Abstract
The purpose of this study was to investigate the physico-chemical properties (free acidity, pH, aw, ash content, moisture content, color (L*, a*, b*, hue-angle, chroma and yellow index), fructose, glucose and sucrose content) and textural parameters (viscosity, hardness, adhesion, springiness, cohesiveness, chewiness and gumminess) of 50 samples of honey of different botanical origin (acacia, polyfloral, honeydew, sunflower and tilia). In order to achieve the authentication of the honey samples analyzed, their data have been subjected to linear discriminant analysis (LDA) and principal component analysis (PCA).The PCA and LDA have proved the possibility of honey authentication using the physico-chemical and textural properties. LDA classified correctly 92.0% of the honeys based on their botanical origin, using the cross validation. In the LDA projection, the textural parameters (chewiness, hardness, cohesiveness, springiness) dominated the two functions.
Keywords: Authentication, Texture parameters, Physico-chemical properties, Chemometrics
Introduction
Honey is known as the oldest natural sweetener with real medicinal qualities and a high nutritional value (Kaygusuz et al. 2016). From the chemical point of view, honey consists of approximately 80% sugars (the main sugars are monosaccharides—fructose and glucose) and other substances such as: proteins, vitamins, minerals, enzymes (Escriche et al. 2011, 2014; Oroian et al. 2017; Oroian and Ropciuc 2017). The honey characteristics (e.g. composition, flavor, aroma and color) are influenced by the botanical and geographical origin, climate and honeybee involved in its production. In the same time the weather conditions and processing and keeping conditions influence honey quality (Escuredo et al. 2014; Tornuk et al. 2013; da Silva et al. 2016).
In the last decade consumers prefer more monofloral honeys than polyfloral ones, which are much cheaper than monofloral honeys. A honey can be labeled as monofloral if it complies with some requirements established by the international food standards; the sample must contain whole or predominant pollen from a floral source and present some specific proprieties (e.g. aroma, flavor, color, composition) so as to belong to the monofloral category (Codex Alimentarius 2001). The limited produced quantity and high price make honey be one of the most adulterated products. The knowledge of the quality and authentication parameters of honeys is useful for honey adulteration detection (Puscas et al. 2013; da Silva et al. 2016).
Many papers on honey authentication based on physico-chemical properties have been published in the last decades (Escriche et al. 2011, 2014; Kato et al. 2014; Castro-Vázquez et al. 2014; Ciaccheri et al. 2015; Tuberoso et al. 2014; Beitlich et al. 2014; Oroian et al. 2015; Svečnjak et al. 2015; Gok et al. 2015). Up to the present there have been no other attempts to authenticate honeys using physico-chemical and their textural parameters.
The purpose of this work was to authenticate different honey types (acacia, polyfloral, honeydew, sunflower and tilia) in function of their botanical origin using physico-chemical, textural parameters and chemometrics [linear discriminant analysis (LDA) and principal component analysis (PCA)].
Materials and methods
Materials
Fifty different samples of different honey types (acacia, polyfloral, honeydew, sunflower and tilia) have been bought from local beekeepers of Suceava County, Romania. Each sample of honey was of 500 g. The 50 samples of honey are of five different botanical origins: 10 samples of acacia, eight samples of tilia, 12 samples of polyfloral, nine samples of honeydew and 11 samples of sunflower.
Physico-chemical properties
The physico-chemical parameters analyzed were: moisture content, pH, Brix concentration, refraction index, ash content and electrical conductivity have been determined using International Honey Commission (2009) methods. The water activity was measured using a water activity meter AquaLab Lite (Decagon, USA).
The Konica CR400 cromametre was used for color determination (Konica Minolta, Japan). The color intensity, hue angle and yellow index (YI) have been computed as:
| 1 |
| 2 |
| 3 |
Honey sugars (fructose, glucose and sucrose) have been determined using an International Honey Commission (2009) method base on a 10ADVP-SHIMADZU HPLC coupled with a refractive index detector. The sugars have been separated on an amino column with 250 × 4.6 mm i.d. (5 µm particle size). Five grams of honey was mixed with 40 ml water, 25 ml methanol and transferred to a 100 ml flask; the solution has been completed to the mark with water. After homogenization, the solution was filtered with a 0.45 µm membrane filter. Mobile phase A—acetonitrile (80%, v/v) and mobile phase B—water (20% v/v), with a flow rate set at 1.3 ml/min. Column oven and detector temperature: 30 °C, the injection volume: 10 µl. The results for each sugar were calculated as g/100 g honey.
Texture profile analysis (TPA)
Since the texture parameters are influenced by air bubbles and crystals (Oroian 2015), the honey samples have been warmed at 55 °C in a water bath to dissolve the crystals. Thus, to remove the air bubbles, the samples have been kept prior to the analysis in flasks at 30 °C (Oroian 2012).
The TPA was carried out at 25 °C with Mark 10 Texture Analyzer (Mark 10 Corporation, USA). The samples have been placed into a flask with a 70 mm diameter while the disc probe was of 50 mm. The speed was set at 150 mm/min. The samples have been placed into a flask with a 25 mm high. During the procedure, the disc probe penetrated the sample to a 12.5 mm depth for two times. The texture parameters analyzed generated from the texture profile analysis were the following: viscosity (V), hardness (H), adhesion (A), springiness (S), cohesiveness (Co), chewiness (Ch) and gumminess (G) (Chen and Opara 2013).
Statistical analysis
The statistical analyses used in the present study are the following: one-factor analysis of variance (ANOVA), Linear discriminant analysis (LDA) and Principal component analysis (PCA). The Unscramble × 10.1 (CAMO Process AS, Oslo, Norway) was used for the statistical analysis as well.
Results and discussion
The physico-chemical parameters (moisture content, electrical conductivity, pH, aw, free acidity, refraction index, °Brix concentration, ash, colour parameters (L*, a*, b*, chroma, hue angle, yellow index), fructose, glucose and sucrose content) of 50 honey samples analyzed in this study are presented in Table 1.
Table 1.
