Abstract
The oxidative stability and fatty acid composition of groundnut seed oil (GSO) exposed to microwaves were evaluated during heating at 170 °C. During heating, the oxidative indices such as free fatty acid, peroxide value, p-anisidine value, TOTOX, thiobarbituric acid value, specific extinctions, and color value were increased. The increments were found to be higher in unroasted seed oils compared to roasted ones indicating lower release of lipid oxidation products in roasted GSO. After 9 h heating, the relative content of polyunsaturated fatty acid (PUFA) decreased to 89.53% and that of saturated fatty acid (SFA) increased to 117.46% in unroasted sample. The relative content of PUFA decreased to 92.05% and that of SFA increased to 105.76% in 7.5 min roasted sample after 9 h of heating. However, the roasting process slowed down the oxidative deterioration of PUFA. With increased heating times, an appreciable loss was more apparent in the triacylglycerol species OLL and OOL in unroasted samples compared to roasted ones. In FTIR, the peak intensities in unroasted samples were markedly changed in comparison with roasted samples during heating. The roasting of groundnut seed prior to the oil extraction reduced the oxidative degradation of oil samples; thereby increasing heat stability.
Keywords: Groundnut seed oil, Oxidation stability, Fatty acid composition, Triacylglycerol composition, Roasting
Introduction
Groundnuts or peanuts (Arachis hypogaea L.) are the second most harvested legume in the world behind soybeans, providing an important nutrient source to the world’s population (Craft et al. 2010). They are a great source of nutrients and edible oil, that are grown in almost all tropical and sub-tropical countries of the world (Arya et al. 2016; Bhatti et al. 2010). A lot of groundnuts may be consumed raw, roasted, pureed, or in a variety of the other processed forms, and constitute as a multi-million-dollar crop worldwide with numerous potential dietary benefit (Yu et al. 2005). The impacts of various processing methods in preparing oilseeds for human consumption are of utmost importance. Roasting is a high temperature and short time processing method which enhances the oxidative stability, antioxidant activity, and level of saturated and unsaturated fatty acids in oils (Ali et al. 2016a, b; El Anany 2015). Microwave processing of foods is a recent development, which is gaining momentum in household and large scale food applications.
Oxidation is one of the leading causes of oil deterioration which results in off flavors and rancidity. In general, the oxidative stability of an oil depends on its fatty acid and triacylglycerol composition, as well as the composition of minor constituents with antioxidative properties (Nederal et al. 2012). In oxidation process, the triglyceride structure undergoes changes with the consequence of the formation of low-molecular-weight off-flavour compounds, which can make oil unacceptable to consumers or for industrial use as a food ingredient (Choe and Min 2006; Guillen and Goicoechea 2009). The properties of a particular oil are determined mainly by the abundance of different TAG molecular species (Yoshida et al. 2001). In addition, a complex pattern of thermolytic and oxidative reactions with the formation of some decomposition products, occurs during heating of oil. These reactions lead to the changes in physical and chemical properties of oil (Ali et al. 2016b; Vaidya and Choe 2011). Monitoring the changes in properties of oils during heating is an effective method to assess thermal oxidation changes in the oils. The effects of heating on the oxidative stability and composition of roasted pumpkin seed oil (Ali et al. 2016b) and roasted mustard seed oil (Vaidya and Choe 2011) were investigated. To date, some studies have been conducted on the oxidative deterioration and chemical composition of microwave roasted GSO without applying any thermal treatment of oil (Raigar et al. 2017; Smith et al. 2014; Jittrepotch et al. 2010). However, so far no research has been reported on how heat treatment affects oxidative stability, fatty acid composition, and triacylglycerol species of microwave roasted groundnut seed oil, which are the main objectives in the present study.
Materials and methods
Freshly harvested and dried groundnuts (2 kg, Arachis hypogaea L.) were obtained from Oilseeds Research Centre of BARI, Gazipur, Dhaka. The total weight of the 30 groundnut seeds was 16.09 g, and length and diameter of each seed were in average 1.35 and 0.83 cm, respectively. The matured and healthy nuts were selected and stored at 4 °C in a sealed plastic bag. The various chemicals and reagents used were of analytical grade or better. Thiobarbituric acid was procured from HiMedia Laboratories (Mumbai, India), and acetic acid and standards were from Sigma-Aldrich Co. (St. Louis, MO, USA). All other chemicals and solvents were from Merck (Darmstadt, Germany or Mumbai, India) unless otherwise stated.
Roasting and extraction protocol
A domestic size microwave oven (MS 3042G, LG, China) at 2450 MHz with power output of 340 W was used. Whole groundnuts (40 g) were placed in single layer in a Pyrex petri dish (12-cm diameter) and roasted by microwave for 2.5, 5, or 7.5 min after covering the dish based on trial results. The roasting treatment was carefully optimized in such a way that it resulted in optimum roasting without burning. After roasting, the nuts were allowed to cool to ambient temperature and were manually shelled to obtain seeds. The raw or roasted seeds were reduced to fine powder and moisture contents were determined by AOAC (2005) official method 930.15. The powder was mixed with n-hexane, at a sample to solvent ratio of 1:10 w/v. The mixture was homogenized with a homogenizer (Polytron PT 2100, Switzerland) at a speed of 15,000 rpm for 20 min, and filtered through a Whatman No. 4 filter paper using a Buchner funnel. The residue was re-extracted twice with the same solvent. The filtrates were combined and dried in a rotary vacuum evaporator at 45 °C. The oil thus obtained was weighed and stored into capped glass bottles at a temperature below −16 °C for further studies.
