Abstract
Moringa oleifera (Moringaceae) is a specie of significant importance because of its multiple nutraceutical properties, that has led to increase in its consumption. The seeds contain a high percentage of protein (37.48%). However, little is known about the bioactive properties of these proteins and peptides, especially those generated by enzymatic hydrolysis. The objective of this study was to evaluate the biofunctional properties of total hydrolysates (TH) and peptide fractions from protein isolates of moringa seeds. Isoelectric protein isolates were prepared and TH were obtained by digestion with trypsin, chymotrypsin and pepsin–trypsin for 2.5 and 5 h. TH were fractioned by ultrafiltration (UF) with a 10 kDa membrane to generate the peptide fractions. In all treatments, the antioxidant capacity was significantly higher in peptide fractions > 10 kDa with 5 h of hydrolysis. The results showed that the fraction > 10 kDa of pepsin–trypsin digested for 5 h presented a better Angiotensin Converting Enzyme inhibition (ACE-I) activity with an IC50 of 0.224 μg/μl. Also, antidiabetic activity was enhanced in pepsin–trypsin treatment with 5 h of hydrolysis showing an IC50 of 0.123 μg/μl. Finally, this study showed that hydrolysates of moringa seed proteins had excellent in vitro nutraceutical potential.
Keywords: Moringa oleifera, Protein isolates, Bioactive peptides, Antioxidant, Anti-hypertensive, Anti-diabetic activity
Introduction
Moringa oleifera is a perennial tree and the best known and most widely distributed species of the Moringaceae family (Anwar et al. 2007). Its leaves contain β-carotene, vitamin C, flavonoids, phenolic compounds, fatty acids such as omega 3 and 6, calcium and potassium; the seed are high in carbohydrates, lipids and proteins which makes up 33–60% of their dry wight (Kasolo et al. 2010; Vats and Gupta 2017). This species has been widely used because of its chronic diseases preventing properties (including antispasmodic, antidiuretic, anti-hypertensive, anti-ulcer, anti-oxidant, anticarcinogenic, antidiabetic, antimicrobial and anticholesterolemic effects) (Anwar et al. 2007; Mehta et al. 2011; Jung 2014). The species may still be explored for other properties such as the presence of peptides with biofunctional properties.
In recent years, legumes and oilseeds have been explored for production of protein isolates with functional properties (Segura-Campos et al. 2013). In this sense, protein isolates are used as food supplements and have the potential to generate bioactive peptides through in vitro or digestive proteolysis (Kitts and Weiler 2003; Lopez-Sanchez et al. 2016). Peptides can be produced naturally by the gastrointestinal digestion process but they can also be obtained in vitro by using proteolytic enzymes such as pepsin, trypsin or chymotrypsin (Segura-Campos et al. 2013; Lopez-Sanchez et al. 2016). The biofunctional properties of these peptides are related to regulatory functions in the cardiovascular, digestive, immune and nervous systems, depending on the composition and structure of the amino acid. Their nutraceutical properties include antioxidant capacity to counteract lipid peroxidation (Singh et al. 2014), an antihypertensive function associated with ACE inhibition, an antidiabetic property associated with α-amylase activity (Nair et al. 2013), an anti-inflammatory property, as well as cell aging retardants, antimicrobials and others (Rebello et al. 2014).
Beyond its use as food, production and consumption of moringa have been boosted by products derived from its leaves and seeds. However, no study has demonstrated their biofunctional properties. Based on the above, the objective of this study was to characterize the in vitro biofunctional properties (antioxidant, antihypertensive and antidiabetic activities) of the TH and peptides fractions of protein isolates from moringa seed flour.
Materials and methods
Preparation of moringa flour
Seeds of M. oleifera were provided by the Centro de Agricultura Protegida at the Facultad de Agronomía UANL in Mexico. Seeds were ground and the flour was sieved through a 50–150 μm meshes. Fine flour was defatted with 1:3 (flour:hexane) ratio by stirring 1 h and then filtrating to eliminate fatty residues. The recovered flour was dried and washed several times with water to remove polysaccharides. Finally, it was dried in an oven at 55 °C during 3 h prior to grinding again into a fine powder (150 μm).
Proximate analysis
The determination of moisture percentage, ash, total protein, fat and crude fiber contents were performed at the Laboratory of Food, Drug and Toxicology of the Facultad de Ciencias Quimicas, UANL, San Nicolas de los Garza, Nuevo Leon, Mexico following AOAC protocols (1995).
Moisture percentage was determined according to the AOAC 950.46B protocol based on the kiln drying method. Briefly, 2 g sample in a pre-weighed dish was placed in a drying oven at 100–102 °C for 16–18 h. After this time, the sample was cooled in a desiccator and the difference in weight was used to calculate the moisture percentage of the sample.
Ash content was determined following the AOAC 938.08 protocol based on the analysis of inorganic residues after ignition or complete oxidation of organic matter. For this, 2 g of previously dried samples were placed in a muffle at 550 °C for 2–3 h. The ash percentage was calculated by weighting the inorganic residue.
Protein content in the flour was determined by the AOAC 928.08 protocol, which is based on determination of total nitrogen by the Kjeldahl method. For this determination, 2 g of samples was digested with sulfuric acid in presence of catalysts; the digested samples were neutralized with concentrated NaOH to collect the resulting ammonium, and subsequently, the nitrogen present in the sample was determined through titration. To calculate the % protein in the sample, nitrogen-to-protein conversion factors were used.
