through retroperitoneal space, to lateral aspect of the vertebral bodies. | L5 and above | Groin and thigh pain. Lumbar nerve root injury. | Allows for insertion of graft with large footprint. ALL maintained. Posterior structures left intact when performed in isolation. | The presence of an access surgeon may be advisable for those less familiar with the approach. Non-trivial rate of iatrogenic transient neurologic deficits. Two approaches necessary if desire 360° fusion. |
| AxiaLIF | Paracoccygeal incision, through plane between peritoneum and sacrum. Cylindrical cage inserted through drill path in sacrum to cross disc space. | L5–S1 | Rectal perforation, pelvic hematoma, sacral fracture. | Avoids mobilization of abdominal viscera or neurovascular structures. | Limited adoption due to unfamiliar approach, substantial side effect profile, and small body of literature. |
ALIF anterior lumbar interbody fusion, ALL anterior longitudinal ligament, PLIF posterior lumbar interbody fusion, TLIF transforaminal lumbar interbody fusion, LLIF lateral lumbar interbody fusion, AxiaLIF axial lumbar interbody fusion
Posterior lumbar interbody fusion (PLIF) is accomplished via the standard posterior approach. After wide laminectomy and partial bilateral facetectomy, the neural elements are retracted to either side, allowing for disc space preparation and insertion of an interbody device on each side of the intervertebral space [32, 35]. The benefit of this technique is that it utilizes an approach that is familiar to the spine surgeon, who can achieve circumferential fusion without the added morbidity and operative time associated with performing two surgical approaches under the same anesthetic. The primary drawback is related to the need for significant dural retraction, which may result in incidental durotomy or nerve root injuries [32, 36•, 37].
Transforaminal lumbar interbody fusion (TLIF) involves placement of a single interbody device into the midline of the intervertebral space via a posterior or posterolateral approach and was first described for use in the setting of spondylolisthesis [38]. A unilateral laminotomy and partial facetectomy are performed on the side of the patient’s symptoms to produce a posterolateral window for disc space preparation and interbody device insertion [32]. A single “banana”-shaped or rectangular interbody device can be inserted into the midline from a unilateral approach to support the entire segment [32]. This technique exhibits the advantages of PLIF without the risks associated with neural retraction [37, 39]. It does, however, require a unique set of surgical instruments to which the surgeon must become accustomed, especially when performed in a minimally invasive fashion [32]. Furthermore, access to the disc space may be challenging due to the narrow-working corridor and steep angle inherent to spondylolisthesis cases [40].
Lateral lumbar interbody fusion (LLIF) is a relatively newer technique that gains access to the lumbar spine via a lateral approach through the retroperitoneal fat and psoas muscle [41]. Similar to ALIF, this approach allows for insertion of an interbody device with a large footprint and spares the posterior osseous and ligamentous structures when performed in a stand-alone fashion. However, LLIF also retains the anterior longitudinal ligament, which adds rigidity to the segment [42]. Retention of these osseous and ligamentous structures is especially desirable in the setting of degenerative spondylolisthesis, where lack of restraints may exacerbate spinal destabilization and contribute to failure of instrumentation [42]. LLIF avoids many of the access-related complications associated with ALIF but comes with its own set of risks and limitations. The location of the iliac wing precludes exposure of the L5–S1 disc space and may make L4–5 surgery difficult. Access to the upper lumbar spine may need to be accomplished via an intercostal approach [43]. Complications include post-operative groin and thigh pain as well as injury to the lumbar nerve roots and plexus [17, 44, 45]. Intra-operative neurological monitoring and frequent fluoroscopic imaging are utilized in an attempt to minimize these risks [41]. Additionally, some surgeons employ the assistance of a vascular access surgeon.
Axial lumbar interbody fusion (AxiaLIF), an interbody fusion strategy which was never widely adopted, utilizes the plane separating the peritoneal contents from the sacrum and is intended almost exclusively for arthrodesis of the L5–S1 space, although two-level fusions have been performed (L4–S1) [46]. A small paracoccygeal incision is made, and a cannulated drill is passed from the sacral promonotory through the sacrum to create a path to the L5–S1 disc space. Disc space preparation is performed via this channel and then an axially directed implant is inserted [46]. A pedicle screw reduction system may be used to perform spondylolisthesis reduction prior to the AxiaLIF portion of the procedure [40]. The purported benefit of AxiaLIF is that it avoids mobilization of abdominal viscera or neurovascular structures [47]. However, limited high-quality research exists regarding surgical outcomes and complications [48]. Rectal perforation, pelvic hematoma, and sacral fracture have been reported, and the technique has largely fallen out of favor [49–51].