Thermal oxidation of samples
The roasted or unroasted oil samples (80 g) were taken into 100 ml beakers and placed in an electric oven at 170 °C, in order to accelerate the lipid oxidation and thermal degradation. Oil samples were withdrawn at intervals of 0, 3, 6 and 9 h for analyses.
Oxidative indices
American Oil Chemists’ Society official methods (AOCS 1987) were employed for determining free fatty acid content (method Ca 5a-40), peroxide value (method Cd 8-53) and thiobarbituric acid value (method Cd 19-90). Specific extinctions (method p2.15) at 233 and 269 nm (E1%233 and E1%269) and p-anisidine value (method p2.4) of the samples were measured using a spectrophotometer (T 60, PG Instruments, Leicestershire, UK) according to PORIM (1995) test methods. Total oxidation or TOTOX value was calculated as: TOTOX = 2PV + p-AV (Wan et al. 2009).
Colour development
As an index of colour development (Yoshida et al. 1999), the absorbance at 420 nm of 5.0% (w/v) solutions of oils in chloroform was measured by a spectrophotometer (T 60U, PG Instruments, Leicestershire, UK).
Fatty acid composition (FAC)
Fatty acids of the oil samples were transesterified to their corresponding methyl esters following PORIM (1995) test method p3.4 prior to analysis by gas chromatography. The FAC was evaluated by the rapid method of AOCS (1987) Cd 14c-94 using a gas chromatography (7890A, Agilent Technologies, USA) furnished with a polar SP™ (Supelco, Bellefonte, PA) capillary column (0.25 mm i.d. × 60 m × 0.2 µm) and a flame ionization detector. The carrier gas was nitrogen at 70 psi. The oven temperature was set as follows: initial temperature of 100 °C, and programmed to increase to 250 °C at 10 °C/min. Methyl esters were quantified by comparing the retention times and peak area of the unknowns with known FAME standard (Supelco, Bellefonte, PA).
Triacylglycerol (TAG) composition
The TAGs were analyzed by injecting 1 μl sample solution (40 μl sample dissolved in 1960 μl of 75:25, v/v of chloroform: acetone solution) through a Rheodyne valve into a HPLC system (Agilent 1260 Infinity, USA). The TAG was separated using packed Poroshell 120 EC-C18 (Agilent, USA) column (50 mm × 4.6 mm i.d. × 2.7 μm) using acetone/acetonitrile (65:35, v/v) mobile phase at a flow rate of 1 ml/min. Detection of the TAG was done by Evaporative Light Scattering Detector (ELSD). Identification of TAG was performed by comparing with the standards (Sigma-Aldrich Co., St. Louis, MO, USA).
FT-IR spectroscopy
The infrared spectra were measured with the help of a Fourier Transform Spectroscopy (IRAffinity-1S, Shimadzu Corporation, Kyoto, Japan) equipped with a high sensitivity pyroelectric detector (deuterated l-alanine doped triglycine sulphate) and connected to software of the lab solutions DBIR operating system. Samples were applied to a sodium chloride cell and periodical scans (15 scans, 4 cm−1 resolution) were obtained in the spectral range of 850–4000 cm−1. The spectra corrected against the background spectrum of air, were recorded as absorbance values at each data point.
Statistical analysis
All data were expressed as the mean and standard deviation (SD) and were subjected to one way analysis of variance (ANOVA). Mean values were compared at p < 0.05 significant level by Duncan’s multiple range test using IBM SPSS 22 statistics.
Results and discussion
Moisture content of groundnut seed was 5.20% (DM) and decreased to 4.10, 3.40 and 3.10% (DM) with increasing roasting times 2.5, 5 and 7.5 min respectively. The crude oil levels were in the range of 39.89% (DM) for unroasted sample to 42.31% (DM) for 7.5 min roasted one. The yield of crude oil increased with increasing roasting times. Wroniak et al. (2016) also reported that the oil content in rapeseed increased as a result of seed roasting process.
Oxidative indices
The accumulation of free fatty acid (FFA) in GSO was increased with increasing roasting and heating times (Fig. 1a). The increase in FFA content for unroasted sample was higher than that of the roasted samples throughout the heating period. A similar trend was followed by Kim et al. (2014) during storage of microwave treated rice bran. At the end of 9 h heating, the amount of FFA was highest (p < 0.05) in unroasted sample (6.72%) compared to 7.5 min roasted one (5.33%). The unroasted samples had significantly (p < 0.05) higher peroxide value (PV) than that of roasted samples at all corresponding heating times, indicating a higher extent of hydroperoxides in unroasted ones (Fig. 1b). The PV increased up to the 6 h for all samples and then decreased until the end of the heating. A similar trend was followed by Ali et al. (2016b) for pumpkin seed oil during thermal oxidation.
Fig. 1.