Fat content in the flour was obtained following the Mexican Standard NMX-F-615 protocol. The ethereal extract was obtained with 2 g of sample extracted in a Soxhlet extractor for 4–6 h. Percentage of fat was calculated from the weight of the container with the fat extracted using petroleum ether minus the weight of the fat-free container at constant weight.
Finally, the raw fiber contained was determined with the AOAC 962.09 protocol. The percentage of crude fiber was calculated after lignition as the loss of dry residue, which was obtained after digestion of 2 g of sample with sulfuric acid and NaOH under specific conditions. The difference in weight after calcination indicates the amount of fiber present.
Preparation of protein isolates
Proteins isolates were obtained by the alkaline extraction and isoelectric precipitation method reported by Salcedo-Chávez (2002). Extraction was performed at different pH (9, 10 and 11) using a 1 M NaOH in a ratio of 1:10 (flour: deionized water) and stirring at 4 °C for 1 h. These mixtures were centrifuged at 10,000g for 15 min at 4 °C. The supernatant was recovered and distributed in three equal parts. For the isoelectric protein precipitation, pH was adjusted to 4, 4.5 and 5 with 1 M HCl. The samples were centrifuged at 10,000g for 30 min and the supernatant removed. Protein isolates were lyophilized, labeled indicating the pH and the isoelectric point as (pH, Ip) and kept at − 20 °C until subsequent analysis.
Protein quantification
Protein concentration was determined using Bradford reagent (Bradford 1976) and a standard curve prepared with a 2 mg/ml stock solution of bovine serum albumin (BSA). A volume of 30 μl of each sample was used and 1 ml of Bradford’s solution was added. The mixture was reposed for 5 min and then absorbance was determinates at 595 nm.
In vitro enzymatic hydrolysis
Peptide fractions were prepared with three proteolytic enzymes: porcine pepsin, bovine trypsin and bovine chymotrypsin (Sigma-Aldrich, St. Louis, MO, USA).
The hydrolysis treatments were performed with at 1:5 (w/w) ratio (enzyme:substrate) with trypsin, chymotrypsin and a sequential digestion using pepsin–trypsin (to simulate gastrointestinal fluids) for 2.5 and 5 h of hydrolysis. Fifty mg of lyophilized protein isolates was dissolved in deionized water at 1:10 ratio (protein:water), the solution pH was adjusted to pH 2.0 for pepsin digestion and pH 7.8 for trypsin and chymotrypsin digestions. Then 10 mg of digestive enzymes were dissolved and added to the previous solution and digestion was performed at 37 °C for 2.5 and 5 h. The pH and temperature were monitored during the entire digestion process. The reaction was stopped by heating at 80 °C for 10 min and then centrifuged at 7000g for 10 min; the supernatant was recovered and labeled as total hydrolysate (TH). A volume of 15 ml of TH was fractionated by UF using a 10 kDa membrane (Amicon Ultra-system 15 MWCO) (Millipore, Billerica, MA, USA); the sample was centrifuged at 5000g for 15 min and the peptide sample retained on the membrane was labeled as the peptide fraction > 10 kDa and the peptides sample that passed through the membrane was denominated peptide fraction < 10 kDa. Both TH and peptide fractions were collected and stored at −20 °C for subsequent analysis.
Protein analysis by SDS-PAGE
The protein profile of isolates (pH 11, 4) and UF samples (fraction > 10 kDa) were visualized on 12% SDS-PAGE (Laemmli 1970). A total of 40 µg of protein sample in tricine buffer were loaded. Electrophoresis was run at 80 V for 20 min for the first step to concentrate the sample in the gel well and then at 120 V for 80 min to resolve proteins according their molecular weight.
ABTS radical scavenging assay
Antioxidant activity was measured using a modified Re et al. (1999) method. Solutions of ABTS at 7 mM and 2.45 mM potassium persulfate were prepared and mixed. After 12 h this reagent was dissolved in absolute ethanol to an absorbance of 0.700 at 734 nm. A Trolox® standard curve at different concentrations: 20, 40, 60, 80 and 100 ppm was prepared. Reactions were prepared with 50 µl of each sample or standard and 0.950 ml of reagent; absorbance at 734 nm was determined after 1 min. The activity was calculated after sample blank subtraction and was expressed as Trolox equivalents (mM ET)/ mg of protein.
ACE inhibition activity
Antihypertensive property was evaluated as ACE-I activity according to the Hayakari et al. (1978) protocol. The ACE was extracted from rabbit lungs and dissolved in 0.1 M buffer of KH2PO4, pH 8.3 and His-His-Leu (HHL) tripeptide was used as substrate. Absorbance at 382 nm was determined in the supernatant obtained after centrifugation at 10,000g for 10 min. The percentage of ACE inhibition was obtained by difference in absorbance between the reactions of enzyme-substrate (ES) and enzyme-substrate-inhibitor (ESI) where the inhibitor was the TH and peptide fractions (µg/µl), captopril was used as inhibition control. Finally, the IC50 was determined by regression analysis of ACE inhibition (%) versus protein fraction concentration (µg/µl). Calculated as follows:
where CES = Control Enzyme-Substrate; CSE = Control-Sample-Enzyme.