Comparison of Approaches
There have been numerous studies evaluating the merits and disadvantages of the above IBF techniques, but the heterogeneous nature of these trials and the paucity of direct head-to-head comparisons have made it difficult to define an optimal approach. Existing evidence-based clinical guidelines have deemed the existing evidence inadequate to identify a preferred IBF technique [8••, 9, 10••]. The available literature, some of which was not available at the time that the aforementioned guidelines were developed, is described below as well as summarized in Table 2.
Table 2.
Summary of direct comparisons between interbody fusion techniques in degenerative lumbar spondylolisthesis reported in the literature
| Study | Level of evidence | Description | Results | Conclusion |
|---|---|---|---|---|
| Yan et al. 2008 [52] | III | Retrospective cohort study comparing PLIF (n = 85) versus TLIF (n = 91) for single-level fusion in Grade I–II DLS. PSSI performed in all cases. Minimum two-year follow-up. | All patients achieved fusion. No instances of cage extrusion. Both techniques resulted in pain improvement, good or excellent JOA scores, and increased disc and foraminal height, but there were no significant differences between the two techniques. Similar complication profile. | Both PLIF and TLIF provide good medium-term outcomes with similar safety profiles. No significant differences identified. |
| Liu et al. 2015 [53•] | III | Retrospective cohort study comparing TLIF (n = 101) and PLIF (n = 125) in treatment of 1- or 2-level Grade I–II DLS. PSSI performed in all cases. Immediate peri-operative timeframe only. | Operative time, intra-operative blood loss, and allogenic blood transfusion rates significantly higher in PLIF group, which also had a significantly higher rate of dural tear, post-operative nerve root dysfunction, and reoperation (due to infection and nerve root injury). Similar improvement in pain and functional outcomes 1 week post-operatively. | PLIF and TLIF produce similar short-term functional improvements, but PLIF is associated with significantly more operative morbidity and peri-operative complications. |
| Pawar et al. 2015 [36•] | III | Retrospective matched cohort study comparing LLIF (n = 39) versus PLIF (n = 39) for 1- or 2-level DLS. PSSI performed in all cases. Average 16.1 month follow-up in LLIF group and 21 months in PLIF group. | Similar operative time between groups, but significantly less average blood loss and dural tear incidence with LLIF. Restoration of forminal height, disc height, and lumbar lordosis significantly better in LLIF group. No permanent iatrogenic neurologic deficits in either group. Significantly greater improvement in ODI scores with LLIF, but no other significant differences in clinical outcome scores between groups. | LLIF resulted in improved radiologic outcomes with fewer intra-operative complications than PLIF. LLIF may also result in greater improvements in disability. |
| Norton et al. 2015 [20•] | III | Analysis of 48,911 patients undergoing fusion for DLS in a large national inpatient database from 2001 through 2010. Compared complications of ALIF versus combined group of PLIF and TLIF patients. | Compared to the combined PLIF and TLIF group, patients undergoing ALIF had a significantly lower rate of acute blood loss anemia but a significantly higher incidence of GI complications. | Interpretation difficult due to lack of granularity in surgical groups and complication categories. No post-discharge outcomes to guide decision making. |
| Sembrano et al. 2016 [54•], and Isaacs et al. 2016 [55•] | II | Two-year outcomes of a prospective cohort study comparing LLIF (n = 29) to MIS TLIF (n = 26) in treatment of 1- or 2-level Grade I–II DLS. Clinical and radiographic findings reported in separate manuscripts. | Significantly less blood loss with LLIF. No difference in operative time or hospital length of stay. 31% of LLIF patients (and no TLIF patients) had mild, transient post-operative hip flexion weakness. Similar improvements in pain, disability and quality of life scores. Greater post-operative increase in central canal area with TLIF. Less subsidence in the LLIF group at two-year follow-up. All LLIF patients and 96% of TLIF patients had evidence of fusion on computed tomography (p = 0.448). | Both LLIF and MIS TLIF were effective with few significant differences identified between them. Fusion rates and patient-reported outcomes comparable. |
PLIF posterior lumbar interbody fusion, TLIF transforaminal lumbar interbody fusion, DLS degenerative lumbar spondylolisthesis, PSSI posterior segmental spinal instrumentation, JOA Japanese Orthopedic Association, LLIF lateral lumbar interbody fusion, MIS minimally invasive surgical, VAS visual analogue scale, ODI Oswestry Disability Index, ALIF anterior lumbar interbody fusion, GI gastrointestinal
In 2008, Yan et al. reported the results of a retrospective cohort study comparing PLIF versus TLIF for single-level fusion in Grade I–II DLS [52]. All IBFs were performed in conjunction with posterior segmental spinal instrumentation (PSSI). The minimum two-year follow-up was achieved in 85 patients undergoing PLIF and 91 undergoing TLIF. There were no instances of cage extrusion in either group, and all patients achieved fusion. The complication profiles of the two groups were similar, with radiculitis in three PLIF and two TLIF patients and one instance of screw loosening in each group. Both techniques generally resulted in good or excellent Japanese Orthopedic Association (JOA) scores (83.5% of PLIF and 84.6% of TLIF patients). Visual analogue scale (VAS) scores decreased by 4.24 in the PLIF group and by 4.34 in the TLIF group, both of which were highly statistically significant findings (p = <0.001). The percent slippage improved significantly between the pre-operative and initial post-operative radiographs in both groups—from an average of 30.1 to 7.3% with PLIF and from an average of 31.4 to 7.4% with TLIF. This did not change significantly between initial post-operative and final follow-up imaging. Finally, both IBF techniques were successful in imparting a significant increase in intervertebral space and foraminal height. Notably, there were no significant differences between the two techniques in any of the clinical or radiographic measures. Results of this study suggest that PLIF and TLIF exhibit similar safety profiles, and both provide good medium-term clinical and radiographic outcomes.