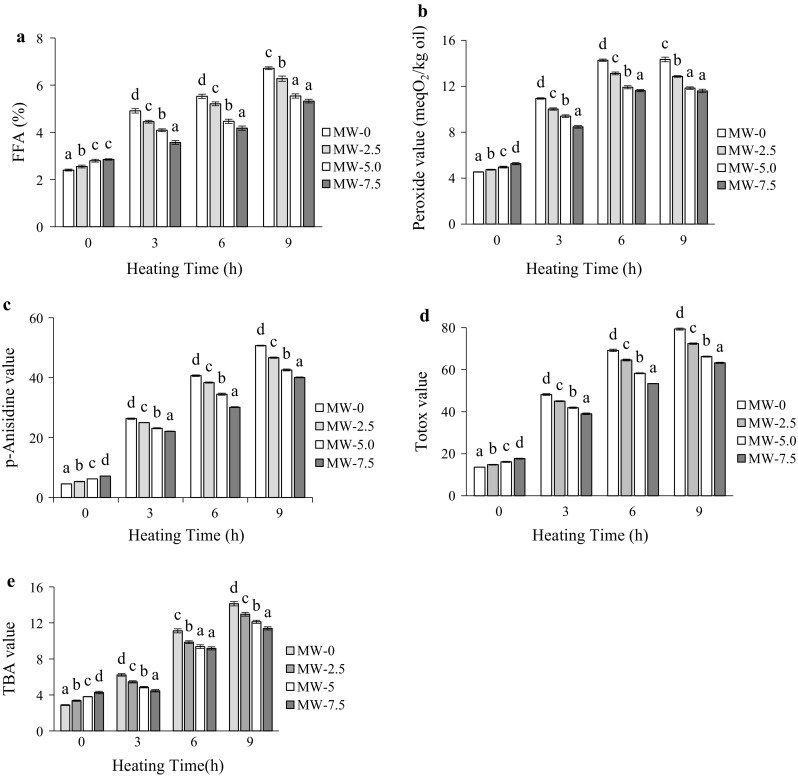
Change in chemical characteristics of unroasted (MW-0) and roasted (MW-2.5, roasted at 2.5 min; MW-5, roasted at 5 min and MW-7.5, roasted at 7.5 min) GSO during heating at 170 °C. a FFA, b peroxide value, c p-anisidine value, d TOTOX value, and e TBA value. Each value is the mean ± standard deviation of triplicate determinations. Values in each heating grouping with different letters on bar, are significantly different (p < 0.05)
The p-Anisidine value (p-AV) in GSO increased with heating time and was found to be significantly higher (p < 0.05) in unroasted samples at each heating period (Fig. 1c). Moreover, this increment was high at earlier phase and low at later phase of heating. At the end of 9 h heating, the p-AVs reached to 50.70, 46.68, 42.56, and 40.08 for 0, 2.5, 5, and 7.5 min roasted samples, respectively. Seed roasting slowed down the rate of formation of secondary oxidation products as reflected by p-AV in GSO during heating at elevated temperature. Cai et al. (2013) also confirmed that the oil extracted from roasted pine nut contain lower secondary oxidation products as compared to the oil from unroasted nut. Figure 1d shows that TOTOX values in roasted and unroasted oil samples increased with prolonged oxidation time. Significant differences (p < 0.05) in TOTOX values in groundnut samples were found after 9 h of oxidation test. The TOTOX values, from lowest to highest, were displayed in samples roasted for 7.5, 5, 2.5, and 0 min. The lower TOTOX values of the roasted samples indicated more stable to oxidative rancidity than the control. Generally, thiobarbituric acid (TBA) content showed significant increasing trends during the thermal treatment for all samples, but no regular pattern of increase was observed (Fig. 1e). A sharp increase in TBA values was noticed up to 6 h heating, followed by the rate of increment decreases. At the end of 9 h heating, unroasted sample exhibited the highest TBA (14.13), while roasted sample exhibited the least (11.39). This might be due to the volatilization of secondary oxidation products or their further breakdown. The increasing trend in oxidation of unroasted oil samples, as illustrated in TBA values, compared with roasted ones, was similar to that obtained for oxidation products as reflected in PV and p-AV values.
Roasting had a remarkable effect on ultraviolet absorptions at 233 (E1%233) and 269 nm (E1%269) in all samples (Fig. 2a, b). Significant differences (p < 0.05) in ultraviolet absorptions in GSOs were observed after 9 h of oxidation test. The levels of conjugated dienes and trienes were however highest in unroasted samples, with lowest level detected in 7.5 min roasted samples at all heating periods. These lower levels are the indications of good oxidative stability of roasted samples compared to unroasted ones. Color formation in the oil was influenced by the extent of roasting and heating conditions employed (Fig. 2c). With an increase in roasting and heating times, browning substances were developed, resulting in a significant (p < 0.05) increase of the absorbance at 420 nm. The Maillard reactions lead to the formation of browning substances in several thermally processed foods (Koehler and Odell 1970). The absorbance values for unroasted and 7.5 min roasted seed oils, increased from 0.45 and 0.52 to 0.88 and 0.67, respectively, after 9 h of heating. Longer seed roasting times had a much higher effect on further improvements in oxidative stability. Thus, the present results lend support to earlier finding, which indicated an increase in the colour of oils with increasing roasting temperature of cashew nut (Chandrasekara and Shahidi 2011).
Fig. 2.