α-Amylase inhibition activity
Antidiabetic activity was related to the inhibition of the α-amylase according to the method reported by Nair et al. (2013). Human amylase was dissolved in 0.2 mM buffer NaH2PO4 at pH 6.8; 100 µl of human enzyme (1 U/ml) was incubated with 1 µl of the peptide fraction for 10 min at room temperature. Then 100 µl of 1% starch were added to each sample and incubated at 37 °C for 30 min. Subsequently 100 µl of 3,5-dinitrosalicylic acid (DNS) was added and heated at 90 °C for 5 min. Then samples were cooled on ice and diluted with 900 µl of deionized water after recording the absorbance at 540 nm. Percentage of inhibition was established by difference in absorbance between the reactions of enzyme-substrate (ES) and enzyme-substrate-inhibitor (ESI) where the inhibitor was the total hydrolysate and peptide fractions (µg/µl), calculated as:
IC50 was determined by regression analysis of α-amylase inhibition (%) versus protein fraction concentration (µg/µl), calculated as follows:
Statistical analysis
All extractions, hydrolyses and determinations were performed in triplicate. To evaluate the possible differences between treatments a one way analysis of variance (P ≤ 0.05) was used and the means were compared by the Tukey’s test with the statistical package SPSS statistical software.
Results and discussion
Preparation of protein isolates
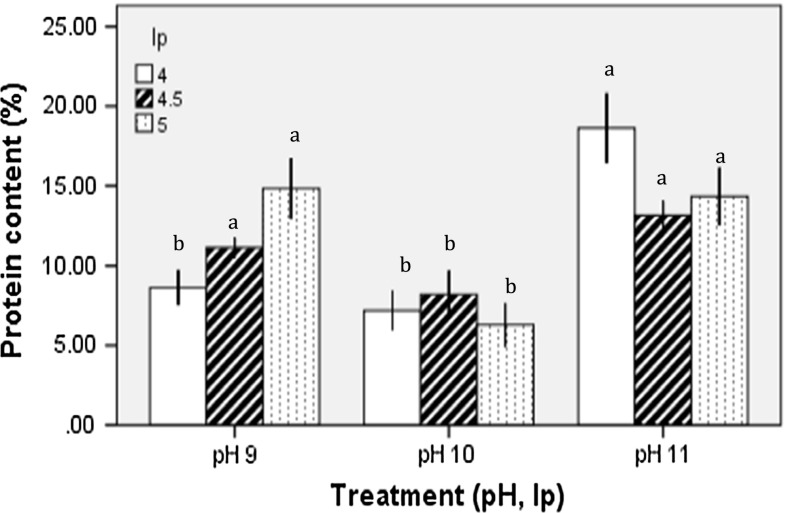
Quantification of proteins showed that percentages varied between treatments, revealing two statistically different groups. The best results were obtained at high extraction pH. The protein isolated with pH (11, 4 treatment showed 19.8% of protein, while pH 9, 5) yielded 15.9% and the (pH 11, 5) showed 14.3% with no significant difference (p < 0.05) between these treatments (Fig. 1). The pH effect on isoelectric precipitation did not exhibit a characteristic pattern between or within treatments suggesting no significant correlation between the protein concentrations in relation to isoelectric precipitation.
Fig. 1.
Protein content (%) of different protein isolates obtained with alkaline extraction pH 9, 10, and 11 and isoelectric precipitation (4, 4.5 and 5). Different letters in the bars indicate statistical difference (p < 0.05)
Although it has been widely reported that the alkaline extraction and isoelectric precipitation allows efficient protein separation (Pedroche et al. 2004), and despite reports on legumes with proteins yields of 50–90%, in our study protein content varied between 6.3 and 19.89%. These relatively low yields could be related to the native content and biochemical characteristics of the proteins present in moringa seeds.
Despite the fact that proteins precipitate at a certain pH, there are other elements affecting their solubility. The pH can alter the total charge and electrostatic interactions of the protein; thus, their conformation may be modified by the presence of ionizable amino acid residues such as arginine, histidine and aspartic acid. Ijarotimi et al. (2013) reported an amino acid profile in raw moringa seed flour (g/100 g of crude protein) that included glutamic acid (17.87 g), aspartic acid (15.70 g), arginine (8.28 g), isoleucine (3.83 g), phenylalanine (4.23 g) and leucine (3.27 g) as the most abundant amino acids. In the case of moringa seeds, coagulant properties related to low molecular weight peptides (6.5–13 kDa) have been reported with isoelectric points between 10 and 11, which are associated with stabilization of high positive charges (Ghebremichael et al. 2005; Anwar et al. 2007). Also, among other possible factors affecting the availability of protein is the formation of complexes with additional biomolecules. Since it has been reported that proteins such as lectins have conglomeration capacity these proteins can bind to carbohydrates affecting their solubility, therefore also explaining the low protein content in the protein isolates of moringa seeds (Santos et al. 2012). It has also been proposed that the solubility of the moringa protein at alkaline pH may be diminished by their native content or by their high lipid content, affecting the extraction efficiency. Based on the above, it would be relevant to study other methods of extraction and fractionation.
Proximate analysis of protein isolates and defatted flour of moringa
Legumes and oilseeds with a high protein content are good sources of protein isolates. The proximate composition of whole defatted flour was 37.48% of total protein, while the isolated lyophilized protein (pH 11, 4) was 49.67, 12.9% more than the flour (Table 1). Moringa seeds can thus be an important source of protein and compete with other protein-rich legumes such as soybeans (Glycine max, 39% protein content) (Rosenthal et al. 2001), and other important seeds such as amaranth (Amaranthus sp.) 12–19%, and chia (Salvia hispanica) (15.95–26%) (Coates 2011).
Table 1.