Physico-chemical properties of the honeys analysed
| Sample | Moisture Content (%) | Electrical conductivity (µS/cm) | pH | aw | Free Acidity (meq acid/k) | Refraction index | °Brix | Ash (%) |
|---|---|---|---|---|---|---|---|---|
| Acacia | 17.28 ± 0.01 | 136.10 ± 3.10 | 4.81 ± 0.01 | 0.50 ± 0.01 | 4.10 ± 0.21 | 1.49 ± 0.01 | 81.30 ± 0.10 | 0.07 ± 0.01 |
| Acacia | 18.12 ± 0.02 | 148.70 ± 4.10 | 4.75 ± 0.01 | 0.52 ± 0.01 | 4.50 ± 0.21 | 1.49 ± 0.01 | 80.50 ± 0.10 | 0.07 ± 0.01 |
| Acacia | 16.20 ± 0.01 | 116.60 ± 0.80 | 4.34 ± 0.01 | 0.48 ± 0.01 | 29.50 ± 1.20 | 1.50 ± 0.01 | 82.20 ± 0.10 | 0.06 ± 0.01 |
| Acacia | 17.60 ± 0.03 | 137.40 ± 1.20 | 4.74 ± 0.01 | 0.54 ± 0.01 | 5.10 ± 0.80 | 1.49 ± 0.01 | 80.90 ± 0.10 | 0.07 ± 0.01 |
| Acacia | 17.48 ± 0.01 | 176.70 ± 3.10 | 4.46 ± 0.01 | 0.54 ± 0.01 | 6.50 ± 0.10 | 1.49 ± 0.01 | 81.10 ± 0.10 | 0.09 ± 0.01 |
| Acacia | 17.00 ± 0.02 | 140.80 ± 1.90 | 4.45 ± 0.01 | 0.55 ± 0.01 | 6.20 ± 0.20 | 1.49 ± 0.01 | 81.50 ± 0.10 | 0.07 ± 0.01 |
| Acacia | 19.68 ± 0.01 | 134.90 ± 2.91 | 4.45 ± 0.01 | 0.51 ± 0.01 | 6.20 ± 0.10 | 1.50 ± 0.01 | 82.50 ± 0.10 | 0.07 ± 0.01 |
| Acacia | 15.84 ± 0.02 | 192.80 ± 1.60 | 4.03 ± 0.01 | 0.60 ± 0.01 | 10.50 ± 0.30 | 1.49 ± 0.01 | 79.10 ± 0.10 | 0.09 ± 0.01 |
| Acacia | 15.72 ± 0.02 | 184.50 ± 1.70 | 4.06 ± 0.01 | 0.57 ± 0.01 | 11.00 ± 0.30 | 1.49 ± 0.01 | 79.20 ± 0.10 | 0.09 ± 0.01 |
| Acacia | 15.32 ± 0.01 | 197.30 ± 1.90 | 4.40 ± 0.01 | 0.50 ± 0.01 | 7.20 ± 0.50 | 1.50 ± 0.01 | 83.30 ± 0.10 | 0.10 ± 0.01 |
| Tilia | 18.40 ± 0.02 | 423.00 ± 2.90 | 5.34 ± 0.01 | 0.55 ± 0.01 | 3.90 ± 0.10 | 1.49 ± 0.01 | 80.20 ± 0.10 | 0.11 ± 0.01 |
| Tilia | 18.84 ± 0.03 | 419.60 ± 3.10 | 5.36 ± 0.01 | 0.52 ± 0.01 | 3.40 ± 0.20 | 1.49 ± 0.01 | 79.80 ± 0.10 | 0.11 ± 0.01 |
| Tilia | 18.68 ± 0.02 | 775.50 ± 4.00 | 5.01 ± 0.01 | 0.58 ± 0.01 | 15.10 ± 0.70 | 1.48 ± 0.01 | 78.00 ± 0.10 | 0.38 ± 0.01 |
| Tilia | 17.20 ± 0.01 | 453.70 ± 3.50 | 5.18 ± 0.01 | 0.55 ± 0.01 | 6.10 ± 0.20 | 1.49 ± 0.01 | 81.30 ± 0.10 | 0.22 ± 0.01 |
| Tilia | 17.56 ± 0.02 | 667.60 ± 3.20 | 5.07 ± 0.01 | 0.56 ± 0.01 | 9.40 ± 0.30 | 1.49 ± 0.01 | 81.20 ± 0.10 | 0.33 ± 0.01 |
| Tilia | 17.48 ± 0.03 | 682.30 ± 3.10 | 6.39 ± 0.01 | 0.52 ± 0.01 | 4.00 ± 0.10 | 1.49 ± 0.01 | 81.30 ± 0.10 | 0.33 ± 0.01 |
| Tilia | 16.92 ± 0.02 | 670.50 ± 1.90 | 6.28 ± 0.01 | 0.52 ± 0.01 | 4.20 ± 0.10 | 1.49 ± 0.01 | 81.80 ± 0.10 | 0.33 ± 0.01 |
| Tilia | 15.40 ± 0.01 | 702.30 ± 2.90 | 5.48 ± 0.01 | 0.53 ± 0.01 | 6.90 ± 0.20 | 1.50 ± 0.01 | 83.20 ± 0.10 | 0.34 ± 0.01 |
| Polyfloral | 17.84 ± 0.02 | 109.90 ± 1.90 | 5.40 ± 0.01 | 0.54 ± 0.01 | 5.20 ± 0.10 | 1.49 ± 0.01 | 50.60 ± 0.10 | 0.05 ± 0.01 |
| Polyfloral | 15.43 ± 0.02 | 706.80 ± 1.40 | 4.66 ± 0.01 | 0.50 ± 0.01 | 21.90 ± 0.20 | 1.50 ± 0.01 | 83.20 ± 0.10 | 0.35 ± 0.01 |
| Polyfloral | 17.12 ± 0.01 | 340.30 ± 2.10 | 4.11 ± 0.01 | 0.54 ± 0.01 | 14.70 ± 0.30 | 1.49 ± 0.01 | 81.40 ± 0.10 | 0.17 ± 0.01 |
| Polyfloral | 15.53 ± 0.02 | 489.50 ± 3.10 | 4.55 ± 0.01 | 0.52 ± 0.01 | 11.10 ± 0.10 | 1.50 ± 0.01 | 83.00 ± 0.10 | 0.24 ± 0.01 |
| Polyfloral | 17.12 ± 0.01 | 420.30 ± 3.50 | 3.88 ± 0.01 | 0.54 ± 0.01 | 33.10 ± 0.10 | 1.49 ± 0.01 | 80.90 ± 0.10 | 0.21 ± 0.01 |
| Polyfloral | 19.64 ± 0.02 | 465.10 ± 3.60 | 4.20 ± 0.01 | 0.56 ± 0.01 | 16.40 ± 0.30 | 1.49 ± 0.01 | 78.70 ± 0.10 | 0.23 ± 0.01 |
| Polyfloral | 17.40 ± 0.02 | 360.10 ± 1.90 | 4.61 ± 0.01 | 0.55 ± 0.01 | 9.80 ± 0.10 | 1.49 ± 0.01 | 81.00 ± 0.10 | 0.18 ± 0.01 |
| Polyfloral | 16.20 ± 0.02 | 352.90 ± 1.80 | 4.07 ± 0.01 | 0.53 ± 0.01 | 28.50 ± 0.30 | 1.50 ± 0.01 | 82.50 ± 0.10 | 0.17 ± 0.01 |
