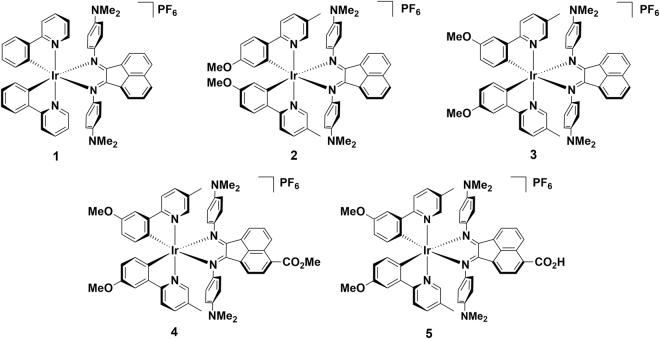
Abstract
We report the synthesis, UV-vis absorption, electrochemical characterisation, and DFT studies of five panchromatic, heteroleptic iridium complexes (four of which are new) supported by Ar-BIAN ligands. In particular, the synthesis of an ester-functionalised Ar-BIAN ligand was carried out by a mechanochemical milling approach, which was advantageous over conventional metal templating solution methods in terms of reaction time and product purity. The introduction of ester and carboxylate functionalities at the bay region of the acenaphthene motif increases each ligand’s π-accepting capacity and imparts grafting capabilities to the iridium complexes. These complexes have absorption profiles that surpass the renowned N3 dye [Ru(dcbpy)2(NCS)2] (dcbpy = 4,4′-dicarboxy-2,2′-bipyridine), making them of interest for solar-energy-harvesting applications.
Introduction
There is growing recognition of the need for alternative sources of energy to reduce our reliance on fossil fuels and overcome the challenges of global climate change and environmental pollution. Solar energy is one of the sustainable alternatives, and several promising approaches to use sunlight include the generation of electricity with photovoltaics, the production of solar fuels and chemicals by artificial photosynthesis, and the storage of solar thermal energy1. One of the established technologies that can be exploited for both photovoltaics and artificial photosynthesis is the dye-sensitised solar cell (DSSC)2, which has lately been adapted for the production of solar chemicals in a dye-sensitised photoelectrosynthesis cell (DSPEC)3–6. The workhorse behind both the DSSCs and the DSPECs are the photosensitisers that absorb UV to NIR radiation2–6, but notably, the dyes predominantly comprise heteroleptic ruthenium complexes (e.g. the celebrated N3 dye) or complicated porphyrin systems7,8. On the contrary, iridium-based photosensitisers have received greater attention in the nascent field of photoredox catalysis for organic syntheses9–13. Iridium complexes have potential benefits of higher thermal and chemical stability, similar quantum yields, and longer-lived excited states in comparison to ruthenium compounds14, and iridium systems have been successfully deployed in numerous light harvesting and emitting applications15–19. Despite this, there has been a dearth of reports on iridium-based photosensitisers in DSSCs or DSPECs14.
Our team has sought to expand the library of potential candidates for light harvesting by developing iridium and other metal complexes composed of bis(arylimino)acenaphthene (Ar-BIAN) ligands that are highly modular and can be readily synthesised from commercially available reagents20–24. The Ar-BIANs are known to be versatile π-acceptors and have been employed for hydroamination and hydrogenation catalysis with iridium complexes25–27. We surmised that the Ar-BIAN ligand can be functionalised with an electron-withdrawing anchoring functionality such as a carboxylate, with the dual function of facilitating grafting on the surface of metal oxides, and also enhancing the absorption profile. Herein, we describe the synthesis of the first Ar-BIAN ligand possessing methyl carboxylate and carboxylic acid substituents in the bay region of the acenaphthene motif via a mechanochemical milling approach, in which reactions are induced by mechanical energy through ball milling and grinding28.
Although solution-based methods have traditionally been utilised for chemical synthesis by default, there has been increasing interest in recent years towards mechanochemical synthesis, partly because it has been shown to reduce reaction times and can sometimes provide almost quantitative yields29. A specific mechanochemical technique is ball milling, in which reaction vessels are oscillated from side-to-side30. This motion creates impact between the ball-bearing inside the reaction vessel, the chemical contents, and the walls of the vessel, providing energy input to drive chemical reactions. The speed and milling time, together with the size of the ball bearing in the reaction vessel are adjustable and can be systematically varied30. Mechanochemical synthesis through ball milling is a more eco-friendly, essentially solvent-free approach that has been exceptionally effective to access metastable compounds and materials that may react with coordinating solvents31. We present optical absorption spectroscopy, electrochemical measurements, and density functional theory (DFT) calculations of a series of five complexes (Fig. 1) to highlight how we have achieved panchromatic light absorption with modest effects on the electrochemical potentials, indicating that the carboxylate on the Ar-BIAN ligand can potentially be employed in DSSCs and DSPECs.
Figure 1.
Complexes investigated in this study.
Results and Discussion
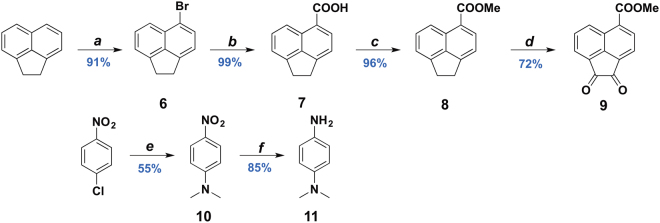
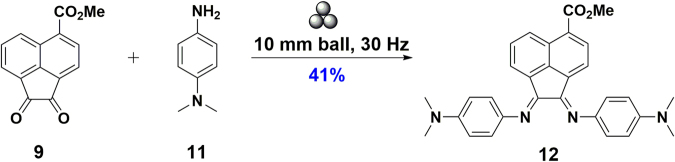
The synthesis of 5-carboxymethylacenaphthoquinone, 9, and N,N-dimethyl-4-phenylenediamine, 11, is outlined in Fig. 2. Although compounds 6, 10, and 11 are commercially available, we elected to synthesise them from much more affordable precursors. Acenaphthene was regioselectively monobrominated at the 5-position using N-bromosuccinimide in acetonitrile (MeCN)32. The vital carboxymethyl group was installed in two steps. Lithiation of 6 and quenching with dry ice33 yielded 7, following which 7 was esterified using thionyl chloride and methanol to afford 8 in excellent yield34. Oxidation of 8 with chromium trioxide in acetic anhydride following the procedure of Pei and co-workers35 resulted in the formation of an inseparable mixture of dione 9 and a second similarly symmetric product, putatively identified as the enediol by 1H NMR spectroscopy. Initially, we attempted to prepare ligand 12 via solution methods through a ZnCl2 templated condensation between 9 and 11 36, the latter of which was obtained in two steps from 4-chloronitrobenzene via nucleophilic aromatic substitution with DMF37, followed by reduction of the nitro group to the corresponding amine38. This templating method with ZnCl2 was deemed necessary because acid-catalysed condensation reactions do not work well with sterically hindered ketones and amines. Furthermore, Cenini et al. showed that the driving force for this double condensation is the precipitation of the metal Ar-BIAN complex. In cases where the final metal complex did not precipitate out, the product was formed in only minute amounts39. However, in the present case, attempts at removal of the Zn salts often led to hydrolysis, hindering purification of the ligand. Instead, ligand 12 was successfully obtained by mechanochemical synthesis through an acetic acid-catalysed condensation between 9 and 11 (Fig. 3). Mechanochemical synthesis through ball milling has been found to be remarkably effective for the solid-state synthesis of both organic and inorganic molecules, including an indium Ar-BIAN complex from our team, and has been especially advantageous for the facile synthesis and purification of 12 here23.
Figure 2.