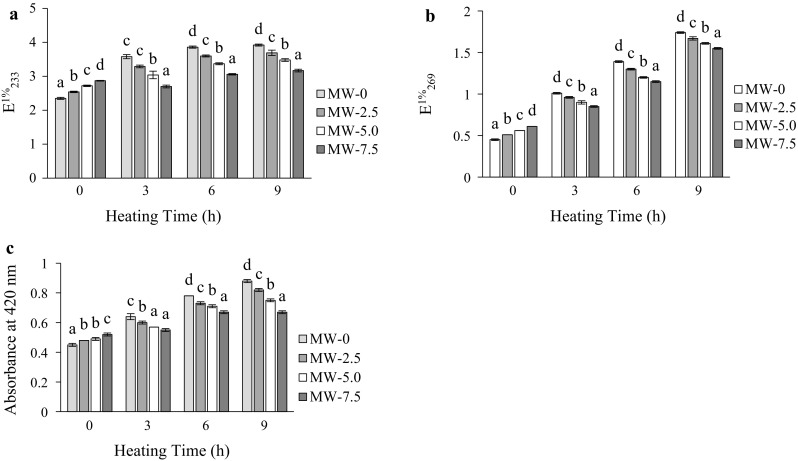
Change in specific extinctions at 232 (a) and 269 nm (b), and in colour (absorbance at 420 nm) (c), of unroasted (MW-0) and roasted (MW-2.5, roasted at 2.5 min; MW-5, roasted at 5 min and MW-7.5, roasted at 7.5 min) GSO during heating at 170 °C. Each value is the mean ± standard deviation of triplicate determinations. Values in each heating grouping with different letters on bar, are significantly different (p < 0.05)
Fatty acid composition (FAC)
Changes in the FAC of the roasted and unroasted GSOs before heating are typified in Table 1. The primary fatty acids of fresh GSO were C18:1 (42.58%), C18:2 (34.69%), and C16:0 (13.53%) acids while C18:0, C18:3, C20:0, C22:0, and C24:0 present in concentrations < 4%. These three major fatty acids comprised 90.80% of the total fatty acids in GSO, which almost same to previously reported data (Siddeeg and Xia 2015). There was almost no change in FAC of GSO when prepared by microwave roasting until 7.5 min. Vaidya and Choe (2011) reported that the FAC did not change with roasting in mustard seed oil. As can be seen in Table 2, total SFA, MUFA and PUFA were: 22.24, 42.58 and 35.18%, respectively for raw and 22.73, 42.28 and 34.97% respectively for 7.5 min roasted samples before thermal treatment. During heating at 170 °C the percentage of PUFA tended to decrease, whereas the percentage of SFA increased in all samples, probably due to PUFA degradation. A similar trend was found by Ali et al. (2013) during thermooxidative degradation of canola oil. In unroasted samples, relative content of PUFA decreased to 89.53%, while of SFA increased to 117.46% after 9 h of heating. On the other hand, at the end of 9 h heating, the relative contents of PUFA decreased to 92.05%, while of SFA increased to 105.76% in 7.5 min roasted samples. However, the change in relative contents of PUFA or SFA was high in unroasted samples compared to that in roasted samples throughout the heating period. These results are in accordance with those reported for pumpkin seed (Ali et al. 2016b) and mustard seed (Vaidya and Choe 2011) oils during heating. Moreover, the ratio of polyunsaturated to saturated fatty acids (P/S) as reflected a valid indicator of evaluating oil oxidation (Lee et al. 2007), of all samples declined with increasing thermal oxidation time. The smallest change (decrease) in P/S ratio belonged to the roasted samples. This means that oxidation process progressed more rapidly in unroasted samples as compared to roasted ones during heating.
Table 1.
Fatty acid composition (%) of unroasted and roasted groundnut seed oils before heating
| Fatty acids | Roasting time (min) | |||
|---|---|---|---|---|
| 0 | 2.5 | 5.0 | 7.5 | |
| C16:0 | 13.53 ± 0.02 | 13.58 ± 0.08 | 13.79 ± 0.11 | 13.38 ± 0.01 |
| C18:0 | 3.36 ± 0.02 | 3.53 ± 0.01 | 3.58 ± 0.01 | 3.68 ± 0.001 |
| C18:1 | 42.58 ± 0.07 | 41.99 ± 0.02 | 42.37 ± 0.04 | 42.28 ± 0.04 |
| C18:2 | 34.69 ± 0.06 | 34.53 ± 0.06 | 34.29 ± 0.05 | 34.45 ± 0.02 |
| C18:3 | 0.49 ± 0.00 | 0.56 ± 0.00 | 0.52 ± 0.01 | 0.52 ± 0.01 |
| C20:0 | 1.01 ± 0.05 | 1.20 ± 0.01 | 1.14 ± 0.03 | 1.19 ± 0.003 |
| C22:0 | 1.71 ± 0.03 | 2.06 ± 0.03 | 1.93 ± 0.03 | 2.00 ± 0.01 |
| C24:0 | 2.56 ± 0.05 | 2.57 ± 0.01 | 2.38 ± 0.06 | 2.49 ± 0.02 |
| ∑Saturated fatty acids | 22.24 | 22.93 | 22.82 | 22.73 |
| ∑Monounsaturated fatty acids | 42.58 | 41.99 | 42.37 | 42.28 |
| ∑Polyunsaturated fatty acids | 35.18 | 35.09 | 34.80 | 34.97 |
Each value is the mean ± standard deviation of triplicate determinations
Table 2.
