Abstract
Nowadays, fingerprinting of food became one of the most perspective analytical tools to resolve a problem of food quality and authentication, especially in difficult cases like differentiation of fruit varieties. In this work, plum brandies distillated by the same technology from 25 plum cultivars were distinguished by comprehensive two-dimensional gas chromatographic analysis and sensory evaluation. The number of identified compounds in studied samples varied between 91 and 195 depending on the plum variety. Enriched volatile organic compounds (VOC) profile was identified for the samples received from “Chrudimer”, “Cacak Fruitful” and “Hanita” plum varieties, whereas in the case of “Gabrovská”, “Presenta”, Elena” and “President” plum varieties, the VOC profile was significantly reduced. From qualitative point of view, the particular plum brandies showed differences in the presence of unsaturated fusel alcohols (e.g. 3-methyl-3-buten-1-ol, trans-3-hexenol), unsaturated aldehydes (2-butenal, 2-nonenal), monoterpene derivatives (e.g. linalool acetate, geraniol acetate) and lactones, which were mainly detected at the trace level.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2900-5) contains supplementary material, which is available to authorized users.
Keywords: Plum brandy, Plum cultivar, Comprehensive two-dimensional gas chromatography, Sensory analysis, GCxGC–TOF-MS
Introduction
Plum orchards are widely grown worldwide especially in Central Europe and Asian regions, and their areas are ranked on the 2nd place after apple among all pome and stone fruit crops. Nearly over 2000 named varieties of plums have been established with a dozen different species (Gómez-Plaza and Ledbetter 2010). Prunus salicina (Japanese plum) and Prunus domestica (European plum) are the most common cultivated plum species. At the same time, wild plums are mainly applied as donors to enhance resistance and ecological adaptability of cultivars, for instance Prunus cerasifera originated from central Asia, Prunus americana and Prunus nigra derived from the North America. Various plum cultivars differ significantly in their properties such as climatic adaptation, harvest yield or fruit characteristics, e.g. fruit size, shape, colour, taste and flavour. For example “Italian Prune”, “Hanita” and “Harbella” are considered as good donors for flavour and fine acid content (Hartmann and Neumüller 2009), while cultivars “Katinka”, “Tegera” and “Cacak Beauty” are found as effective parents to improve firmness of the plum fruit.
Prunus domestica plums are commonly consumed fresh but more frequently are used for production of alcoholic beverages and syrups. Alcoholic beverages, particularly plum distillates are known for their production traditions and appears under different names depending on geographic region, e.g. rakia (Balkan countries), pálinka (Hungary), Slivovica or Slivovitz in Easter and Central European countries. The quality of received alcoholic beverages mostly depends on fruit conditions, fermentation and distillation technologies. Influence of different yeasts (isolated from plums fruits, plum musts and commercial strains) on the fermentation and chemical composition of plum brandies has been studied by Satora and Tuszyński (2010). In comparison to commercial yeast strains, the lower amounts of higher alcohols were obtained in the samples fermented with indigenous strains. Simultaneously, effect of maturation process and botanic species of wood used for maturation was investigated for these beverages (Pecić et al. 2012). It was found that wood origin mainly defined the composition of phenols, whereas the time of maturation influenced their concentration. In addition, the impact of cultivars on the Volatile Organic Compounds (VOC) composition of alcoholic beverage could be an issue of critical study, because variable chemical profile was shown for several types of fruits, such as apples (Lasekan et al. 2013), pears (Chen et al. 2007), cherries (Serradilla et al. 2012), raspberries (Malowicki et al. 2008), or elderberries (Veberic et al. 2009). For example, in case of apple distillates it was demonstrated that concentration of ethyl octanoate, hexyl 2-methylbutanoate, 1-hexanol, benzaldehyde and furfural was correlated with apple variety (Versini et al. 2009). On the other hand, the content of propanol, 2-methyl-1-propanol and 2-phenylethanol (with pleasant rose flavour) varied in apple wines produced from 3 different apple varieties (Satora et al. 2008). The characteristic VOC profile was identified for Italian distillates produced from wild and three cultivated pears. The main differences were found in content of farnesene isomers, citronellol and methyl and ethyl unsaturated decanoate isomers (Versini et al. 2012). The concentration of amyl alcohols changed significantly in spirits received for black current cultivars and red currant cultivars (Vulić et al. 2012).
The small differences in VOC profiles were detected among various plum species, e.g. aldehydes were dominant in P. domestica and P. spinosa, whereas esters were prevailing for P. salicina, their hybrids and P. ussuriensis (Chai et al. 2012). Moreover, deviations in fermentable sugars (the total of glucose, fructose, and sucrose), sorbitol content, dry matter content and titratable acidity were shown for Prunus domestica varieties (Bohačenko et al. 2010). On the contrary, the ratio of the content of 2-methyl-1-propanol and 3-methyl-1-butanol could be a marker of variety recognition used for production of plum brandies (Spaho et al. 2013). At the same time, volatile fingerprinting is more global approach of food authentication in comparison with the classical techniques (stable isotope analysis, determination of particular markers). This approach could be useful in complicated situations like distinguishing varieties of the same fruit.
The GC–MS is used for determination of VOC profiles, but recently introduced comprehensive two-dimensional gas chromatography (GCxGC) is characterized with enhanced separation efficiency and better resolution power. Thus, it is especially suitable for analysis of volatile organic profile of high-complexity samples such as alcoholic beverages (Vyviurska et al. 2015; Cardeal and Marriott 2009; Villière et al. 2012; Weldegergis et al. 2011; Robinson et al. 2011). This higher efficiency of separation is achieved due to application of two separation columns with different polarity of stationary phases available to resolve structural and geometrical isomers. Simultaneously, high data acquisition rate and a small internal volume of detector used in GCxGC offer increased sensitivity of measurements. In our study, plum brandies produced from 25 Prunus domestica plum cultivars were analysed using comprehensive two-dimensional gas chromatography to establish influence of plum variety on the final volatile composition of plum brandies.
Materials and methods
Chemicals