Proximal analysis of defatted flour and Moringa oleifera protein isolates
| Determination (%) | Defatted flour | Protein isolate pH 11, pI 4 |
|---|---|---|
| Protein | 37.48 | 49.67 |
| Ashes | 3.73 | 2.37 |
| Ether extract | 26.54 | 32.65 |
| Raw fiber | 5.65 | 7.78 |
| Moisture | 5.32 | 1.9 |
The fiber content was lower (5.6%) compared with other reported species; up to 15% in Phaseolus lunatus, 23% in P. vulgaris, 13% in Chenopodium quinoa, 33.6% in S. hispanica, and 11.6% in Lupinus albus (Torruco-Uco et al. 2009). Finally, in our study, because the protein isolated with the (pH 11, 4) treatment had the highest yield, it was used for digestion and further analysis.
Total hydrolysates and peptide fractions of protein from moringa
The most common protocol for producing hydrolysates and bioactive peptides is based on in vitro hydrolysis with proteolytic enzymes that simulate the gastrointestinal process, these include pepsin, trypsin and chymotrypsin. The hydrolysis with multiple proteolytic enzymes can generate peptide fragments that have a wide range of actions. For this reason, the bioactive peptides released have been extensively studied in terms of their biofunctional or nutraceutical potential. A nutraceutic is defined as any bioactive agent that, in addition to nutrition, also contributes to enhancing human health (Tavano 2013).
The peptide fractions > 10 kDa had higher protein concentration that the fractions < 10 kDa, indicating that most of the peptides were retained on the 10 kDa membrane. Therefore, only the fractions > 10 kDa were analyzed. Based on the amino acid composition of moriga seed protein reported by Ijarotimi et al. (2013), a considerable amount of peptide can be generated, since trypsin cleaves peptidic bonds next to alkaline residues and chymotrypsin cleaves phenylalanine and leucine residues. Also, with pepsin–trypsin digestion a higher concentration and variety of peptides was expected, due to a broader spectrum of catalytic sites, because pepsin digest on phenylalanine, tyrosine and leucine, while trypsin cleaves in lysine and arginine residues.
SDS-PAGE of hydrolysates and peptide fractions
Under non-reducing conditions, the protein profile of isolates (pH 11, 4) showed bands with molecular weights between 6 and 50 kDa (Fig. 2a). Peptide fractions > 10 kDa digested for 2.5 h with trypsin and chymotrypsin had electrophoretic patterns with bands of 15, 20, 45 and 50 kDa, similar to the profile of total protein isolates but with less intense bands probably due to a partial digestion. In lane 4 the fraction digested with pepsin–trypsin for 2.5 h has a profile with less intense bands between 15 and 20 kDa, possibly due to greater digestion compared with the other treatments (Fig. 2a). Under reducing conditions using β-mercaptoethanol, the band of 50 kDa (as observed under non-reducing conditions) was reduced to bands with 20, 25 and 30 kDa molecualr weight (Fig. 2b). The protein profile observed in our research are almost similar to profile previously reported in moringa seed proteins. For example, 66 kDa protein was found in SDS-PAGE (Agrawal et al. 2007). Also, cationic dimeric pepetide ranging between 6.5–13 kDa with coagulant properties and isoelectric points between 10 and 11 were reported by Ghebremichael et al. (2005).
Fig. 2.
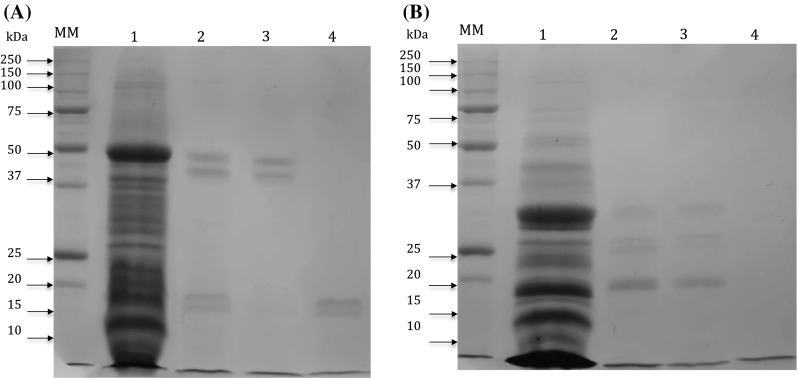
Polyacrylamide gel electrophoresis profile in 12% SDS-PAGE. a Non-reducing conditions. b Reducing conditions with β-mercaptoethanol. Lane MM) indicates the molecular weight marker. Lane 1: The protein isolates at pH 11, 4 treatment (40 μg/μl). Lane 2: Fractions > 10 kDa digested with trypsin for 2.5 h. Lane 3: Fractions > 10 kDa digested with chymotrypsin for 2.5 h; Lane 4: Fractions > 10 kDa digested with pepsin–trypsin for 2.5 h
Antioxidant activity of total hydrolysate and peptide fractions
It has been widely reported that food protein hydrolysates act as multifunctional antioxidants inhibiting a wide range of oxidation pathways. Generally, antioxidant and other functional properties are correlated with the specificity of the proteolytic enzyme and the time and degree of hydrolysis (Zhao et al. 2012). Our analysis showed low antioxidant activity with an ET of 1.69 mM/mg of protein (data not shown) in the total protein isolate (pH 11, 4). It can be seen that there was a difference between the ET values in the TH and the peptide fractions relative to hydrolysis time. Also, antioxidant capacity was significantly higher in the peptide fraction > 10 kDa with respect to the TH. The peptide fraction > 10 kDa with trypsin–pepsin was highest, having an ET of 27.43 mM/mg protein with 5 h of hydrolysis, followed by 18.26 mM/mg of protein after 2.5 h of digestion; while that with chymotrypsin was 17.89 mM/mg after 5 h of hydrolysis (Table 2).