Changes in saturated, monounsaturated and polyunsaturated fatty acid composition of groundnut seed oils during heating
| Roasting time (min) | Heating time (h) | Fatty acid composition (%) | |||
|---|---|---|---|---|---|
| Saturated fatty acids | Monounsaturated fatty acids | Polyunsaturated fatty acids | P/S | ||
| 0 | 0 | 22.24 (100.00) | 42.58 (100.00) | 35.18 (100.00) | 1.58 |
| 3 | 23.06 (103.68) | 42.38 (99.54) | 34.56 (98.23) | 1.49 | |
| 6 | 25.53 (114.79) | 42.12 (98.93) | 32.35 (91.95) | 1.26 | |
| 9 | 26.12 (117.46) | 42.38 (99.53) | 31.50 (89.53) | 1.20 | |
| 2.5 | 0 | 22.93 (100.00) | 41.99 (100.00) | 35.09 (100.00) | 1.53 |
| 3 | 22.20 (96.82) | 40.01 (95.29) | 34.79 (99.16) | 1.56 | |
| 6 | 23.46 (102.30) | 44.22 (105.33) | 32.32 (92.12) | 1.38 | |
| 9 | 24.19 (105.49) | 44.16 (105.18) | 31.65 (90.20) | 1.31 | |
| 5.0 | 0 | 22.82 (100.00) | 42.37 (100.00) | 34.80 (100.00) | 1.52 |
| 3 | 22.19 (97.21) | 43.19 (101.93) | 34.62 (99.47) | 1.56 | |
| 6 | 24.04 (105.35) | 43.54 (102.74) | 32.42 (93.15) | 1.35 | |
| 9 | 23.98 (105.08) | 43.99 (103.82) | 32.03 (92.04) | 1.33 | |
| 7.5 | 0 | 22.73 (100.00) | 42.28 (100.00) | 34.97 (100.00) | 1.54 |
| 3 | 22.82 (100.37) | 42.34 (100.14) | 34.84 (99.63) | 1.52 | |
| 6 | 23.51 (103.42) | 43.55 (103.01) | 32.94 (94.19) | 1.40 | |
| 9 | 24.04 (105.76) | 43.78 (103.54) | 32.19 (92.05) | 1.34 | |
Number in parenthesis is relative % of saturated, monounsaturated and polyunsaturated fatty acids based on the initial saturated, monounsaturated and polyunsaturated fatty acids content before heating. P/S-ratio of polyunsaturated to saturated fatty acids. Each value is the mean of triplicate determinations
Triacylglycerol (TAG) composition
The typical changing patterns in the TAG species (P, palmitic; M, myristic; O, oleic; L, linoleic) from GSO evaluated by HPLC are shown in Table 3. Major TAG species were OOL (25.65%), OLL (23.86%), POL (19.59%), MPP (9.31%), OOO (9.21%) and PLL-MOL (5.17%). The other species PLP, SOO, POO, and POS were minor components (< 2%). In this study, microwave roasting did not affect significantly on the TAG composition, as only a small amount of species containing more than four double bonds was present in TAGs. Yoshida et al. (2003) also demonstrated no significant loss in TGA species of peanut by microwave roasting. However, The OLL, PLL-MOL, and OOL levels in GSO were decreased whilst the MPP and OOO levels increased with increasing heating time. At the end of 9 h heating, the amount of OLL, OOL, and PLL-MOL was significantly (p < 0.05) higher and that of OOO significantly (p < 0.05) lower in 7.5 min roasted samples compared to unroasted ones. The changes in POL and MPP were found to be insignificant after 9 h of heating. Of the above species, most significant reduction was detected in OLL; the amounts of it in unroasted and 7.5 min roasted samples decreased from 23.86 to 16.88% and from 23.23 to 18.30%, respectively, by 9 h heating at 170 °C. The above results indicated the changes in TAG species became more pronounced in unroasted samples compared to roasted ones. These results would depend on differences in the amounts of TAGs composed of oleic and linoleic acids (Yoshida and Takagi 1999). The obtained results concur with previously published data (with few exception) for pumpkin seed oil (Ali et al. 2016b). In this study, the higher stability of the TAG species in the roasted seed oil, as compared to the unroasted seed oil could have contributed to reduced oxidation in the roasted seed oil versus the unroasted seed oil.
