Abstract
In recent years, hygienic handling of fishery waste is demanded owing to the fact that the fishery waste is an ideal raw material for the preparation of bioactive compounds. In the present study, the effect of pre-processing storage (at 4 ± 2 °C) of whole tilapia waste (WTW) on the properties of its protein hydrolysate derived using pepsin was evaluated. Fish protein hydrolysates (FPH) were prepared from 0, 24 and 48 h old ice stored WTW and designated as FPH-0, FPH-1, and FPH-2, respectively. Total amino acids, total essential amino acids and total hydrophobic amino acids of FPH samples increased with the storage period of raw material (WTW). Antioxidant activities such as DPPH (2, 2 diphynyl-1-picrylhydrazyl) free radical scavenging activity and ferric reducing power of FPH samples were dose dependent. FPH-0 had better antioxidant properties including linoleic acid peroxidation inhibition activity than FPH-1 and FPH-2. The DNA nicking assay revealed the protective effect of FPH preparations against Fenton’s reaction mediated oxidative damage. FPH-2 had better emulsifying properties and foaming stability whereas the FPH-0 had relatively good foaming capacity. SDS–PAGE indicated the presence of peptides ranging from 116 to < 14.4 kDa in FPH-0 and less than 18 kDa in FPH-1 and FPH-2. The present study, clearly demonstrated that whole tilapia waste can effectively be converted to FPH and could be a potential ingredient in functional food and as a rich source of high-quality protein in animal feed formulations.
Keywords: Antioxidant, Amino acid composition, Functional properties, Bioactive fish protein hydrolysate, Tilapia fish waste, DNA nicking assay
Introduction
Worldwide, fish protein hydrolysate (FPH) is one of the most researched fish products of the last decade. The greater attention emerging towards FPH is due to their bioactive properties and growing global market of nutraceuticals and functional food products. All over the world, governing bodies are strictly enforcing laws and regulations on aquatic food processing industries for appropriate disposal of waste to protect the environment. This necessitated proper management and better utilization of fish processing waste which comprises of head, skin, trimmings, fins, frames and viscera (Dekkers et al. 2011). The aforementioned causes and the ease of production process together boosted FPH production from fish processing waste. The use of FPH could be as a functional ingredient in health food formulations, as feed supplements for animals or as ingredients in microbiological media (Fallah et al. 2015).
Among the cultured fish species, tilapia is the most widespread and well adapted to different farming techniques and climate conditions. Tilapia production has increased in the recent past and the global tilapia production has been projected to reach about 7.3 MT by 2030 (World Bank Report 2013). At present, in developing countries like India, government encourages fish farmers to farm tilapia, in particular, GIFT (Genetically Improved farmed Tilapia). GIFT is expected to have high export potential to the US, African countries and also to Japan. The major form of processed tilapia is fillet and the filleting operation yields 30–40% edible meat and produces 60–70% of the initial raw material as waste (Clement and Lovell 1994).
Different FPH preparations such as FPH from tilapia by-products (head, frames, and tail) obtained by using alcalase (Roslan et al. 2014), alkaline-treated tilapia muscle hydrolysates using Cryotin F, Protease A Amano, Protease N Amano, Flavourzyme, and Neutrase (Raghavan and Kristinsson 2008), tilapia protein isolate hydrolysate using alcalase, flavourzyme, papain, protamex by one and two-step hydrolysis, have been reported for their antioxidant properties (Yarnpakdee et al. 2015). Antioxidant properties studied were radical scavenging, reducing power and lipid peroxidation inhibition. Antioxidant properties of FPH have been gaining importance as the long term use of synthetic antioxidants in the food system has raised certain health concerns and there is a continuous search for alternative natural antioxidants (Chalamaiah et al. 2012). However, whole waste (skin, frame, head, trimmings and viscera) of tilapia as such has not been studied for hydrolysate preparation and their properties. This approach would simplify and also ensure effective utilization of tilapia waste and could avoid the sorting line for different waste materials. The inclusion of visceral mass in the waste will hasten the spoilage/autolysis during storage. Generally, refrigerated or iced or frozen storage of raw material is practised prior to FPH preparation. However, changes in the properties of protein hydrolysates because of the storage period of raw material (fish waste) not reported frequently. Hence, in the present study, the effect of ice storage period of whole tilapia waste on antioxidant, functional properties (emulsifying and foaming) and amino acid composition of WTW protein hydrolysates was investigated. Pepsin was used as a hydrolysis enzyme which has the specificity towards hydrophobic amino acids (Elavarasan and Shamasundar 2016).
Materials and methods
Raw material
Tilapia (Oreochromis niloticus) fish waste was collected from two local stations namely, Thoppumpady fish market, Cochin and ICAR-CIFT, Fish Processing Plant, Cochin, Kerala State, India and brought to the laboratory in iced condition at the ratio of 1:1(w/w). Fish waste collected be comprised of head, skin, trimmings, fins, frames and visceral waste. The collected fish waste was divided into three lots. One lot was used immediately to prepare the hydrolysates (FPH-0); the other two lots were stored at 4 °C. Hydrolysates were prepared after 24 and 48 h of storage and were designated as FPH-1 and FPH-2.
Methods
Preparation of protein hydrolysates
Fish waste was rinsed in potable water briefly and chopped manually using a knife. The chopped whole tilapia waste was mixed with distilled water at a ratio of 1:2 and ground into paste using a household warring blender (MX-AC350, Super Mixer Grinder, Panasonic, Panasonic Appliances India Co., Ltd, India). The homogenate obtained was adjusted to pH 2.5 using 2 M HCl. Homogenate was pre-incubated at 37 °C for 5 min to attain the temperature equilibrium with occasional stirring. Hydrolysis reaction was initiated by adding pepsin (from porcine gastric mucosa, powder, ≥ 250 units/mg solid, from Sigma-Aldrich, MO, USA) at an enzyme to substrate ratio of 1% (w/w). Hydrolysis was carried out at 37 °C for 3 h and the reaction was terminated by heating the mixture in a boiling water bath (Julabo TW20, Germany) for 15 min. After cooling the mixture to room temperature, the pH was adjusted to 7 using 2M NaOH. The mixture was filtered through a muslin cloth and centrifuged (Thermo Fisher, HERAEUS MULTIFUGE 3SR+, Germany) at 10,000 rpm for 15 min to remove the fine solids. The supernatant obtained was subjected to spray drying using a spray dryer (SM Scientech, SMST, Machine No.-16, India). The inlet temperature, outlet temperature, and the feeding rate were 180, 80 °C and 20 rpm, respectively. Spray dried hydrolysates were stored under desiccated conditions till further analyses were carried out.