| Polyfloral | 16.96 ± 0.01 | 464.00 ± 2.10 | 4.05 ± 0.01 | 0.54 ± 0.01 | 26.80 ± 0.20 | 1.49 ± 0.01 | 81.70 ± 0.10 | 0.23 ± 0.01 |
| Polyfloral | 16.83 ± 0.02 | 504.20 ± 2.60 | 4.00 ± 0.01 | 0.54 ± 0.01 | 32.00 ± 0.40 | 1.49 ± 0.01 | 81.90 ± 0.10 | 0.25 ± 0.01 |
| Polyfloral | 17.48 ± 0.02 | 469.10 ± 2.40 | 4.33 ± 0.01 | 0.57 ± 0.01 | 37.10 ± 0.50 | 1.49 ± 0.01 | 81.20 ± 0.10 | 0.23 ± 0.01 |
| Polyfloral | 17.00 ± 0.01 | 495.10 ± 2.60 | 4.57 ± 0.01 | 0.54 ± 0.01 | 13.40 ± 0.20 | 1.49 ± 0.01 | 81.60 ± 0.10 | 0.24 ± 0.01 |
| Honeydew | 14.44 ± 0.02 | 923.30 ± 3.90 | 5.10 ± 0.01 | 0.53 ± 0.01 | 16.60 ± 0.20 | 1.50 ± 0.01 | 84.10 ± 0.10 | 0.45 ± 0.01 |
| Honeydew | 16.76 ± 0.01 | 1276.80 ± 4.20 | 4.25 ± 0.01 | 0.51 ± 0.01 | 12.10 ± 0.30 | 1.48 ± 0.01 | 81.70 ± 0.10 | 0.63 ± 0.01 |
| Honeydew | 17.04 ± 0.02 | 1091.80 ± 2.10 | 4.19 ± 0.01 | 0.53 ± 0.01 | 11.80 ± 0.10 | 1.49 ± 0.01 | 81.30 ± 0.10 | 0.54 ± 0.01 |
| Honeydew | 15.84 ± 0.01 | 1157.00 ± 2.60 | 5.06 ± 0.01 | 0.52 ± 0.01 | 20.00 ± 0.30 | 1.50 ± 0.01 | 82.60 ± 0.10 | 0.57 ± 0.01 |
| Honeydew | 16.60 ± 0.02 | 937.80 ± 2.80 | 5.16 ± 0.01 | 0.54 ± 0.01 | 17.00 ± 0.40 | 1.50 ± 0.01 | 81.90 ± 0.10 | 0.46 ± 0.01 |
| Honeydew | 15.92 ± 0.01 | 843.70 ± 3.10 | 4.53 ± 0.01 | 0.54 ± 0.01 | 16.50 ± 0.50 | 1.50 ± 0.01 | 82.40 ± 0.10 | 0.41 ± 0.01 |
| Honeydew | 17.20 ± 0.02 | 823.10 ± 2.50 | 5.09 ± 0.01 | 0.58 ± 0.01 | 17.10 ± 0.20 | 1.49 ± 0.01 | 81.30 ± 0.10 | 0.40 ± 0.01 |
| Honeydew | 16.52 ± 0.01 | 1015.00 ± 2.40 | 5.17 ± 0.01 | 0.56 ± 0.01 | 16.80 ± 0.30 | 1.50 ± 0.01 | 82.00 ± 0.10 | 0.50 ± 0.01 |
| Honeydew | 16.44 ± 0.02 | 1003.00 ± 2.60 | 5.15 ± 0.01 | 0.54 ± 0.01 | 16.80 ± 0.10 | 1.50 ± 0.01 | 82.10 ± 0.10 | 0.49 ± 0.01 |
| Sunflower | 19.60 ± 0.01 | 281.10 ± 1.90 | 3.99 ± 0.01 | 0.54 ± 0.01 | 14.60 ± 0.10 | 1.49 ± 0.01 | 79.00 ± 0.10 | 0.14 ± 0.01 |
| Sunflower | 19.16 ± 0.03 | 631.40 ± 2.10 | 4.84 ± 0.01 | 0.60 ± 0.01 | 9.90 ± 0.20 | 1.49 ± 0.01 | 78.40 ± 0.10 | 0.31 ± 0.01 |
| Sunflower | 19.48 ± 0.01 | 359.00 ± 2.10 | 4.11 ± 0.01 | 0.57 ± 0.01 | 12.00 ± 0.10 | 1.49 ± 0.01 | 79.10 ± 0.10 | 0.18 ± 0.01 |
| Sunflower | 18.20 ± 0.02 | 346.90 ± 2.60 | 4.07 ± 0.01 | 0.58 ± 0.01 | 13.60 ± 0.30 | 1.49 ± 0.01 | 79.20 ± 0.10 | 0.17 ± 0.01 |
| Sunflower | 16.16 ± 0.01 | 353.20 ± 1.70 | 4.17 ± 0.01 | 0.51 ± 0.01 | 14.40 ± 0.40 | 1.50 ± 0.01 | 82.40 ± 0.10 | 0.17 ± 0.01 |
| Sunflower | 15.80 ± 0.02 | 416.40 ± 1.60 | 4.45 ± 0.01 | 0.49 ± 0.01 | 11.90 ± 0.10 | 1.50 ± 0.01 | 82.70 ± 0.10 | 0.20 ± 0.01 |
| Sunflower | 19.89 ± 0.01 | 261.50 ± 1.20 | 4.01 ± 0.01 | 0.57 ± 0.01 | 13.00 ± 0.20 | 1.48 ± 0.01 | 77.90 ± 0.10 | 0.13 ± 0.01 |
| Sunflower | 17.16 ± 0.02 | 248.10 ± 1.10 | 4.05 ± 0.01 | 0.52 ± 0.01 | 15.20 ± 0.10 | 1.49 ± 0.01 | 81.40 ± 0.10 | 0.12 ± 0.01 |
| Sunflower | 17.64 ± 0.01 | 336.70 ± 1.60 | 4.06 ± 0.01 | 0.54 ± 0.01 | 15.50 ± 0.20 | 1.49 ± 0.01 | 81.10 ± 0.10 | 0.17 ± 0.01 |
| Sunflower | 17.12 ± 0.02 | 339.90 ± 1.80 | 4.02 ± 0.01 | 0.52 ± 0.01 | 16.80 ± 0.10 | 1.49 ± 0.01 | 80.90 ± 0.10 | 0.17 ± 0.01 |
| Sunflower | 17.60 ± 0.01 | 232.90 ± 2.10 | 4.26 ± 0.01 | 0.56 ± 0.01 | 6.30 ± 0.10 | 1.49 ± 0.01 | 80.80 ± 0.10 | 0.11 ± 0.01 |
| SSample | L* | a* | b* | Chroma (C*) | Hue angle | Yellow index | Fructose (g/100 g) | Glucose (g/100 g) |
|---|---|---|---|---|---|---|---|---|
| Acacia | 45.10 ± 0.25 | − 0.67 ± 0.15 | 15.11 ± 0.05 | 15.12 ± 0.09 | 3.74 ± 0.20 | 47.87 ± 0.81 | 48.31 ± 0.45 | 22.06 ± 0.45 |
| Acacia | 45.20 ± 0.29 | − 0.70 ± 0.21 | 15.06 ± 0.15 | 15.08 ± 0.08 | − 1.14 ± 0.21 | 47.60 ± 0.21 | 47.78 ± 0.65 | 23.71 ± 0.25 |