Table 2.
Antioxidant activity by the ABTS method. Percent inhibition and mM Equivalents of Trolox (ET) of total hydrolysates and peptide fractions > 10 kDa
| Treatment | % Inhibition | mM ET/mg protein |
|---|---|---|
| TH, trypsin, 2.5 h | 14.32 | 4.19c |
| TH, trypsin, 5 h | 21.74 | 5.26c |
| Fraction > 10KDa, 2.5 h | 24.74 | 6.77c |
| Fraction > 10KDa, 5 h | 32.81 | 7.84c |
| TH, chymotrypsin, 2.5 h | 16.63 | 5.22c |
| TH, chymotrypsin, 5 h | 20.69 | 6.02c |
| Fraction > 10KDa, 2.5 h | 35.32 | 7.82c |
| Fraction > 10KDa, 5 h | 37.87 | 17.89b |
| TH, pepsin–trypsin, 2.5 h | 25.4 | 8.85c |
| TH, pepsin-trypsin, 5 h | 35.57 | 13.86b |
| Fraction > 10KDa, 2.5 h | 29.15 | 18.26b |
| Fraction > 10KDa, 5 h | 29.30 | 27.43a |
Different letter superscripts indicate statistical difference (P < 0.05)
The samples with the lowest antioxidant activity were those digested with trypsin; there were no significant differences between the peptide fraction and the TH. The highest were the peptide fractions with 6.77 and 7.84 mM ET/mg of protein and 2.5 and 5 h of hydrolysis, respectively (Table 2).
Finally, in the pepsin–trypsin digestion, significant differences (p < 0.05) were observed between TH at 2.5 and 5 h of hydrolysis (8.85 and 13.86 mM) and the peptide fractions > 10 kDa (18.26 and 27.43 mM). This shows that, depending on the proteolytic enzyme and the time of hydrolysis, it is possible to generated peptides with appropriate size and sequence for a specific bioactivity. Antioxidant activities in protein hydrolysates vary widely. For example, in legumes such as P. vulgaris, a hydrolysate fraction > 10 kDa obtained with pepsin and pancreatin resulted in a Trolox Equivalent Antioxidant Capacity (TEAC) of 170.48 mM/mg protein (Ruiz-Ruiz et al. 2013), while in Mucuna pruriens hydrolysates with pepsin a TEAC of 3.42 mM/mg was obtained (Segura-Campos et al. 2014). Likewise, Li et al. (2008) reported that a low molecular weight hydrolyzed protein fraction from Cicer arietinum seeds had antioxidant activity of 86% at a concentration of 1 mg/ml, which was higher than our results. It has been previously reported that peptides derived from proteins rich in hydrophobic amino acid residues exhibit high antioxidant properties (Chen et al. 1998). In the same way, moringa seed proteins have the potential to generate peptides with the composition and sequences required, depending on the enzyme used. Protein hydrolysates with low molecular weight and high concentration of hydrophobic amino acids residues have been associated with high hydroxyl free radicals trapping activity (Li et al. 2008). This coincides with the protein molecular weight and content of amino acids in moringa protein composition. Finally, it would be interesting to characterize the peptide sequences obtained in our study to confirm its potential.
Antihypertensive propertie of total hydrolysates and peptide fractions
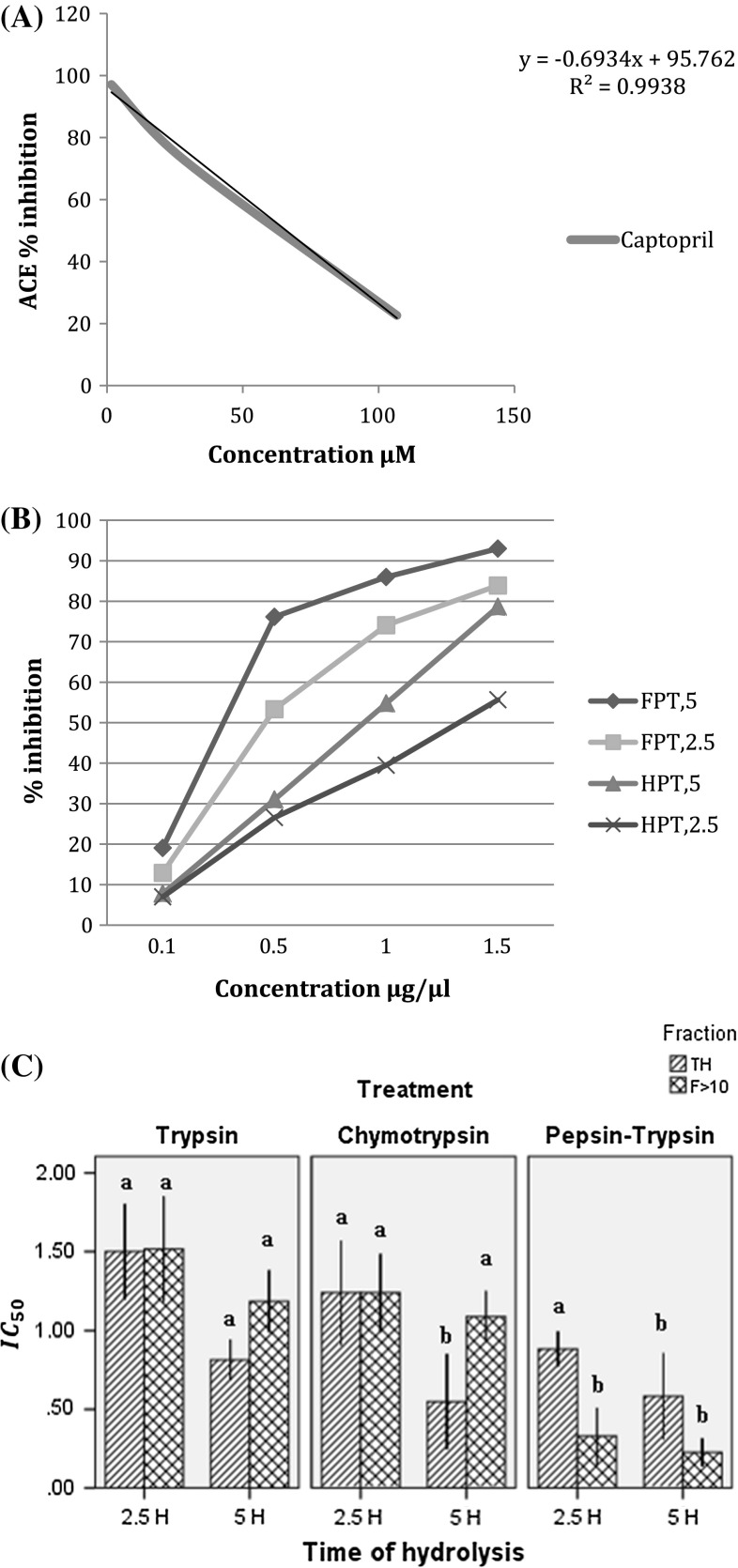