Pentane used for liquid–liquid extraction was obtained from Merck (Darmstadt, Germany), anhydrous NaCl was supplied from Microchem (Pezinok, Slovakia), a mixture for alkanes (C7–C30) used for calculation of retention indices was purchased from Supelco (Belleforte, PA, USA). Solution of benzophenone in ethanol with concentration 1.6 mg l−1 was applied as an internal standard solution. The standards of chemical listed in Table 2 were obtained from Sigma-Aldrich (St. Louis, USA), Microchem (Pezinok, Slovakia), Merck KGaA (Darmstadt, Germany), SAFC Biosciences (Lenexa, USA).
Table 2.
Compound which identification confirmed with authentic standards in the plum brandies with GCxGC–TOFMS
| DBFFAPxBPX-50 | RI | HP-5xBPX-50 | Compounds | Area (%)f | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT | RI | SL1 | SL2 | SL3 | SL4 | SL5 | SL6 | SL7 | SL8 | SL9 | SL10 | SL11 | SL12 | SL13 | |||
| 1D (min) | 2D (s) | ||||||||||||||||
| 9.6 | 1.54 | 984 | 596 | 2,3-Butanedionea | 0.28 | 0.24 | 0.42 | 0.56 | 0.21 | 0.05 | 0.08 | 0.66 | 0.58 | ||||
| 11.8667 | 1.46 | 1085 | 591 | 2-Butanola,e | 0.66 | 0.21 | 5.70 | 0.03 | |||||||||
| 12.4 | 2.89 | 1028 | 804 | Ethyl butanoatea,e | 0.31 | 0.92 | 0.47 | 0.30 | 0.49 | 0.54 | 0.31 | 0.57 | 0.10 | 0.84 | 0.90 | 0.78 | 0.17 |
| 13.0667 | 3.37 | 1051 | 851 | Ethyl 2-methylbutanoatea,e | 0.09 | 0.04 | 0.32 | 0.04 | 0.20 | 0.03 | 0.02 | ||||||
| 13.6 | 1.39 | 1038 | 581 | 1-Propanolb,e | 9.10 | 8.25 | 13.51 | 8.04 | 17.44 | 13.53 | 9.37 | 16.10 | 1.21 | 16.65 | 13.95 | 11.33 | 26.33 |
| 13.7333 | 1.91 | 1063 | 697 | 2,3-Pentanedionea,e | 0.06 | 0.02 | 0.02 | 0.06 | 0.08 | 0.08 | 0.03 | 0.13 | 0.04 | ||||
| 14.2667 | 2.83 | 1074 | 816 | Butyl acetatec,e | 1.59 | 0.33 | 0.04 | 0.01 | 0.61 | ||||||||
| 16.8 | 1.48 | 1091 | 669 | 2-Methyl-1-propanola,e | 2.31 | 11.95 | 17.94 | 22.34 | 14.79 | 23.52 | 10.93 | 10.38 | 22.13 | 3.32 | 17.15 | 12.84 | 16.18 |
| 16.9333 | 3.27 | 1122 | 880 | 3-Methylbutyl acetate d,e | 10.28 | 9.47 | 12.08 | 9.32 | 1.79 | 9.63 | 3.32 | 7.33 | 1.37 | 7.37 | 3.83 | 11.55 | 3.06 |
| 17.6 | 3.62 | 1133 | 906 | Ethyl pentanoatea,e | 0.04 | 0.03 | 0.03 | 0.03 | |||||||||
| 17.6 | 3.63 | 1133 | 854 | Ethyl 3-methylbutanoatea,e | 0.03 | 0.60 | 0.06 | 0.05 | 0.03 | ||||||||
| 17.7333 | 1.62 | 1108 | 701 | 3-Pentanola | 0.03 | ||||||||||||
| 18.6667 | 4.65 | 1160 | 992 | Myrcenea | 0.03 | ||||||||||||
| 19.0667 | 1.58 | 1122 | 701 | 2-Pentanola,e | 0.20 | 0.12 | 0.01 | 0.13 | 0.01 | 0.13 | 0.11 | ||||||
| 20.8 | 3.47 | 1178 | 925 | Methyl hexoatea,e | 0.08 | 0.12 | 0.15 | 0.04 | 0.04 | 0.05 | 0.08 | 0.10 | 0.11 | 0.10 | > 0.01 | ||
| 21.0667 | 3.07 | 1176 | 891 | 2-Heptanonea | > 0.01 | ||||||||||||
| 21.3333 | 1.46 | 1157 | 678 | 1-Penten-3-ola | 0.11 | 0.10 | 0.04 | 0.11 | 0.10 | 0.05 | 0.09 | 0.10 | 0.05 | ||||
| 23.8667 | 1.61 | 1200 | 732 | 2-Methyl-1-butanola,e | 1.45 | 7.77 | 8.61 | 4.47 | 9.86 | 0.12 | 6.58 | 8.82 | 6.49 | 3.40 | |||
| 24.1333 | 4.14 | 1224 | 1000 | Ethyl hexoatea,e | 1.42 | 3.05 | 2.73 | 0.89 | 1.33 | 2.77 | 2.30 | 3.10 | 2.70 | 3.16 | 4.27 | 3.76 | 1.32 |
| 24.2667 | 1.73 | 1201 | 730 | 3-Methyl-1-butanola,e | 15.39 | 15.01 | 1.43 | 13.45 | 4.51 | 8.20 | 36.29 | 15.61 | 3.19 | 8.15 | 13.62 | 14.88 | 10.06 |
| 24.9333 | 1.4 | 1202 | 640 | 2-Methyl-2-propen-1-ol | |||||||||||||
| 25.0667 | 1.74 | 1215 | 802 | 2-Hexanola,e | 0.07 | 0.02 | |||||||||||
| 26 | 2.77 | 1248 | 892 | Styrened,e | 0.32 | 0.03 | 0.11 | 0.08 | 0.02 | 0.04 | 0.36 | 0.10 | 0.10 | 0.06 | 0.05 | ||
| 26.2667 | 4.56 | 1258 | 1059 | 3-Methylbutyl butanoatea,e | 0.02 | ||||||||||||
| 26.9333 | 1.62 | 1243 | 766 | 1-Pentanola,e | 0.98 | 0.18 | 0.25 | 0.20 | 0.33 | 0.40 | 0.24 | 0.29 | 0.14 | 0.29 | 0.16 | 0.31 | 0.18 |
| 26.9333 | 1.53 | 1241 | 727 | 3-Methyl-3-buten-1-ola | 0.31 | 0.09 | 0.10 | 0.04 | 0.17 | 0.04 | 0.09 | 0.07 | 0.04 | 0.05 | 0.04 | 0.08 | 0.05 |
| 30.9333 | 1.68 | 1305 | 840 | 4-Methyl-1-pentanola,e | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | 0.05 | 0.02 | 0.02 | 0.06 | 0.02 | 0.03 | 0.03 | 0.03 |
| 31.3333 | 1.91 | 1313 | 903 | 2-Heptanola,e | 0.17 | 0.03 | 0.07 | 0.08 | 0.03 | 0.03 | 0.05 | 0.27 | 0.08 | 0.13 | 0.04 | 0.12 | |
| 31.3333 | 4.2 | 1324 | 1102 | Ethyl heptanoatea,e | 0.09 | 0.03 | 0.04 | 0.03 | 0.03 | 0.06 | 0.02 | 0.03 | 0.06 | 0.05 | 0.02 | 0.01 | |
| 32.4 | 4.35 | 1339 | 1111 | cis-Rose oxidea,e | 0.01 | ||||||||||||
| 32.9333 | 1.67 | 1335 | 813 | Ethyl 2-hydroxypropanoatea,e | 0.59 | 1.71 | 0.57 | 0.52 | 1.04 | 1.35 | 1.73 | 1.83 | 1.28 | 1.51 | |||
| 33.4667 | 1.8 | 1343 | 870 | 1-Hexanolb,e | 2.87 | 1.17 | 4.23 | 0.55 | 1.32 | 1.49 | 0.49 | 2.01 | 0.94 | 2.08 | 0.73 | 2.52 | 0.97 |
| 34.1333 | 3.79 | 1366 | 1115 | Heptyl acetatea,e | 0.07 | ||||||||||||
| 34.1333 | 1.69 | 1355 | 852 | trans-3-Hexen-1-ola | 0.04 | ||||||||||||
| 35.2 | 3.5 | 1381 | 1094 | 2-Nonanonea,e | 0.10 | > 0.01 | 0.02 | 0.02 | 0.02 | ||||||||
| 35.2 | 3.85 | 1378 | 1126 | Methyl octanoatea,e | 0.19 | 0.43 | 0.44 | 0.12 | 0.18 | 0.12 | 0.33 | 0.28 | 0.42 | 0.50 | 0.28 | 0.17 | 0.13 |
| 35.3333 | 1.68 | 1375 | 859 | cis-3-Hexen-1-ola,e | 0.22 | 0.03 | 0.05 | 0.08 | 0.04 | 0.09 | 0.04 | 0.04 | 0.07 | 0.04 | |||
| 36 | 2.16 | 1385 | 997 | 3-Octanola,e | 0.04 | 0.01 | 0.03 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.03 | 0.01 | 0.01 | ||
| 36.9333 | 1.63 | 1398 | 871 | trans-2-Hexen-1-old | |||||||||||||
| 37.4667 | 1.63 | 1407 | 867 | cis-2-Hexen-1-old | 0.02 | 0.01 | 0.01 | 0.01 | |||||||||
| 37.7333 | 2.08 | 1413 | 1003 | 2-Octanola | 0.06 | 0.02 | 0.04 | 0.01 | |||||||||
| 38.1333 | 4.42 | 1426 | 1198 | Ethyl octanoatea,e | 1.62 | 0.42 | 2.99 | 2.77 | 6.33 | 6.85 | 7.92 | 3.02 | 10.32 | 9.31 | 6.85 | 6.56 | 4.95 |
| 38.2667 | 3.39 | 1426 | 1090 | α,p-Dimethylstyrened | 0.01 | ||||||||||||
| 38.8 | 2.69 | 1428 | 1072 | Linalool oxidea,e | 0.17 | 0.11 | 0.20 | 0.01 | 0.03 | > 0.01 | 0.06 | 0.03 | 0.02 | 0.01 | 0.02 | 0.02 | |
| 39.6 | 1.9 | 1443 | 981 | 1-Octen-3-ola,e | 0.14 | 0.05 | 0.14 | 0.03 | 0.02 | 0.03 | 0.04 | 0.07 | 0.02 | 0.08 | 0.02 | 0.04 | 0.01 |
| 40 | 1.88 | 1449 | 974 | 1-Heptanola,e | 0.31 | 0.09 | 0.28 | 0.06 | 0.07 | 0.15 | 0.07 | 0.10 | 0.04 | 0.17 | 0.11 | 0.09 | 0.06 |
| 40.2667 | 1.14 | 1443 | 685 | Acetic acida,e | 4.07 | 3.11 | 2.25 | 0.57 | 4.93 | 1.53 | 0.26 | 2.91 | 2.48 | 2.27 | 2.50 | 0.69 | 2.17 |
| 40.5333 | 1.96 | 1455 | 989 | 6-Methyl-5-hepten-2-old | 0.01 | > 0.01 | > 0.01 | ||||||||||
| 40.9333 | 1.68 | 1461 | 832 | Furfurala,e | 0.77 | 1.15 | 1.24 | 1.15 | 2.05 | 0.09 | 1.00 | 0.62 | 1.55 | 0.92 | 1.53 | 1.42 | 1.32 |
| 42.2667 | 3.76 | 1487 | 1206 | Decanala,e | 0.04 | 0.02 | 0.01 | 0.02 | 0.03 | 0.03 | 0.02 | 0.02 | |||||
| 43.3333 | 1.87 | 1497 | 914 | 2-Acetylfurana | 0.01 | 0.01 | > 0.01 | > 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | |||
| 43.8667 | 2.26 | 1510 | 1104 | 2-Nonanola,e | 0.18 | 0.01 | > 0.08 | 0.04 | 0.03 | 0.03 | 0.67 | 0.05 | 0.25 | 0.02 | |||
| 44 | 2.26 | 1513 | 959 | Benzaldehydea,e | 6.77 | 2.92 | 4.57 | 6.40 | 7.48 | 1.91 | 4.31 | 3.39 | 6.30 | 2.39 | 2.13 | 2.03 | 0.74 |
| 44.5333 | 4.46 | 1527 | 1296 | Ethyl nonylatea,e | 0.62 | 0.58 | 0.77 | 0.33 | 0.59 | 0.53 | 0.15 | 0.45 | 0.18 | 0.80 | 0.52 | 0.17 | 0.34 |
| 44.5333 | 3.07 | 1524 | 1161 | trans-2-Nonenald,e | 0.01 | > 0.01 | 0.01 | ||||||||||