| Acacia | 48.08 ± 0.22 | − 1.23 ± 0.23 | 7.37 ± 0.35 | 7.47 ± 0.04 | 3.13 ± 0.05 | 21.89 ± 0.21 | 42.80 ± 0.85 | 27.10 ± 1.01 |
| Acacia | 47.20 ± 0.28 | − 1.96 ± 0.26 | 9.70 ± 0.21 | 9.89 ± 0.05 | 0.23 ± 0.25 | 29.35 ± 0.82 | 43.40 ± 1.05 | 27.90 ± 1.21 |
| Acacia | 47.15 ± 0.21 | − 1.93 ± 0.29 | 11.58 ± 0.29 | 11.74 ± 0.09 | 3.46 ± 0.15 | 35.09 ± 0.71 | 47.88 ± 1.15 | 28.07 ± 0.75 |
| Acacia | 46.47 ± 0.29 | − 1.54 ± 0.20 | 10.39 ± 0.22 | 10.51 ± 0.12 | − 2.00 ± 0.65 | 31.95 ± 0.12 | 45.00 ± 1.02 | 31.00 ± 0.60 |
| Acacia | 44.62 ± 0.31 | − 0.97 ± 0.22 | 10.39 ± 0.16 | 10.44 ± 0.19 | − 0.33 ± 0.15 | 33.28 ± 0.09 | 47.62 ± 1.01 | 23.84 ± 0.76 |
| Acacia | 44.01 ± 0.12 | − 0.17 ± 0.21 | 13.66 ± 0.15 | 13.66 ± 0.19 | − 0.33 ± 0.25 | 44.34 ± 0.08 | 48.08 ± 0.45 | 24.55 ± 1.01 |
| Acacia | 44.08 ± 0.20 | − 0.15 ± 0.15 | 13.67 ± 0.20 | 13.67 ± 0.20 | − 13.44 ± 0.35 | 44.31 ± 0.20 | 41.80 ± 0.65 | 28.20 ± 0.45 |
| Acacia | 44.44 ± 0.23 | − 0.88 ± 0.05 | 12.66 ± 0.26 | 12.69 ± 0.19 | 0.33 ± 0.21 | 40.71 ± 0.20 | 43.60 ± 0.65 | 28.60 ± 0.97 |
| Tilia | 44.10 ± 0.24 | − 0.11 ± 0.06 | 15.77 ± 0.21 | 15.77 ± 0.30 | − 0.36 ± 0.20 | 51.09 ± 0.25 | 40.00 ± 0.55 | 29.25 ± 0.54 |
| Tilia | 32.37 ± 0.21 | − 0.17 ± 0.09 | 14.57 ± 0.22 | 14.57 ± 0.24 | 3.96 ± 0.26 | 64.32 ± 0.25 | 43.14 ± 0.95 | 30.67 ± 0.68 |
| Tilia | 43.37 ± 0.28 | 0.42 ± 0.02 | 16.17 ± 0.25 | 16.17 ± 0.10 | − 6.68 ± 0.27 | 53.25 ± 0.19 | 44.39 ± 0.40 | 34.52 ± 0.42 |
| Tilia | 43.74 ± 0.18 | − 0.57 ± 0.05 | 14.06 ± 0.29 | 14.07 ± 0.26 | − 3.32 ± 0.28 | 45.91 ± 0.27 | 42.99 ± 0.50 | 34.51 ± 0.68 |
| Tilia | 41.26 ± 0.23 | 1.17 ± 0.03 | 14.23 ± 0.24 | 14.28 ± 0.09 | − 2.47 ± 0.21 | 49.28 ± 0.15 | 40.24 ± 0.95 | 32.65 ± 0.54 |
| Tilia | 41.43 ± 0.20 | 1.75 ± 0.09 | 15.33 ± 0.28 | 15.43 ± 0.08 | − 1.37 ± 0.20 | 52.87 ± 0.16 | 42.00 ± 0.85 | 33.14 ± 0.42 |
| Tilia | 40.21 ± 0.25 | 1.63 ± 0.10 | 14.57 ± 0.22 | 14.66 ± 0.20 | − 1.96 ± 0.26 | 51.76 ± 0.23 | 40.87 ± 0.95 | 32.58 ± 0.21 |
| Tilia | 40.88 ± 0.26 | 1.71 ± 0.11 | 14.37 ± 0.21 | 14.47 ± 0.16 | − 0.61 ± 0.21 | 50.21 ± 0.18 | 42.26 ± 1.05 | 33.55 ± 0.46 |
| Polyfloral | 44.87 ± 0.23 | − 1.08 ± 0.05 | 13.73 ± 0.20 | 13.77 ± 0.05 | 0.40 ± 0.21 | 43.70 ± 0.16 | 35.71 ± 1.05 | 34.51 ± 0.98 |
| Polyfloral | 34.09 ± 0.24 | 6.97 ± 0.01 | 9.20 ± 0.05 | 11.54 ± 0.09 | 0.26 ± 0.22 | 38.57 ± 0.14 | 36.21 ± 1.10 | 35.50 ± 1.06 |
| Polyfloral | 40.33 ± 0.19 | 4.01 ± 0.03 | 15.21 ± 0.06 | 15.73 ± 0.12 | 1.31 ± 0.21 | 53.87 ± 0.18 | 34.98 ± 1.20 | 33.96 ± 1.09 |
| Polyfloral | 41.11 ± 0.19 | 2.11 ± 0.04 | 15.93 ± 0.09 | 16.07 ± 0.16 | 0.33 ± 0.23 | 55.37 ± 0.16 | 36.84 ± 0.95 | 36.12 ± 1.20 |
| Polyfloral | 40.00 ± 0.24 | 4.37 ± 0.06 | 14.67 ± 0.18 | 15.30 ± 0.20 | 4.60 ± 0.233 | 52.38 ± 0.21 | 35.61 ± 0.95 | 34.57 ± 1.09 |
| Polyfloral | 43.43 ± 0.22 | 1.67 ± 0.05 | 17.15 ± 0.26 | 17.23 ± 0.26 | 0.91 ± 0.21 | 56.42 ± 0.22 | 34.31 ± 0.48 | 34.57 ± 1.45 |
| Polyfloral | 39.79 ± 0.27 | 2.26 ± 0.02 | 13.53 ± 0.23 | 13.72 ± 0.26 | − 3.41 ± 0.15 | 48.59 ± 0.19 | 33.64 ± 0.35 | 32.98 ± 0.87 |
| Polyfloral | 40.06 ± 0.28 | 3.20 ± 0.03 | 14.76 ± 0.35 | 15.10 ± 0.18 | 0.10 ± 0.29 | 52.62 ± 0.25 | 37.21 ± 0.29 | 36.13 ± 0.95 |
| Polyfloral | 38.52 ± 0.23 | 5.33 ± 0.04 | 13.38 ± 0.41 | 14.40 ± 0.16 | − 1.37 ± 0.24 | 49.61 ± 0.21 | 34.98 ± 0.98 | 34.29 ± 0.32 |
| Polyfloral | 38.89 ± 0.29 | 4.96 ± 0.06 | 13.72 ± 0.35 | 14.59 ± 0.17 | − 2.54 ± 0.21 | 50.40 ± 0.19 | 35.14 ± 1.02 | 34.12 ± 0.54 |
| Polyfloral | 40.59 ± 0.24 | 2.35 ± 0.07 | 14.70 ± 0.15 | 14.89 ± 0.16 | − 5.28 ± 0.23 | 51.75 ± 0.21 | 37.65 ± 1.01 | 36.20 ± 0.69 |