Synthesis of 5-carboxymethylacenaphthoquinone, 9, and N,N-dimethyl-4-phenylenediamine, 11. (a) 1 equiv. NBS/MeCN, RT, 19 h; (b) i. 1.5 equiv. n-BuLi, −78 °C 30 min; ii. CO2(s), RT; (c). 2.5 equiv. SOCl2/MeOH, reflux, 19 h; (d) i. 7.8 equiv. CrO3/Ac2O, 110 °C; ii. conc. HCl, 0 °C; (e) 10 equiv. KOH/DMF, 155 °C, 19 h; (f) 3 equiv. NaBH4, 20 mol% Cu(acac)2/1:1 v/v i-PrOH:EtOH, 35 °C, N2, 9 h.
Figure 3.
Mechanochemical synthesis of a methyl ester-modified Ar-BIAN ligand. The symbol for mechanical milling above the arrow of the equation has been proposed by Hanusa et al.31.
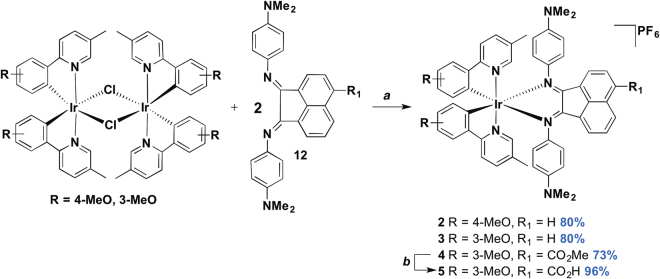
In parallel, 2-(3′-anisyl)-5-methylpyridine (3MeO-5Meppy) and 2-(4′-anisyl)-5-methylpyridine (4MeO-5Meppy) were obtained in 47 and 95% yield, respectively, through the Suzuki coupling of 2-bromo-5-methylpyridine and the corresponding arylboronic acids36,40. The corresponding chloro-bridged iridium(III) dimers were then isolated in 68 and 71% yield, respectively, following the procedure of Watts and co-workers41. We had previously reported 1 20, a reference compound for our current manuscript, and we adopted a similar protocol to synthesise mononuclear complexes 2–4. The new Ir complexes were prepared in good yields through cleavage of the corresponding iridium dimer, [Ir(C^N)2(μ-Cl)]2, with the corresponding Ar-BIAN ligands (Fig. 4). Saponification of the methyl ester in 4 afforded complex 5 almost quantitatively. The identity and purity of 2–5 were confirmed by 1H and 13C NMR spectroscopy, melting points, and high-resolution mass spectrometric (HRMS) analyses.
Figure 4.
Synthesis of iridium(III) complexes 2–5. (a) i. DCM, 50 °C, 19 h; ii. NH4PF6 (aq); (b) 2.7 equiv. NaOH/1:1 MeCN:H2O v/v, 85 °C, 22 h.
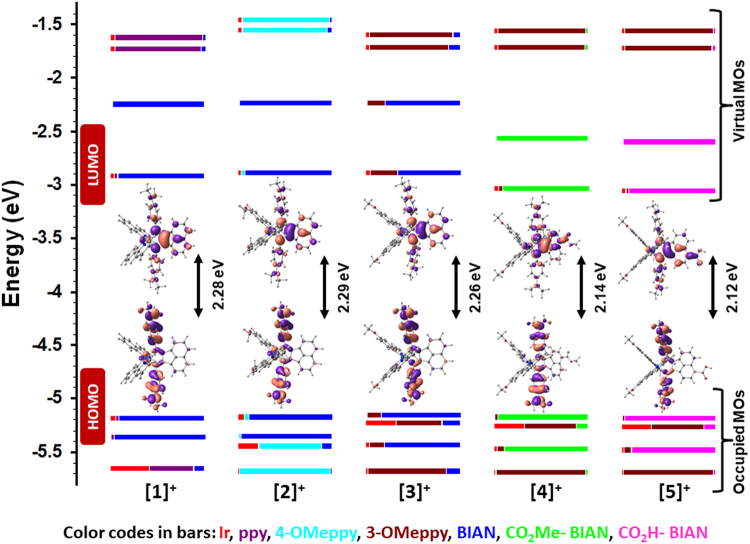
To assess the light absorption behavior among our iridium(III) complexes, the UV-vis-NIR absorption spectra were collected in acetonitrile (MeCN) solution and are illustrated in Fig. 5. Some of the significant absorption band maxima along with their corresponding molar extinction coefficients are collected in Table 1. Overlays of experimentally observed and theoretically predicted absorption data by TD-DFT are shown in Supplementary Figs S23–S27. In all of these complexes, the HOMO resides on the N,N-dimethylaniline part of the Ar-BIAN ligand and the LUMO resides on the acenaphthylene-1,2-diimine core of the Ar-BIAN ligand (Fig. 6). The intense bands in the UV region at ~225 nm are assigned to be spin-allowed ligand-centered (1LC) 1(π-π*) transitions37. For 1, the band at 258 nm is an admixture of 1LC, (1(πAr-BIAN-πAr-BIAN*)) and singlet ligand-ligand charge transfer (1LLCT) (Ar-BIAN(π) to ppy(π*)) transitions (Supplementary Table S1). For complexes 2–5, the bands at ~260 nm consist primarily of 1LLCT (Ar-BIAN(π)to ppy(π*) for 2) or 1LC (1(πppy-πppy*) for 3–5), with minor contributions from singlet metal-to-ligand charge-transfer (1MLCT) (Ir(dπ) to ppy(π*) for 2–5) (Supplementary Tables S1–S5). Similarly, the bands at ~310 nm of 1–5 also possess varying contributions of 1LC, 1LLCT, and 1MLCT transitions (Supplementary Tables S1–S5). Despite the structural variation on both sets of ligands in 1–5, the energies of these higher-energy absorption bands differ very little across the series.
Figure 5.
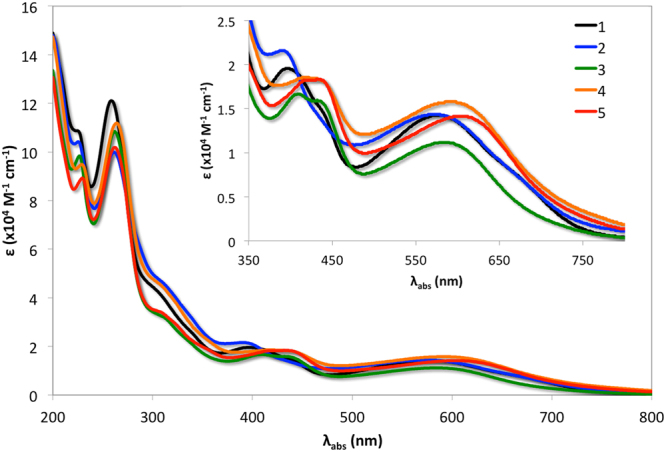
Electronic absorption spectra of 1–5 recorded in MeCN at 298 K. Inset: Expanded UV-vis-NIR absorption spectra from 350–800 nm.
Table 1.
Spectroscopic data for 1–5.
| Complex | λabs / nm (ε / 104 M−1 cm−1)a |
|---|---|
| 1 | 224 (10.9); 258 (12.1); 310 (sh,b 4.02); 396 (1.95); 576 (1.42); 675 (sh,b 0.71) |
| 2 | 225 (10.4); 261 (9.99); 310 (sh,b 4.62); 390 (2.15); 570 (1.43); 665 (sh,b 0.78) |
| 3 | 227 (9.82); 262 (10.8); 312 (sh,b 3.18); 409 (1.66); 432 (1.59); 585 (1.11) |
| 4 | 229 (9.48); 264 (11.2); 309 (sh,b 4.48); 416 (1.85); 590 (1.57) |
| 5 | 229 (8.91); 262 (10.2); 310 (sh,b 3.37); 427 (1.82); 604 (1.41) |
aAbsorption spectra recorded in aerated MeCN at 298 K. Absorbance values were collected over a concentration range of 8.78 × 10−2 to 3.51 × 101 µM, and the molar extinction coefficients (ε) were determined by assuming the complexes obeyed the Beer-Lambert law. bShoulder.
