Abstract
This work was designed to obtain the valuable compounds with antioxidant, anti-proliferative and anti-inflammatory activities from Astragalus chinensis. Ethyl acetate fraction obtained from A. chinensis L.f. had significant antioxidant, anti-proliferative and anti-inflammatory activities. Subsequently, five single compounds were separated and purified, which were identified as formononetin (1), rhamnocitrin (2), calycosin (3), β-daucosterol (4), rhamnocitrin-3-O-β-d-glucoside (5). The results displayed that formononetin and rhamnocitrin exhibited significant cytotoxicity actions against tumor cell lines. Calycosin exerted the strongest anti-inflammatory effect of inhibition effects on NO production in macrophages.
Keywords: Astragalus chinensis L.f., Antioxidant, Antitumor, Anti-inflammatory, Formononetin, Calycosin
Introduction
Astragalus chinensis L.f., the dried ripe seed of Astragalus complanatus Bunge, which belongs to Leguminosae, has been regarded as raw material of health food in China. It is extensively distributed in northern China and called sha-yuan-zi in Chinese. China Pharmacopoeia (1990 edition) recorded Astragalus chinensis L.f. with its healthcare functions and the clinical usage of its prescription (Zhang et al. 2013).
In the past 10 years, much research has been conducted to identify the chemical constituents from Astragalus chinensis L.f. and investigate their biological activities and pharmacological effects (Li et al. 2005). Reports indicated that Astragalus chinensis L.f. had the functions of tonifying kidney, protecting hepato and restraining urination (Xue et al. 2008). Besides, it also has been used as antihypertensive drugs in clinic (Liu et al. 2005). These results showed that many of these pharmacological activities are attributed to its main constituents such as complanatoside A, complanatoside B, astragalin, myricomplanoside, neocomplanoside and so on. Favonoids from Astragalus chinensis L.f. also showed good biological activity (Zhang et al. 2005; Zhang et al. 2010; Ou et al. 2007). Nowadays, many types of food products involved in Astragalus chinensis L.f. have been developed, such as commercial Astragalus chinensis L.f. extracts, candies and so on.
The present research investigated the effective ingredients in Astragalus chinensis L.f., which possess the antioxidant, anti-proliferative and anti-inflammatory activities that used to evaluate the potential nutritive value of Astragalus chinensis L.f., especially the individual compounds from it. It is hoped that, with the report of the present research, Astragalus chinensis L.f. may become a more available material in the development of functional foods.
Materials and methods
Plant material
The dried Astragalus chinensis L.f., identified as the dry ripe seeds of Astragalus complanatus Bunge, was bought from Guangzhou medicamentarius, and were authenticated by professor Hao Gang of South China Agricultural University, where voucher specimens (voucher specimen number 38276) were kept. Samples were crushed in a cutting mill to powder for further use.
Reagents
1, 1-diphenyll-2-2-pricylhydrazyl (DPPH), vitamin C (VC), trichloroacetic acid (TCA), penicillin, streptomycin, MTT, 5-fluorouracil, lipopolysaccharide (LPS) were obtained from Sigma. Fetal bovine serum (FBS), DMEM medium and trypsin–EDTA were obtained from Gibco.
Extraction, isolation and purification of bioactive constituents
Firstly, Astragalus chinensis L.f. was extracted with 95% ethanol and the extracting solution was concentrated and evaporated. Next, four fractions were obtained by organic solvent extraction. Ethyl acetate fraction exhibited the best effects, which was chosen to be further isolated and purified.