A 2015 retrospective cohort study by Liu et al. compared TLIF (n = 101) and PLIF (n = 125) in treatment of Grade I–II DLS [53•]. The study was limited to one- or two-level fusions, and PSSI was performed in all cases. In addition to being somewhat larger than the Yan et al. study, Liu et al. focused more closely on intra-operative events and the immediate post-operative period (within 1 week of surgery). Compared to patients in the TLIF group, those in the PLIF group had significantly longer operative times (242 ± 67 versus 188 ± 46 min, p = 0.037), more intra-operative blood loss (483 ± 403 versus 308 ± 385 mL, p = 0.035), and higher allogenic blood transfusion rates (19.2 versus 4.9%, p = 0.001). Furthermore, those in the PLIF group had a significantly greater rate of dural tear (12 versus 3.9%, p = 0.030), post-operative iatrogenic nerve root dysfunction (9.6 versus 1.9%, p = 0.018), and reoperation (10.4 versus 1.9%, p = 0.001). Reoperations were for infection and nerve root injury. Functional outcomes, as determined by the Kirkaldy-Willis criteria, improved similarly between groups (p = 0.64). Compared to pre-operative baselines, VAS scores decreased by an average of 4.24 in the PLIF group and 4.34 in the TLIF group (p = 0.32) by 1 week post-operatively. Curiously, the reported mean pre- and post-operative average VAS scores as well as standard deviations for both groups were exactly the same between this and the 2008 Yan et al. study despite being different patient populations at different time points. It seems unlikely that these values would be identical to the reported hundredth of a point, which may cast some doubt on this and other results reported in the Liu et al. manuscript. This aside, these findings suggest that PLIF and TLIF produce similar short-term functional improvements, but PLIF is associated with significantly more operative morbidity and peri-operative complications.
PLIF and LLIF were compared in a study published by Pawar et al. in 2015 [36•]. This retrospective matched cohort study investigated the effects of each of these techniques in conjunction with PSSI for one- or two-level DLS. There were 39 patients in each group, with an average follow-up of 16.1 months for the LLIF patients and 21 months for the PLIF patients. Operative time was similar between groups, but average blood loss was significantly less in the LLIF than the PLIF group (438 versus 750 min, p < 0.01), and the incidence of dural tear was significantly lower with LLIF (0 versus 5, p = 0.014). The LLIF group also experienced significantly greater restoration of foraminal height, intervertebral space height, and lumbar lordosis. There were no permanent iatrogenic neurologic deficits in either group. There was significantly greater decline in disability in the LLIF group as measured by the Oswestry Disability Index (− 19.5 versus − 7.7, p = 0.001), but no other significant differences in clinical outcome scores (Short Form 12 Physical & Mental Component Summary, VAS) between groups. These results suggest that LLIF results in improved radiologic outcomes with fewer intra-operative complications than PLIF. LLIF may also produce greater improvements in disability.
Norton et al. published an analysis of the National Inpatient Sample (NIS) database with a focus on the treatment of patients with DLS [20•]. The study, also released in 2015, examined inpatient hospital data from the decade spanning 2001 through 2010. Authors reported complication rates by surgical technique, but were forced to lump TLIF and PLIF together (P/TLIF) due to limitations of administrative coding data. Similarly, they were unable to discriminate between ALIF and LLIF. Authors found that patients undergoing interbody fusion via an anterior or lateral approach were significantly less likely to develop acute blood loss anemia but were at significantly higher risk of gastrointestinal complications than those undergoing P/TLIF. However, the study’s utility in informing clinical decision making is limited by the lack of granularity in surgical groups and complication categories as well as the absence of post-discharge outcomes.