Table 3.
Change in major triacylglycerol species in groundnut seed oils during heating
| Heating time (h) | Roasting time (min) | TAG composition (%) | |||||
|---|---|---|---|---|---|---|---|
| OLL | OOL | POL | PLL-MOL | MPP | OOO | ||
| 0 | 0 | 23.86 ± 0.04b | 25.65 ± 0.13a | 19.59 ± 0.20a | 5.17 ± 0.08b | 9.31 ± 0.10a | 9.21 ± 0.15a |
| 2.5 | 23.44 ± 0.42ab | 25.94 ± 0.33a | 19.48 ± 0.11a | 4.96 ± 0.01a | 9.52 ± 0.23ab | 9.47 ± 0.20a | |
| 5.0 | 23.28 ± 0.11ab | 25.85 ± 0.13a | 19.65 ± 0.22a | 5.03 ± 0.01a | 9.86 ± 0.14b | 9.38 ± 0.08a | |
| 7.5 | 23.23 ± 0.21a | 25.76 ± 0.16a | 19.53 ± 0.26a | 5.03 ± 0.04a | 9.60 ± 0.17ab | 9.64 ± 0.28a | |
| 3 | 0 | 21.19 ± 1.18a | 24.72 ± 0.93a | 19.19 ± 0.09a | 4.89 ± 0.15a | 10.73 ± 0.35c | 10.19 ± 0.33b |
| 2.5 | 22.22 ± 0.13ab | 25.73 ± 0.05a | 19.55 ± 0.15ab | 4.91 ± 0.02a | 10.15 ± 0.08b | 9.55 ± 0.31a | |
| 5.0 | 22.47 ± 0.10ab | 25.55 ± 0.06a | 19.64 ± 0.27b | 5.05 ± 0.09ab | 9.96 ± 0.08ab | 9.69 ± 0.14ab | |
| 7.5 | 22.75 ± 0.10b | 25.60 ± 0.11a | 19.78 ± 0.16b | 5.21 ± 0.04b | 9.64 ± 0.08a | 9.59 ± 0.05a | |
| 6 | 0 | 18.33 ± 0.18a | 23.22 ± 0.14a | 19.50 ± 0.16a | 4.83 ± 0.05ab | 11.39 ± 0.22b | 11.41 ± 0.21c |
| 2.5 | 18.49 ± 0.16ab | 23.12 ± 0.12a | 19.11 ± 0.19ab | 4.89 ± 0.04b | 11.14 ± 0.01ab | 11.00 ± 0.06ab | |
| 5.0 | 18.81 ± 0.10b | 23.54 ± 0.53a | 19.01 ± 0.04a | 4.84 ± 0.07ab | 11.43 ± 0.14b | 11.16 ± 0.11bc | |
| 7.5 | 19.23 ± 0.12c | 23.68 ± 0.10a | 18.71 ± 0.32a | 4.75 ± 0.06a | 10.91 ± 0.22a | 10.78 ± 0.18a | |
| 9 | 0 | 16.88 ± 0.90a | 22.72 ± 0.50a | 18.71 ± 0.76a | 4.54 ± 0.15a | 11.66 ± 0.41a | 12.82 ± 1.08b |
| 2.5 | 17.45 ± 0.36ab | 23.43 ± 0.44ab | 18.99 ± 0.71a | 4.75 ± 0.07b | 11.96 ± 0.07a | 11.59 ± 0.23ab | |
| 5.0 | 18.09 ± 0.10b | 23.39 ± 0.17ab | 19.26 ± 0.06a | 4.76 ± 0.02b | 11.78 ± 0.18a | 11.47 ± 0.19ab | |
| 7.5 | 18.30 ± 0.01b | 23.63 ± 0.06b | 19.26 ± 0.41a | 4.83 ± 0.01b | 11.61 ± 0.16a | 11.21 ± 0.22a | |
Each value in the table represents the mean of triplicate determinations ± SD; values within a column with the same letters are not significantly different at p < 0.05
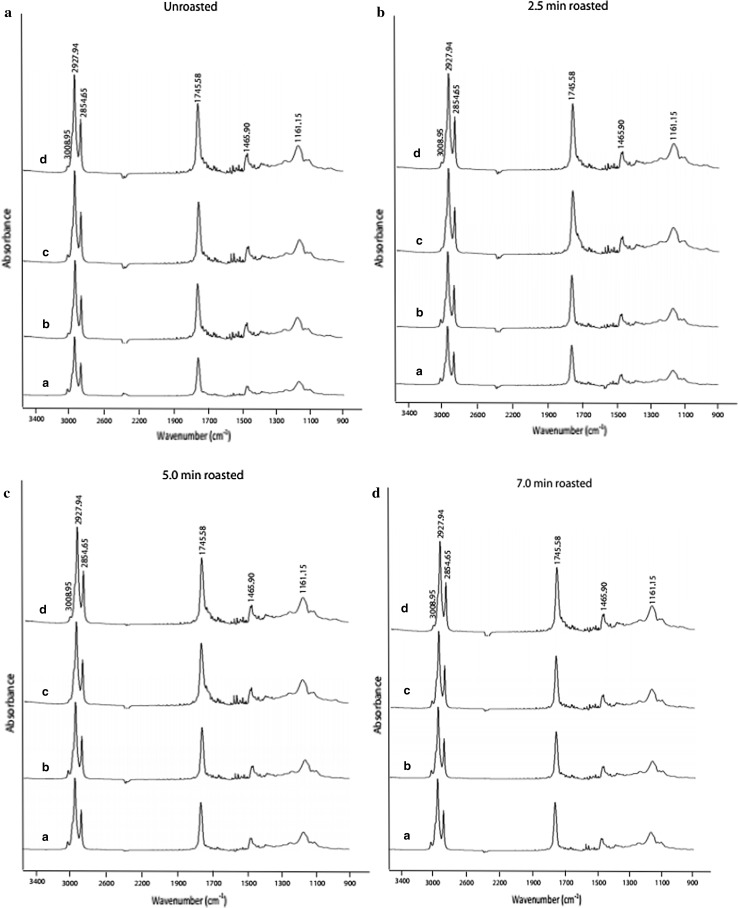
Evaluation by FT-IR
The significant information on the oxidative status of oils can be obtained by studying the frequency and absorbance values of several bands of infrared spectra. An increase or decrease in some of the wave number regions was observed in this study. However, only the regions which were generated for the certain oil oxidation products, were evaluated. Figure 3 shows most significant bands (Lerma-Garcia et al. 2010; Guillen and Cabo 1997): band near 3008 cm−1 due to the cis double-bond stretching vibration; bands near 2927 and 2854 cm−1 due to the asymmetric and symmetric stretching vibration of the aliphatic CH2 functional group; band at 1745 cm−1 due to the ester carbonyl functional group of the triglycerides; band near 1465 cm−1 due to the bending vibrations of CH2 and CH3 aliphatic groups; band near 1377 cm−1 due to bending vibrations of CH2 groups; band near 1161 cm−1 associated with the stretching vibration of the C–O ester groups.
Fig. 3.