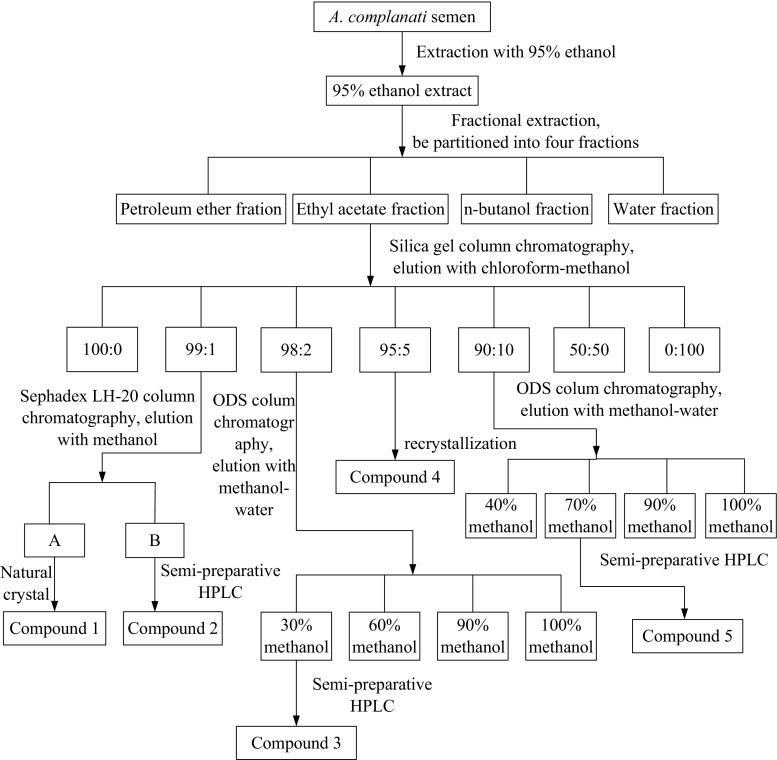
Ethyl acetate fraction was directly loaded to a silica gel column and eluted with different concentrations of chloroform–methanol solution (100:0, 99:1, 98:2, 95:5, 90:10, 50:50, 0:100, v/v). The eluates were divided into several fractions on the basis of qualitative analysis. Fraction 99:1 was further loaded on a Sephadex LH-20 column and eluted with 100% methanol to yield compound 1 (natural crystal), and compound 2 was obtained by semi-preparative HPLC. Fraction 98:2 was further purified by a ODS column chromatography to obtain four fractions (30, 60, 90 and 100% methanol fraction, respectively). Compound 3 was obtained from the 30% methanol fraction, which was further purified by semi-preparative HPLC. Compound 4 was obtained from the fraction 95:5 through recrystallization method. Fraction 90:10 was applied on a ODS column to obtain four fractions (40, 70, 90 and 100% methanol fraction, respectively). The 70% methanol fraction was further purified by semi-preparative HPLC, and then compound 5 was obtained. Figure 1 illustrates the above separation and purification process of Astragalus chinensis L.f. in a flow diagram.
Fig. 1.
The isolation and purification process of Astragalus chinensis L.f.
Chemical structure analysis
The chemical structures of isolated pure compounds were analyzed and identified according to EI-MS, 1H-NMR, 13C-NMR and DEPT135-NMR spectra analyses.
DPPH radical scavenging activity
The effects of four fractions on DPPH radical scavenging were performed on the basis of previous method with small changes (Prathapan et al. 2011; Muniz-Marquez et al. 2013). 0.5 mL of different samples at a concentration of 30, 50, 100, 300 and 500 μg/mL were added to 150 μM DPPH· solution. Then incubate the samples in dark for 30 min, and the absorbance was obtained at 517 nm.
Total reducing power ability
The effects of samples on the reducing power ability were performed on basis of previous method (Kapoor et al. 2013). Firstly, different samples at a concentration of 50, 100, 200, 400 and 800 μg/mL were added to phosphate buffer and K3Fe(CN)6. Then the reaction mixture was incubated at 50 °C for 20 min. Next, 10% TCA was mixed with the mixture and it was centrifuged for 10 min. After that, the upper layer solution was added to 0.1% FeCl3. Then the optical density was obtained at 700 nm. The increase of absorbance value reflected the reducing power ability.
Inhibition activity on human cervical carcinoma cell HeLa
The anti-proliferative activities of four fractions and four pure compounds were tested by MTT assay with human cervical carcinoma cell HeLa in vitro, respectively, in which 5-FU was considered as the positive control. Briefly, exponential growth phase HeLa cells were cultured in complete DMEM medium, and they were put in 96-well plates and incubated at 37 °C overnight. Then the supernatant media were discarded and 100 μL complete medium containing different concentrations of samples were added and incubated at 37 °C for 24 h. After then, 100 μL of media containing 10% MTT (5 mg/mL) was added to each well, followed by incubation for 4 h at 37 °C. Then, the supernatant was discarded and 150 μL of DMSO was added to dissolve formazan crystals. The optical density (OD) was measured at 490 nm. Cell proliferation inhibition rate was calculated using the following formula (Wijesinghe et al. 2013):
Anti-inflammatory assays
The effects of samples on anti-inflammatory activities were investigated with macrophages. Cells were cultured in complete DMEM medium. The Griess reaction was used to measure NO production, and the cells were activated with LPS on basis of Hsu et al. (2013). After that, absorbance was measured at 540 nm and nitrite levels in the samples were calculated from a standard curve with known concentrations of sodium nitrite. NO inhibition rate was calculated using the following formula:
Statistical analysis
Data were analyzed by ANOVA and presented as mean ± SD. Significant differences were performed by variance-Duncan’s multiple range test (p ≤ 0.05). The letters in the figures indicated the significant differences between different fractions or compounds.