Two-year outcomes of a prospective cohort study comparing LLIF to minimally invasive surgical (MIS) TLIF in the treatment of one- or two-level Grade I-II DLS were published in 2016 [54•, 55•]. In total, 29 patients underwent LLIF and 26 underwent TLIF. Percutaneous pedicle screws were inserted bilaterally for both groups, but direct decompression was only performed in the TLIF patients. Investigators found that blood loss was lower in the LLIF group, with intra-operative blood loss of less than 100 mL of occurring in 73% of LLIF versus 27% of TLIF cases (p < 0.01). Mean operative time and hospital length of stay did not differ between groups. Post-operative hip flexion weakness was only observed in the LLIF group (31% of patients, p < 0.001), but it resolved within 6 months in all cases. There were few sensory or distal motor deficits in the study—all were transient, and no significant difference between groups was identified. One pseudoarthrosis was identified and revised (TLIF group); no other revisions were performed. Pain, disability, and quality of life scores improved significantly over baseline in both groups with minimal statistically significant intra-group differences. Radiographically, disc height was significantly improved in both groups at all time points, but less subsidence was observed in the LLIF group at two-year follow-up (p = 0.002). There was greater post-operative increase in central canal area in the TLIF than the LLIF group (43.1 versus 4.1 mm2, p = 0.028). All LLIF patients and 96% of TLIF patients had evidence of fusion on computed tomography (p = 0.448). Ultimately, authors concluded that both techniques are reasonable treatment options as they provide high fusion rates and significant and sustained clinical improvements over a two-year timeframe.
Synthesizing this rather limited data remains difficult. Based on the existing publications, however, TLIF may generally be a better option than PLIF for DLS, especially in light of the increased operative morbidity and peri-operative complications observed with PLIF. Similarly, LLIF appears superior to PLIF due to improved radiologic outcomes, fewer intra-operative complications, and potentially greater improvements in disability. The data comparing LLIF to TLIF are currently less conclusive. Unfortunately, no direct comparison to date has included AxiaLIF in the study groups, and the single study containing ALIF patients has limited clinical utility at distinguishing between IBF techniques. In addition to the above, the decision on which approach to utilize should continue to depend on individual patient factors and surgeon familiarity.
Conclusions
Interbody fusion boasts a number of potential benefits in the treatment of DLS, but the limited existing data neither consistently support its use nor conclusively identify an optimal technique. Additional research is required to conclusively define the role of IBS in this setting.
In our practice, fusion for DLS is performed via a posterior approach using segmental pedicle screw fixation without IBF for the majority of cases. Indications for the addition of an interbody device are the following: the presence of frank instability, a large or mobile disc space, degenerative scoliosis, post-laminectomy spondylolisthesis, recurrent herniated nucleus pulposis (HNP) at the L4–5 level, and laterally based HNP with exiting nerve root compression. The latter is a relatively uncommon indication due to the fact that DLS typically compresses the traversing root in the lateral recess and less commonly affects the exiting root. When IBF is indicated, our preferred technique is TLIF with PSSI (Figs. 1 and 2), which allows for anterior support via a posterior approach with minimal nerve root retraction.
Fig. 1.

Pre-operative radiographic and magnetic resonance images of a 44-year-old male with L4–5 degenerative spondylolisthesis and severe L4–5 facet arthrosis, central stenosis, and foraminal stenosis. Note the presence of maintained disc height and degenerative scoliosis, indications for interbody fusion
Fig. 2.
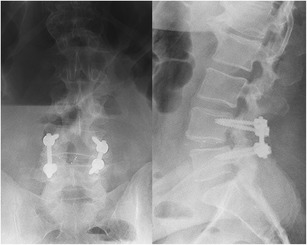
Post-operative radiographs status post L4–5 instrumented posterior spinal fusion and transforaminal lumbar interbody fusion
Compliance with Ethical Standards
Conflict of Interest
Peter B. Derman declares that he has no conflict of interest.
Todd J. Albert received royalties from DePuy and Biomet; owns stock or stock options in Vertech, In Vivo Therapeutics, Paradigm Spine, Biomerix, Breakaway Imaging, Crosstree, Invuity, Pioneer, Gentis, ASIP, and PMIG; and serves as a consultant to DePuy, Biomet, Facetlink, and Spinicity.