| 45.6 | 1.16 | 1527 | 694 | Propanoic acida | 0.03 | 0.02 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | ||
| 45.6 | 2.22 | 1539 | 1100 | Linaloola,e | 0.17 | 0.01 | 0.22 | 0.06 | 0.05 | 0.03 | 0.03 | 0.12 | 0.09 | 0.01 | 0.13 | 0.12 | 0.04 |
| 45.8667 | 3.6 | 1546 | 1258 | Linalool acetatea | 0.03 | ||||||||||||
| 46.1333 | 2.02 | 1548 | 1072 | 1-Octanola,e | 0.31 | 0.20 | 0.21 | 0.10 | 0.11 | 0.16 | 0.09 | 0.28 | 0.13 | 0.16 | 0.13 | 0.19 | 0.12 |
| 46.6667 | 4.49 | 1562 | 1293 | Bornyl acetated | 0.13 | ||||||||||||
| 47.2 | 6.46 | 1575 | 1426 | Caryophyllened,e | 0.10 | ||||||||||||
| 47.6 | 1.94 | 1568 | 963,6 | 5-Methyl furfurala | > 0.01 | 0.01 | > 0.01 | ||||||||||
| 48.1333 | 4.11 | 1581 | 1326 | Methyl decanoatea,e | 0.25 | 0.62 | 0.75 | 0.10 | 0.32 | 0.08 | 0.34 | 0.29 | 0.72 | 0.70 | 0.30 | 0.11 | 0.16 |
| 48.4 | 2.87 | 1588 | 1179 | 4-Terpinenola,e | 0.09 | > 0.01 | 0.02 | 0.02 | 0.02 | ||||||||
| 48.9333 | 2.07 | 1593 | 985 | Benzonitrilea | 0.01 | ||||||||||||
| 50.2667 | 2.38 | 1618 | 1147 | β-Terpineola | 0.01 | ||||||||||||
| 50.5333 | 1.88 | 1620 | 916 | Butyrolactonea | 0.02 | > 0.01 | > 0.01 | > 0.01 | > 0.01 | 0.01 | 0.01 | > 0.01 | 0.01 | > 0.01 | > 0.01 | ||
| 50.5333 | 1.2 | 1620 | 812 | Butanoic acida | 0.01 | 0.05 | 0.03 | 0.02 | > 0.01 | 0.03 | 0.04 | 0.02 | 0.04 | ||||
| 50.6667 | 4.74 | 1625 | 1397 | Ethyl decanoatea,e | 5.44 | 0.66 | 0.67 | 3.13 | 3.50 | 5.13 | 3.12 | 2.02 | 12.86 | 6.92 | 3.24 | 5.50 | 8.86 |
| 50.9333 | 3.19 | 1631 | 1264 | trans-2-Decenald,e | 0.02 | 0.01 | > 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | > 0.01 | |||||
| 51.2 | 2.22 | 1633 | 1044 | Benzeneacetaldehydea,e | 0.08 | 0.02 | 0.05 | 0.02 | 0.02 | ||||||||
| 51.6 | 2.34 | 1640 | 1067 | Acetophenonea,e | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | |
| 52 | 3.81 | 1652 | 1355 | Citronellyl acetatea,e | 0.03 | ||||||||||||
| 52.1333 | 2.17 | 1653 | 1175 | 1-Nonanola,e | 0.79 | 0.37 | 0.69 | 0.39 | 0.29 | 0.82 | 0.13 | 0.57 | 0.18 | 0.44 | 0.31 | 0.37 | 0.71 |
| 52.5333 | 1.36 | 1656 | 857 | 2-Furanmethanola | 0.01 | > 0.01 | > 0.01 | > 0.01 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 |
| 53.0667 | 4.17 | 1661 | 1413 | Decyl acetatea,e | 0.02 | 0.05 | 0.02 | 0.01 | 0.01 | ||||||||
| 53.0667 | 2.63 | 1671 | 1152 | p-Vinylanisolea,e | 0.30 | 1.34 | 0.50 | 0.11 | 0.01 | 0.52 | 0.84 | 0.41 | 0.10 | 0.53 | 0.01 | ||
| 53.2 | 2.55 | 1670 | 1181 | Diethyl butanedioatea,e | 0.56 | 0.08 | 0.11 | 0.03 | 0.14 | 0.05 | 0.31 | 0.73 | 1.30 | 0.12 | 2.08 | 0.05 | 0.32 |
| 54 | 2.46 | 1669 | 1192 | α-Terpineola,e | 0.05 | 0.08 | 0.08 | 0.02 | 0.01 | 0.01 | 0.34 | 0.17 | 0.03 | 0.12 | 0.03 | ||
| 54.4 | 2.62 | 1692 | 1216 | trans,trans-2,4-Nonadienala | > 0.01 | ||||||||||||
| 54.5333 | 2.17 | 1691 | 1054 | γ-Hexalactonea | > 0.01 | > 0.01 | > 0.01 | ||||||||||
| 55.8667 | 2.51 | 1719 | 1165 | Phenylmethyl acetatea,e | 0.88 | 0.01 | 0.05 | 0.03 | 0.04 | 0.01 | 0.01 | 0.04 | 0.01 | 0.01 | 0.01 | 0.01 | > 0.01 |
| 57.3333 | 3.53 | 1748 | 1384 | Geraniol acetatee | 0.04 | ||||||||||||
| 57.6 | 2.51 | 1751 | 1180 | Methyl benzeneacetatea | 0.01 | ||||||||||||
| 57.7333 | 1.62 | 1749 | 915 | 2(5H)-Furanonea | 0.01 | > 0.01 | > 0.01 | 0.02 | 0.02 | 0.03 | 0.01 | 0.02 | 0.04 | 0.01 | 0.02 | 0.01 | 0.02 |
| 57.7333 | 2.06 | 1725 | 1177 | Epoxylinalole | 0.01 | > 0.01 | > 0.01 | > 0.01 | |||||||||
| 57.7333 | 2.28 | 1754 | 1273 | 1-Decanola,e | 0.06 | 0.03 | 0.03 | 0.01 | 0.02 | 0.03 | 0.01 | 0.06 | 0.03 | 0.03 | 0.02 | 0.02 | 0.01 |
| 58 | 2.13 | 1758 | 1229 | β-Citronellola,e | 0.09 | > 0.01 | > 0.01 | 0.01 | > 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | |||
| 58.2667 | 2.5 | 1766 | 1197 | Methyl salicylatea,e | 0.03 | 0.09 | 0.13 | > 0.01 | 0.14 | 0.10 | 0.01 | 0.06 | 0.01 | 0.07 | 0.01 | 0.02 | 0.09 |
| 58.8 | 3.83 | 1776 | 1396 | α-Damasconea | 0.01 | ||||||||||||
| 59.4667 | 2.2 | 1787 | 1262 | cis-4-Decen-1-ola,e | 0.01 | 0.08 | 0.01 | 0.02 | 0.01 | > 0.01 | |||||||
| 59.6 | 4.52 | 1790 | 1530 | Citronellyl butyratea | 0.02 | ||||||||||||
| 59.7333 | 2.1 | 1790 | 1229 | Nerola,e | 0.06 | 0.01 | 0.03 | 0.03 | 0.01 | 0.01 | |||||||
| 59.7333 | 4.34 | 1787 | 1526 | Methyl dodecanoatea,e | 0.24 | 0.31 | 0.40 | 0.04 | 0.16 | 0.04 | 0.20 | 0.16 | 0.54 | 0.45 | 0.15 | 0.09 | 0.14 |
| 60 | 2.77 | 1798 | 1273 | Ethyl salicylatea,e | 0.24 | 0.07 | 0.17 | 0.03 | 0.19 | 0.13 | 0.03 | 0.10 | 0.04 | 0.07 | 0.04 | 0.03 | 0.09 |
| 60.4 | 3.57 | 1805 | 1388 | β-Damascenonea,e | 0.10 | 0.04 | 0.04 | 0.01 | 0.03 | 0.01 | 0.02 | 0.02 | 0.05 | 0.02 | 0.05 | 0.03 | |
| 60.5333 | 1.72 | 1808 | 1075 | 1-Phenylethanola,e | > 0.01 | ||||||||||||
| 61.8667 | 4.86 | 1829 | 1595 | Ethyl dodecanoatea,e | 2.15 | 10.03 | 1.38 | 1.52 | 5.24 | 2.92 | 1.51 | 1.16 | 4.11 | 4.03 | 6.10 | 1.50 | 7.61 |
| 62.2667 | 2.07 | 1838 | 1256 | Geraniola,e | 0.11 | 0.05 | > 0.01 | 0.04 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | ||||
| 62.6667 | 4.27 | 1883 | 1539 | Geraniol butyratea | > 0.01 | ||||||||||||
| 63.7333 | 1.59 | 1869 | 1034 | Benzyl alcohola,e | 0.47 | 0.12 | 0.19 | 0.07 | 0.13 | 0.02 | 0.16 | 0.09 | 0.18 | 0.08 | 0.07 | 0.01 | 0.08 |
| 65.4667 | 1.76 | 1901 | 1124 | 2-Phenylethanola,e | 0.26 | 0.29 | 0.21 | 0.03 | 0.07 | 0.11 | 0.12 | 0.13 | 0.29 | 0.15 | 0.20 | 0.02 | 0.32 |
| 65.6 | 2.41 | 1905 | 1260 | γ-Octalactonea,e | 0.01 | > 0.01 | > 0.01 | > 0.01 | |||||||||
| 67.3333 | 1.4 | 1941 | 1159 | 2-Ethylhexoic acida | 0.01 | 0.01 | > 0.01 | 0.01 | 0.01 | 0.01 | |||||||
| 69.8667 | 2.88 | 1991 | 1567 | Nerolidola,e | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | > 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | > 0.01 | ||
| 70.1333 | 4.56 | 1994 | 1727 | Methyl tetradecanoatea,e | 0.05 | 0.03 | 0.04 | 0.03 | 0.02 | 0.01 | 0.02 | 0.02 | 0.06 | 0.03 | 0.02 | 0.04 | 0.03 |
| 71.0667 | 2.51 | 2017 | 1368 | γ-Nonanolactonea,e | 0.02 | > 0.01 | |||||||||||
| 71.3333 | 1.95 | 2024 | 1287 | 4-Ethylguaiacola,e | > 0.01 | 0.01 | |||||||||||
| 72.1333 | 4.97 | 2035 | 1796 | Ethyl tetradecanoatea,e | 1.10 | 0.81 | 0.92 | 0.33 | 0.49 | 0.60 | 0.56 | 0.55 | 0.97 | 0.79 | 0.61 | 0.97 | 0.71 |
| 72.6667 | 1.4 | 2051 | 1174 | Octanoic acida,e | 0.21 | 0.17 | 0.22 | 0.03 | 0.04 | 0.02 | 0.01 | 0.13 | 0.21 | 0.07 | 0.11 | 0.03 | |
| 73.4667 | 2.51 | 2068 | 1390 | Methyl cinnamatea | 0.20 | > 0.01 | > 0.01 | ||||||||||
| 73.4667 | 2.61 | 2069 | 1287 | δ-Nonalactonea | > 0.01 | ||||||||||||
| 75.0667 | 4.65 | 2097 | 1829 | Methyl pentadecanoatea,e | 0.01 | ||||||||||||
| 75.8667 | 2.71 | 2120 | 1468 | Ethyl cinnamatea,e | 0.47 | 0.23 | 0.02 | 0.01 | 0.06 | 0.02 | 0.05 | 0.09 | 0.08 | 0.03 | 0.07 | 0.01 | 0.01 |
| 76.4 | 2.61 | 2133 | 1474 | γ-Decalactonea,e | 0.03 | 0.01 | 0.02 | 0.02 | > 0.01 | 0.01 | |||||||
| 77.6 | 1.98 | 2159 | 1362 | Eugenola,e | 0.06 | 0.04 | 0.03 | > 0.01 | 0.01 | > 0.01 | 0.01 | 0.01 | > 0.01 | > 0.01 | 0.01 | ||
| 79.7333 | 4.77 | 2202 | 1929 | Methyl hexanoatea,e | 0.14 | 0.04 | 0.03 | 0.03 | 0.05 | 0.06 | 0.04 | 0.04 | 0.05 | 0.04 | 0.06 | 0.03 | 0.04 |
| 81.3333 | 5.21 | 2241 | 1997 | Ethyl hexanoatea,e | 1.62 | 0.72 | 0.51 | 0.36 | 0.67 | 1.16 | 0.55 | 0.54 | 0.61 | 0.59 | 1.05 | 0.47 | 0.58 |