Results and discussion
DPPH radical scavenging activity
In this study, DPPH radical scavenging activities of four fractions were tested and the results were compared. As seen in Fig. 2a, the DPPH radical-scavenging capacity of all samples change in a dose-dependent manner. The scavenging ability of ethyl acetate fraction significantly increased with the increase in concentration and was stronger than that of other three fractions at all concentrations. The scavenging effect of ethyl acetate reached about 90% at the concentration of 500 μg/mL, which was on the verge of VC. In addition, n-butyl alcohol fraction showed a certain scavenging effect, while petroleum ether and water fraction showed low scavenging activity. Besides, the estimated IC50 values of the four fractions extracted from Astragalus chinensis L.f. and the positive control were obtained. VC exhibited remarkable IC50 value (31.96 μg/mL), and the IC50 values of four fractions (in the order of petroleum ether fraction, ethyl acetate fraction, water fraction and n-butyl alcohol fraction) were > 500, 95.69, > 500 and 229.55 μg/mL, respectively, which could be seen that ethyl acetate fraction displayed a better effect among the four fractions.
Fig. 2.
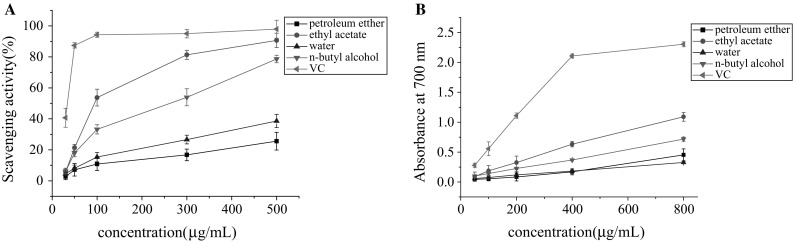
Antioxidant activity of the four fractions from Astragalus chinensis L.f. a DPPH radical scavenging ability. b Total Reducing power
Total reducing power
The reducing power of chemical compounds may be used as an available indicator of the potential antioxidant activity. Figure 2b shows the reducing power of four fractions. The higher absorbance reflects the stronger reducing power. VC has very strong antioxidant ability that used as positive control. In this work, the value of all samples was much lower than that of VC. However, ethyl acetate fraction showed the best effect among the four fractions, which were higher than that of the other three fractions at various concentrations. According to reducing power assays, the activities of the four fractions decreased in the order of ethyl acetate fraction > n-butyl alcohol fraction > water fraction > petroleum ether fraction, which was similar to that observed for the DPPH assays.
The results mentioned above demonstrated that ethyl acetate fraction had a noticeable effect on inhibiting the formation of DPPH free radical and a better reducing power ability among these four fractions. Although none of the samples had stronger activities than VC at the same concentration, it implied that ethyl acetate fraction isolated from Astragalus chinensis L.f. was valuable for further separation and purification.
Structure identification
The chemical structures of five compounds isolated from A. chinensis L.f. were identified according to EI-MS and NMR spectra analyses, which are listed below and displayed in Fig. 3.
Fig. 3.
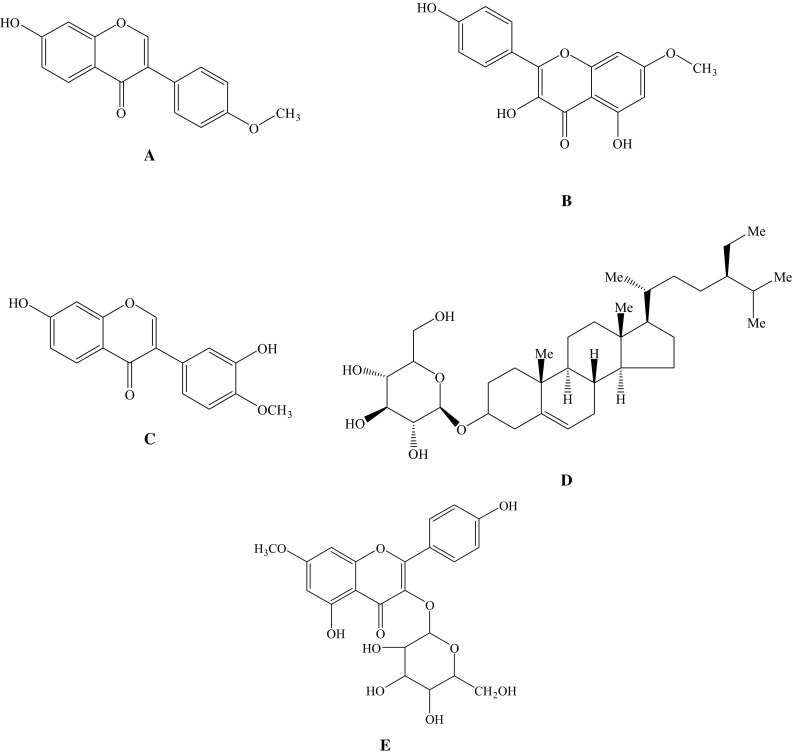
Chemical structures of the compounds isolated from Astragalus chinensis L.f. a: rhamnocitrin-3-O-β-d-glucoside; b: calycosin; c: formononetin; d: rhamnocitrin; e: β-daucosterol
Compound 1 was white powder, molecular formula C16H12O4, ESI–MS (m/z) [M-H]− 267. On the basis of 1H-NMR spectral, four hydrogen atoms conducted a form of AA′XX′ spin coupling system, which at δH 7.5 (2H, d, J = 8.6 Hz) and δH 6.98 (2H, d, J = 8.5 Hz), showed that there was a benzene ring structure. It showed 2-H signal of isoflavone and –OCH3 signal, which were at δH 8.30 (1H, s) and δH 3.77 (3H, s), respectively. It was identified as formononetin with a purity of 98% (Fig. 3a) (Wang et al. 2014).