| Polyfloral | 36.83 ± 0.22 | 4.12 ± 0.01 | 11.40 ± 0.55 | 12.12 ± 0.12 | − 2.54 ± 0.24 | 44.22 ± 0.18 | 35.97 ± 0.45 | 35.26 ± 0.65 |
| Honeydew | 21.02 ± 0.24 | 4.16 ± 0.03 | 5.92 ± 0.15 | 7.23 ± 0.10 | 0.15 ± 0.26 | 40.22 ± 0.16 | 40.98 ± 0.49 | 34.21 ± 0.69 |
| Honeydew | 20.16 ± 0.23 | 7.68 ± 0.04 | 5.55 ± 0.25 | 9.48 ± 0.09 | 1.14 ± 0.27 | 39.31 ± 0.18 | 39.01 ± 0.85 | 36.54 ± 0.61 |
| Honeydew | 19.60 ± 0.19 | 5.81 ± 0.09 | 4.16 ± 0.21 | 7.15 ± 0.15 | 1.15 ± 0.20 | 30.30 ± 0.16 | 37.51 ± 0.67 | 35.21 ± 1.00 |
| Honeydew | 19.80 ± 0.23 | 4.48 ± 0.15 | 4.75 ± 0.22 | 6.53 ± 0.16 | 0.56 ± 0.15 | 34.30 ± 0.15 | 35.96 ± 1.06 | 32.98 ± 1.05 |
| Honeydew | 21.52 ± 0.26 | 5.65 ± 0.19 | 6.90 ± 0.29 | 8.92 ± 0.18 | 0.36 ± 0.05 | 45.82 ± 0.09 | 40.21 ± 1.08 | 36.40 ± 0.98 |
| Honeydew | 25.94 ± 0.27 | 5.47 ± 0.17 | 10.27 ± 0.23 | 11.64 ± 0.60 | − 0.32 ± 0.01 | 56.59 ± 0.23 | 40.69 ± 1.44 | 34.52 ± 0.54 |
| Honeydew | 22.31 ± 0.21 | 6.08 ± 0.13 | 7.23 ± 0.28 | 9.44 ± 0.12 | 0.40 ± 0.01 | 46.30 ± 0.11 | 38.69 ± 0.45 | 35.69 ± 0.50 |
| Honeydew | 22.26 ± 0.24 | 6.46 ± 0.12 | 7.55 ± 0.29 | 9.94 ± 0.50 | 0.43 ± 0.02 | 48.45 ± 0.26 | 39.21 ± 0.52 | 36.97 ± 0.69 |
| Honeydew | 22.15 ± 0.27 | 6.16 ± 0.23 | 7.13 ± 0.27 | 9.42 ± 0.16 | 0.44 ± 0.05 | 45.97 ± 0.15 | 39.64 ± 0.69 | 36.59 ± 0.78 |
| Sunflower | 31.70 ± 0.41 | 2.31 ± 0.19 | 15.58 ± 0.15 | 15.75 ± 0.17 | 2.08 ± 0.25 | 70.20 ± 0.21 | 40.10 ± 0.67 | 36.50 ± 0.65 |
| Sunflower | 31.34 ± 0.21 | 1.46 ± 0.17 | 15.29 ± 0.20 | 15.36 ± 0.18 | 0.66 ± 0.05 | 69.68 ± 0.16 | 38.10 ± 0.65 | 35.10 ± 0.59 |
| Sunflower | 40.80 ± 0.25 | 1.63 ± 0.18 | 15.63 ± 0.12 | 15.72 ± 0.16 | 10.86 ± 0.25 | 54.74 ± 0.22 | 38.91 ± 0.42 | 34.74 ± 0.69 |
| Sunflower | 43.02 ± 0.31 | 1.55 ± 0.10 | 17.54 ± 0.05 | 17.61 ± 0.15 | − 0.38 ± 0.05 | 58.25 ± 0.11 | 37.62 ± 0.75 | 34.20 ± 1.02 |
| Sunflower | 36.75 ± 0.34 | 2.78 ± 0.13 | 12.03 ± 0.19 | 12.35 ± 0.14 | 0.41 ± 0.05 | 46.78 ± 0.09 | 39.40 ± 0.69 | 36.15 ± 0.98 |
| Sunflower | 41.77 ± 1.20 | 2.45 ± 0.15 | 15.93 ± 0.18 | 16.12 ± 0.13 | 5.06 ± 0.25 | 54.48 ± 0.21 | 37.98 ± 0.75 | 34.65 ± 1.03 |
| Sunflower | 43.33 ± 2.20 | 1.33 ± 0.10 | 17.90 ± 0.16 | 17.95 ± 0.12 | 0.81 ± 0.06 | 59.03 ± 0.09 | 40.89 ± 0.89 | 36.51 ± 0.89 |
| Sunflower | 39.54 ± 0.90 | 2.85 ± 0.09 | 13.49 ± 0.05 | 13.78 ± 0.09 | − 0.02 ± 0.01 | 48.73 ± 0.06 | 40.61 ± 0.76 | 37.29 ± 1.05 |
| Sunflower | 41.31 ± 0.10 | 2.48 ± 0.08 | 15.77 ± 0.09 | 15.96 ± 0.18 | 17.01 ± 0.25 | 54.52 ± 0.21 | 41.69 ± 0.55 | 38.25 ± 1.08 |
| Sunflower | 39.71 ± 0.90 | 2.23 ± 0.20 | 14.83 ± 0.08 | 15.00 ± 0.16 | 2.63 ± 0.25 | 53.35 ± 0.22 | 39.35 ± 2.05 | 36.74 ± 0.69 |
| Sunflower | 45.22 ± 0.50 | 1.78 ± 0.07 | 18.24 ± 0.05 | 18.32 ± 0.17 | − 0.97 ± 0.25 | 57.61 ± 0.23 | 38.91 ± 0.95 | 35.30 ± 0.78 |
The honey moisture content ranged between 14.44 and 19.89%. None of the samples exceeded the limit of 20% established by the Codex Alimentarius (2001). Out of the five botanical origins of honeys, it can be observed that the highest moisture content was observed for sunflower honeys, while the honeydew honeys presented the lowest levels. The difference of moisture content according to their origin was significant (P < 0.05). A higher concentration than 20% will accelerate the fermentation process during honey storage (Oroian 2012). The moisture content of the honeys analyzed were similar to those reported earlier (Bath and Singh 1999; Oroian et al. 2013; Escriche et al. 2011; Flores et al. 2015). Singh and Bath (1997) reported similar moisture content for Trifolium and Eucalyptus lanceolatus honeys, but the higher moisture content for Brassica juncea honey.