Changes of FT-IR spectra of GSO extracted from unroasted and roasted groundnut seeds during heating. a 0 h heating, b 3 h heating, c 6 h heating, d 9 h heating
The intensity (absorbances) near 3008 cm−1 (shoulder) remained almost unaltered or suffers a very slow shifting toward smaller values during oxidative stress. The bands 2927 and 2854 cm−1 experienced a clear increase in absorbance that indicate some chemical changes due to advanced state of oxidation of samples (Valdés et al. 2015). The prominent peak at 1745 cm−1 corresponds to the carbonylic compounds generated from the hydroperoxide decompositions during heating (Mariod et al. 2012); the intensity of it tended to increase with oxidative treatment. Valdés et al. (2015) also observed the increments in absorbance intensity at the bands near 2927, 2854, and 1745 cm−1 during accelerated oxidation of blanched, roasted or fried almonds. The band near 1161 cm−1 suffers changes during the oxidation process and increase its intensity. Liang et al. (2013) observed a similar trend at band 1163 cm−1 in walnut oils during heating. The peak intensities (absorbencies) of unroasted samples were markedly changed in comparison with roasted samples indicating a clear effect of heating on oxidative state of oil. During heating, the absorbance values (data not shown) of all peaks increased (with exception at 3008 cm−1) and these increments were higher in unroasted oils, indicating the oxidation proceeded more rapidly in the unroasted samples compared to roasted ones.
Conclusion
The observed changes in oxidative indices revealed that the oil samples from roasted seed were more resistant to the formation of lipid oxidation products compared to unroasted ones. The exposure of groundnut seeds to microwaves caused no significant loss or change in the content of fatty acids or TAG species in seed oils. During heating at 170 °C, both roasted and unroasted seed oils become oxidized, with degradation of PUFA and formation of some undesirable and harmful compounds. Thus, the markedly slower rate of degradation of PUFA in roasted samples during heating probably provided protection against lipid oxidation. TGA and FTIR data also indicate a higher extent of oxidation in unroasted samples. The present results indicated that microwave roasting enhanced the oxidative stability of oil and significantly modify the composition of oil.
References
- Ali MA, Nouruddeen ZB, Muhamad II, Latip RA, Othman NH, Mahmood NAN. Impact of palm olein addition on the thermooxidative degradation of canola oil during frying. Chiang Mai J Sci. 2013;40:643–655. [Google Scholar]
- Ali A, Islam A, Pal Tarun K. The effect of microwave roasting on the antioxidant properties of the Bangladeshi groundnut cultivar. Acta Sci Pol Technol Aliment. 2016;15:429–438. doi: 10.17306/J.AFS.2016.4.41. [DOI] [PubMed] [Google Scholar]
- Ali MA, Nargis A, Othman NH, Noor AF, Sadik G, Hossen J. Oxidation stability and compositional characteristics of oils from microwave roasted pumpkin seeds during thermal oxidation. Int J Food Prop. 2016 [Google Scholar]
- AOAC (2005) Official methods of analysis of AOAC international, Association of Official Agricultural Chemists, AOAC International, VA, USA
- AOCS . Official methods and recommended practices of the American Oil Chemists’ Society. 4. Champaign: AOCS press; 1987. [Google Scholar]
- Arya SS, Salve AR, Chauhan S. Peanuts as functional food: a review. J Food Sci Technol. 2016;53:31–41. doi: 10.1007/s13197-015-2007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti IA, Ashraf S, Shahid M, Asi MR, Mehboob S. Quality index of oils extracted from g-irradiated peanuts (Arachis hypogaea L.) of the golden and bari varieties. Appl Radiat Isot. 2010;68:2197–2201. doi: 10.1016/j.apradiso.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Cai L, Cao A, Aisikaer G, Ying T. Influence of kernel roasting on bioactive components and oxidative stability of pine nut oil. Eur J Lipid Sci Technol. 2013;115:556–563. doi: 10.1002/ejlt.201200337. [DOI] [Google Scholar]
- Chandrasekara N, Shahidi F. Oxidative stability of cashew oils from raw and roasted nuts. J Am Oil Chem Soc. 2011;88:1197–1202. doi: 10.1007/s11746-011-1782-3. [DOI] [Google Scholar]
- Choe E, Min DB. Mechanisms and factors for edible oil oxidation. Compr Rev Food Sci F. 2006;5:169–186. doi: 10.1111/j.1541-4337.2006.00009.x. [DOI] [Google Scholar]
- Craft BD, Kosińska A, Amarowicz R, Pegg RB. Antioxidant properties of extracts obtained from raw, dry-roasted, and oil-roasted US peanuts of commercial importance. Plant Food Hum Nutr. 2010;65:311–318. doi: 10.1007/s11130-010-0160-x. [DOI] [PubMed] [Google Scholar]
- El Anany AM. Nutritional composition, antinutritional factors, bioactive compounds and antioxidant activity of guava seeds (Psidium myrtaceae) as affected by roasting processes. J Food Sci Technol. 2015;52:2175–2183. doi: 10.1007/s13197-013-1242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillen MD, Cabo N. Characterization of edible oils and lard by Fourier transform infrared spectroscopy. Relationships between composition and frequency of concrete bands in the fingerprint region. J Am Oil Chem Soc. 1997;74:1281–1286. doi: 10.1007/s11746-997-0058-4. [DOI] [Google Scholar]