| 81.4667 | 2.72 | 2248 | 1578 | γ-Undecanolactonea | 0.02 | ||||||||||||
| 82.2667 | 1.51 | 2267 | 1392 | Decanoic acida,e | 0.19 | 0.08 | 0.09 | 0.02 | 0.03 | 0.02 | 0.01 | 0.06 | 0.09 | 0.03 | 0.06 | 0.02 | 0.05 |
| 85.6 | 2.63 | 2338 | 1724 | Farnesola | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | |||||||
| 86.2667 | 2.82 | 2364 | 1682 | γ-Dodecalactonea,e | 0.02 | 0.01 | > 0.01 | 0.01 | 0.02 | ||||||||
| 89.8667 | 5.33 | 2447 | 2195 | Ethyl octadecanoatea,e | 0.01 | 0.01 | 0.01 | ||||||||||
| 94.1333 | 1.67 | 2557 | 1401 | Vanillina,e | > 0.01 | > 0.01 | 0.01 | > 0.01 | |||||||||
| DBFFAPxBPX-50 | RI | HP-5xBPX-50 | Compounds | Area (%)f | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT | RI | SL14 | SL15 | SL16 | SL17 | SL18 | SL19 | SL20 | SL21 | SL22 | SL23 | SL24 | SL25 | |||
| 1D (min) | 2D (s) | |||||||||||||||
| 9.6 | 1.54 | 984 | 596 | 2,3-Butanedionea | 0.38 | 0.10 | 0.10 | 0.28 | 0.03 | 0.43 | 0.01 | 0.70 | 0.46 | 0.08 | ||
| 11.8667 | 1.46 | 1085 | 591 | 2-Butanola,e | 8.34 | 14.79 | 6.46 | 0.08 | 29.00 | |||||||
| 12.4 | 2.89 | 1028 | 804 | Ethyl butanoatea,e | 0.37 | 0.06 | 0.77 | 0.72 | 0.56 | 0.50 | 1.01 | 0.22 | 0.92 | 0.42 | ||
| 13.0667 | 3.37 | 1051 | 851 | Ethyl 2-methylbutanoatea,e | 0.19 | 0.09 | 0.02 | 0.19 | 0.050 | |||||||
| 13.6 | 1.39 | 1038 | 581 | 1-Propanolb,e | 16.75 | 9.62 | 11.65 | 14.97 | 12.14 | 22.23 | 18.17 | 22.59 | 15.99 | 20.02 | 20.03 | 23.00 |
| 13.7333 | 1.91 | 1063 | 697 | 2,3-Pentanedionea,e | 0.11 | 0.01 | 0.01 | 0.03 | 0.11 | |||||||
| 14.2667 | 2.83 | 1074 | 816 | Butyl acetatec,e | 0.01 | 1.47 | 0.55 | 0.06 | ||||||||
| 16.8 | 1.48 | 1091 | 669 | 2-Methyl-1-propanola,e | 12.40 | 11.26 | 11.45 | 14.17 | 13.47 | 11.69 | 15.15 | 17.00 | 15.26 | 4.28 | 13.39 | 9.27 |
| 16.9333 | 3.27 | 1122 | 880 | 3-Methylbutyl acetate d,e | 3.10 | 1.12 | 5.28 | 1.73 | 1.13 | 7.94 | 4.05 | 0.50 | 2.40 | 2.76 | 9.30 | 0.09 |
| 17.6 | 3.62 | 1133 | 906 | Ethyl pentanoatea,e | 0.06 | 0.03 | 0.05 | 0.01 | 0.02 | 0.04 | 0.01 | |||||
| 17.6 | 3.63 | 1133 | 854 | Ethyl 3-methylbutanoatea,e | 0.19 | 0.03 | ||||||||||
| 17.7333 | 1.62 | 1108 | 701 | 3-Pentanola | 0.04 | 0.01 | ||||||||||
| 18.6667 | 4.65 | 1160 | 992 | Myrcenea | ||||||||||||
| 19.0667 | 1.58 | 1122 | 701 | 2-Pentanola,e | 0.01 | 0.01 | 0.20 | 0.23 | 0.01 | 0.03 | 0.02 | |||||
| 20.8 | 3.47 | 1178 | 925 | Methyl hexoatea,e | 0.01 | 0.15 | 0.05 | 0.08 | 0.07 | 0.19 | 0.02 | 0.03 | 0.14 | 0.05 | ||
| 21.0667 | 3.07 | 1176 | 891 | 2-Heptanonea | 0.05 | |||||||||||
| 21.3333 | 1.46 | 1157 | 678 | 1-Penten-3-ola | 0.04 | 0.09 | 0.08 | 0.06 | 0.06 | 0.03 | 0.05 | 0.03 | 0.03 | |||
| 23.8667 | 1.61 | 1200 | 732 | 2-Methyl-1-butanola,e | 10.39 | 10.58 | 10.60 | 0.02 | 1.36 | 3.39 | 0.53 | 7.12 | 25.80 | 3.89 | 0.05 | |
| 24.1333 | 4.14 | 1224 | 1000 | Ethyl hexoatea,e | 1.10 | 0.58 | 3.71 | 1.32 | 3.23 | 3.77 | 8.16 | 1.01 | 1.20 | 6.34 | 2.64 | 0.18 |
| 24.2667 | 1.73 | 1201 | 730 | 3-Methyl-1-butanola,e | 5.39 | 11.39 | 3.90 | 27.50 | 19.79 | 13.89 | 4.71 | 20.27 | 29.98 | 3.16 | 20.62 | 17.53 |
| 24.9333 | 1.4 | 1202 | 640 | 2-Methyl-2-propen-1-ol | 0.01 | |||||||||||
| 25.0667 | 1.74 | 1215 | 802 | 2-Hexanola,e | ||||||||||||
| 26 | 2.77 | 1248 | 892 | Styrened,e | 0.04 | 0.06 | 0.02 | 0.22 | 0.38 | 0.06 | 0.01 | 0.68 | 0.07 | |||
| 26.2667 | 4.56 | 1258 | 1059 | 3-Methylbutyl butanoatea,e | ||||||||||||
| 26.9333 | 1.62 | 1243 | 766 | 1-Pentanola,e | 0.34 | 0.32 | 0.49 | 0.36 | 0.29 | 0.25 | 0.08 | 0.14 | 0.10 | 0.08 | 0.10 | 0.43 |
| 26.9333 | 1.53 | 1241 | 727 | 3-Methyl-3-buten-1-ola | 0.04 | 0.06 | 0.10 | 0.02 | 0.06 | 0.04 | 0.02 | 0.03 | 0.03 | 0.02 | 0.04 | |
| 30.9333 | 1.68 | 1305 | 840 | 4-Methyl-1-pentanola,e | 0.02 | 0.01 | 0.02 | 0.01 | 0.04 | 0.02 | ||||||
| 31.3333 | 1.91 | 1313 | 903 | 2-Heptanola,e | 0.02 | 0.08 | 0.11 | 0.11 | 0.04 | 0.06 | 0.67 | 0.02 | 0.11 | |||
| 31.3333 | 4.2 | 1324 | 1102 | Ethyl heptanoatea,e | 0.07 | 0.08 | 0.04 | 0.02 | 0.04 | 0.04 | 0.02 | 0.03 | 0.02 | 0.02 | ||
| 32.4 | 4.35 | 1339 | 1111 | cis-Rose oxidea,e | ||||||||||||
| 32.9333 | 1.67 | 1335 | 813 | Ethyl 2-hydroxypropanoatea,e | 0.88 | 0.65 | 2.72 | 0.39 | 0.60 | 0.37 | ||||||
| 33.4667 | 1.8 | 1343 | 870 | 1-Hexanolb,e | 1.48 | 1.68 | 1.76 | 1.64 | 1.16 | 1.70 | 0.38 | 0.93 | 0.46 | 1.42 | 0.97 | 1.49 |
| 34.1333 | 3.79 | 1366 | 1115 | Heptyl acetatea,e | ||||||||||||
| 34.1333 | 1.69 | 1355 | 852 | trans-3-Hexen-1-ola | ||||||||||||
| 35.2 | 3.5 | 1381 | 1094 | 2-Nonanonea,e | 0.07 | 0.01 | 0.01 | |||||||||
| 35.2 | 3.85 | 1378 | 1126 | Methyl octanoatea,e | 0.15 | 0.30 | 0.07 | 0.25 | 0.28 | 0.97 | 0.09 | 0.09 | 0.53 | 0.19 | 0.02 | |
| 35.3333 | 1.68 | 1375 | 859 | cis-3-Hexen-1-ola,e | 0.03 | 0.04 | 0.10 | 0.03 | 0.16 | 0.08 | 0.02 | 0.01 | 0.05 | 0.04 | 0.08 | |
| 36 | 2.16 | 1385 | 997 | 3-Octanola,e | 0.01 | 0.04 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | |||
| 36.9333 | 1.63 | 1398 | 871 | trans-2-Hexen-1-old | 0.01 | |||||||||||
| 37.4667 | 1.63 | 1407 | 867 | cis-2-Hexen-1-old | 0.02 | 0.01 | ||||||||||
| 37.7333 | 2.08 | 1413 | 1003 | 2-Octanola | 0.02 | 0.01 | 0.02 | |||||||||
| 38.1333 | 4.42 | 1426 | 1198 | Ethyl octanoatea,e | 4.54 | 2.86 | 4.33 | 1.83 | 2.32 | 8.79 | 10.58 | 4.83 | 4.21 | 4.84 | 11.25 | 0.79 |
| 38.2667 | 3.39 | 1426 | 1090 | α,p-Dimethylstyrened | ||||||||||||
| 38.8 | 2.69 | 1428 | 1072 | Linalool oxidea,e | 0.03 | 0.01 | 0.08 | 0.02 | 0.01 | > 0.01 | 0.02 | 0.03 | 0.12 | |||
| 39.6 | 1.9 | 1443 | 981 | 1-Octen-3-ola,e | 0.02 | 0.05 | 0.05 | 0.07 | 0.02 | 0.04 | 0.02 | 0.02 | 0.02 | 0.01 | ||
| 40 | 1.88 | 1449 | 974 | 1-Heptanola,e | 0.10 | 0.11 | 0.15 | 0.07 | 0.09 | 0.08 | 0.04 | 0.05 | 0.03 | 0.05 | 0.04 | 0.09 |
| 40.2667 | 1.14 | 1443 | 685 | Acetic acida,e | 2.10 | 0.94 | 1.06 | 1.32 | 0.74 | 0.46 | 1.15 | 0.58 | 0.81 | 0.83 | 1.09 | 0.49 |
| 40.5333 | 1.96 | 1455 | 989 | 6-Methyl-5-hepten-2-old | > 0.01 | |||||||||||
| 40.9333 | 1.68 | 1461 | 832 | Furfurala,e | 0.93 | 1.14 | 0.44 | 0.31 | 0.57 | 0.32 | 1.13 | 0.40 | 0.52 | 0.30 | 0.39 | 0.34 |
| 42.2667 | 3.76 | 1487 | 1206 | Decanala,e | 0.04 | 0.01 | 0.03 | 0.04 | 0.03 | |||||||
| 43.3333 | 1.87 | 1497 | 914 | 2-Acetylfurana | 0.01 | 0.01 | > 0.01 | |||||||||
| 43.8667 | 2.26 | 1510 | 1104 | 2-Nonanola,e | 0.02 | 0.10 | 0.23 | 0.03 | 0.05 | 0.04 | 0.09 | 0.23 | 0.03 | 0.08 | ||
| 44 | 2.26 | 1513 | 959 | Benzaldehydea,e | 4.35 | 6.07 | 4.92 | 10.74 | 2.28 | 0.11 | 1.28 | 2.64 | 2.88 | 2.27 | 1.16 | 3.54 |
| 44.5333 | 4.46 | 1527 | 1296 | Ethyl nonylatea,e | 3.16 | 0.59 | 0.22 | 0.08 | 0.25 | 0.55 | 0.41 | 0.96 | 0.09 | 0.40 | 0.29 | 0.07 |
| 44.5333 | 3.07 | 1524 | 1161 | trans-2-Nonenald,e | > 0.01 | |||||||||||
| 45.6 | 1.16 | 1527 | 694 | Propanoic acida | 0.01 | 0.01 | 0.01 | > 0.01 | ||||||||
| 45.6 | 2.22 | 1539 | 1100 | Linaloola,e | 0.06 | 0.11 | 0.01 | 0.07 | 0.04 | 0.02 | 0.03 | 0.09 | 0.03 | 0.03 | 0.01 | 0.10 |
| 45.8667 | 3.6 | 1546 | 1258 | Linalool acetatea | ||||||||||||
| 46.1333 | 2.02 | 1548 | 1072 | 1-Octanola,e | 0.13 | 0.09 | 0.18 | 0.08 | 0.09 | 0.08 | 0.10 | 0.10 | 0.05 | 0.08 | 0.07 | 0.09 |
| 46.6667 | 4.49 | 1562 | 1293 | Bornyl acetated | ||||||||||||