Compound 2 was yellow acicular crystal, m.p. 221-222 °C. ESI–MS m/z: 323 [M+Na]+, 301 [M+H]+, 299 [M-H]−, indicated that its molecular weight was 300. In the 1H-NMR spectral, it showed kaempferol signals, which were at δH 12.47 (1H, s), δH 8.09 (2H, d, J = 8.9 Hz), δH 6.95 (2H, d, J = 8.9 Hz), δH 6.73(1H, d, J = 1.6 Hz) and δH 6.35 (1H, d, J = 1.6 Hz). There was an extra –OCH3 signal in comparison with kaempferol, which was at δH 3.86 (3H, s). There were 14 carbon signals in 13C-NMR spectral. It could also be concluded to have symmetrical structure fragments combined with 1H-NMR and DEPT135-NMR spectral, which were at δC 129.5 (C–H) and δC 115.4 (C–H). Its molecular formula was C16H12O6. Comparing the data with the literature, the compound was identified as rhamnocitrin with a purity of 98% (Fig. 3b) (Omosa et al. 2014).
Compound 3 was obtained as white crystal. It showed brownish red macula at UV254 nm, fluorescent at UV365 nm, but no color in an atmosphere of sulfuric acid agent. It can be concluded to have 12 hydrogen atom and 16 carbon atom on the base of the NMR spectral. Combined with ESI–MS (m/z) [M-H]− 283, the molecular formula was C16H12O5. In the 1H-NMR spectral, it showed 2-H signal of isoflavone and –OCH3 signal, which were at δH 8.28 (1H, s) and δH 3.79 (3H, s). In addition, there was a trisubstituted aromatic ring protons signals at δH 7.95 (1H, d, J = 8.8 Hz), δH 6.94 (1H, dd, J = 8.8, 2.0 Hz) and δH 6.92 (1H, d, J = 2.0 Hz) that were ABX system, which stand for H-5, H-6, H-8, respectively. There were 7 methyne carbons, 4 quaternary carbons attached to the oxygen, 1 carbonyl carbons, 3 quaternary carbons and 1 carbon attached to methoxyl. It showed the 4-C of isoflavone signal at δC 174.55. It was identified as calycosin with a purity of 96% (Fig. 3c) (Jiang et al. 2014a, b).
Compound 4 was obtained as white powder, m.p. 283–283 °C. It was soluble in chloroform. ESI–MS (m/z) [M-H]− 576. The color was amaranth when placed in 10% sulfuric acid–ethanol condition. On the basis of the rate of flow (Rf) value in the application of thin layer chromatography with various developing solvent systems (chloroform/methanol 85:15, Rf = 0.43; BAW 4:1:1, Rf = 0.57; chloroform/methanol/water 9:1:0.1, Rf = 0.45), and color rendering together with reference substance β-daucosterol, the data were the same (Zhang et al. 2014a, b). The compound was identified as β-daucosterol with a purity of 99% (Fig. 3d).
Compound 5 was obtained as yellow acicular crystal, m.p. 198–200 °C. There was no fluorescence at 365 nm in UV lamp, while it appeared yellow under an atmosphere of AlCl3-MeOH solution and sulfuric acid–ethanol solution, which show it belongs to flavonoids. ESI–MS m/z: 947 [2M+Na]+, 485 [M+Na]+, 461 [M-H]−, indicated that its molecular weight was 462. In the secondary dissociation mass spectrum, m/z: 299 [M-H-162]−, it shows that there may be hexose fragment in the structure. It displayed the similar signals in the 1H-NMR spectra compared with the compound 2 (rhamnocitrin), and the difference is that there is saccharous terminal hydrogen signal at δH 5.47 (1H, s, J = 7.2 Hz) and other hydrogen signals of saccharide at δH 5.0–2.0. Combined with mass spectrum, it was concluded to be glucoside of rhamnocitrin. There were a series of carbon signals of glucose in the 13C-NMR spectra, which were at δC 100.9 (C–H, anomeric carbon), 77.5 (C–H), 76.4 (C–H), 74.2 (C–H), 69.9 (C–H), 60.0 (−CH2), and further confirmed that this compound was the glucoside of rhamnocitrin. The molecular formula was speculated as C22H22O11 according to the EI-MS, 1H-NMR, 13C-NMR and DEPT135 NMR spectra. Comparing the data with the literature, the compound was identified as rhamnocitrin-3-O-β-d-glucoside (RHG) with a purity of 96% (Fig. 3e) (Zhou et al. 2009).
