Abstract
This is a first kind of study on genotype diversity of starches of Moth Bean an underutilized pulse of India. Physicochemical properties like amylose content (7.8–21.4%), swelling power (11–13.5 g/g), solubility (5.9–9.0%) of starches were observed to differ significantly among the six moth bean starches. Swelling power of all the moth bean starches was observed to increase in the temperature range of 55–95 °C. Scanning electron microscopy indicated polyhedral, irregular shape of granule. X-ray diffraction studies indicated a ‘C’ type crystalline structure and the starches differed significantly in relative crystallinity (17–34%) which affected significantly retro gradation tendencies of the starches. Peak viscosity of starches varied significantly and ranged between 4580 and 5087 cP. Resistant starch content of starches also varied significantly among the cultivars and ranged between 57.3 and 75.6%.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2930-z) contains supplementary material, which is available to authorized users.
Keywords: Moth bean starch, Amylose, Pasting, Retrogradation, FTIR, X-ray diffraction, Digestibility
Introduction
India is the worlds’ largest producer and consumer of pulses and of moth bean. It produces around 22–25% of the total world produce of pulses (FAO 2010). Legumes are important nutritional source in developing countries. Chickpea, pigeonpea, lentil, urdbean, mungbean, lablab bean, moth bean, horse gram, pea grass pea, cowpea and faba bean are the major pulse crops grown in India. Legumes however, are said to have low protein and starch digestibility (Ghavidel and Prakash 2007) which may serve as source for making resistant starches using physical, chemical and enzymatic methods.
Moth bean is considered to be native crop of India and Pakistan and is grown during the kharif season. In the event of the grim situation of water shortages and rising agricultural input prices Moth bean is an ideal crop to grow since it requires very low inputs (no or little water) and is grown in arid and semi arid regions of South Asia and India like Rajashtan, Maharastara, Madhya Pradesh and some parts of Uttar Pradesh and Punjab (Sathe and Venkatachalam 2007). The production of moth bean was 2.77 lakh tonnes in India during 2012–2015 (http://dpd.dacnet.nic.in). It is also grown in Mynammar, Srilanka, China, Africa and Southern and South-Western USA where it is cultivated mainly in the drier areas for different purposes like feed, fodder and pasture crop. Starch constitutes major component (45–65%) of almost all pulses and affects texture appearance of products made from them (Norton et al. 1985). Starch granules when heated in water swell and then rupture, releasing amylose which gets solubulized in water. Starch gelatinization can be described as a process of swelling of starch granules in water and destruction of molecular organization and crystallinity loss (Noda et al. 2004). On storage of starch gel retrogradation occurs and leads to reorganization of starch chains and a gradual change from amorphous state to ordered state (Bao et al. 2007). Starches from most of the legumes seed contain higher amylose content (30–45%) which results into higher gelatinization temperature and retrogradation leading to low digestibility (Guillon and Champ 2002). Due to high retrogradation tendency pulse starches are not digested easily in body leading to low glycemic index (Singh 2017). The pulse starch granules usually exhibit oval and round shapes with indentation. Native starch is semi crystalline and the pulse starches exhibit the C-type polymorph which is considered to be a mixture of A- and B-type polymorphs forms in packing density and structure (Hoover et al. 2010). According to Bogracheva et al. (1998) the centers and periphery of legume starch granules are relatively richer in B- type and A-type polymorphs, respectively and this ratio may depend on legume genotype. Pasting properties of starches are studied using Rapid Visco Analyzer, Brookfields Viscometer, Anton Paar rheometer. Pasting of starches is cooking of the starch dispersion in water using controlled heating and cooling cycles to asses various viscosity and short term retrogradation behaviour. Pasting of starches involves development of viscosity following gelatinization and consists of events like granule swelling, solubulization, rupture, leaching of amylose and bond formation between leached amylose and water.
Starch finds its uses in many food products as texture agent, stabilizer, bulking agent. Pulse starches can be good alternate to cross linked starches in food products as these are observed to posses stability towards heat and shearing actions (Singh 2017). There has been a constant search for finding new sources of starches. To the best of our knowledge there has been no or very less literature on physicochemical, structural, crystalline properties, thermal properties and digestibility of starches isolated from different cultivars of moth bean. This is in fact a first time detailed study on the starch diversity of moth bean. The objective of the present research was to investigate genotype diversity among moth bean cultivars of India.
Materials and methods
Materials
Six notified varieties namely RMO-40, RMO-225, RMO-257, RMO-423, RMO-435 and RMb-25 of Moth Bean seeds were procured from AINRP on Arid Legumes, Agriculture Research Station, SK Rajasthan Agricultural University, Bikaner (Rajasthan).