Determination of in vitro antioxidant properties
DPPH free radical scavenging activity
DPPH free radical scavenging activity of protein hydrolysates was evaluated as per the method described by Yen and Wu (1999). Solutions of fish protein hydrolysates were prepared by dissolving them in double distilled water at 0.5, 1.0, 1.5, 2.0 and 2.5 mg/mL concentration. A known volume of 1.5 mL of each sample was added to 1.5 mL of 0.1 mM DPPH in 99.50% ethanol and the solution was mixed systematically in a high-speed vortex mixture. Then the sample was kept under the dark condition for 30 min at room temperature. The change in color as a result of radical scavenging was measured at 517 nm using double beam spectrophotometer (UV–VIS-1601 spectrophotometer, Shimadzu). Appropriate control was prepared using double distilled water and ethanol mixture. BHA and BHT were used as positive controls at different concentrations viz. 50, 100, 150, 200 and 250 ppm. DPPH radical scavenging activity was calculated using the following equation
Further, the EC50 (concentration required to exhibit 50% radical scavenging activity) value of different FPH preparations was calculated using linear regression analysis from the plot of DPPH free radical scavenging activity versus concentration.
Ferric reducing antioxidant power assay
The ferric reducing capacity of protein hydrolysates was assessed according to the method of Oyaiza (1986). An aliquot of 1 mL sample (20, 30, 40, 50 mg of hydrolysate/mL) was thoroughly mixed with 2.5 mL of 0.2 M phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide. Then, the reaction mixture was incubated at 50 °C for 30 min, which was followed by addition of 2.5 mL of 10% trichloroacetic acid. At the end, 2.5 mL of solution mixture was taken out and mixed with 2.5 mL of distilled water and 0.5 mL of 0.1% ferric chloride solution. The final reaction mixture was incubated for 10 min before recording the absorbance at 700 nm using spectrophotometer (UV–VIS-1601 spectrophotometer, Shimadzu, Japan). BHA and BHT at different concentrations (50, 100, 150, 200, 250 ppm) were used as positive controls. Results are expressed as absorbance at 700 nm. Higher the absorbance at 700 nm indicates higher ferric reducing power.
Linoleic acid peroxidation inhibition activity
Linoleic acid peroxidation inhibition activity of fish protein hydrolysates was determined according to the method of Osawa and Namiki (1985). Accurately 400 mg of protein hydrolysates were weighed and dissolved in 10 mL of 50 mM phosphate buffer (pH 7.0), then 0.13 mL of linoleic acid and 10 mL of 95% ethanol were added to the solution and made up to to 25 mL with distilled water. The solution mixture was incubated at 40 ± 1 °C for 5 days in a hot-air oven (dark room conditions were maintained by covering the assay tubes with aluminium foil and thick paper). An aliquot of 0.1 mL of the reaction mixture was pipetted out and mixed with 4.7 mL ethanol (75% v/v) followed by 0.1 mL of ammonium thiocyanate (30% w/v) and 0.1 mL ferrous chloride solution (20 mM prepared in 3.5% HCl). After incubating for 3 min, the color developed was measured at 500 nm using a spectrophotometer. The natural antioxidant, α-tocopherol, was used as the internal standard. The capacity to inhibit the peroxide formation in linoleic acid was calculated as follows:
DNA nicking assay
DNA nicking assay was performed using pCRII™TOPO plasmid (Sigma, Invitro-gen) by the method of Lee et al. (2002). FPH samples were dissolved in deionized distilled water at a concentration of 2 mg/mL. FPH solution was mixed with plasmid DNA (0.5 µg/well) and incubated for 10 min at room temperature. After incubation, 10 µL of Fenton’s reagent was added. Further, the reaction mixture was incubated at 37 °C for 35 min. An aliquot of 10 µL of sample was loaded onto 1% (w/v) agarose gel to assess the impairment of plasmid DNA (Jemil et al. 2014).
SDS–polyacrylamide gel electrophoresis (SDS–PAGE)
Peptides present in the hydrolysates were profiled using sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) techniques. SDS–PAGE was performed according to the method described by Laemmli (1970). Spray dried FPH samples (50 mg mL−1) were dissolved in 1 mL of SDS (5%) solution. Proteins were extracted by incubating the mixture in a boiling water bath for 30 min.
Solubilized samples of FPH were mixed at 1:1 (v/v) ratio with the sample buffer containing 0.5 M Tris–HCl, pH 6.8, 4% SDS, 20% glycerol and 10% β-mercapethanol. The mixture was heat treated in a boiling water bath for 2 min before loading the sample. An aliquot of 10 µL of samples were loaded onto polyacrylamide gel. The concentration of separating and stacking gels were 12.5 and 5%, respectively.
Electrophoresis was carried out at a constant voltage of 90 V using a Mini-Protein II unit (Bio-Rad Laboratories, Inc., Richmond, CA, USA). At the end of the run, gels were removed carefully and stained in Coomassie Blue G-250 (0.05% (w/v)) prepared in 7.5% (v/v) acetic acid and destained with 7.5% (v/v) acetic acid. A wide-range protein molecular weight marker (Thermo Scientific Pierce unstained protein molecular weight marker, 116–14.4 kDa) was used to determine the approximate molecular weight of FPH samples.
Amino acids analysis
Amino acid composition of protein hydrolysate samples was determined after hydrolyzing with acid and derivatization with O-phthalaldehyde according to the method of Ishida et al. (1981). Shimadzu chromatograph LC-10AT VP high-performance liquid chromatography (HPLC) equipped with quaternary pump, 20 µL injection valve, ion exchange column and a fluorescence detector, was used. The separation of the amino acid mixture in the column was achieved using mobile phase A (sodium citrate and ethanol with pH 3.5) and B (sodium citrate and NaOH with pH 9.8). The elution was carried at a fixed flow rate of 0.4 ml/min, while the column temperature was maintained at 60 °C. The amino acids were identified and quantified by comparison of their retention times and area under the curve with those of standards (Sigma). The results were expressed in terms of g of amino acid/kg of protein hydrolysates.