The deterioration of honey can be shown by the free acidity level. This parameter is characterized by the presence of different substances such as organic acids, lactone and some inorganic ions [e.g. phosphates, sulphates and chlorides (Oroian and Ropciuc 2017)]. The free acidity maximum level for mono and polyfloral honeys was of 40 meq acid/kg, while for honeydew honeys the level was 50 meq acid/kg (Codex Alimentarius 2001). Higher values than those established may stand for fermentation of sugars in organic acids (da Silva et al. 2016). In this study neither mono and polyfloral nor the honeydew honeys have exceeded the maximum allowable level. The honey acidity was in the same range with those reported by Oroian and Ropciuc (2017), Bath and Singh (1999) and Singh and Bath (1997).
The pH values of honey samples were in the acidic nature, ranging between 3.88 and 6.39. The acid nature of honey was due to the presence of amino acids and organic acids (Oroian 2012). The pH values of the analyzed honeys were consistent showed to those reported for honeys from Algeria (Khalil et al. 2012), India (Singh and Bath 1997; Nanda et al. 2009) and Spain (Oroian et al. 2013). Tilia and acacia samples the lowest pH values, while the honeydew and polyfloral honeys showed the highest.
The electrical conductivity of honey (Table 1) is a parameter correlated to acidity and ash content (Yücel and Sultanoglu 2013). A value of the electrical conductivity higher than 800 µS/cm is specific to honeydew honeys (International Honey Commission 2009). Honeydew honeys had the highest electrical conductivity (1276.80 µS/cm) and the lowest electrical conductivity was observed for acacia sample (136.10 µS/cm). The botanical origin of honeys influenced significantly the electrical conductivity (P < 0.05).
Ash content is a one of the quality parameters of honey which is expressed by the mineral content. The values of ash content is not established by the Codex Alimentarius standards (2001), but according to the scientific literature, the average ash content in honey ranged from 0.02 to 1.03% (Chakir et al. 2011). Ash content of the honey samples analyzed ranged between 0.05 and 0.63%. The acacia honeys presented the lowest ash content, while the honeydew exhibited the highest.
Water activity of honey is influenced by the molar concentration of the soluble species especially by those which have high molecular mass (e.g. proteins, aminoacids, enzymes, vitamins, minerals or aroma compounds), but there were present in low concentrations in honey and cannot contribute significantly. For this reason, honey water activity was influenced mainly by the fructose and glucose concentrations and to lesser extent by sucrose (Sagona et al. 2017). The water activity ranged between 0.48 and 0.60 (P < 0.05). The values were similar to honeys analyzed by Sagona et al. (2017).
The honey color was an important parameter used for its authentication and is considered the first attribute of a honey. The color of honeys ranged from colorless to black (Belay et al. 2015). The color parameters of the honey analyzed are shown in Table 1. In terms of luminosity (L*) it can be observed that acacia honeys and tilia present the highest values. The highest values of L* indicated that the honeys were translucent (acacia honeys), while low values of L* indicated that the honey were dark (honeydew honeys). The lowest chroma (C*) value was observed for honeydew, while the sunflower had the highest values. Sunflower honeys presented the highest yellow index. The color parameters were different accordingly to the botanical origins (P < 0.001). The differences in terms of color between the different honey types are due to the chemical composition and variety (Oroian 2012). The color parameters were in agreement with those reported earlier (Habib et al. 2014; Escriche et al. 2014). According to the Pearson correlation, the L* was correlated negatively with ash content (− 0.850**), electrical conductivity (− 0.855**) and positively with sucrose content (0.698**). The a* was correlated positively with free acidity (0.527**), electrical conductivity (0.778**), ash content (0.780**) and glucose content (0.627**), while b* is correlated positively with the moisture content (0.461**), and negatively with the electrical conductivity (− 0.597**) and ash content (− 0.600**).
Honey sugars’ concentrations are influenced by the botanical origin, and they are produced by honey-bees from nectar sucrose, the latter one being transformed into glucose and fructose through enzymatic hydrolysis by the bees (da Silva et al. 2016). Codex Alimentarius (2001) established that the concentration of fructose and glucose must be higher than 60% in all honey samples. In our study all the honeys had a glucose and fructose concentration higher than 60%. The highest concentrations of fructose were determined in acacia honeys (Table 1). The polyfloral honeys had the highest concentration of glucose while honeydew did not present any concentration of sucrose.
The textural properties of honeys have been determined using a texturemetre device, and the parameters measured were: viscosity (V), hardness (H), adhesion (A), springiness (S), cohesiveness (Co), chewiness (Ch) and gumminess (G). The textural parameters of honey have not been reported till now as a part of honey authentication to the author’s knowledge. A typical TPA profile for honey samples is shown in Fig. 1. The evolution of the textural parameters of honey according to the botanical origin is shown in Fig. 2. The honeydew honeys exhibit the highest hardness, viscosity, gumminess and chewiness. The botanical origin does not influence cohesiveness and springiness (P > 0.05). The textural parameters are highly correlated between them, as follows: hardness with viscosity (r = 0.911**), hardness with adhesion (r = 0.853**), hardness with gumminess (r = 0.979**), hardness with chewiness (r = 0.930**), viscosity with adhesion (r = 0.909**), viscosity with gumminess (r = 0.889**), viscosity with chewiness (r = 0.874**), adhesion with gumminess (r = 0.837**), springiness with chewiness (r = 0.790**), cohesiveness with gumminess (r = 0.496**), cohesiveness with chewiness (r = 0.487**) and gumminess with chewiness (r = 0.955**), respectively. Regarding the correlation of the textural parameters with physico-chemical properties, the textural parameters are negatively correlated with water activity and moisture content. The moisture content influences negatively the evolution of the textural parameters because water has the ability of modifying the speed of molecules (Oroian 2012).
Fig. 1.
Honey texture profile
Fig. 2.

Honey texture parameters evolution
Chemometrics
PCA (principal component analysis) and LDA (linear discriminant analysis) applied to the physico-chemical (pH, aw, free acidity, ash content, moisture content, color (L*, a*, b*, hue-angle, chroma and yellow index (YI)), fructose, glucose and sucrose content) and textural (viscosity (V), hardness (H), adhesion (A), springiness (S), cohesiveness (Co), chewiness (Ch) and gumminess (G)) parameters of 50 honey samples. PCA and LDA have been applied to achieve whether the physico-chemical and textural parameters can be used for the discrimination of honeys based on their botanical origin.