Starch isolation
Starch from Moth Bean seeds was isolated using wet milling scheme partly adopted from Schoch and Maywald (1968) which consists of dipping 500 g of moth bean seeds in 1 litre of water containing 0.25% sodium hydroxide (w/v) and kept overnight at 4 °C. The steeping solution was then removed and the seeds washed three four times with water until the neutralization occurred. The seeds were then ground using a blender for 2–3 min. The slurry was passed through a 200-mesh screen. The material remaining on the sieve was washed with water to effect starch-protein to pass through the sieve. Grinding and filtering were done on this material again. After rinsing, the material remaining on the sieve was discarded. The filtrate was allowed to sediment for half to one hour. The filtrate was centrifuged at 5000 rpm for 10 min to separate protein from starch. The grey colored lighter protein-rich layer was obtained and removed carefully using a spatula. Centrifugation was again repeated with the solid material remaining in the tubes by adding water. The aim was to achieve a pure white layer of starch. The starch obtained was dried in hot air oven at 45 °C for 24 h.
Amylose content
The method of Williams et al. (1970) was used to determine the amylose content of the isolated moth bean starches. 20 mg of starch was dissolved in 10 ml of KOH (0.5 N) with continuous stirring. It was then transferred to volumetric flask and distilled water was added to make the volume to 100 ml. 10 ml of starch solution was then taken into volumetric flask (50 ml) and 5 ml of 0.1 N HCl was added. 0.5 ml of sublimed iodine reagent was then added. Distilled water was then added upto 50 ml mark. The absorbance of the solution was measured at 625 nm. A control solution was also prepared without starch. Standard curves were also prepared using different mixtures of amylose and amylopectin.
Swelling power and solubility
Swelling power and solubility of moth bean starches were found out using 2% aqueous suspension of the starch by the method of Leach et al. (1959). This measurement was done at five temperatures viz 55, 65, 75, 85 and 95 °C and the graphs were plotted for understanding the trend of change with temperature.
Morphological properties
Scanning electron micrographs of moth bean starches were obtained with a scanning microscope (ZEISS EVO Series Scanning Electron Microscope Model EVO15). An acceleration potential of 15 kV was used during micrography.
X-ray diffraction
X-ray diffractograms of starch granules (exposed to 100% relative humidity for 3 days) were studied by a copper anode X-ray tube using a Rigaku Corporation, Tokyo manufactured Analytical Diffractometer. The diffractometer was operated at 30 mA and 40 kV with a scanning speed of goniometer of 4o/min. The method of Nara and Komiya (1983) was employed to calculate the relative crystallinity of moth bean starches.
FTIR
FT-IR spectra moth bean starches were performed on a Shimadzu FT-IR instrument (Shimadzu corporation, Kyoto, Japan). Potassium bromide (KBr) disc with 1% finely ground starch powder was used for the analysis. Parameters of FTIR spectra were wave number between 400 and 4000 cm−1; resolution of 4 cm−1 and 32 scans for each sample analysis.
Thermal properties
Gelatinization and retro gradation are important parameters influencing usage of starches in food products. Thermal properties of native starches viz transition temperatures and gelatinization and retro gradation enthalpies were obtained using a differential scanning calorimeter, DSC-821e of Mettler Toledo, Switzerland equipped with a thermal analysis data station and with the method reported in Singh et al (2009). The parameters reported are onset temperature (To), peak temperature (Tp), conclusion temperature (Tc), enthalpy of gelatinization (ΔHgel), enthalpy of retrogradation (ΔHret). The percentage retrogradation (%R) was also reported as the ratio of enthalpy of retrogradation to the enthalpy of gelatinization multiplied by 100.
Pasting properties
Rapid Visco Analyser (RVA-4, Newport Scientific, Warriewood, Austrailia) (Newport scientific 1998). was employed to test the pasting properties of moth bean. The method of the analysis was RVA™ Crosslinked and Substituted Method, No. 9, Version 4 as reported in Newport Scientific Manual. Starch (3 g, 14% moisture basis) was weighed itself in the empty aluminum canister provided with RVA machine which was followed by addition of distilled water to make total sample weight of 28 g. A heating and cooling cycle was programmed with the samples being held at 50 °C for 1 min, heating to 95 °C in 3.7 min followed by holding at 95 °C for 2.5 min before cooling to 50 °C in 3.8 min, and then again holding at 50 °C for 2 min. Parameters obtained were : peak viscosity (PV); hot paste viscosity (HPV) (minimum viscosity at 95 °C); final viscosity (FV) (final viscosity at 50 °C); breakdown (BD) (= PV − HPV); set back (SB) (= CPV − HPV); Pasting temperature (PT). All samples were analyzed in triplicate.
Starch digestibility
In vitro starch digestibility of moth bean starches was investigated using the procedure of Englyst et al. (1992). The resistant starch (RS) was nutritional fraction which was undigested after 120 min of enzyme hydrolysis.
Statistical analysis
The data shown here are mean of the triplicate measurements and the standard deviations are less than ± 5%. ANOVA analysis was utilized to study the data statistically and the differences between the means were located with Tukey’s multiple comparison test (p < 0.05).