- Guillen MD, Goicoechea E. Oxidation of corn oil at room temperature: Primary and secondary oxidation products and determination of their concentration in the oil liquid matrix from 1H nuclear magnetic resonance data. Food Chem. 2009;116:183–192. doi: 10.1016/j.foodchem.2009.02.029. [DOI] [Google Scholar]
- Jittrepotch N, Kongbangkerd T, Rojsuntornkitti K. Influence of microwave irradiation on lipid oxidation and acceptance in peanut (Arachis hypogaea L.) seeds. Int Food Res J. 2010;17:173–179. [Google Scholar]
- Kim SM, Chung HJ, Lim ST. Effect of various heat treatments on rancidity and some bioactive compounds of rice bran. J Cereal Sci. 2014;60:243–248. doi: 10.1016/j.jcs.2014.04.001. [DOI] [Google Scholar]
- Koehler PE, Odell GV. Factors affecting the formation of pyrazine compounds in sugar-amine reaction. J Agric Food Chem. 1970;18:895–898. doi: 10.1021/jf60171a041. [DOI] [Google Scholar]
- Lee J, Kim M, Choe E. Antioxidant activity of lignan compounds extracted from roasted sesame oil on the oxidation of sunflower oil. Food Sci Biotechnol. 2007;16:981–987. [Google Scholar]
- Lerma-Garcia MJ, Ramis-Ramos G, Herrero-Martinez JM, Simo-Alfonso EF. Authentication of extra virgin olive oils by Fourier transform infrared spectroscopy. Food Chem. 2010;118:78–83. doi: 10.1016/j.foodchem.2009.04.092. [DOI] [Google Scholar]
- Liang P, Chen C, Zhao S, Ge F, Liu D, Liu B, Fan Q, Han B, Xiong X. Application of fourier transform infrared spectroscopy for the oxidation and peroxide value evaluation in virgin walnut oil. J Spectrosc. 2013;2013:1–5. [Google Scholar]
- Mariod AA, Ahmed SY, Abdelwahab SI, Cheng SF, Eltom AM, Yagoub SO, Gouk SW. Effects of roasting and boiling on the chemical composition, amino acids and oil stability of safflower seeds. Int J Food Sci Technol. 2012;47:1737–1743. doi: 10.1111/j.1365-2621.2012.03028.x. [DOI] [Google Scholar]
- Nederal S, Skevin D, Kraljic K, Obranovic M, Papesa S, Bataljaku A. Chemical composition and oxidative stability of roasted and cold pressed pumpkin seed oils. J Am Oil Chem Soc. 2012;89:1763–1770. doi: 10.1007/s11746-012-2076-0. [DOI] [Google Scholar]
- PORIM . PORIM test methods. Bandar Baru Bangi: Palm Oil Research Institute of Malaysia; 1995. [Google Scholar]
- Raigar RK, Upadhyay R, Mishra HN. Optimization of microwave roasting of peanuts and evaluation of its physicochemical and sensory attributes. J Food Sci Technol. 2017;54:2145–2155. doi: 10.1007/s13197-017-2654-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddeeg A, Xia W. Oxidative stability, chemical composition and organoleptic properties of seinat (Cucumis melo var. tibish) seed oil blends with peanut oil from China. J Food Sci Technol. 2015;52:8172–8179. doi: 10.1007/s13197-015-1889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AL, Perry JJ, Marshall JA, Yousef AE, Barringer SA. Oven, microwave, and combination roasting of peanuts: comparison of inactivation of salmonella surrogate Enterococcus faecium, color, volatiles, flavor, and lipid oxidation. J Food Sci. 2014;79:S1584–S1594. doi: 10.1111/1750-3841.12528. [DOI] [PubMed] [Google Scholar]
- Vaidya B, Choe E. Effects of seed roasting on tocopherols, carotenoids, and oxidation in mustard seed oil during heating. J Am Oil Chem Soc. 2011;88:83–90. doi: 10.1007/s11746-010-1656-0. [DOI] [Google Scholar]
- Valdés A, Beltrán A, Karabagias I, Badeka A, Kontominas MG, Garrigós MC. Monitoring the oxidative stability and volatiles in blanched, roasted and fried almonds under normal and accelerated storage conditions by DSC, thermogravimetric analysis and ATR-FTIR. Eur J Lipid Sci Technol. 2015;117:1199–1213. doi: 10.1002/ejlt.201400384. [DOI] [Google Scholar]
- Wan TW, Bahruddin S, Boey PL. Determination of TOTOX value in palm oleins using a FI-potentiometric analyzer. Food Chem. 2009;113:285–290. doi: 10.1016/j.foodchem.2008.06.082. [DOI] [Google Scholar]
- Wroniak M, Rekas A, Siger A, Janowicz M. Microwave pretreatment effects on the changes in seeds microstructure, chemical composition and oxidative stability of rapeseed. LWT Food Sci Technol. 2016;68:634–641. doi: 10.1016/j.lwt.2016.01.013. [DOI] [Google Scholar]
- Yoshida H, Takagi S. Microwave roasting and molecular species of triacylglycerols in soybean embryonic axes. J Am Oil Chem Soc. 1999;76:1065–1071. [Google Scholar]
- Yoshida H, Takagi S, Mitsuhashi S. Tocopherol distribution and oxidative stability of oils prepared from the hypocotyl of soybeans roasted in microwave oven. J Am Oil Chem Soc. 1999;6:915–920. doi: 10.1007/s11746-999-0106-3. [DOI] [Google Scholar]
- Yoshida H, Hirakawa Y, Abe S. Roasting influences on molecular species of triacylglycerols in sunflower seeds (Helianthus annuus L.) Food Res Int. 2001;34:613–619. doi: 10.1016/S0963-9969(01)00079-5. [DOI] [Google Scholar]
- Yoshida H, Hirakawa Y, Tomiyama Y, Mizushina Y. Effects of microwave treatment on the oxidative stability of peanut (Arachis hypogaea) oils and the molecular species of their triacylglycerols. Eur J Lipid Sci Technol. 2003;105:351–358. doi: 10.1002/ejlt.200390073. [DOI] [Google Scholar]
- Yu J, Ahmenda M, Goktepe I. Effects of processing methods and extraction solvents on concentration and antioxidant activity of peanut skin phenolics. Food Chem. 2005;90:199–206. doi: 10.1016/j.foodchem.2004.03.048. [DOI] [Google Scholar]