ACE activity increases in blood pressure in the organism and is related to the production of a vasoconstrictor angiotensin II derived from angiotensin I. ACE-I was calculated based on the IC50 which indicates the peptide concentration necessary to inhibit 50% ACE activity (Hartmann and Meisel 2007). In our study an IC50 for captopril of 0.016 μM was found, similar to the 0.019 μM reported by Li et al. (2005), who used the conventional method of 0.022 μM (Fig. 3a). Based on our results, we concluded that proteolytic enzyme and hydrolysis time played an important role in ACE- inhibiting activity (Fig. 3b). Higher antihypertensive activity was observed with pepsin–trypsin being enhanced in the peptide fractions (Fig. 3b) with an IC50 value of 0.226 μg/μl for the fraction > 10 kDa digested for 5 h (Fig. 3c), while in the TH the IC50 value was 0.582 μg/μl (Fig. 3c). However at 2.5 h of hydrolysis, the peptide fractions showed an IC50 value of 0.33 and 0.883 μg/μl in the TH (Fig. 3c). In the treatments with trypsin and chymotrypsin at 2.5 h the TH and the peptide fractions showed similar inhibitory capacity with an IC50 ranging between 1.5 and 1.2 μg/μl, respectively. On the other hand, at 5 h of hydrolysis, the TH was better than the peptide fraction with 0.81 and 0.548 μg/μl for trypsin and chymotrypsin respectively, while the fraction > 10 kDa showed IC50 values of 1.185 and 1.088 μg/μl (Fig. 3c). This may be due to the fact, that during ultrafiltration, the peptides < 10 kDa present in the TH were eliminated and these may play an important role in ACE-I activity. Usually small peptides are those that potentially have better capacity. It was observed that the time of hydrolysis had a significant influence since, in all cases, inhibition increased proportionally to time. In the case of sequential hydrolysis with pepsin–trypsin, we observed an enhancing effect on ACE inhibition, which could be associated with the broad diversity of peptides generated by a possible synergistic effect between the two enzymes. According to Jung et al. (2005), peptides generated with trypsin and chymotrypsin may have residues of Val, Ala, Leu, Pro, Tyr, Phe, His or Trp in the terminal C and so it is suggested that these can inhibit ACE with high affinity.
Fig. 3.
Percent inhibition of captopril (a). Percent Inhibition of the hydrolysates with pepsin–trypsin and peptide fractions > 10 KDa for 2.5 and 5 h (b) IC50 (μg/μl) of the total hydrolysates (TH) and peptide fraction >10 with 2.5 and 5 h of hydrolysis (c)
α-Amylase inhibition of the peptide fractions
α-amylase is an enzyme responsible for the hydrolysis of starch carbohydrates in the digestive system. Its inhibition is related to decrements in blood glucose levels (Visvanathan et al. 2016). The highest inhibitory activity was found in the treatment with pepsin–trypsin where the peptide fractions showed an IC50 of 0.195 and 0.123 μg/μl for 2.5 h and 5 h of hydrolysis respectively (Table 3). In contrast hydrolysates with trypsin showed the lowest α-amylase inhibitory activity with an IC50 value of 0.505 and 0.664 μg/μl for 2.5 and 5 h of hydrolysis.
Table 3.