Results and discussion
Physico-chemical properties
Table 1 highlights the physicochemical properties of starches isolated from six moth bean cultivars. Amylose content of six starches varied widely and ranged between 7.8% (RMO-423) and 21.4% (RMO-225). Liu et al. (2015) and Simsek et al. (2009) showed higher amylose content values in the range of 33.2–33.6 and 35.0–41.1% for field pea and pea starches, respectively. Sandhu and Lim (2008) also showed higher amylose content values in the range 23.6–29.8% for for various legumes’ (black gram, chickpea, mung bean, lentil, field pea and pigeon pea) starches of India, respectively. The difference with other studies may be due to difference in legume type, method of determination and climatic, agronomic conditions, and genetics.
Table 1.
Physiochemical properties and digestibility of moth starches isolated from six bean cultivars
| Sample name | Amylose content (%) | Swelling power (g/g) | Solubility (%) | Loose density (g/ml) | Packed density (g/ml) | Resistant starch content |
|---|---|---|---|---|---|---|
| RMO-423 | 7.8a ± 0.1 | 11.0a ± 1.4 | 5.9a ± 0.8 | 0.784e ± 0.01 | 0.905d ± 0.01 | 61.8b ± 1.3 |
| RMO-257 | 11.8b ± 0.1 | 13.5c ± 0.1 | 7.6c ± 0.1 | 0.744d ± 0.01 | 0.855c ± 0.01 | 75.8d ± 1.1 |
| RMb-25 | 13.5c ± 0.2 | 13.2c ± 0.3 | 9.1d ± 0.1 | 0.740 cd ± 0.02 | 0.868c ± 0.03 | 71.2c ± 1.4 |
| RMO-435 | 13.9c ± 0.1 | 11.2a ± 0.1 | 6.7b ± 0.2 | 0.671a ± 0.01 | 0.782a ± 0.01 | 72.6c ± 0.9 |
| RMO-40 | 16.3d ± 0.2 | 13.1c ± 0.4 | 6.1ab ± 0.8 | 0.725c ± 0.01 | 0.805ab ± 0.01 | 57.3a ± 1.2 |
| RMO-225 | 21.4e ± 0.2 | 12.5b ± 0.1 | 7.2c ± 0.4 | 0.700b ± 0.01 | 0.812b ± 0.01 | 61.5b ± 1.4 |
Results are mean value of determination ± standard deviation
Pulse starches usually exhibit higher amylose content however Forsyth et al. (2002) observed amylose content in the range of 11.6–23.6% for Yam Bean starches which is close to the values in our study.
Loose and packed densities of starches varied between 0.671 g/ml (RMO-435)–0.789 g/ml (RMO-423) and between 0.782 g/ml (RMO-435)–0.905 g/ml (RMO-435). Oladebeye et al. (2009) reported a bulk density value of 0.76 g/g for sweet potato starch. The density measurement depends upon many factors like amylose content, starch chain lengths, granule size, intergranular porosity and packing.
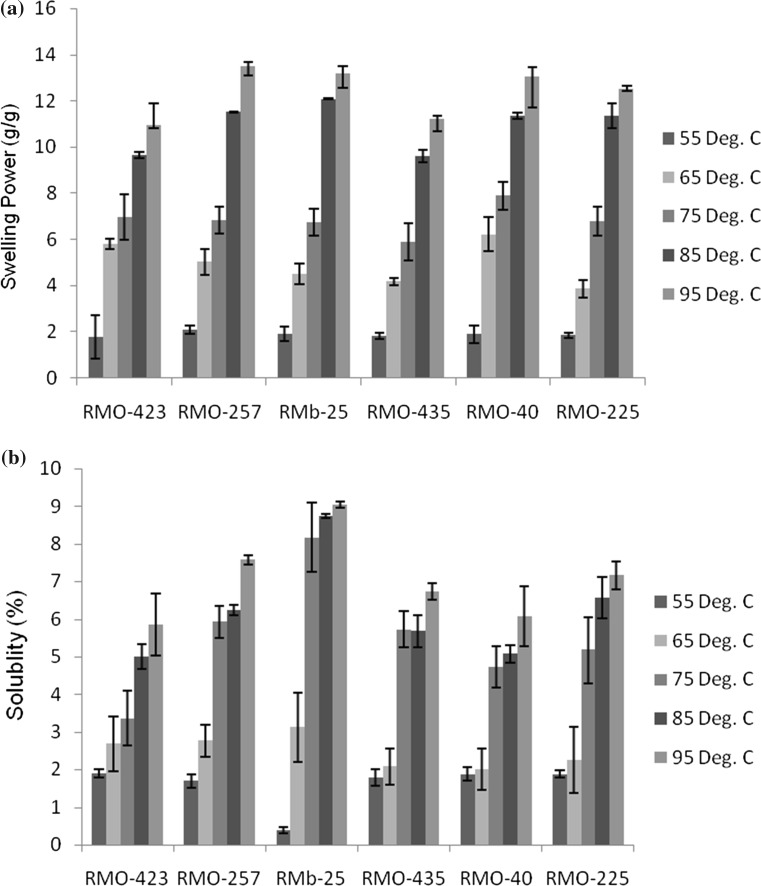
During heating of starch in water crystalline structure is destroyed and hydroxyl groups of amylose and amylopectin form hydrogen bonds with water molecules which is termed swelling (Simsek et al. 2009). Swelling power indicates both inter and intragranular swelling. Swelling power at 95 °C varied between 11 g/g (RMO-423) and 13.5 g/g (RMO-257). Joshi et al. (2013) indicated that lentil starch has lower swelling power than corn and potato starches and attributed this to higher gelatinization temperature of lentil starch. It was observed that swelling power of all starches increased with temperature (Fig. 1a). The increase in swelling power with temperature