Chemical score of FPHs and indispensable amino acid index (IAAI)
Indispensable Amino Acid Index (IAAI) and Chemical scores of FPH preparations from tilapia whole waste were assessed according to the method of Hardy and Barrows (2002). IAAI is calculated as the ratio of the indispensable amino acid in the protein hydrolysate (PH) to the indispensable amino acids in whole egg protein (WEP) as follows:
Chemical scores were calculated using the following equation:
Determination of functional properties
Emulsifying properties
Emulsifying properties were estimated as per the method given by Pearce and Kinsella (1978). Initially, 30 mL of 1% FPH solution was mixed with 10 mL of vegetable oil. The solution was pre-sheared at 9000 rpm for 30 s and the homogenization was continued at 20,000 rpm for 1 min in a high-speed homogenizer. An aliquot of 50 µL emulsion was pipetted out from the bottom of the container at different time interval (0 and 10 min) and mixed with 5 mL of 0.1% SDS (sodium dodecyl sulfate) solution. The resultant mixture was briefly vortexed and the turbidity was measured at 500 nm using a spectrophotometer. Both emulsion activity index (EAI) and emulsion stability index (ESI) were calculated as follows
where, A500 is an absorbance at 500 nm; l is path length; Φ is oil volume fraction (0.25), and C is protein concentration.
where, ΔA = A0 – A10 and Δt = 10 min
Foaming properties
Foaming capacity and stability of fish protein hydrolysates were assessed according to the method of Sathe and Salunkhe (1981). An aliquot of 20 mL of FPH solution (0.5%) was subjected to whipping at a speed of 14,000 rpm for 2 min at room temperature in a high-speed homogenizer (Bio-Gen PRO200/PRO250 Homogenizer, PRO Scientific Inc, USA) to incorporate the air. The whipped sample was transferred to a graduated measuring cylinder. The volume before whipping and after whipping was recorded. Foaming capacity was calculated using the following equation:
Foams were allowed to stand at room temperature for 30 min and final volume was recorded. Using the following equation, the foame stability was calculated.
Statistical analysis
All experiments were carried out in triplicates. Homogeneity of variance was tested using ANOVA to know the existence of significant difference between the treatments. Significance between mean values of different samples was estimated by Duncan’s multiple range test using a statistical software (SPSS Version 16).
Results and discussion
In the present study, pepsin enzyme was applied to convert the whole tilapia waste into protein hydrolysate. The raw material i.e. whole tilapia waste was stored at iced condition (4 ± 2 °C) for a different periods (0, 24 and 48 h). As utilization of fishery by-products are gaining importance, it is essential to study the effect of ice storage period of raw material on the properties of the end product. Whole tilapia waste protein hydrolysates prepared were evaluated for changes in amino acid composition, antioxidant activity, and functional properties in order to validate the potential of whole tilapia waste protein hydrolysates as an ingredient in animal feed and in functional food formulations.
Antioxidant properties
DPPH free radical scavenging activity
DPPH free radical scavenging activity of FPH was tested at different concentrations viz. 0.5, 1.0, 1.5, 2.0 and 2.5 mg/mL and the results are presented in Table 1. The EC50 value (the concentration at which 50% of the initial radicals scavenged) of FPH-0, FPH-1, and FPH-2 were 1.349, 2.062 and 2.067 mg/mL, respectively. The lower EC50 value indicates higher radical scavenging potential. The DPPH free radical scavenging activities of all the FPH were dose dependent. FPH prepared from the fresh sample (FPH-0) exhibited higher radical scavenging activity compared to the FPH prepared after 24 (FPH-1) and 48 h (FPH-2) of ice storage. This could be due to the difference in the sequence of peptides released from fresh and ice stored samples. In the present study, it is also evidenced by the SDS–PAGE pattern that the ice stored samples underwent more hydrolysis and released smaller peptides compared to the fresh samples. In addition, amino acid composition of FPH from fresh sample was found to have more Tyr, Phe, His, Lys and Arg and these amino acids have been well correlated to the higher radical scavenging activity (You et al. 2010). There exists a lack of consensus on the size of the peptides and the corresponding antioxidant activity in the reported studies. Some studies have confirmed that the smaller peptides have higher antioxidant potential whereas others have reported lower activity for the smaller peptides (Ajibola et al. 2011; Girgih et al. 2011). However, synthetic antioxidants such as BHA and BHT showed higher radical scavenging activity compared to FPH samples. Similar findings with reference to concentration have been reported (Ktari et al. 2012; Nasri et al. 2013). Results clearly indicated that all the FPH containing a mixture of peptides have the ability to transfer electrons and could be effectively used to terminate the free radical-induced chain reaction in oxidation process.
Table 1.
The effect of chilled storage and concentration on DPPH free radical-scavenging activity of fish protein hydrolysates prepared from tilapia fish waste
| Sample | Concentration | ||||
|---|---|---|---|---|---|
| 0.5 mg/ml | 1.0 mg/ml | 1.5 mg/ml | 2.0 mg/ml | 2.5 mg/ml | |
| FHP-0 | 29.48a ± 0.55 | 45.89a ± 6.06 | 52.36b ± 1.12 | 64.79b ± 0.78 | 73.62b ± 1.14 |
| FPH-1 | 32.10a ± 4.03 | 40.25a ± 1.88 | 34.17a ± 2.05 | 47.84a ± 2.25 | 60.01a ± 4.61 |
| FPH-2 | 33.20a ± 3.03 | 38.65a ± 1.83 | 35.72a ± 0.64 | 52.52a ± 2.21 | 56.09a ± 1.88 |
| 50 ppm | 100 ppm | 150 ppm | 200 ppm | 250 ppm | |
| BHA | 78.81b ± 0.39 | 84.42b ± 0.72 | 89.48d ± 0.03 | 90.38d ± 0.63 | 92.53c ± 0.12 |
| BHT | 41.92b ± 0.06 | 46.60a ± 0.55 | 71.61c ± 0.11 | 79.17c ± 1.05 | 80.44b ± 0.24 |
Different superscript (a, b, c, d) in the column indicate significant difference (p < 0.05) among the FPH samples and standard antioxidant. Sample was also compared with standard antioxidants. (Duncan’s multiple range test, α = 0.05). Values are expressed as mean ± SE (n = 6)
Ferric reducing antioxidant power (FRAP)
Ferric reducing antioxidant power (FRAP) of FPH samples were evaluated at different concentration ranging from 20 to 50 mg/mL with the interval of 10 mg/mL and the results are depicted in Table 2. Reducing power increased significantly with the increase in the concentration of hydrolysate in all three FPH preparations (FPH-0, FPH-1, FPH-2). Among the FPH samples, FPH-0 showed better reducing power than the FPH-1 and FPH-2. FRAP is often used to measure the ability of fish protein hydrolysates to reduce the ferric ions to ferrous form (Khantaphant and Benjakul 2008). Fish protein hydrolysates with higher reducing power have better abilities to donate the electrons. The reducing power of FPH samples was comparable with the synthetic antioxidants such as BHA and BHT. Results obtained were in agreement with the published reports (Klompong et al. 2007; Elavarasan and Shamasundar 2016). The reducing power of FPH could be used to reduce DNA damage, mutagenesis, carcinogenesis, and inhibition of pathogenic bacterial growth (Gulcin et al. 2010).