| 47.2 | 6.46 | 1575 | 1426 | Caryophyllened,e | 0.06 | |||||||||||
| 47.6 | 1.94 | 1568 | 963,6 | 5-Methyl furfurala | ||||||||||||
| 48.1333 | 4.11 | 1581 | 1326 | Methyl decanoatea,e | 0.25 | 0.13 | 0.21 | 0.04 | 0.30 | 0.37 | 1.80 | 0.17 | 0.13 | 0.26 | 0.23 | 0.04 |
| 48.4 | 2.87 | 1588 | 1179 | 4-Terpinenola,e | 0.02 | 0.06 | 0.01 | 0.01 | > 0.01 | 0.05 | ||||||
| 48.9333 | 2.07 | 1593 | 985 | Benzonitrilea | 0.01 | |||||||||||
| 50.2667 | 2.38 | 1618 | 1147 | β-Terpineola | ||||||||||||
| 50.5333 | 1.88 | 1620 | 916 | Butyrolactonea | > 0.01 | 0.01 | > 0.01 | |||||||||
| 50.5333 | 1.2 | 1620 | 812 | Butanoic acida | 0.02 | 0.02 | ||||||||||
| 50.6667 | 4.74 | 1625 | 1397 | Ethyl decanoatea,e | 6.99 | 6.30 | 7.86 | 1.38 | 12.25 | 2.66 | 2.76 | 10.80 | 2.14 | 0.71 | 2.20 | 1.15 |
| 50.9333 | 3.19 | 1631 | 1264 | trans-2-Decenald,e | 0.01 | > 0.01 | > 0.01 | > 0.01 | > 0.01 | |||||||
| 51.2 | 2.22 | 1633 | 1044 | Benzeneacetaldehydea,e | 0.02 | |||||||||||
| 51.6 | 2.34 | 1640 | 1067 | Acetophenonea,e | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | |
| 52 | 3.81 | 1652 | 1355 | Citronellyl acetatea,e | ||||||||||||
| 52.1333 | 2.17 | 1653 | 1175 | 1-Nonanola,e | 0.94 | 0.30 | 0.53 | 0.14 | 0.32 | 0.35 | 0.30 | 0.51 | 0.06 | 0.08 | 0.15 | 0.22 |
| 52.5333 | 1.36 | 1656 | 857 | 2-Furanmethanola | 0.01 | 0.02 | > 0.01 | > 0.01 | > 0.01 | > 0.01 | 0.01 | > 0.01 | 0.01 | 0.01 | 0.01 | > 0.01 |
| 53.0667 | 4.17 | 1661 | 1413 | Decyl acetatea,e | 0.01 | 0.03 | 0.01 | |||||||||
| 53.0667 | 2.63 | 1671 | 1152 | p-Vinylanisolea,e | 0.05 | 0.01 | 0.65 | 0.07 | 1.13 | 1.13 | 0.13 | 0.01 | 0.01 | 0.06 | 0.09 | 0.01 |
| 53.2 | 2.55 | 1670 | 1181 | Diethyl butanedioatea,e | 0.08 | 2.60 | 0.02 | 0.15 | 0.05 | 0.03 | 0.04 | 0.15 | 0.06 | 0.08 | 0.04 | 0.02 |
| 54 | 2.46 | 1669 | 1192 | α-Terpineola,e | 0.03 | 0.04 | 0.14 | 0.02 | 0.01 | 0.01 | 0.05 | 0.01 | 0.01 | 0.03 | ||
| 54.4 | 2.62 | 1692 | 1216 | trans,trans-2,4-Nonadienala | ||||||||||||
| 54.5333 | 2.17 | 1691 | 1054 | γ-Hexalactonea | ||||||||||||
| 55.8667 | 2.51 | 1719 | 1165 | Phenylmethyl acetatea,e | 0.02 | 0.43 | 0.03 | 0.03 | 0.02 | 0.01 | > 0.01 | 0.01 | 0.02 | 0.09 | ||
| 57.3333 | 3.53 | 1748 | 1384 | Geraniol acetatee | ||||||||||||
| 57.6 | 2.51 | 1751 | 1180 | Methyl benzeneacetatea | 0.01 | |||||||||||
| 57.7333 | 1.62 | 1749 | 915 | 2(5H)-Furanonea | 0.01 | 0.02 | 0.01 | > 0.01 | > 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | |||
| 57.7333 | 2.06 | 1725 | 1177 | Epoxylinalole | > 0.01 | |||||||||||
| 57.7333 | 2.28 | 1754 | 1273 | 1-Decanola,e | 0.02 | 0.03 | 0.03 | 0.01 | 0.01 | 0.01 | 0.03 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 |
| 58 | 2.13 | 1758 | 1229 | β-Citronellola,e | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | ||||||
| 58.2667 | 2.5 | 1766 | 1197 | Methyl salicylatea,e | 0.05 | > 0.01 | 0.08 | 0.01 | 0.03 | 0.03 | 0.02 | 0.01 | 0.01 | 0.01 | 0.20 | 0.04 |
| 58.8 | 3.83 | 1776 | 1396 | α-Damasconea | > 0.01 | |||||||||||
| 59.4667 | 2.2 | 1787 | 1262 | cis-4-Decen-1-ola,e | 0.01 | |||||||||||
| 59.6 | 4.52 | 1790 | 1530 | Citronellyl butyratea | ||||||||||||
| 59.7333 | 2.1 | 1790 | 1229 | Nerola,e | 0.01 | |||||||||||
| 59.7333 | 4.34 | 1787 | 1526 | Methyl dodecanoatea,e | 0.11 | 0.08 | 0.15 | 0.01 | 0.18 | 0.21 | 0.60 | 0.07 | 0.08 | 0.32 | 0.04 | |
| 60 | 2.77 | 1798 | 1273 | Ethyl salicylatea,e | 0.12 | 0.10 | 0.18 | 0.03 | 0.06 | 0.04 | 0.03 | 0.04 | 0.05 | 0.04 | 0.09 | |
| 60.4 | 3.57 | 1805 | 1388 | β-Damascenonea,e | 0.03 | 0.04 | 0.03 | 0.03 | 0.01 | 0.02 | 0.03 | 0.03 | ||||
| 60.5333 | 1.72 | 1808 | 1075 | 1-Phenylethanola,e | ||||||||||||
| 61.8667 | 4.86 | 1829 | 1595 | Ethyl dodecanoatea,e | 5.59 | 3.22 | 1.13 | 0.61 | 1.47 | 2.79 | 7.20 | 1.75 | 2.01 | 2.45 | 1.50 | 1.02 |
| 62.2667 | 2.07 | 1838 | 1256 | Geraniola,e | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | > 0.01 | 0.01 | > 0.01 | 0.01 | 0.02 | ||
| 62.6667 | 4.27 | 1883 | 1539 | Geraniol butyratea | ||||||||||||
| 63.7333 | 1.59 | 1869 | 1034 | Benzyl alcohola,e | 0.07 | 0.74 | 0.16 | 0.13 | 0.07 | 0.02 | 0.02 | 0.05 | 0.01 | 0.01 | 0.01 | 0.18 |
| 65.4667 | 1.76 | 1901 | 1124 | 2-Phenylethanola,e | 0.16 | 0.13 | 0.12 | 0.03 | 0.07 | 0.03 | 0.17 | 0.04 | 0.09 | 0.01 | 0.05 | 0.03 |
| 65.6 | 2.41 | 1905 | 1260 | γ-Octalactonea,e | ||||||||||||
| 67.3333 | 1.4 | 1941 | 1159 | 2-Ethylhexoic acida | 0.01 | |||||||||||
| 69.8667 | 2.88 | 1991 | 1567 | Nerolidola,e | 0.01 | > 0.01 | > 0.01 | > 0.01 | ||||||||
| 70.1333 | 4.56 | 1994 | 1727 | Methyl tetradecanoatea,e | 0.02 | 0.03 | 0.03 | 0.02 | 0.01 | 0.04 | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | |
| 71.0667 | 2.51 | 2017 | 1368 | γ-Nonanolactonea,e | > 0.01 | |||||||||||
| 71.3333 | 1.95 | 2024 | 1287 | 4-Ethylguaiacola,e | > 0.01 | |||||||||||
| 72.1333 | 4.97 | 2035 | 1796 | Ethyl tetradecanoatea,e | 0.64 | 0.79 | 0.78 | 0.09 | 0.51 | 0.48 | 0.87 | 0.58 | 0.46 | 0.57 | 0.46 | 0.26 |
| 72.6667 | 1.4 | 2051 | 1174 | Octanoic acida,e | 0.07 | 0.07 | 0.03 | 0.02 | 0.02 | 0.01 | 0.05 | 0.02 | 0.02 | 0.01 | 0.03 | 0.01 |
| 73.4667 | 2.51 | 2068 | 1390 | Methyl cinnamatea | ||||||||||||
| 73.4667 | 2.61 | 2069 | 1287 | δ-Nonalactonea | ||||||||||||
| 75.0667 | 4.65 | 2097 | 1829 | Methyl pentadecanoatea,e | ||||||||||||
| 75.8667 | 2.71 | 2120 | 1468 | Ethyl cinnamatea,e | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.06 | 0.08 | 0.02 | 0.01 | 0.04 | 0.04 | 0.01 |
| 76.4 | 2.61 | 2133 | 1474 | γ-Decalactonea,e | > 0.01 | |||||||||||
| 77.6 | 1.98 | 2159 | 1362 | Eugenola,e | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | > 0.01 | > 0.01 | > 0.01 | > 0.01 | > 0.01 | ||
| 79.7333 | 4.77 | 2202 | 1929 | Methyl hexanoatea,e | 0.05 | 0.11 | 0.05 | 0.01 | 0.02 | 0.02 | 0.02 | 0.08 | 0.03 | 0.01 | 0.02 | 0.03 |
| 81.3333 | 5.21 | 2241 | 1997 | Ethyl hexanoatea,e | 0.69 | 1.35 | 0.64 | 0.41 | 0.41 | 0.41 | 0.29 | 1.32 | 0.49 | 0.16 | 0.42 | 0.47 |
| 81.4667 | 2.72 | 2248 | 1578 | γ-Undecanolactonea | ||||||||||||
| 82.2667 | 1.51 | 2267 | 1392 | Decanoic acida,e | 0.03 | 0.04 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 |
| 85.6 | 2.63 | 2338 | 1724 | Farnesola | 0.01 | |||||||||||
| 86.2667 | 2.82 | 2364 | 1682 | γ-Dodecalactonea,e | 0.01 | |||||||||||
| 89.8667 | 5.33 | 2447 | 2195 | Ethyl octadecanoatea,e | 0.01 | |||||||||||
| 94.1333 | 1.67 | 2557 | 1401 | Vanillina,e | ||||||||||||
RT retention time, RI retention index
aSigma-Aldrich (St. Louis, USA)
bMicrochem (Pezinok, Slovakia)
cMerk KGaA (Darmstadt, Germany)
dSAFC Biosciences (Lenexa, USA)
ecompounds separated on the column setup HP-5 xBPX-50
fArea (%)—the ratio of the compounds area to total peaks areas of identified compounds
Samples
The plums of different varieties were obtained from several botanical gardens (Table 1). The all plums were fermented by the same technology under pseudo-anaerobic conditions with addition of 0.5 kg of sucrose to 100 kg of fruits and using the same type of yeast strains. Fermented beverages were distillated by pot still method, and then diluted to final ethanol content 50–52% (v/v). The received brandies were kept for maturation in glass bottles for 2–3 years.
Table 1.