was more rapid after 75 °C. This can be attributed to increase in interactions between starch chains. Wang et al. (2014) also observed a rapid increase of swelling power of pea and lentil starches in the range of 65–95 °C. These authors reported that the rapid increase in swelling power of starches in the stated temperature range was due to the melting of starch crystallites. Solubility of starches also showed increase with temperature (Fig. 1b). Both swelling and solubility are affected by factors like amylose content, viscosity patterns, the molecular weight of amylopectin, fine structure amylose leaching, granule organization and interaction among glucan chains (Tester and Morrison 1990; Lin et al. 2012; Simsek et al. 2009).
Fig. 1.
a Swelling power of starches isolated from six different moth bean cultivars. b Solubility of of starches separated from six different moth bean cultivars
Morphological properties
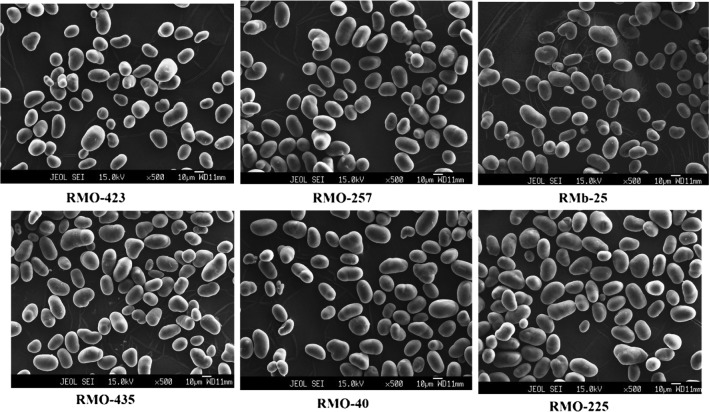
Size and shape of starch granules have their importance in adsorption, reactions and texture imparting properties. Moth bean starch granules were irregular, elongated, polyhedral as well as lobed shape without any fissures (Fig. 2). The starch granules were found to be of two types small sized and large sized. Pores or pin holes were however absent in the scanning electron micrographs. Spherical starch granules have been also reported in black bean (Hoover et al. 2010). Maaran et al. (2014) also showed irregular shaped sized granules of starches isolated from rice bean, navy bean, lablabbean with some roughness and indentation on surfaces. However, Singh et al. (2004) and Martínez et al. (2011) reported intact, large oval to spherical shape granules of chickpea and bean starches without any fissure. The size and shape characteristics of starches have been hypothesized to be influenced by plant genetics, climatic, agronomic conditions, the biochemistry of the chloroplast or amyloplast, membranes and the physical properties of the plastids (Badenhuizen 1969).
Fig. 2.
SEM of starches isolated from six different moth bean cultivars
X-ray diffraction
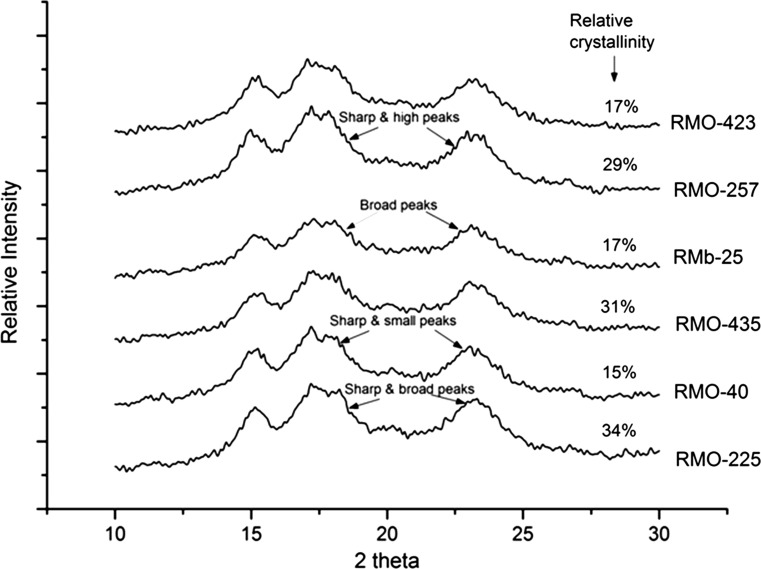
The packing of starch chains can lead to different extent of crystallinity in starches from different genotypes and X-ray diffraction of starches can give an idea about the difference. All the six moth bean starches showed a typical ‘C’ X-ray pattern (Simsek et al. 2009) with peaks at 2θ = 14.3°, 17.1° and 22.5° (Fig. 3). However, an additional peak at 2θ = 5.6 was observed by Liu et al. (2015) for pea starches which they assigned to “B’ type polymorph. Starches differed significantly in the relative crystallinity ranging from 15% (RMO-257) to 34% (RMO-225). Relative crystallinity differences among starches from six moth bean cultivars could be due to differences in amylose content, crystallite size, polymorphic content and different orientations of double helices within the crystalline domains (Ambigaipalan et al. 2011).