Human and Animal Rights and Informed Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required. This article does not contain any studies with animals performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Treatment of Lumbar Degenerative Pathology
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Jacobsen S, Sonne-Holm S, Rovsing H, Monrad H, Gebuhr P. Degenerative lumbar spondylolisthesis: an epidemiological perspective: the Copenhagen Osteoarthritis Study. Spine. 2007;32(1):120–125. doi: 10.1097/01.brs.0000250979.12398.96. [DOI] [PubMed] [Google Scholar]
- 2.Kelleher MO, Timlin M, Persaud O, Rampersaud YR. Success and failure of minimally invasive decompression for focal lumbar spinal stenosis in patients with and without deformity. Spine. 2010;35(19):E981–E987. doi: 10.1097/BRS.0b013e3181c46fb4. [DOI] [PubMed] [Google Scholar]
- 3.Kepler CK, Vaccaro AR, Hilibrand AS, Anderson DG, Rihn JA, Albert TJ, et al. National trends in the use of fusion techniques to treat degenerative spondylolisthesis. Spine. 2014;39(19):1584–1589. doi: 10.1097/BRS.0000000000000486. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder GD, Kepler CK, Kurd MF, Vaccaro AR, Hsu WK, Patel AA, Savage JW. Rationale for the Surgical Treatment of Lumbar Degenerative Spondylolisthesis. Spine (Phila Pa 1976). 2015;40(21):E1161–6. [DOI] [PubMed]
- 5.Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am. 1991;73(6):802–808. doi: 10.2106/00004623-199173060-00002. [DOI] [PubMed] [Google Scholar]
- 6.Bridwell KH, Sedgewick TA, O’Brien MF, Lenke LG, Baldus C. The role of fusion and instrumentation in the treatment of degenerative spondylolisthesis with spinal stenosis. J Spinal Disord. 1993;6(6):461–472. doi: 10.1097/00002517-199306060-00001. [DOI] [PubMed] [Google Scholar]
- 7.Kornblum MB, Fischgrund JS, Herkowitz HN, Abraham DA, Berkower DL, Ditkoff JS. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine. 2004;29(7):726–733. doi: 10.1097/01.BRS.0000119398.22620.92. [DOI] [PubMed] [Google Scholar]
- 8.Mummaneni PV, Dhall SS, Eck JC, Groff MW, Ghogawala Z, Watters WC, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 11: interbody techniques for lumbar fusion. J Neurosurg Spine. 2014;21(1):67–74. doi: 10.3171/2014.4.SPINE14276. [DOI] [PubMed] [Google Scholar]
- 9.Gibson JNA, Waddell G. Surgery for degenerative lumbar spondylosis: updated Cochrane Review. Spine. 2005;30(20):2312–20. doi: 10.1097/01.brs.0000182315.88558.9c. [DOI] [PubMed] [Google Scholar]
- 10.Matz PG, Meagher RJ, Lamer T, Tontz WL, Annaswamy TM, Cassidy RC, et al. Guideline summary review: an evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spondylolisthesis. Spine J Off J North Am Spine Soc. 2016;16(3):439–448. doi: 10.1016/j.spinee.2015.11.055. [DOI] [PubMed] [Google Scholar]
- 11.Oda I, Abumi K, Yu B-S, Sudo H, Minami A. Types of spinal instability that require interbody support in posterior lumbar reconstruction: an in vitro biomechanical investigation. Spine. 2003;28(14):1573–1580. [PubMed] [Google Scholar]
- 12.McAfee PC, DeVine JG, Chaput CD, Prybis BG, Fedder IL, Cunningham BW, et al. The indications for interbody fusion cages in the treatment of spondylolisthesis: analysis of 120 cases. Spine. 2005;30(6 Suppl):S60–S65. doi: 10.1097/01.brs.0000155578.62680.dd. [DOI] [PubMed] [Google Scholar]
- 13.Liao J-C, Lu M-L, Niu C-C, Chen W-J, Chen L-H. Surgical outcomes of degenerative lumbar spondylolisthesis with anterior vacuum disc: can the intervertebral cage overcome intradiscal vacuum phenomenon and enhance posterolateral fusion? J Orthop Sci Off J Jpn Orthop Assoc. 2014;19(6):851–859. doi: 10.1007/s00776-014-0618-z. [DOI] [PubMed] [Google Scholar]
- 14.Ha K-Y, Na K-H, Shin J-H, Kim K-W. Comparison of posterolateral fusion with and without additional posterior lumbar interbody fusion for degenerative lumbar spondylolisthesis. J Spinal Disord Tech. 2008;21(4):229–234. doi: 10.1097/BSD.0b013e3180eaa202. [DOI] [PubMed] [Google Scholar]