Plum fruits
| Code | Plum variety | Fruit source |
|---|---|---|
| SL1 | Chrudimer | Moravský Žižkov, Czech Republic |
| SL2 | Cacak Fruitful | Vilémov, Czech Republic |
| SL3 | Cacak Early | Vilémov, Czech Republic |
| SL4 | Cacak Beauty | Horné Obdokovce, Slovak Republic |
| SL5 | Stanley | Kyjov, Czech Republic |
| SL6 | Tolar | Moravský Žižkov, Czech Republic |
| SL7 | Blue free | Moravský Žižkov, Czech Republic |
| SL8 | Top King | Slup, Czech Republic |
| SL9 | Haganta | Slup, Czech Republic |
| SL10 | Hanita | Kyjov, Czech Republic |
| SL11 | Top Taste | Moravský Žižkov, Czech Republic |
| SL12 | Topper | Veselé pri Piešťanoch, Slovak Republic |
| SL13 | Tophit | Veselé pri Piešťanoch, Slovak Republic |
| SL14 | Švestka domácí | Dambořice, Czech Republic |
| SL15 | Durancie | Kyjov, Czech Republic |
| SL16 | Katinka | Veselé pri Piešťanoch, Slovak Republic |
| SL17 | Gabrovská | Starý Poddvorov, Czech Republic |
| SL18 | Carpatin | Moravský Žižkov, Czech Republic |
| SL19 | Vlaška | Moravský Žižkov, Czech Republic |
| SL20 | Top Gigant | Veselé pri Piešťanoch, Slovak Republic |
| SL21 | Presenta | Dambořice, Czech Republic |
| SL22 | President | Slup, Czech Republic |
| SL23 | Firense | Moravský Žižkov, Czech Republic |
| SL24 | Amers | Moravský Žižkov, Czech Republic |
| SL25 | Elena | Týn u Přerova, Czech Republic |
Sensory analysis
Quality of plum brandies aroma was characterized by 7 certified distillate tasters in blind sensory evaluation. Evaluation took place in authorized sensory laboratory under conditions recommended for distillates (30 ml of distillate poured into ISO XL5 wine taster glasses; serving temperature 12 °C). Aroma of each plum brandy sample was evaluated in two repetitions by Quantitative Descriptive Analysis. Tasters have evaluated positive fruity like odours as well as possible off flavours by ten-point scale (0 representing not perceived and 10 representing extreme value of odour descriptor perception). Intensities of following major attributes were evaluated: plum, marzipan, plum jam, stones, spicy flavour, sweetness, green leaves, fusel alcohols, volatile acidity, and solvent. Other variable odour descriptors were evaluated as well: apple, sour cherry, cherry, apricot, banana, pear, citrus, beet, caramel, conifer, and nutmeg. Spider web diagrams characterizing aroma of individual spirits were constructed based on average values of means obtained by the evaluation.
Sample preparation
Liquid–liquid extraction (LLE)
In total, 30 ml of sample previously mixed with NaCl (2 g) and 100 µl benzophenone used as an internal standard solution, was extracted with three 10 ml portions of pentane in a separation funnel. The combined pentane extracts were concentrated using the Kuderna-Danish apparatus at 55 °C to final volume of 1 ml. After then, the extract was removed into a volumetric flask and filled to the final volume of 2 ml with pentane. An amount of 1 μl of the sample was injected into GC in splitless mode.
Solid phase microextraction (SPME)
The HS-SPME procedure was performed using Gerstel multipurpose sampler with SPME fibres coated with 100 µm of polydimethylsiloxane (PDMS), 65 µm of polydimethylsiloxane-divinylbenzene (PDMS/DVB), 85 µm of polyacrylate (PA), 85 µm of (CAR/PDMS), and 50/30 µm of divinylbenzene-carboxene-polydimethylsiloxane (DVB/CAR/PDMS). Both 3 ml of plum brandy and 3 ml of distilled water were combined in a single 20 ml capped glass vial, and 0.5 g of sodium chloride and 20 µl of the internal standard solution were added. The vial with sample was heated at 60 °C for 30 min with stirring at a rate of 400 rpm. The SPME fibre was exposed to the headspace for 30 min, and then transferred to the injector for desorption at 220 °C for 2 min.
Gas chromatography–mass spectrometry analysis
The gas chromatography analysis was performed on Pegasus 4D GCxGC–TOFMS (LECO Corporation, St. Joseph, MI, USA), consisting of Agilent 6890 gas chromatograph (Agilent, Agilent J&W Column, Agilent Technologies, Palo Alto, CA, USA), a dual stage jet cryogenic modulator based on liquid N2, time of flight mass spectrometer (LECO Corporation, St. Joseph, MI, USA) and Gerstel MPS2 autosampler (Gerstel, Mülheim, Germany). The column setup contained a 30 m × 0.25 mm ID column coated with 0.25 μm film thickness of polyethylene glycol modified by nitroterephthalic acid phase (DB-FFAP from Agilent, Agilent J&W Column, Agilent Technologies, Palo Alto, CA, USA) or HP-5 column (Agilent, Agilent J&W Column, Agilent Technologies, Palo Alto, CA, USA) of 30 m × 0.25 mm ID coated with 0.25 μm film thickness of (5%-phenyl)-methylpolysiloxane stationary phase in the first dimension, and BPX-50 column (SGE Analytical Science, Victoria, Australia) 1.5 m × 0.1 mm ID coated with 0.1 μm film thickness of 50% diphenyl–50% dimethyl polyphenylene-siloxane in the second dimension. The injector port temperature was set at 220 °C, while the helium carrier gas flow rate was kept constant at 1 ml/min. Oven temperature was 40 °C was kept for 10 min then programmed to 240 °C with a rate of 2 °C/min and kept for 5 min. The modulator trapping was operated at 30 °C higher temperature compared to actual oven temperature with a modulation period of 8 s. The second column was operated at 10 °C higher temperature offset than the primary column, and the transfer line was heated at 240 °C. The temperature of ion source of TOFMS was hold at 230 °C, and detector voltage was set at 1700 V during the analysis. Energy of 70 eV was used for electron impact ionization source. The signal acquisition rate of 100 spectra/s within a mass range of m/z 29–450 was applied. The measured chromatograms were evaluated using LECO ChromaTOF 4.21 software and US National Institutes of Standards and Technology (NIST08) mass library. Identification of compounds were additionally confirmed by comparison of retention factors and mass fragmentation patterns of authentic standards.
Results and discussions
Sensory analysis
Sensory evaluation results indicated significant differences in the flavour profile of analyzed plum brandies (the supplementary 1). It should be noted that despite a marked plum aroma, samples were also described with additional fruity aroma notes (e.g. apple, sour and sweet cherries, apricot, banana, pear, citrus etc.) and the other aroma notes such as beet, caramel, conifer, and nutmeg (Fig. 1). The plum brandies of varieties “Chrudimer”, “Presenta” and “Blue Free” were highly estimated by panellists which seemed quite promising for further beverage production. These samples showed similar sensory profile defined as strong “plum” aroma, odour of “marzipan”, additional fruit or flower notes with some “stones”, “spicy flavours” and “sweetness” attributes. Whereas sensory characteristics like “dimness”, “fusel alcohols” and “acidity” resulted in poor aroma quality of the plum brandies made from varieties “Cacak Fruitful” and “Top Taste”.
Fig. 1.
Aroma profiles of the selected plum brandies
Sample preparation
Liquid–liquid extraction into pentane and solid-phase microextraction with different coating material were used as sample preparation techniques for extraction of volatile organic compounds from a plum brandy samples. Overall, 60 compounds were common to both methods, while 34 additional compounds were extracted by LLE, and 15–20 compounds were only obtained by SPME. A wider variety of alcohols like 2-pentanol, 3-pentanol, 2-hexanol, terpenoid alcohols (borneol, cis- and trans-carveols) and perillyl alcohol was detected in pentane extracts. Additionally, a SPME profile was enriched with 2-butanol, 2,3-butanediol, caryophyllene, butyrolactone, menthol, citronellol and carvone. Five types of SPME fibres such as PDMS, PA, CAR/PDMS, DVB/PDMS and DVB/CAR/PDMS were used in the experimental study. The efficiency of SPME extraction is generally controlled by several factors including polarity, volatility and shape of an analyte, structure of fibre coating, and complexity of sample (Pawliszyn 2012). In the case of PA and PDMS polymeric coatings, absorption is a dominant mechanism associated with thickness of fibre coating as well as polarity of compounds. Consequently, non-polar terpenes (e.g. γ-terpinene and myrcene) were more effectively extracted by PDMS fibre, and polar PA layer provided additional sorption of 2,3-butanediol and butyrolactone. For DVB/PDMS, CAR/PDMS and DVB/CAR/PDMS fibres, adsorption of analytes is controlled by coating porosity, where DVB is a mesoporous polymer and CAR is mainly microporous molecular sieve. In our case, CAR/PDMS fibre was not so effective for extraction of esters, especially for fatty acids with long carbon chain (e.g. ethyl 2-methylbutyrate, ethyl pentanoate, methyl undecanoate, ethyl pentadecanoate, methyl hexadecanoate). DVB/PDMS layer showed poor affinity for alcohols and carbonyl compounds with lower molecular weight (e.g. 2,3-butanedione, 2-alcohols C5–C8, cis-3-hexen-1-ol, trans-2-hexenal). Since a triple phase of DVB/CAR/PDMS coating combines the characteristics of both adsorbents, which could be particularly seen in the case of 2-butanol and carvone, its capacity was worse compared to the fibres with a single adsorbent. Simultaneously, this fibre covered a wider variety of analytes and was used for the further studies.
Analysis of plum brandies produced from different plum varieties
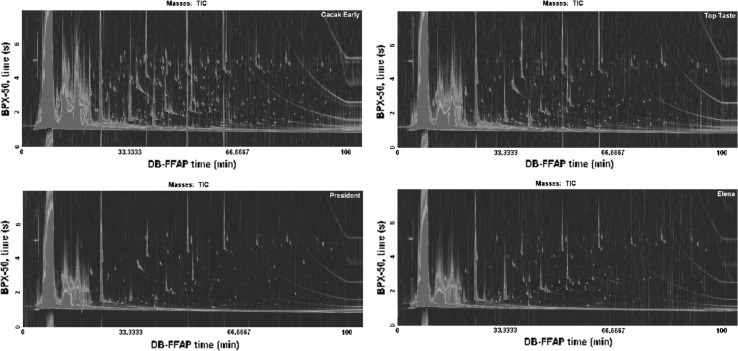
The plum brandies were analyzed on two sets of column combinations, HP-5–BPX-50 (conventional “nonpolar × medium-polar” setup) and DBFFAP–BPX-50 (reversed “polar × medium-polar” setup). It was found that 2D separation space of the “reversed” combination of columns was more exploited with better peak distribution (Fig. 2). Furthermore, the received ordered chromatographic profile for the most common classes of compounds considerably simplified the interpretation of chromatograms. These column setups were discussed in detail in our previous study (Vyviurska et al. 2015). In general, from 91 to 195 compounds were tentatively identified in plum brandies, and among them 124 compounds were confirmed with authentic standards (Table 2). The concentration of compounds confirmed was calculated as relative content of compounds in area % (ratio of the compound TIC area to total TIC peaks areas of identified compounds). The richest VOC profile was observed for the sample produced from “Chrudimer”, “Cacak Fruitful”, “Cacak Early”, “Hanita” plum varieties, while distillates from “Gabrovská”, “Presenta”, “Amers”, “Elena” and “President” varieties showed poorest VOC profile. The VOC profiles of some plum brandies are shown on Fig. 3 and in supplementary 2.
Fig. 2.
GCxGC–TOFMS chromatograms of the plum brandy distilled from “Chrudimer” plum variety with the column combinations of DB-FFAP and BPX-50 (a), and HP-5 and BPX-50 (b)
Fig. 3.