Fig. 3.
X-ray diffraction pattern of starches isolated from six different moth bean cultivars
FTIR
The infrared spectra of six moth bean starches are presented in Supplementary Fig. 1. The samples showed peaks above 900 cm−1 (skeletal mode vibrations of α-1,4 glycosidic linkage), between 1100 and 1500 cm−1 (the fingerprint region), between 1500–1700 cm−1 and near 3000 cm−1 (C–H stretching region) and between 3000 and 3600 cm−1 (O–H stretching region) (Joshi et al. 2013). Zhang and Han (2006) have assigned the broad band observed between 3000 and 3600 cm−1 to the vibration stretches of hydroxyl groups between starch molecules. The peak at or near 1660 cm−1 has been ascribed to the scissor vibrations of –OH from water present in the amorphous area of starch (Bernardino-Nicanor et al. 2017).
The bands appearing between 860 and 770 cm−1 have been reported to be indicative of α glycosidic linkages of starch (Capek et al. 2010; Bernardino-Nicanor et al. 2017). The peak near 1150 cm−1 was missing in three starches, namely RMb-25, RMO-423 and RMO-435 and this peak is attributed to C–O and CH2 stretching. Hence it can be said that starches differed in crystallinity range and short range order.
Thermal properties
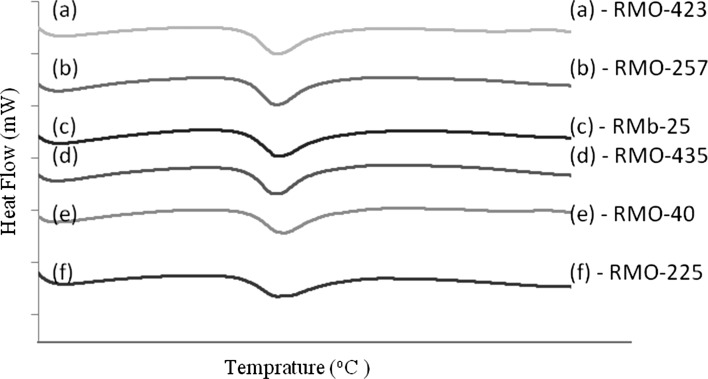
Thermal properties of starches isolated from six moth bean cultivars are shown in Fig. 4. T o, and T p of starches did not show significant variation. Differences in Tc and To may be due to the heterogeneity of the starch crystallites within the granules, however the starches might have differed in organization, order and stability of crystallites as we find significant variation in T c and ΔHgel of the starches. Gelatinization temperatures are hypothesized to be affected by the molecular architecture of the crystalline region which results due to the distribution of amylopectin short chains (DP6-11) and not by the ratio of amorphous to crystalline regions, which in turn corresponds to the amylose/amylopectin ratio (Noda et al. 2004). The difference in completion of gelatinization of six starches can be attributed to difference/delay in second stages of gelatinization which consist of transformation of rapid helix to coil transition. The first stage of gelatinization which consists of slower side by side dissociation of double helices might have continued with same pace in six starches (Waigh et al. 2000). ΔHgel of starches varied between 11.4 J/g (RMO-257) and 15.07 J/g (RMO-423). The value of ΔHgel is said to be dependent on the quality and quanity of crystallinity, degree of organization, stability and perfection of crystals, loss of molecular order (Cooke and Gidley 1992; Tester and Morrison 1990).
Fig. 4.