- 15.Vamvanij V, Ferrara LA, Hai Y, Zhao J, Kolata R, Yuan HA. Quantitative changes in spinal canal dimensions using interbody distraction for spondylolisthesis. Spine. 2001;26(3):E13–E18. doi: 10.1097/00007632-200102010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Macki M, Bydon M, Weingart R, Sciubba D, Wolinsky J-P, Gokaslan ZL, et al. Posterolateral fusion with interbody for lumbar spondylolisthesis is associated with less repeat surgery than posterolateral fusion alone. Clin Neurol Neurosurg. 2015;138:117–123. doi: 10.1016/j.clineuro.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Marchi L, Abdala N, Oliveira L, Amaral R, Coutinho E, Pimenta L. Stand-alone lateral interbody fusion for the treatment of low-grade degenerative spondylolisthesis. ScientificWorldJournal. 2012;2012:456346. doi: 10.1100/2012/456346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao PJ, Ghent F, Phan K, Lee K, Reddy R, Mobbs RJ. Stand-alone anterior lumbar interbody fusion for treatment of degenerative spondylolisthesis. J Clin Neurosci. 2015;22(10):1619–24. [DOI] [PubMed]
- 19.Gille O, Challier V, Parent H, Cavagna R, Poignard A, Faline A, et al. Degenerative lumbar spondylolisthesis. Cohort of 670 patients, and proposal of a new classification. Orthop Traumatol Surg Res. 2014;100(6):S311–S315. doi: 10.1016/j.otsr.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Norton RP, Bianco K, Klifto C, Errico TJ, Bendo JA. Degenerative spondylolisthesis: an analysis of the nationwide inpatient sample database. Spine. 2015;40(15):1219–1227. doi: 10.1097/BRS.0000000000000987. [DOI] [PubMed] [Google Scholar]
- 21.Abdu WA, Lurie JD, Spratt KF, Tosteson ANA, Zhao W, Tosteson TD, et al. Degenerative spondylolisthesis: does fusion method influence outcome? Four-year results of the spine patient outcomes research trial. Spine. 2009;34(21):2351–2360. doi: 10.1097/BRS.0b013e3181b8a829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker JF, Errico TJ, Kim Y, Razi A. Degenerative spondylolisthesis: contemporary review of the role of interbody fusion. Eur J Orthop Surg Traumatol Orthop Traumatol. 2016; [DOI] [PubMed]
- 23.Li Y-C, Yang S-C, Chen H-S, Kao Y-H, Tu Y-K. Impact of lumbar instrumented circumferential fusion on the development of adjacent vertebral compression fracture. Bone Jt J. 2015;97–B(10):1411–1416. doi: 10.1302/0301-620X.97B10.34927. [DOI] [PubMed] [Google Scholar]
- 24.Lee JC, Kim Y, Soh J-W, Shin B-J. Risk factors of adjacent segment disease requiring surgery after lumbar spinal fusion: comparison of posterior lumbar interbody fusion and posterolateral fusion. Spine. 2014;39(5):E339–E345. doi: 10.1097/BRS.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 25.Videbaek TS, Egund N, Christensen FB, Grethe Jurik A, Bünger CE. Adjacent segment degeneration after lumbar spinal fusion: the impact of anterior column support: a randomized clinical trial with an eight- to thirteen-year magnetic resonance imaging follow-up. Spine. 2010;35(22):1955–1964. doi: 10.1097/BRS.0b013e3181e57269. [DOI] [PubMed] [Google Scholar]
- 26.Sudo H, Oda I, Abumi K, Ito M, Kotani Y, Hojo Y, et al. In vitro biomechanical effects of reconstruction on adjacent motion segment: comparison of aligned/kyphotic posterolateral fusion with aligned posterior lumbar interbody fusion/posterolateral fusion. J Neurosurg. 2003;99(2 Suppl):221–228. doi: 10.3171/spi.2003.99.2.0221. [DOI] [PubMed] [Google Scholar]
- 27.Heo Y, Park JH, Seong HY, Lee YS, Jeon SR, Rhim SC, Roh SW. Symptomatic adjacent segment degeneration at the L3-4 level after fusion surgery at the L4-5 level: evaluation of the risk factors and 10-year incidence. Eur Spine J. 2015;24(11):2474–80. [DOI] [PubMed]
- 28.Eismont FJ, Norton RP, Hirsch BP. Surgical management of lumbar degenerative spondylolisthesis. J Am Acad Orthop Surg. 2014;22(4):203–213. doi: 10.5435/JAAOS-22-04-203. [DOI] [PubMed] [Google Scholar]
- 29.Ikard RW. Methods and complications of anterior exposure of the thoracic and lumbar spine. Arch Surg. 2006;141(10):1025–1034. doi: 10.1001/archsurg.141.10.1025. [DOI] [PubMed] [Google Scholar]