Table 2.
Ferric reducing antioxidant power (FRAP) activity and Linoleic acid peroxidation inhibition (LAPI) activity of fish protein hydrolysates prepared from tilapia fish waste under different condition
| Sample | FRAP | LAPI | |||
|---|---|---|---|---|---|
| 20 mg/ml | 30 mg/ml | 40 mg/ml | 50 mg/ml | 40 mg/ml | |
| FHP-0 | 0.70c ± 0.01 | 0.82c ± 0.01 | 1.05c ± 0.02 | 1.30c ± 0.03 | 55.49b ± 1.65 |
| FPH-1 | 0.55b ± 0.03 | 0.71b ± 0.01 | 1.01c ± 0.03 | 1.20c ± 0.02 | 43.57a ± 2.30 |
| FPH-2 | 0.54b ± 0.03 | 0.70b ± 0.01 | 1.06c ± 0.07 | 1.08b ± 0.03 | 43.65a ± 2.86 |
| 50 ppm | 100 ppm | 150 ppm | 200 ppm | ||
| BHA | 0.53b ± 0.02 | 0.65a ± 0.01 | 0.81b ± 0.03 | 1.27c ± 0.04 | – |
| BHT | 0.31a ± 0.01 | 0.60a ± 0.04 | 0.64a ± 0.01 | 0.91a ± 0.03 | – |
| α-Tocopherol | – | – | – | – | 73.51c ± 1.96 |
Different superscript (a, b, c) in the column indicate significant difference (p < 0.05) among the FPH samples and standard antioxidant. Sample was also compared with standard antioxidants. (Duncan’s multiple range test, α = 0.05). Values are expressed as mean ± SE (n = 6)
Linoleic acid peroxidation inhibition activity
Linoleic acid peroxidation inhibition (LAPI) activity of FPH was tested at a single concentration (40 mg/mL). The natural antioxidant, α-Tocopherol (200 ppm) was used as a positive control. Results are presented in Table 2. FPH-0 had a higher inhibitory effect (55.49) towards lipid peroxidation than the FPH-1 (43.57%) and FPH-2 (43.65%). However, α-tocopherol had higher lipid peroxidation inhibitory activity than FPH samples. Oxidation in any food product will adversely affect the sensory and nutritional quality. In the past, reports have shown that protein hydrolysates have the ability to act as antioxidants against lipid peroxidation (Elavarasan et al. 2014). Results of the present study clearly add to the evidence that the storage period has negative effect on the antioxidant properties of FPH. The nature of enzyme used, the state of raw material, the sequence of the parent protein, the extent of hydrolysis and hydrolysis conditions and drying method could influence the properties of peptides released including antioxidant properties. In addition, molecular size, amino acid sequence and composition, charge of peptides, steric properties of peptides and the drying method could also play a major role in dictating the antioxidant properties of FPH (Tang et al.2013; Elavarasan and Shamasundar 2016).
DNA protective property
DNA nicking assay was performed to assess the protective effect of FPH against DNA damage and the result is presented in Fig. 1a. Overall, FPH preparations from tilapia whole waste showed a strong protective effect against hydroxyl radicals produced by Fenton’s reaction. Lane 1 (positive control) showed a sharp and intense band of supercoiled DNA and there was no sign of DNA damage indicating the intactness. Lane 2 (negative control) where the DNA was incubated with Fenton’s reagent comprises of hydrogen peroxide and ferric chloride showed a complete degradation of supercoiled DNA. This indicates that the hydroxyl radicals nicked the DNA severely (Klompong et al. 2009). The increasing sharpness of supercoiled DNA band in Lanes 3, 4 and 5 clearly present the evidence that the peptides present in the FPH prepared from whole tilapia waste scavenged the hydroxyl radicals or chelated the Fe2+ and protected the DNA from damage. From the results obtained, it could be inferred that during storage of whole tilapia waste, there could be a chance of generation of new peptides through endogenous enzyme action in addition to the peptides formed during in vitro hydrolysis by pepsin. The ice-stored samples being more susceptible to hydrolysis by pepsin resulted in the formation of more of low molecular weight peptides. This assumption is also supported by the SDS–PAGE patterns of whole tilapia waste protein hydrolysates prepared at different time interval of ice stored whole tilapia waste.
Fig. 1.