Gelatinization characteristics of starches separated from six different moth bean cultivars
Retrogradation of starches is an important factor in their roles as additive in different products as it is a measure of water release of the gel during storage. ΔHret of six moth bean starches were measured and ranged between 3.72 J/g (RMO-257) and 6.35 J/g (RMO-040). Differences in ΔHret can be attributed to the differential strength of lateral association of double helices involving amylopectin chains during storage of gel (Hoover et al. 1994). Percentage retro gradation of moth bean starches ranged between 24.7% (RMO-025) and 43.4% (RM0-225). Collado and Corke (1999) have observed that rate of association of amylose chains during retrogradation may depend on highly swollen granules between adjacent amylose chains. Slow/fast rate of retrogradation in bean starches have been hypothesized to result from slow/fast association of B1 amylopectin chains because of their higher mobility (Martínez et al. 2011). Further the alignment of starch chains during storage and the stability of reformed crystals also affect the retrogradation.
Pasting properties
Table 2 shows the pasting characteristics of six moth bean starches evaluated using RVA. PT of six starches varied between 75.8 °C (RMO-225) and 77.3 °C (RMO-435). Sharma et al. (2015) reported PT of starches separated from seventeen kidney bean starches between 75.4 and 83 °C. The amylose content in their study was also higher and ranged between 17.4 and 40.7% for kidney bean starches. Pasting temperature is the minimum temperature at which viscosity starts increasing with temperature and can be hypothesized to be proportional to resistance towards swelling. PV, FV and BD of starches varied between 4580–5087 cP, 3702–4182 cp, and 2002–2564 cP respectively. Liu et al. (2015) reported lower values of PV (2119–2805 cP) for starches isolated from four field pea cultivars. The pasting properties are affected by various factors like granule swelling, friction between swollen granules, starch chain length, granule size distribution interaction between starch chains within the native granule, amylose leaching and crystallinity (Lin et al. 2012). SB of starches varied between 1140 and 1339 cP. The set-back (SB) viscosity is an indirect measure of short time retrogradation and can be related to the granule rigidity, amylose content, branched chain fraction of amylose, amount and molecular weight of leached amylose, interaction between leached amylose chains, presence of unfragmented swollen granules embedded in the leached amylose network, reassociation of leached amylose (Ambigaipalan et al. 2011; Lin et al. 2012; Wang et al. 2014).
Table 2.
Pasting characteristics of starches isolated from six bean cultivars
| Sample | Pasting temperature (°C) | Peak viscosity (cP) | Hold viscosity (cP) | Final viscosity (cP) | Break down (cP) | Setback (cP) |
|---|---|---|---|---|---|---|
| RMO-423 | 76.00a ± 0.00 | 5087 cd ± 046 | 2662a ± 63 | 3835b ± 22 | 2425bc ± 110 | 1173a ± 41 |
| RMO-257 | 77.00b ± 0.28 | 5164d ± 111 | 2600a ± 00 | 3799a ± 09 | 2564c ± 111 | 1199a ± 09 |
| RMb-25 | 76.05a ± 0.07 | 4707b ± 099 | 2562a ± 89 | 3702a ± 14 | 2145a ± 009 | 1140a ± 74 |
| RMO-435 | 77.25b ± 0.07 | 4877b ± 019 | 2830b ± 25 | 4182c ± 38 | 2003a ± 056 | 1339b ± 45 |
| RMO-40 | 76.10a ± 0.00 | 4580a ± 099 | 2578a ± 06 | 3842b ± 58 | 2002a ± 106 | 1264ab ± 52 |
| RMO-225 | 75.80a ± 0.28 | 4917c ± 048 | 2608a ± 07 | 3815b ± 65 | 2309b ± 041 | 1207ab ± 72 |
Starch digestibility
The RS content of moth bean starches is reported in Table 2 and varied between 57.3% (RMO-040) and 75.8% (RMO-0257). Sandhu and Lim (2008) have reported resistant starch content of 50.3 and 65.2% of mung bean and lentil starches of Indian origin. The difference of in vitro starch digestibility can be said to be affected by factors like genetics, processing of plant source, amylose/amylopectin ratio, degree of crystallinity, branched structure and molecular weight of amylopectin, type of crystalline polymorph, granule diameter, interactions between starch chains (Chung et al. 2006; Liu et al. 2015).
Conclusions
Starches isolated from moth bean cultivars showed significant variations in physicochemical, crystalline and retro gradation properties. The starches when compared with other pulse starches of Indian and international origin showed wide differences in terms of gelatinization behavior and amylose content. Moth bean starches differed in relative crystallinity, which might have led to their different retrogradation behavior The starches showed high viscosity, lower swelling power and can be utilized in food products requiring high thermal stability.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank The Project In Charge, AINRP on Arid Legumes, ARS, Bikaner for providing the moth bean seed samples for the present research. Research Facilities provided by Central Instrumentation Facility of MNNIT are also acknowledged.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2930-z) contains supplementary material, which is available to authorized users.