GCxGC–TOFMS chromatograms of the plum brandies produced from varieties
Alcohols
Similarly to the results obtained by Satora and Tuszyński (2008), alcohols were one of the most common classes of compounds detected in the plum brandies (Fig. 4). Unsaturated and saturated straight-chain C3–C9 alcohols and cis-3-hexanol were also found to be abundant compounds in juices obtained from P. domestica plums (Ismail et al. 1981). The whole homological series of n-alcohols (C3–C10) excluding 1-butanol, as well as branched alcohols e.g. 2-methyl-1-propanol and 3-methyl-1-butanol, aromatic alcohols (benzyl alcohol, 2-phenylethanol) and furfuryl alcohol were present in all studied samples. In alcoholic beverages, a content and type of amino acids mainly determines the composition of higher (fusel) alcohols which are produced by transamination and oxidative deamination processes. Moreover, certain amounts of higher alcohols could be present in plums and plum juice. Aldehydes and ketones are considered as immediate precursors of metabolisms, which are reduced by alcohol dehydrogenases to the corresponding alcohols (Velíšik 2014). The influence of fusel alcohols on aroma of alcoholic beverages depends on their concentration, e.g. fruity notes at optimal levels or a strong, pungent smell at excessive content (Swiegers et al. 2005). 1-Propanol was one of the most abundant n-alcohol identified in the plum brandies, while a content of the other homologous did not exceed 1% of contribution to the VOC profile except for 1-hexanol. The relatively low amount of alcohols with carbon number higher than 5 is considered to be connected with the specificity of alcohol dehydrogenases to the substrate and a decreased rate of reduction processes of corresponding aldehydes (Knee 1993 ) and distillation cut temperature. Overall, the concentration of 1-alcohols was generally higher compared to their 2- and 3-isomers. For example, 2-hexanol, 2-octanol and 3-pentanol were present only in a few samples, especially in distillates from “Chrudimer”, “Top King” and “Švestka domácí” varieties. The presence of 3-pentanol contributed to herbal notes of aroma typical for alcoholic beverages. It should be also mentioned that 2-butanol originated from bacterial action and 1-hexanol, commonly have negative influence on flavour at elevated concentration (Spaho et al. 2013). 1-Hexanol is not a fermentation product, and usually is found in higher concentration in plum fruits (Chai et al. 2012). The structure of branched fusel alcohols is primary determined by parent branched-chain amino acids. Similarly to other alcoholic beverages (Vyviurska et al. 2015; Câmara et al. 2007; García-Llobodanim et al. 2008), 2-methylpropanol (iso-butyl alcohol), 3-methyl-1-butanol (iso-amyl alcohol) and 2-methyl-1-butanol (active amyl alcohol) were detected at highest abundance among branched higher alcohols. However, active amyl alcohol typical for plum fruits (Pino and Quijano 2012) was not present in “Tolar”, “Topper”, “Tophit” and “Presenta” samples. 4-Methyl-1-pentanol (iso-hexyl alcohol) was observed only at the trace level, and not detected in the brandies made from the plum varieties “Stanley” and “President”.
Fig. 4.
The average distribution of main classes of compounds detected in the plum brandies
Unsaturated alcohols arise from reduction of unsaturated aldehydes which are commonly formed by enzymatic cleavage of linoleic and linolenic acids (Serot et al. 2001). In general, unsaturated alcohols, especially C6 compounds, contribute to herbaceous and green notes of alcoholic beverages aroma. 3-Methyl-3-buten-1-ol, 1-penten-3-ol, cis-3-hexen-1-ol, 1-octen-3-ol were the most common unsaturated alcohols detected in the studied plum brandies. On the contrary to cis-3-hexen-1-ol, cis-2-hexen-1-ol was merely observed in the brandies from plum varieties “Cacak Early”, “Cacak Fruitful”, “Katinka”, “Top King” and “Durancie”. Whereas their geometrical isomer trans-2-hexen-1-ol and trans-3-hexen-1-ol were quite specific for “Chrudimer” or “Elena” varieties, respectively. The same observation was found for 2-methyl-2-propen-1-ol identified only in “Durancie” sample. The other detected unsaturated alcohols 6-methyl-5-hepten-2-ol (sulcatol) and cis-4-decen-1-ol were established in a few samples which was mainly fermented from cultivars Chrudimer”, “Cacak Early”, “Hanita”.
Aromatic alcohols such as benzyl alcohol and its higher homologue 2-phenylethanol, originated from phenylalanine through different metabolic processes, were found in all plum brandies. Overall, brandies produced from fermenting fruits with stones are characterized with larger quantities of benzyl alcohol. Despite 2-phenylethanol identified as a component of plum fruit and described with “cooked plum-like” aroma (Ismail et al. 1981), its isomer 1-phenylethanol was detected only in distillate from “Chrudimer” variety. In this case, heterocyclic alcohols were mainly represented by furfuryl alcohol which is a degradation product of sugars.
Esters
In general, esters are produced by yeast during fermentation in a reaction between alcohols and acyl-CoA molecules. They are crucial intermediates for production of free organic acids (Lea and Piggott 2003). During aging of alcoholic beverages formation of esters continues through acidolysis, alcoholysis and transesterification processes (Velíšik 2014). Since acetyl-CoA and ethanol are main products of catabolism of glucose, acetates and ethyl esters were usually dominant esters in the plum brandies. At the same time, ethyl ester of nonanoic acid and methyl cinnamate were reported to be important components of plum fruits (Ismail et al. 1981). Overall, esters significantly contribute to sweet fruity notes of beverages. Among methyl esters, those derivatives of salicylic acid and even chain length saturated C6–C16 fatty acids were found in all samples, whereas methyl esters of pentadecanoic acid and benzeneacetic acid were specific only for “Chrudimer” sample. Similar to the previous results, the ethyl ester profile of the plum brandies generally includes ethyl esters of even chain length saturated C4–C16 carboxylic acids and ethyl esters of nonanoic, cinnamic and salicylic acids, diethyl butanedioate. Plum brandies fermented from cultivars “Chrudimer”, “Cacak Beauty”, “Tolar” and “Gabrovská” also contained ethyl ester of the longest fatty acid chain (C18). Ethyl derivatives of odd number carboxylic acids or branched carboxylic acids such as ethyl pentanoate, ethyl heptanoate, ethyl 2-methylbutanoate and ethyl 3-methylbutanoate were more specific and appeared in some plum brandies. For the majority of samples, ethyl lactate formed during the fermentation from malic acid and ethanol (McKay et al. 2011) was an important part of the ester profile (0.39–2.71%). Similar to its parent alcohol, 3-methylbutyl acetate characterized with strong pear aroma was found at significant level compared to the other esters. At the same, a few specific esters like 3-methylbutyl butanoate and heptyl acetate were typical only for the brandy produced from variety “Chrudimer”.
Carbonyl compounds
The greatest diversity of carbonyl compounds was found for the samples obtained from plum varieties “Cacak Beauty”, “Chrudimer”, “Stanley” and “Cacak Early”. Aldehydes are important intermediates in higher-alcohol production, and conditions that favour higher-alcohol production also support the formation of small quantities of aldehydes (Lea and Piggott 2003). Among aldehydes, benzaldehyde previously found also in plum fruits (Ismail et al. 1981) was detected with high abundance (1–10%) in all the samples. Enzymatic degradation of amygdalin in the fruit stones is the main source of benzaldehyde responsible for bitter almond aroma of the alcoholic beverages. On the contrary, its higher derivatives such as benzeneacetaldehyde with a distinct green flavour and 4-methoxybenzaldehyde as well as aliphatic decanal and trans-2-decenal were significantly less common in studied plum brandies. The other unsaturated aldehydes were specific for the particular samples, e.g. trans-2-nonenal was present in the samples of “Cacak Beauty”, “Chrudimer” and “Vlaška”; trans,trans-2,4-nonadienal was established only in the sample “Cacak Beauty”. Heterocyclic aldehydes formed through dehydrating reactions of monosaccharides were represented by furfural and 5-methylfurfural. The first one furanoid was identified in all samples, whereas its higher homologue 5-methylfurfural was identified in samples “Cacak Fruitful”, “Haganta” and “Tophit”. Methylketones occur in alcoholic beverages as by-products of 2-alcohol synthesis, and in our case, acetophenone predominated among the others. Whereas 2-nonanone was found in one-third of the samples, and 2-heptanone was typical only for the plum brandies received from “Topper” and “Katinka” varieties. Moreover, in the half of the cases, the VOC profile was completed with 2,3-butanedione which had an unpleasant buttery flavour, and 2,3-pentanedione.
Acids
The biosynthesis of fatty acids is more complicated in comparison to other volatile compounds. Usually it occurs during alcoholic fermentation initiated in the yeast cell by formation of acetyl coenzyme A (Berger 2007). In general, 6 carboxylic acids were identified and confirmed with authentic standards, and among them acetic acid was dominant. This fact could be caused by additional production of acetic acid during and/or after fermentation through oxidation of ethanol under aerobic conditions (Berger 2007). The content of the other carboxylic acid was significantly lower (propanoic, butanoic, 2-ethylhexanoic acids) and these compounds were presented only in a few plum brandies. According to Ismail et al. (1981) the composition of carboxylic acids depends mainly on the applied yeast strain and to a lesser extent on the used raw material.
Terpenes and terpenoids
Since terpenes are linked to sugar molecules as non-volatile glycosides in plants, fruit ripen process and conditions of hydrolysis are responsible for the overall aroma perception of alcoholic spirit. As can be noticed, in the plum brandies terpenes were mainly represented by monoterpenes, monoterpene derivatives and sesquiterpenes with sweet floral odours. The presence of myrcene, 4-terpinelol, α-terpineol, linalool, linalool oxide, and nerol was previously mentioned in Prunus domestica plum fruit (Pino and Quijano 2012). Linalool contributing to “plum-like” aroma (Ismail et al. 1981) is the only one of terpenoids found in the all studied samples. Whereas its oxidation product epoxylinalool was less common and more specific for the plum brandies “Chrudimer”, “Cacak Early”, “Cacak Fruitful”, “Katinka”, “Top King”. The other common terpenoids include monoterpenic alcohols (β-citronellol, geraniol, α-terpineol, 4-terpineol), sesquiterpenic alcohols (nerolidol, farnesol) and linalool oxides obtained by cyclisation of epoxylinalool. It is interesting that sesquiterpene caryophyllene was found only in the plum brandies fermented from varieties “Chrudimer” and “Durancie”. Especially enriched terpenoid profile was established for the brandy fermented from plum cultivar “Chrudimer, where the presence of myrcene with strong spicy notes and a wide variety of monoterpenes derivatives (linalool acetate, geraniol acetate, geraniol butyrate, cis-rose oxide, bornyl acetate, citronellyl acetate, citronellyl butyrate) may correlate with pleasant aroma notes of this sample.
Lactones and miscellaneous compounds
Lactones, as important components of fruity notes of fermented alcoholic beverages are formed from amino acids through the processes of oxidative deamination and decarboxylation (Berger 2007). Three lactones, γ-hexalactone, γ-octalactone and γ-decalactone were previously reported to be responsible for plum-like aroma (Ismail et al. 1981). In this study, the brandies obtained from “Elena”, “Presenta” and “Gabrovská” cultivar did not contain lactone compounds at all. A marked dominance of γ-lactones was evident for the majority of samples, as well as a typical feature here was a presence of 2(5H)-furanone and butyrolactone. Sample “Chrudimer” was characterized by the reachest diversity of lactones involving γ-lactones in the C8–C12 range, butyrolactone, γ-crotonolactone and δ-octalactone. In addition, γ-nonanolactone, γ-decalactone and γ-dodecalactone were mainly detected in the VOC profile of the samples obtained from “Cacak” plum cultivars and a few more samples.
Among fragrances formed during degradation of phenolic acids, eugenol was established to be the most common compound. Vanillin and 4-ethylguaiacol were mainly identified in the plum brandies fermented from “Cacak” plum cultivars. A majority of the samples contained styrene with a characteristic terpenic odour, and 4-vinylanisole was present in one-third of the cases. A phenolic flavour p,α-dimethyl styrene and benzonitrile appeared to be quite specific for the plum brandies produced from “Hanita”, “Chrudimer” and “Durancie” plums, respectively. Metabolites of oxidative degradation of carotenoids were generally represented by β-damascenone, whereas α-damascone was detected only in the brandies fermented from “Chrudimer” and “Katinka” plum varieties.