Figure 6.
Calculated frontier Kohn-Sham MOs of 1–5. DFT calculations were performed with the B3LYP/SBKJC-VDZ basis set for Ir(III) and 6–31 G** for C, H, N, and O, using a CPCM (MeCN) solvent model. The orbitals are isocontoured at 0.02.
Inspection of the lower energy region of the spectrum reveals a more notable structure-property relationship. The absorption bands in 1 and 2 between 390–396 and 570–576 nm are shifted bathochromically in 3–5 to 409–427 nm and 585–604 nm, respectively. For 1–3, the set of bands at 390–409 nm are composed of an admixture of 1LLCT (ppy(π) to Ar-BIAN(π*)) and 1MLCT (Ir((dπ)) to Ar-BIAN(π*)) transitions, whereas bands in 4 and 5 at 416 nm and 427 nm, respectively, are described primarily by 1LC (ppy(π) to ppy(π*)) transitions with minor contributions from 1MLCT (Ir((dπ)) to ppy(π*)) transitions (Supplementary Tables S1–S5). The magnitude of the red-shift for the absorption bands around 590 nm increases from 3 to 5, while the bands centered around 400 nm are less sensitive to the functionalisation in the bay region of the acenaphthene core. The bands at 570–576 nm in 1 and 2 are principally 1LLCT (ppy(π) to Ar-BIAN (π*)) in nature with minor contribution from 1MLCT (Ir(dπ) to Ar-BIAN(π*)) (Supplementary Tables S1 and S2). For 3, the band at 585 nm is an admixture of transitions, primarily consisting of 1LC (Ar-BIAN/ppy(π) to Ar-BIAN/ppy(π*)) with minor 1LLCT (Ar-BIAN(π) to ppy(π*)) contributions (Supplementary Table S3). On the other hand, notably, the bands at 590 nm and 604 nm in 4 and 5, respectively, are composed exclusively of intra-ligand charge-transfer transitions (1ILCT) within the Ar-BIAN moiety (Supplementary Tables S4 and S5). Moreover, as the conjugative framework of the ancillary ligand is expanded with the incorporation of the carboxy moiety in 4 and 5, the molar extinction coefficients of the charge-transfer (CT) bands also increase.
The absorption bands at λabs > 600 nm in 1 and 2 are principally 1ILCT and 1LLCT in character, respectively (Ar-BIAN(π) to Ar-BIAN(π*) for 1 and ppy(π) to Ar-BIAN(π*) for 2). Although no distinct bands at λabs > 600 nm could be observed for 3–5, spin-forbidden 3CT transitions at 752–783 nm with very low molar absorptivity are predicted by TD-DFT. Quantification of the light-harvesting capacities of 1–5 and comparison to that of the benchmark dye N3, [Ru(dcbpy)2(NCS)2] consisted of an analysis of the integrated product of their absorption spectra with the AM 1.5 solar irradiance spectrum over the range of 400–800 nm (dcbpy = 4,4′-dicarboxy-2,2′-bipyridine). Complexes 1, 3, and 5 absorb 1.56, 1.47, and 2.19 times more light over this spectral range compared to N3. The light harvesting capacity of 5 is in fact even larger since its absorption profile extends beyond 800 nm.
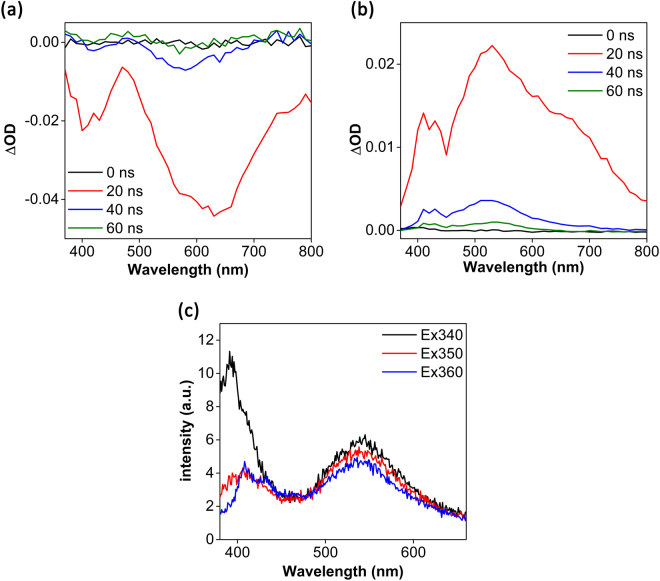
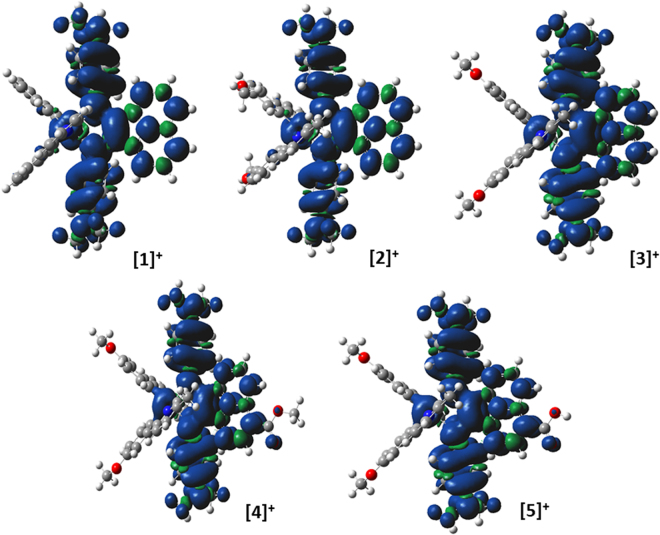
In order to examine the time-resolved photophysical properties of the Ir complexes, steady-state photoluminescence (PL) spectra were first recorded for 4 (in DCM) and 5 (in MeCN since it is insoluble in DCM). For compound 4, weak emission was observed at 540 and 410 nm when the sample was excited at 420 and 340 nm, respectively (Fig. 7). A similar emission profile was also observed when 5 was irradiated at the same wavelengths (Supplementary Fig. S28). We propose that the two emission bands may arise from mixed MLCT/ILCT transitions due to the two different ligand motifs. Both the C^N and Ar-BIAN ligands are π-acceptors, although the π* orbitals of the Ar-BIAN ligand are lower in energy due to more extensive conjugation. Thus, we expect that there could be MLCT/ILCT/LLCT excited states arising from promotion of the Ir d electrons to the two distinct ligand π* orbitals, resulting in radiative recombination from the Ar-BIAN ligand back to Ir, between the C^N and Ar- BIAN ligands, and within the Ar-BIAN ligand at a lower energy. The spin density distribution obtained from unrestricted DFT calculations are predominantly distributed within the Ar-BIAN ligand, with minor contributions from the Ir center (Fig. 8). The overall photoluminescence quantum yield, ϕPL, for 4 was estimated to be 0.03% in DCM, using the comparative method described by Williams et al.42.
Figure 7.
Transient (a) absorption and (b) emission spectra of 0.050 mM solutions of 4 collected in DCM at 298 K. (c) Steady-state photoluminescence spectra of a 0.050 mM solution of 4 collected in DCM at 298 K.
Figure 8.
Triplet spin density distributions of complexes 1–5, obtained from DFT calculations with the UB3LYP/SBKJC-VDZ basis set for Ir(III) and 6–31 G** for C, H, N, and O, using a CPCM (MeCN) solvent model. The contours are isovalued at 0.004.