Percent inhibition of α-amylase activity and IC50 of peptides fractions > 10 kDa
| Treatment | % Inhibition | IC50 (μg/μl) |
|---|---|---|
| Trypsin, fraction > 10 KDa, 2.5 h | 32.44 | 0.505a |
| Trypsin, fraction > 10 kda, 5 h | 27.84 | 0.664a |
| Chymotrypsin, fraction > 10 kda, 2.5 h | 58.8 | 0.158b |
| Chymotrypsin, fraction > 10 KDa, 5 h | 56.22 | 0.353a |
| Pepsin–trypsin, fraction > 10 KDa, 2.5 h | 36.31 | 0.195b |
| Pepsin–trypsin, fraction > 10 KDa, 5 h | 38.56 | 0.123b |
Different letter superscripts indicate statistical difference (P < 0.05)
The results also showed that the hydrolysis time for trypsin and chymotrypsin were inversely proportional to its inhibitory potential; inhibition activity was better with 2.5 h than with 5 h of digestion (Table 3). In a report by Yu et al. (2012), an α-amylase inhibiting peptide obtained from albumin had the sequence lysine–leucine–proline–glycine–phenylalanine. This coincides with another study with pinto beans where a similar amino acid residues sequence was obtained, indicating that combinations of these amino acid residues have the potential to inhibit α-amylase activity. Other studies have suggested that dipeptides (proline–proline, cysteine–cysteine, leucine–leucine and glycine–glycine) have high potential for antidiabetic activity, but the peptides were not identified. This problem will be considered for future research. Another important property of biological peptides is the positioning of amino acid residues at the N- or C-terminal ends (Najafian and Babji 2014) since peptides with antidiabetic activity have been reported to possess an N-terminal of glycine or phenylalanine and a C-terminal of phenylalanine or leucine. Although the position of the amino acids in our work is not known, it is possible that several peptides have this type of terminal because of the enzymes used.
Conclusion
Moringa oleifera proteins exhibit better functional properties when they are digested sequentially with pepsin–trypsin for longer than 5 h, which increases their nutraceutical activities as antioxidants, antihypertensives and antidiabetics. Also, peptides fractions > 10 kDa possess antidiabetic properties superior to their antihypertensive properties, evidencing the influence of the peptide composition of fractions on diverse properties. In recent years, moringa has given great importance in the prevention of different chronic degenerative diseases. For this reason, in future research characterization of the potential health benefits of peptides present in the proteins of moringa seed flour could contribute to promoting its consumption.
Acknowledgements
This work was supported by Programa de Apoyo a la Investigación Científica y Tecnológica (PAICYT) of Universidad Autonoma de Nuevo Leon (Grant Number CT303-15).
References
- Agrawal H, Shee C, Sharma AK. Isolation of a 66KDa protein with coagulation activity from seeds of Moringa oleifera. Res J Agric Biol Sci. 2007;3(5):418–421. [Google Scholar]
- Anwar F, Latif S, Ashraf M, Gilani AH. Moringa oleifera: a food plant with multiple medicinal uses. Phytother Res. 2007;21(1):17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis. 16. Arlington: Association of Official Analytical Chemists; 1995. pp. 1–45. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen HM, Muramoto K, Yamauchi F, Fujimoto K, Nokihara K. Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J Agric Food Chem. 1998;46(1):49–53. doi: 10.1021/jf970649w. [DOI] [PubMed] [Google Scholar]
- Coates W. Protein content, oil content and fatty acid profiles as potential criteria to determine the origin of commercially grown chia (Salvia hispanica L.) Ind Crops Prod. 2011;34(2):1366–1371. doi: 10.1016/j.indcrop.2010.12.007. [DOI] [Google Scholar]
- Ghebremichael KA, Gunaratna KR, Henriksson H, Brumer H, Dalhammar G. A simple purification and activity assay of the coagulant protein from Moringa oleifera seed. Water Res. 2005;39(11):2338–2344. doi: 10.1016/j.watres.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Hayakari M, Kondo Y, Izumi H. A rapid and simple spectrophotometric assay of angiotensin-converting enzyme. Anal Biochem. 1978;84(2):361–369. doi: 10.1016/0003-2697(78)90053-2. [DOI] [PubMed] [Google Scholar]
- Ijarotimi OS, Adeoti OA, Ariyo O. Comparative study on nutrient composition, phytochemical, and functional characteristics of raw, germinated, and fermented Moringa oleifera seed flour. Food Sci Nutr. 2013;1(6):452–463. doi: 10.1002/fsn3.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung IL. Soluble extract from Moringa oleifera leaves with a new anticancer activity. PLoS ONE. 2014;9(4):e95492. doi: 10.1371/journal.pone.0095492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung WK, Park PJ, Byun HG, Moon SH, Kim SK. Preparation of hoki (Johnius belengerii) bone oligophosphopeptide with a high affinity to calcium by carnivorous intestine crude proteinase. Food Chem. 2005;91(2):333–340. doi: 10.1016/j.foodchem.2004.06.016. [DOI] [Google Scholar]
- Kasolo JN, Bimenya GS, Ojok L, Ochieng J, Ogwal-Okeng JW. Phytochemicals and uses of Moringa oleifera leaves in Ugandan rural communities. J Med Plants Res. 2010;4(9):753–757. [Google Scholar]