Formononetin, rhamnocitrin, calycosin, RHG are important compounds that could be found in several plants. There were some biological activities of these isolated compounds that were reported before. Formononetin had significant antioxidant and estrogenic effects, and the estrogenic effect was not in a dose-dependent manner (Mu et al. 2009). Tu et al. indicated that rhamnocitrin not only protect low-density lipoprotein (LDL) from oxidation but also prevent atherogenesis through suppressing macrophage uptake of oxLDL (Tu et al. 2007). Guo et al. suggested calycosin protected endothelial cells from hypoxia-induced barrier impairment, as well as improving cytoskeleton remodeling (Fan et al. 2003). However, no systematic report regarding its antitumor activity against the proliferation of HeLa cells and anti-inflammatory activity could be found. Besides, β-daucosterol is a very common compound widely distributed in plants, and much work about its biological activities have been investigated, so we make no further study on this compound (Jiang et al. 2014a, b; Choi et al. 2012; Lee et al. 2007).
Antitumor activity of various factions and purified compounds
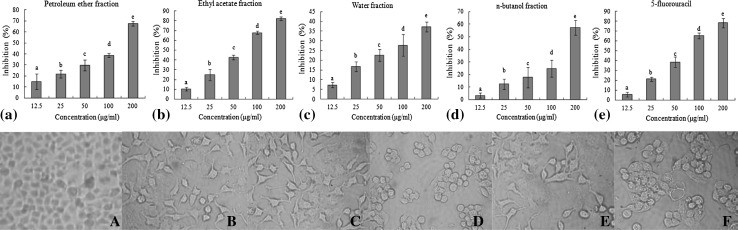
The anti-proliferative effect of samples was performed by MTT assay. Different fractions showed different inhibition effects on HeLa cells (Fig. 4). Petroleum ether and n-butanol demonstrated moderate anticancer activity with the highest inhibition rate 67.60 and 57.36% at 200 μg/mL (Fig. 4a, c). In particular, ethyl acetate fraction exhibited a better antitumor activity, which could significantly inhibit the HeLa cells at concentrations of 12.5–200 μg/mL (Fig. 4b). The inhibition rate of ethyl acetate fraction significantly increased with the concentration increasing and was stronger than that of 5-fluorouracil (positive control) at each concentration. Whereas, water fraction showed low inhibition effect (Fig. 4d). Generally speaking, petroleum ether fraction contains much oil and fatty acid, which are not the main components we desired. Ethyl acetate fraction is rich in medium polar components such as flavonoids, alkaloids, coumarins and organic acids, which are the main bioactive ingredients contained in medical plants and exerts the anti-proliferation activity on HeLa cells. The experimental results showed that the active ingredients on antitumor were concentrated in the Ethyl acetate fraction after 95% ethanol was partitioned into four fractions. As a consequence, the ethyl acetate fraction was made for further isolation and purification to obtain bioactive compounds.
Fig. 4.
Effects of four fractions on the growth and survival of HeLa cells. The column graphs (a–e) showed the in vitro inhibition ratio of HeLa cells by four fractions at different concentrations for 24 h. The cell images (A–F) showed the cell growth morphology of A control group, B petroleum ether fraction (200 μg/mL), C ethyl acetate fraction (200 μg/mL), D n-butanol fraction (200 μg/ml), E water fraction (200 μg/mL) and F 5-fluorouracil (200 μg/mL), respectively, observed with invert microscope. Letters a–e refer to significant differences within a fraction/compound by one-way analysis of variance-Duncan’s multiple range test (p ≤ 0.05)
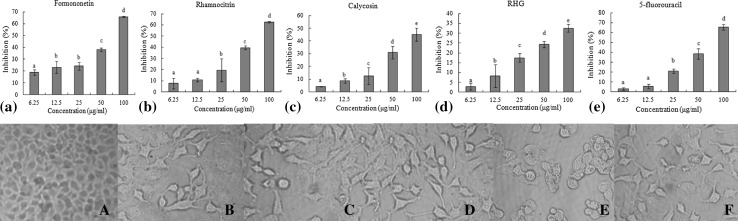
Among these four compounds isolated and purified from ethyl acetate fraction, formononetin and rhamnocitrin showed a stronger inhibition effect on the HeLa cells, of which formononetin exhibited a closer inhibition activity to 5-fluorouracil at the concentration of 25–100 μg/mL (Fig. 5). Calycosin showed moderate activities at 100 μg/mL with the inhibition rate was 45.14%. RHG did not display a significant inhibition effect on the HeLa cells, and its inhibition rate increased slowly with the increase of the concentration.