To obtain additional insights into the excited state characteristics of these Ir complexes, nanosecond transient absorption and emission spectroscopic measurements were conducted (Fig. 7). In each time resolved optical spectroscopic experiment, the sample was probed by a broadband xenon lamp beam before and after 5–8 ns pulses. In the transient absorption spectra, the detected intensity of the transmitted signal is presented as ∆OD, which is the logarithm of the ratio of the light intensity from the probe beam after laser excitation to the intensity before laser excitation. Hence, a positive ∆OD refers to increased absorption while a negative ∆OD refers to reduced absorption/emission of the excited state relative to the ground state. The transient emission spectra show two broad emission bands with maxima at 410 nm and 530 nm when irradiated by a 355 nm laser pulse, matching the spectral profile derived from the steady-state PL experiments. On the other hand, the transient absorption spectra reveal two bands with negative ∆OD peaked at 410 and 630 nm, which results from a superposition of both the excited state emission and the ground state bleach, since a fraction of the molecules has been promoted to the excited state by the laser pulse. We attempted to estimate the excited [Ir]+* lifetime at 520 nm (and other wavelengths), but the lifetime turned out to be shorter than the time-resolution of our instrument (Supplementary Fig. S29). Nevertheless, the steady-state and time-resolved spectroscopic studies confirmed that both 4 and 5 exhibit weak PL with time-scales consistent with fluorescence. There is overlap between the PL and the absorption spectra for both 4 and 5, concurring with our previous assignment that the spin-allowed fluorescence arises from the 1ILCT/1MLCT/1LLCT absorptions at higher energy, whereas the lowest energy absorption bands are due to spin-forbidden CT transitions (λabs > 600 nm). As reported by Tkachenko et al., the MLCT excited state for complexes with Ar-BIAN ligands decays to an intra-molecular Ar-BIAN triplet state, which typically decays back to the ground state on the order of picoseconds43. This is likely due to the substantial spatial overlap of the HOMO and LUMO at the bis(arylimine) part of the ligand, which facilitates ultrafast recombination. Presumably, the π-accepting orbitals become more localised on the acenaphthene bay region, which provides better spatial separation of the electron from Ir after mixed MLCT/ILCT/LLCT transitions, thus leading to sufficiently long-lived, radiative singlet photoexcited states. There was little solvent dependence observed for 4 (Fig. 7 and Supplementary Fig. S30), suggesting that ligand dissociation or exciplex formation with MeCN did not occur upon photoexcitation. Gratifyingly too, these observations validate our attempts to improve the excited state photophysical properties, grafting abilities, and applicability of the Ar-BIAN Ir complexes in DSSCs and DSPECs.
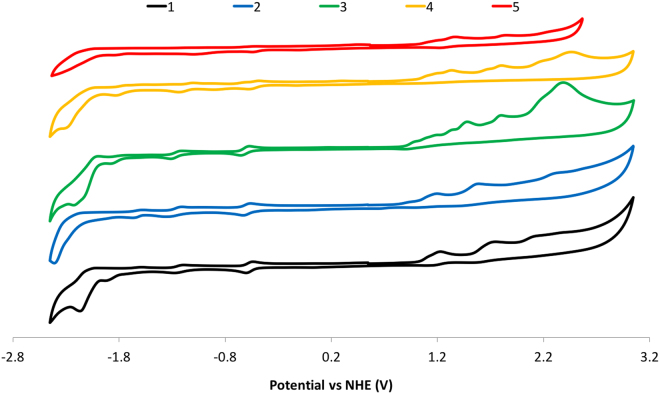
Cyclic voltammetry studies of 1–5 were conducted in order to further probe their ground state electronic behavior. The cyclic voltammograms (CVs) are shown in Fig. 9 and the observed redox couples are summarised in Table 2. The CV of 1, previously reported by us20, exhibits a reversible first reduction wave at −0.57 V versus NHE and a second quasi-reversible wave at −1.23 V, both ascribed to reduction of the Ar-BIAN ligand. The series of oxidation waves in 1 are all irreversible. We had previously assigned the first oxidation wave at 1.22 V to be localised on the N,N-dimethylaniline fragment, while the second oxidation consisted of contributions from the C^N ligands and the IrIV/IrIII redox couple. The incorporation of an electron-donating MeO group leads to a cathodic shift in both the reduction and oxidation waves20,21,24,37. The oxidation waves are more substantively affected with substitution at the 3-position resulting in a 0.13 V shift of E 1 ox to lower potential in 3 compared to a more modest 0.03 V shift in 2 with the 4MeO-5Meppy C^N ligand. The lower energies calculated for the HOMO of 2 (EHOMO = −5.18 eV) compared to that of 3 (EHOMO = −5.15 eV) are in good agreement with the higher anodic potentials measured for 2 compared to that of 3 (Table 2). Addition of the electron-withdrawing ester functionality in 4 results in an anodic shift of both the oxidation and the reduction waves. Hydrolysis of the ester to a carboxylic acid as in 5 anodically shifts E 1 ox further to 1.33 V, but does not dramatically affect E 1 red. Thus, peripheral substitution of the iridium complexes in 2–5 does not appear to alter the nature of the first oxidation and reduction processes. In comparison, the important redox processes in the N3 dye in methanol are found at 1.13 and −0.89 V, resulting in an electrochemical gap of 2.02 V, which is significantly larger than that found experimentally for 5 7.
Figure 9.
CV traces for 1–5 recorded at 298 K at 50 mV/s in MeCN solution with 0.10 M (n-Bu4N)PF6 as the supporting electrolyte.
Table 2.
Summary of electrochemical data for 1–5.
| Compound | E 1/2 /V vs NHE (ΔE p /mV)a | ΔEd/V | EHOMO e/eV | ELUMO e/eV | E 0,0 f/eV | E (S+/S*)g/V | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| E 1 ox | E 2 ox | E 1 red | E 2 red | |||||||
| 1 | 1.22b | 1.73b | −0.57 (70)c | −1.23 (60)b | 1.82 | −5.19 | −2.91 | 2.28 | 1.66 | −0.44 |
| 2 | 1.19b | 1.57b | −0.60 (65)c | −1.28 (131)b | 1.82 | −5.18 | −2.89 | 2.29 | 1.60 | −0.41 |
| 3 | 1.09b | 1.51b | −0.61 (75)c | −1.28 (65)b | 1.74 | −5.15 | −2.89 | 2.26 | 1.66 | −0.57 |
| 4 | 1.21b | 1.38b | −0.53 (81)c | −1.17 (127)b | 1.77 | −5.18 | −3.04 | 2.14 | 1.55 | −0.34 |
| 5 | 1.33b | 1.83b | −0.52 (78)b | −1.05 h | 1.89 | −5.18 | −3.06 | 2.12 | 1.55 | −0.27 |
aCV traces recorded at 298 K at 50 mV/s in MeCN solution with 0.1 M (n-Bu4N)PF6. Values are in V vs. NHE (Fc+/Fc vs. NHE = 0.63 V). The numbers in parentheses refer to ΔEp, which is the difference between the anodic and cathodic peak potentials38. A non-aqueous Ag+/Ag electrode (silver wire in a solution of 0.1 M AgNO3 in MeCN) was used as the pseudo reference electrode; a glassy-carbon electrode was used for the working electrode, and a Pt electrode was used as the counter electrode. bIrreversible. Epa reported for oxidation peak potentials and Epc for reduction peak potentials. cQuasireversible. dΔE = ΔEredox = E1 ox(pa)-E1 red(pc). Note that the E1 ox(pa) and E1 red(pc) are distinct from E1ox and E1red, respectively, in the table since the latter are averaged values for the quasi-reversible redox waves. eDFT calculations were performed with the B3LYP/SBKJC-VDZ basis set for Ir(III) and 6–31 G** for C, H, N, and O, using a CPCM (MeCN) solvent model. fE0,0 is estimated from the onset of the absorption spectrum at ca. 10% intensity. gCalculated from E(S+/S*) = E(S+/S) − E0,0. hE2 red (pc).