References
- Ambigaipalan P, Hoover H, Donner E, Liu Q, Jaiswal S, Chibbar R. Structure of faba bean, black bean and pinto bean at different levels of granule organization and their physicochemical properties. Food Res Int. 2011;44:2962–2974. doi: 10.1016/j.foodres.2011.07.006. [DOI] [Google Scholar]
- Badenhuizen NP. The biogenesis of starch granules in higher plants. New York: Appleton Crofts; 1969. [Google Scholar]
- Bao J, Sun M, Corke H. Analysis of genotypic diversity in starch thermal and retrogradation properties in nonwaxy rice. Carbohydr Polym. 2007;67:174–181. doi: 10.1016/j.carbpol.2006.05.011. [DOI] [Google Scholar]
- Bernardino-Nicanor A, Acosta-García G, Güemes-Vera N, Montañez-Soto JL, de los Ángeles Vivar-Vera M, González-Cruz L. Fourier transform infrared and Raman spectroscopic study of the effect of the thermal treatment and extraction methods on the characteristics of ayocote bean starches. J Food Sci Technol. 2017;54(4):933–943. doi: 10.1007/s13197-016-2370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogracheva TY, Morris VJ, Ring SG, Hedley CL. The granular structure of C-type pea starch and its role in gelatinization. Biopolymers. 1998;45:323–332. doi: 10.1002/(SICI)1097-0282(19980405)45:4<323::AID-BIP6>3.0.CO;2-N. [DOI] [Google Scholar]
- Capek P, Drábik M, Turjan J (2010) Characterization of starch and its mono and hybrid derivatives by thermal analysis and FT-IR spectroscopy. J Therm Anal Calorim 99:667–673
- Chung H-J, Lim HS, Lim S-T. Effect of partial gelatinization and retrogradation on the enzymatic digestion of waxy rice starch. J Cereal Sci. 2006;43:353–359. doi: 10.1016/j.jcs.2005.12.001. [DOI] [Google Scholar]
- Collado LS, Corke H. Heat-moisture treatment effects on sweet potato starches differing in amylose content. Food Chem. 1999;65:339–346. doi: 10.1016/S0308-8146(98)00228-3. [DOI] [Google Scholar]
- Cooke D, Gidley MJ. Loss of crystalline and molecular order during starch gelatinization: origin of enthalpic transition. Carbohydr Res. 1992;227:103–112. doi: 10.1016/0008-6215(92)85063-6. [DOI] [Google Scholar]
- Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr. 1992;46:S33–S50. [PubMed] [Google Scholar]
- FAO – Food and Agriculture Organization of United Nations (2010) FAOSTAT Statistics database-agriculture. FAO, Rome, Italy
- Forsyth JL, Ring SG, Noel TR, Parker R, Cairns P, Findlay K. Characterization of starch from tubers of yam bean (Pachyrhizus ahipa) J Agric Food Chem. 2002;50:361–367. doi: 10.1021/jf0108922. [DOI] [PubMed] [Google Scholar]
- Ghavidel RA, Prakash J. The impact of germination and dehulling on nutrients, antinutrients, in vitro iron and calcium bioavailability and in vitro starch and protein digestibility of some legume seeds. LWT Food Sci Technol. 2007;40:1292–1299. doi: 10.1016/j.lwt.2006.08.002. [DOI] [Google Scholar]
- Guillon F, Champ MM. Carbohydrate fractions of legumes: uses in human nutrition and potential for health. Br J Nutr. 2002;88:293–306. doi: 10.1079/BJN2002720. [DOI] [PubMed] [Google Scholar]
- Hoover R, Vasanthan T, Senanayake NJ, Martin AM. The effects of defatting and heat-moisture treatment on retrogradation of starch gels from wheat, oat, potato and lentil. Carbohydr Res. 1994;261:13–24. doi: 10.1016/0008-6215(94)80002-2. [DOI] [Google Scholar]
- Hoover R, Hughes T, Chung HJ, Liu Q. Composition, molecular structure, properties, and modification of pulse starches: a review. Food Res Int. 2010;43:399–413. doi: 10.1016/j.foodres.2009.09.001. [DOI] [Google Scholar]
- Joshi M, Aldred P, McKnight S, Panozzo JF, Kasapis S, Adhikari R, Adhikari B. Physicochemical and functional characteristics of lentil starch. Carbohydr Polym. 2013;92(2013):1484–1496. doi: 10.1016/j.carbpol.2012.10.035. [DOI] [PubMed] [Google Scholar]
- Leach HW, McCowen LD, Schoch TJ. Structure of the starch granule. I. Swelling and solubility patterns of various starches. Cereal Chem. 1959;36:534–544. [Google Scholar]
- Lin JH, Pan CL, Hsu YH, Singh H, Chang YH. Influence of moisture content on the degradation of waxy and normal corn starches acid-treated in methanol. Food Hydrocolloid. 2012;26:370–376. doi: 10.1016/j.foodhyd.2011.02.020. [DOI] [Google Scholar]