- 30.Nourian AA, Cunningham CM, Bagheri A, Bruffey JD, Eastlack RK. Effect of anatomic variability and level of approach on perioperative vascular complications with anterior lumbar interbody fusion. Spine (Phila Pa 1976). 2016;41(2):E73–7. [DOI] [PubMed]
- 31.Malham GM, Parker RM, Ellis NJ, Blecher CM, Chow FY, Claydon MH. Anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2: a prospective study of complications. J Neurosurg Spine. 2014;21(6):851–860. doi: 10.3171/2014.8.SPINE13524. [DOI] [PubMed] [Google Scholar]
- 32.Cole CD, McCall TD, Schmidt MH, Dailey AT. Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskelet Med. 2009;2(2):118–126. doi: 10.1007/s12178-009-9053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steiger F, Becker H-J, Standaert CJ, Balague F, Vader J-P, Porchet F, et al. Surgery in lumbar degenerative spondylolisthesis: indications, outcomes and complications. A systematic review. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2014;23(5):945–973. doi: 10.1007/s00586-013-3144-3. [DOI] [PubMed] [Google Scholar]
- 34.Fantini GA, Pappou IP, Girardi FP, Sandhu HS, Cammisa FP. Major vascular injury during anterior lumbar spinal surgery: incidence, risk factors, and management. Spine. 2007;32(24):2751–2758. doi: 10.1097/BRS.0b013e31815a996e. [DOI] [PubMed] [Google Scholar]
- 35.Lara-Almunia M, Gomez-Moreta JA, Hernandez-Vicente J. Posterior lumbar interbody fusion with instrumented posterolateral fusion in adult spondylolisthesis: description and association of clinico-surgical variables with prognosis in a series of 36 cases. Int J Spine Surg. 2015;9:22. doi: 10.14444/2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.• Pawar AY, Hughes AP, Sama AA, Girardi FP, Lebl DR, Cammisa FP. A comparative study of lateral lumbar interbody fusion and posterior lumbar interbody fusion in degenerative lumbar spondylolisthesis. Asian Spine J. 2015;9(5):668–74. This retrospective matched cohort study compared LLIF (n = 39) to PLIF (n = 39) for 1- or 2-level DLS. Authors found that LLIF resulted in improved radiologic outcomes (restoration of foraminal height, disc height, and lumbar lordosis) with less average blood loss and fewer intra-operative dural tears than PLIF. There was also significantly greater improvement in ODI scores with LLIF, but no other significant differences in clinical outcome scores between groups. [DOI] [PMC free article] [PubMed]
- 37.Zhang Q, Yuan Z, Zhou M, Liu H, Xu Y, Ren Y. A comparison of posterior lumbar interbody fusion and transforaminal lumbar interbody fusion: a literature review and meta-analysis. BMC Musculoskelet Disord. 2014;15:367. doi: 10.1186/1471-2474-15-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harms J, Rolinger H. A one-stager procedure in operative treatment of spondylolistheses: dorsal traction-reposition and anterior fusion (author’s transl) Z Für Orthop Ihre Grenzgeb. 1982;120(3):343–347. doi: 10.1055/s-2008-1051624. [DOI] [PubMed] [Google Scholar]
- 39.Chrastil J, Patel AA. Complications associated with posterior and transforaminal lumbar interbody fusion. J Am Acad Orthop Surg. 2012;20(5):283–291. doi: 10.5435/JAAOS-20-05-283. [DOI] [PubMed] [Google Scholar]
- 40.Tender GC, Miller LE, Block JE. Percutaneous pedicle screw reduction and axial presacral lumbar interbody fusion for treatment of lumbosacral spondylolisthesis: A case series. J Med Case Rep. 2011;5:454. doi: 10.1186/1752-1947-5-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J Off J North Am Spine Soc. 2006;6(4):435–443. doi: 10.1016/j.spinee.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Fogel GR, Turner AWL, Dooley ZA, Cornwall GB. Biomechanical stability of lateral interbody implants and supplemental fixation in a cadaveric degenerative spondylolisthesis model. Spine. 2014;39(19):E1138–E1146. doi: 10.1097/BRS.0000000000000485. [DOI] [PubMed] [Google Scholar]