Conclusions
Overall, we have presented a series of five panchromatic iridium complexes (four of which are new) coordinated by Ar-BIAN ligands. The Ir complexes have absorption profiles that surpass the renowned N3 dye, and both the electrochemical measurements and DFT calculations support the existence of MLCT, LLCT, and ILCT mixed states that account for the low energy optical absorption bands. Most significantly, in contrast to traditional solution methods, mechanochemical ball milling enabled the synthesis of new Ar-BIAN ligands in which ester and carboxylate functionalities are present at the bay region of the acenaphthene motif. This increases the ligands′ π-accepting capacities and imparts grafting capabilities to our iridium complexes. Our ongoing efforts include the introduction of these iridium Ar-BIAN compounds into DSSCs and more generally, the creation of new Ar-BIAN copper and other metal complexes that can be incorporated into DSPECs to produce solar fuels and chemicals.
Methods
Compound 5-carboxy-1,2-dihydroacenaphthene, 7
To a −78 °C (60 mL) solution of 6 (3.00 g, 12.8 mmol, 1.0 equiv.) in diethyl ether, 1.6 M n-BuLi (12 mL, 19 mmol, 1.5 equiv.) was added dropwise over 30 min. The reaction mixture was stirred an additional 30 min at −78 °C. The solution was then allowed to warm to RT and stirred for an additional 1 h. The reaction was quenched with dry ice and a white precipitate formed, which was separated by vacuum filtration to obtain the desired product. The crude product was purified by recrystallisation using aqueous ethanol, and collected as an off-white solid (2.51 g). Yield: 99%. Mp: 217 °C. 1 H NMR (400 MHz, D 2 O) δ (ppm): 7.91 (d, J = 8.37 Hz, 1 H), 7.57 (d, J = 7.05 Hz, 1 H), 7.30 (t, J = 7.58 Hz, 1 H), 7.06 (t, J = 6.85 Hz, 2 H), 3.04 (s, 4 H). 13 C NMR (100 MHz, D 2 O) δ (ppm): 171.4, 149.4, 146.7, 138.9, 130.9, 129.0, 128.8, 128.4, 121.2, 119.5, 118.6, 29.9, 29.7. HRMS (EI, 70 eV): [M-H]− Calculated: (C13H9O2) 197.0597; Found: 197.0603. This compound has been twice previously reported where characterisation was limited to a melting point (Mp44: 207–211 °C and Mp45: 214–218 °C).
Compound 5-carboxymethyl-1,2-dihydroacenaphthene, 8
To a stirred solution of 7 (3.00 g, 15.1 mmol, 1.0 equiv.) in 50 mL of MeOH cooled in an ice bath was added dropwise, SOCl2 (1.8 mL, 38 mmol, 2.5 equiv.) over 30 min. The reaction mixture was then allowed to warm to RT, before it was heated to reflux for 19 h. The MeOH was evaporated under reduced pressure and H2O was then added (50 mL). The product was extracted with DCM (50 mL), and the organic phase was dried over MgSO4, filtered under vacuum, and concentrated under reduced pressure to obtain the desired product as a brownish solid (2.45 g). Yield: 96%. Mp: 74 °C. 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.63 (d, J = 8.37 Hz, 1 H), 8.29 (d, J = 7.35 Hz, 1 H), 7.59 (dd, J = 6.91, 8.56 Hz, 1 H), 7.35 (d, J = 6.87 Hz, 1 H), 7.30 (dd, J = 1.25, 7.37 Hz, 1 H) 3.99 (s, 3 H), 3.42 (s, 4 H). 13 C NMR (100 MHz, CDCl 3 ) δ (ppm): 168.1, 153.2, 146.5, 139.8, 133.3, 130.4, 130.0, 122.2, 122.1, 120.2, 118.6, 52.03, 30.7, 30.6. The characterisation matches that previously reported46,47.
Compound 5-methylcarboxylate-1,2-dioxo-1,2-dihydroace-naphthylene, 9
Compound 8 (1.63 g, 7.67 mmol. 1.0 equiv.) was dissolved in 50 mL of acetic anhydride at 110 °C. CrO3 (6.0 g, 60 mmol, 7.8 equiv.) was added carefully to the stirred solution over a period of 1 h. The resulting green suspension was stirred at 110 °C for a further 1 h and poured onto crushed ice. Concentrated HCl (10 mL) was added and the mixture was filtered. The yellow precipitate was washed with water and dried in vacuum. The crude product (R f of 0.15, DCM on silica) was purified by column chromatography with silica gel using MeOH/DCM (2.5:97.5%). A yellow flaky solid (1.31 g) was collected. Yield: 72%. Mp: 189 °C. 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 9.32 (dd, J = 8.68, 19.9 Hz, 1 H), 8.58 (m, 2 H), 8.16 (dd, J = 7.16, 29.0 Hz, 1 H), 7.95 (m, 1 H), 4.08 (s, 3 H). 13 C NMR (100 MHz, CDCl 3 ) δ (ppm): 188.2, 187.4, 166.2, 145,9, 132.9, 132.5, 132.0, 131.2, 130.3, 129.7, 129.3, 122.8, 121.0, 53.0. HR-MS (EI, 70 eV): [M + Na]+ Calculated: (C14H8O4Na) 263.0315; Found: 263.0324.
Compound (1E,2E)-methyl-1,2-bis((4-(dimethylamino)phen-yl)imino)-1,2-dihydroacenaph-thylene-5-carboxylate, 4-Me2N Ph-CO2MeBIAN, 12
A stainless steel grinder jar was dried in an oven at 120 °C overnight prior to use. The grinder jar was charged with 9 (0.072 g, 0.30 mmol), N,N-dimethyl-4-phenylenediamine (0.094 g, 0.67 mmol), acetic acid (4.3 µL, 0.075 mmol, 25 mol%), and Na2SO4 (0.043 g, 0.30 mmol), and equipped with a 10 mm stainless steel ball. The contents in the jar were then ground for 4 h at 30 Hz. The resultant purple gel was suspended in dichloromethane (DCM), filtered, and the filtrate was concentrated to dryness and rinsed with cyclohexane. The purple reside was recrystallised from diethyl ether (Et2O) and the isolated yield was 0.054 g (41%). 1 H NMR (400 MHz, CDCl 3) δ (ppm): 8.90 (d, J = 8.4 Hz, 1 H), 8.16 (d, J = 7.6 Hz, 1 H), 7.51 (t, J = 8.0 Hz, 1 H), 7.32–7.35 (m, 2 H), 7.10–7.14 (m, 4 H), 6.82–6.86 (m, 4 H), 3.99 (s, 3 H), 3.04 (s, 6 H), 3.03 (s, 6 H). 13 C{ 1 H} NMR (100 MHz, CDCl 3 ) δ (ppm): 166.9, 160.3, 159.7, 148.8, 148.5, 141.6, 141.5, 141.2, 133.3, 132.0, 130.0, 129.7, 129.3, 128.2, 127.2, 123.7, 121.9, 120.8, 120.5, 113.4, 113.1, 52.4, 41.1, 41.0. HRMS (ESI + , m/z): [M + H]+ Calculated: (C30H29N4O2) 477.2291; Found 477.2268. Anal. Calcd. for C30H28N4O2: C, 75.61; H, 5.92; N, 11.76; Found: C, 75.89; H, 5.57; N, 11.29.
General procedure for the synthesis of Ir(III) bis[(C^N)-N,C2′]-N,N’-bis(phenylmino)acenaphthene (Ar-BIAN) hexafluoro-phosphate complexes 1–4
The iridium(III) dimer [(C^N)2Ir(μ-Cl)]2 (0.080 mmol, 1.0 equiv.) and bis(arylimino)acenaphthene (Ar-BIAN) ligand (0.16 mmol, 2.0 equiv.) were solubilised in 12 mL of dry DCM. The mixture was degassed repeatedly, placed under N2, and heated to 50 °C for 19 h. Over the course of the reaction, the mixture darkened in color from the initial yellow. The solution was cooled to RT and the solvent was removed under reduced pressure. The crude solid was re-dissolved in a minimum amount of MeOH and added slowly to an aqueous solution of NH4PF6 (10 mL, 6.13 mmol, 1 g/10 mL) under gentle stirring. The first drop caused the precipitation of a dark-colored solid. The solid suspension was conserved at 0 °C for 2 h, collected on a Buchner funnel, and the resulting solid was washed with water and Et2O. The residue was dried in vacuo to obtain a dark brown/green solid. The complexes were then crystallised in dichloromethane/diisopropylether (50:50) by slow evaporation. Complexes 1–4 were obtained using this protocol.