Conclusion
Complete analysis of the plum brandies produced from 25 plum cultivars was carried out with comprehensive two-dimensional gas chromatography and sensory evaluation. Two sample preparation procedures were tested and compared, namely liquid–liquid extraction and solid phase microextraction. Regarding to quantity and diversity of compounds, extraction with pentane was more efficient, however 2-butanol, 2,3-butanediol, caryophyllene, butyrolactone, menthol, citronellol and carvone were sorbed only by SPME fibre. As the result, triple phase DVB/CAR/PDMS fibre coating with combined properties was applied for determination of the VOC profile of the plum brandy. The combination of DBFFAP–BPX-50 columns was more appropriate for analysis of plum brandies than HP-5–BPX-50 column setup, because of better separation characteristics. Overall, the analysed plum brandies were distinguished by the presence of a number of identified compounds, since 91–195 compounds were tentatively identified in the samples, and 124 of them were confirmed by analysis of authentic standards. Alcohols e.g. 1-propanol, 2-propanol, 3-methyl-1-butanol, 1-hexanol, ethyl esters of even chain length saturated C6–C12 carboxylic acids, acetic acid and benzaldehyde were dominant components of the VOC profile of the plum brandies. Specific compounds found only in the particular samples mainly belongs to unsaturated fusel alcohols (e.g. 2-methyl-2-propen-1-ol, trans-3-hexen-1-ol and 1-octen-3-ol), unsaturated aldehydes (trans-2-nonenal, trans,trans-2,4-nonadienal), monoterpene derivatives (e.g. linalool acetate, geraniol acetate, geraniol butyrate). Furthermore, it should be noted that the brandy produced from “Chrudimer” plum variety was characterized by broad variety of γ-lactones and terpenoids, whereas the samples obtained from “Elena”, “Presenta” and “Gabrovská” cultivars did not include any lactone compounds. The variation of composition of the plum brandies were confirmed by the sensory evaluation, and the samples described by strong “plum” aroma, odour of “marzipan”, additional fruit or flower notes (e.g. the plum brandies fermented from the cultivars “Chrudimer”, “Presenta”, “Blue Free”) were highly estimated by panellists.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (TIFF 10998 kb)
Acknowledgements
This work was supported by VEGA Grant No. 1/0573/14 and Slovak Research and Development Agency under Contract No. APVV-15-0355.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2900-5) contains supplementary material, which is available to authorized users.
References
- Berger RG. Flavour and fragrances: chemistry, bioprocessing and sustainability. Berlin: Springer; 2007. [Google Scholar]
- Bohačenko I, Pinkrová J, Komárková J, Paprštein F. Selected processing characteristics of new plum cultivars grown in the Czech Republic. Hort Sci. 2010;37:39–45. [Google Scholar]
- Câmara JS, Marques JC, Perestrelo RM, Rodrigues F, Oliveira L, Andrade P, Caldeira M. Comparative study of the whisky aroma profile based on headspace solid phase microextraction using different fibre coatings. J Chromatogr A. 2007;1150:198–207. doi: 10.1016/j.chroma.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Cardeal ZL, Marriott PJ. Comprehensive two-dimensional gas chromatography–mass spectrometry analysis and comparison of volatile organic compounds in Brazilian cachaça and selected spirits. Food Chem. 2009;112:747–755. doi: 10.1016/j.foodchem.2008.06.057. [DOI] [Google Scholar]
- Chai Q, Wu B, Liu W, Wang L, Yang C, Wang Y, Fang J, Liu Y, Li S. Volatiles of plums evaluated by HS-SPME with GC–MS at the germplasm level. Food Chem. 2012;130:432–440. doi: 10.1016/j.foodchem.2011.05.127. [DOI] [Google Scholar]
- Chen J, Wang Z, Wu J, Wang Q, Hu X. Chemical compositional characterization of eight pear cultivars grown in China. Food Chem. 2007;104:268–275. doi: 10.1016/j.foodchem.2006.11.038. [DOI] [Google Scholar]
- García-Llobodanim L, Ferrando M, Gűell C, López F. Pear distillates: influence of the raw material used on final quality. Eur Food Res Technol. 2008;228:75–82. doi: 10.1007/s00217-008-0908-9. [DOI] [Google Scholar]
- Gómez-Plaza E, Ledbetter C. The flavor of plums. In: Hui YH, editor. Handbook of fruit and vegetable flavors. New Jersey: Wiley; 2010. pp. 415–431. [Google Scholar]
- Hartmann W, Neumüller M. Plum breeding. In: Priyadarshan PM, Jain SM, editors. Breeding plantation tree crops: temperate species. New York: Springer; 2009. pp. 161–231. [Google Scholar]
- Ismail HM, Williams AA, Tucknott OG. The flavour of plums (Prunus domestica L.). An examination of the aroma components of plum juice from the cultivar victoria. J Sci Food Agric. 1981;32:613–619. doi: 10.1002/jsfa.2740320614. [DOI] [Google Scholar]
- Knee M. Pome fruits. In: Seymour GB, Taylor JE, Tucker GA, editors. Biochemistry of fruit ripening. 1. Dordrecht: Springer; 1993. pp. 325–346. [Google Scholar]
- Lasekan O, Khatib A, Juhari H, Patiram P, Lasekan S. Headspace solid-phase microextraction gas chromatography–mass spectrometry determination of volatile compounds in different varieties of African star apple fruit (Chrysophillum albidum) Food Chem. 2013;141:2089–2097. doi: 10.1016/j.foodchem.2013.05.081. [DOI] [PubMed] [Google Scholar]
- Lea AGH, Piggott J. Fermented beverage production. New York: Springer; 2003. [Google Scholar]
- Malowicki SMM, Martin R, Qian MC. Volatile composition in raspberry cultivars grown in the pacific northwest determined by stir bar sorptive extraction–gas chromatography–mass spectrometry. J Agric Food Chem. 2008;56:4128–4133. doi: 10.1021/jf073489p. [DOI] [PubMed] [Google Scholar]
- McKay M, Buglass AJ, Lee CG. Fermented Beverages: beers, ciders, wines and related drinks. In: Buglass AJ, editor. Handbook of alcoholic beverages: technical, analytical and nutritional aspects. 1. West Succex: Wiley; 2011. pp. 96–112. [Google Scholar]
- Pawliszyn J. Handbook of Sold Phase Microextraction. London: Elsevier; 2012. [Google Scholar]
- Pecić S, Veljović M, Despotović S, Leskošek-Čukalović I, Jadranin M, Tešević V, Nikšić M, Nikićević N. Effect of maturation conditions on sensory and antioxidant properties of old Serbian plum brandies. Eur Food Res Technol. 2012;232:479–487. [Google Scholar]
- Pino JA, Quijano CE. Study of the volatile compounds from plum (Prunus domestica L. cv. Horvin) and estimation of their contribution to the fruit aroma. Food Sci Technol (Campinas) 2012;32:76–83. doi: 10.1590/S0101-20612012005000006. [DOI] [Google Scholar]
- Robinson AL, Boss PK, Heymann H, Solomon PS, Trengove D. Development of a sensitive non-targeted method for characterizing the wine volatile profile using headspace solid-phase microextraction comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. J Chromatogr A. 2011;1218:504–517. doi: 10.1016/j.chroma.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Satora P, Tuszyński T. Chemical characteristics of Śliwowica Łacka and other plum brandies. J Sci Food Agricul. 2008;88:167–174. doi: 10.1002/jsfa.3067. [DOI] [Google Scholar]
- Satora P, Tuszyński T. Influence of indigenous yeasts on the fermentation and volatile profile of plum brandies. Food Microb. 2010;27:418–424. doi: 10.1016/j.fm.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Satora P, Sroka P, Duda-Chodak A, Tarko T, Tuszyński T. The profile of volatile compounds and polyphenols in wines produced from dessert varieties of apples. Food Chem. 2008;111:513–519. doi: 10.1016/j.foodchem.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Serot T, Prost C, Visan L, Burcea M. Identification of the main odor-active compounds in musts from French and Romanian Hybrids by three olfactometric methods. J Agric Food Chem. 2001;49:1909–1914. doi: 10.1021/jf0012291. [DOI] [PubMed] [Google Scholar]
- Serradilla MJ, Martín A, Ruiz-Moyano S, Hernández A, López-Corrales M, de Guía CM. Physicochemical and sensorial characterisation of four sweet cherry cultivars grown in Jerte Valley (Spain) Food Chem. 2012;133:41551–41559. doi: 10.1016/j.foodchem.2012.02.048. [DOI] [Google Scholar]
- Spaho N, Dürr P, Grba S, Velagić-Habul E, Blesić M. Effects of distillation cut on the distribution of higher alcohols and esters in brandy produced from three plum varieties. J Inst Brew. 2013;119:48–56. doi: 10.1002/jib.62. [DOI] [Google Scholar]
- Swiegers JH, Bartowsky EJ, Henschke PA, Pretoriu IS. Yeast and bacterial modulation of wine aroma and flavour. Aust J Grape Wine Res. 2005;11:139–173. doi: 10.1111/j.1755-0238.2005.tb00285.x. [DOI] [Google Scholar]
- Veberic R, Jakopic J, Stampar F, Schmitzer V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009;114:511–515. doi: 10.1016/j.foodchem.2008.09.080. [DOI] [Google Scholar]
- Velíšik J. Flavour-active compounds. In: Velíšik J, editor. The chemistry of food. West Sussex: Wiley; 2014. pp. 449–656. [Google Scholar]
- Versini G, Franco MA, Mosera S, Barchettia P, Manca G. Characterisation of apple distillates from native varieties of Sardinia island and comparison with other Italian products. Food Chem. 2009;113:1176–1183. doi: 10.1016/j.foodchem.2008.08.003. [DOI] [Google Scholar]
- Versini G, Franco MA, Moser S, Manca G. Characterization of pear distillate from wild and cultivated varieties in Sardinia. Int J Food Sci Tech. 2012;47:2519–2531. doi: 10.1111/j.1365-2621.2012.03130.x. [DOI] [Google Scholar]
- Villière A, Arvisenet G, Lethuaut L, Prost C, Sérot T. Selection of a representative extraction method for the analysis of odourant volatile composition of French cider by GC–MS–O and GC×GC–TOF-MS. Food Chem. 2012;131:1561–1568. doi: 10.1016/j.foodchem.2011.10.008. [DOI] [Google Scholar]
- Vulić T, Nikićević N, Stanković L, Veličković M, Todosijević M, Popović B, Urošević I, Stanković M, Beraha I, Tešević VV. Chemical and sensorial characteristics of fruit spirits produced from different black currant (Ribes nigrum l.) and red currant (Ribes rubrum l.) cultivars. Maced J Chem Chem Eng. 2012;31:217–227. [Google Scholar]
- Vyviurska O, Pysarevska S, Janoškova N, Špánik I. Comprehensive two-dimensional gas chromatographic analysis of volatile organic compounds in distillate of fermented Sorbus domestica fruit. Open Chem. 2015;13:96–104. doi: 10.1515/chem-2015-0007. [DOI] [Google Scholar]
- Weldegergis BT, Crouch AM, Górecki T, de Villiers A. Solid phase extraction in combination with comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry for the detailed investigation of volatiles in South African red wines. Anal Chim Acta. 2011;701:98–111. doi: 10.1016/j.aca.2011.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (TIFF 10998 kb)