Fig. 5.
Effects of four purified compounds on the growth and survival of HeLa cells. The column graphs (a–e) showed the in vitro inhibition ratio of HeLa cells by four purified compounds at different concentrations for 24 h. The cell images (A–F) showed the cell growth morphology of A control group, B RHG (100 μg/mL), C calycosin (100 μg/mL), D formononetin (100 μg/mL), E rhamnocitrin (100 μg/mL) and F 5-fluorouracil (100 μg/mL), respectively, observed with invert microscope. Letters a-e refer to significant differences within a fraction/compound by one-way analysis of variance-Duncan’s multiple range test (p ≤ 0.05)
Formononetin is a natural isoflavone that can be found in the roots of many medical plants. The in vitro and in vivo studies showed that formononetin inhibited the cell migration and invasion of breast cancer through the decreasing expression of MMP-2 and MMP-9 (Zhou et al. 2014). A similar result indicated that formononetin, isolated from the red clover, exerted the anticarcinogenic effect on prostatic adenocarcinoma cell line (Zhang et al. 2014a, b). In the present study, formononetin showed an obvious inhibition of HeLa cells, suggesting that it would be a novel drug carrier for carcinoma of uterine cervix. It is considered that higher concentrations of formononetin caused cell cycle arrest at certain phase and inhibited the proliferation of HeLa cells by up-regulating the expression of cell apoptotic protein and down-regulating the expression of cell proliferation protein within signaling pathway in a dose-dependent manner, which resulted in induced apoptosis in HeLa cells.
Anti-inflammatory activity of various factions and purified compounds
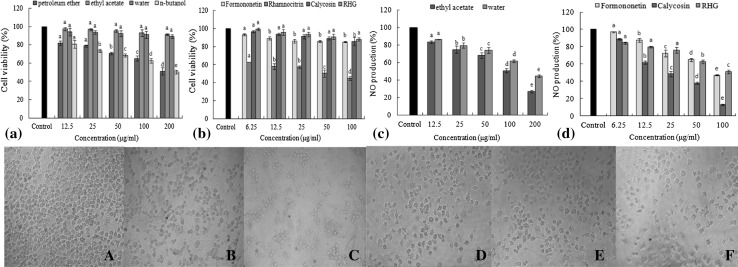
As seen in Fig. 6a, some significant toxic effects were observed on the cells treated with these fractions. Petroleum ether fraction and n-butanol fraction both exhibited a regular change of toxic effect on cells with increasing concentration. The highest cell viability was about 80% at 12.5 μg/mL, and the lowest cell viability was only about 50% with the concentration was 200 μg/mL. Therefore, these two fractions should not be assayed for the anti-inflammatory effect. The ethyl acetate fraction and water fraction both improved the cell viability. When treated with ethyl acetate fraction and water fraction, the highest cell viability were 91.20% and 89.07%, respectively. The same method was used to analyze the purified compounds (Fig. 6b). Results suggested that the growth of RAW 264.7 cell lines had been a little affected by ethyl acetate fraction, water fraction, RHG, calycosin, formononetin, and the cell viability was almost not influenced by them, which could be applied to the anti-inflammatory activity.
Fig. 6.
Effects of four fractons and four purified compounds on macrophages. The column graphs (a–d) showed the effect of samples on the vitality of macrophages and the inhibitory effect on NO production of samples at different concentrations. The cell images (A–F) showed the cell growth morphology of A control group, B ethyl acetate fraction (200 μg/mL), C water fraction (200 μg/mL), D Formononetin (100 μg/mL), E Calycosin (100 μg/mL) and F RHG (100 μg/mL), respectively, observed with invert microscope. Letters a–e refer to significant differences within a fraction/compound by one-way analysis of variance-Duncan’s multiple range test (p ≤ 0.05)
The anti-inflammatory activity of samples was quantitatively analyzed on the basis of the NO2−concentration that in order to reflect the inhibition of NO production. Ethyl acetate fraction and water fraction dose-dependently inhibited NO production with the inhibition rate reached 73.10 and 55.39% at the concentration of 200 μg/mL, indicating that these two fractions both had a certain anti-inflammatory activity at concentrations of 12.5-200 μg/mL, especially for the ethyl acetate fraction (Fig. 6c). Among the three purified compounds, RHG and formononetin did not display a stronger inhibitory activity, but a moderate anti-inflammatory activity with the increasing concentration. Calycosin showed the strongest inhibitory effect at 100 μg/mL with the lowest inhibition rate of 12.80% (Fig. 6d).