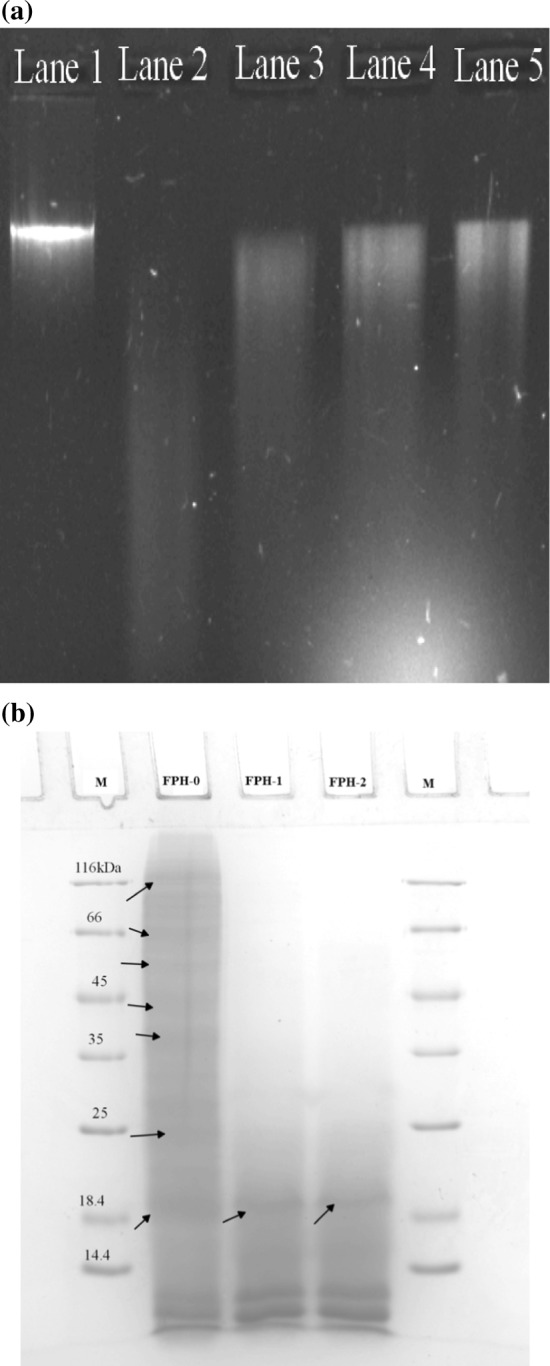
a Agarose gel electrophoresis pattern of the plasmid pCRII™TOPO incubated with Fenton’s reagent in the presence and absence of FPHs. Lane 1: untreated control: native pCRII™TOPO DNA (0.5 μg); Lane 2: DNA sample incubated with Fenton’s reagent; Lanes 3, 4, 5: DNA sample incubated with Fenton’s reagent in the presence of FPH-0, FPH-1 and FPH-2. b SDS–PAGE patterns of protein hydrolysates prepared from tilapia fish whole waste
SDS–PAGE of whole tilapia waste protein hydrolysates
Peptides present in different FPH preparations of tilapia waste were profiled using SDS–PAGE technique and is presented in Fig. 1b. FPH-0 had multiple peptides with molecular weight ranging from 116 to < 14.4 kDa (Lane 1). The high molecular weight peptides in FPH disappeared when the storage period of raw material increased. In other words, the peptides formed in FPH-1 and FPH-2 were largely of low molecular weight peptides with the molecular weight of < 18.4 kDa. It could be speculated that high molecular weight peptides in the parent protein might have been degraded initially by endogenous enzymes and peptides formed further might have been more vulnerable to proteolysis by pepsin. The study opens a novel idea that if fish waste proteins were digested initially by the process of autolysis and subsequently hydrolysed by digestive proteases-like pepsin, it would result in a higher extent of hydrolysis. The peptides formed could mainly be sourced to the proteins like myosin, actin, troponin, tropomyosin and collagen for the reason that the fish waste like head, frames, fins, skin and viscera are mainly comprised of these proteins.
Amino acid composition
Amino acid profiles of FPH-0, FPH-1, and FPH-2 are presented in Table 3. Amino acid content was compared with the amino acid profile of fish meal and soybean meal (retrieved from the literature). In general, FPH preparations have free amino acids and short chain peptides. Amino acid profile is more important to understand their nutritional properties and also related to their functional characteristics and nutraceutical value of FPH (Chalamaiah et al. 2012). Total amino acids (TAA), total essential amino acids (TEAA) and total hydrophobic amino acids (THBAA) in FPH increased with storage period of raw material. Similarly, the ratio of THAA/TAA and TEAA/TAA also increased. Glycine was the abundant amino acid while cysteine was the limiting amino acid in all the FPH preparations. It is worthy to mention that the visceral tissue, bone, and skin are rich in collagen which contains about 33% glycine (Hema et al. 2014). Among the essential amino acids, arginine content was the highest in FPH-0, whereas leucine was the highest in the case of FPH-1 and FPH-2. Results indicated that the pattern of releasing of peptides from protein differed with the ice storage period of raw material. Amino acids such as tyrosine, phenylalanine, histidine, lysine and arginine were higher in FPH-0 compared to FPH-1 and FPH-2. It has been reported that the presence of hydrophobic amino acids and one or more residues of histidine, proline, methionine, cysteine, tyrosine, tryptophan and phenylalanine could improve the antioxidant activity of peptides present in FPH (You et al. 2010). Chemical score and indispensable amino acid index of FPH indicate their nutritional quality. In the present investigation, the chemical score of all the FPH preparation indicated that the hydrolysates obtained were better than the fishmeal and soybean meal (Table 4). This confirmed that the FPH preparation can be used as a source of high-quality proteins particularly in animal feed formulations.
Table 3.
Amino acid profile of FPH preparation from tilapia whole waste is compared with other feed ingredients used in aquaculture
| Amino acids | FPH-0* | FPH-1* | FPH-2* | Fishmeal† | Soybean meal† | Human requirement (adult) |
|---|---|---|---|---|---|---|
| Essential (g/kg) | ||||||
| Arginine | 42.25 ± 0.33# | 41.11 ± 0.34# | 41.66 ± 0.32# | 37.8 | 30.5 | – |
| Histidine | 19.23 ± 0.34c | 17.20 ± 0.23b | 15.57 ± 0.02a | 14.0 | 12.5 | 16 |
| Methionine | 10.87 ± 0.18a | 12.58 ± 0.31b | 12.41 ± 0.09b | 18.0 | 4.7 | 17 |
| Isoleucine | 14.10 ± 0.14# | 15.05 ± 0.07# | 15.33 ± 0.33# | 21.5 | 17.5 | 13 |
| Lysine | 26.54 ± 0.25c | 25.11 ± 0.12b | 23.01 ± 0.01a | 39.8 | 25.7 | 16 |
| Leucine | 36.84 ± 0.11a | 46.43 ± 0.98b | 47.82 ± 0.75c | 37.7 | 29.1 | 19 |
| Phenylalanine | 15.89 ± 0.36# | 14.83 ± 0.33# | 14.86 ± 0.45# | 18.1 | 19.1 | – |
| Threonine | 21.51 ± 0.24a | 23.90 ± 0.05c | 22.94 ± 0.08b | 19.9 | 15.1 | 9 |
| Valine | 26.51 ± 0.21# | 27.75 ± 0.31# | 27.36 ± 0.11# | 25.9 | 19.0 | 13 |
| Tryptophan | NA | NA | NA | 3.9 | 8.0 | – |
| Non essential (g/kg) | ||||||
| Tyrosine | 10.61 ± 0.35# | 8.95 ± 0.32# | 6.31 ± 0.61# | – | – | |
| Aspartic acid | 60.27 ± 0.37a | 73.39 ± 0.31b | 76.04 ± 0.61c | – | – | |
| Glutamic acid | 98.00 ± 0.55a | 139.14 ± 1.32b | 145.88 ± 0.39c | – | – | |
| Glycine | 122.84 ± 0.33a | 149.33 ± 0.33b | 153.74 ± 0.31c | – | – | |
| Alanine | 78.71 ± 0.81a | 115.63 ± 0.34b | 118.71 ± 0.35c | – | – | |
| Proline | 41.56 ± 0.32a | 41.86 ± 0.41a | 44.64 ± 0.33b | – | – | |
| Cysteine | 0.95 ± 0.02a | 3.85 ± 0.38b | 1.46 ± 0.32a | – | – | |
| Serine | 26.46 ± 0.19a | 32.35 ± 0.25c | 30.55 ± 0.22b | – | – | |
| TEAA | 213.74 ± 2.93# | 223.95 ± 2.72# | 220.95 ± 3.12# | – | – | – |
| TAA | 653.13 ± 4.85a | 788.46 ± 5.42b | 798.27 ± 1.50b | – | – | |
| THBAA | 355.68 ± 2.71a | 428.08 ± 2.66b | 438.01 ± 2.98c | – | – | |
| THBAA/TAA | 54.46 ± 0.03# | 54.29 ± 0.04# | 54.87 ± 0.23# | – | – | |
| TEAA/TAA | 32.72 ± 0.21b | 28.40 ± 0.15a | 27.68 ± 0.29a | – | – | |
Values of the present investigation are expressed in mean (n = 3)
NA Not analysed
#Not significant
*Present work data
†Data retrieved from Halver (1995)
Different superscript (a, b, c) in the row indicate significant difference (p < 0.05) among the FPH samples. Values are expressed as mean ± SE (n = 3)
Table 4.