Iridium(III)bis[2-phenyl-pyridinato-N,C2′]-N,N’-bis(4-N,N-di-methylphenylimino)acenaph-thenehexafluorophos-phate: [Ir(ppy)2(4-NMe2PhBIAN)]+(PF6)−, 1
Black crystals (0.146 g). Yield: 86%. The characterization matches that previously reported21.
Iridium (III) bis[4-methoxy-2-phenyl-5-methylpyridinato-N,C2′]-N,N’-bis(4-N,N-dimethyl-phenylimino)acenaphth-ene hexafluorophosphate: [Ir(4-MeO-5-Me-ppy)2(4-NMe2Ph-BI-AN)]+(PF6)−, 2: Black crystals (0.073 g). Yield: 80%. Mp: 337–339 °C (turned into dark liquid). 1 H NMR (500 MHz, CD 3 CN) δ (ppm): 8.39–8.34 (m, 2 H), 8.21 (d, J = 8.2 Hz, 2 H), 7.68 (d, J = 1.5 Hz, 2 H), 7.65 (s, 2 H), 7.57–7.53 (m, 2 H), 7.44 (d, J = 7.3 Hz, 2 H), 7.32 (d, J = 8.6 Hz, 2 H), 6.36 (dd, J = 8.6, 2.6 Hz, 10 H), 5.45 (s, 2 H), 3.50 (s, 6 H), 2.89 (s, 12 H), 2.28 (s, 6 H). 13 C NMR (125 MHz, CDCl 3 ) δ (ppm): 169.74, 165.16, 160.15, 150.75, 150.45, 148.60, 144.51, 143.45, 139.26, 136.83, 134.17, 132.07, 131.81, 130.68, 128.89, 127.70, 125.37, 123.54, 118.36, 117.32, 111.34, 107.02, 54.96, 40.51, 18.62. HRMS (EI, 70 eV): [M-PF6]+ Calculated: (C54H50IrN6O2) 1007.3623; Found: 1007.3646.
Iridium (III) bis[3-methoxy-2-phenyl-5-methylpyridinato-N,C2′]-N,N’-bis(4-N,N-dimethyl-phenylimino)acenaphth-ene hexafluorophosphate: [Ir(3-MeO-5-Me-ppy)2(4-NMe2Ph-BIAN)]+(PF6)−, 3
Black crystals (0.116 g). Yield: 80%. Mp: 354 °C (turned into dark liquid). 1 H NMR (500 MHz, CD 3 CN) δ (ppm): 8.41 (d, J = 1.7 Hz, 2 H), 8.21 (d, J = 8.2 Hz, 2 H), 7.78–7.67 (m, 5 H), 7.55 (t, J = 7.8 Hz, 2 H), 7.47 (d, J = 7.3 Hz, 2 H), 6.95 (d, J = 2.7 Hz, 2 H), 6.43–6.27 (m, 8 H), 5.90 (d, J = 8.3 Hz, 2 H), 5.45 (s, 1 H), 3.66 (s, 6 H), 2.89 (s, 12 H), 2.30 (s, 6 H). 13 C NMR (125 MHz, CDCl 3 ) δ (ppm): 169.79, 165.25, 156.29, 150.33, 149.15, 144.46, 143.53, 139.30, 138.46, 134.04, 133.52, 131.82, 131.73, 130.58, 128.87, 127.81, 123.46, 119.11, 116.78, 111.33, 109.21, 55.63, 40.47, 18.67. HRMS (EI, 70 eV): [M-PF6]+ Calculated: (C54H50IrN6O2) 1007.3623; Found: 1007.3625.
Iridium (III) methyl-bis[3-methoxy-2-phenyl-5-methyl-pyridinato-N,C2′]-N,N’-bis(4-N,N-di-methylphenyl-imino)ace-naphthene-5-carboxylate hexafluorophosphate: [Ir(3-MeO-5-Me-ppy)2(4-NMe2Ph-BIAN-CO2Me)]+(PF6)−, 4
Black crystals (0.095 g). Yield: 73%. Mp: 353–355 °C (turned into dark liquid). 1 H NMR (500 MHz, CD 3 CN) δ (ppm): 9.07 (dd, J = 8.7, 0.7 Hz, 1 H), 8.41 (dd, J = 1.9, 1.0 Hz, 2 H), 8.22 (d, J = 7.7 Hz, 1 H), 7.78–7.72 (m, 4 H), 7.67 (dd, J = 8.7, 7.4 Hz, 2 H), 7.63–7.57 (m, 2 H), 7.57–7.52 (m, 1 H), 6.98 (d, J = 2.6 Hz, 2 H), 6.52–6.05 (m, 8 H), 5.92 (dd, J = 9.8, 8.3 Hz, 2 H), 3.99 (s, 3 H), 3.69 (d, J = 2.4 Hz, 6 H), 2.93 (d, J = 5.7 Hz, 12 H), 2.33 (s, 6 H). 13 C NMR (125 MHz, CDCl 3 ) δ (ppm): 169.25, 168.03, 166.52, 165.14, 156.40, 150.70, 150.40, 149.10, 149.03, 144.37, 143.58, 143.51, 139.46, 139.40, 138.09, 133.94, 133.64, 132.93, 131.93, 131.75, 131.73, 130.43, 130.35, 130.11, 128.50, 128.35, 123.58, 121.78, 119.17, 116.85, 116.79, 111.28, 111.20, 109.19, 55.63, 52.84, 40.45, 40.42, 18.65. HRMS (EI, 70 eV): [M-PF6]+ Calculated: (C56H52IrN6O4) 1065.3678; Found: 1065.3668.
Synthesis of Ir(III) bis[3-methoxy-2-phenyl-5-methylpyridinato-N,C2′]-N,N′-bis(4-N,N-dimethylphenyl-imino)acenaphthene-5-carboxylate hexafluorophosphate, 5: Complex [Ir(3-MeO-5-Me-ppy)2(4-NMe2Ph-BIAN-CO2Me)]+ (PF6)− (4) (0.050 g, 0.040 mmol, 1.0 equiv.) and NaOH (0.0050 g, 0.12 mmol, 2.7 equiv.) were dissolved with 6 mL H2O and 6 mL MeCN. The mixture was degassed three times, placed under N2, and heated to 85 °C for 22 h. The solution was cooled to RT and quenched with 1.2 mL of 0.10 N aqueous HCl. After careful removal of the solvent, the product was extracted with DCM (3 × 15 mL), evaporated to dryness, and the crude solid was re-dissolved in a minimum amount of MeOH. To this solution was slowly added a solution of NH4PF6 (10 mL, 6.13 mmol, 1 g/10 mL) under gentle stirring. Upon the first drop addition of aqueous NH4PF6 solution, the appearance of a greenish black precipitate was observed and continued until the addition was complete. Then, the greenish black solid suspension was conserved for 3 h at 0 °C and collected by a Buchner funnel filtration following washing with water and Et2O. The resulting residue was dried in vacuo to obtain a greenish dark solid powder. The crude product was recrystallised in dichloromethane/diisopropylether (50:50) by slow evaporation to obtain greenish black crystals. Greenish black crystals (0.047 g). Yield: 96%. Mp: 359–360 °C (turned into dark liquid). 1 H NMR (400 MHz, CD 3 CN) δ (ppm): 9.12 (d, J = 8.6 Hz, 1 H), 8.42 (bs, 2 H), 8.25 (d, J = 7.7 Hz, 1 H), 7.76 (d, J = 2.1 Hz, 5 H), 7.65 (m, 2 H), 7.60–7.52 (m, 2 H), 6.98 (d, J = 2.6 Hz, 2 H), 6.47–6.24 (m, 8 H), 5.97–5.88 (m, 2 H), 3.69 (d, J = 1.7 Hz, 6 H), 2.94 (s, 12 H), 2.33 (s, 6 H). 13 C NMR (125 MHz, CD 3 CN) δ (ppm): 170.44, 169.38, 166.52, 164.41, 156.27, 150.49, 150.29, 144.72, 144.10, 144.06, 139.16, 137.92, 137.88, 134.23, 134.19, 132.74, 132.58, 130.10, 130.02, 129.41, 128.99, 128.09, 127.04, 123.21, 121.59, 118.90, 118.89, 115.86, 115.83, 111.13, 111.11, 109.99, 108.97, 54.99, 39.61, 39.58, 17.33, 17.31. HR-MS (EI, 70 eV): [M-PF 6 ] + Calculated: (C55H50IrN6O4) 1051.3521; Found: 1051.3519.