Calycosin, a bioactive compound isolated from Radix astragali, showed significant anticancer and anti-inflammatory activities. It could regulate and control cell cycle and apoptosis, as well as show a certain effect on the treatment of leukemia (Jin et al. 2010; Zhang et al. 2012). Although this study showed that calycosin had a fine anti-inflammatory activity, exhibiting its great potentiality as a therapeutic drug, the mechanism of its anti-inflammatory activity should be further investigated in future.
Conclusion
The antioxidant activity of the ethyl acetate fraction from A. chinensis L.f. were stronger than other fractions, which may be due to the most abundant flavonoids compounds in the fraction. Subsequently, five pure compounds were identified from ethyl acetate fraction, and then their anti-proliferative and anti-inflammatory activities were compared. Petroleum ether and ethyl acetate both exhibited effectively anti-proliferative activity. Water fraction and ethyl acetate fraction exerted a better anti-inflammatory activity. Among the four pure compounds, formononetin and calycosin were the bioactive compounds, which had obvious cytotoxicities against HeLa cells, as well as effective anti-inflammatory activities. The results obtained in this work might be beneficial to the application of Astragalus chinensis L.f. in food and drug enterprises.
Acknowledgements
This research was supported by the Science and Technology Project of Guangzhou City (201604020150), the National Natural Science Foundation of China (21702156), the Hubei Natural Science Foundation (2017CFB200) and the Scientific Research Foundation of Wuhan institute of technology (K201759).
References
- Choi JN, Choi YH, Lee JM, Noh IC, Park JW, Choi WS, Choi JH. Anti-inflammatory effects of beta-sitosterol-beta-d-glucoside from Trachelospermum jasminoides (Apocynaceae) in lipopolysaccharide-stimulated RAW 264.7 murine macrophages. Nat Prod Res. 2012;26:2340–2343. doi: 10.1080/14786419.2012.654608. [DOI] [PubMed] [Google Scholar]
- Fan Y, Wu DZ, Gong YQ, Zhou JY, Hu ZB. Effects of calycosin on the impairment of barrier function induced by hypoxia in human umbilical vein endothelial cells. Eur J Pharmacol. 2003;481:33–40. doi: 10.1016/j.ejphar.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Hsu CL, Fang SC, Yen GC. Anti-inflammatory effects of phenolic compounds isolated from the flowers of Nymphaea mexicana Zucc. Food Funct. 2013;4:1216–1222. doi: 10.1039/c3fo60041f. [DOI] [PubMed] [Google Scholar]
- Jiang LH, Yang NY, Yuan XL, Zou YJ, Zhao FM, Chen JP, Wang MY, Lu DX. Daucosterol promotes the proliferation of neural stem cells. J Steroid Biochem. 2014;140:90–99. doi: 10.1016/j.jsbmb.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Jiang ZP, Wei GM, Li Z, Liu YH, Wang ZD. Flavonoids from the roots of Astragalus membranaceus (Fisch.) Bge. prevent development of diabetic nephropathy in vitro. Lat Am J Pharm. 2014;33:339–343. [Google Scholar]
- Jin S, Zhang QY, Kang XM. Daidzein induces MCF-7 breast cancer cell apoptosis via the mitochondrial pathway. Ann Oncol. 2010;21:263–268. doi: 10.1093/annonc/mdp499. [DOI] [PubMed] [Google Scholar]
- Kapoor IPS, Singh B, Singh G, De Heluani CS, De Lampasona MP, Catalan CAN. Chemical composition and antioxidant activity of essential oil and oleoresins of nutmeg (Myristica fragrans Houtt.) fruits. Int J Food Prop. 2013;16:1059–1070. doi: 10.1080/10942912.2011.576357. [DOI] [Google Scholar]
- Lee JH, Lee JY, Park JH, Jung HS, Kim JS, Kang SS, Kim YS, Han Y. Immunoregulatory activity by daucosterol, a beta-sitosterol glycoside, induces protective Th1 immune response against disseminated Candidiasis in mice. Vaccine. 2007;25:3834–3840. doi: 10.1016/j.vaccine.2007.01.108. [DOI] [PubMed] [Google Scholar]
- Li JX, Xue B, Chai Q, Liu ZX, Zhao AP, Chen LB. Antihypertensive effect of total flavonoid fraction of Astragalus complanatus R.Brown in hypertensive rats. Chin J Physiol. 2005;48:101–106. [PubMed] [Google Scholar]