Chemical score of amino acids and indispensable amino acid index (IAAI) of FPH preparation compared with reference feed ingredients (soybean meal and fish meal)
| Amino acids | Chemical score | Whole egg amino acid (g kg−1) | ||||
|---|---|---|---|---|---|---|
| FPH-0† | FPH-1† | FPH-2† | Soybean meal‡ | Fishmeal‡ | ||
| Arginine | 64.01 ± 0.49# | 62.29 ± 0.52# | 63.12 ± 0.48# | 46.2 | 57.3 | 66 |
| Histidine | 80.12 ± 1.40c | 71.67 ± 1.45b | 64.86 ± 1.32a | 52.1 | 58.3 | 24 |
| Methionine | 27.16 ± 0.79a | 31.44 ± 0.78b | 31.04 ± 0.86b | 11.8 | 45.0 | 40 |
| Isoleucine | 18.31 ± 0.45# | 19.55 ± 0.42# | 19.91 ± 0.43# | 22.8 | 27.9 | 77 |
| Lysine | 37.92 ± 0.47c | 35.87 ± 0.49b | 32.87 ± 0.49a | 36.7 | 43.3 | 70 |
| Leucine | 40.04 ± 0.36a | 50.47 ± 0.26b | 51.97 ± 0.57c | 31.6 | 41.0 | 92 |
| Phenylalanine | 25.22 ± 0.57# | 23.54 ± 0.53# | 23.58 ± 0.51# | 30.3 | 28.7 | 63 |
| Threonine | 50.03 ± 0.41a | 55.57 ± 0.12b | 53.35 ± 0.18c | 35.1 | 46.3 | 43 |
| Valine | 36.82 ± 0.48# | 38.54 ± 0.22# | 37.99 ± 0.87# | 26.4 | 36.0 | 72 |
| Tryptophan | NA | NA | NA | 53.3 | 26.0 | 15 |
| IAAI | 379.64 ± 5.57# | 388.94 ± 5.14# | 378.70 ± 4.98# | 346.3 | 409.8 | |
#Not significant
†Present work
‡Data retrieved from Halver (1995)
Different superscript (a, b, c) in the row indicate significant difference (p < 0.05) among the FPH samples. Values are expressed as mean ± SE (n = 3)
Functional properties
Emulsifying properties
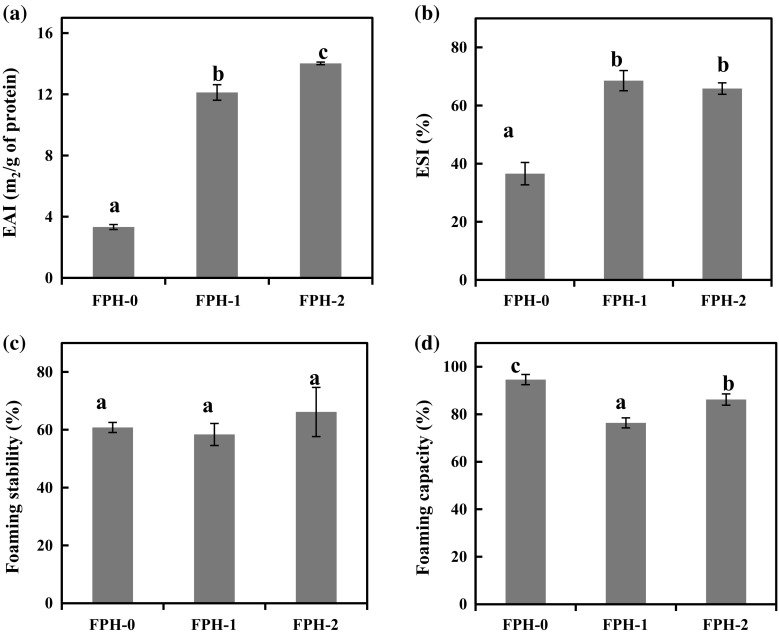
Emulsifying activity index (EAI) and emulsion stability index (ESI) of FPH preparations were determined and the results are presented in Fig. 2a, b. FPH-0, FPH-1, and FPH-2 differed significantly in EAI and ESI (p < 0.05). Among the FPH preparations, the highest EAI and ESI were recorded for FPH-2. This could be due to the formation of low molecular weight peptides in the ice stored samples used for hydrolysis reaction. The state of raw material used for hydrolysis plays a major role in dictating the properties of the hydrolysates. Structures of native proteins are expected to be altered upon storage at low temperature that in turn could affect the exposure of susceptible sites for the enzyme activity. On the other hand, one cannot rule out the possibilities of formation of peptides due to the action of endogenous enzymes in ice stored raw material. Further, the low EAI of FPH prepared from fresh fish waste (FPH-0) could be due to the inability of large molecular weight peptides to reduce the interfacial tension at the oil–water interface. Rahali et al. (2000) have reported that amino acid sequence plays a vital role at the oil–water interface. In addition to the peptide chain length, the ratio of hydrophilicity to hydrophobicity is more important for exhibiting superior emulsion properties. Further, Kato et al. (1985) have stated that flexibility of peptides also plays a decisive role in influencing the emulsifying properties. The results of the present study are comparable with the reported studies (Klompong et al. 2007; Elavarasan et al. 2014).