Data availability
Supplementary Information available: General procedures, experimental details, photophysical and electrochemical characterisation protocols, 1H and 13C NMR spectra, and computational details. The data supporting this study are available at: 10.17630/c28fddad-6877-4c31-80fa-f88600b735ae.
Electronic supplementary material
Acknowledgements
E.Z-C thanks EPSRC (EP/M02105X/1) and NSERC for financial support. F.G. and H.S.S. would like to thank A*STAR AME IRG (A1783c0003) for financial support. F.G. also thanks NTU start-up grant (M4080552) and MOE Tier 1 grant (M4011441). H.S.S. is supported by a NTU start-up grant (M4081012), MOE Tier 1 grants (M4011611), and the Nanyang Assistant Professorship (M4081154). C. H. acknowledges the Région Bretagne for funding. H.S.S. also thanks the Solar Fuels Laboratory at NTU and the Singapore-Berkeley Research Initiative for Sustainable Energy (SinBeRISE) CREATE Programme.
Author Contributions
K. Hasan contributed to the synthesis of all the iridium complexes, UV-vis measurements, and cyclic voltammetry studies. J. Wang contributed to the synthesis of ester functionalised Ar-BIAN ligand, steady-state photoluminescence measurements, and time-resolved transient spectroscopic studies. A.K. Pal contributed to the synthesis of the complexes and DFT calculations for all the compounds. C. Hierlinger contributed to the synthesis of complexes. E. Zysman-Colman, F. García, and H.S. Soo planned and designed the experiments. All authors contributed to writing and editing the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-14996-4.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lewis NS. Research opportunities to advance solar energy utilisation. Science. 2016;351:353. doi: 10.1126/science.aad1920. [DOI] [PubMed] [Google Scholar]
- 2.Pashaei B, Shahroosvand H, Graetzel M, Nazeeruddin MK. Influence of ancillary ligands in dye-sensitised solar cells. Chem. Rev. 2016;116:9485–9564. doi: 10.1021/acs.chemrev.5b00621. [DOI] [PubMed] [Google Scholar]
- 3.Alibabaei L, Sherman BD, Norris MR, Brennaman MK, Meyer TJ. Visible photoelectrochemical water splitting into H2 and O2 in a dye-sensitised photoelectrosynthesis cell. Proc. Natl. Acad. Sci. USA. 2015;112:5899–5902. doi: 10.1073/pnas.1506111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farnum BH, Wee KR, Meyer TJ. Self-assembled molecular p/n junctions for applications in dye-sensitised solar energy conversion. Nat. Chem. 2016;8:845–852. doi: 10.1038/nchem.2536. [DOI] [PubMed] [Google Scholar]
- 5.Morseth ZA, et al. Ultrafast dynamics in multifunctional Ru(II)-loaded polymers for solar energy conversion. Acc. Chem. Res. 2015;48:818–827. doi: 10.1021/ar500382u. [DOI] [PubMed] [Google Scholar]
- 6.Youngblood WJ, Lee SHA, Maeda K, Mallouk TE. Visible light water splitting using dye-sensitised oxide semiconductors. Acc. Chem. Res. 2009;42:1966–1973. doi: 10.1021/ar9002398. [DOI] [PubMed] [Google Scholar]
- 7.Nazeeruddin MK, et al. Conversion of light to electricity by cis-X2bis(2,2′-bipyridyl-4,4′-dicarboxylate)ruthenium(II) charge-transfer sensitisers (X = Cl−, Br−, I−, CN−, and SCN−) on nanocrystalline titanium dioxide electrodes. J. Am. Chem. Soc. 1993;115:6382–6390. doi: 10.1021/ja00067a063. [DOI] [Google Scholar]
- 8.Yella A, et al. Porphyrin-sensitised solar cells with cobalt (II/III)-based redox electrolyte exceed 12 percent efficiency. Science. 2011;334:629–634. doi: 10.1126/science.1209688. [DOI] [PubMed] [Google Scholar]
- 9.Karkas MD, Porco JA, Stephenson CRJ. Photochemical approaches to complex chemotypes: Applications in natural product synthesis. Chem. Rev. 2016;116:9683–9747. doi: 10.1021/acs.chemrev.5b00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prier CK, Rankic DA, MacMillan DWC. Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw MH, Shurtleff VW, Terrett JA, Cuthbertson JD, MacMillan DWC. Native functionality in triple catalytic cross-coupling: sp3 C-H bonds as latent nucleophiles. Science. 2016;352:1304–1308. doi: 10.1126/science.aaf6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen JD, Matsuura BS, Stephenson CRJ. A photochemical strategy for lignin degradation at room temperature. J. Am. Chem. Soc. 2014;136:1218–1221. doi: 10.1021/ja4113462. [DOI] [PubMed] [Google Scholar]
- 13.Narayanam JMR, Stephenson CRJ. Visible light photoredox catalysis: Applications in organic synthesis. Chem. Soc. Rev. 2011;40:102–113. doi: 10.1039/B913880N. [DOI] [PubMed] [Google Scholar]
- 14.Mayo EI, et al. Cyclometalated iridium(III)-sensitised titanium dioxide solar cells. Photochem. Photobiol. Sci. 2006;5:871–873. doi: 10.1039/b608430c. [DOI] [PubMed] [Google Scholar]
- 15.Lee J, et al. Deep blue phosphorescent organic light-emitting diodes with very high brightness and efficiency. Nat. Mater. 2016;15:92–98. doi: 10.1038/nmat4446. [DOI] [PubMed] [Google Scholar]
- 16.Yersin H, Rausch AF, Czerwieniec R, Hofbeck T, Fischer T. The triplet state of organo-transition metal compounds. Triplet harvesting and singlet harvesting for efficient OLEDs. Coord. Chem. Rev. 2011;255:2622–2652. doi: 10.1016/j.ccr.2011.01.042. [DOI] [Google Scholar]
- 17.Henwood AF, Zysman-Colman E. Luminescent iridium complexes used in light-emitting electrochemical cells (LEECs) Top. Curr. Chem. 2016;374:36. doi: 10.1007/s41061-016-0036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baranoff E, et al. Cyclometallated iridium complexes as sensitisers for dye-sensitised solar cells. Chem. Asian J. 2010;5:496–499. doi: 10.1002/asia.200900429. [DOI] [PubMed] [Google Scholar]
- 19.Baranoff E, Yum JH, Graetzel M, Nazeeruddin MK. Cyclometallated iridium complexes for conversion of light into electricity and electricity into light. J. Organomet. Chem. 2009;694:2661–2670. doi: 10.1016/j.jorganchem.2009.02.033. [DOI] [Google Scholar]
- 20.Hasan K, Zysman-Colman E. Panchromic cationic iridium(III) complexes. Inorg. Chem. 2012;51:12560–12564. doi: 10.1021/ic301998t. [DOI] [PubMed] [Google Scholar]