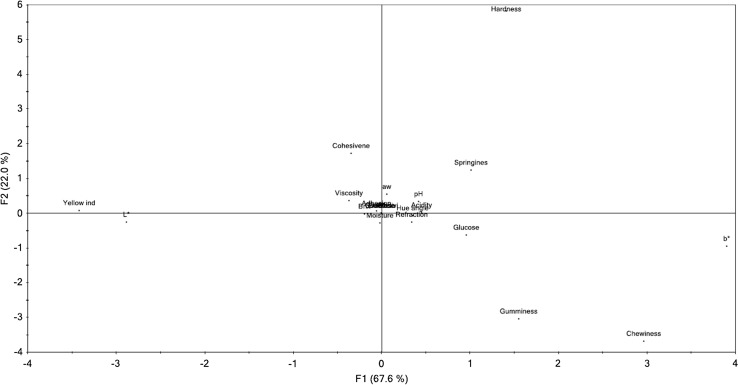
PCA was used to see the influence of physico-chemical and textural parameters on the honey botanical origin using a descriptive point of view. PCA scores and loadings are presented in the Figs. 3 and 4. The PC1 and PC2 explained 99% of the variations; the PC1 explains 98%, while the PC2 explains 1% of the variations. The PC2 divides the samples acacia and sunflower of tilia and honeydew, while the polyfloral honeys are placed near to the coordinates origins. The groups were well defined and the samples were grouped according to their botanical origin. The parameters which influenced more the projection were placed in the outer ellipse, while the samples placed in the inner ellipse did not influence to great extent.
Fig. 3.
Principal component analysis of the multi-elements scores of acacia (A), sunflower (S), tilia (T), polyfloral (P) and honeydew honeys (H)
Fig. 4.
Principal component analysis of the physicochemical and textural parameters: H—hardness, V—viscosity, Sp—springiness, Co—cohesiveness, Gu—gumminess, Ch—chewiness, pH, aw, A—free acidity, RI—refraction index, M—moisture content, C—conductivity, Ac—ash content, L*, a*, b*, C*, ha—hue angle, YI—yellow index, F—fructose, G—glucose, S—sucrose
LDA was applied to physico-chemical and textural properties using the stepwise method. The correct classification of the honeys based on their botanical origin has been achieved for 96.0% of the samples using the original grouped, while in the case of the cross-validated grouped the correct classification could be achieved for 92% of the samples. Table 2 presents the classification of the honeys using these two methods. The LDA resulted into two canonical functions with the eigen values 23.041 and 7.519. Total variance of 67% was explained by the function 1, while 22.0% was explained by function 2. It can be observed in Fig. 5 the projection of the samples of honeydew, tilia and acacia honeys being well defined as groups, while sunflower and polyfloral are mixed in some region. The b* parameter (F1 = 3.901, F2 = − 0.952) dominated the first function, followed closely by chewiness (F1 = 2.966, F2 = − 3.694). The hardness parameter (F1 = 1.412, F2 = 5.818) dominated the second discriminant function, followed by cohesiveness (F1 = − 0.341, F2 = 1.724) and springiness (F1 = 1.011, F2 = 1.231) (Fig. 6).
Table 2.
Classification results of the analysed honeys using the LDA
| Model | Honey type | Predicted group membership % | Total | ||||
|---|---|---|---|---|---|---|---|
| Acacia | Tilia | Polyfloral | Honeydew | Sunflower | |||
| Original group | Acacia | 100 | 0 | 0 | 0 | 0 | 100 |
| Tilia | 0 | 100 | 0 | 0 | 0 | 100 | |
| Polyfloral | 0 | 8.30 | 83.30 | 0 | 8.30 | 100 | |
| Honeydew | 0 | 0 | 0 | 100 | 0 | 100 | |
| Sunflower | 0 | 0 | 0 | 0 | 100 | 100 | |
| Cross-validated | Acacia | 100 | 0 | 0 | 0 | 0 | 100 |
| Tilia | 0 | 75.0 | 12.5 | 0 | 12.50 | 100 | |
| Polyfloral | 0 | 8.30 | 83.20 | 0 | 8.30 | 100 | |
| Honeydew | 0 | 0 | 0 | 100 | 0 | 100 | |
| Sunflower | 0 | 0 | 9.09 | 0 | 90.91 | 100 | |
Fig. 5.
Linear discriminant score plot of honeys analysed
Fig. 6.
Linear discriminant score loadings
Conclusion
The present paper approaches a new perspective of honey authentication based on physico-chemical, textural parameters and chemometrics. The physico-chemical parameters of honeys are in agreement with other honeys presented in the literature. The PCA and LDA have proved the possibility of authenticating honeys by the use of the physico-chemical and textural properties. Thus, by means of the LDA it was achieved a 92.0% correct classification of honeys using the cross validation grouped, and 96.0% correct classification of honeys using the original validation grouped. In the LDA projection, the textural parameters (chewiness, hardness, cohesiveness, springiness) dominated the two functions.
Acknowledgements
This work was supported by a grant of the Romanian National Authority for Scientific Research and Innovation, CNCS – UEFISCDI, Project Number PN-II-RU-TE-2014-4-0110.
References
- Bath PK, Singh N. A comparison between Helianthus annuus and Eucalyptus lanceolatus honey. Food Chem. 1999;67(4):389–397. doi: 10.1016/S0308-8146(99)00132-6. [DOI] [Google Scholar]
- Beitlich N, Koelling-Speer I, Oelschlaegel S, Speer K. Differentiation of Manuka honey from Kanuka honey and from jelly bush honey using HS-SPME-GC/MS and UHPLC-PDA-MS/MS. J Agric Food Chem. 2014;62(27):6435–6444. doi: 10.1021/jf501818f. [DOI] [PubMed] [Google Scholar]
- Belay A, Solomon WK, Bultossa G, Adgaba N, Melaku S. Botanical origin, colour, granulation, and sensory properties of the Harenna forest honey, Bale, Ethiopia. Food Chem. 2015;167:213–219. doi: 10.1016/j.foodchem.2014.06.080. [DOI] [PubMed] [Google Scholar]
- Castro-Vázquez L, Leon-Ruiz V, Alañon ME, Pérez-Coello MS, González-Porto AV. Floral origin markers for authenticating Lavandin honey (Lavandula angustifolia x latifolia). Discrimination from Lavender honey (Lavandula latifolia) Food Control. 2014;37:362–370. doi: 10.1016/j.foodcont.2013.09.003. [DOI] [Google Scholar]
- Chakir A, Romane A, Marcazzan GL, Ferrazzi P. Physicochemical properties of some honeys produced from different plants in Morocco. Arab J Chem. 2016;9:946–954. doi: 10.1016/j.arabjc.2011.10.013. [DOI] [Google Scholar]
- Chen L, Opara UL. Texture measurement approaches in fresh and processed foods—a review. Food Res Int. 2013;51(2):823–835. doi: 10.1016/j.foodres.2013.01.046. [DOI] [Google Scholar]
- Ciaccheri L, Mignani AG, Mencaglia AA, Di Sanzo R, Carabetta S, Russo MT. Nondestructive and rapid authentication of honey using dispersive raman spectroscopy. AISEM Ann Conf. 2015;18:1–4. [Google Scholar]
- Codex Alimentarius (2001) Revised codex standards for honey. Codex Standard 12-1981, Rev. 2