Fig. 2.
Functional properties of tilapia fish whole waste protein hydrolysates. a Emulsion activity index; b emulsion stability index; c foaming capacity; d foaming stability
Foaming properties
Foaming properties of FPH preparations are presented in Fig. 2c, d. FPH-0 had significantly higher foaming capacity (94.61%) than FPH-1 (76.36%) and FPH-2 (86.20%). Foam is an immiscible system in which water is the continuous phase and air is the discontinuous phase. Substances which have the ability to reduce the interfacial tension at air–water interface form foams. Foaming stability was found to be higher in FPH-2 (66.15%) compared to FPH-0 (60.79%) and FPH-1 (58.36%). The surface tension at the air–water interface is lowered by the proteins/peptides which leads to the formation of a stable foam (Surowka and Fik 1992). Generally, foam formation follows three major steps, transportation, penetration and restructuring of the molecules at the air–water interface (Klompong et al. 2007). In addition, Mutilangi et al. (1996) have reported that foaming stability of compounds mainly depend on the protein–protein interaction within the matrix. In the present study, there is no direct relationship between the aging of the sample (storage period of raw material) and foaming properties of FPH. From the results, it could be inferred that the foaming capacity is favored by the presence of large molecular weight peptides in the FPH while that of the foaming stability is favored by relatively low molecular weight peptides. This postulate is also supported by the peptide profile of FPH preparations as revealed by SDS–PAGE analysis.
Conclusion
Manipulating protein hydrolysis process with reference to intended use of end products necessitates having better understanding of the properties of protein hydrolysate as influenced by pre-processing preservation methods like ice storage. In the present study, fish protein hydrolysates were prepared from 0 (FPH-0), 24 (FPH-1) and 48 h (FPH-2) old ice stored whole tilapia waste. FPH-0 had higher antioxidant and foaming capacity, whereas, FPH-1 and FPH-2 samples had better emulsifying properties and foaming stability. Amino acid analysis, chemical score and indispensable amino acid index of FPH samples clearly showed that the fish protein hydrolysates from whole tilapia waste were nutritionally superior. As the hydrolysate samples possessed antioxidant, functional and also high nutritional value, it is proposed to find its application in animal feed industry as a bio-functional protein ingredient. There is also an ample scope for the whole tilapia waste protein hydrolysate to be used as a nutraceutical ingredient in health food formulations. The conversion of whole tilapia waste into protein hydrolysates through a biochemical process like protein hydrolysis using exogenous enzymes paves the way for better and maximum utilization of tilapia fish co-products resulting in higher returns to the processor and also help to achieve the sustainability in fish processing sector.
Acknowledgements
The authors gratefully acknowledge the Director, ICAR-Central Institute of Fisheries Technology, the Director, Central Marine Fisheries Research Institute, Cochin for providing the necessary facilities. The authors also wish to express their gratitude to technical staff of Biochemistry and Nutrition Division, CIFT for the support rendered during the research.
References
- Ajibola CF, Fashakin JB, Fagbemi TN, Aluko RE. Effect of peptide size on antioxidant properties of African yam bean seed (Sphenostylis stenocarpa) protein hydrolysate fractions. Int J Mol Sci. 2011;12:6685–6702. doi: 10.3390/ijms12106685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalamaiah M, Dinesh KB, Hemalatha R, Jyothirmayi T. Fish protein hydrolysates: proximate composition, amino acid composition, antioxidant activities and applications: a review. Food Chem. 2012;135:3020–3038. doi: 10.1016/j.foodchem.2012.06.100. [DOI] [PubMed] [Google Scholar]
- Clement S, Lovell RT. Comparison of culture Nile tilapia (Oreochromis niloticus) and channel catfish (Ictalurus punctatus) Aquaculture. 1994;119:299–310. doi: 10.1016/0044-8486(94)90184-8. [DOI] [Google Scholar]
- Dekkers E, Raghavan S, Kristinsson HG, Marshall MR. Oxidative stability of Mahi mahi red muscle dipped in tilapia protein hydrolysates. Food Chem. 2011;124:640–645. doi: 10.1016/j.foodchem.2010.06.088. [DOI] [Google Scholar]
- Elavarasan K, Shamasundar BA. Effect of oven drying and freeze drying on the antioxidant and functional properties of protein hydrolysates derived from freshwater fish (Cirrhinus mrigala) using papain enzyme. J Food Sci Technol. 2016;53:1303–1311. doi: 10.1007/s13197-015-2084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elavarasan K, Naveen Kumar V, Shamasundar BA. Antioxidant and functional properties of fish protein hydrolysates from fresh water carp (Catla catla) as influenced by the nature of enzyme. J Food Process Preserv. 2014;38:1207–1214. doi: 10.1111/jfpp.12081. [DOI] [Google Scholar]
- Fallah M, Bahram S, Javadian SR. Fish peptone development using enzymatic hydrolysis of silver carp byproducts as a nitrogen source in Staphylococcus aureus media. Food Sci Nutr. 2015;3:153–157. doi: 10.1002/fsn3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgih AT, Udenigwe CC, Aluko RE. In vitro antioxidant properties of hemp seed protein hydrolysate fractions. J Am Oil Chem Soc. 2011;88:381–389. doi: 10.1007/s11746-010-1686-7. [DOI] [Google Scholar]
- Gulcin I, Huyut Z, Elmastas M, Aboul-Enein HY. Radical scavenging and antioxidant activity of tannic acid. Arab J Chem. 2010;3:43–53. doi: 10.1016/j.arabjc.2009.12.008. [DOI] [Google Scholar]
- Halver JE. Use of INFIC data base for amino acid requirements studies. J Appl Ichthyol. 1995;11:129–140. doi: 10.1111/j.1439-0426.1995.tb00014.x. [DOI] [Google Scholar]