- 43.Karikari IO, Nimjee SM, Hardin CA, Hughes BD, Hodges TR, Mehta AI, et al. Extreme lateral interbody fusion approach for isolated thoracic and thoracolumbar spine diseases: initial clinical experience and early outcomes. J Spinal Disord Tech. 2011;24(6):368–375. doi: 10.1097/BSD.0b013e3181ffefd2. [DOI] [PubMed] [Google Scholar]
- 44.Khajavi K, Shen A, Lagina M, Hutchison A. Comparison of clinical outcomes following minimally invasive lateral interbody fusion stratified by preoperative diagnosis. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2015;24(Suppl 3):322–330. doi: 10.1007/s00586-015-3840-2. [DOI] [PubMed] [Google Scholar]
- 45.Hrabalek L, Adamus M, Gryga A, Wanek T, Tucek P. A comparison of complication rate between anterior and lateral approaches to the lumbar spine. Biomed Pap Med Fac Univ Palacký Olomouc Czechoslov. 2014;158(1):127–132. doi: 10.5507/bp.2012.079. [DOI] [PubMed] [Google Scholar]
- 46.Issack PS, Kotwal SY, Boachie-Adjei O. The axial transsacral approach to interbody fusion at L5-S1. Neurosurg Focus. 2014;36(5):E8. doi: 10.3171/2014.2.FOCUS13467. [DOI] [PubMed] [Google Scholar]
- 47.Marotta N, Cosar M, Pimenta L, Khoo LT. A novel minimally invasive presacral approach and instrumentation technique for anterior L5-S1 intervertebral discectomy and fusion: technical description and case presentations. Neurosurg Focus. 2006;20(1):E9. doi: 10.3171/foc.2006.20.1.10. [DOI] [PubMed] [Google Scholar]
- 48.Schroeder GD, Kepler CK, Vaccaro AR. Axial interbody arthrodesis of the L5-S1 segment: a systematic review of the literature. J Neurosurg Spine. 2015;23(3):314–9. [DOI] [PubMed]
- 49.Lindley EM, McCullough MA, Burger EL, Brown CW, Patel VV. Complications of axial lumbar interbody fusion. J Neurosurg Spine. 2011;15(3):273–279. doi: 10.3171/2011.3.SPINE10373. [DOI] [PubMed] [Google Scholar]
- 50.Mazur MD, Duhon BS, Schmidt MH, Dailey AT. Rectal perforation after AxiaLIF instrumentation: case report and review of the literature. Spine J Off J North Am Spine Soc. 2013;13(11):e29–e34. doi: 10.1016/j.spinee.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 51.Botolin S, Agudelo J, Dwyer A, Patel V, Burger E. High rectal injury during trans-1 axial lumbar interbody fusion L5-S1 fixation: a case report. Spine. 2010;35(4):E144–E148. doi: 10.1097/BRS.0b013e3181ca7351. [DOI] [PubMed] [Google Scholar]
- 52.Yan D-L, Pei F-X, Li J, Soo C-L. Comparative study of PILF and TLIF treatment in adult degenerative spondylolisthesis. Eur Spine J. 2008;17(10):1311–1316. doi: 10.1007/s00586-008-0739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.• Liu J, Deng H, Long X, Chen X, Xu R, Liu Z. A comparative study of perioperative complications between transforaminal versus posterior lumbar interbody fusion in degenerative lumbar spondylolisthesis. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2015;This retrospective cohort study compared TLIF (n = 101) and PLIF (n = 125) in the treatment of 1- or 2-level Grade I–II DLS. Authors reported that PLIF and TLIF produce similar short-term functional improvements, but PLIF is associated with significantly more operative morbidity (blood loss, dural tear) and peri-operative complications (nerve root dysfunction, allogenic blood transfusion, reoperation due to infection and nerve root injury). The groups experienced similar improvement in pain and functional outcomes 1 week post-operatively.
- 54.Sembrano JN, Tohmeh A, Isaacs R, SOLAS Degenerative Study Group two-year comparative outcomes of mis lateral and mis transforaminal interbody fusion in the treatment of degenerative spondylolisthesis: part I: clinical findings. Spine. 2016;41(Suppl 8):S123–S132. doi: 10.1097/BRS.0000000000001471. [DOI] [PubMed] [Google Scholar]
- 55.Isaacs RE, Sembrano JN, Tohmeh AG, SOLAS Degenerative Study Group Two-year comparative outcomes of mis lateral and mis transforaminal interbody fusion in the treatment of degenerative spondylolisthesis: part II: radiographic findings. Spine. 2016;41(Suppl 8):S133–S144. doi: 10.1097/BRS.0000000000001472. [DOI] [PubMed] [Google Scholar]