- Liu CY, Gu ZL, Zhou WX, Guo CY. Effect of Astragalus complanatus flavonoid on anti-liver fibrosis in rats. World J Gastroenterol. 2005;37:5782–5786. doi: 10.3748/wjg.v11.i37.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu H, Bai YH, Wang ST, Zhu ZM, Zhang YW. Research on antioxidant effects and estrogenic effect of formononetin from Trifolium pratense (red clover) Phytomedicine. 2009;16:314–319. doi: 10.1016/j.phymed.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Muniz-Marquez DB, Martinez-Avila GC, Wong-Paz JE, Belmares-Cerdaa R, Rodriguez-Herrera R, Aguilar CN. Ultrasound-assisted extraction of phenolic compounds from Laurus nobilis L. and their antioxidant activity. Ultrason Sonochem. 2013;20:1149–1154. doi: 10.1016/j.ultsonch.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Omosa LK, Amugune B, Ndunda B, Milugo TK, Heydenreich M, Yenesew A, Midiwo JO. Antimicrobial flavonoids and diterpenoids from Dodonaea angustifolia. S Afr J Bot. 2014;91:58–62. doi: 10.1016/j.sajb.2013.11.012. [DOI] [Google Scholar]
- Ou LN, Zhang JJ, Zhang YJ, Yang XL, Zeng FY, Li W. Compare with the content of companatoside A of commercial semen A. complanati in different districts. Chin J Pharm Anal. 2007;27:742–745. [Google Scholar]
- Prathapan A, Cherian OL, Nampoothiri SV, Mini S, Raghu KG. In vitro antiperoxidative, free radical scavenging and xanthine oxidase inhibitory potentials of ethyl acetate fraction of Saraca ashoka flowers. Nat Prod Res. 2011;25:298–309. doi: 10.1080/14786419.2010.510472. [DOI] [PubMed] [Google Scholar]
- Tu YC, Lian TW, Yen JH, Chen ZT, Wu MJ. Antiatherogenic effects of kaempferol and rhamnocitrin. J Agric Food Chem. 2007;55:9969–9976. doi: 10.1021/jf0717788. [DOI] [PubMed] [Google Scholar]
- Wang H, Mei WL, Guo ZK, Xia ZF, Zhong HM, Dai HF. Chemical constituents of Dalbergia odorifera. J Chin Materia Medica. 2014;39:1625–1629. [PubMed] [Google Scholar]
- Wijesinghe WAJP, Jeon YJ, Ramasamy P, Wahid MEA, Vairappan CS. Anticancer activity and mediation of apoptosis in human HL-60 leukaemia cells by edible sea cucumber (Holothuria edulis) extract. Food Chem. 2013;139:326–331. doi: 10.1016/j.foodchem.2013.01.058. [DOI] [PubMed] [Google Scholar]
- Xue B, Li JX, Chai Q, Liu ZX, Chen LB. Effect of total flavonoid fraction of Astragalus complanatus R.Brown on angiotensin II-induced portal-vein contraction in hypertensive rats. Phytomedicine. 2008;15:759–762. doi: 10.1016/j.phymed.2007.11.030. [DOI] [PubMed] [Google Scholar]
- Zhang JJ, Yan XL, Zhang YJ. Determination of complanatoside A in semen A. complanati by HPLC. J Chin Materia Medica. 2005;30:600–602. [PubMed] [Google Scholar]
- Zhang QA, Fan XH, Zhang ZQ, Ma XY, Chen Y, Wang J. Study on the phenolic determination of extracts from semen A. complanati. Food Sci. 2010;31:178–181. [Google Scholar]
- Zhang D, Zhuang Y, Pan J. Investigation of effects and mechanisms of total flavonoids of Astragalus and calycosin on human erythroleukemia cells. Oxid Med Cell Longev. 2012;20:1–5. doi: 10.1155/2012/209843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QA, Fan XH, Li T, Zhang ZQ, Liu YK, Li XP. Optimization of ultrasound extraction for flavonoids from semen A. complanati and its identification by HPLC-DAD-MS/MS. Int J Food Sci Technol. 2013;48:1970–1976. doi: 10.1111/ijfs.12178. [DOI] [Google Scholar]
- Zhang TT, Lu CL, Jiang JG. Bioactivity evaluation of ingredients identified from the fruits of Amomum tsaoko Crevost et Lemaire, a Chinese spice. Food Funct. 2014;5:1747–1754. doi: 10.1039/C4FO00169A. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bi LY, Ye Y, Chen J. Formononetin induces apoptosis in PC-3 prostate cancer cells through enhancing the Bax/Bcl-2 ratios and regulating the p38/Akt pathway. Nutr Cancer. 2014;66:656–661. doi: 10.1080/01635581.2014.894098. [DOI] [PubMed] [Google Scholar]
- Zhou GX, Lu CL, Wang HS, Yao XS. An acetyl flavonol from Nervilia fordii (Hance) Schltr. J Asian Nat Prod Res. 2009;11:498–502. doi: 10.1080/10286020902893074. [DOI] [PubMed] [Google Scholar]
- Zhou R, Xu L, Ye M, Liao M, Du H, Chen H. Formononetin inhibits migration and invasion of MDA-MB-231 and 4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through PI3K/AKT signaling pathways. Horm Metab Res. 2014;46:753–760. doi: 10.1055/s-0034-1376977. [DOI] [PubMed] [Google Scholar]