- da Silva PM, Gauche C, Gonzaga LV, Costa ACO, Fett R. Honey: chemical composition, stability and authenticity. Food Chem. 2016;196:309–323. doi: 10.1016/j.foodchem.2015.09.051. [DOI] [PubMed] [Google Scholar]
- Escriche I, Kadar M, Juan-Borrás M, Domenech E. Using flavonoids, phenolic compounds and headspace volatile profile for botanical authentication of lemon and orange honeys. Food Res Int. 2011;44(5):1504–1513. doi: 10.1016/j.foodres.2011.03.049. [DOI] [Google Scholar]
- Escriche I, Kadar M, Juan-Borrás M, Domenech E. Suitability of antioxidant capacity, flavonoids and phenolic acids for floral authentication of honey. Impact of industrial thermal treatment. Food Chem. 2014;142:135–143. doi: 10.1016/j.foodchem.2013.07.033. [DOI] [PubMed] [Google Scholar]
- Escuredo O, Dobre I, Fernández-González M, Seijo MC. Contribution of botanical origin and sugar composition of honeys on the crystallization phenomenon. Food Chem. 2014;149:84–90. doi: 10.1016/j.foodchem.2013.10.097. [DOI] [PubMed] [Google Scholar]
- Flores MSR, Escuredo O, Seijo MC. Assessment of physicochemical and antioxidant characteristics of Quercus pyrenaica honeydew honeys. Food Chem. 2015;166:101–106. doi: 10.1016/j.foodchem.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Gok S, Severcan M, Goormaghtigh E, Kandemir I, Severcan F. Differentiation of Anatolian honey samples from different botanical origins by ATR-FTIR spectroscopy using multivariate analysis. Food Chem. 2015;170:234–240. doi: 10.1016/j.foodchem.2014.08.040. [DOI] [PubMed] [Google Scholar]
- Habib HM, Al Meqbali FT, Kamal H, Souka UD, Ibrahim WH. Physicochemical and biochemical properties of honeys from arid regions. Food Chem. 2014;153:35–43. doi: 10.1016/j.foodchem.2013.12.048. [DOI] [PubMed] [Google Scholar]
- International Honey Commission (2009) Harmonised methods of the international honey commission
- Kato Y, Fujinaka R, Ishisaka A, Nitta Y, Kitamoto N, Takimoto Y. Plausible authentication of manuka honey and related products by measuring leptosperin with methyl syringate. J Agric Food Chem. 2014;62(27):6400–6407. doi: 10.1021/jf501475h. [DOI] [PubMed] [Google Scholar]
- Kaygusuz H, Tezcan F, Erim FB, Yildiz O, Sahin H, Can Z, Kolayli S. Characterization of Anatolian honeys based on minerals, bioactive components and principal component analysis. LWT Food Sci Technol. 2016;68:273–279. doi: 10.1016/j.lwt.2015.12.005. [DOI] [Google Scholar]
- Khalil MI, Moniruzzaman M, Boukra L, Benhanifia M, Islam MA, Islam MN, Gan SH. Physicochemical and antioxidant properties of Algerian honey. Molecules. 2012;17(9):11199–11215. doi: 10.3390/molecules170911199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda V, Singh B, Kukreja VK, Bawa AS. Characterisation of honey produced from different fruit plants of northern India. Int J Food Sci Technol. 2009;44(12):2629–2636. doi: 10.1111/j.1365-2621.2009.02094.x. [DOI] [Google Scholar]
- Oroian M. Physicochemical and rheological properties of Romanian honeys. Food Biophys. 2012;7(4):296–307. doi: 10.1007/s11483-012-9268-x. [DOI] [Google Scholar]
- Oroian M. Influence of temperature, frequency and moisture content on honey viscoelastic parameters–neural networks and adaptive neuro-fuzzy inference system prediction. LWT Food Sci Technol. 2015;63(2):1309–1316. doi: 10.1016/j.lwt.2015.04.051. [DOI] [Google Scholar]
- Oroian M, Ropciuc S. Honey authentication based on physicochemical parameters and phenolic compounds. Comput Electron Agric. 2017;138:148–156. doi: 10.1016/j.compag.2017.04.020. [DOI] [Google Scholar]
- Oroian M, Amariei S, Escriche I, Gutt G. Rheological aspects of Spanish honeys. Food Biotechnol. 2013;6(1):228–241. [Google Scholar]
- Oroian M, Amariei S, Leahu A, Gutt G. Multi-element composition of honey as a suitable tool for its authenticity analysis. Pol J Food Nutri Sci. 2015;65(2):93–100. [Google Scholar]
- Oroian M, Ropciuc S, Buculei A. Romanian honey authentication based on physico-chemical parameters and chemometrics. J Food Measur Charact. 2017;11(2):719–725. doi: 10.1007/s11694-016-9441-x. [DOI] [Google Scholar]
- Puscas A, Hosu A, Cimpoiu C. Application of a newly developed and validated high-performance thin-layer chromatographic method to control honey adulteration. J Chromatogr A. 2013;1272:132–135. doi: 10.1016/j.chroma.2012.11.064. [DOI] [PubMed] [Google Scholar]
- Sagona S, Bozzicolonna R, Nuvoloni R, Cilia G, Torracca B, Felicioli A. Water activity of fresh bee pollen and mixtures of bee pollen-honey of different botanical origin. LWT Food Sci Technol. 2017;84:595–600. doi: 10.1016/j.lwt.2017.06.015. [DOI] [Google Scholar]
- Singh N, Bath PK. Quality evaluation of different types of Indian honey. Food Chem. 1997;58(1):129–133. doi: 10.1016/S0308-8146(96)00231-2. [DOI] [Google Scholar]
- Svečnjak L, Bubalo D, Baranović G, Novosel H. Optimization of FTIR-ATR spectroscopy for botanical authentication of unifloral honey types and melissopalynological data prediction. Eur Food Res Techn. 2015;240(6):1101–1115. doi: 10.1007/s00217-015-2414-1. [DOI] [Google Scholar]
- Tornuk F, Karaman S, Ozturk I, Toker OS, Tastemur B, Sagdic O, Kayacier A. Quality characterization of artisanal and retail Turkish blossom honeys: Determination of physicochemical, microbiological, bioactive properties and aroma profile. Ind Crops Prod. 2013;46:124–131. doi: 10.1016/j.indcrop.2012.12.042. [DOI] [Google Scholar]
- Tuberoso CIG, Jerković I, Sarais G, Congiu F, Marijanović Z, Kuś PM. Color evaluation of seventeen European unifloral honey types by means of spectrophotometrically determined CIE chromaticity coordinates. Food Chem. 2014;145:284–291. doi: 10.1016/j.foodchem.2013.08.032. [DOI] [PubMed] [Google Scholar]
- Yücel Y, Sultanoğlu P. Characterization of Hatay honeys according to their multi-element analysis using ICP-OES combined with chemometrics. Food Chem. 2013;140(1):231–237. doi: 10.1016/j.foodchem.2013.02.046. [DOI] [PubMed] [Google Scholar]