- Liu C, Wang S, Copeland L, Wang S. Physicochemical properties and in vitro digestibility of starches from field peas grown in China. LWT Food Sci Technol. 2015;64:829–836. doi: 10.1016/j.lwt.2015.06.060. [DOI] [Google Scholar]
- Maaran S, Hoover R, Donner E, Liu Q. Composition, structure, morphology and physicochemical properties of lablab bean, navy bean, rice bean, tepary bean and velvet bean starches. Food Chem. 2014;152:491–499. doi: 10.1016/j.foodchem.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Martínez MO, Bello-Pérez LA, Whitney K, Osorio-Díaz P, Simsek S. Starch characteristics of bean (Phaseolus vulgaris L.) grown in different localities. Carbohydr Polym. 2011;85:54–64. doi: 10.1016/j.carbpol.2011.01.043. [DOI] [Google Scholar]
- Nara S, Komiya T. Studies on the relationship between water-saturated state and crystallinity by the diffraction method for moistened potato starch. Starch /Stärke. 1983;35:407–410. doi: 10.1002/star.19830351202. [DOI] [Google Scholar]
- Newport Scientific . Applications manual for the rapid visco™ analyser. Warriewood, Australia: Newport Scientific; 1998. [Google Scholar]
- Noda T, Tsuda S, Mori M, Takigawa S, Matsuura-Endo C, Saito K. The effect of harvest dates on the starch properties of various potato cultivars. Food Chem. 2004;86:119–125. doi: 10.1016/j.foodchem.2003.09.035. [DOI] [Google Scholar]
- Norton G, Bliss FA, Bressani R. Biochemical and nutritional attributes of grain legumes. In: Summerfield RJ, Roberts EH, editors. Grain legume crops. London: Collins; 1985. pp. 73–114. [Google Scholar]
- Oladebeye AO, Oshodi AA, Oladebeye AA. Comparative Studies on the pasting properties of starches of corms and cormels of two cocoyam (Colocasia esculenta) cultivars. J Chem Technnol. 2009;2:260–264. [Google Scholar]
- Sandhu KS, Lim ST. Digestibility of legume starches as influenced by their physical and structural properties. Carbohydr Polym. 2008;71:245–252. doi: 10.1016/j.carbpol.2007.05.036. [DOI] [Google Scholar]
- Sathe SK, Venkatachalam M. Fractionation and biochemical characterization of moth bean (Vigna aconitifolia L.) proteins. LWT Food Sci Technol. 2007;40:600–610. doi: 10.1016/j.lwt.2006.03.021. [DOI] [Google Scholar]
- Schoch TJ, Maywald EC. Preparation and properties of various legume starches. Cereal Chem. 1968;45:564–573. [Google Scholar]
- Sharma S, Singh N, Virdi AS, Rana JC. Himalayan kidney bean germplasm: grain-flour characteristics, structural-functional properties and in-vitro digestibility of starches. Food Res Int. 2015;77:498–505. doi: 10.1016/j.foodres.2015.08.030. [DOI] [Google Scholar]
- Simsek S, Tulbek M, Yao Y, Schatz B. Starch characteristics of dry peas (Pisum sativum L.) grown in the USA. Food Chem. 2009;115:832–838. doi: 10.1016/j.foodchem.2008.12.093. [DOI] [Google Scholar]
- Singh N. Pulses: an overview. J Food Sci Technol. 2017;54(4):853–857. doi: 10.1007/s13197-017-2537-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Sandhu KS, Kaur M. Characterization of starches separated from Indian chickpea (Cicer arietinum L.) cultivars. J Food Eng. 2004;63:441–449. doi: 10.1016/j.jfoodeng.2003.09.003. [DOI] [Google Scholar]
- Singh H, Sodhi NS, Singh N. Structure and functional properties of acid thinned sorghum starch. Int J Food Prop. 2009;12:713–725. doi: 10.1080/10942910801995614. [DOI] [Google Scholar]
- Tester RF, Morrison WR. Swelling and gelatinization of cereal starches. Cereal Chem. 1990;67:558–563. [Google Scholar]
- Waigh TA, Kato KL, Donald AM, Gidley MJ, Clarke CJ, Riekel C. Side-chain liquid crystalline model for starch. Starch/Starke. 2000;52:450–460. doi: 10.1002/1521-379X(200012)52:12<450::AID-STAR450>3.0.CO;2-5. [DOI] [Google Scholar]
- Wang N, Warkentin TD, Vandenberg B, Bing DJ. Physicochemical properties of starches from various pea and lentil varieties, and characteristics of their noodles prepared by high temperature extrusion. Food Res Int. 2014;55:119–127. doi: 10.1016/j.foodres.2013.10.043. [DOI] [Google Scholar]
- Williams PC, Kuzina FD, Hlynka I. A rapid colorimetric procedure for estimating the amylose content of starches and flours. Cereal Chem. 1970;47:411–420. [Google Scholar]
- Zhang Y, Han JH. Plasticization of pea starch films with monosaccharides and polyols. J Food Sci. 2006;37:253–261. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.