- 21.Hasan K, Zysman-Colman E. The effect of aryl substitution on the properties of a series of highly absorptive cationic iridium(III) complexes bearing ancillary bis(arylimino)acenaphthene ligands. Eur. J. Inorg. Chem. 2013;2013:4421–4429. doi: 10.1002/ejic.201300583. [DOI] [Google Scholar]
- 22.Kee JW, et al. Development of bis(arylimino)acenaphthene (BIAN) copper complexes as visible light harvesters for potential photovoltaic applications. Inorg. Chem. Front. 2016;3:651–662. doi: 10.1039/C5QI00221D. [DOI] [Google Scholar]
- 23.Wang J, et al. A multi-step solvent-free mechanochemical route to indium(III) complexes. Dalton Trans. 2016;45:7941–7946. doi: 10.1039/C6DT00978F. [DOI] [PubMed] [Google Scholar]
- 24.O’brien C, Wong MY, Cordes DB, Slawin AMZ, Zysman-Colman E. Cationic platinum(II) complexes bearing aryl-BIAN ligands: Synthesis and structural and optoelectronic characterisation. Organometallics. 2015;34:13–22. doi: 10.1021/om5006512. [DOI] [Google Scholar]
- 25.Gray K, Page MJ, Wagler J, Messerle BA. Iridium(II) Cp* complexes for the efficient hydroamination of internal alkynes. Organometallics. 2012;31:6270–6277. doi: 10.1021/om300550k. [DOI] [Google Scholar]
- 26.Kennedy DF, Messerle BA, Smith MK. Synthesis of Cp* iridium and rhodium complexes containing bidentate sp2-n-donor ligands and counter-anions [Cp*MCl3]−. Eur. J. Inorg. Chem. 2007;2007:80–89. doi: 10.1002/ejic.200600735. [DOI] [Google Scholar]
- 27.Kennedy DF, Messerle BA, Rumble SL. Application of UV-vis spectroscopy to high throughput screening of hydroamination catalysts. New J. Chem. 2009;33:818–824. doi: 10.1039/b820357c. [DOI] [Google Scholar]
- 28.Takacs L. The historical development of mechanochemistry. Chem. Soc. Rev. 2013;42:7649–7659. doi: 10.1039/c2cs35442j. [DOI] [PubMed] [Google Scholar]
- 29.James SL, et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012;41:413–447. doi: 10.1039/C1CS15171A. [DOI] [PubMed] [Google Scholar]
- 30.Garay AL, Pichon A, James SL. Solvent-free synthesis of metal complexes. Chem. Soc. Rev. 2007;36:846–855. doi: 10.1039/b600363j. [DOI] [PubMed] [Google Scholar]
- 31.Rightmire NR, Hanusa TP. Advances in organometallic synthesis with mechanochemical methods. Dalton Trans. 2016;45:2352–2362. doi: 10.1039/C5DT03866A. [DOI] [PubMed] [Google Scholar]
- 32.Zysman-Colman E, Arias K, Siegel JS. Synthesis of arylbromides from arenes and N-bromosuccinimide (NBS) in acetonitrile - a convenient method for aromatic bromination. Can. J. Chem. 2009;87:440–447. doi: 10.1139/V08-176. [DOI] [Google Scholar]
- 33.Gilman H, Langham W, Moore FW. Some interconversion reactions of organolithium compounds. J. Am. Chem. Soc. 1940;62:2327–2335. doi: 10.1021/ja01866a020. [DOI] [Google Scholar]
- 34.Hosangadi BD, Dave RH. An efficient general method for esterification of aromatic carboxylic acids. Tetrahedron Lett. 1996;37:6375–6378. doi: 10.1016/0040-4039(96)01351-2. [DOI] [Google Scholar]
- 35.Ding L, Ying HZ, Zhou Y, Lei T, Pei J. Polycyclic imide derivatives: Synthesis and effective tuning of lowest unoccupied molecular orbital levels through molecular engineering. Org. Lett. 2010;12:5522–5525. doi: 10.1021/ol1024103. [DOI] [PubMed] [Google Scholar]
- 36.Hasan K, et al. Tuning the emission of cationic iridium (III) complexes towards the red through methoxy substitution of the cyclometalating ligand. Sci. Rep. 2015;5:12325. doi: 10.1038/srep12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasan K, Zysman-Colman E. Synthesis, UV–vis and CV properties of a structurally related series of bis(arylimino)acenaphthenes (Ar-BIANs) J. Phys. Org. Chem. 2013;26:274–279. doi: 10.1002/poc.3081. [DOI] [Google Scholar]
- 38.Pavlishchuk VV, Addison AW. Conversion constants for redox potentials measured versus different reference electrodes in acetonitrile solutions at 25 degrees C. Inorg. Chim. Acta. 2000;298:97–102. doi: 10.1016/S0020-1693(99)00407-7. [DOI] [Google Scholar]
- 39.Gasperini M, Ragaini F, Cenini S. Synthesis of Ar-BIAN ligands (Ar-BIAN = bis(aryl)acenaphthenequinonediimine) having strong electron-withdrawing substituents on the aryl rings and their relative coordination strength toward palladium(0) and -(II) complexes. Organometallics. 2002;21:2950–2957. doi: 10.1021/om020147u. [DOI] [Google Scholar]
- 40.Vandromme, L., Reissig, H. U., Groper, S. & Rabe, J. P. Practical routes to 2,6-disubstituted pyridine derivatives. Eur. J. Org. Chem., 2049–2055 (2008).
- 41.Sprouse S, King KA, Spellane PJ, Watts RJ. Photophysical effects of metal-carbon.sigma. bonds in ortho-metalated complexes of iridium(III) and rhodium(III) J. Am. Chem. Soc. 1984;106:6647–6653. doi: 10.1021/ja00334a031. [DOI] [Google Scholar]
- 42.Williams ATR, Winfield SA, Miller JN. Relative fluorescence quantum yields using a computer-controlled luminescence spectrometer. Analyst. 1983;108:1067–1071. doi: 10.1039/an9830801067. [DOI] [Google Scholar]
- 43.Papanikolaou PA, Tkachenko NV. Probing the excited state dynamics of a new family of Cu(I)-complexes with an enhanced light absorption capacity: Excitation-wavelength dependent population of states through branching. Phys. Chem. Chem. Phys. 2013;15:13128–13136. doi: 10.1039/c3cp50838b. [DOI] [PubMed] [Google Scholar]
- 44.Morrison DC. Synthesis of DL-beta-(5-acenaphthenyl)alanine1. J. Org. Chem. 1958;23:33–34. doi: 10.1021/jo01095a011. [DOI] [Google Scholar]
- 45.Richter HJ. 5-acenaphtheneacetic acid. J. Am. Chem. Soc. 1953;75:2774–2775. doi: 10.1021/ja01107a516. [DOI] [Google Scholar]
- 46.Adonin NY, Ryabinin VA, Starichenko VF. Interaction of substituted 5-bromoacenaphthenes with catalytic reductive system of NiCl2-2,2′-bipyridyl (or 1,10-phenanthroline)-Zn. Zh. Org. Khim. 1999;35:938–940. [Google Scholar]
- 47.Masahiro M, Keisuke I, Ken-ichi K, Masaaki Y. An observation on carboxylation of 4H-cyclopenta[d,e,f]phenanthrene. Bull. Chem. Soc. Jpn. 1988;61:2063–2066. doi: 10.1246/bcsj.61.2063. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supplementary Information available: General procedures, experimental details, photophysical and electrochemical characterisation protocols, 1H and 13C NMR spectra, and computational details. The data supporting this study are available at: 10.17630/c28fddad-6877-4c31-80fa-f88600b735ae.