- Kitts DD, Weiler K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr Pharm Des. 2003;9(16):1309–1323. doi: 10.2174/1381612033454883. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li GH, Liu H, Shi YH, Le GW. Direct spectrophotometric measurement of angiotensin I-converting enzyme inhibitory activity for screening bioactive peptides. J Pharm Biomed Anal. 2005;37(2):219–224. doi: 10.1016/j.jpba.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Li Y, Jiang B, Zhang T, Mu W, Liu J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH) Food Chem. 2008;106(2):444–450. doi: 10.1016/j.foodchem.2007.04.067. [DOI] [Google Scholar]
- Lopez-Sanchez J, Ponce-Alquicira E, Pedroza-Islas R, de la Peña-Diaz A, Soriano-Santos J. Effects of heat and pH treatments and in vitro digestion on the biological activity of protein hydrolysates of Amaranthus hypochondriacus L. grain. J Food Sci. 2016 doi: 10.1007/s13197-016-2428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S, Rai PK, Rai NK, Rai AK, Bicanic D, Watal G. Role of spectral studies in detection of antibacterial phytoelements and phytochemicals of Moringa oleifera. Food Biophys. 2011;6(4):497–502. doi: 10.1007/s11483-011-9231-2. [DOI] [Google Scholar]
- Nair SS, Kavrekar V, Mishra A. In vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. Eur J Exp Biol. 2013;3(1):128–132. [Google Scholar]
- Najafian L, Babji AS. Production of bioactive peptides using enzymatic hydrolysis and identification antioxidative peptides from patin (Pangasius sutchi) sarcoplasmic protein hydolysate. J Funct Foods. 2014;9:280–289. doi: 10.1016/j.jff.2014.05.003. [DOI] [Google Scholar]
- Pedroche J, Yust MM, Lqari H, Girón-Calle J, Alaiz M, Vioque J, Millán F. Brassica carinata protein isolates: chemical composition, protein characterization and improvement of functional properties by protein hydrolysis. Food Chem. 2004;88(3):337–346. doi: 10.1016/j.foodchem.2004.01.045. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rebello CJ, Greenway FL, Finley JW. A review of the nutritional value of legumes and their effects on obesity and its related co-morbidities. Obes Rev. 2014;15(5):392–407. doi: 10.1111/obr.12144. [DOI] [PubMed] [Google Scholar]
- Rosenthal A, Pyle DL, Niranjan K, Gilmour S, Trinca L. Combined effect of operational variables and enzyme activity on aqueous enzymatic extraction of oil and protein from soybean. Enzyme Microb Technol. 2001;28(6):499–509. doi: 10.1016/S0141-0229(00)00351-3. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ruiz J, Dávila-Ortiz G, Chel-Guerrero L, Betancur-Ancona D. Angiotensin I-converting enzyme inhibitory and antioxidant peptide fractions from hard-to-cook vean enzymatic hydrolysates. J Food Biochem. 2013;37(1):26–35. doi: 10.1111/j.1745-4514.2011.00594.x. [DOI] [Google Scholar]
- Salcedo-Chávez B, Osuna-Castro JA, Guevata-Lara F, Dominguez-Dominguez J, Paredes-López O. Optimization of the isoelectric precipitation method to obtain protein isolates from amaranth (Amarathus cruentus) seeds. J Agric Food Chem. 2002;50(22):6515–6520. doi: 10.1021/jf020522t. [DOI] [PubMed] [Google Scholar]
- Santos AF, Paiva PM, Teixeira JA, Brito AG, Coelho LC, Nogueira R. Coagulant properties of Moringa oleifera protein preparations: application to humic acid removal. Environ Technol. 2012;33(1):69–75. doi: 10.1080/09593330.2010.550323. [DOI] [PubMed] [Google Scholar]
- Segura-Campos MR, Salazar-Vega IM, Chel-Guerrero LA, Betancur-Ancona DA. Biological potential of chia (Salvia hispanica L.) protein hydrolysates and their incorporation into functional foods. LWT Food Sci Technol. 2013;50(2):723–731. doi: 10.1016/j.lwt.2012.07.017. [DOI] [Google Scholar]
- Segura-Campos MR, Tovar-Benítez T, Chel-Guerrero L, Betancur-Ancona D. Functional and bioactive properties of Velvet bean (Mucuna pruriens) protein hydrolysates produced by enzymatic treatments. J Food Meas Charact. 2014;8(2):61–69. doi: 10.1007/s11694-013-9165-0. [DOI] [Google Scholar]
- Singh BP, Vij S, Hati S. Functional significance of bioactive peptides derived from soybean. Peptides. 2014;54:171–179. doi: 10.1016/j.peptides.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Tavano OL. Protein hydrolysis using proteases: an important tool for food biotechnology. J Mol Catal B Enzyme. 2013;90:1–11. doi: 10.1016/j.molcatb.2013.01.011. [DOI] [Google Scholar]
- Torruco-Uco J, Chel-Guerrero L, Martínez-Ayala A, Dávila-Ortíz G, Betancur-Ancona D. Angiotensin-I converting enzyme inhibitory and antioxidant activities of protein hydrolysates from Phaseolus lunatus and Phaseolus vulgaris seeds. LWT-Food Sci Technol. 2009;42(10):1597–1604. doi: 10.1016/j.lwt.2009.06.006. [DOI] [Google Scholar]
- Vats S, Gupta T. Evaluation of bioactive compounds and antioxidant potential of hydroethanolic extract of Moringa oleifera Lam. from Rajasthan. India. Physiol Mol Biol Plants. 2017;23(1):239–248. doi: 10.1007/s12298-016-0407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvanathan R, Jayathilake C, Liyanage R. A simple microplate-based method for the determination of α-amylase activity using the glucose assay kit (GOD method) Food Chem. 2016;211:853–859. doi: 10.1016/j.foodchem.2016.05.090. [DOI] [PubMed] [Google Scholar]
- Yu Z, Yin Y, Zhao W, Liu J, Chen F. Anti-diabetic activity peptides from albumin against α-glucosidase and α-amylase. Food Chem. 2012;135(3):2078–2085. doi: 10.1016/j.foodchem.2012.06.088. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Xiong H, Selomulya C, Chen XD, Zhong H, Wang S, Zhou Q. Enzymatic hydrolysis of rice dreg protein: effects of enzyme type on the functional properties and antioxidant activities of recovered proteins. Food Chem. 2012;134(3):1360–1367. doi: 10.1016/j.foodchem.2012.03.033. [DOI] [PubMed] [Google Scholar]