- Hardy RW, Barrows FT. Diet formulation and manufacture. In: Halver JE, Hardy RW, editors. Fish nutrition. London: Academic Press; 2002. p. 587. [Google Scholar]
- Hema GS, Shyni K, Mathew S, Ninan G, Joshy CG. Comparison of collagen extracted from skin of double spotted queenfish and Malabar grouper. Fishery Technol. 2014;51:93–97. [Google Scholar]
- Ishida Y, Fujita T, Arai K. New detection and separation method for amino acid by high performance liquid chromatography. J Chromatogr. 1981;204:143–148. doi: 10.1016/S0021-9673(00)81650-7. [DOI] [PubMed] [Google Scholar]
- Jemil I, Jridi M, Nasri R, Ktari N, Salem RBSB, Mehiri M, Hajji M, Nasri M. Functional, antioxidant and antibacterial properties of protein hydrolysates prepared from fish meat fermented by Bacillus subtilis A26. Process Biochem. 2014;49:963–972. doi: 10.1016/j.procbio.2014.03.004. [DOI] [Google Scholar]
- Kato A, Komatsu K, Fujimoto K, Kobayashi K. Relationship between surface functional properties and flexibility of proteins detected by protease susceptibility. J Agric Food Chem. 1985;33:931–934. doi: 10.1021/jf00065a039. [DOI] [Google Scholar]
- Khantaphant S, Benjakul S. Comparative study on the proteases from fish pyloric caeca and the use for production of gelatin hydrolysate with antioxidative activity. Comp Biochem Phys B. 2008;151:410–419. doi: 10.1016/j.cbpb.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Klompong V, Benjakul S, Kantachote D, Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102:1317–1327. doi: 10.1016/j.foodchem.2006.07.016. [DOI] [Google Scholar]
- Klompong V, Benjakul S, Yachai M, Visessanguan W, Shahidi F, Hayes KD. Amino acid composition and antioxidative peptides from protein hydrolysates of yellow stripe trevally (Selaroides leptolepis) J Food Sci. 2009;74:126–133. doi: 10.1111/j.1750-3841.2009.01047.x. [DOI] [PubMed] [Google Scholar]
- Ktari N, Jridi M, Bkhairia I, Sayari N, Ben Salah R, Nasri M. Functionalities and antioxidant properties of protein hydrolysates from muscle of zebra blenny (Salaria basilisca) obtained with different crude protease extracts. Food Res Int. 2012;49:747–756. doi: 10.1016/j.foodres.2012.09.024. [DOI] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee JC, Kim HR, Kim J, Jang YS. Antioxidant property of an ethanol extract of the stem of Opuntia ficus-indica var. saboten. J Agric Food Chem. 2002;50:6490–6496. doi: 10.1021/jf020388c. [DOI] [PubMed] [Google Scholar]
- Mutilangi WAM, Panyam D, Kilara A. Functional properties of hydrolysates from proteolysis of heat-denatured whey protein isolate. J Food Sci. 1996;61:270–275. doi: 10.1111/j.1365-2621.1996.tb14174.x. [DOI] [Google Scholar]
- Nasri R, Younes I, Jridi M, Trigui M, Bougatef A, Nedjar-Arroume N, Dhulster P, Nasri M, Karra-Châabouni M. ACE inhibitory and antioxidative activities of Goby (Zosterissessor ophiocephalus) fish protein hydrolysates: effect on meat lipid oxidation. Food Res Int. 2013;54:552–561. doi: 10.1016/j.foodres.2013.07.001. [DOI] [Google Scholar]
- Osawa T, Namiki M. Natural antioxidants isolated from Eucalyptus leaf waxes. J Agric Food Chem. 1985;33:777–780. doi: 10.1021/jf00065a001. [DOI] [Google Scholar]
- Oyaiza M. Studies on products of browning reaction: antioxidative activity of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Pearce KN, Kinsella JE. Emulsifying properties of proteins evaluation of a turbidimetric technique. J Agric Food Chem. 1978;26:716–723. doi: 10.1021/jf60217a041. [DOI] [Google Scholar]
- Raghavan S, Kristinsson HG. Antioxidative efficacy of alkali-treated Tilapia protein hydrolysates: a comparative study of five enzymes. J Agric Food Chem. 2008;56:1434–1441. doi: 10.1021/jf0733160. [DOI] [PubMed] [Google Scholar]
- Rahali V, Chobert JM, Haertle T, Gueguen J. Emulsification of chemical and enzymatic hydrolysates of b-lactoglobulin: characterization of the peptides adsorbed at the interface. Nahrung. 2000;44:89–95. doi: 10.1002/(SICI)1521-3803(20000301)44:2<89::AID-FOOD89>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Roslan J, Yunos KFM, Abdullah N, Kamal SMM. Characterization of fish protein hydrolysate from Tilapia (Oreochromis Niloticus) by-product. Agric Agric Sci Proc. 2014;2:312–319. [Google Scholar]
- Sathe SK, Salunkhe DK. Functional properties of the Great Northern Bean (Phaseolus vulgaris L.) proteins: emulsion, foaming, viscosity and gelation properties. J Food Sci. 1981;46:71–74. doi: 10.1111/j.1365-2621.1981.tb14533.x. [DOI] [Google Scholar]
- Surowka K, Fik M. Studies on the recovery of proteinaceous substances from chicken heads. I. An application of neutrase to the production of protein hydrolysate. Int J Food Sci Technol. 1992;27:9–20. doi: 10.1111/j.1365-2621.1992.tb01173.x. [DOI] [Google Scholar]
- Tang WL, Zhang M, Adhikari B, Mujumdar AS. Effects of preparation and drying methods on the antioxidant activity of enzymatically hydrolyzed porcine placenta hydrolysates. Dry Technol. 2013;31:1600–1610. doi: 10.1080/07373937.2013.808660. [DOI] [Google Scholar]
- World Bank . Fish to 2030: prospects for fisheries and aquaculture. Washington: World Bank; 2013. [Google Scholar]
- Yarnpakdee S, Benjakul S, Kristinsson HG, Kishimura H. Antioxidant and sensory properties of protein hydrolysate derived from Nile tilapia (Oreochromis niloticus) by one- and two-step hydrolysis. J Food Sci Technol. 2015;52:3336–3349. doi: 10.1007/s13197-014-1672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen G, Wu J. Antioxidant and radical scavenging properties of extract from Ganoderma tsugae. Food Chem. 1999;65:375–379. doi: 10.1016/S0308-8146(98)00239-8. [DOI] [Google Scholar]
- You L, Zhao M, Regenstein JM, Ren J. Purification and identification of antioxidative peptides from loach (Misgurnus anguillicaudatus) protein hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Res Int. 2010;43:1167–1173. doi: 10.1016/j.foodres.2010.02.009. [DOI] [Google Scholar]